Abstract
The COVID-19 pandemic has affected millions of patients across the globe. Multiple studies, national and international governmental data have shown important sex and gender differences in the incidence and outcomes of patients with COVID-19. These differences are not only attributed to the differences in age and comorbid conditions but likely a combination of factors, including hormonal differences, immune response, inflammatory markers and behavioral attitudes, among others. In this review, we discuss the studies addressing sex- and gender-specific differences in COVID-19 infections with a focus on the potential pathophysiological mechanisms of these differences.
Keywords: Sex, Gender, Disparities, COVID-19, Outcomes
Highlights
-
•
Sex- and gender-specific differences are found in COVID-19 infection and outcomes.
-
•
These differences are likely attributed to a combination of factors.
-
•
Factors include hormonal differences, immune response, and inflammatory markers.
-
•
Differences in attitudes and behaviors also impact infection and outcomes.
-
•
Further studies of sex-disaggregated as well as gender-specific data are needed.
1. Introduction
The novel coronavirus disease (COVID-19) pandemic has affected millions of patients in the United States (US) and worldwide [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]]. Studies have shown that older age and certain comorbidities are associated with higher infection severity and mortality in patients with COVID-19 infection, partially explaining the higher severity and case fatality of COVID-19 infections observed in men; with men being twice as likely to die from COVID-19 compared with women [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]]. However, data also show that differences in the disease severity and outcomes exist based on sex and gender, independent of age and comorbidity profiles, not only in the US but also in various countries across the globe [[4], [5], [6],[13], [14], [15], [16], [17], [18]]. The exact mechanisms of these differences are not completely understood and likely represent a complex interplay of multiple factors. In this review, we discuss these sex and gender differences, highlighting the recent studies reporting sex-specific comorbidity profiles and outcomes.
2. Risk factors
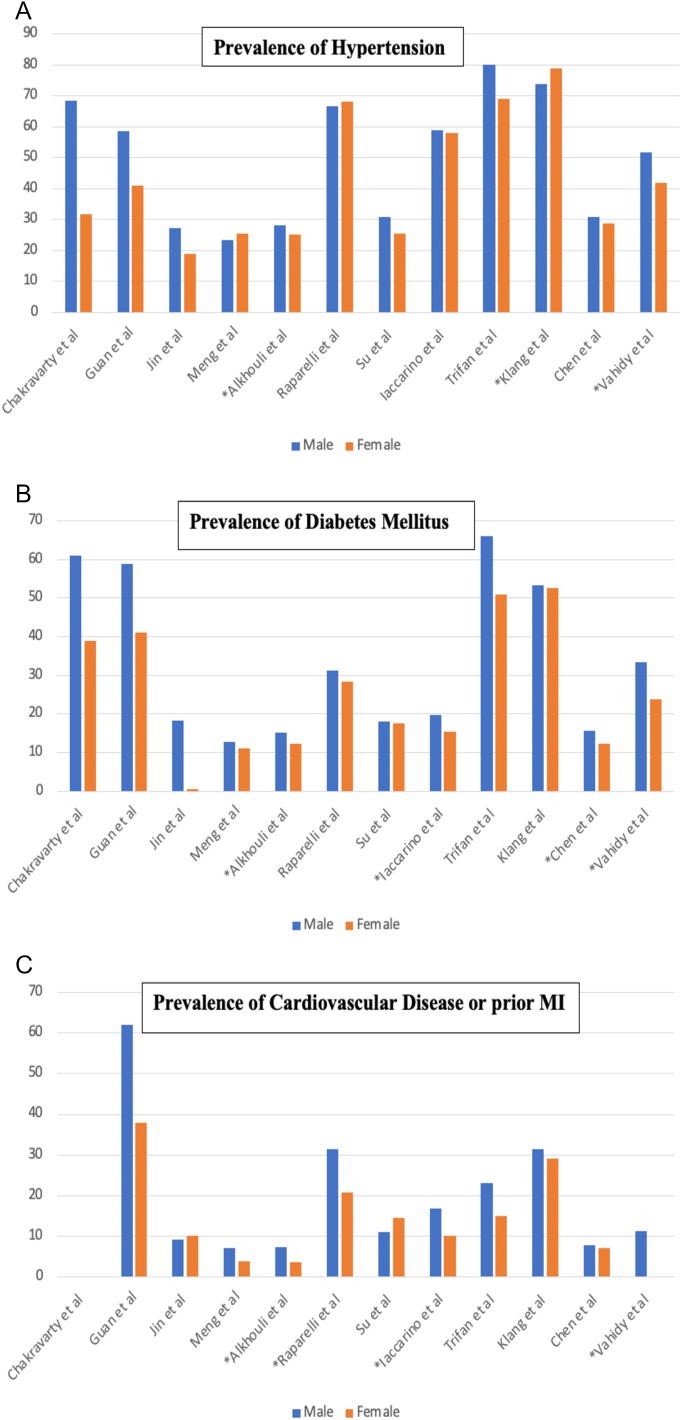
