Abstract
The novel SARS-CoV-2 has infected over 48 million persons around the world. Children have been spared with regards to symptoms and sequelae of this highly infectious virus and in those with neurologic issues, the virus has not been present in the cerebrospinal fluid. Here, the authors present the first case of metabolic stroke-like episode with SARS-CoV-2 present in the cerebrospinal fluid in a child with a FARS2 deficiency. This report suggests a possible association of SARS-COV-2 infection and metabolic stroke-like episode, even in the absence of a phenotype classically associated with metabolic stroke-like episodes.
Keywords: FARS2, Mitochondrial, Stroke, SARS-CoV-2, Pediatric, Cerebrospinal fluid
Abbreviations: PCR, (polymerase chain reaction)
1. Introduction
Mutations in the FARS2 gene yield a set of mitochondrial disorders associated with dysfunctional mitochondrial phenylanyl-tRNA synthetase 2 [1]. The clinical phenotypes are variable, ranging from infantile-onset epileptic mitochondrial encephalopathy to later-onset spastic paraplegia [2]. The epileptic phenotype is the more severe of the two, with developmental delay, seizure onset within the first year of life, and death in early childhood frequently reported in the literature [1]. Although a mitochondrial disorder, mutations in the FARS2 gene have not been reported to be associated with metabolic stroke-like episodes, as observed in other mitochondrial disorders such as Mitochondrial Encephalopathy, Lactic Acidosis, and Stroke Like Episodes (MELAS) [1,2].
There have been multiple reports of neurologic manifestations associated with the novel coronavirus (SARS-CoV-2) although children seem to have infrequent complications [3,4]. In persons with neurologic complications of SARS-CoV-2, it appears that the inflammatory cascade, as opposed to the virus itself, is causative for the pathology observed, particularly in cerebrovascular accidents [[5], [6], [7], [8]].
Here the authors report a novel case of metabolic stroke-like episodes in a child with FARS2 gene- associated combined oxidative phosphorylation deficiency type 14 with spastic-paraparesis following infection with SARS-CoV-2 with both active serum and CSF PCR positivity.
2. Case report
An 11-year-old male with history of FARS2-related combined oxidative phosphorylation deficiency type 14, spastic paraparesis, and mild developmental delay presented with increased work of breathing, poor oxygen saturation, and lactic acidosis (peak 85.3 mg/dL). He was noted to be SARS-CoV-2 PCR positive on nasopharyngeal swab. His pulmonary distress coincided with acute changes to his neurologic status. On examination, he displayed a new onset gaze preference towards the left, along with opsoclonic eye movements and global aphasia. Pertinent laboratory values are displayed in Table 1. Urgent neuroimaging revealed discrete areas of restricted diffusion in the periaqueductal gray matter and dorsal midbrain as well as the bilateral red nuclei (Fig. 1). Given concern for active infection associated metabolic stroke, a lumbar puncture was performed (Table 1). Notably, the patient had no pleocytosis, although had elevated lactic acid and a positive SARS-CoV-2 PCR in the CSF.
Table 1.
Serum lab findings on admission and lumbar puncture results.
| Serum lab | Value | Reference value |
|---|---|---|
| WBC | 12.07 K/uL | 4.31–11 |
| HGB | 9.5 g/dL | 10.8–13.4 |
| HCT | 28.5% | 32.2–39.8 |
| PLTE | 342 K/uL | 342–369 |
| Sodium | 140 mEq/L | 135–145 |
| Potassium | 4.6 mEq/L | 3.6–5 |
| Chloride | 108 mEq/L | 98–107 |
| CO2 total | 18 mEq/L | 22–30 |
| Creatinine | 0.90 mg/dL | 0.7–1.50 |
| Glucose level | 121 mg/dL | 60–115 |
| Lactic Acid | Range: 33.7–85.3 mg/dL Mean 50.72 mg/dL |
6.3–18.9 |
| AST | 39 units/L | 15–46 |
| ALT | 43 units/L | (<44 |
| CK | 66 units/L | 57–374 |
| Alk phos | 146 units/L | 60–280 |
| PT | 12.1 s ( | 8.8–12.5 |
| PT-INR | 1.1 | (>4 |
| PTT | 25 s | 25–39 |
| D-Dimer | 525 ng/mL | (>570 |
| CSF Lab | Value | Reference Value |
| RBC | 221 Cell/mm3 | 0–5 |
| WBC | 2 Cell/mm3 | 0–4 |
| Glucose | 52 mg/dL | 37–75 |
| Protein | 31 mg/dL | 12–60 |
| Lactate | 30.6 mg/dL | 0–20 |
| Pyruvate | 0.75 mg/dL | 0.50–1.70 |
| Amino Acid CSF | Citrulline 6.1 uMOL/L Alanine 47.5 uMOL/L Isoleucine C 9.5 uMOL/L Leucine 21.8 uMOL/L |
1–5 10–34 2–6 8–18 |
| Paraneoplastic Panel | Negative | Negative |
| Film Array Meningitis/Encephalitis Panela | Negative | Negative |
| SARS-COV-2 PCR | Positive | Negative |
Bold indicates the Abnormal values.
