Abstract
Objective:
Endocrine Society guidelines recommend adrenal venous sampling (AVS) in primary aldosteronism (PA) if adrenalectomy is considered. We tested whether functional imaging of adrenal cortex with 11C-metomidate (11C-MTO) could offer a noninvasive alternative to AVS in the subtype classification of PA.
Design:
We prospectively recruited 58 patients with confirmed PA who were eligible for adrenal surgery.
Methods:
Subjects underwent AVS and 11C-MTO-PET without dexamethasone pretreatment in random order. The lateralization of 11C-MTO-PET and adrenal CT were compared with AVS in all subjects and in a prespecified adrenalectomy subgroup in which the diagnosis was confirmed with immunohistochemical staining for CYP11B2.
Results:
In the whole study population, the concordance of AVS and 11C-MTO-PET was 51% and did not differ from that of AVS and adrenal CT (53%). The concordance of AVS and 11C-MTO-PET was 55% in unilateral and 44% in bilateral PA. In receiver operating characteristics analysis, the maximum standardized uptake value ratio of 1.16 in 11C-MTO-PET had an AUC of 0.507 (P = n.s.) to predict allocation to adrenalectomy or medical therapy with sensitivity of 55% and specificity of 44%. In the prespecified adrenalectomy subgroup, AVS and 11C-MTO-PET were concordant in 10 of 19 subjects with CYP11B2-positive adenoma and in 6 of 10 with CYP11B2-positivity without an adenoma.
Conclusions:
The concordance of 11C-MTO-PET with AVS was clinically suboptimal, and did not outperform adrenal CT. In a subgroup with CYP11B2-positive adenoma, 11C-MTO-PET identified 53% of cases. 11C-MTO-PET appeared to be inferior to AVS for subtype classification of PA.
Introduction
Untreated primary aldosteronism (PA) increases the risk of mortality and cardiovascular, renal, and metabolic events beyond the risk caused by essential hypertension of comparable severity (1, 2, 3). Targeted treatments with adrenalectomy or medical therapy can prevent these adverse events and possibly provide a reversal of end-organ damage. However, in large series, antihypertensive medical therapy with a mineralocorticoid receptor antagonist (MRA) appeared to be inferior to adrenal surgery in reducing the risk of mortality, cardiovascular events, atrial fibrillation and decline of renal function, and quality of life (4, 5, 6, 7).
Determination of the optimal treatment for PA critically relies on subtype classification (1, 8). Most patients with PA are middle-aged or older and anatomical imaging with adrenal CT cannot distinguish unilateral aldosterone-producing adenomas (APAs) from nonfunctioning adrenal nodules, lateralization of aldosterone secretion from hyperplastic adrenal glands, or aldosterone-producing cell clusters (APCCs) that become more prevalent with age (9, 10). Therefore, the guidelines (8) recommend PA subtyping with bilateral adrenal venous sampling (AVS) in patients who are considered for adrenal surgery.
In experienced centers the diagnostic performance of AVS is excellent (8, 11, 12). The disadvantages include technical difficulties in non-specialized centers, potential vascular complications, and relatively high costs from the procedure and laboratory measurements (1). Currently, the standardized procedure and interpretation of AVS results are under discussion (1, 13).
11C-metomidate PET (11C-MTO-PET) CT is a potentially promising method since 11C-metomidate traces 11β-hydroxylase (CYP11B2) activity in the adrenal cortex (14, 15). Recent case series of PA patients suggest that 11C-MTO-PET may provide clinical benefit in PA subtype classification (16, 17).
In the present prospective study, our objective was to evaluate the diagnostic power of 11C-MTO-PET imaging compared with AVS in establishing or excluding lateralization of aldosterone production in patients with confirmed PA. A secondary prespecified objective included analysis of the performance of 11C-MTO-PET compared with AVS to detect lateralization in the subgroup of adrenalectomy patients in whom biochemical and medical outcome, as well as immunohistochemical analysis of aldosterone synthase (CYP11B2) of the adrenal samples, were available.
