Abstract
Anti-N-methyl-D-aspartate receptor encephalitis is a clinical condition characterized by acute behavioral and mood changes, abnormal movements, autonomic instability, seizures, and encephalopathy. We describe a 7-year-old boy diagnosed with autoimmune encephalitis due to NMDAR antibody in association with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease 2019) (COVID-19), without pulmonary involvement or fever. The patient presented with acute ataxia, rapidly developed encephalopathy, and autoimmune encephalitis was suspected. Steroid treatment was withheld because of lymphopenia and intravenous immunoglobulin was started. The absence of clinical response prompted plasmapheresis and, when lymphocyte counts improved, pulse steroid treatment was applied. The latter was followed by significant improvement and the patient was discharged in a conscious and ambulatory state. Autoimmune encephalitis should be considered in the presence of neurological symptoms accompanying SARS-CoV-2 infection and steroid treatment should be preferred unless limited by contraindications.
Keywords: COVID-19, Anti-NMDA receptor encephalitis, Ataxia, Pediatric
Introduction
Anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis is a clinical condition characterized by sudden changes in the level of consciousness, behavior, or mood; new onset seizures; abnormal movements; and autonomic instability [1]. Viral infections constitute its most frequent precipitating factor in children [2, 3]. The pandemic of severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) has been associated with various neurological complications attributed to neural infection, vascular complications, or the inflammatory reaction. Four adult cases of SARS-CoV-2-associated anti-NMDAR encephalitis and only one young child have been reported to date [4–7].
We present a pediatric case with anti-NMDAR encephalitis and SARS-CoV-2 infection confirmed by viral PCR.
Case presentation
A 7-year old boy was admitted to our hospital because he had experienced unsteady gait for the prior 4 days. He had no complaints of headache, fever, or antecedent infection. Medical and family histories were unremarkable. A general physical examination was normal. He had ataxia and wide-based gait. Deep tendon reflexes could not be elicited. On the second day, he developed somnolence and seizures, and levetiracetam was started (Fig. 1). Peripheral blood biochemistry and erythrocyte sedimentation rate were normal; C-reactive protein (CRP) was 20 mg/L (0–5 mg/L); absolute lymphocyte count was 700/mm3 (2.25–8.89/mm3) (Fig. 2). Brain magnetic resonance imaging (MRI) was normal. Cerebrospinal fluid (CSF) analysis was normal for protein, glucose, lactate, and pyruvate levels and IgG index; no cells or oligoclonal bands were observed, and serology for Epstein-Barr virus (EBV), Herpes simplex virus type 1 and type 2 (HSV-1 and -2), and Borrelia burgdorferi was negative. Treatment was started for possible encephalitis with acyclovir, ceftriaxone, and clarithromycin. CSF bacterial culture and PCR for HSV-1 and HSV-2 were negative. On the third day of admission, the SARS-CoV-2 rtPCR test from the throat swab was reported to be positive. The patient had no fever or respiratory symptoms, but lymphopenia persisted. Awake and sleep EEGs were encephalopathic with widespread delta waves (Fig. 3). Persistent lymphopenia and increasing creatinine and CRP levels prompted the use of plasmapheresis and intravenous immune human globulin (IVIg) to the treatment, with no significant benefit. On the 8th day of admission, choreiform movements in the hands and feet, tongue protrusion, bruxism, lip smacking, agitation, catatonia, echolalia, and tachycardia were observed. A test of the CSF for anti-NMDAR IgG was positive. Three courses of plasmapheresis were performed in the first hospital week. The patient’s lymphopenia and creatinine values started to normalize and methylprednisolone 30 mg/kg/day for 5 days followed by 20 mg/kg for 2 days, and IVIg 2 g/kg over 5 days were applied, followed by prednisolone 2 mg/kg p.o. His level of consciousness, oral intake, and involuntary movements gradually improved within 2 weeks after beginning prednisolone. A focal seizure occurred on day 26 in hospital: a second brain MRI was normal and clobazam and topiramate were added. The patient was discharged ambulating but mildly ataxic, with the plan of slow oral prednisolone taper, antiepileptic treatment, and repeat IVIg if necessary.