Several studies have shown the sex-specific differences in the prevalence and comorbidity profile between men and women infected with COVID-19 (Fig. 1) [[2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]]. Some studies reported a higher incidence of COVID-19 infection in men [3,4,11,13], while other studies did not show a difference in incidence of COVID-19 infections [2,[5], [6], [7], [8], [9], [10],[12], [13], [14], [15]]. This could be attributed to higher risk of exposure in men [8], access to healthcare and testing availability among other factors [13]. Not only do the reported comorbidities differ between men and women, but they also differ based on the population being studied (Fig. 1) [[2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]]. In general, men had higher prevalence of smoking, lung disease and cardiovascular disease, while women had higher prevalence of obesity and kidney disease (Table 1). Moreover, a few studies assessed the adjusted sex-specific outcomes in these patients (Table 1) [[4], [5], [6],13]. Interestingly, even after adjusting for these comorbidities, men still had higher rates of mortality or severe infection compared to women [[4], [5], [6],13]. In addition to mortality, studies have shown that men with COVID-19 were at higher risk for intensive care unit (ICU) admissions and were more likely to have disease progression and undergo mechanical ventilation [8,9,12,13,15]. In a study by Iaccarino et al., male sex was a predictor for ICU admissions in COVID-19 patients [9]. They also found in sex-specific analysis that obesity, chronic kidney disease and hypertension were associated with higher rates of ICU admission among men, whereas obesity and heart failure were associated with higher rates of ICU admission among women [9]. These observational studies further support the need to understand the effects of sex and gender on COVID-19 infections.
Fig. 1.
Bar graphs showing sex-stratified prevalence of co-morbidities in COVID-19 patients. Asterisks in panels A-C indicate significant statistical differences by P < 0.05. Panel A: Bar graph showing sex-stratified prevalence of hypertension in patients with COVID-19 infections. Panel B: Bar graph showing sex-stratified prevalence of diabetes mellitus in patients with COVID-19 infections. Panel C: Bar graph showing sex-stratified prevalence of cardiovascular disease or prior myocardial infarction in patients with COVID-19 infections. Chakravarty et al. did not report prevalence of cardiovascular disease or prior myocardial infarction.
Table 1.
