Graphical abstract
Keywords: SARS-CoV-2, COVID-19, Type I interferon deficiency, Hyperinflammation, Inflammatory cytokines, Thrombosis, NETosis, Treatment, Combination therapies, Interferon-alpha2, Interferon-beta, JAK1/2 inhibitor, Ruxolitinib, Baricitinib, Hydroxyurea, Statins
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) elicits an interferon (IFN) deficiency state, which aggravates the type I interferon deficiency and slow IFN responses, which associate with e.g. aging and obesity. Additionally, SARS-CoV-2 may also elicit a cytokine storm, which accounts for disease progression and ultimately the urgent need of ventilator support. Based upon several reports, it has been argued that early treatment with IFN-alpha2 or IFN-beta, preferentially in the early disease stage, may prohibit disease progression. Similarly, preliminary studies have shown that JAK1/2 inhibitor treatment with ruxolitinib or baricitinib may decrease mortality by dampening the deadly cytokine storm, which – in addition to the virus itself - also contributes to multi-organ thrombosis and multi-organ failure. Herein, we describe the rationale for treatment with IFNs (alpha2 or beta) and ruxolitinib emphasizing the urgent need to explore these agents in the treatment of SARS-CoV-2 – both as monotherapies and in combination. In this context, we take advantage of several safety and efficacy studies in patients with the chronic myeloproliferative blood cancers (essential thrombocythemia, polycythemia vera and myelofibrosis) (MPNs), in whom IFN-alpha2 and ruxolitinib have been used successfully for the last 10 (ruxolitinib) to 30 years (IFN) as monotherapies and most recently in combination as well. In the context of these agents being highly immunomodulating (IFN boosting immune cells and JAK1/2 inhibitors being highly immunosuppressive and anti-inflammatory), we also discuss if statins and hydroxyurea, both agents possessing anti-inflammatory, antithrombotic and antiviral potentials, might be inexpensive agents to be repurposed in the treatment of SARS-CoV-2.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection emerged in Wuhan, China 2019 [1,2]. Since then, SARS-CoV-2 has spread rapidly across the globe to become as the most devastating pandemic in more than a century. The clinical spectrum of COVID-19 ranges from asymptomatic carriers, upper respiratory tract disease to severe COVID-19 pneumonia, requiring mechanical ventilation [[1], [2], [3]]. In severely afflicted patients in need of ventilator support, the mortality rate is high - in particular in elderly patients and in patients with a severe comorbidity burden, including cardiovascular diseases, metabolic syndrome, type II diabetes mellitus and other inflammation-mediated comorbidities [[1], [2], [3]]. In addition to the above risk factors, virus strain and virus load have been shown to be determinant for the severity of the COVID-19 infection. The mortality rate in the COVID-19 infection is significantly higher than the latest influenza pandemic with the influenza virus H1N1 in 2009 [4]. Importantly, the most influential pathogenic factor determinant for the high mortality rate is an overwhelming hyperimmune response elicited by the SARS-CoV-2, giving rise to an immense cytokine storm – a hyperinflammation syndrome – that may ultimately culminate in acute refractory respiratory syndrome and fatal multi-organ failure [[5], [6], [7], [8], [9]]. Autopsy studies have unraveled that the COVID-19 infection is also associated with a massive thromboembolic disease burden [[10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]], which not only involves the pulmonary vessels but diffusely and markedly impacts the vascular system due to microthrombotic angiopathy and endothelial dysfunction, the latter being described as “endothelialitis” [12].
Several clinical trials are currently investigating the best drugs to be used in the treatment of the COVID-19 infection. Most clinical trials include various anti-virus agents, either as monotherapy or in combination [25,26]. One of these anti-virus agents – remdesevir –was recently reported to reduce time to recovery but without a significant reduction in mortality [[27], [28], [29], [30], [31]]. Indeed, one study concluded that remdesivir was not associated with statistically significant clinical benefits [29], and a critical reappraisal of the use of remdesevir in COVID-19 afflicted patients concluded that it is far too premature to identify remdesivir as a life-saving intervention during the COVID-19 pandemic [32]. Based upon the preliminary results reported and without in-depth analysis of the safety profile, remdesevir was given a conditional marketing authorization in the EU on 3 July 2020 and was licensed by FDA on 22 October 2020 for the treatment of the COVID-19 infection with the recommendation to be used as early as possible during the COVID-19 infection [30,31].
In midst November 2020, WHO advised remdesevir not to be used in the treatment of hospitalized patients with COVID-19 infection due to a lack of evidence that remdesivir improves significant outcomes such as reduced mortality, need for mechanical ventilation, and time to clinical improvement [33]. Based on an analysis of 82 trials, the Copenhagen Trial Unit, Rigshospitalet Denmark, has concluded that remdesevir might benefit COVID-19 patients, but the certainty of evidence was low [34].
Surprisingly, only a few studies have investigated the potential role of the oldest anti-virus agent –interferon (IFN) – in the treatment of patients with the COVID-19 infection [[35], [36], [37]], mainly using inhaled IFN-beta [[38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54]]. Since the initial results of remdesevir as a useful drug in reducing mortality in COVID-19 afflicted patients [[27], [28], [29], [30]] do not hold true after in depth analysis of all remedesvir trials and the drug is today not being recommended by WHO, there is an urgent need to investigate other drugs with the potential to dampen the cytokine storm [[1], [2], [3],[7], [8], [9]].
In this context, a Janus Kinase (JAK) 1/2 inhibitor was suggested as a candidate drug, since it efficiently blocks the JAK- STAT (Signal Transduction and Transcription)) pathway and thereby the production and release of several potent inflammatory cytokines from different hyperactivated immune cells [[5], [6], [7], [8], [9]]. Therefore, several studies were subsequently launched, investigating the role of JAK1/2 inhibitor treatment - either as monotherapy or in various combinations - in the treatment of patients with the COVID-19 infection [25,26,[55], [56], [57], [58], [59], [60], [61], [62]]. These studies are based upon the mechanisms underlying the anti-inflammatory and immunosuppressive activity of the oldest JAK1/2 inhibitor, ruxolitinib, [[63], [64], [65], [66], [67], [68], [69]] and the immense hyperinflammation state which may be elicited by the COVID-19 virus [[7], [8], [9],[70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93]]. The urgent need to intensify investigations of JAK1/2 inhibitors in the treatment of severely COVID-19 afflicted patients has most recently been put in perspective as the efficacy of the other drug for treatment of severe COVID-19 – dexamethasone – [94] only associates with low to very low evidence after in depth analysis by among others WHO and The Copenhagen Trial Unit [33,34]. The excessive cytokine storm in severely afflicted patients with respiratory failure may not only associate with virus-induced hemophagocytosis [4,[7], [8], [9]] but also in several patients may contribute significantly to the pronounced thrombogenic state and the thrombotic microangiopathy [[10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]]. Therefore, targeting the cytokine storm by potent JAK1/2 inhibition [[7], [8], [9],26,[55], [56], [57], [58], [59], [60], [61], [62]] may likely also reduce the risk of life-threatening thromboembolic disease burden by dampening inflammation-mediated in vivo leukocyte, platelet and endothelial activation and activation of the coagulation system as well. Furthermore, since not only platelets and endothelial cells are involved in COVID-19 induced thrombogenesis and the diffuse thrombotic microangiopathy, but also neutrophils and neutrophil extracellular trap formation (NETosis) are of paramount importance as well [[95], [96], [97], [98], [99], [100], [101]], targeting the myeloid compartment and NETosis might be equally important. In this context, IFN-alpha2 or IFN-beta might be highly efficacious, dampening the inflammasome [102] and NETosis [103] together with a JAK1/2 inhibitor, which also impairs NET formation [104], and at the same time IFN potently inhibiting virus replication [[36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54]]. Based upon experimental, clinical, molecular and immunological studies, we herein describe the rationales and perspectives for treating the COVID-19 infection with IFN-alpha2 or IFN-beta in the early COVID-19 disease phase, and a JAK1/2 inhibitor in the later disease stage, either as monotherapies or in combination (IFN + JAK1/2 inhibitor). In addition, we describe inexpensive old drugs to be repurposed in the treatment of the COVID-19 infection – statins and hydroxyurea –due to their anti-inflammatory, antithrombotic and antiviral capabilities.
2. Rationale for treatment of COVID-19 patients with a JAK1/2 inhibitor
2.1. Does treatment with a JAK1/2 inhibitor have the potential to improve clinical outcome in the severely afflicted patient with coronavirus infection?
Several lines of evidence support the rationale for the use of JAK1/2 inhibition to extinguish the fire in COVID-19 pneumonia.
1. As alluded to above, acute respiratory failure [1,2] is among others explained by virus-mediated severe hyperinflammation in the lungs as part of a hyperinflammatory cytokine storm syndrome [[7], [8], [9]].
2. JAK1/2 inhibition with ruxolitinib has been successfully used for the last 10 years in the treatment of the chronic blood cancers - myelofibrosis and polycythemia vera (MPNs) [105]. Chronic inflammation is an important driving force for development and progression of these blood cancers, which accordingly have been described as “A Human Inflammation Model “[[106], [107], [108]]. Within hours/days, ruxolitinib alleviates inflammation-mediated symptoms and large spleens are being reduced within weeks to months. Several patients also suffer inflammatory connective tissue diseases, which are also markedly improved by treatment with ruxolitinib [109]. The highly beneficial effects of ruxolitinib treatment in MPN-patients are due to a rapid decline in elevated circulating inflammatory cytokines, which have been shown to impact prognosis and add important information in predicting prognosis in MPNs [110,111].
3. In several COVID-19 patients, this cytokine storm is also associated with secondary hemophagocytic lymphohistocytosis syndrome (HLS) – a severe hyperinflammatory syndrome which unfortunately in several patients is characterized by a fulminant and fatal hypercytokinaemia, low blood cell counts due to hemophagocytosis and multiorgan failure [4,[112], [113], [114], [115], [116]]. Murine models of HLS have confirmed the importance of inflammatory cytokines for the development of HLS [117], and the efficacy of JAK1/2 inhibition for its immediate resolution [[118], [119], [120], [121], [122], [123]]. Importantly, several clinical studies have shown ruxolitinib to be highly efficacious in HLS [[124], [125], [126], [127], [128], [129], [130]], being most recently confirmed in several patients in a single center study [129]. Of note, in several of these patients, the cytokine storm was refractory to high-dose glucocorticoids [[124], [125], [126], [127], [128], [129]], which neither previously were WHO recommended for the treatment of the acute respiratory distress syndrome (ARDS) nor imminent multi-organ failure consequent to excessive hyperinflammation in intensive-care patients [131,132].
4. Several single arm studies have already shown that JAK1/2 inhibitor treatment - either as monotherapy or in various combinations – may benefit patients with the COVID-19 infection [25,26,[55], [56], [57], [58], [59], [60], [61], [62]]. As addressed above, the rationales for these studies are based upon the mechanisms underlying the anti-inflammatory and immunosuppressive effects of ruxolitinib [[63], [64], [65], [66], [67], [68], [69]], and the immense hyperinflammation state which is elicited by the COVID-19 virus [[7], [8], [9],[70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93]].
5. Baricitinib - another JAK1/2 inhibitor – is being used for patients with rheumatoid arthritis and in addition to dampening inflammation, this agent may also affect cellular viral entry in COVID-19 [6]. Most recently, a large randomised trial has demonstrated combination therapy, baricitinib and remdesevir, to be superior to remdesevir alone in reducing recovery time and accelerating clinical improvement among patients with COVID-19, in particular among those receiving high-flow oxygen or noninvasive ventilation [133].
6. JAK 1/2 inhibition is being used in several other chronic inflammatory diseases, in which they often are highly efficaceous in controlling disease activity [134].
7. Severe graft-versus-host disease (GVHD) has been shown to respond favourably to ruxolitinib, even in patients otherwise refractory to high-dose steroids [135], being attributed to its highly potent immunosuppressive and anti-inflammatory capabilities. Accordingly, ruxolitinib is today FDA approved for the treatment of GVHD. Highly intriguing, a COVID-19 infection in an allogeneic hematopoietic stem cell transplant patient was attenuated on ruxolitinib treatment [57].
8. As alluded to above, most recent studies have shown that NETosis is deeply involved in thrombogenesis and organ damage in COVID-19 afflicted patients [[95], [96], [97], [98], [99], [100], [101]]. Ruxolitinib inhibits NET formation in patients with MPN [104] and may likely do so in patients with COVID-19 as well.
9. By inhibiting B-cells, ruxolitinib may decrease the production of autoantibodies against IFN and thereby prohibit the aggravation of the IFN deficiency state, which is also being elicited by the COVID-19 virus itself [136]. The rationales for treatment of COVID-19 with JAK1/2 inhibitors are summarized in Table 1 .
Table 1.
Rationales for Treatment with a JAK1/2 Inhibitor in Patients with COVID-19.
 |
Abbreviations: IFN = Interferon; HLS = Hemophagocytic Lymphohistiocytosis Syndrome; GVHD = Graft Versus Host Disease.
3. Rationale for treatment of the COVID-19 infection with Interferon-alpha2 or beta
Type I IFNs-alpha2/beta are potent antiviral agents, which have both direct inhibitory effects on viral replication and also support and enhance the immune response to improve clearance of the virus infection [[35], [36], [37]]. Treatment of hospitalized SARS-CoV patients with IFN-alpha2 during the SARS-CoV outbreak in Toronto in 2003 showed an accelerated resolution of lung abnormalities [137]. Several rationales and studies support the contention that IFN-alpha2a/2b or IFN-beta may also impact the clinical course of the COVID-19 infection [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53]].
1 SARS-CoV-2 was found to be more susceptible to type I interferons than SARS-CoV [41].
2. In an uncontrolled, exploratory study of patients moderately afflicted by COVID-19, Zhou et al. showed that nebulized IFN-alpha2b therapy shortened duration of viral shedding and reduced markers of acute inflammation such as CRP and interleukin (IL) IL-6, which correlated with the shortened virus shedding [49]. The importance of IFN-mediated reduction in virus shedding was underscored in the context of dampening population spread [49]. In addition, this important first study of IFN-alpha2 in COVID-19 afflicted patients highlighted that the concurrent reduction in inflammatory markers, including IL-6, might also influence the detrimental impact of this cytokine upon prognosis [49] but also emphasized that targeting the cause (SARS-CoV-2) by IFN-alpha2b might be a more rational approach than targeting the symptoms by blocking the IL-6 receptor, using e.g tocilizumab or sarilumab [49].
3. A multicenter study of the safety and efficacy of early intervention with IFN showed that early IFN therapy was associated with favourable responses in COVID-19 patients [48].
4. In a prospective randomised trial adding IFN-beta by injection to the anti-virus agents lopinavir-ritonavir, and ribavirin in patients with mild or moderate disease at the time of enrolment displayed superiorty in the IFN-arm in regard to a significant reduction in duration of virus shedding and significant differences in outcomes as well. It is important to note that IFN-beta was given in the combination group only to patients who were enrolled less than 7 days after onset of symptoms [45,46].
5. Nebulized IFN-beta inhalation therapy was associated with decreased mortality in Chinese COVID-19 patients and has also demonstrated safety and efficacy in other studies with a rapid viral clearance in concert with clinical improvement [138,139]. The rationales for using nebulized IFN-beta inhalation therapy have recently been reviewed [139].