Subjects and Methods
Study design and participants
We recruited 58 eligible consecutive patients with PA who were referred to endocrinology units in Helsinki, Tampere, and Turku University Hospitals between February 2012 and December 2015. Patients fulfilling the criteria for confirmed PA according to the 2008 Endocrine Society guidelines (18) who were willing and eligible for possible adrenalectomy were included (Fig. 1). Inclusion criteria were age between 20 and 70 years, good general health enabling possible adrenalectomy, and a BMI of less than 35 kg/m2. The exclusion criteria are presented in the Supplementary material (see section on supplementary materials given at the end of this article). A prespecified post hoc, blinded adrenal CT analysis was performed by a single experienced specialist in abdominal radiology (E.L.).
Figure 1.

Patient selection, excluded subjects, and allocation to adrenalectomy or medical therapy. AVS, adrenal venous sampling; 11C-MTO-PET, 11C-metomidate positron emission tomography; LI, lateralization index.
All subjects underwent AVS and 11C-MTO-PET imaging in random order. Subjects with lateralization of aldosterone secretion in AVS were allocated to adrenal surgery (adrenalectomy group). In case of unsuccessful AVS, concordant findings suggesting single adrenal adenoma on 11C-MTO-PET and adrenal CT justified adrenal surgery. The postoperative outcome was evaluated about 3 months after adrenalectomy. For those treated with medical therapy (medical therapy group), medicine and blood pressure data were collected after lateralization studies for comparison. We applied retrospectively the PASO consensus criteria for a surgical cure (19). The detailed blood pressure, daily defined dose (DDD) of antihypertensive medication, and biochemical cut points are described in the PASO study (19).
All subjects provided written informed consent. The study protocol was approved by the ethics committee of Turku University Hospital and the study was registered in the ClinicalTrials.gov database (NCT01567111). The study was undertaken in accordance with the Declaration of Helsinki. Patients received written information describing AVS and 11C-MTO-PET procedures, including benefits and predictable complications.
Methods
11C-metomidate positron emission tomography
All PET scans were performed in Turku PET Centre and the detailed description of the imaging is shown in the Supplementary material.
Images were analyzed with Advantage Workstation (version 4.7, GE Healthcare). PET lateralization was defined as metabolic activity localized to an anatomic adenoma compared to anatomic adenomas without activity and/or >15% difference in SUVmax values between the adrenal glands. In some patients, clear anatomic adenomas could not be visualized, but there was a difference in 11C-MTO uptake between the adrenal glands, or similar adrenal gland activity with clear anatomic adenoma also showing metabolic activity was found.
Follow-up 11C-metomidate PET CT using dexamethasone suppression
According to previous studies, cortisol production (CYP11B1 activity) by cortical tumor may cause positive 11C-MTO uptake in PET CT (15, 16). To test the reproducibility of the PET scans, we investigated the 11C-MTO-PET CT during dexamethasone (DXM) pretreatment (0.5 mg every 6 h for 3 days) in a subgroup of seven patients.
Adrenal venous sampling
All AVS studies were conducted at Tampere University Hospital. Cosyntropin infusion and detailed methodology are described in the Supplementary material.
The selectivity of AVS on both sides was confirmed by an adrenal vein to peripheral cortisol ratio of greater than 5:1. An aldosterone to cortisol ratio greater than 4:1 on the dominant side compared with the non-dominant side confirmed the diagnosis of unilateral hyperaldosteronism. Ratios between 3:1 and 4:1 presented a zone of overlap, where lateralization was interpreted as positive according to a contralateral suppression index of <1.0.
Pathological and immunohistochemical analysis
Diagnostic H&E stained adrenal slides were reviewed centrally in the Helsinki University Hospital by a single pathologist with special expertise in endocrine pathology (HL). One or two representative blocks per case were selected with the following criteria: (a) adenoma coupled with normal adrenal cortex and (b) hyperplasia presenting with a dominant nodule. Immunohistochemical labeling was performed with previously described primary antibodies CYP11B1 (11β-hydroxylase, dilution 1:5) and CYP11B2 (aldosterone synthase, dilution 1:3000) (20). Each sample was categorized as APA or non-APA based on immunohistochemistry, as described in Fig. 2 (21, 22, 23). For detailed immunohistochemical methods, please see the Supplementary material.
Figure 2.
