Abstract
Background
The COVID-19 pandemic has necessitated the adoption of protocols to minimize risk of periprocedural complications associated with SARS-CoV-2 infection. This typically involves a preoperative symptom screen and nasal swab RT-PCR test for viral RNA. Asymptomatic patients with a negative COVID-19 test are cleared for surgery. However, little is known about the rate of postoperative COVID-19 positivity among elective surgical patients, risk factors for this group and rate of complications.
Methods
This prospective multicenter study included all patients undergoing elective surgery at 170 Veterans Health Administration (VA) hospitals across the United States. Patients were divided into groups based on first positive COVID-19 test within 30 days after surgery (COVID[-/+]), before surgery (COVID[+/−]) or negative throughout (COVID[−/−]). The cumulative incidence, risk factors for and complications of COVID[-/+], were estimated using univariate analysis, exact matching, and multivariable regression.
Results
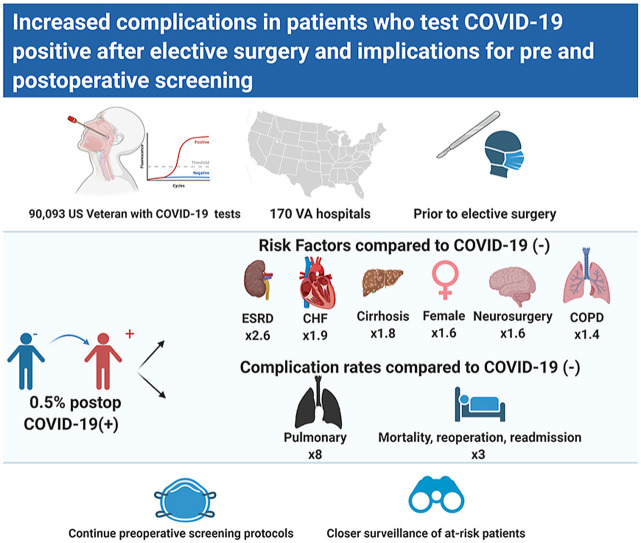
Between March 1 and December 1, 2020 90,093 patients underwent elective surgery. Of these, 60,853 met inclusion criteria, of which 310 (0.5%) were in the COVID[-/+] group. Adjusted multivariable logistic regression identified female sex, end stage renal disease, chronic obstructive pulmonary disease, congestive heart failure, cancer, cirrhosis, and undergoing neurosurgical procedures as risk factors for being in the COVID[-/+] group. After matching on current procedural terminology code and month of procedure, multivariable Poisson regression estimated the complication rate ratio for the COVID[-/+] group vs. COVID[−/−] to be 8.4 (C.I. 4.9–14.4) for pulmonary complications, 3.0 (2.2, 4.1) for major complications, and 2.6 (1.9, 3.4) for any complication.
Discussion
Despite preoperative COVID-19 screening, there remains a risk of COVID infection within 30 days after elective surgery. This risk is increased for patients with a high comorbidity burden and those undergoing neurosurgical procedures. Higher intensity preoperative screening and closer postoperative monitoring is warranted in such patients because they have a significantly elevated risk of postoperative complications.
Keywords: COVID-19 testing, Postoperative complications, Elective surgical procedure
Graphical abstract
Introduction
A nationwide moratorium on elective surgery was imposed in March 2020. The imperatives included preservation of hospital resources for patients requiring hospitalization due to COVID-19 and a perceived risk of COVID-19 transmission to patients if they underwent surgery. Given the potential for additional COVID-19 surges and future new pandemics, data-driven solutions are needed that balance the need for critical hospital capacity while maintaining essential elective surgical procedures.
We have found that postoperative pulmonary, ischemic, and thrombotic complications are increased in patients who are COVID-19 positive prior to elective surgery, though at lower rates than patients undergoing emergency surgery.1, 2, 3 Recent data suggest that during the current pandemic, elective cancer surgery can be performed safely in COVID-19 negative patients.4 , 5 Therefore current guidelines recommend preoperative screening of patients with the SARS-CoV-2 polymerase chain reaction (PCR) test prior to elective surgery.6 , 7 It has been assumed that an asymptomatic patient with a negative COVID-19 PCR test prior to surgery is definitively uninfected. However, very little is known about the rate at which COVID-19 negative patients may turn positive after elective surgery. Even less is known about the risk factors for or complications of COVID-19 positivity in the postoperative period. COVID-19 negative patients may test positive after elective surgery for several reasons. First, the sensitivity and specificity of the SARS-CoV-2 RNA PCR test is only 90%, and can therefore present false negative results in around 10% of instances.8 , 9 Second, patients being planned for elective surgery are, by definition, asymptomatic for COVID-19, and the negative predictive value of the PCR test in such individuals during periods of high community prevalence may be associated with a higher pre-test probability of infection.10 Third, older individuals may have a longer incubation period11 with slower transition to a detectable viral load.12 Finally, COVID-19 infection may be acquired in the postoperative period.
Patients who undergo elective surgery during their COVID-19 incubation period or who acquire COVID-19 while recovering from surgery may have worse outcomes than those who do not.2 , 13 In addition, tracking the rates of COVID-19 positivity in the postoperative period provides a metric for the effectiveness of transmission-prevention policies in the hospital. Therefore, we used a nationwide dataset incorporating all 170 hospitals and 1074 clinical centers within the Veterans Affairs (VA) healthcare system to evaluate the incidence, risk factors and outcome of COVID-19 negative patients undergoing elective surgery, who tested positive in the postoperative period.