6. IFN may also impact regression of lung fibrosis after COVID-19 [140], and SARS-CoV-2 has been shown not to elicit a significant induction of types I, II, or III interferons in ex-vivo infected human lung tissues as compared with 2003 SARS-CoV [141]. Indeed, SARS-CoV-2 seems to have developed a distinct machinery, which is able to shut down host IFN production, which subsequently has been convincingly demonstrated in several studies [[142], [143], [144]]. Thus, Blanco-Melo et al. showed that SARS-CoV-2 induced only a very weak type 1 and III IFN response, which was juxtaposed to a cytokine storm with highly elevated serum IL-6 and TNF-α levels [142]. Similarly, in a comprehensive study of inflammatory and immunological signatures in COVID-19 patients, Hadjadj et al. reported low IFN-alpha2 plasma levels [144]. Low IFN-alpha2 levels preceded clinical deterioration, and distinct patterns of circulating IFN-alpha2 characterized each disease grade. Furthermore, IFN activity in serum of severe or critical ill patients was also lower than that of mild-to-moderate ill patients [144]. In all three studies, serum IFN-beta concentrations were undetectable as well [[131], [132], [133]]. Since type I IFN deficiency is associated with hyperinflammation driven by NF-κB and lower viral clearance, their results definitely support the contention that the early use of IFN - likely in combination with targeted anti-inflammatory therapies, which will be addressed below - may overcome SARS-CoV-2 infection by preventing rapid virus spreading and the subsequent damaging cytokine storm [42,145]. This may also hold true for patients with severe infection, since the most severe cases of COVID-19 were featured by impaired IFN-alpha2 production and higher virus loads [142,143].
As noted above, type I IFN production may not only be reduced or exhausted in the severely afflicted patients [142,143] but indeed being an early event, elicited by the SARS-CoV-2 itself, since recent cellular and animal studies have shown that SARS-CoV-2 inhibits type I and III IFN induction [146].
7. IFN beta decreases virus-induced lung fibrosis in a mouse model, which might improve outcomes of COVID-19 patients severely afflicted by ARDS [140,141].
8. Most recently, novel genetic mechanisms of critical illness in COVID-19 have been unravelled in an extensive genome-wide association study (GWAS) in 2244 critically ill UK COVID-19 patients [147]. Highly intriguing, increased expression of the interferon receptor subunit IFNAR2, which is critical for an adequate response to viral infections was found to reduce the risk of severe COVID-19, implying IFNAR2 to have a protective role against severe COVID-19. It was concluded that IFN treatment of COVID-19 patients may reduce the risk of critical illness [147]. The critical role of IFNAR2 in the context of protecting against severe COVID-19 is substantiated by the reported associations between loss-of function mutations in IFNAR2 and severe virus diseases [148,149], including severe COVID-19 [150].
9. “High-risk profile” patients for serious disease and mortality from COVID-19, e.g. the elderly and obese patients [151] have been shown to have impaired IFN-responses [152,153]. Thus, elderly people and obese patients may in particular benefit from early treatment with IFN. The concept of an association between a type I IFN deficiency state and susceptibility to COVID-19 infection and seriousness of the disease is supported by the mild courses of the COVID-19 in most children, who have a much more robust type I IFN response than the elderly [154].
10. IFN has been shown to normoregulate or significantly downregulate upregulated thromboinflammatory genes, including PAD4 in patients with MPNs [103]. Normo- or downregulation of thromboinflammatory genes by IFN may likely decrease NETosis formation in patients with COVID-19 infection as well and accordingly the increased risk of thrombosis. Since oxidative stress is also closely associated with thrombogenesis by several mechanisms [155], it is important to note that IFN-alpha2 has been shown to significantly downregulate upregulated oxidative stress genes and upregulate downregulated anti-oxidative defence genes in patients with MPNs [156].
11. In a mouse model of arthritis, IFN-alpha2 decreased the production of several cytokines, including IL-6, IL-12, and tumor necrosis factor alpha (TNF-α)) and increased the serum levels of the anti-inflammatory cytokine transforming growth factor beta (TGF-β) after antigen stimulation [157]. IFN-alpha2 also elicited an early macrophage-derived production of TGF-β combined with a later increase in CD4 + T cells producing TGF-β [157]. Interestingly, in the early phase after immmunisation, presence of IFN-alpha2 inhibited production of IL-12 and TNF-α whereas IFN-γ, including macrophages producing IFN-γ was inhibited at later times [157]. The inhibitory effect of type I IFNs on Th1 immunity (IL-12, TNF-α, IL-1β, and IFN-γ signaling) has also been recorded in monocytes [158]. Taken into account the prominent role of the monocyte-macrophage system in the development of tissue damage during the COVID-19 infection [[159], [160], [161], [162], [163]], the observations of the impact of IFN-alpha2 upon cytokine levels additionally support the early administration of IFN-alpha2 in COVID-19 afflicted patients, thereby likely decreasing elevated levels of several highly important cytokines (IL-1β, IL-6, IL-12, TNF-α, and IFN-γ) which are considered of utmost importance for the development of the cytokine storm and ultimately multi-organ failure. The early inhibition of Th1-promoting cytokines, especially IL-12, by IFN-alpha2 may also be timely and of utmost importance when considering that IFN-alpha2 might lower the increased number of T-helper cells (CD4+) during the COVID-19 infection and thereby also decreasing the production of the Th1 cytokine IFN-γ. Importantly, IFN-alpha2 has been shown to induce a marked increase in circulating CD4(+)CD25(+)Foxp3(+) T cells (Tregs) [164]. TGF-β is an important mediator for development of both Tregs [165] and their immunosuppressive capacity. Of note, TGF-β, in contrast to all other analyzed cytokines, was significantly increased in mice treated with IFN-alpha2 [157]. Highly interesting, type I IFN also negatively regulates CD8 + T cell responses through IL-10-producing CD4 + T regulatory cells [166].
12. Type I IFNs exert a variety of effects on monocytes, including rapid differentiation of monocytes into activated dendritic cells, which – together with IFN-induced activation of NK-cells – is considered of utmost importance for the rapid development of a robust antiviral response [[167], [168], [169]]. Based upon the above observations, we hypothesize that the administration of IFN-alpha2 or IFN-beta in COVID-19 patients induces a tolerogenic state by a concert of actions, including inhibition of virus replication, inhibition of proinflammatory cytokines, especially early IL-6 production, early enhancement of TGF-β-producing macrophages, resulting in fewer IFN-γ- and IL-17-producing CD4 + T cells and at a later stage development of TGF-β-producing CD4 + T cells. Rationales for therapy with IFN-alpha2 or beta in patients with COVID-19 are summarized in Table 2 .
Table 2.
Rationales for Treatment with Interferon-alpha2 and Interferon-beta in Patients with COVID-19.
 |
Abbreviations: IFN = Interferon; HLS = Hemophagocytic Lymphohistiocytosis Syndrome; ND = No Data; ET = Essential Thrombocythemia; PV = Polycythemia vera.
4. Rationale for combination therapy with interferon and JAK1/2 inhibitor in COVID-19
Since several experimental and clinical studies have demonstrated both IFNs (-alpha2 or -beta) and JAK1/2 inhibitor therapy to inhibit several disease-promoting mechanisms in patients with COVID-19 as summarized in all the above mentioned rationales for monotherapy with IFNs and JAK1/2 inhibitors, it is tempting to speculate whether a combination of these agents might indeed be superior to single agent therapies. In the context of combination therapy with an activator of antiviral immunity (IFN) and an inhibitor of antiviral signaling (JAK/STAT inhibitor) one may wonder, whether JAK/STAT inhibition might not impair the efficacy of IFN. However, our clinical trials in MPN-patients have shown that these two agents actually act in synergy, implying an enhanced efficacy of this combination therapy. As addressed below, these highly interesting and encouraging findings may be explained by several mechanisms, including the fact that e.g ruxolitinib has a half-life of only a few hours leaving an open window of several hours per day for IFN-signalling. Other mechanisms might be that JAK/STAT inhibition dampens inflammation, which has been reported to impair IFN-signalling by degradation of the IFN-receptor (please, see below). Indeed, we believe that the avenue is already open for pilot studies combining IFN and JAK1/2 inhibitor treatment in well-designed trials, thereby prohibiting virus replication and virus shedding (IFNs) in concert with a massive boosting of virtually all immune cells (IFNs) and concurrently dampening the hyperinflammation (IFNs and JAK1/2 inhibition), which may ultimatively elicit the life-threatening cytokine storm. Importantly, inflammation is associated with impaired efficacy of IFN-alpha2 [170]. All effects of IFN-alpha2 and beta are elicited through interaction with the type I IFN receptors which consists of IFNAR1 and IFNAR2 chains, and inflammation-mediated downregulation of IFNAR1 is associated with refractoriness to IFN [171]. In this context, it is important to note that the inflammatory cytokines interleukin 1-α (IL1-α) and TNF-α, which are elevated in COVID-19 patients in the hyperinflammatory stage, stimulate IFNAR1 degradation and accordingly attenuate IFN-alpha2 signaling [170]. Similarly, unresponsiveness to IFN-alpha2 in hepatitis patients may be explained by oxidative stress, impairing IFN-alpha2 signaling [172]. As noted previously, COVID-19 afflicted patients display increased levels of several inflammatory cytokines, including IL1-α and TNF-α, the highest levels being reported in patients with imminent respiratory failure. Thus, in this perspective, treating COVID-19 patients with IFN-alpha2 at the earliest disease-stage possible, when the inflammatory state is less pronounced, seems to be a more rational approach than initiating IFN-alpha2 later when the inflammatory load is increasing and therefore the efficacy of IFN is declining and potentially harmful. The early intervention with IFN has recently been supported by mathematical modelling studies of the COVID-19 infection, showing that the earlier IFN is instituted the better the treatment response (92). These model simulation studies also support the concept that anti-inflammatory or antiviral treatments combined with IFN are effective in reducing the duration of the viral plateau phase and in diminishing the time to recovery (92).
The rationales of studies on combination therapy with JAK1/2 inhibitor and IFN (COMBI) are also encouraged by Danish safety and efficacy studies of COMBI in patients with myelofibrosis and polycythemia vera, displaying highly encouraging results with rapid resolution of inflammation-mediated MPN symptoms and improvement in disease activity of other inflammatory diseases in MPN-patients as well [173]. These studies have demonstrated IFN-alpha2 and ruxolitinib to exert synergistic effects, implying reduced dosages of both IFN and ruxolitinib to obtain normalization of elevated leukocyte and platelet counts. Accordingly, we envisage COMBI in COVID-19 to be disease modifying in terms of shortening of virus shedding, shortening of time with symptoms, reduction in symptom score and severity and ultimately in reducing the risk of complications, including thromboses, and risk of disease progression towards terminal multi-organ failure. Rationales for combination therapy with IFN-alpha2 or beta and JAK1/2 inhibitor (COMBI) in patients with COVID-19 are summarized in Table 3 .
Table 3.
Rationales for Combination Therapy with Interferon-alpha2 or beta and JAK1/2 inhibitor (COMBI) in Patients with COVID-19.
 |
5. Urgent questions on the role of treatment with interferon-alpha2, JAK1/2 inhibitor, statins and hydroxyurea and factors of potential importance for the phenotype of the COVID-19 infection in individual patients
5.1. Does interferon-alpha2 protect against COVID-19 infection?
Interferon-alpha2 has been used for decades in the treatment of hepatitis B and C [174]. In addition, it has been used previously in the treatment of HIV-infection [174]. As addressed above, several studies have been performed or are ongoing, investigating the safety and efficacy of IFN in the treatment of infection with COVID-19, either as monotherapy or in combination with other anti-viral agents [[45], [46], [47], [48], [49], [50], [51], [52], [53], [54],139,[142], [143], [144]]. During the last 30 years, IFN-alpha2 has been used in the treatment of patients with MPNs [106]. Within weeks to months, IFN-alpha2 normalizes elevated blood cell counts. The mechanisms of action of IFN-alpha2 in MPNs are likely – among others - attributed to a potent enhancement and boosting of virtually all immune cells (dendritic cells, NK-cells, T cells and B-cells as well). It is a clinical experience that MPN-patients being treated with IFN-alpha2 more rarely are afflicted by infections, including viral infections, but no larger studies have explored this potential effect of IFN-alpha2. From current registry studies on all Danish IFN-alpha2 treated patients (estimated to be approximately 1000 patients) with collection of data on the frequency of COVID-19 infection, the severity of the COVID-19 infection in terms of the need of hospitalization and need of mechanical ventilation, we envisage to provide highly important information on the frequency of COVID-19 infection in IFN-alpha2 treated patients and whether treatment with IFN-alpha2 might actually protect against COVID-19 infection and reduce the risk of serious or critical disease.
5.2. Does JAK1/2 inhibitor treatment protect against development of a life-threatening cytokine storm in COVID-19 afflicted patients?
Taking into account the reduction of inflammatory cytokines in MPN patients being treated with ruxolitinib and in patients with rheumatoid arthritis on treatment with baricitinib, it is tempting to speculate whether these patients may actually be protected against severe COVID-19 infection – an issue, which has most recently been addressed in patients with MPNs [175]. Since ruxolitinib impairs cellular immunity by depressing virtually all immune cells, one might anticipate ruxolitinib-treated MPN patients to be prone to a more severe course of the COVID-19 infection. Therefore, the COVID-19 Treatment Guidelines Panel does not recommend the use of JAK1/2 inhibitors for COVID-19 except in clinical trials, arguing that the broad immunosuppressive effects might exaggerate the COVID-19 infection [176]. In regard to MPN, the guidelines for treating COVID-19 infection among others note that ruxolitinib may predispose the patients to severe COVID-19 infection [177]. For reasons given above, we believe that ruxolitinib may actually benefit MPN-patients with COVID-19 infection, reducing their risk of developing a cytokine storm and accordingly severe and critical disease [178]. Furthermore, by dampening the hyperinflammatory state in MPN-patients, ruxolitinib may also reduce the increased risk of thromboembolism, elicited by the COVID-19 infection per se [[10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]] and adding to the inherent risk of thrombosis associated with MPN. Therefore, we do not recommend discontinuation of ruxolitinib in COVID-19 afflicted patients with MPN, since the COVID-19 infection and the concurrent hyperinflammatory state may be deleterious and more easily elicit a withdrawal syndrome with rapid clinical deterioration and a high mortality rate [179]. Most recently, this clinical dilemma has also been described in an elderly female patient with myelofibrosis, being treated with ruxolitinib and developing severe illness when ruxolitinib was discontinued and immediately improved when ruxolitinib was restarted. Intriguingly, adding IFN-alpha2 cleared the virus infection [180].