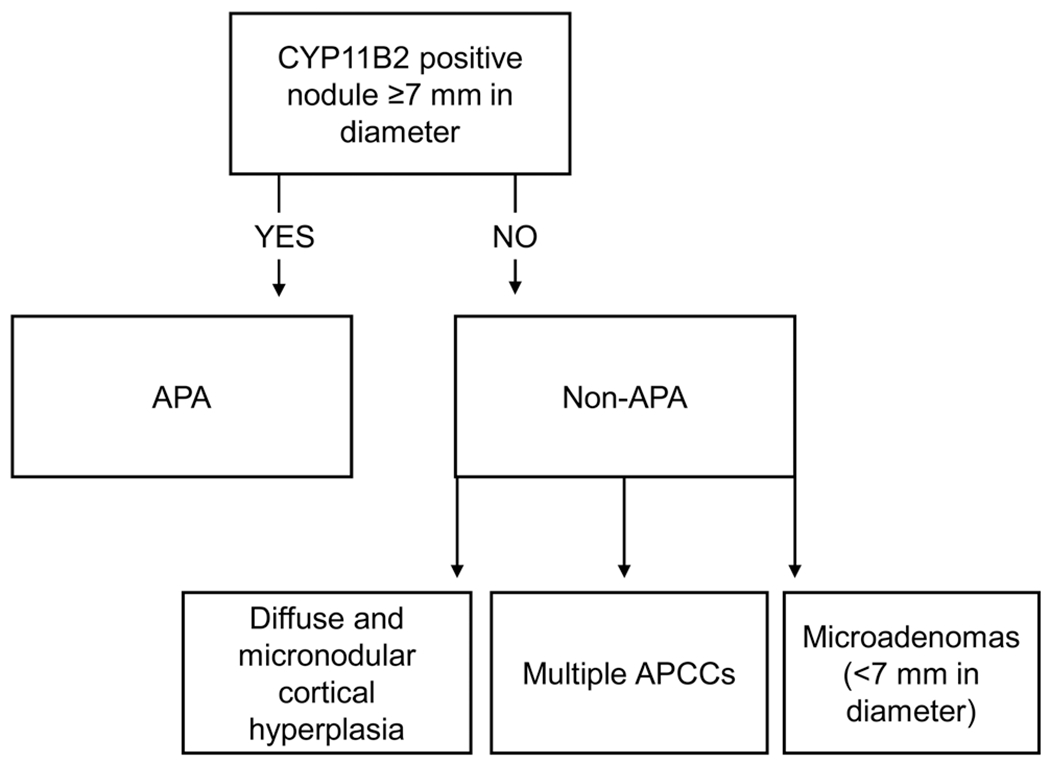
CYP11B2-based categorization of adrenal gland samples.
Statistical analysis
Power calculation for the study and detailed statistical methods are presented in the Supplementary material. We analyzed all patients who completed the follow-up (Fig. 1). The test performance characteristics of AVS, CT, and 11C-MTO-PET to lateralize adrenal aldosterone secretion were analyzed in the whole study population and in a prespecified subgroup of patients who underwent adrenalectomy. A receiver operating characteristics (ROC) curve was constructed from the pairs of sensitivity and specificity measured at each SUVmax ratio between dominant and non-dominant adrenal SUVmax. The ROC analysis was conducted, including all subjects and using allocation to the operation or medical therapy as the standard.
Results
Altogether, 55 subjects were included in the study (Fig. 1). A total of 34 displayed significant lateralization of aldosterone production and underwent unilateral adrenalectomy. The remaining 20 subjects without lateralization received medical therapy. Based on AVS, surgery was recommended to one subject who opted for medical therapy due to discordant lateralization studies and was included in the medical therapy group. Table 1 displays the baseline characteristics for all subjects in the study and clinical, biochemical data at follow-up during medical therapy or after adrenalectomy. Fourteen subjects had adequately controlled diabetes and four had the previous history of stable cardiovascular disease.
Table 1.
Patient characteristics. Data are presented as number, mean and standard deviation, or median and interquartile range. SUVmax indicates the standardized uptake value (SUV) of the dominant adrenal and SUVmax-ratio indicates the ratio between the dominant and non-dominant adrenal gland in 11C-metomidate positron emission CT. Follow-up data were evaluated about 3 months after adrenalectomy or at a follow-up visit after intensification of the medical therapy.