Methods
Participants and study design
The VA Health Administration provides care to more than 9 million Veterans throughout the US. We conducted a nationwide multicenter prospective study of all patients undergoing elective surgery at VA facilities across the US. Information was obtained from the VINCI (VA Informatics and Computing Infrastructure), the VA corporate data warehouse that stores in real time, all the data entered into the VA’s electronic medical record.14 We identified 90,093 patients undergoing elective surgery between March 1, 2020 and December 1, 2020. Data describing the patient demographics, medical risk factors, surgical procedures and postoperative complications occurring within 30 days after surgery were extracted. Our exposure was a laboratory confirmed positive SARS-CoV-2 quantitative RT-PCR test within 30 days after surgery in patients who had tested negative on preoperative screening. Laboratory testing for SARS-CoV-2 infection was uniform across VA facilities. Electronic medical record documentation of SARS-CoV-2 positive results and dates of testing were also available for the small number of patients who were tested at a non-VA facility and were included in the analysis. To meet inclusion criteria, patients had to have tested negative for SARS-CoV-2 during their preoperative screening occurring before surgery. Patients were then divided into three groups. In the first group (COVID[−/−]), patients did not have a positive COVID-19 test within 30 days before surgery and their preoperative screening, nor a positive test within 30 days after surgery. Asymptomatic patients not tested in the postoperative period were presumed to be COVID-19 negative. In the second group (COVID[+/−]), patients had at least one positive COVID-19 test within 30 days before surgery and their negative preoperative screening, but no positive tests within 30 days after surgery. Asymptomatic patients not tested in the postoperative period were presumed to be COVID-19 negative. In the third group (COVID[-/+]), patients did not have a positive test within 30 days before surgery and their preoperative screening but tested positive within 30 days after their surgery. The study was reviewed and approved by the University of Maryland Institutional Review Board and the Baltimore VA Research and Development Committee and was exempted from the need for informed consent.
Data collection
Data describing the patient’s age, sex, race (white, black, and other), ethnicity (Hispanic or Latino), body mass index (BMI), and the American Society of Anesthesiologists (ASA) physical status classification (I through VI) were obtained.15 We excluded patients classified as ASA classes V and VI because there were too few patients in these classes for meaningful comparisons. Medical comorbidities were determined by reviewing International Classification of Disease system-10 (ICD-10) codes identified at inpatient and outpatient encounters in the two-years prior to the index surgical procedure and included congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), liver cirrhosis, human immunodeficiency virus (HIV), cancer, end stage renal disease (ESRD), chronic hepatitis, and diabetes. Smoking status was classified as current smoker, past smoker, never smoker and unknown. For each surgery, Current Procedural Terminology (CPT) codes were examined to determine procedural data (from the full code) and organ system on which the procedure was performed (from the first two digits of the code). We excluded patients whose CPT codes corresponded to miscellaneous, mediastinum/diaphragm, lymphatic, gynecological, maternity care, fine needle aspiration procedures, endocrine, and auditory operations because there were too few patients in these categories for meaningful comparisons. Anesthesia type used during the procedure (general or other [composite of sedation, spinal, epidural and local]), was also recorded.
Outcome measures
Primary outcome measures were COVID-19 positive test in the postoperative period, recorded up to 30-days after the index elective surgical procedure and four composite measures: major complications (mortality, readmission or reoperation), pulmonary complications (pneumonia, Acute Respiratory Distress Syndrome [ARDS], or need for post-operative mechanical ventilation), ischemic complications (myocardial infarction [MI] or stroke), and a composite of any complication (pulmonary complications, ischemic complications, major complications, septic shock, deep vein thrombosis, or pulmonary embolism). Hospital length of stay was a secondary outcome. ICD-10 diagnosis codes were used to identify the outcome measures, and a combination of ICD-10 and CPT codes were used to establish the need for mechanical ventilation.
Statistical analysis
We compared the demographic, clinical and procedural characteristics of the three groups: COVID [−/−], COVID[+/−], and COVID[-/+] using frequencies and percentages, means and standard deviations, or medians and interquartile ranges (IQR), as appropriate. Comparisons of categorical data were performed using Pearson’s test or Fisher’s Exact test when cell sizes were 5 or less. Student’s t-test was used to compare normally distributed continuous data between groups, and the Mann-Whitney-U tests were used for non-normally distributed continuous data. ANOVA was used to compare means from all three groups for normally distributed continuous data, and Kruskal-Wallis Rank Sum tests were used for non-normally distributed continuous data. The statistical analysis was organized around three aims.
The first aim was to identify the incidence of COVID-19 negative patients becoming COVID-19 positive after surgery (COVID[-/+]). We determined the number of patients in the COVID[-/+] group and divided this number by the total number of patients in the COVID[-/+] and COVID[−/−] groups, assuming that patients in the COVID[+/−] group were no longer at risk for additional infection.
The second aim was to identify risk factors for COVID-19 negative patients becoming COVID-19 positive after surgery (COVID[-/+]). We used multivariable logistic regression to adjust for those covariates that had a difference in distribution on bivariate comparison of the COVID[−/−], COVID[+/−] groups with a p value ≤ 0.2. These included sex, body mass index, smoking status (never, former, current, unknown), end stage renal disease (ESRD), chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), cancer, liver cirrhosis, diabetes, cardiovascular surgery, gastrointestinal surgery, and neurological surgery.