Summary of studies reporting adjusted outcomes startified by sex.
| Author | Population, number of patients | Major findings |
|---|---|---|
| Jin et al. [4] | Single center study from China, 43 patients | Of the deceased 37 patients, 70.3% were men and 29.3% were women. Men were more prone to death and severe infections despite similar susceptibility and comorbidities (p = 0.016) |
| Meng et al. [5] | Severely ill Covid-19 patients, 168 patients | Sex-specific trend for critically-ill condition was observed: males: OR = 3.824, 95% CI: 1.279–11.435; females: OR = 2.992, 95% CI: 0.937–9.558, even after adjusting for covariates including age, respiratory symptoms, days from illness onset to the first admission and the pathogens identified. |
| Alkhouli et al. [6] | Multinational data of COVID-19 patients from United States and other countries, 14,712 patients | After propensity matching, all-cause mortality remained significantly higher in men than in women (8.13% vs 4.60%; odds ratio, 1.81; 95% CI, 1.55 to 2.11; P < 0.001). |
| Vahidy et al. [13] | Diverse US metropolitan area, 14,992 COVID-19 patients | After adjusting, length of stay, ICU admissions, mechanical ventilation and in-hospital mortality were higher in men. |
Abbreviations: CI (Confidence Interval), ICU (Intensive Care Unit), OR (Odds Ratio).
Additionally, based on the WHO data, among 55 countries providing sex-disaggregated data on the pandemic cases, 48 countries showed disproportionately higher deaths and higher case fatality rates among men with COVID-19 infection [[14], [15], [16], [17]]. It is important to note that despite efforts to encourage reporting sex-disaggregated data, only 74 out of 187 countries on the Global Health 5050 provided sex-specific data, with some countries providing partial sex-specific data [18]. With that being said, more efforts, both on the local and the international levels, are needed to ensure accurate and comprehensive reporting of sex-specific data.
3. Role of biological factors
Sex differences refer to the biological attributes, including hormonal, immune and inflammatory response to infection, that potentially influence the severity and outcomes of COVID-19 infections [1,[19], [20], [21], [22]]. Estrogens promote both innate and adaptive immune responses, potentially leading to faster clearance of pathogens, less severe symptoms in women and more robust immune response to vaccines [19,20,22]. In addition, estrogen is associated with decreased expression of angiotensin-converting enzyme 2 (ACE2) receptors, which are the functional receptors for SARS-CoV-2 to enter host target cells [19,20]. On the other hand, testosterone is associated with suppressive effects on immune function, which may explain the greater susceptibility to infectious diseases observed in men [19,20]. Moreover, reduction in testosterone levels in aging men has been associated with increased proinflammatory cytokine levels and potential higher risk of “cytokine storms”, which may contribute to worse COVID-19 progression and severity in older men [[19], [20], [21], [22]]. These cytokines include C-reactive protein (CRP) and various interleukins [19,20,22]. The increase in pro-inflammatory response is combined with preferential sex-specific T cell activation in the early phase of COVID-19 infection, which is robust even in older women, and declines significantly in men with aging, making older men at higher risk for COVID-19 infections [22]. A study showed that early elevation in CRP >15 mg/L was a marker of disease severity, and a level > 200 mg/L was independently associated with five times the odds of mortality [20]. Men with severe COVID-19 had higher CRP concentrations compared with women, independent of age and comorbidities [19,20].
4. Role of gender
Besides these potential sex-based differences, gender constitutes complex social construct incorporating social role, identity, and relations, and may influence infectious disease exposure and risk [[22], [23], [24], [25]]. The initial public health response to the pandemic required fundamental changes in individual behavior, such social distancing and wearing masks [[22], [23], [24], [25], [26]]. A study of 21,649 participants in the US showed that women were more likely to perceive COVID-19 as a very serious health problem, to agree with restraining public policy measures, and to comply with these measures [23]. Moreover, another study showed that ethnic minorities, including Black, Latina and/or Asian patients, were more likely to report wearing a mask compared with White men [26]. By contrast, men in general had higher prevalence of high-risk behaviors, including smoking and alcohol consumption, were more likely to work in high-risk jobs, including driving, which increase their risk of exposure to the infection or present later when symptoms are worse, potentially explaining, at least in part, the higher severity of infections in men and consequent outcomes [[22], [23], [24], [25]].