5.3. Does interferon-alpha2 treatment of the COVID-19 infected patient have the potential to prohibit hospitalization?
Based upon the promising results from several trials on the safety and efficacy of IFN-treatment in COVID-19 afflicted patients, it is tempting to speculate whether patients, already being treated with IFN might have a reduced risk of developing severe COVID-19 infection. As previously addressed, this information will be retrieved from current registry studies of the clinical outcome of COVID-19 infection in patients being treated with IFN (class examples: MPN, multiple sclerosis, hepatitis B or C). If so, this observation will further support the urgent need for prospective trials on the safety and efficacy of pegylated IFN (either alpha2 or beta) in reducing the symptom burden and time to recovery from COVID-19 infection. In this context, the need of hospitalization might also be reduced. The beneficial impact of a favorable outcome of such an IFN-intervention in the early stage of COVID-19 will not only be measurable at the individual level but also at the socioeconomic level in terms of a huge reduction in costs for hospitalisations and when considering that patients may more rapidly recover and return to the labour market. Taking into account that treatment with pegylated IFNs e.g IFN-alpha2a (Pegasys), IFN-alpha2b (Besremi) or IFN-beta-1a (Plegridy) (two injections with one week’s interval (Pegasys) or with two weeks intervals (Besremi and Plegridy) is likely sufficient to boost immune cells and restore the temporary immunodeficiency state induced by the COVID-19 infection) accounts about 2000 DKK (example: Pegasys 90 ug per week) equivalent to approximately 290 USD and the devastating consequences of the COVID-19 pandemic, we believe such trials to be highly relevant and timely and to have a cost-effectiveness profile that calls for their urgent launching. Such an out-patient IFN- schedule is also much more cost-effective than remdesevir, which requires adherence to hospital due to daily intravenous injections for approximately 10 days. Furthermore, early treatment with IFN might also reduce the risk of debilitating long-term consequences of COVID-19, including e.g mental diseases, chronic fibrosing lung disease, chronic heart failure or chronic kidney disease [181].
5.4. Does statin treatment improve the outcome of the COVID-19 infection?
Statins are not only cholesterol-lowering agents but possess a broad range of so-called pleiotrophic effects, which include potent anti-inflammatory and anti-thrombotic capabilities. Accordingly, several studies and reviews have focused upon the potential of statins as an adjuvant to current therapies of the COVID-19 infection, which has most recently been extensively reviewed [182,183].
The potential beneficial effects of statins in COVID-19 patients not only include anti-inflammatory and anti-thrombotic effects by blocking several molecular mechanisms, including NF-κB and NLRP3 inflammasomes, and thereby calming the cytokine storm in critical COVID-19 patients. Statins may also modulate virus entry, acting on the SARS-CoV-2 receptors, ACE2 and CD147, and/or lipid rafts engagement [183]. Importantly, statins may also decrease the risk of thrombosis by modulating coagulation and fibrinolytic pathways. Thus, statins enhance the fibrinolytic activity within the vessel wall as reflected by the findings of reduced levels of plasminogen activator inhibitor-I (PAI-I) and increased levels of tissue plasminogen activator (t-PA) within smooth muscle and endothelial cells. Additionally, statins have been shown to inhibit tissue factor expression by monocytes and macrophages. Statins also strongly enhance endothelial anticoagulant and fibrinolytic properties by increasing thrombomodulin expression and function in human endothelial cells [183]. All these statin effects may counteract the COVID-19 associated thrombogenic state by the combined effects of impaired virus entry into cells, inhibition of thrombogenic activated blood cells (leukocytes, monocytes, thrombocytes) and endothelial cells (decreasing COVID-19 induced “endothelialithis“?) and enhancing anticoagulant and fibrinolytic properties of the inflamed endothelium (182,183). Since statins also enhance the efficacy of IFN and JAK1/2 inhibition with ruxolitinib, the avenue is opened for well -designed studies of treating COVID-19 patients with a combination of these agents – very similar to the rationales for treating the “inflammatory“ myeloid neoplasias – MPNs – with these repurposed anti-inflammatory agents in a combinatorial approach [106]. Rationales for the potential benefits of statins in patients with COVID-19 are summarized in Table 4 . Repurposing the old drugs – IFN (-alpha2 or beta) and statins – with a JAK1/2 inhibitor for the treatment of COVID-19 infection is illustrated in Fig. 1 .
Table 4.
Rationales for Treatment with Statins in Patients with COVID-19.
 |
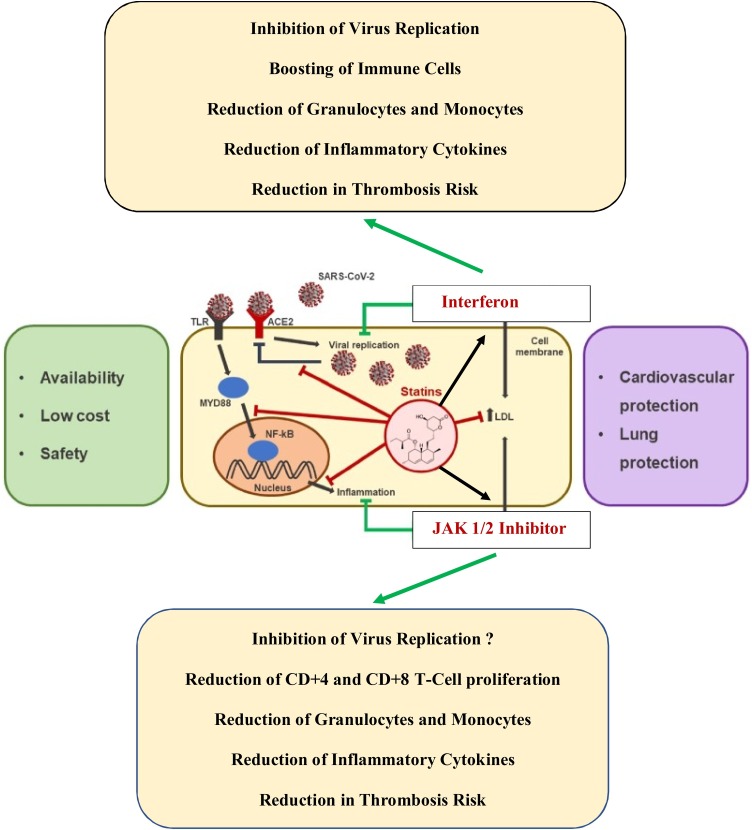
Fig. 1.
Repurposing old drugs – interferon (-alpha2 or beta) and statins – with a JAK1/2 inhibitor for the treatment of COVID-19 infection. The old drugs, interferon and statins, are safe and cost-effective in the treatment of COVID-19 as compared to remdesevir, which requires hospitalization and daily injections for 8-10 days. Combination therapy with interferon (alpha2) and JAK1/2 inhibitor (ruxolitinib) has been shown to exhibit synergistic effects in the treatment of the Philadelphia-negative myeloproliferative neoplasms (essential thrombocythemia, polycythemia vera and myelofibrosis). By early administration of these agents, it is envisaged that virus replication is immediately blocked and concurrent incipient inflammation exhausted by JAK1/2 inhibition. Thereby the vicious virus-induced inflammation circle is interrupted. Statins are potent anti-inflammatory agents, which enhance JAK-STAT- signaling and accordingly the efficacy of interferon. Statins also enhance the efficacy of JAK1/2 inhibitor (ruxolitinib) (Modified from Vincenzo Castiglione et al. Statins in COVID-19 infection. European Heart Journal - Cardiovascular Pharmacotherapy (2020) 6, 258–259.
5.5. Does hydroxyurea treatment improve the outcome of a COVID-19 infection?
Hydroxyurea is a nonalkylating antineoplastic and antiviral hydroxylated urea analog, which for several decades has been used for a variety of hematological, oncological and infectious diseases. Today, it is primarily being used in the treatment of MPNs and sickle-cell anemia (SCA). Hydroxyurea inhibits ribonucleotide phosphatase reductase in proliferating cells and lowers within a few days elevated cell counts [184]. In addition, hydroxyurea also potently lowers elevated levels of inflammatory cytokines in another “inflammation model” for thrombosis development—SCA— which shares several thrombosis promoting mechanisms with the COVID-19 infection and MPNs, including in vivo activation of leukocytes and platelets and in vivo activation of endothelial cells as well [185].
Hydroxyurea reduces the thrombosis risk including the risk of SCA crisis, which -like COVID-19 - is yet another hyperinflammatory thrombogenic syndrome, tightly associated with exacerbation of the chronic inflammatory state in SCA. Thus, circulating inflammatory cytokines (eg, TNF-α, IL-8, IL-1β, and IL-6) are markedly elevated in SCA and hydroxyurea significantly lowers them all. Hydroxyurea not only affects monocyte subsets, but also the ability of the cells to produce pro-inflammatory cytokines [186], thereby decreasing the inflammatory state considered contributary to the increased risk of thrombosis in SCA patients [185]. Similarly, it is intriguing to consider, if hydroxyurea might calm the cytokine storm in critically ill COVID-19 patients by immediate reduction in elevated inflammation-driven cell counts, which also account for elevated inflammatory cytokines, the devasting, life-threatening thrombogenic state and accordingly being predictors of a grim outcome. By the immediate reduction in elevated cell counts and impact upon neutrophils and monocytes, it is tempting to speculate, whether hydroxyurea might also reduce NET formation in COVID-19 afflicted patients and by this mechanism reduce the risk of thrombosis as well.
Hydroxyurea has also previously been used as an antiviral agent. Thus, in vitro studies of HIV-infected lymphocytes have shown that hydroxyurea inhibits viral DNA synthesis, interacts synergistically with nucleoside reverse transcriptase inhibitors and increases the antiviral activity of didanosine. Hydroxyurea in combination with didanosine has been shown to produce potent and sustained viral suppression in patients with HIV infection. As in HIV-infection, hydroxyurea may also in COVID-19 attenuate viral rebound by decreasing CD4 T cell proliferation, as well as preventing the exhaustion of CD8 T cells (for Reviews: 187,188). Indeed, the cytostatic effect of hydroxyurea on both CD4 and CD8 T cells may also prohibit the cytokine storm by reducing COVID-19 induced immune system overactivation, thus preventing both CD8 T cell exhaustion and CD4 T cell depletion and thereby the ultimate collapse of the immune system in COVID-19 [189,190]. Rationales for the potential benefits of hydroxyurea in patients with COVID-19 are summarized in Table 5 . Repurposing the old drugs - IFN- (alpha or beta), statins and hydroxyurea -for the treatment of COVID-19 infection is illustrated in Fig. 2 (Table 6 ).
Table 5.
Rationales for Treatment with Hydroxyurea in Patients with COVID-19.
 |
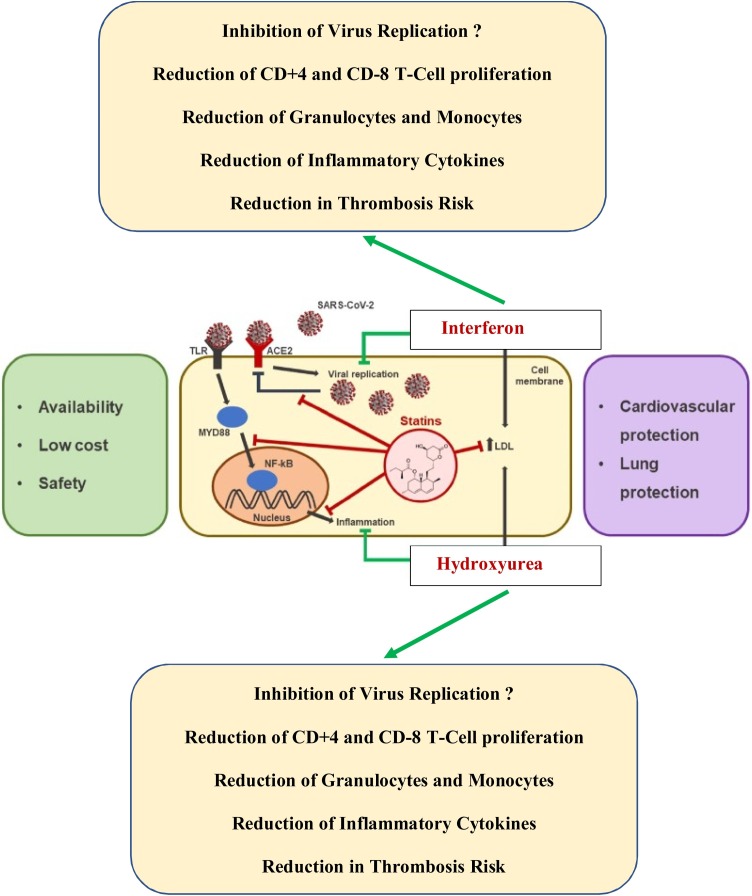
Fig. 2.
Repurposing old drugs – interferon (-alpha2 or beta) and statins – with hydroxyurea for the treatment of COVID-19 infection. The old drugs, interferon, statins and hydroxyurea, are safe and cost-effective in the treatment of COVID-19 as compared to remdesevir, which requires hospitalization and daily injections for 8-10 days. Combination therapy with interferon (alpha2) and hydroxyurea targets the myeloid compartment with a rapid reduction in granulocytes and monocytes (hydroxyurea), which may be of utmost importance in dampening lung damage and accordingly reducing the risk of ARDS being characterized by a massive influx of neutrophils and monocytes. By early administration of these agents, it is envisaged that virus replication is immediately blocked and concurrent incipient inflammation exhausted by the combined actions of these three agents. Thereby, the vicious virus-induced inflammation circle is interrupted. Statins are potent anti-inflammatory agents, which enhance JAK-STAT- signaling and accordingly the efficacy of interferon. Statins also enhance the efficacy of JAK1/2 inhibitor (ruxolitinib) (Modified from Vincenzo Castiglione et al. Statins in COVID-19 infection. European Heart Journal - Cardiovascular Pharmacotherapy (2020) 6, 258–259).
Table 6.
Similarities and Differences between Interferon alpha2, Interferon-beta, JAK 1/2 Inhibitor, Hydroxyurea and Statins in regard to Clinical, Biochemical and Immunological Markers in COVID-19. Prospects for Combination Therapies.
 |
Abbreviations: ND = No data.
5.6. Do the weak Type I responses in the elderly and obese predispose to severe and critical COVID-19?
Regarding the weak type I IFN responses, induced by the the SARS-CoV-2, they have been demonstrated to be associated with longer time to obtain virus clearance and more severe hyperinflammation [142,143]. Therefore, it is intriguing to consider, whether the weak type I IFN responses are compensated by immune mechanisms, which unfortunately then associate with a cytokine storm [139]. In this context, it might be anticipated that early treatment with IFN may prohibit disease progression by several mechanisms, including both a direct inhibition of virus replication and thereby the virus-promoting IFN-deficiency state but also indirectly by dampening the immune mechanisms that elicit the ensuing hyperinflammation.
“High-risk profile” patients for serious disease and mortality from COVID-19, e.g. the elderly and obese patients [151] have been shown to have impaired IFN-responses [152,153]. Thus, elderly people and obese patients may in particular benefit from early treatment with IFN. The concept of an association between a type I IFN deficiency state and susceptibility to COVID-19 infection and seriousness of the disease is supported by the mild courses of the COVID-19 in most children, who have a much more robust type I IFN response than the elderly [154].
5.7. An association between clonal hematopoiesis of indeterminate protential (CHIP) in the elderly and risk of serious COVID-19 infection?
