Dear Editor
The advent of vaccines against COVID-19 has been major stride forward towards quelling the pandemic, with India leading from the front by successfully running the world’s largest vaccination campaign. Currently Covishield (AZD1222) and Covaxin (BBV152) are in circulation in India. Recently there are speculations, stray newspaper reports and preprints on vascular thromboembolic catastrophes post-vaccination, especially with AstraZeneca vaccine [[1], [2], [3]]. There is single case report of myocardial infarction (MI) after the Moderna vaccine [4] and deep vein thrombosis following second dose of Pfizer vaccine [5]. Here, we report a case of MI following Covishield vaccination with the sole purpose of generating awareness among clinicians regarding this possible rare major adverse event and unfurling the possible pathophysiology behind it.
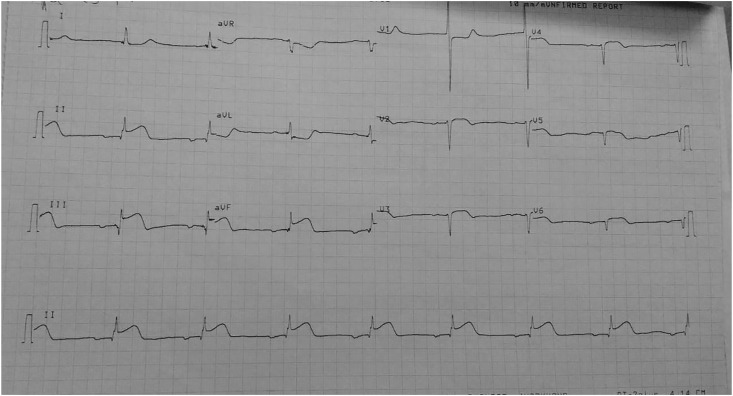
A previously healthy 63-year-old man presented to the emergency with complaint of chest pain for last 6 hours. He was non-hypertensive, non-diabetic, and devoid of any other traditional risk factors of cardiovascular diseases including smoking. He had history of taking first dose of Covishield vaccine 2 days back and since then he developed uneasiness, dizziness and excessive sweating. On examination, he was hemodynamically stable, with 12 lead electrocardiogram (ECG) showing ST elevation in leads II, III and aVF (Fig. 1 ) suggestive of acute ST elevated inferior wall myocardial infarction (STEIWMI). Serum creatine kinase-MB (CK-MB) and cardiac troponin-I (cTnI) were >150 IU/L (n, <9) and 49.28 ng/ml (n, <0.04) respectively. 2-D echocardiography showed inferior wall hypokinesia with left ventricular ejection fraction of 50%. As our centre did not have the facility of emergency percutaneous coronary intervention (PCI), he was thrombolysed with 1.5 million IU streptokinase, and anti-platelets and anti-anginal drugs were administered according to standard protocol. Routine investigations (complete hemogram, liver function test, renal function test, lipid profile, thyroid function test, fasting and post-prandial blood sugar, glycated hemoglobin, urine examination, serum electrolytes, chest X-ray, ultrasonography whole abdomen) were within normal limit except eosinophilia (absolute eosinophil count- 1860/dl; n, 20–500), aspartate aminotransferase 184 IU/L (n, 5–40) and lactate dehydrogenase 1647 IU/ml (n, 200–450). Rest of his hospital stay was uneventful and after 5 days he was discharged and referred to a PCI-capable centre.
Fig. 1.
12 leads ECG showing ST elevation in inferior leads.
As at this point there is no experiment done to specifically investigate the incidence of MI among COVID-19 vaccine recipients, some hypotheses can be put forward. Firstly, Greinacher et al. suggested vaccine induced prothrombotic immune thrombocytopenia, an entity similar to heparin induced thrombocytopenia as the reason behind thrombotic phenomenon post-vaccination [3]. Secondly, Boivin et al. stated demand-supply mismatch in a frail heart post-vaccination [4]. Thirdly, transfection of platelets by mRNA or viral vector-based vaccine may be remote possibility [6]. Fourthly, it can be vasospastic allergic myocardial infarction in response to vaccine, termed as Kounis syndrome [7,8].
At this juncture, it would be premature to draw a causal relationship between COVID-19 vaccine and MI. Considering vaccination is being administered en mass and MI is relatively a common emergency, it can merely be a coincidence or even an idiosyncratic reaction. Thus, each case should be reviewed with extreme caution before communicating with the patient’s attendant and lay public. Thus, these reports including the present one should never discourage the vaccine rollout, but monitoring of evolving data should be carried on by manufacturers and independent authorities before coming to a definitive conclusion [9].
Authors’ contribution
All authors were involved in managing the patient bedside. SC wrote the first draft which was then critically reviewed and modified by UKO, BV and AT. All authors agreed upon the final form of the article.
Funding
Nil.
Consent
Written consent has been taken for using the medical data of the patient for academic purpose while maintaining full anonymity.
Declaration of competing interest
Nil.
References
- 1.Wise J. Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ. 2021;372:n699. doi: 10.1136/bmj.n699. [DOI] [PubMed] [Google Scholar]
- 2.The Hindu. says Health Dept; 2021. Ballari resident, who took coronavirus vaccine, died due to heart attack.https://www.thehindu.com/news/national/karnataka/ballari-resident-who-took-coronavirus-vaccine-died-due-to-heart-attack-says-health-dept/article33606623.ece (accessed on April 2nd, 2021) [Google Scholar]
- 3.Grienacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P., Eichinger S. A prothrombotic thrombocytopenic disorder resembling heparin-induced thrombocytopenia following coronavirus-19 vaccination. Res Square. 2021 doi: 10.21203/rs.3.rs-362354/v1. [DOI] [Google Scholar]
- 4.Boivin Z., Martin J. Untimely myocardial infarction or COVID-19 vaccine side effect. Cureus. 2021;13 doi: 10.7759/cureus.13651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carli G., Nichele I., Ruggeri M., Barra S., Tosetto A. Deep vein thrombosis (DVT) occurring shortly after the second dose of mRNA SARS-CoV-2 vaccine. Intern Emerg Med. 2021 Mar 9:1–2. doi: 10.1007/s11739-021-02685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merchant H. COVID vaccines and thrombotic events: is mRNA translation and spike protein synthesis by platelets a real possibility? BMJ. 2021 https://www.bmj.com/content/372/bmj.n699/rr-20 [Google Scholar]
- 7.Kounis N.G., Mazarakis A., Tsigkas G., Giannopoulos S., Goudevenos J. Kounis syndrome: a new twist on an old disease. Future Cardiol. 2011;7:805–824. doi: 10.2217/fca.11.63. [DOI] [PubMed] [Google Scholar]
- 8.Kounis N.G., Koniari I., de Gregorio C., Velissaris D., Petalas K., Brinia A. Allergic reactions to current available COVID-19 vaccinations: pathophysiology, causality, and therapeutic considerations. Vaccines. 2021;9:221. doi: 10.3390/vaccines9030221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahase E. Covid-19: AstraZeneca vaccine is not linked to increased risk of blood clots, finds European Medicine Agency. BMJ. 2021;372:n774. doi: 10.1136/bmj.n774. [DOI] [PubMed] [Google Scholar]