5. Sex Differences in Long-Term COVID-19 Manifestations
Despite the higher mortality in men, women tend to be at higher risk for long-term COVID-19 manifestations [27]. In a study by the International Severe Acute Respiratory and emerging Infections Consortium (ISARIC) of 327 hospitalized patients who were discharged alive and had confirmed or high likelihood of COVID-19 infection, the investigators found that women younger than 50 years old were five times less likely to report “feeling recovered”, twice as likely to report worse fatigue, seven times more likely to report breathlessness, and more likely to have greater disability at follow up versus men hospitalized with COVID-19 of similar age. The disability the investigators looked at in this study usually affected memory, mobility, communication, vision, or hearing [27]. More than half of the patients did not fully recover at 7-month follow up with persistent symptoms reported by 93.3%, most commonly fatigue and breathlessness [27].
6. COVID-19 in Pregnancy
There seem to be some differences in COVID-19 infections among pregnant women with COVID-19 compared with non-pregnant women [28]. In a meta-analysis of 77 cohort studies including 11,432 pregnant or recently pregnant women with suspected or confirmed COVID-19, the investigators found that pregnant women with COVID-19 were less likely to have symptoms compared with similar age non-pregnant women, but they had increased risk for ICU admission [28]. In addition, pregnant women with COVID-19 were more likely to have preterm birth and their newborns were more likely to be in a neonatal ICU. It is important to mention that some risk factors are associated with increased risk of severe COVID-19, including older age, overweight or obesity, and presence of pre-existing comorbidities, such as hypertension or diabetes [28].
7. Evidence from clinical trials
Only a few therapeutic interventions have proven effective for hospitalized COVID-19 patients in large-scale randomized trials [29,30]. Since women are less frequently hospitalized with COVID-19, in both trials, women only represented ~1/3 of the participants. Without more representation of women in randomized trials, our estimates to compare the efficacy of therapies might be inadequate [31]. It is worth mentioning that not only were women under-represented in clinical trials, but some observational studies suggested that sex disparities also exist in the treatment of COVID-19 patients. For example, in one study including >3300 patients from China, women were less likely to receive antiviral therapy (p = 0.025) and glucocorticoids treatment (p < 0.0001), however this could be attributed to the fact that women were less sick [12]. Reassuringly, in the large-scale trials assessing the efficacy of vaccines for asymptomatic individuals, there has been adequate representation of women in both trials [32,33]. In a recent multicenter trial enrolling 4488 patients which has yet to undergo peer review, colchicine reduced the composite of mortality or hospitalization for COVID-19. Interestingly, the effect seemed to be more pronounced among men (odds ratio 0.67, 95% confidence interval 0.48–0.95) versus women (odds ratio 1.07, 95% confidence interval 0.70–1.65), which further highlights that there might be sex-based differences in the responses to therapies [34].
8. Conclusion
In conclusion, many studies continue to highlight the sex and gender-based differences in the severity and outcomes of COVID-19 infections. More studies assessing the sex-disaggregated data are needed, to address numerous knowledge gaps. These include suspected source of infection, comorbidities, immune response, and individual behaviors, among other variables. Such data would inform our understanding of the complex interactions between these variables and their impact on the disease severity and sex-specific outcomes (Fig. 2). Future studies addressing sex and gender disparities in COVID-19 infection are encouraged to help generate evidence-based recommendations for decision-making in order to limit these gaps in the healthcare system. Finally, ongoing and future randomized trials need to consider adequate representation for both sexes in order to investigate sex-specific differences in therapies.
Fig. 2.
Summary of potential factors influencing sex- and gender-specific outcomes in COVID-19 infections.
CRediT authorship contribution statement
Lina Ya'qoub, MD: Conceptualization, methodology, investigation, writing original draft, critical revision.
Islam Y. Elgendy, MD: Conceptualization, methodology, investigation, writing original draft, critical revision.
Carl J. Pepine, MD: Critical review and revision, mentorship.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Dr. George Abela served as Guest Editor in Chief for this manuscript.