The third aim was to quantify the complication rates in each group relative to the group that remained COVID-19 negative before and after surgery (COVID[−/−]). To allow a comparison of complications in the three groups, a two-step analysis was performed. We created two datasets, one with COVID[−/−] and COVID[+/−] patients and another with COVID[−/−] and COVID[-/+] patients. We then performed two separate exact matches in a 3:1 ratio (3 COVID[−/−]:1 COVID[+/−] and 3 COVID[−/−]:1 COVID[-/+]) on the CPT code to reduce confounding by procedural complexity, and on the month when surgery was performed to account for differences in improved understanding of COVID-19 over time. With each matched dataset, we used Poisson regression to predict the rate of each composite outcome measure by COVID-19 status (COVID[+/−] vs COVID[−/−] and COVID[-/+] vs COVID[−/−]). We identified confounders as variables that had a different distribution by COVID status (COVID[+/−] vs COVID[−/−] and COVID[-/+] vs COVID[−/−]) at a p value ≤ 0.2 within each matched dataset. This process allowed the set of appropriate confounders to vary for each of the eight models (one model for each outcome in both of the matched datasets). The exact model specifications are presented in Supplementary Table 1 .
A two-sided p-value of ≤0.05 was considered statistically significant. Statistical analyses were performed using R version 4.0.1 (R Core Team, Vienna, Austria).
Results
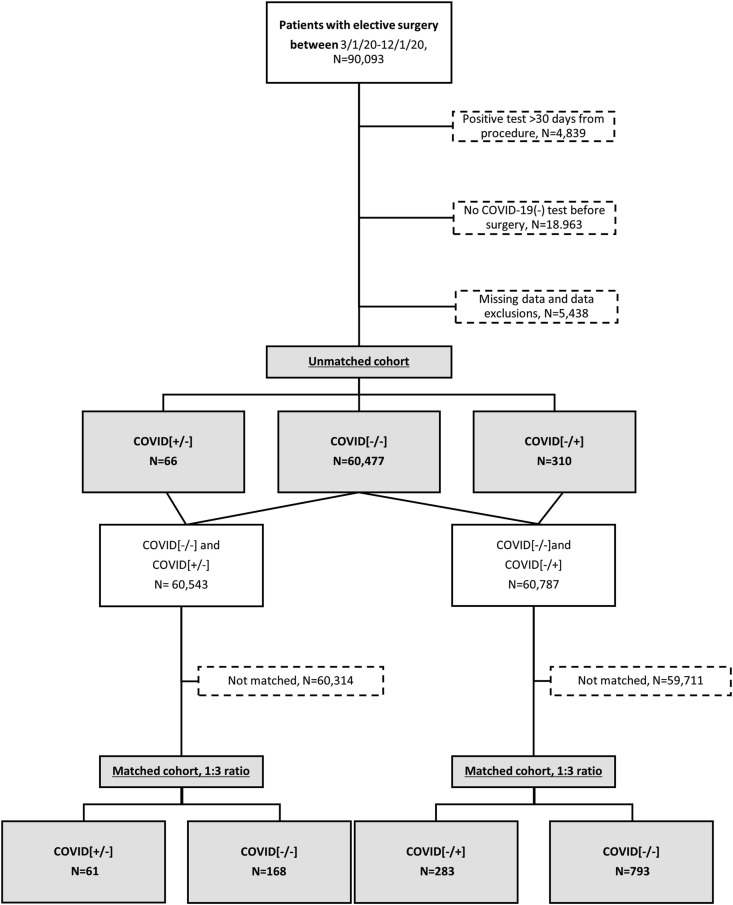
Between March 1 and December 1, 2020, 90,093 patients underwent at least one elective surgical procedure at a VA facility and had a COVID-19 status in the VA electronic records at any time. Patients were excluded if they did not have a laboratory confirmed COVID-19 negative test before their elective surgery (n = 18,963), if their COVID[+] test was not within 30 days before or after their index procedure (n = 4839), or if they did not have complete data (n = 5438). Among the remaining 60,853 patients, 66 were COVID[+/−], 310 were COVID[-/+], and 60,477 were COVID[−/−] (Fig. 1 ). During the study period of 3/1/20-12/1/20, the incidence of testing COVID-19 positive within 30 days after an elective surgery when the patient was COVID-19 negative within 30 days before surgery, was 5 per 1000 procedures.
Fig. 1.
Consort Diagram of the two cohorts analyzed (unmatched and matched). Visual abstract. Created withBioRender.com.
Comparisons of demographic, clinical and procedural characteristics between the COVID[−/−], COVID[+/−] and COVID[-/+] groups are presented in Table 1 . Mean age, sex, and distribution of race and ethnicity did not differ significantly across the three groups. The COVID[-/+] had a higher mean BMI and higher proportion of patients with CHF, COPD, liver cirrhosis, ESRD, and diabetes than the other two groups. Both the COVID[+/−] group and the COVID[-/+] groups had more neurological procedures that the COVID[−/−] group.
Table 1.