| All subjects | Medical therapy baseline | Medical therapy follow-up | Adrenalectomy baseline | After adrenalectomy follow-up | |
|---|---|---|---|---|---|
| Number (male/female) | 55 (40/15) | 21 (17/4) | – | 34 (23/11) | – |
| Age (years) | 54.7 ± 8.5 | 55.2 ± 7.8 | – | 54.3 ± 9.0 | – |
| BMI (kg/m2) | 30.5 ± 5.3 | 31.1 ± 5.0 | – | 30.2 ± 5.6 | – |
| Systolic BP (mmHG) | 154 ± 19 | 153 ± 22 | 139 ± 13 | 155 ± 18 | 133 ± 12*** |
| Diastolic BP (mmHG) | 94 ± 10 | 95 ± 10 | 88 ± 10 | 94 ± 10 | 80 ± 8*** |
| Duration of treated hypertension (years) | 12 (7.8–25) | 22 (8.8–25) | – | 10 (5.8–16.3)* | – |
| Antihypertension medication, DDD | 4.5 (2.7–6.0) | 4.5 (2.9–6.3) | 4.3 (3.3–5.3) | 4.0 (2.5–5.8) | 2.0 (0–3.3)*** |
| Antihypertension medication (n) | 2.7 (2–6) | 3 (2–3) | 3 (2–3) | 2.5 (2–3.25) | 1 (0–2)*** |
| Serum aldosterone (pmol/L) | 685 (467–1010) | 567(421–871) | – | 765 (519–1217) | 82 (30–208)*** |
| PRA (pmol/L/min) | 0.2 (<0.2–0.2) | <0.2 (<0.2–0.2) | – | <0.2 (<0.2–0.2) | 0.6 (0.2–2.1)* |
| ARR, PRA | 2825 (1767–4701) | 2600 (1630–4007) | – | 3440 (1950–4773) | 140 (25–567)*** |
| DRC (mU/L) | 5.0 (2.5–10.1) | 8.5 (5.5–16.8) | – | 2.5 (1.7–3.2) | 10 (5–21)* |
| ARR, DRC | 117 (65–338) | 91 (34–119) | – | 338 (93–1771) | 12.3 (6.0–21.4) |
| 24 h U-aldosterone (nmol) | 60 (51–96) | 59 (50–68) | – | 70 (55–112) | 11 (8–24)* |
| Plasma potassium (mmol/L) | 2.95 ± 0.41 | 3.14 ± 0.44 | 4.35 ± 0.21 | 2.86 ± 0.38** | 4.05 ± 0.47*** |
| SUVmax (g/mL) | 23.7 ± 8.8 | 21.7 ± 12.2 | – | 25.4 ± 9.2 | – |
| SUVmax ratio | 1.49 (1–6.39) | 1.51 (1.00–6.39) | 1.48 (1–3.83) | – | |
| Lateralization index | 27.37 (1.07–159.23) | 1.95 (1.07–4.55) | 43.47 (3.00–159.23)*** | – | |
| Contralateral suppression index | 1.29 (0.02–4.21) | 2.47 (1.19–4.21) | 0.52 (0.02–1.51)*** | – |
Asterisks indicate significant differences between medical therapy vs adrenalectomy at baseline or before vs postadrenalectomy in the adrenalectomy group:
P < 0.05,
P < 0.01,
P < 0.001.
ARR, aldosterone-renin ratio; BP, blood pressure; DDD, daily defined dose; DRC, direct renin concentration; PRA, plasma renin activity; SUV, standardized uptake value.
Clinical outcome in the medical therapy group
A total of 21 patients with PA were treated with medical therapy. Use of MRA increased from 29 to 95% in the medical therapy group at follow-up. The number of antihypertensive medications and their DDD remained constant at follow-up. Compared with the adrenalectomy group, the number of medications and the DDD were significantly higher in the medical therapy group (P < 0.001 for both). Systolic blood pressure decreased, and plasma potassium concentration increased significantly (P = 0.013 and P = 0.028) but diastolic blood pressure did not change (P = 0.062) from the baseline (Table 1). With medical therapy diastolic blood pressure decreased less than after adrenalectomy (P = 0.004) but no difference was found in systolic blood pressure decrease between the groups (Table 1).
Clinical, biochemical, and immunohistochemical outcome of adrenalectomized patients
The characteristics before and after operation in the 34 adrenalectomized patients are shown in Table 1. Blood pressure, the number of antihypertensive medications and DDD decreased, and plasma potassium concentration increased significantly after adrenalectomy. Of the 34 operated subjects, 10 (29%) were able to stop all antihypertensive medications, 12 (35%) used one drug, and none used MRAs.