In the perspective of the elderly, age-related “inflammatory mutations “– inflammaging and immunaging – might also add to the increased risk of a detrimental course of the COVID-19 infection and, indeed, other infections as well. Thus, the JAK2V617F mutation is a thrombosis promotor per se, which might aggravate the inherent risk of thrombosis elicited by the COVID-19 infection. In addition, the JAK2V617F mutation is a generator of reactive oxygen species (ROS) which similarly may “fuel the fire“. Importantly, as alluded to above, the JAK2V617F mutation also facilitates NETosis formation, which is closely associated with the development of thrombosis. In regard to CHIP, the JAK2V617F mutation is much more prevalent in the background population than previously reported with an estimate of approximately 3.2 % [191], implying a massive underdiagnosis of MPNs (550.000 US citizens with undiagnosed MPNs and an inherent risk of serious COVID-19 infection?). Other inflammatory mutations, which appear with aging such as TET2, DNMT3A and ASXL1, may also be particular risk factors for severe COVID-19 to be addressed in future studies.
5.8. An association between an antecedent elevated inflammatory state and increased morbidity and mortality during the COVID-19 infection?
Not only elderly and obese people but also patients with chronic inflammatory diseases (e.g rheumatoid arthritis, inflammatory bowel diseases (IBD), cardiovascular diseases, type II DM, hematological and non-hematological cancers) have an increased risk of developing serious COVID-19 disease [[192], [193], [194], [195], [196]]. It is tempting to speculate, whether the increased morbidity and mortality during the COVID-19 infection are more dependent upon the antecedent inflammatory state – the fire is already turned on -, implying an increased risk of rapid development of an excessive and life-threatening cytokine storm, than being dependent upon the immunodeficiency state, which may associate with the diseases per se (e.g cancer) or consequent to immunosuppressive treatment. In this context, cytokine inhibitors might actually protect patients against severe COVID-19 infection. Indeed, a most recent study has shown a low prevalence of COVID-19 seroconversion in patients with IBD being treated with cytokine inhibitors [197]. This highly important observation may reflect that cytokine inhibitor treatment actually may at least partially protect from SARS-CoV-2 infection [197].
5.9. Is the monocyte the common link to explain the impact of inflammation upon the severity of the COVID-19 infection?
As alluded to above, the monocyte-macrophage cells have a predominant role in the immunopathology of the SARS-CoV-2 infection [198]. Thus, these cells are considered drivers of the cytokine storm, in which IL-6 may have a particular role, since this cytokine is an essential factor in controlling monocyte activation, differentiation of monocytes to macrophages, and switching the differentiation of monocytes from dendritic cells with antigen-presenting functions to inflammatory macrophages [199].
The metabolic syndrome, obesity, type II diabetes mellitus and cardiovascular diseases are classic risk factors for severity in COVID-19 patients. These conditions and diseases are all closely related to perturbation of the monocyte compartment, implying a marked shift towards a pro-inflammatory phenotype. This shift might contribute greatly to the development of low-grade inflammation, which is recorded in patients with the above diseases [200]. IL-6 has been highlighted as a driver of “metabolic inflammation” [200], and elevated IL-6 levels are significantly associated with an increased risk of severe COVID-19 infection [84].
5.10. Does loss of function of the IL-6 receptor protect against a severe course of COVID-19. Lessons from studies in patients with MPNs?
Chronic inflammation is considered to be a highly important driving force for clonal evolution and disease progression in patients with MPNs [[106], [107], [108]]. The concept of chronic low-grade inflammation as an independent risk factor for the JAK2V617F somatic mutation and MPN has most recently been substantiated in a Mendelian randomization approach in the Copenhagen General Population Study with 107,969 individuals [201]. This large study confirmed that an anti-inflammatory loss-of-function polymorphism in the IL6R gene (marked by rs4537545) reduces risk of JAK2V617F somatic mutation and myeloproliferative neoplasm. Based upon these findings, it was proposed that agents blocking the IL-6R signaling might prevent or retard progression of MPN. Since elevated levels of inflammatory cytokines, in particular IL-6, are driving the life-threatening cytokine storm in COVID-19 patients, this loss-of-function polymorphism in the IL6R gene might reduce the risk of developing a severe and potentially critical cytokine storm in MPNs – an association which deserves to be explored in future studies.
5.11. What is the Role of the Renin-Angiotensin System (RAS) in the Pathobiology of the COVID-19 Infection and how does Interferon-alpha2 /beta and JAK1−2 Inhibitors impact the RAS system?
Angiotensin Converting Enzyme 2 (ACE2) is the main host cell receptor for human pathogenic coronaviruses (SARS-CoV, MERS and SARS-CoV-2) and plays an important role in the entry of the virus into the cell, and for viral spreading and pathogenesis [[202], [203], [204], [205]]. ACE2 is widely distributed in human tissues and is considered a determinant factor of the pathophysiology of COVID-19. Since studies have highlighted that IFNs might enhance expression of ACE2, it has been suggested that IFN therapy could potentially exacerbate COVID-19 by upregulating ACE2 [206]. Importantly, however, a most recent study has shown that antiviral activity of type I, II and III IFNs counterbalances ACE2 inducibility and indeed restricts SARS-CoV-2 [207]. Accordingly, ACE2 is not induced by IFN [208,209].
Human monocytes exhibit different expression of ACE type 1 and 2 [210] and are considered to be directly involved in the regulation of vascular homeostasis, and monocytes are involved in the development of acute coronary syndromes. Thus, SARS-CoV-2 activation of monocytes and perturbation of RAS through ACE2-monocyte activation may trigger acute coronary syndromes in predisposed COVID-19 patients [211]. JAK1/2 inhibitor treatment impairs monocyte activation and production of inflammatory cytokines from activated monocytes [212,213], thereby indirectly impairing cytokine-mediated activation of ACE2 [214]. In addition, JAK inhibitors have been shown to curb the activation of ACE2 and IFN-stimulated transcriptomes in human airway epithelium [215].
5.12. Is the JAK2V617F mutation associated with an increased risk of serious COVID-19 in the younger population?
Considering the above notions on a potential association between CHIP and risk of serious COVID-19 infection in the elderly, it is relevant to consider, whether severe and critical COVID-19 infection in younger people may also associate with acquisition of the JAK2V617F mutation and perhaps a particular JAK2 46/1 haplotype, which may associate with an exaggerated myelomonocytic response to infectious agents and impaired defence against infection [216]. Highly intriguing, a most recent study has provided evidence that the JAK2-mutant clone may manifest as CHIP for a decade or more before presenting as an overt MPN [217]. These findings are supported by another most recent study that provides evidence for MPN to originate from driver mutation acquisition (JAK2V617F) very early in life, even before birth, with life-long clonal expansion and evolution [218]. Accordingly, the scientific platform for this hypothesis is robust and studies on this association are urgently needed.
6. Discussion and perspectives
Every day patients with COVID-19 pneumonia are dying due to refractory respiratory failure in the intensive care units (ICU) worldwide. Despite launching of vaccination programmes worldwide, the scenario with desperately ill patients fighting for their lives will continue and many thousands will succumb. Today we know that the high mortality rate is mainly attributed to a deadly cytokine storm, which develops consequent to an extreme virus-induced hyperstimulation of the immune system. After failure of both remdesevir and dexamethasone to reduce mortality in COVID-19 [33,34] and accordingly no longer being WHO-recommended, no specific and effective evidence-based treatment, reducing mortality is available for patients suffering acute respiratory failure due to COVID-19 pneumonia other than ventilatory support and antibiotic treatment for complicating bacterial infections. Taking into account that mortality in COVID-19 pneumonia is driven by a severe hyperinflammatory state, it is time for rethinking the urgent need to institute highly potent anti-inflammatory treatment with a JAK1/2 inhibitor in severely afflicted patients with COVID-19 pneumonia [[5], [6], [7], [8]], taking into account the increasing number of studies, showing JAK1/2 inhibition with ruxolitinib to be safe and efficaceous in these patients [26,[55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69]].
We have severel years’s experience with JAK1/2 inhibitors in the treatment of chronic inflammatory diseases, but also in another acute potentially lethal hyperinflammation syndrome, which is also elicited by virus - the virus-associated HLS [4,[124], [125], [126], [127], [128], [129]]. In this context, preliminary data indicate that the cytokine storm in COVID-19 pneumonia is no exception and may be associated with HLS. Unfortunately, this hyperinflammatory syndrome is in several patients characterized by a fulminant and fatal hypercytokinaemia, low blood cell counts due to hemophagocytosis and multiorgan failure. Importantly, as stressed above, ruxolitinib is highly efficacious in HLS and may rescue patients from acute refractory respiratory and multiorgan failure [[124], [125], [126], [127], [128], [129]]. The efficacy of ruxolitinib in calming the cytokine storm has been thoroughly investigated in animal models of HLS [[118], [119], [120], [121], [122], [123]], showing that the mechanisms of action involve inhibition of among others CD8 + T cells and important inflammatory cytokines, including IFN γ [[148], [149], [150], [151], [152]], being of utmost importance for the development of HLS [122,123]. Most lately, NETosis has attracted great interest in the pathogenesis of organ damage in COVID-19 afflicted patients [[95], [96], [97], [98], [99], [100], [101]], with NETs contributing both to ARDS and immunothrombosis in COVID-19 [101]. Accordingly, it has been argued that NETs might be a potential target in COVID-19 [95]. In this context, it is highly intriguing that ruxolitinib potently impairs NETosis by inhibiting the protein, PAD4, which is required in NET formation [104]. Most recently, it has been announced that the large randomized ruxolitinib trial, launched by Incyte/Novartis in March 2020, has failed in displaying efficacy in severely afflicted COVID-19 patients. In this trial, a very low ruxolitinib dose was initially used (5 mg x 2 /day), which may explain the lack of efficacy [219]. This dosage is far less than the dosage of ruxolitinib 20 mg x 2 /day, which is being used as the starting dosage in patients with myelofibrosis to quell hyperinflammation and the cytokine storm.
Type I interferons (IFN-Is) are the oldest known anti-virus agents and as alluded to above, the track-record of studies on the safety and efficacy of IFN-alpha2 or IFN-beta in the treatment of virus diseases is very long, the most important and succesful stories being in the treatment of patients with hepatitis B and C [174]. Similarly, IFNs have been used for decades and successfully in the treatment of patients with multiple sclerosis (IFN-beta) and several cancers, including chronic myelogenous leukemia and MPNs [106]. Unfortunately, several viruses have developed highly effective mechanisms to avoid the antiviral activities of IFN [220] and as noted above this holds true for SARS-CoV-2, which has developed mechanisms to eliminate the capability of IFNs to induce innate immunity, implying an impaired production of IFN-beta and an impaired IFN-I induced signaling. Taking into account that the COVID-19 infection induces a type I IFN-deficiency state with impairment of anti-virus defence mechanisms and potently induces NETosis formation enhancing virus spreading, disease progression and multi-organ thrombosis, respectively, there is an urgent need to revive the oldest anti-virus agent - IFN - in the treatment of the COVID-19 infection [[221], [222], [223], [224]]. Indeed, all the observations given above support the contention that IFN treatment is a rational approach to start or improve the antiviral response of COVID-19 patients, not only patients in the early disease phase but also together with a JAK1/2 inhibitor in those being severely afflicted by COVID-19, in whom Type 1 IFN deficiency is prominent as well [143,224].
Despite all the anti-virus mechanisms of action of IFNs, current knowledge on type I IFN-deficiency with inhibition of IFN-beta production by SARS-CoV-2 in the early stage of the COVID-19 infection and decades of experience with IFN-alpha2 and IFN-beta in the treatment of hepatitis B and C, multiple sclerosis, chronic myelogenous leukemia and MPNs, one may wonder why these agents have not received much more attention in the treatment of SARS-CoV-2 and not already been worldwide implemented in the fight against the COVID-19 pandemic.
Taking into account current knowledge on a type I IFN deficiency state during the COVID-19 infection and our knowledge on concurrent increased production of pro-inflammatory cytokines, the time is ripe to launch studies combining type I IFN and a JAK1/2 Inhibitor. Similar to IFNs, hydroxyurea and statins are old drugs – used for several decades – as cytoreductive and cholesterol lowering agents, respectively. In the early HIV-era, hydroxyurea was used in the treatment of HIV and demonstrated synergistic effects with e.g nucleoside reverse transcriptase inhibitors [187,188]. Based upon its cytoreductive, anti-inflammatory and antithrombotic capabilities, it may be a highly efficacious agent in the treatment of COVID-19 patients with leukocytosis and imminent ARDS due to a rising potentially life-threatening cytokine storm [[184], [185], [186], [187], [188], [189]]. In this context, the hydroxyurea-specific myeloid targets - monocytes and macrophages – may be of particular importance. Similarly, lipophilic statins may benefit patients with COVID-19 consequent to their anti-inflammatory, antithrombotic and fibrinolytic effects [182,183]. Studies are urgently needed to explore the efficacy of these repurposing, safe and inexpensive drugs.
In conclusion, taking into account that mortality in COVID-19 pneumonia is driven by a severe hyperinflammatory state, there is an urgent need to institute JAK1/2 inhibition in severely afflicted COVID-19 patients [5,6,[55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79]]. This obligation is even more urgent since virus-associated HLS is likely massively underdiagnosed in severe COVID-19 pneumonia. Thus, during the severe influenza A (H1N1) pandemic in 2009, virus-associated HLS was recorded in 36 % of critically ill patients and a major contributor to death in 89 %, contrasting a mortality rate of 25 % in the remaining patients without virus-associated HLS [4].
We have a huge amount of knowledge from so many lessons in the last 5–10 years on the safety and efficacy of JAK1/2 inhibitor treatment in several inflammatory diseases. We have an ethical obligation to translate this knowledge into immediate action to meet the urgent need to rescue severely afflicted patients, who otherwise will die in a cytokine storm and several likely with undiagnosed and untreated virus-associated HLS. The time is not to “watch and wait “for the large randomised study in patients severely afflicted in a cytokine storm, which we know will be calmed by JAK1/2 inhibition. This is our ethical dilemma, which we hope to share with many other physicians caring for these patients. We believe that saving lifes with early intervention with IFN-alpha2 or beta in combination with JAK1/2 inhibition are superior to obtaining the highest level of evidence in randomized trials for the efficacy and safety of JAK1/2 inhibition in COVID-19 afflicted patients. We have this data already from several single-arm ruxolitinib and baricitinib trials emphasizing the safety and efficacy of rational immunosuppressive treatment that directly targets the life-threatening cytokine storm and thereby is foreseen to rescue a large number of COVID-19 afflicted patients.
Such early intervention may have several important perspectives. First, several patients being in the time window just before urgent need of ventilation may improve and thereby not neeed ventilation. Second, the time on ventilation may likely be reduced. Third, by shortening the time exposure for severe pulmonary hyperinflammation, we foresee that the risk of inflammation-induced permanent lung fibrosis and other chronic debilitating consequences of the COVID-19 infection may be similarly markedly reduced. In addition to saving lives, the ultimate outcome of early intervention with IFN and a JAK1/2 inhibitor will be an increase in ventilator capacity which in several countries worldwide will be life-safing per se with the implication that ventilators will be available in ICU to other patient categories and elective operation programs. From a socio-economic perspective, such effforts are highly cost-effective. In the future, the COVID-19 pandemic may have the post-script that it was the human inflammation model which opened the horizon for rethinking how to treat hyperinflammation syndromes with JAK1/2 inhibitors- not only in the setting of virus-associated HLS but also the hyperinflammation which follows other infections, including the sepsis syndrome. Thereby, the COVID-19 infection has opened the avenue for clinical studies on the safety and efficacy of JAK1/2 inhibtion in the setting of hyperinflammation syndromes in the ICUs.