Demographic, clinical, and procedural characteristics of patients undergoing elective procedures by COVID-19 status. Values are presented as count (%) unless otherwise indicated.
| COVID[−/−] N = 60,477 |
COVID[-/+] N = 310 |
COVID[+/−] N = 66 |
p-values |
|||
|---|---|---|---|---|---|---|
| Three Way | COVID[-/+] vs. COVID[−/−] | COVID[+/−] vs. COVID[−/−] | ||||
| Demographic Characteristics | ||||||
| Age (median[IQR], years) | 67.0 [58.0,73.0] | 67.0 [59.0,73.8] | 67.0 [53.5,72.0] | 0.40 | 0.31 | 0.36 |
| Sex (% Female) | 5302 (8.8) | 36 (11.6) | 6 (9.1) | 0.21 | 0.10 | 1 |
| Racea | ||||||
| White | 44,822 (74.1) | 228 (73.5) | 41 (62.1) | 0.24 | 0.77 | 0.08 |
| Black | 11,593 (19.2) | 58 (18.7) | 19 (28.8) | |||
| Other | 4062 (6.7) | 24 (7.7) | 6 (9.1) | |||
| Ethnicity (% Hispanic or Latino) | 3772 (6.2) | 21 (6.8) | 4 (6.1) | 0.93 | 0.79 | 1 |
| Body mass index (median[IQR]) | 29.2 [25.7,33.3] | 30.1 [26.4,34.1] | 29.8 [27.1,33.6] | 0.03 | 0.02 | 0.31 |
| Clinical Characteristics | ||||||
| Smoking | ||||||
| Never | 19,413 (32.1) | 101 (32.6) | 21 (31.8) | 0.10 | 0.04 | 0.57 |
| Former | 26,742 (44.2) | 152 (49.0) | 25 (37.9) | |||
| Current | 12,581 (20.8) | 45 (14.5) | 17 (25.8) | |||
| Unknown | 1741 (2.9) | 12 (3.9) | 3 (4.5) | |||
| Congestive heart failure | 4836 (8.0) | 58 (18.7) | 10 (15.2) | <0.001 | <0.001 | 0.06 |
| Chronic obstructive pulmonary disease | 12,536 (20.7) | 91 (29.4) | 13 (19.7) | 0.001 | <0.001 | 0.96 |
| Liver cirrhosis | 1525 (2.5) | 17 (5.5) | 4 (6.1) | 0.001 | 0.002 | 0.15 |
| Human immunodeficiency virus | 569 (0.9) | 3 (1.0) | 2 (3.0) | 0.21 | 1 | 0.26 |
| Cancer | 20,582 (34.0) | 128 (41.3) | 28 (42.4) | 0.01 | 0.01 | 0.19 |
| End stage renal disease | 1521 (2.5) | 26 (8.4) | 1 (1.5) | <0.001 | <0.001 | 0.90 |
| Chronic hepatitis | 184 (0.3) | 2 (0.6) | 1 (1.5) | 0.12 | 0.57 | 0.51 |
| Diabetes | 21,231 (35.1) | 139 (44.8) | 21 (31.8) | 0.001 | <0.001 | 0.67 |
| Procedural Characteristics | ||||||
| ASA class III or IVb | 46,065 (76.2) | 245 (79.0) | 47 (71.2) | 0.32 | 0.27 | 0.42 |
| Anesthesia (% General) | 29,795 (49.3) | 152 (49.0) | 33 (50.0) | 0.99 | 0.98 | 1 |
| Organ system undergoing surgeryc | ||||||
| Musculoskeletal | 13,035 (21.6) | 64 (20.6) | 12 (18.2) | 0.74 | 0.75 | 0.61 |
| Gastrointestinal | 11,429 (18.9) | 44 (14.2) | 14 (21.2) | 0.10 | 0.04 | 0.75 |
| Ophthalmological | 10,650 (17.6) | 49 (15.8) | 6 (9.1) | 0.14 | 0.45 | 0.10 |
| Urological | 10,197 (16.9) | 60 (19.4) | 12 (18.2) | 0.49 | 0.27 | 0.90 |
| Cardiovascular | 4372 (7.2) | 30 (9.7) | 4 (6.1) | 0.24 | 0.12 | 0.90 |
| Integumentary | 4088 (6.8) | 19 (6.1) | 6 (9.1) | 0.68 | 0.74 | 0.61 |
| Neurological | 3746 (6.2) | 29 (9.4) | 10 (15.2) | 0.001 | 0.03 | 0.01 |
| Respiratory | 2960 (4.9) | 15 (4.8) | 2 (3.0) | 0.78 | 1 | 0.68 |
Abbreviations.
ASA, American Society of Anesthesiologists.
COVID[−/−], Patients who did not have a positive COVID-19 test within 30 days before surgery and their preoperative screening, nor a positive test within 30 days after surgery.
COVID[-/+], Patients who did not have a positive test within 30 days before surgery and their preoperative screening, but tested positive within 30 days after their surgery.
COVID[+/−], Patients who had at least one positive COVID-19 test within 30 days before surgery and their preoperative screening, but no positive tests within 30 days after surgery.
CPT, Current procedural terminology.
IQR, Interquartile range.
FNA, Fine-needle aspiration.
Other includes Asian, American Indian or Alaskan Native, Native Hawaiian or Other Pacific Islander.
ASA classes V and VI were excluded and the others were grouped due to low proportions.