Individual clinical and biochemical complete, partial, and absent cure responses (19) are shown in Table 2. Biochemical cure could be determined in 32 of the 34 operated subjects. None in the adrenalectomy group showed complete absence of clinical or biochemical cure and those with absent response according to the consensus criteria showed clinically meaningful improvement in either plasma aldosterone concentration, blood pressure, or DDD that did not reach the limit of the consensus guideline (19).
Table 2.
Individual data of the adrenalectomy group showing the results from immunohistochemical analysis, histological size of an APA, adrenal venous sampling (AVS), 11C-metomidate (11C-MTO) positron emission tomography (PET), and biochemical and clinical improvement according to (19).
| Histology based on CYP11B2 | APA size, mm | Operation side | APCC, n | AVS, LI | AVS, side | SUVmax Right | SUVmax Left | SUVmax ratio | PET, side | PET finding | AVS vs PET | Biochemical improvement | Clinical improvement |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| APA | 13 | Left | 1 | 159.9 | L | 6.7 | 25.8 | 3.38 | L | Adenoma | Concordant | Complete | Partial |
| APA | 12 | Left | 2 | NA | NA | 8.8 | 20 | 2.28 | L | Adenoma | NA | Complete | Complete |
| APA | 15 | Left | 0 | 23.4 | L | 9 | 19.6 | 2.19 | L | Adenoma | Concordant | Complete | Complete |
| APA | 14 | Left | 1 | 59.5 | L | 23.2 | 47.8 | 2.06 | L | Adenoma | Concordant | Complete | Complete |
| APA | 14 | Left | 0 | 151.8 | L | 11 | 21.7 | 1.98 | L | Adenoma | Concordant | Complete | Partial |
| Non-APA | - | Right | 4 | 34.7 | R | 46.4 | 23.6 | 1.97 | R | Adenoma | Concordant | Absent* | Partial |
| Non-APA | - | Right | 5 | NA | NA | 25.7 | 13.5 | 1.9 | R | Adenoma | NA | Complete | Partial |
| APA | 29 | Right | 1 | 105.5 | R | 37.9 | 21.5 | 1.76 | R | Adenoma | Concordant | Complete | Partial |
| Non-APA | - | Left | 2 | 7.2 | L | 9.2 | 16.2 | 1.66 | L | Adenoma | Concordant | Complete | Absent# |
| APA | 7 | Left | 0 | NA | NA | 14.1 | 22.2 | 1.57 | L | Hyperplasia L | NA | Complete | Absent# |
| Non-APA | - | Right | 4 | 3 | R | 17.1 | 11.1 | 1.54 | R | Hyperplasia R | Concordant | Complete | Partial |
| Non-APA | - | Left | 5 | 5.3 | L | 13.8 | 20.6 | 1.49 | L | Adenoma | Concordant | Complete | Partial |
| Non-APA | - | Left | 0 | 5.2 | L | 12.7 | 17.7 | 1.39 | L | Hyperplasia L | Concordant | Partial | Partial |
| APA | 16 | Left | 0 | 66.4 | L | 20.9 | 28.9 | 1.38 | L | Adenoma | Concordant | Complete | Complete |
| APA | 10 | Right | 1 | 128.9 | R | 26.3 | 19.3 | 1.36 | R | Adenoma | Concordant | Complete | Partial |
| APA | 13 | Right | 0 | 54.2 | R | 17.7 | 24 | 1.36 | L | Hyperplasia L | Discordant | Not done | Partial* |
| APA | 8 | Right | 3 | 13.3 | R | 22.6 | 17.4 | 1.3 | R | Adenoma (suspicion) | Concordant | Complete | Partial |
| APA | 13 | Right | 2 | 29.2 | R | 17.4 | 13.9 | 1.25 | R | Adenoma | Concordant | Complete | Complete |
| APA | 12 | Left | 0 | 14.2 | L | 42.1 | 34 | 1.24 | R | Adenoma R/Hyperplasia L | Discordant | Not done | Partial* |
| APA | 7 | Right | 1 | NA | NA | 28.7 | 34.2 | 1.19 | L | Adenoma L/ Hyperplasia R |
NA | Partial | Partial |
| APA | - | Left | 2 | 19.6 | L | 25.6 | 30.3 | 1.18 | L | Adenoma | Concordant | Complete | Complete |
| Non-APA | - | Left | 5 | 36.6 | L | 20.6 | 24 | 1.17 | L | Hyperplasia L | Concordant | Complete | Complete |
| APA | 14 | Left | 4 | 8.4 | L | 29.2 | 33.5 | 1.15 | None | No activity difference | Discordant | Complete | Complete |