In house PCR film array tests for Escherichia coli K1, Haemophilius influenza, Listeria monocytogenes, Neisseria meningitidis (encaps), Streptococcus agalactiae, Streptococcus pneumoniae, Cytomegalovirus, Enterovirus, Herpes simplex virus 1,2, 6, Human parechovirus, Varicella Zoster Virus, Cryptococcus neoformans/gattii.
Fig. 1.
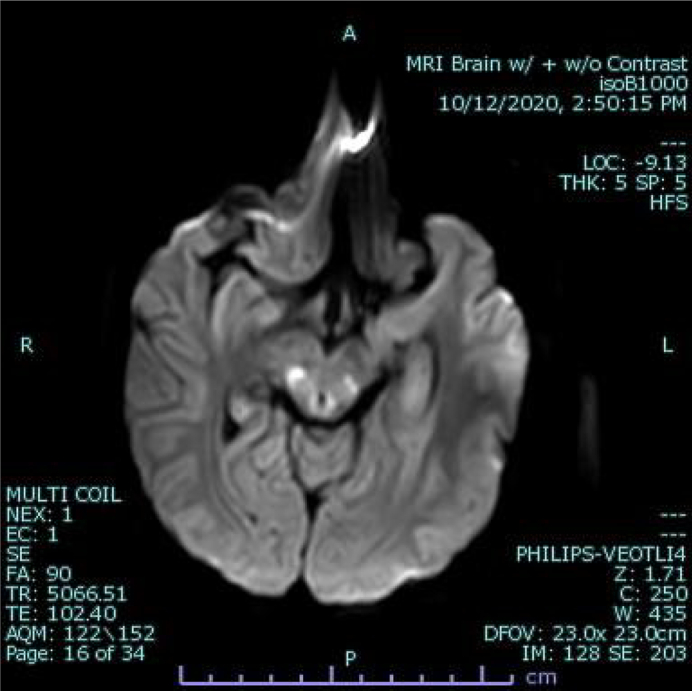
Axial image DWI images demonstrating restricted diffusion in the periaqueductal grey matter and dorsal midbrain.
The ketogenic diet was initiated to correct his metabolic acidosis, with subsequent normalization of his venous blood gas and lactic acid. His hospitalization was complicated by development of a rubral tremor and signs of autonomic dysfunction, including fluctuations in temperature, heart rate, blood pressure, GI motility and hyperhidrosis. Low dose propranolol was trialed, with stabilization of his heart rate and temperature. On discharge, the patient continued to be globally aphasic and have mild but present opsoclonic eye movements.
3. Discussion
The authors report a novel case of SARS-CoV-2 associated metabolic stroke-like episode in a child with FARS2 deficiency. This case is novel for two primary reasons. Firstly, this is the first report of a metabolic stroke-like episode in any child with primary SARS-CoV-2 infection. Although this patient would be considered susceptible due to his mitochondrial gene mutation, FARS2 deficiency (any phenotypic presentation) is not classically associated with metabolic stroke. In this case, the positive CSF PCR for SARS-CoV-2 raises the question of whether active infection or the inflammatory cascade associated with infection was causative or not.
There has been only one documented case of SARS COV2 detected in the CSF of a pediatric patient, a 10-week-old with suspected sepsis [[9], [10], [11], [12]]. Several cases have shown pleocytosis and elevated protein but have failed to detect the virus within the CSF, indicating rapid CSF viral clearance [4,14]. Rather, most pediatric studies have demonstrated abnormalities of serum inflammatory markers, including D-dimer, procalcitonin, creatine kinases, and interleukin-6 [11,13]. As hypothesized in adult studies, it is unclear if the neuro-pathology associated with SARS-CoV-2 is infection triggered or of a post-infectious inflammatory nature as these laboratory disturbances would indicate [5,6,8,9]. Here, the capture of the virus in the CSF indicates the possibility that the actual SARS-CoV-2 virus could have potentially triggered the metabolic failure observed in this patient. This is of particular interest in that this patient's genetic defect is not associated with metabolic stroke-like episodes, making it possible that SARS-CoV-2 infection may have caused metabolic failure itself [14].
An unanswered question in this case is why a patient with FARS2 gene mutation would be susceptible to metabolic stroke-like episode when this has never been reported in the literature. In this case, the patient's hyperlactacidemia was indicative of a severe metabolic crisis, which although infrequently reported in persons with FARS2 gene mutations, is known. It is possible that this systemic metabolic failure caused by the direct infection with SARS-CoV-2 may have increased blood-brain barrier permeability, allowing the virus privileged entry into the central nervous system. In this case, it could be surmised that the presence of lactic acidosis and CSF positivity for SARS-CoV-2 could best be explained in this manner, with the acidotic milieu triggering cytopathy and cell death, seen as restricted diffusion on neuroimaging. The predilection for the dorsal midbrain, while symmetric in a pattern characteristic of mitochondrial disorders, is of unclear significance.