Definition of the organ system is based on the first two digits of the CPT code. The following procedure types were excluded due to low proportions: miscellaneous, mediastinum/diaphragm, operating microscope, lymphatic, gynecological, maternity care, FNA procedures, endocrine, auditory.
We determined risk factors for being in the COVID[-/+] group rather than the COVID[−/−] group. In the multivariable logistic regression, female sex, ESRD, COPD, CHF, cancer, cirrhosis, and undergoing neurosurgical procedures were associated with a significantly increased likelihood of being in the COVID[-/+] group (Table 2 ).
Table 2.
Risk factors for testing COVID positive within 30 days after an elective procedure.
| Risk factors | Odds Ratio (95% CI)a |
|---|---|
| End stage renal disease | 2.6 (1.6, 4.1) |
| Congestive heart failure | 1.9 (1.4, 2.6) |
| Liver cirrhosis | 1.8 (1.1, 3.0) |
| Undergoing neurological surgery | 1.6 (1.1, 2.4) |
| Female sex | 1.6 (1.1, 2.2) |
| Chronic obstructive pulmonary disease | 1.4 (1.1, 1.8) |
| Unknown smoking status (REF never smoker) | 1.4 (0.7, 2.5) |
| Cancer | 1.3 (1.0, 1.6) |
| Diabetes | 1.2 (0.9, 1.5) |
| Former smoker (REF never smoker) | 1.0 (0.8, 1.3) |
| Body mass index | 1.0 (1.0, 1.0) |
| Undergoing cardiovascular surgery | 0.9 (0.6, 1.3) |
| Undergoing gastrointestinal surgery | 0.8 (0.6, 1.1) |
| Current smoker (REF never smoker) | 0.7 (0.5, 1.0) |
Abbreviations.
CI, Confidence intervals.
COVID[−/−], Patients who did not have a positive COVID-19 test within 30 days before surgery and their preoperative screening, nor a positive test within 30 days after surgery.
COVID[-/+], Patients who did not have a positive test within 30 days before surgery and their preoperative screening, but tested positive within 30 days after their surgery.
REF, Reference.
Odds ratios of testing COVID[-/+] within 30 days after an elective procedure are presented from logistic regression on unmatched data relative to testing COVID[−/−]. There were 310 patients in the COVID[-/+] group and 60,477 patients in the COVID[−/−] group.
To quantify the primary and secondary outcome measures in patients that tested COVID-19 positive after surgery, and in those that tested positive before surgery, relative to the group that remained COVID-19 negative before and after surgery, we created two new datasets. One dataset was developed by matching each COVID[-/+] patient to up to 3 COVID[−/−] patients; the other dataset matched each COVID[+/−] patient to up to 3 COVID[−/−] patients. For some patients only 1 or 2 exact matches could be found. This resulted in datasets well-matched with respect to demographic, clinical, and procedural characteristics that were appropriate for performing comparisons of outcome measures (Supplementary Tables 2 and 3). Compared to those that remained negative before and after surgery, more patients who tested positive after surgery had major (COVID[-/+] vs. COVID[−/−] 32.9 vs 9.3%), pulmonary (23.0 vs 2.3%), ischemic (4.2 vs 1.9%) or any (41.0 vs 13.5%) complications, and they stayed longer in the hospital (5.0 [3.0, 8.0] vs 3.0 [2.0, 6.0] days) (Table 3 ). An adjusted multivariable analysis showed that being COVID[-/+] was associated with significantly higher rates of 30-day pulmonary complications (Rate ratio = 8.44, 95% CI [4.94,14.41]), major complications (3.00 [2.18,4.12]), and any complications (2.57 [1.95,3.38]), relative to COVID[−/−] (Table 4 ). Patients testing positive before surgery did not show more complications than those that remained negative before and after surgery (Table 3). An adjusted multivariable analysis did not yield any differences in rate ratios of primary or secondary endpoints between the COVID[−/−] group and the COVID[+/−] groups (Table 4).
Table 3.
Primary and secondary 30-day outcomes among patients who underwent an elective procedure by COVID-19 status. Values are presented as count (%) unless otherwise indicated.
| 30-day outcomes | COVID[-/+]a N = 283 |
COVID[−/−] N = 793 |
p-value | COVID[+/−]b N = 61 |
COVID[−/−] N = 168 |
p-value |
|---|---|---|---|---|---|---|
| Pulmonary complications | 65 (23.0) | 18 (2.3) | <0.001 | 3 (4.9) | 8 (4.8) | 1 |
| Major complications | 93 (32.9) | 74 (9.3) | <0.001 | 9 (14.8) | 19 (11.3) | 0.64 |
| Ischemic complications | 12 (4.2) | 15 (1.9) | 0.05 | 2 (3.3) | 2 (1.2) | 0.62 |
| Any complications | 116 (41.0) | 107 (13.5) | <0.001 | 12 (19.7) | 25 (14.9) | 0.50 |
| Length of stay (median [IQR]) | 5.0 [3.0, 8.0] | 3.0 [2.0, 6.0] | 0.001 | 3.0 [2.0, 8.5] | 3.0 [2.0, 4.0] | 0.40 |
Abbreviations.
COVID[−/−], Patients who did not have a positive COVID-19 test within 30 days before surgery and their preoperative screening, nor a positive test within 30 days after surgery.
COVID[-/+], Patients who did not have a positive test within 30 days before surgery and their preoperative screening, but tested positive within 30 days after their surgery.