References
- 1.Sharma G., Volgman A.S., Michos E.D. Sex differences in mortality from COVID-19 pandemic: are men vulnerable and women protected? JACC Case Rep. 2020;2:1407–1410. doi: 10.1016/j.jaccas.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakravarty D., Nair S.S., Hammouda N., et al. Sex differences in SARS-CoV-2 infection rates and the potential link to prostate cancer. Commun. Biol. 2020;3:374. doi: 10.1038/s42003-020-1088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.J., Liang W.H., Zhao Y., et al. China Medical Treatment Expert Group for COVID-19 Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin J.M., Bai P., He W., et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front. Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng Y., Wu P., Lu W., et al. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: a retrospective study of 168 severe patients. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alkhouli M., Nanjundappa A., Annie F., et al. Sex differences in case fatality rate of COVID-19: insights from a multinational registry. Mayo Clin. Proc. 2020;95:1613–1620. doi: 10.1016/j.mayocp.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raparelli V., Palmieri L., Canevelli M., et al. Italian National Institute of Health COVID-19 Mortality Group Sex differences in clinical phenotype and transitions of care among individuals dying of COVID-19 in Italy. Biol. Sex Differ. 2020;11:57. doi: 10.1186/s13293-020-00334-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su W., Qiu Z., Zhou L., et al. Sex differences in clinical characteristics and risk factors for mortality among severe patients with COVID-19: a retrospective study. Aging (Albany NY) 2020;12:18833–18843. doi: 10.18632/aging.103793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iaccarino G., Grassi G., Borghi C., et al. Gender differences in predictors of intensive care units admission among COVID-19 patients: the results of the SARS-RAS study of the Italian Society of Hypertension. PLoS One. 2020;15 doi: 10.1371/journal.pone.0237297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trifan G., Goldenberg F.D., Caprio F.Z., et al. Characteristics of a diverse cohort of stroke patients with SARS-CoV-2 and outcome by sex. J. Stroke Cerebrovasc. Dis. 2020;29 doi: 10.1016/j.jstrokecerebrovasdis.2020.105314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klang E., Soffer S., Nadkarni G., et al. Sex differences in age and comorbidities for COVID-19 mortality in urban New York City. S.N. Compr. Clin. Med. Aug. 2020;9:1–4. doi: 10.1007/s42399-020-00430-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J., Bai H., Liu J., et al. Distinct clinical characteristics and risk factors for mortality in female inpatients with coronavirus disease 2019 (COVID-19): a sex-stratified, large-scale cohort study in Wuhan, China. Clin. Infect. Dis. 2020;71:3188–3195. doi: 10.1093/cid/ciaa920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vahidy F.S., Pan A.P., Ahnstedt H., et al. Sex differences in susceptibility, severity, and outcomes of coronavirus disease 2019: cross-sectional analysis from a diverse US metropolitan area. PLoS One. 2021;16 doi: 10.1371/journal.pone.0245556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burki T. The indirect impact of COVID-19 on women. Lancet Infect. Dis. 2020;20:904–905. doi: 10.1016/S1473-3099(20)30568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dehingia N., Raj A. Sex differences in COVID-19 case fatality: do we know enough? Lancet Glob. Health. 2021;9:e14–e15. doi: 10.1016/S2214-109X(20)30464-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peckham H., Gruijter N.M. de, Raine C., et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020;11:6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spagnolo P.A., Manson J.E., Joffe H. Sex and gender differences in health: what the COVID-19 pandemic can teach us. Ann. Intern. Med. 2020;173:385–386. doi: 10.7326/M20-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Global Health 5050, the sex, gender and COVID-19 project. The COVID-19 Sex-Disaggregated Data Tracker. Available at: https://globalhealth5050.org/the-sex-gender-and-covid-19-project/dataset/. Accessed Feb 1, 2021.