Fig. 1.
Clinical features and treatment over time
Fig. 2.
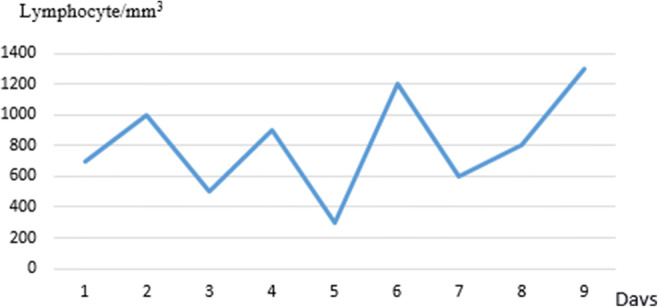
Lymphocyte counts over time
Fig. 3.
Widespread delta waves on the EEG performed on the 2nd day of hospitalization
Discussion
As of when this written, over 37 million COVID-19 cases and one million deaths have been reported globally (WHO COVID-19 Dashboard). The neurological complications are explained by various pathogenic mechanisms: direct viral injury, systemic inflammatory response syndrome, para- and post-infectious inflammatory or immune-mediated reactions triggered by virus, in particular cross-reacting antibodies against host antigens [8–11]. Monti et al. [6] reported a 50-year-old patient with status epilepticus, anti-NMDAR antibody, and COVID-19 rtPCR positivity but no respiratory or systemic symptoms; Panariello et al. [4] described a 23-year-old boy with acute psychosis due to anti-NMDAR encephalitis, also with no respiratory system involvement. Younger patients with anti-NMDAR encephalitis and COVID-19 positivity were reported by Burr et al. [5] and Moideen et al. [7] in 23 months and 17-year-old patients, respectively, the latter with acute psychosis. Our patient, unlike those in other reports, manifested with acute ataxia, only to become encephalopathic over days.
The NR1A subunit of the NMDAR, the target of the autoantibodies, is expressed in the neocortex, hippocampus, and also cerebellum in humans [12, 13]. The expression level of the NMDAR is subject to age-dependent changes; its function and response to exogenous factors such as stress or steroids can also change during development, which can explain different clinical manifestations of anti-NMDAR encephalitis according to age and developmental status [14].
.The diagnosis of autoimmune encephalitis was strongly considered at our patient’s initial presentation. The marked lymphopenia precluded steroid treatment and IVIg was started first along with an antiviral drug and antibiotics. Lymphopenia is a well-known finding in Covid-19 infection [15]. Lymphocyte counts started to increase and pulse steroid treatment was started on the 10th day of admission, resulting in more rapid clinical improvement. Despite a few days’ delay, our patient’s diagnosis and treatment beginning within 4 days of symptoms can still be considered as relatively early. Although his clinical picture worsened in the first week, the normal MRI findings at admission and follow-up and prompt response to treatment also support timely diagnosis and intervention in this case.
We believe this report can serve as an example of pediatric autoimmune encephalitis associated with COVID-19 and contribute to the clinical perspective, management, and treatment of neurological complications observed during the pandemic.
Acknowledgements
I would like to thank to Prof. Dr. Cetin Okuyaz and Prof. Dr. Ilknur Erol for their support during the follow-up process of this case.
Abbreviations
- Anti-NMDAR
Anti-N-methyl-D-aspartate receptor
- CRP
C-reactive protein
- CSF
Cerebro spinal fluid
- COVID-19
Coronavirus disease 2019
- IVIG
Intravenous immunoglobulin
- MRI
Magnetic resonance imaging
- Plx
Plasmapheresis
Declarations
Ethics approval and consent to participate
The presentation of the case was approved by the guardians of the patient. Informed consent was obtained.
Conflict of interest
The authors declare that they have no conflict of interest to disclose. There is no funding support available for this study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Esra Sarigecili, Email: sarigeciliesra@gmail.com.
Ilknur Arslan, Email: ilknurtolunay@gmail.com.