COVID[+/−], Patients who had at least one positive COVID-19 test within 30 days before surgery and their preoperative screening, but no positive tests within 30 days after surgery.
CPT, Current procedural terminology.
IQR, Interquartile range.
30-day Pulmonary complications: pneumonia, acute respiratory distress syndrome, ventilation.
30-day Major complications: mortality, readmission, reoperation.
30-day Ischemic complications: ischemic stroke, myocardial infarction.
30-day Any complications: mortality, readmission, reoperation, pneumonia, acute respiratory distress syndrome, ventilation, stroke, myocardial infarction, deep vein thrombosis, pulmonary embolism, septic shock.
Patients were exactly matched on CPT code and month of elective procedure in the following ratio: 1 COVID[-/+]: 3 COVID[−/−].
Patients were exactly matched on CPT code and month of elective procedure in the following ratio: 1 COVID[+/−]: 3 COVID[−/−].
Table 4.
Adjusted rate ratios and rates of primary 30-day outcomes among patients who underwent an elective procedure.a
| 30-day outcomes | Rate Ratio (95% CI) of COVID[-/+] vs. COVID[−/−]b | Rate per 100 procedures COVID[-/+] | Rate Ratio (95% CI) of COVID[+/−] vs. COVID[−/−]c | Rate per 100 procedures COVID[+/−] |
|---|---|---|---|---|
| Pulmonary complications | 8.4 (4.9, 14.4) | 0.5 (0.1, 3.8) | 0.9 (0.2, 3.5) | 3.1 (0.8, 12.1) |
| Major complications | 3.0 (2.2, 4.1) | 4.4 (1.6, 11.6) | 1.4 (0.6,3.1) | 14.9 (7.2, 30.9) |
| Ischemic complications | 1.2 (0.5, 2.6) | 0.02 (0.001, 0.8) | 2.0 (0.3, 14.1) | 1.8 × 10−8 (0, ∞) |
| Any complications | 2.6 (1.9, 3.4) | 5.0 (2.1, 11.8) | 1.2 (0.6, 2.4) | 12.4 (5.9, 26.2) |
Abbreviations.
CI, Confidence interval.
COVID[−/−], Patients who did not have a positive COVID-19 test within 30 days before surgery and their preoperative screening, nor a positive test within 30 days after surgery.
COVID[-/+], Patients who did not have a positive test within 30 days before surgery and their preoperative screening, but tested positive within 30 days after their surgery.
COVID[+/−], Patients who had at least one positive COVID-19 test within 30 days before surgery and their preoperative screening, but no positive tests within 30 days after surgery.
CPT, Current procedural terminology.
30-day Major complications: mortality, readmission, reoperation.
30-day Pulmonary complications: pneumonia, acute respiratory distress syndrome, ventilation.
30-day Ischemic complications: ischemic stroke, myocardial infarction.
30-day Any complications: mortality, readmission, reoperation, pneumonia, acute respiratory distress syndrome, ventilation, stroke, myocardial infarction, deep vein thrombosis, pulmonary embolism, septic shock.
Log-linear Poisson regressions predicted the rate of each complication by COVID-19 status (models described on Supplementary Tables 1 and 2).
Patients were exactly matched on CPT code and month of elective procedure in a ratio of 1:3, COVID[-/+] N = 283 vs. COVID[−/−] N = 793.
Patients were exactly matched on CPT code and month of elective procedure in a ratio of 1:3, COVID[+/−] N = 61 vs. COVID[−/−] N = 168.
Discussion
We present one of the first systematic analyses of types of complications, their rates and their risk factors of COVID-19 negative patients who tested COVID-19 positive within 30 days after undergoing elective surgery (the COVID[-/+] group). The cumulative incidence of turning COVID-19 positive after elective surgery is low, approximately 5 per 1000 procedures performed. Female patients, those undergoing neurosurgical procedures, and patients with preexisting CHF, COPD, cancer, cirrhosis, or ESRD are more likely to test COVID-19 positive after elective surgery. These patients have a higher rate of major (mortality, readmission, reoperation), pulmonary (ARDS, pneumonia or mechanical ventilation), and any (pulmonary and major complications, MI, stroke, septic shock, DVT, PE) complications, and longer hospital stays compared to those that remain COVID-19 negative. The COVID[+/−] did not have a higher complication rate on any of the composite outcomes compared to the COVID[−/−] group.
Given the infrequency with which patients turn COVID-19 positive after elective surgery, it has not been possible to identify risk factors for or complications in this subgroup, from small single-center studies. The incidence of patients turning COVID-19 positive after surgery is also difficult to determine from the literature due to variability in the way preoperative COVID-19 testing has been performed and reported. Once we pooled standardized nationwide data across all VA facilities, our study showed that the rate of conversion from COVID-19 negative to positive after elective surgery was low. When COVID-19 status was not tested for before surgery, the rate of testing positive after surgery has been reported as high as 3.2% compared to 0.5% when it is confirmed preoperatively.5 , 16, 17, 18 This is likely because several assumed negative patients were, in fact, infected. We found that high BMI, CHF, COPD, cancer, cirrhosis, and ESRD were associated with testing COVID-19 positive after elective surgery. These are well-known risk factors for COVID-19 infection. However, other factors that are traditionally thought to be associated with risk for SARS-CoV-2 infection, such as race, ethnicity, and diabetes, did not contribute to risk of postoperative infection. More research is needed to understand this finding.