Authorship
Contribution: H.C.H. conceived and wrote the paper, with all coauthors contributing to revision and improvement.
Funding statement
None.
Declaration of Competing Interest
H.C.H. has received a research grant from Novartis and in the Advisory Board for AOP Orphan. The remaining authors declare no competing financial interests.
Acknowledgements
None.
Biography

Professor Hans Hasselbalch Professor Hans Hasselbalch is Consultant Hematologist at the Department of Hematology, Roskilde Hospital, University of Copenhagen. He is former chairman of the Danish Study Group of Chronic Myeloid Neoplasms and member of the Nordic Myeloproliferative Study Group (NMPD). For many years, his professional interests have focused upon chronic myeloid neoplasms (essential thrombocythemia (ET), polycythemia vera (PV) and myelofibrosis (MF) (MPNs). His main research in MPNs is focusing upon integrated molecular (eg. gene expression profiling, epigenetics, SNPs) and immune cell studies in MPN patients before and during treatment with interferon-alpha2 (IFN-alpha2) and novel targeted therapies, including the JAK1−2 inhibitor Ruxolitinib and Histone Deacetylase inhibitor (HDACi) treatment. In this regard, Hans Hasselbalch is Principal Investigator (PI) of a Danish Multicenter Study on low-dose IFN-alpha2 in the treatment of MPNs (DALIAH) and was PI on an International Multicenter Study on the HDACi Vorinostat in the treatment of PV and ET. In 2012, Hasselbalch published in Blood his hypothesis on chronic inflammation as the trigger and driver of clonal evolution, premature atherosclerosis and second cancers in MPNs. This paper has been followed by several others and today chronic inflammation is considered a major driving force for disease progression in MPNs, having a great impact on the mutational landscape in MPNs. In this regard Hasselbalch and his MPN research have as the first shown that smoking –a huge chronic inflammation load – is a risk factor for the development of MPNs. Hasselbalch’s hypothesis prompted a Danish Multicenter Trial - The COMBI-Trial -, in which patients with PV and hyperproliferative MF are treated with a combination of pegylated interferon-alpha2 (Pegasys or PegIntron) and ruxolitinib. The results from this study are highly impressive and encouraging, published in Cancer Medicine (12 months data) and Haematologica (24 months data). This study has been followed by a safety and efficacy study of COMBI in newly diagnosed PV patients. Other research areas within MPNs – all conducted as part of Ph.D theses - include “National Registry Studies on The Co-Morbidity Burden in MPNs”, “The Role of Reactive Oxygen Species in MPNs “, “Immune Cell Studies in CALR-Mutated Patients with The Potential of Vaccination Therapy”, “Quality of Life Studies in MPNs”, and The Danish Multicenter Study of The Safety and Efficacy of Pegylated Interferon Alpha2 in MPNs (DALIAH). Hans Hasselbalch is chairman of a Clinical Academic Group (CAG) - CAG-ZIRI - within Greater Copenhagen Health Science Partners (GCHSPs), selected as a CAG in 2020. In 2021, Hans Hasselbalch has been awarded the Hagedorn Prize for excellent research within the chronic blood cancers - MPN.
References
- 1.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China. A descriptive study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30211-7. Published online January 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;(395):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatraju P.K., Ghassemieh B.J., Nichols M., et al. Covid-19 in critically ill patients in the Seattle region - case series. N. Engl. J. Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beutel G., Wiesner O., Eder M., et al. Virus-associated hemophagocytic syndrome as a major contributor to death in patients with 2009 influenza A (H1N1) infection. Crit. Care. 2011;(15):1–8. doi: 10.1186/cc10073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stebbing J., Phelan A., Griffin I., et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet. 2020 doi: 10.1016/S1473-3099(20)30132-8. Published online February 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson A., et al. Baricitinib as potential treatment for 2019-nCOV acute respiratory disease. Lancet. 2020;395:e30–31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta P., McAuley D., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;28(March (395)):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. 10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarzi-Puttini P., Giorgi V., Sirotti S., et al. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin. Exp. Rheumatol. 2020;38(2):337–342. [PubMed] [Google Scholar]
- 9.Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging [published online ahead of print, 2020 Mar 27] J. Clin. Invest. 2020 doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wichmann D., Sperhake J.P., Lütgehetmann M., et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19 [published online ahead of print, 2020 May 6] Ann. Intern. Med. 2020:M20–2003. doi: 10.7326/M20-2003. [DOI] [PubMed] [Google Scholar]
- 11.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19 [published online ahead of print, 2020 May 21] N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2015432. doi:10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sardu C., Gambardella J., Morelli M.B., Wang X., Marfella R., Santulli G. Hypertension, thrombosis, kidney failure, and diabetes: is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J. Clin. Med. 2020;9(5):E1417. doi: 10.3390/jcm9051417. Published 2020 May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Nieuwkoop C. COVID-19 associated pulmonary thrombosis. Thromb. Res. 2020;191:151. doi: 10.1016/j.thromres.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up [published online ahead of print, 2020 Apr 15] J. Am. Coll. Cardiol. 2020 doi: 10.1016/j.jacc.2020.04.031. S0735-1097(20)35008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magro C., Mulvey J.J., Berlin D., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases [published online ahead of print, 2020 Apr 15] Transl. Res. 2020 doi: 10.1016/j.trsl.2020.04.007. S1931-5244(20)30070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell C.M., Kahwash R. Will complement inhibition be the new target in treating Covid-19-related systemic thrombosis? Circulation. 2020;141(22):1739–1741. doi: 10.1161/CIRCULATIONAHA.120.047419. [DOI] [PubMed] [Google Scholar]
- 19.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kollias A., Kyriakoulis K.G., Dimakakos E., Poulakou G., Stergiou G.S., Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br. J. Haematol. 2020;189(5):846–847. doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terpos E., Ntanasis-Stathopoulos I., Elalamy I., et al. Hematological findings and complications of COVID-19 [published online ahead of print, 2020 Apr 13] Am. J. Hematol. 2020 doi: 10.1002/ajh.25829. doi:10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hess D.C., Eldahshan W., Rutkowski E. COVID-19-Related stroke. Transl. Stroke Res. 2020;11(3):322–325. doi: 10.1007/s12975-020-00818-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiezia L., Boscolo A., Poletto F., et al. COVID-19-Related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb. Haemost. 2020;120(6):998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.(a) Kashi M., Jacquin A., Dakhil B., et al. Severe arterial thrombosis associated with Covid-19 infection [published online ahead of print, 2020 May 16] Thromb. Res. 2020;(192):75–77. doi: 10.1016/j.thromres.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahn D.G., Shin H.J., Kim M.H., et al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19) J. Microbiol. Biotechnol. 2020;30(3):313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tu Y.F., Chien C.S., Yarmishyn A.A., et al. A review of SARS-CoV-2 and the ongoing clinical trials. Int. J. Mol. Sci. 2020;21(7):2657. doi: 10.3390/ijms21072657. Published 2020 Apr 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grein J., Ohmagari N., Shin D., et al. Compassionate use of remdesivir for patients with severe Covid-19 [published online ahead of print, 2020 Apr 10] N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007016. NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldman J.D., Lye D.C.B., Hui D.S., et al. Remdesivir for 5 or 10 days in patients with severe Covid-19 [published online ahead of print, 2020 May 27] N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2015301. doi:10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y., Zhang D., Du G., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial [published correction appears in Lancet. 2020 May 30;395(10238):1694] Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of Covid-19 - preliminary report [published online ahead of print, 2020 May 22] N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007764. NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 31.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of Covid-19 - final report [published online ahead of print, 2020 Oct 8] N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007764. NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brouqui P., Giraud-Gatineau A., Raoult D. Critical reappraisal of remdesivir investigational trials in COVID-19. New Microbes New Infect. 2020;38 doi: 10.1016/j.nmni.2020.100745. Published 2020 Sep 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dyer O. Covid-19: remdesivir has little or no impact on survival, WHO trial shows. BMJ. 2020;19(October (371)) doi: 10.1136/bmj.m4057. m4057PMID: 33077424. [DOI] [PubMed] [Google Scholar]
- 34.Juul S., Nielsen E.E., Feinberg J., Siddiqui F., Jørgensen C.K., Barot E., Nielsen N., Bentzer P., Veroniki A.A., Thabane L., Bu F., Klingenberg S., Gluud C., Jakobsen J.C. Interventions for treatment of COVID-19: a living systematic review with meta-analyses and trial sequential analyses (the LIVING Project) PLoS Med. 2020;17(September (9)) doi: 10.1371/journal.pmed.1003293. Erratum in: PLoS Med. 2020 Dec 29;17(12):e1003517. PMID: 32941437; PMCID: PMC7498193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Müller U., Steinhoff U., Reis L.F., Hemmi S., Pavlovic J., Zinkernagel R.M., Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;24(5167):1918–1921. doi: 10.1126/science.8009221. 264. [DOI] [PubMed] [Google Scholar]
- 36.Samuel C.E. Antiviral actions of interferons. Clin. Microbiol. Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang B.X., Fish E.N. Global virus outbreaks: interferons as 1st responders. Semin. Immunol. 2019;43 doi: 10.1016/j.smim.2019.101300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez M.A. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob. Agents Chemother. 2020:1–18. doi: 10.1128/AAC.00399-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fragkou P.C., Belhadi D., Peiffer-Smadja N., Moschopoulos C.D., Lescure F.X., Janocha H., Karofylakis E., Yazdanpanah Y., Mentré F., Skevaki C., Laouénan C., Tsiodras S., ESCMID Study Group for Respiratory Viruses Review of trials currently testing treatment and prevention of COVID-19. Clin. Microbiol. Infect. 2020;26(August (8)):988–998. doi: 10.1016/j.cmi.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov. Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 41.Lokugamage K.G., Schindewolf C., Menachery V.D. SARS-CoV-2 sensitive to type I interferon pretreatment. BioRxiv. 2020 doi: 10.1101/2020.03.07.982264. [DOI] [Google Scholar]
- 42.Nile S.H., Nile A., Qiu J., Li L., Jia X., Kai G. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons [published online ahead of print, 2020 May 7] Cytokine Growth Factor Rev. 2020 doi: 10.1016/j.cytogfr.2020.05.002. S1359-6101(20)30070-30078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sallard E., Lescure F.X., Yazdanpanah Y., Mentre F., Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19 [published online ahead of print, 2020 Apr 7] Antiviral Res. 2020;(178) doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andreakos E., Tsiodras S. COVID-19: lambda interferon against viral load and hyperinflammation [published online ahead of print, 2020 Apr 25] EMBO Mol. Med. 2020 doi: 10.15252/emmm.202012465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shalhoub S. Interferon beta-1b for COVID-19. Lancet. 2020;395(10238):1670–1671. doi: 10.1016/S0140-6736(20)31101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hung I.F., Lung K.C., Tso E.Y., et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Lababidi R.M., Mooty M., Bonilla M.F., Salem N.M. Treatment of severe pneumonia due to COVID-19 with peginterferon alfa 2a. IDCases. 2020;21 doi: 10.1016/j.idcr.2020.e00837. Published 2020 May 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang N., Zhan Y., Zhu L., et al. Retrospective multicenter cohort study shows early interferon therapy is associated with favorable clinical responses in COVID-19 patients. Cell Host Microbe. 2020;28(3):456–464. doi: 10.1016/j.chom.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou Q., Chen V., Shannon C.P., et al. Interferon-alpha2b treatment for COVID-19. Front. Immunol. 2020;11:1061. doi: 10.3389/fimmu.2020.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng F., Zhou Y., Zhou Z., et al. SARS-CoV-2 clearance in COVID-19 patients with Novaferon treatment: a randomized, open-label, parallel-group trial. Int. J. Infect. Dis. 2020;99:84–91. doi: 10.1016/j.ijid.2020.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davoudi-Monfared E., Rahmani H., Hhalill H., et al. A randomized clinical trial of the efficacy and safety of interferon beta-1a in treatment of severe COVID-19. Antimicrob. Agents Chemother. 2020;64(9) doi: 10.1128/AAC.01061-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rahmani H., Davoudi-Monfared E., Nourian A., Khalili H., Hajizadeh N., Jalalabadi N.Z., Fazeli M.R., Ghazaeian M., Yekaninejad M.S. Interferon β-1b in treatment of severe COVID-19: a randomized clinical trial. Int. Immunopharmacol. 2020;88(November) doi: 10.1016/j.intimp.2020.106903. Epub 2020 Aug 24. PMID: 32862111; PMCID: PMC7445008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pereda R., Gonzalez D., Rivero H.B., et al. Therapeutic effetiveness of interferon-alpha2b against COVID-19: the Cuban experience. J. Interferon Cytokine Res. 2020;40(9):438–442. doi: 10.1089/jir.2020.0124. [DOI] [PubMed] [Google Scholar]