- 19.Hampton T. Insight on sex-based immunity differences, with COVID-19 implications. JAMA. 2020;324:1274. doi: 10.1001/jama.2020.17378. [DOI] [PubMed] [Google Scholar]
- 20.Haitao T., Vermunt J.V., Abeykoon J., et al. COVID-19 and sex differences: mechanisms and biomarkers. Mayo Clin. Proc. 2020;95:2189–2203. doi: 10.1016/j.mayocp.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsilidis K.K., Rohrmann S., McGlynn K.A., et al. Association between endogenous sex steroid hormones and inflammatory biomarkers in US men. Andrology. 2013;1:919–928. doi: 10.1111/j.2047-2927.2013.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi T., Iwasaki A. Sex differences in immune responses. Science. 2021;371:347–348. doi: 10.1126/science.abe7199. [DOI] [PubMed] [Google Scholar]
- 23.Galasso V., Pons V., Profeta P., et al. Gender differences in COVID-19 attitudes and behavior: panel evidence from eight countries. Proc. Natl. Acad. Sci. U. S. A. 2020;117:27285–27291. doi: 10.1073/pnas.2012520117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.C.P. Tadiri, T. Gisinger, A. Kautzy-Willer, et al.; GOING-FWD Consortium, The influence of sex and gender domains on COVID-19 cases and mortality. CMAJ 192 (2020) E1041-E1045. doi: 10.1503/cmaj.200971. [DOI] [PMC free article] [PubMed]
- 25.Abate B.B., Kassie A.M., Kassaw M.W., et al. Sex difference in coronavirus disease (COVID-19): a systematic review and meta-analysis. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-040129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hearne B.N., Niño M.D. Understanding how race, ethnicity, and gender shape mask-wearing adherence during the COVID-19 pandemic: evidence from the COVID Impact Survey. J. Racial Ethn. Health Disparities Jan. 2021;19:1–8. doi: 10.1007/s40615-020-00941-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sigfrid L. Long covid in adults discharged from UK hospitals after covid-19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. medRxiv 2021 [preprint] 2021 doi: 10.1101/2021.03.18.21253888. bmj | BMJ 2021;372:n829 | doi: 10.1136/bmj.n829 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allotey J., Stallings E., Bonet M., Yap M., Chatterjee S., Kew T., Debenham L., Llavall A.C., Dixit A., Zhou D., Balaji R., Lee S.I., Qiu X., Yuan M., Coomar D., van Wely M., van Leeuwen E., Kostova E., Kunst H., Khalil A., Tiberi S., Brizuela V., Broutet N., Kara E., Kim C.R., Thorson A., Oladapo O.T., Mofenson L., Zamora J., Thangaratinam S. for PregCOV-19 Living Systematic Review Consortium. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020 Sep 1;370 doi: 10.1136/bmj.m3320. PMID: 32873575; PMCID: PMC7459193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, Diaz JV, et al., Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 324 (2020) 1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed]
- 30.Beigel J.H., Tomashek K.M., Dodd L.E., et al. ACTT-1 Study Group Members, Remdesivir for the treatment of Covid-19 - final report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elgendy I.Y., Spall H.G.C. Van, Mamas M.A. Cardiogenic shock in the setting of acute myocardial infarction: history repeating itself? Circ. Cardiovasc. Interv. 2020;13 doi: 10.1161/CIRCINTERVENTIONS.120.009034. [DOI] [PubMed] [Google Scholar]
- 32.Polack F.P., Thomas S.J., Kitchin N., et al. C4591001 Clinical Trial Group, Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.L.R. Baden, H.M. El Sahly, B. Essink, et al.; COVE Study Group, Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384 (2021) 403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed]
- 34.J.C. Tardif, N. Bouabdallaoui, P.L. L'Allier, et al., Efficacy of colchicine in non-hospitalized patients with COVID-19. medRxiv Preprint. doi: 10.1101/2021.01.26.21250494. [DOI]