We also found that COVID-19 infection occurring early after elective surgery resulted in a large increase in postoperative complications. This is a unique group of patients demonstrating 8.4 times the rate of pulmonary complications, 3 times the rate of major complication, and 2.6 times the rate of any complication. This was in contrast to the low complication rates observed in patients that were COVID-19 positive before their elective surgery. This is consistent with our previous findings that overall, complications occur less frequently in COVID-19 positive patients undergoing elective surgery compared to emergency surgery.1 There has been indirect evidence of a dramatically elevated risk of complications in patients that turn COVID-19 positive after surgery.2 , 3 , 13 Reports of increased perioperative complications during the early stages of the pandemic likely comprised of patients similar to the COVID[-/+] group in our study.2 , 3 While we are not aware of other studies that make a direct comparison between outcome measures in different patient groups by timing of COVID-19 positivity (before or after surgery), a large multicenter international cohort study identified a 32% risk of pulmonary complications and 38% risk of mortality associated with testing COVID-19 positive within 7 days before and 30 days after surgery.13 We have previously shown that an elevated risk for pulmonary complications can persist for greater than 30 days after surgery in patients who test positive before elective, urgent or emergency procedures surgery.1
There are several possible reasons for a patient to express a COVID-19 infection within 30 days after their procedure. First, the SARS-CoV-2 RT-PCR may not detect the virus if tested too soon after exposure to COVID-19 when the viral replication phase of infection has not commenced.19 Second, the quality of the nasal swab sample can affect the specificity of the test. Third, the patient may acquire an infection during their hospital stay or after returning home. Early reports from Wuhan suggested that up to 44% of COVID-19 infections were nosocomial.20 While it is difficult to determine the overall rate of hospital SARS-CoV-2 transmission, several single and multicenter studies in the US have shown that the implementation of universal masking (for staff and patients) makes the likelihood of hospital-acquired infection to be low.21 Alternatively, patients may be exposed to COVID-19 in the community after discharge. It is also theoretically possible that the stress of surgery would make individuals with compromised immune systems more susceptible to nosocomial or community-acquired COVID-19 infection in the early postoperative period. These data highlight the continued importance of preoperative testing protocols for SARS-CoV-2 infection and provide criteria for more stringent preoperative screening and postoperative surveillance for patients with CHF, COPD, cancer, cirrhosis, or ESRD. The impact of these policies will be critical in the face of potential future surges or pandemics.
This study represents a nationwide cohort with broad representation of patients from the early stages of the COVID-19 pandemic through its recovery period. While this increases generalizability, we acknowledge the limitations inherent to an administrative database. The study sample of Veterans comprises primarily older male patients (approximately 10% were women) with a high comorbidity burden. Racial and ethnic minorities are represented in a higher proportion than are typically seen in other reports. Overall, these individuals are at higher risk of COVID-19 infection and complications from surgery than the average population. Exact matching and multivariable regression were used to account for differences in procedural complexity and month of the pandemic between groups. However, there may be unmeasured confounders that are not accounted for in the analysis. Only 30-day outcomes were presented since we are still early in the course of the pandemic; however, data collection continues, and we intend to report longer term outcomes in the near future. Finally, universal testing was not implemented early during the pandemic and these patients had to be excluded from the analysis. While losing those patients reduced power, it did not lead to selection bias as the exclusion was independent of factors that could modify our outcome measures. Overall, the available data provides valuable information that will help us better understand the clinical course of patients undergoing elective surgery during different phases of the pandemic and guide care and resource allocation.
Conclusion
We found that PCR screening has minimized postoperative COVID-19 infection and COVID-19 related complications in a majority of elective surgical patients. Our results do highlight risk factors for postoperative COVID-19 infection. A high index of suspicion for postoperative COVID-19 positivity should be maintained among women, those undergoing neurosurgical procedures, and patients with COPD, CHF, cirrhosis, cancer or ESRD. A positive COVID-19 test in the postoperative period is associated with an elevated complication rate. Based on these findings, preoperative assessment of patients undergoing elective surgery should include an assessment of likelihood for preoperative as well as postoperative COVID-19 infection. For individuals at risk for being in the COVID [-/+] group, the findings may warrant repeated PCR tests on separate days before surgery, closer postoperative follow-up for symptoms, and stricter guidelines on prevention in the postoperative period. Future studies will need to evaluate the protective effect of preoperative vaccination.
Contributors
The co-authors contributed to study conception, protocol development, data collection, data interpretation, and critical revision of the manuscript. BKL is the guarantor.
Declaration of competing interests
We declare no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amjsurg.2021.04.005.
Data sharing
Data sharing requests will be considered upon written request to the corresponding author. Deidentified participant data or other prespecified data will be available subject to a written proposal and a signed data sharing agreement with the Veterans Affairs Informatics and Computing Infrastructure.
Funding sources
This study was funded by Veterans Affairs awards HSRD C19-20-407, RRD RX000995 and CSRD CX001621, and NIH awards NS080168, NS097876 and AG000513 (BKL); National Institutes of Health awards AG028747, DK072488, and Baltimore VA Medical Center GRECC (JDS); National Institutes of Health T32 AG00262 (NKP).
The views expressed are those of the authors and not necessarily those of the Veterans Affairs or the National Institutes of Health.