Habibe Koc Ucar, Email: hkocselanik@gmail.com.
Umit Celik, Email: ucelik32@gmail.com.
References
- 1.Garg D, Mohammad SS, Sharma S. Autoimmune encephalitis in children: an update. Indian Pediatr. 2020;57:662–670. doi: 10.1007/s13312-020-1896-5. [DOI] [PubMed] [Google Scholar]
- 2.Prüss H, Finke C, Höltje M, Hofmann J, Klingbeil C, Probst C, Borowski K, Ahnert-Hilger G, Harms L, Schwab JM, Ploner CJ, Komorowski L, Stoecker W, Dalmau J, Wandinger KP. N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol. 2012;72:902–911. doi: 10.1002/ana.23689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prüss H. Postviral autoimmune encephalitis: manifestations in children and adults. Curr Opin Neurol. 2017;30:327–333. doi: 10.1097/WCO.0000000000000445. [DOI] [PubMed] [Google Scholar]
- 4.Panariello A, Bassetti R, Radice A, Rossotti R, Puoti M, Corradin M, Moreno M, Percudani M. Anti-NMDA receptor encephalitis in a psychiatric Covid-19 patient: a case report. Brain Behav Immun. 2020;87:179–181. doi: 10.1016/j.bbi.2020.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burr T, Barton C, Doll E, Lakhotia A, Sweeney M. NMDA-receptor encephalitis associated with COVID-19 infection in a toddler. Pediatr Neurol. 2020;114:75–76. doi: 10.1016/j.pediatrneurol.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monti G, Giovannini G, Marudi A, Bedin R, Melegari A, Simone AM, Simone AM, Santangelo M, Pignatti A, Bertellini E, Trenti T, Meletti S. Anti-NMDA receptor encephalitis presenting as new onset refractory status epilepticus in COVID-19. Seizure. 2020;81:18–20. doi: 10.1016/j.seizure.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moideen S, Thomas R, Kumar SPN, Uvais NA, Katshu MZUH. Psychosis in a patient with anti-NMDA-receptor antibodies experiencing significant stress related to COVID-19. Brain Behav Immun. 2020;7:100125. doi: 10.1016/j.bbih.2020.100125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franke C, Ferse C, Kreye J, Reincke M, Sanchez-Sendin E, Rocco A, et al (2020) High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Preprint at medRxiv. [DOI] [PMC free article] [PubMed]
- 10.Kreye J, Reincke SM, Prüss H. Do cross-reactive antibodies cause neuropathology in COVID-19? Nat Rev Immunol. 2020;20:645–646. doi: 10.1038/s41577-020-00458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Consiglio CR, Cotugno N, Sardh F, Landegren N, Palma P, Brodin P. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183:1–14. doi: 10.1016/j.cell.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scherzer CR, Landwehrmeyer GB, Kerner JA, Counihan TJ, Kosinski CM. Standart D.G. Expression of N-methyl-D-aspartate receptor subunit mRNAs in the human brain: hippocampus and cortex. J Comp Neurol. 1998;5:75–90. doi: 10.1002/(SICI)1096-9861(19980105)390:1<75::AID-CNE7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 13.Scherzer CR, Landwehrmeyer GB, Kerner JA, Standaert DG, Hollingsworth ZR, Daggett LP, Standaert DG, Hollingsworth ZR, Daggett LP, Veliçelebi G, Penney JB, Jr, Young AB. Cellular distribution of NMDA glutamate receptor subunit mRNAs in the human cerebellum. Neurobiol Dis. 1997;4:35–46. doi: 10.1006/nbdi.1997.0136. [DOI] [PubMed] [Google Scholar]
- 14.Lee PR, Brady D, Koenig JI. Corticosterone alters N-methyl-D-aspartate receptor subunit mRNA expression before puberty. Brain Res Mol Brain Res. 2003;4:55–62. doi: 10.1016/S0169-328X(03)00180-3. [DOI] [PubMed] [Google Scholar]
- 15.Guan WJ, Ni Z, Hu Y, Liang WH, Ou C, He JT, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]