- 54.Fu W., Liu Y., Liu L., et al. An open-label, randomized trial of the combination of IFN-kappa plus TFF2 with standard care in the treatment of patients with moderate COVID-19. EClinicalMedicine. 2020;27 doi: 10.1016/j.eclinm.2020.100547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giudice V., Pagliano P., Vatrella A., Masullo A., Poto S., Polverino B.M., Gammaldi R., Maglio A., Sellitto C., Vitale C., Serio B., Cuffa B., Borrelli A., Vecchione C., Filippelli A., Selleri C. Combination of ruxolitinib and eculizumab for treatment of severe SARS-CoV-2-Related acute respiratory distress syndrome: a controlled study. Front. Pharmacol. 2020;5(June(11)):857. doi: 10.3389/fphar.2020.00857. PMID: 32581810; PMCID: PMC7291857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Satarker S., Tom A.A., Shaji R.A., Alosious A., Luvis M., Nampoothiri M. JAK-STAT pathway inhibition and their implications in COVID-19 therapy. Postgrad. Med. 2020;27(November) doi: 10.1080/00325481.2020.1855921. Epub ahead of print. PMID: 33245005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neubauer A., Wiesmann T., Vogelmeier C.F., Mack E., Skevaki C., Gaik C., Keller C., Figiel J., Sohlbach K., Rolfes C., Renz H., Wulf H., Burchert A. Ruxolitinib for the treatment of SARS-CoV-2 induced acute respiratory distress syndrome (ARDS) Leukemia. 2020;34(August (8)):2276–2278. doi: 10.1038/s41375-020-0907-9. Epub 2020 Jun 17. PMID: 32555296; PMCID: PMC7298698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foss Fm, Rubinowitz A., Landry Ml, Isufi I., Gowda L., Seropian S., Perreault S., Shenoi Sv. Attenuated novel SARS coronavirus 2 infection in an allogeneic hematopoietic stem cell transplant patient on ruxolitinib. Clin. Lymphoma Myeloma Leuk. 2020;20(November (11)):720–723. doi: 10.1016/j.clml.2020.06.014. Epub 2020 Jun 25. PMID: 32727701; PMCID: PMC7316063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D’Alessio A., Del Poggio P., Bracchi F., Cesana G., Sertori N., Di Mauro D., Fargnoli A., Motta M., Giussani C., Moro P., Vitale G., Giacomini M., Borra G. Low-dose ruxolitinib plus steroid in severe SARS-CoV-2 pneumonia. Leukemia. 2020;10(November (1–4)) doi: 10.1038/s41375-020-01087-z. Epub ahead of print. PMID: 33173161; PMCID: PMC7654848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cao Y., Wei J., Zou L., Jiang T., Wang G., Chen L., Huang L., Meng F., Huang L., Wang N., Zhou X., Luo H., Mao Z., Chen X., Xie J., Liu J., Cheng H., Zhao J., Huang G., Wang W., Zhou J. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J. Allergy Clin. Immunol. 2020;146(July (1)):137–146. doi: 10.1016/j.jaci.2020.05.019. e3Epub 2020 May 26. PMID: 32470486; PMCID: PMC7250105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.La Rosée F., Bremer H.C., Gehrke I., et al. The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation. Leukemia. 2020;34(7):1805–1815. doi: 10.1038/s41375-020-0891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vannucchi A.M., Sordi B., Morettini A., Nozzoli C., Poggesi L., Pieralli F., Bartoloni A., Atanasio A., Miselli F., Paoli C., Loscocco G.G., Fanelli A., Para O., Berni A., Tassinari I., Zammarchi L., Maggi L., Mazzoni A., Scotti V., Falchetti G., et al. Compassionate use of JAK1/2 inhibitor ruxolitinib for severe COVID-19: a prospective observational study. Leukemia. 2020:1–13. doi: 10.1038/s41375-020-01018-y. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rojas P., Sarmiento M. JAK/STAT pathway inhibition may Be a promising therapy for COVID-19-Related hyperinflammation in hematologic patients. Acta Haematol. 2020;29(July):1–5. doi: 10.1159/000510179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gozzetti A., Capochiani E., Bocchia M. The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19. Leukemia. 2020;34(October(10)):2815–2816. doi: 10.1038/s41375-020-01038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.England J.T., Abdulla A., Biggs C.M., Lee A.Y.Y., Hay K.A., Hoiland R.L., Wellington C.L., Sekhon M., Jamal S., Shojania K., Chen L.Y.C. Weathering the COVID-19 storm: lessons from hematologic cytokine syndromes. Blood Rev. 2020;15(May) doi: 10.1016/j.blre.2020.100707. Epub ahead of print. PMID: 32425294; PMCID: PMC7227559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heidel F., Hochhaus A. Holding CoVID in check through JAK? The MPN-approved compound ruxolitinib as a potential strategy to treat SARS-CoV-2 induced systemic hyperinflammation. Leukemia. 2020;34(July (7)):1723–1725. doi: 10.1038/s41375-020-0898-6. Epub 2020 Jun 11. PMID: 32528040; PMCID: PMC7288260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seif F., Aazami H., Khoshmirsafa M., Kamali M., Mohsenzadegan M., Pornour M., Mansouri D. JAK inhibition as a new treatment strategy for patients with COVID-19. Int. Arch. Allergy Immunol. 2020;181(6):467–475. doi: 10.1159/000508247. Epub 2020 May 11. PMID: 32392562; PMCID: PMC7270061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miao Y., Fan L., Li J.Y. Potential treatments for COVID-19 related cytokine storm - beyond corticosteroids. Front. Immunol. 2020;16(June (11)):1445. doi: 10.3389/fimmu.2020.01445. PMID: 32612616; PMCID: PMC7308586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yeleswaram S., Smith P., Burn T., Covington M., Juvekar A., Li Y., Squier P., Langmuir P. Inhibition of cytokine signaling by ruxolitinib and implications for COVID-19 treatment. Clin. Immunol. 2020;218(September) doi: 10.1016/j.clim.2020.108517. Epub 2020 Jun 23. PMID: 32585295; PMCID: PMC7308779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jamilloux Y., Henry T., Belot A., et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun. Rev. 2020;19(7) doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khadke S., Ahmed N., Ahmed N., Ratts R., Raju S., Gallogly M., de Lima M., Sohail M.R. Harnessing the immune system to overcome cytokine storm and reduce viral load in COVID-19: a review of the phases of illness and therapeutic agents. Virol. J. 2020;17(October (1)):154. doi: 10.1186/s12985-020-01415-w. PMID: 33059711; PMCID: PMC7558250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luo W., Li Y.X., Jiang L.J., Chen Q., Wang T., Ye D.W. Targeting JAK-STAT signaling to control cytokine release syndrome in COVID-19. Trends Pharmacol. Sci. 2020;41(August (8)):531–543. doi: 10.1016/j.tips.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weisberg E., Parent A., Yang P.L., Sattler M., Liu Q., Liu Q., Wang J., Meng C., Buhrlage S.J., Gray N., Griffin J.D. Repurposing of kinase inhibitors for treatment of COVID-19. Pharm. Res. 2020;37(August (9)):167. doi: 10.1007/s11095-020-02851-7. PMID: 32778962; PMCID: PMC7417114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elli E.M., Baratè C., Mendicino F., Palandri F., Palumbo G.A. Mechanisms underlying the anti-inflammatory and immunosuppressive activity of ruxolitinib. Front. Oncol. 2019;9:1186. doi: 10.3389/fonc.2019.01186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Banerjee S., Biehl A., Gadina M., Hasni S., Schwartz D. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77:521–546. doi: 10.1007/s40265-017-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Esmaeilzadeh A., Elahi R. Immunobiology and immunotherapy of COVID-19: a clinically updated overview. J. Cell. Physiol. 2020;6(October) doi: 10.1002/jcp.30076. doi: 10.1002/jcp.30076. Epub ahead of print. PMID: 33022076; PMCID: PMC7675260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goker Bagca B., Biray Avci C. The potential of JAK/STAT pathway inhibition by ruxolitinib in the treatment of COVID-19. Cytokine Growth Factor Rev. 2020;54(August):51–62. doi: 10.1016/j.cytogfr.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Owji H., Negahdaripour M., Hajighahramani N. Immunotherapeutic approaches to curtail COVID-19. Int. Immunopharmacol. 2020;88(November) doi: 10.1016/j.intimp.2020.106924. Epub 2020 Aug 21. PMID: 32877828; PMCID: PMC7441891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang J., Jiang M., Chen X., Montaner L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020;108(July (1)):17–41. doi: 10.1002/JLB.3COVR0520-272R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20(June (6)):363–374. doi: 10.1038/s41577-020-0311-8. Epub 2020 Apr 28. PMID: 32346093; PMCID: PMC7187672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rehman M., Tauseef I., Aalia B., Shah S.H., Junaid M., Haleem K.S. Therapeutic and vaccine strategies against SARS-CoV-2: past, present and future. Future Virol. 2020;(July) doi: 10.2217/fvl-2020-0137. doi: 10.2217/fvl-2020-0137. Epub 2020 Jul 27. PMCID: PMC7386380. [DOI] [Google Scholar]
- 82.Sarkar C., Mondal M., Torequl Islam M., Martorell M., Docea A.O., Maroyi A., Sharifi-Rad J., Calina D. Potential therapeutic options for COVID-19: current status, challenges, and future perspectives. Front. Pharmacol. 2020;15(September (11)) doi: 10.3389/fphar.2020.572870. PMID: 33041814; PMCID: PMC7522523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iwasaki M., Saito J., Zhao H., Sakamoto A., Hirota K., Ma D. Inflammation triggered by SARS-CoV-2 and ACE2 augment drives multiple organ failure of severe COVID-19: molecular mechanisms and implications. Inflammation. 2020;8(October):1–22. doi: 10.1007/s10753-020-01337-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Copaescu A., Smibert O., Gibson A., Phillips E.J., Trubiano J.A. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J. Allergy Clin. Immunol. 2020;146(September(3)):518–534. doi: 10.1016/j.jaci.2020.07.001. e1PMID: 32896310; PMCID: PMC7471766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Innes A.J., Cook L.B., Marks S., Bataillard E., Crossette-Thambiah C., Sivasubramaniam G., Apperley J., Milojkovic D. Ruxolitinib for tocilizumab-refractory severe COVID-19 infection. Br. J. Haematol. 2020;190(August (4)):e198–e200. doi: 10.1111/bjh.16979. Epub 2020 Jul 26. PMID: 32593183; PMCID: PMC7361819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Portsmore S., Tran Nguyen T.N., Beacham E., Neelakantan P. Combined IL-6 and JAK/STAT inhibition therapy in COVID-19-related sHLH, potential game changer. Br. J. Haematol. 2020;190(August (4)):525–528. doi: 10.1111/bjh.16966. Epub 2020 Jul 22. PMID: 32584421; PMCID: PMC7361602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saha R.P., Sharma A.R., Singh M.K., Samanta S., Bhakta S., Mandal S., Bhattacharya M., Lee S.S., Chakraborty C. Repurposing drugs, ongoing vaccine, and new therapeutic development initiatives against COVID-19. Front. Pharmacol. 2020;19(August (11)):1258. doi: 10.3389/fphar.2020.01258. PMID: 32973505; PMCID: PMC7466451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chugh H., Awasthi A., Agarwal Y., Gaur R.K., Dhawan G., Chandra R. A comprehensive review on potential therapeutics interventions for COVID-19. Eur. J. Pharmacol. 2020;20(November) doi: 10.1016/j.ejphar.2020.173741. Epub ahead of print. PMID: 33227287; PMCID: PMC7677683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kumar M., Al Khodor S. Pathophysiology and treatment strategies for COVID-19. J. Transl. Med. 2020;18(September (1)):353. doi: 10.1186/s12967-020-02520-8. PMID: 32933536; PMCID: PMC7491044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schett G., Manger B., Simon D., Caporali R. COVID-19 revisiting inflammatory pathways of arthritis. Nat. Rev. Rheumatol. 2020;16(August (8)):465–470. doi: 10.1038/s41584-020-0451-z. Epub 2020 Jun 19. PMID: 32561873; PMCID: PMC7304381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoang T.N., Pino M., Boddapati A.K., Viox E.G., Starke C.E., Upadhyay A.A., et al. Baricitinib treatment resolves lower-airway macrophage inflammation and neutrophil recruitment in SARS-CoV-2-infected rhesus macaques. Cell. 2021;184(January(2)):460–475. doi: 10.1016/j.cell.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang S., Pan Y., Wang Q., Miao H., Brown A.N., Rong L. Modeling the viral dynamics of SARS-CoV-2 infection. Math. Biosci. 2020;328(October) doi: 10.1016/j.mbs.2020.108438. Epub 2020 Aug 6. PMID: 32771304; PMCID: PMC7409942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A., tenOever B.R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–1045. doi: 10.1016/j.cell.2020.04.026. e9. Epub 2020 May 15. PMID: 32416070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.RECOVERY Collaborative Group, Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N. Engl. J. Med. 2020;17(July) doi: 10.1056/NEJMoa2021436. NEJMoa2021436Epub ahead of print. PMID:32678530;PMCID:PMC7383595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 2020;217(6) doi: 10.1084/jem.2020065226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu P.P., Blet A., Smyth D., Li H. The science underlying COVID-19: implications for the cardiovascular system [published online ahead of print, 2020 Apr15] Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047549. doi:10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 97.Arisan E.D., Uysal-Onganer P., Lange S. Putative roles for peptidylarginine deiminases in COVID-19. Int. J. Mol. Sci. 2020;21(June (13)):4662. doi: 10.3390/ijms21134662. PMID: 32629995; PMCID: PMC7370447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arcanjo A., Logullo J., Menezes C.C.B., et al. The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19) Sci. Rep. 2020;10:19630. doi: 10.1038/s41598-020-76781-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zuo Y., Kanthi Y., Knight J.S. Vol. 5. 2020. p. e138999. (Neutrophil Extracellular Traps in COVID-19 JCI Insight). (11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Veras F.P., Pontelli M.C., Silva C.M., et al. SARS-CoV-2–triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 2020;217(12) doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Middleton E.A., He X.Y., Denorme G., et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guarda G., Braun M., Staehli F., et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34(2):213–223. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 103.Skov V., Riley C., Thomassen M., et al. Significantly upregulated thromboinflammatory genes are normoregulated or significantly downregulated during treatment with interferon-alpha2 in patients with Philadelphia-negative chronic myeloproliferative neoplasms. Blood. 2019;134(Supplement1):2978. [Google Scholar]
- 104.Wolach O., Sellar R.S., Martinod K., et al. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci. Transl. Med. 2018;10(April(436)) doi: 10.1126/scitranslmed.aan8292. PMID: 29643232; PMCID: PMC6442466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barbui T., et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32(May (5)):1057–1069. doi: 10.1038/s41375-018-0077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hasselbalch H.C. Perspectives on chronic inflammation in essential thrombocythemia, polycythemia vera, and myelofibrosis: is chronic inflammation a trigger and driver of clonal evolution and development of accelerated atherosclerosis and second cancer? Blood. 2012;119(14):3219–3225. doi: 10.1182/blood-2011-11-394775. [DOI] [PubMed] [Google Scholar]