Funding
Veterans Affairs awardsHSRD C19-20-407, RRD RX000995 and CSRD CX001621, and NIH awards NS080168, NS097876 and AG000513 (BKL); National Institutes of Health awards AG028747, DK072488, and Baltimore VA Medical Centre GRECC (JDS); National Institutes of Health T32 AG00262 (NKP).
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lal B.K., Prasad N.K., Englum B.R., et al. Periprocedural complications in patients with SARS-CoV-2 infection compared to those without infection: a nationwide propensity-matched analysis. Am J Surg. 2020;(S0002-9610-1) doi: 10.1016/j.amjsurg.2020.12.024. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doglietto F., Vezzoli M., Gheza F., et al. Factors associated with surgical mortality and complications among patients with and without coronavirus disease 2019 (COVID-19) in Italy. JAMA Surg. 2020 doi: 10.1001/jamasurg.2020.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lei S., Jiang F., Su W., et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020;21:100331. doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glasbey J.C., Bhangu A. Elective cancer surgery in COVID-19–free surgical pathways during the SARS-CoV-2 pandemic: an international, multicenter, comparative cohort study. J Clin Oncol. October 2020;20 doi: 10.1200/jco.20.01933. JCO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.COVID Surgery Collaborative. Preoperative nasopharyngeal swab testing and postoperative pulmonary complications in patients undergoing elective surgery during the SARS-CoV-2 pandemic. Br J Surg. doi:10.1093/bjs/znaa051. [DOI] [PMC free article] [PubMed]
- 6.American College of Surgeons COVID-19: guidance for triage of non-emergent surgical procedures. https://www.facs.org/covid-19/clinical-guidance/triage
- 7.Royal College of Surgeons Covid-19: good practice for surgeons and surgical teams. https://www.rcseng.ac.uk/standards-and-research/standards-and-guidance/good-practice-guides/coronavirus/
- 8.Buchan B.W., Hoff J.S., Gmehlin C.G., et al. Distribution of SARS-CoV-2 PCR cycle threshold values provide practical insight into overall and target-Specific sensitivity among symptomatic patients. Am J Clin Pathol. 2020;154(4):479–485. doi: 10.1093/AJCP/AQAA133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnaout R., Lee R., Lee G.R., et al. SARS-CoV2 testing: the limit of detection matters. bioRxiv Prepr Serv Biol. 2020:617–667. doi: 10.1101/2020.06.02.131144. [DOI] [Google Scholar]
- 10.Woloshin S., Patel N., Kesselheim A.S. False negative tests for SARS-CoV-2 infection — challenges and implications. N Engl J Med. 2020;383(6) doi: 10.1056/nejmp2015897. [DOI] [PubMed] [Google Scholar]
- 11.Tan W.Y.T., Wong L.Y., Leo Y.S., Toh M.P.H.S. Does incubation period of COVID-19 vary with age? A study of epidemiologically linked cases in Singapore. Epidemiol Infect. 2020;148 doi: 10.1017/S0950268820001995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao A.T., Tong Y.X., Zhang S. False negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: rather than recurrence. J Med Virol. 2020;92(10):1755–1756. doi: 10.1002/jmv.25855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nepogodiev D., Bhangu A., Glasbey J.C., et al. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396(10243):27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Department of Veterans Affairs VA Informatics and computing infrastructure (VINCI) homepage. 2018. https://www.hsrd.research.va.gov/for_researchers/vinci/
- 15.Mayhew D., Mendonca V., Murthy B.V.S. A review of ASA physical status – historical perspectives and modern developments. Anaesthesia. 2019;74(3):373–379. doi: 10.1111/anae.14569. [DOI] [PubMed] [Google Scholar]
- 16.Kasivisvanathan V., Lindsay J., Rakshani-Moghadam S., et al. A cohort study of 30 day mortality after Non-Emergency surgery in a COVID-19 cold site. Int J Surg. 2020;84:57–65. doi: 10.1016/j.ijsu.2020.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kane A.D., Paterson J., Pokhrel S., et al. Peri-operative COVID-19 infection in urgent elective surgery during a pandemic surge period: a retrospective observational cohort study. Anaesthesia. 2020;75(12):1596–1604. doi: 10.1111/anae.15281. [DOI] [PubMed] [Google Scholar]
- 18.Axiotakis L.G., Youngerman B.E., Casals R.K., et al. Risk of acquiring perioperative COVID-19 during the initial pandemic peak: a retrospective cohort study. Ann Surg. 2021;273(1):41–48. doi: 10.1097/SLA.0000000000004586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.George B., McGee J., Giangrasso E., Finkelstein S., Wu S., Glatt A.E. What is the predictive value of a single nasopharyngeal SARS-CoV-2 PCR swab test in a patient with COVID-like symptoms and/or significant COVID-19 exposure? Open Forum Infect Dis. 2020;7(10) doi: 10.1093/ofid/ofaa399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Q., Gao Y., Wang X., et al. Nosocomial infections among patients with COVID-19, SARS and MERS: a rapid review and meta-analysis. Ann Transl Med. 2020;8(10):629. doi: 10.21037/atm-20-3324. 629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richterman A., Meyerowitz E.A., Cevik M. Hospital-acquired SARS-CoV-2 infection: lessons for public Health. JAMA, J Am Med Assoc. 2020;324(21):2155–2156. doi: 10.1001/jama.2020.21399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.