Interestingly, even amongst patients with mitochondrial disorders prone to metabolic stroke-like episodes, there have been no reported cases associated with SARS-CoV-2 to this point. While of great interest in its two novel findings, this case has limitations. This case reports a rare gene mutation which is not generalizable to other individuals with mitochondrial disorders. While these disorders are rare, rates of infection with SARS-CoV-2 across the world have been high. It is possible that there are no reports because persons with mitochondrial or metabolic disorders may be more likely to socially distance than other age-adjusted neurotypical individuals. Although CSF positivity for SARS-CoV-2 was detected, determining if the virus or the inflammatory cascade is responsible for the acute neurologic deterioration incurred is impossible although very suspicious for the former.
This case raises the concerns for SARS-CoV-2 associated metabolic stroke-like episodes in susceptible individuals. Social distancing, mask-wearing, and infectious precautions are critical for at-risk individuals with genetic, metabolic, and mitochondrial disorders.
Conflict of interest disclosures
The authors have no conflicts of interest to disclose.
Funding/support
No funding was secured for this study.
References
- 1.Vantroys E., Larson A., Friederich M. New insights into the phenotype of FARS2 deficiency. Mol. Genet. Metab. 2017;122(4):172–181. doi: 10.1016/j.ymgme.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almannai M., Wang J., Dai H. FARS2 deficiency; new cases, review of clinical, biochemical, and molecular spectra, and variants interpretation based on structural, functional, and evolutionary significance. Mol. Genet. Metab. 2018;125(3):281–291. doi: 10.1016/j.ymgme.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad I., Rathore F.A. Neurological manifestations and complications of COVID-19: a literature review. J. Clin. Neurosci. 2020;77:8–12. doi: 10.1016/j.jocn.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panda P.K., Sharawat I.K., Panda P. Neurological complications of SARS-CoV-2 infection in children: a systematic review and meta-analysis [published online ahead of print, 2020 Sep 10] J. Trop. Pediatr. 2020 doi: 10.1093/tropej/fmaa070. fmaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al Saiegh F., Ghosh R., Leibold A. Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke. J. Neurol. Neurosurg. Psychiatry. 2020;91(8):846–848. doi: 10.1136/jnnp-2020-323522. [DOI] [PubMed] [Google Scholar]
- 6.Espíndola O.M., Brandão C.O., Gomes Y.C.P. Cerebrospinal fluid findings in neurological diseases associated with COVID-19 and insights into mechanisms of disease development [published online ahead of print, 2020 Oct 27] Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.10.044. S1201-9712(20)32247–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J., Jiang M., Chen X. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020;108(1):17–41. doi: 10.1002/JLB.3COVR0520-272R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Z., Kang H., Li S., Zhao X. Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. J. Neurol. 2020;267(8):2179–2184. doi: 10.1007/s00415-020-09929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Destras G., Bal A., Escuret V. Systematic SARS-CoV-2 screening in cerebrospinal fluid during the COVID-19 pandemic. Lancet Microbe. 2020;1(4) doi: 10.1016/S2666-5247(20)30066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Färber K., Stäbler P., Getzinger T. 10 Wochen alter Säugling mit Sepsisverdacht und SARS-CoV-2-Nachweis in liquor und Rachen [suspected sepsis in a 10-week-old infant and SARS-CoV-2 detection in cerebrospinal fluid and pharynx] [published online ahead of print, 2020 Jun 3] Monatsschr Kinderheilkd. 2020:1–4. doi: 10.1007/s00112-020-00942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoang A., Chorath K., Moreira A. COVID-19 in 7780 pediatric patients: a systematic review. EClinicalMedicine. 2020;24:100433. doi: 10.1016/j.eclinm.2020.100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y.H., Jiang D., Huang J.T. SARS-CoV-2 detected in cerebrospinal fluid by PCR in a case of COVID-19 encephalitis. Brain Behav. Immun. 2020;87:149. doi: 10.1016/j.bbi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henry B.M., Benoit S.W., de Oliveira M.H.S. Laboratory abnormalities in children with mild and severe coronavirus disease 2019 (COVID-19): a pooled analysis and review. Clin. Biochem. 2020 Jul;81:1–8. doi: 10.1016/j.clinbiochem.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chhetri S., Khamis F., Pandak N. A fatal case of COVID-19 due to metabolic acidosis following dysregulate inflammatory response (cytokine storm) IDCases. 2020;21 doi: 10.1016/j.idcr.2020.e00829. e00829. Published 2020 May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]