- 107.Hasselbalch H.C. Chronic inflammation as a promotor of mutagenesis in essential thrombocythemia,polycythemia vera and myelofibrosis. A human inflammation model for cancer development? Leuk. Res. 2013;37(2):214–220. doi: 10.1016/j.leukres.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 108.Hasselbalch H.C., Bjørn M.E. MPNs as inflammatory diseases: the evidence, consequences. and Perspectives. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/102476. Epub 2015 Oct 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bjørn M.E., Hasselbalch H.C. The impact of ruxolitinib treatment on inflammation-mediated comorbidities in myelofibrosis and related neoplasms. Clin. Case Rep. 2015;3(6):499–503. doi: 10.1002/ccr3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Øbro N., Grinfeld J., Belmonte M., et al. Longitudinal cytokine profiling identifies GRO-a and EGF as potential biomarkers of disease progression in essential thrombocythemia. HemaSphere. 2020;4:e371. doi: 10.1097/HS9.0000000000000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hasselbalch H.C. Cytokine profiling as a novel complementary tool to predict prognosis in MPNs? Hemasphere. 2020;4(June (3)):e407. doi: 10.1097/HS9.0000000000000407. PMID: 32647805; PMCID: PMC7306306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tothova Z., Berliner N. Hemophagocytic syndrome and critical illness: new insights into diagnosis and management. J. Intensive Care Med. 2015;30(7):401–412. doi: 10.1177/0885066613517076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Buyse S., Teixeira L., Galicier L., et al. Critical care management of patients with hemophagocytic lymphohistiocytosis. Intensive Care Med. 2010;36(10):1695–1702. doi: 10.1007/s00134-010-1936-z. [DOI] [PubMed] [Google Scholar]
- 114.Trottestam H., Horne A., Aricò M., et al. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood. 2011;118(17):4577–4584. doi: 10.1182/blood-2011-06-356261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Birndt S., Schenk T., Heinevetter B., et al. Hemophagocytic lymphohistiocytosis in adults: collaborative analysis of 137 cases of a nationwide German registry. J. Cancer Res. Clin. Oncol. 2020;146(4):1065–1077. doi: 10.1007/s00432-020-03139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Al-Samkari H., Berliner N. Hemophagocytic lymphohistiocytosis. Annu. Rev. Pathol. 2018;13:27–49. doi: 10.1146/annurev-pathol-020117-043625. [DOI] [PubMed] [Google Scholar]
- 117.Crayne C.B., Albeituni S., Nichols K.E., Cron R.Q. The immunology of macrophage activation syndrome. Front. Immunol. 2019;10:119. doi: 10.3389/fimmu.2019.00119. Published 2019 Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jordan M.B., Hildeman D., Kappler J., Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004;104(3):735–743. doi: 10.1182/blood-2003-10-3413. [DOI] [PubMed] [Google Scholar]
- 119.La Rosée P. Alleviating the storm: ruxolitinib in HLH. Blood. 2016;127(13):1626–1627. doi: 10.1182/blood-2016-02-697151. [DOI] [PubMed] [Google Scholar]
- 120.Das R., Guan P., Sprague L., et al. Janus kinase inhibition lessens inflammation and ameliorates disease in murine models of hemophagocytic lymphohistiocytosis. Blood. 2016;127(13):1666–1675. doi: 10.1182/blood-2015-12-684399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Maschalidi S., Sepulveda F.E., Garrigue A., Fischer A., de Saint Basile G. Therapeutic effect of JAK1/2 blockade on the manifestations of hemophagocytic lymphohistiocytosis in mice. Blood. 2016;128(1):60–71. doi: 10.1182/blood-2016-02-700013. [DOI] [PubMed] [Google Scholar]
- 122.Zinter M.S., Hermiston M.L. Calming the storm in HLH. Blood. 2019;134(2):103–104. doi: 10.1182/blood.2019001333. [DOI] [PubMed] [Google Scholar]
- 123.Albeituni S., Verbist K.C., Tedrick P.E., et al. Mechanisms of action of ruxolitinib in murine models of hemophagocytic lymphohistiocytosis. Blood. 2019;134(2):147–159. doi: 10.1182/blood.2019000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Slostad J., Hoversten P., Haddox Cl, Cisak K., Paludo J., Tefferi A. Ruxolitinib as first-line treatment in secondary hemophagocytic lymphohistiocytosis: a single patient experience. Am. J. Hematol. 2018;93(2):E47–E49. doi: 10.1002/ajh.24971. [DOI] [PubMed] [Google Scholar]
- 125.Zandvakili I., Conboy C.B., Ayed A.O., Cathcart-Rake E.J., Tefferi A. Ruxolitinib as first-line treatment in secondary hemophagocytic lymphohistiocytosis: a second experience. Am. J. Hematol. 2018;93(5):E123–E125. doi: 10.1002/ajh.2506. [DOI] [PubMed] [Google Scholar]
- 126.Goldsmith S.R., Saif Ur Rehman S., Shirai C.L., Vij K., DiPersio J.F. Resolution of secondary hemophagocytic lymphohistiocytosis after treatment with the JAK1/2 inhibitor ruxolitinib. Blood Adv. 2019;3(23):4131–4135. doi: 10.1182/bloodadvances.2019000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sin J.H., Zangardi M.L. Ruxolitinib for secondary hemophagocytic lymphohistiocytosis: first case report. Hematol. Stem Cell Ther. 2019;12(3):166–170. doi: 10.1016/j.hemonc.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 128.Trantham T., Auten J., Muluneh B., Van Deventer H. Ruxolitinib for the treatment of lymphoma-associated hemophagocytic lymphohistiocytosis: a cautionary tale [published online ahead of print, 2019 Oct 1] J. Oncol. Pharm. Pract. 2019 doi: 10.1177/1078155219878774. [DOI] [PubMed] [Google Scholar]
- 129.Ahmed A. Ruxolitinib in adult patients with secondary haemophagocytic lymphohistiocytosis: an open-label, single-centre, pilot trial. Lancet Haematol. 2019;6(December (12)):e630–e637. doi: 10.1016/S2352-3026(19)30156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.La Rosée P., Horne A., Hines M., et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465–2477. doi: 10.1182/blood.2018894618. [DOI] [PubMed] [Google Scholar]
- 131.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395(10225):683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R., Ghazaryan V., et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N. Engl. J. Med. 2020;11(December) doi: 10.1056/NEJMoa2031994. NEJMoa2031994 Epub ahead of print. PMID: 33306283; PMCID: PMC7745180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schwartz D.M., Kanno Y., Villarino A., Ward M., Gadina M., O’Shea J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017;17(1):78. doi: 10.1038/nrd.2017.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gomez V.E., Garcia-Gutierrez V., Corral L.L., et al. Ruxolitinib in refractory acute and chronic graft-versus-host disease:a multicenter survey study. Bone Marrow Trans. 2020;55:641–648. doi: 10.1038/s41409-019-0731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bastard P., Rosen L.B., Zheng Q., et al. Autoantibodies against type I IFN in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4585. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Loutfy M., Blatt L.M., Siminovitch K.A., Ward S., Wolff B., Lho H., et al. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome. JAMA. 2003;290:3222–3228. doi: 10.1001/jama.290.24.3222. [DOI] [PubMed] [Google Scholar]
- 138.Mary A., Hénaut L., Schmit J.L., Lanoix J.P., Brazier M. Therapeutic options for coronavirus disease 2019 (COVID-19) - modulation of Type I interferon response as a promising strategy? Front. Public Health. 2020;15(May (8)):185. doi: 10.3389/fpubh.2020.00185. PMID: 32574289; PMCID: PMC7243823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mary A., Hénaut L., Macq P.Y., Badoux L., Cappe A., Porée T., Eckes M., Dupont H., Brazier M. Rationale for COVID-19 treatment by nebulized Interferon-β-1b-Literature review and personal preliminary experience. Front. Pharmacol. 2020;30(November (11)) doi: 10.3389/fphar.2020.592543. PMID: 33329000; PMCID: PMC7734101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chu H., Chan J.F.-W., Wang Y., et al. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa410. published online April 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.George P.M., Wells A.U., Jenkins R.G. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir. Med. 2020;8(August (8)):807–815. doi: 10.1016/S2213-2600(20)30225-3. Epub 2020 May 15. PMID: 32422178; PMCID: PMC7228727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–1045. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Trouillet-Assant S., Viel S., Gaymard A., Pons S., Richard J.-C., Perret M., et al. Type I IFN immunoprofiling in COVID-19 patients. J. Allergy Clin. Immunol. 2020;146:206–208. doi: 10.1016/j.jaci.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McKechnie J.L., Blish C.A. The innate immune system: fighting on the front lines or fanning the flames of COVID-19? Cell Host Microbe. 2020;27(6):863–869. doi: 10.1016/j.chom.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Park A., Iwasaki A. Type I and Type III interferons - induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27(June (6)):870–878. doi: 10.1016/j.chom.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pairo-Castineira E., et al. Genetic mechanisms of critical illness in Covid-19. Nature. 2020 doi: 10.1038/s41586-020-03065-y. [DOI] [PubMed] [Google Scholar]
- 148.Hambleton S., Goodbourn S., Young D.F., Dickinson P., Mohamad S.M.B., Valappil M., et al. STAT2 deficiency and susceptibility to viral illness in humans. Proc. Natl. Acad. Sci. U.S.A. 2013;110:3053–3058. doi: 10.1073/pnas.1220098110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Duncan C.J.A., Mohamad S.M.B., Young D.F., Skelton A.J., Leahy T.R., Munday D.C., et al. Human ifnar2 deficiency: lessons for antiviral immunity. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aac4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J., et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;23(October (6515)) doi: 10.1126/science.abd4570. Epub 2020 Sep 24. PMID: 32972995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mei Q., Wang A., Yang Y., Li M., Wang F., Du F., et al. Survival factors and metabolic pathogenesis in elderly patients (≥65) with COVID-19: a multicenter study of 223 cases Res. Square. 2020 doi: 10.21203/rs.3.rs-23199/v1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Abb J., Abb H., Deinhardt F. Age-related decline of human interferon alpha and interferon gamma production. Blut. 1984;48:285–289. doi: 10.1007/BF00320399. [DOI] [PubMed] [Google Scholar]
- 153.Teran-Cabanillas E., Montalvo-Corral M., Caire-Juvera G., Moya-Camarena S.Y., Hernández J. Decreased interferon-α and interferon-β production in obesity and expression of suppressor of cytokine signaling. Nutrition. 2013;29:207–212. doi: 10.1016/j.nut.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 154.Trouillet-Assant S., Viel S., Ouziel A., Boisselier L., Rebaud P., Basmaci R., et al. Type I interferon in children with viral or bacterial infections. Clin. Chem. 2020;66:802–808. doi: 10.1093/clinchem/hvaa089. [DOI] [PubMed] [Google Scholar]
- 155.Wang Q., Zennadi R. Oxidative stress and thrombosis during aging: the roles of oxidative stress in RBSs in venous thrombosis. Int. J. Mol. Sci. 2002:4259. doi: 10.3390/ijms21124259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Skov V., Riley C., Thomassen M., et al. Interferon-alpha2 significantly downregulates upregulated oxidative stress genes and upregulates downregulated anti-oxidative defence genes in patients with polycythemia vera and associated neoplasms. Blood. 2018;132(Supplement 1):4326. [Google Scholar]
- 157.Chalise J., Narendra S., Paudyal B., Magnusson M. Interferon alpha inhibits antigen-specific production of proinflammatory cytokines and enhances antigen-specific transforming growth factor beta production in antigen-induced arthritis. Arthritis Res. Ther. 2013;15(October (5)):R143. doi: 10.1186/ar4326. PMID: 24286140; PMCID: PMC3978460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.de Paus R.A., van Wengen A., Schmidt I., Visser M., Verdegaal E.M., van Dissel J.T., van de Vosse E. Inhibition of the type I immune responses of human monocytes by IFN-alpha and IFN-beta. Cytokine. 2013;61:645–655. doi: 10.1016/j.cyto.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 159.Gómez-Rial J., Rivero-Calle I., Salas A., Martinón-Torres F. Role of Monocytes/Macrophages in Covid-19 pathogenesis: implications for therapy. Infect. Drug Resist. 2020;22(July (13)):2485–2493. doi: 10.2147/IDR.S258639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gómez-Rial J., Currás-Tuala M.J., Rivero-Calle I., Gómez-Carballa A., Cebey-López M., Rodríguez-Tenreiro C., Dacosta-Urbieta A., Rivero-Velasco C., Rodríguez-Núñez N., Trastoy-Pena R., Rodríguez-García J., Salas A., Martinón-Torres F. Increased serum levels of sCD14 and sCD163 indicate a preponderant role for monocytes in COVID-19 immunopathology. Front. Immunol. 2020;23(September (11)) doi: 10.3389/fimmu.2020.560381. PMID: 33072099; PMCID: PMC7538662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Martinez F.O., Combes T.W., Orsenigo F., Gordon S. Monocyte activation in systemic Covid-19 infection: assay and rationale. EBioMedicine. 2020;59(September) doi: 10.1016/j.ebiom.2020.102964. Epub 2020 Aug 30. PMID: 32861199; PMCID: PMC7456455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jafarzadeh A., Chauhan P., Saha B., Jafarzadeh S., Nemati M. Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID-19: lessons from SARS and MERS, and potential therapeutic interventions. Life Sci. 2020;15(September (257)) doi: 10.1016/j.lfs.2020.118102. Epub 2020 Jul 18. PMID: 32687918; PMCID: PMC7367812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20(June (6)):355–362. doi: 10.1038/s41577-020-0331-4. Epub 2020 May 6. Erratum in: Nat Rev Immunol. 2020 Jun 2;: PMID: 32376901; PMCID: PMC7201395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Riley C.H., Jensen M.K., Brimnes M.K., Hasselbalch H.C., Bjerrum O.W., Straten P.T., IM Svane. Increase in circulating CD4(+)CD25(+)Foxp3(+) T cells in patients with Philadelphia-negative chronic myeloproliferative neoplasms during treatment with IFN-alpha. Blood. 2011;118:2170–2173. doi: 10.1182/blood-2011-03-340992. [DOI] [PubMed] [Google Scholar]
- 165.Chen W., Jin W., Hardegen N., Lei K.J., Li L., Marinos N., McGrady G., Wahl S.M. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dikopoulos N., Bertoletti A., Kroger A., Hauser H., Schirmbeck R., Reimann J. Type I IFN negatively regulates CD8+ t cell responses through IL-10-producing CD4+ t regulatory 1 cells. J. Immunol. 2005;174:99–109. doi: 10.4049/jimmunol.174.1.99. [DOI] [PubMed] [Google Scholar]
- 167.Lee A.J., Chen B., Chew M.V., Barra N.G., Shenouda M.M., Nham T., et al. Inflammatory monocytes require type I interferon receptor signaling to activate NK cells via IL-18 during a mucosal viral infection. J. Exp. Med. 2017;214(April (4)):1153–1167. doi: 10.1084/jem.20160880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Borges R.C., Hohmann M.S., Borghi S.M. Dendritic cells in COVID-19 immunopathogenesis: insights for a possible role in determining disease outcome. Int. Rev. Immunol. 2020;16(November):1–18. doi: 10.1080/08830185.2020.1844195. Epub ahead of print. PMID: 33191813. [DOI] [PubMed] [Google Scholar]
- 169.Pantel A., Teixeira A., Haddad E., Wood E.G., Steinman R.M., Longhi M.P. Direct type I IFN but not MDA5/TLR3 activation of dendritic cells is required for maturation and metabolic shift to glycolysis after poly IC stimulation. PLoS Biol. 2014;12(January (1)) doi: 10.1371/journal.pbio.1001759. Epub 2014 Jan 7. PMID: 24409099; PMCID: PMC3883643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wei-Chun H.F., Qian J., Liu C., Liu J., Lokshin A.E., Baker D.P., Rui H., Fuchs S.Y. Inflammatory signaling compromises cell responses to interferon. Oncogene. 2012;31(2):161–172. doi: 10.1038/onc.2011.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Messina J.L., Yu H., Riker A.I., Munster P.N., Jove R.I., Daud A.I. Activated STAT-3 in melanoma. Cancer Control. 2008;15:196–201. doi: 10.1177/107327480801500302. [DOI] [PubMed] [Google Scholar]
- 172.Bona D.D., Cippitelli M., Fionda C., Camma C., Licata A., Santoni A., Craxi A. Oxidative stress inhibits IFN-alpha2-induced antiviral gene expression by blocking the JAK-STAT pathway. J. Hepatol. 2006;45:271–279. doi: 10.1016/j.jhep.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 173.Sørensen A.L., Mikkelsen S.U., Knudsen T.A., Bjørn M.E., Andersen C.L., Bjerrum O.W., Brochmann N., et al. Ruxolitinib and interferon-α2 combination therapy for patients with polycythemia vera or myelofibrosis: a phase II study. Haematologica. 2019;(December):26. doi: 10.3324/haematol.2019.235648. pii: haematol.2019.235648 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li S.F., Gong M.J., Zhao F.R., Shao J.J., Xie Y.L., Zhang Y.G., Chang H.Y. Type I interferons: distinct biological activities and current applications for viral infection. Cell. Physiol. Biochem. 2018;51(5):2377–2396. doi: 10.1159/000495897. [DOI] [PubMed] [Google Scholar]
- 175.Kamaz B., Mullally A. COVID-19 and myeloproliferative neoplasms: some considerations. Leukemia. 2020;26(October):1–3. doi: 10.1038/s41375-020-01070-8. Epub ahead of print. PMID: 33106624; PMCID: PMC7586362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.NIH . 2020. COVID-19 Treatment Guidelines.https://covid19treatmentguidelines.nih.gov/ [Google Scholar]
- 177.Harrison C., Ferrara F., Rea D. COVID-19 and myeloid malignancies: managing patients during the pandemic. Presented at: Virtual 25th EHA Congress. 2020 June 11. [Google Scholar]
- 178.Breccia M., Piciocchi A., De Stefano V., et al. COVID-19 in Philadelphia-negative myeloproliferative disorders: a GIMEMA survey. Leukemia. 2020;34(10):2813–2814. doi: 10.1038/s41375-020-01032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Barbui T., Vannucchi A.M., Alvarez-Larran A., et al. High mortality rate in COVID-19 patients with myeloproliferative neoplasms after abrupt withdrawal of ruxolitinib. Leukemia. 2021;7(January):1–9. doi: 10.1038/s41375-020-01107-y. Epub ahead of prnt. PMID:33414483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Frankel A.E., Reddy R., DeSuza K.R., et al. 2021. Response to Pegylated Interferon in a COVID-19 Positive Elderly Woman with Primary Myelofibrosis Treated with Ruxolitinib. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yelin D., Wirtheim E., Vetter P., Kalil A.C., Bruchfeld J., Runold M., Guaraldi G., Mussini C., Gudiol C., Pujol M., Bandera A., Scudeller L., Paul M., Kaiser L., Leibovici L. Long-term consequences of COVID-19: research needs. Lancet Infect. Dis. 2020;20(October (10)):1115–1117. doi: 10.1016/S1473-3099(20)30701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kashour T., Halwani R., Arabi Y.M., Sohail M.R., O’Horo J.C., Badley A.D., Tleyjeh I.M. Statins as an adjunctive therapy for COVID-19: the biological and clinical plausibility. Immunopharmacol. Immunotoxicol. 2021;6(January):1–14. doi: 10.1080/08923973.2020.1863984. Epub ahead of print. PMID: 33406943. [DOI] [PubMed] [Google Scholar]
- 183.Rodrigues-Diez R.R., Tejera-Muñoz A., Marquez-Exposito L., Rayego-Mateos S., Santos Sanchez L., Marchant V., et al. Statins: could an old friend help in the fight against COVID-19? Br. J. Pharmacol. 2020;177(November (21):4873–4886. doi: 10.1111/bph.15166. Epub 2020 Jul 15. PMID: 32562276; PMCID: PMC7323198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Spivak J.L., Hasselbalch H. Hydroxycarbamide: a user’s guide for chronic myeloproliferative disorders. Expert Rev. Anticancer Ther. 2011;11(March (3)):403–414. doi: 10.1586/era.11.10. PMID: 21417854. [DOI] [PubMed] [Google Scholar]
- 185.Zahran A.M., Nafady A., Saad K., et al. Effect of hydroxyurea treatment on the inflammatory markers among children with sickle cell disease. Clin. Appl. Thromb. Hemost. 2020;26 doi: 10.1177/1076029619895111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Guarda C.C., Silveira-Mattos P.S.M., Yahouédéhou S.C.M.A., et al. Hydroxyurea alters circulating monocyte subsets and dampens its inflammatory potential in sickle cell anemia patients. Sci. Rep. 2019;9:14829. doi: 10.1038/s41598-019-51339-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lori F., Lisziewicz J. Hydroxyurea: overview of clinical data and antiretroviral and immunomodulatory effects. Antivir Ther. 1999;4(Suppl 3):101–108. PMID: 16021881. [PubMed] [Google Scholar]
- 188.Lisziewicz J., Foli A., Wainberg M., Lori F. Hydroxyurea in the treatment of HIV infection: clinical efficacy and safety concerns. Drug Saf. 2003;26(9):605–624. doi: 10.2165/00002018-200326090-00002. PMID: 12814330. [DOI] [PubMed] [Google Scholar]
- 189.Yang P.H., Ding Y.B., Xu Z., Pu R., Li P., Yan J., et al. Increased circulating level of interleukin-6 and CD8+ T cell exhaustion are associated with progression of COVID-19. Infect. Dis. Poverty. 2020;9(November (1)):161. doi: 10.1186/s40249-020-00780-6. PMID: 33239109; PMCID: PMC7686818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhao Y., Nie H.X., Hu K., Wu X.J., Zhang Y.T., Wang M.M., Wang T., Zheng Z.S., Li X.C., Zeng S.L. Abnormal immunity of non-survivors with COVID-19: predictors for mortality. Infect. Dis. Poverty. 2020;9(August (1)):108. doi: 10.1186/s40249-020-00723-1. PMID: 32746940; PMCID: PMC7396941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cordua S., Kjaer L., Skov V., Pallisgaard N., Hasselbalch H.C., Ellervik C. Prevalence and phenotypes of JAK2 V617F and calreticulin mutations in a Danish general population. Blood. 2019;134(August (5)):469–479. doi: 10.1182/blood.2019001113. [DOI] [PubMed] [Google Scholar]
- 192.Merriman T., et al. Gout, rheumatoid arthritis and the risk of death from COVID-19: an analysis of the UK Biobank. medRxiv. 2020 doi: 10.1101/2020.11.06.20227405. https://www.medrxiv.org/content/10.1101/2020.11.06.20227405v1 [DOI] [Google Scholar]
- 193.Neurath M.F. COVID-19 and immunomodulation in IBD. Gut. 2020;69(July (7)):1335–1342. doi: 10.1136/gutjnl-2020-321269. Epub 2020 Apr 17. PMID: 32303609; PMCID: PMC7211083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liu P.P., Blet A., Smyth D., Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020;142(July (1)):68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. Epub 2020 Apr 15. PMID: 32293910. [DOI] [PubMed] [Google Scholar]
- 195.Glenthøj A., Jakobsen L.H., Sengeløv H., Ahmad S.A., Qvist K., Rewes A., Poulsen C.B., Overgaard U.M., Mølle I., Severinsen M.T., Strandholdt C.N., Maibom J., Kodahl A.R., Ryg J., Ravn P., Johansen I.S., Helsø S.N., Jensen-Fangel S., Kisielewicz J., Wiese L., Helleberg M., Kirk O., Clausen M.R., Frederiksen H. SARS-CoV-2 infection among patients with haematological disorders: severity and one-month outcome in 66 Danish patients in a nationwide cohort study. Eur. J. Haematol. 2021;106(January (1)):72–81. doi: 10.1111/ejh.13519. [DOI] [PubMed] [Google Scholar]
- 196.Sorouri M., Kasaeian A., Mojtabavi H., Radmard A.R., Kolahdoozan S., Anushiravani A., Khosravi B., Pourabbas S.M., Eslahi M., Sirusbakht A., Khodabakhshi M., Motamedi F., Azizi F., Ghanbari R., Rajabi Z., Sima A.R., Rad S., Abdollahi M. Clinical characteristics, outcomes, and risk factors for mortality in hospitalized patients with COVID-19 and cancer history: a propensity score-matched study. Infect Agent Cancer. 2020;15(December (1)):74. doi: 10.1186/s13027-020-00339-y. PMID: 33334375; PMCID: PMC7745169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Simon D., Tascilar K., Krönke G., Kleyer A., Zaiss M.M., Heppt F., et al. Patients with immune-mediated inflammatory diseases receiving cytokine inhibitors have low prevalence of SARS-CoV-2 seroconversion. Nat. Commun. 2020;11(July (1)):3774. doi: 10.1038/s41467-020-17703-6. PMID: 32709909; PMCID: PMC7382482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gómez-Rial J., Rivero-Calle I., Salas A., Martinón-Torres F. Role of Monocytes/Macrophages in Covid-19 pathogenesis: implications for therapy. Infect. Drug Resist. 2020;22(July (13)):2485–2493. doi: 10.2147/IDR.S258639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chomarat P., Banchereau J., Davoust J., Palucka A.K. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat. Immunol. 2000;1(December (6)):510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 200.Sindhu S., Thomas R., Shihab P., Sriraman D., Behbehani K., Ahmad R. Obesity is a positive modulator of IL-6R and IL-6 expression in the subcutaneous adipose tissue: significance for metabolic inflammation. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0133494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pedersen K.M., Çolak Y., Ellervik C., Hasselbalch H.C., Bojesen S.E., Nordestgaard B.G. Loss-of-function polymorphism in IL6R reduces risk of JAK2V617F somatic mutation and myeloproliferative neoplasm: a Mendelian randomization study. EClinicalMedicine. 2020;19(February (21)) doi: 10.1016/j.eclinm.2020.100280. PMID: 32382712; PMCID: PMC7201035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lu R., Zhao X., Li J., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bourgonje A.R., Abdulle A.E., Timens W., et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J. Pathol. 2020;251(3):228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zemlin A.E., Wiese O.J. Coronavirus disease 2019 (COVID-19) and the renin-angiotensin system: a closer look at angiotensin-converting enzyme 2 (ACE2) Ann. Clin. Biochem. 2020;57(September (5)):339–350. doi: 10.1177/0004563220928361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Aleksova A., Ferro F., Gagno G., Cappelletto C., Santon D., Rossi M., Ippolito G., Zumla A., Beltrami A.P., Sinagra G. COVID-19 and renin-angiotensin system inhibition: role of angiotensin converting enzyme 2 (ACE2) - is there any scientific evidence for controversy? J Intern Med. 2020;288(October (4)):410–421. doi: 10.1111/joim.13101. Epub 2020 Jun 8. PMID: 32459372; PMCID: PMC7283873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–1035. doi: 10.1016/j.cell.2020.04.035. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Busnadiego I., Fernbach S., Pohl M.O., Karakus U., Huber M., Trkola A., Stertz S., Hale B.G. Antiviral activity of type I, II, and III interferons counterbalances ACE2 inducibility and restricts SARS-CoV-2. mBio. 2020;11(September (5)):e01928–20. doi: 10.1128/mBio.01928-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Saffern M. ACE2 is not induced by interferon. Nat. Rev. Immunol. 2020;20(September(9)):521. doi: 10.1038/s41577-020-00416-8. PMID: 32737461; PMCID: PMC7394041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Onabajo O.O., et al. Interferons and viruses induce a novel primate-specific isoform dACE2 and not the SARS-CoV-2 receptor ACE2. bioRxiv. 2020 doi: 10.1101/2020.07.19.210955. - DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Rutkowska-Zapala M., Suski M., Szatanek R., et al. Human monocyte subsets exhibit divergent angiotensin I-converting activity. Clin. Exp. Immunol. 2015;181(1):126–132. doi: 10.1111/cei.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shantsila E., Lip G.Y. Monocytes in acute coronary syndromes. Arterioscler. Thromb. Vasc. Biol. 2009;29(10):1433–1438. doi: 10.1161/ATVBAHA.108.180513. [DOI] [PubMed] [Google Scholar]
- 212.Barone M., Catani L., Ricci F., Romano M., Forte D., Auteri G., Bartoletti D., Ottaviani E., Tazzari P.L., Vianelli N., Cavo M., Palandri F. The role of circulating monocytes and JAK inhibition in the infectious-driven inflammatory response of myelofibrosis. Oncoimmunology. 2020;9(June (1)) doi: 10.1080/2162402X.2020.1782575. PMID: 32923146; PMCID: PMC7458658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yeleswaram S., Smith P., Burn T., Covington M., Juvekar A., Li Y., Squier P., Langmuir P. Inhibition of cytokine signaling by ruxolitinib and implications for COVID-19 treatment. Clin. Immunol. 2020;218(September) doi: 10.1016/j.clim.2020.108517. Epub 2020 Jun 23. PMID: 32585295; PMCID: PMC7308779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hennighausen L., Lee H.K. Activation of the SARS-CoV-2 receptor Ace2 by cytokines through pan JAK-STAT enhancers. bioRxiv [Preprint]. 2020;11(May) doi: 10.1101/2020.05.11.089045. 2020.05.11.089045. Update in: Cell Rep. 2020 Sep29;32(13):108199. PMID: 32511315; PMCID: PMC7239044. [DOI] [Google Scholar]
- 215.Lee H.K., Jung O., Hennighausen L. Activation of interferon-stimulated transcriptomes and ACE2 isoforms in human airway epithelium is curbed by Janus kinase inhibitors. Res Sq [Preprint]. 2020;11(December) doi: 10.21203/rs.3.rs-119695/v1. rs.3.rs-119695.PMID:33330857;PMCID: PMC7743079. [DOI] [Google Scholar]
- 216.Hermouet S., Vilaine M. The JAK2 46/1 haplotype: a marker of inappropriate myelomonocytic response to cytokine stimulation, leading to increased risk of inflammation, myeloid neoplasm, and impaired defence against infection? Haematologica. 2011;96(11):1575–1579. doi: 10.3324/haematol.2011.055392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Van Egeren D., Escabi J., Nguyen M., Liu S., Reilly C.R., Patel S., Kamaz B. ASH; 2020. Reconstructing the Lineage Histories and Differentiation Trajectories of Individual Hematopoietic Stem Cells in JAK2-Mutant Myeloproliferative Neoplasms. Abstract 714. Oral and Poster. Basic Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Williams N., Lee J., Moore L., Baxter J.E., Hewinson J., Kevin J., Dawson K.J., et al. ASH; 2020. LBA-1 Driver Mutation Acquisition in Utero and Childhood Followed By Lifelong Clonal Evolution Underlie Myeloproliferative Neoplasms. LBA-1. Late-Breaking Abstracts Session. [Google Scholar]
- 219.A Phase 3 Randomized Double-blind, placebo-controlled multi-center study to assess the efficacy and safety of ruxolitinib in patients with COVID-19 associated cytokine storm (RUXCOVID).”. ClinicalTrials.gov. 2020 https://clinicaltrials.gov/ct2/show/NCT04362137 [Google Scholar]
- 220.Randall R.E., Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 221.Aricò E., Bracci L., Castiello L., Gessani S., Belardelli F. Are we fully exploiting type I Interferons in today’s fight against COVID-19 pandemic? Cytokine Growth Factor Rev. 2020;54:43–50. doi: 10.1016/j.cytogfr.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lee J.S., Park S., Jeong H.W., Ahn J.Y., Choi S.J., Lee H., Choi B., Nam S.K., Sa M., Kwon J.S., Jeong S.J., Lee H.K., Park S.H., Park S.H., Choi S.J.Y., Kim S.H., Jung I., Shin E.C. Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci. Immunol. 2020;5(July (49)) doi: 10.1126/sciimmunol.abd1554. PMID: 32651212; PMCID: PMC7402635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lei X., Dong X., Ma R., Wang W., Xiao X., Tian Z., Wang C., Wang Y., Li L., Ren L., Guo F., Zhao Z., Zhou Z., Xiang Z., Wang J. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat. Commun. 2020;11(July (1)):3810. doi: 10.1038/s41467-020-17665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wang Z., Pan H., Jiang B. Type I IFN deficiency: an immunological characteristic of severe COVID-19 patients. Signal Transduct. Target. Ther. 2020;5(1):198. doi: 10.1038/s41392-020-00306-4. PMID: 32929061; PMCID: PMC7487337. [DOI] [PMC free article] [PubMed] [Google Scholar]