| APA | 15 | Right | 0 | 6.3 | R | 51.4 | 44.7 | 1.15 | None | Hyperplasia both sides | Discordant | Partial | Partial |
| APA | 18 | Left | 2 | 4.8 | L | 18.5 | 20 | 1.08 | None | Hyperplasia both sides | Discordant | Complete | Complete |
| Non-APA | - | Left | 5 | 10.7 | L | 36.5 | 39.1 | 1.07 | None | No activity difference | Discordant | Partial | Partial |
| Non-APA | - | Right | 8 | 5.9 | R | 15.4 | 14.5 | 1.06 | None | No activity difference | Discordant | Complete | Complete |
| APA | - | Left | 3 | 9 | L | 29.4 | 28.3 | 1.04 | None | No activity difference | Discordant | Complete | Partial |
| Non-APA | - | Right | 4 | 13 | R | 20.1 | 19.4 | 1.04 | None | No activity difference | Discordant | Complete | Complete |
| APA | 20 | Left | 1 | 95.6 | L | 20.4 | 21.1 | 1.03 | None | No activity difference | Discordant | Complete | Partial |
| APA | 12 | Left | 4 | 88.5 | L | 31.2 | 32.2 | 1.03 | None | Hyperplasia both sides | Discordant | Complete | Complete |
| Non-APA | - | Right | 5 | 8.1 | R | 23.5 | 24.3 | 1.03 | None | No activity difference | Discordant | Complete | Partial |
| APA | 9 | Left | 2 | 4.3 | L | 34.8 | 34.9 | 1 | None | Hyperplasia both sides | Discordant | Partial | Partial |
| APA | 14 | Right | 0 | 132.5 | R | NA | NA | NA | NA | NA | NA | Complete | Partial |
Data are organized according to descending SUVmax-ratios.
Subjects with contralateral findings in AVS and 11C-MTO-PET.
Subjects with absent clinical or biochemical improvement by PASO criteria (ref. (19)) all showed improvement that did not meet the criteria.
AVS, adrenal venous sampling; L, left; LI, lateralization index; NA, not applicable; R, right; SUV, standardized uptake value.
The immunohistochemical diagnosis was APA in 68% and non-APA in 32% of the cases (Table 2). Based on the immunohistochemical reevaluation of the adrenalectomy samples, the histological H&E classification between adenoma and hyperplasia changed in seven out of 34 (20.6%) samples (Supplementary data). Twenty-five out of 34 samples were found with a median of three APCCs. These subjects had a similar cure rate to those with a non-APA or adenoma.
Adrenal 11C-MTO-PET concordance with adrenal venous sampling
The primary objective of the study was to compare the lateralization between 11C-MTO-PET and AVS (Fig. 3). In the ROC analysis, the SUVmax ratio of dominant vs non-dominant adrenal in 11C-MTO-PET, the area under the curve (AUC) could not significantly predict subject allocation to the adrenalectomy vs medical therapy groups. The cut point of 1.16 for the SUVmax ratio of dominant vs non-dominant adrenal yielded a sensitivity of 55% and specificity of 44%. The AUC for the blinded adrenal CT report for lateralization or no lateralization did not reach statistical significance in the ROC analysis.
Figure 3.

Receiver operating characteristics (ROC) analysis. Pairs of sensitivity and specificity were calculated using 11C-metomidate positron emission tomography (11C-MTO-PET) SUVmax ratio (AUC = 0.507, P = 0.939), Lateralization index (LI) for adrenal venous sampling (AVS) (AUC = 0.990, P < 0.001), and adrenal computed tomography radiology report (lateralizing or bilateral/no findings, AUC = 0.542, P = 0.63) to predict subject allocation to adrenalectomy vs medical therapy groups (n = 48). SUV, standardized uptake value.
In the whole study population, AVS and 11C-MTO-PET demonstrated concordance (lateralization to the same side or no lateralization) in 24/47 (51%) subjects (Fig. 4). Of the discordant studies between AVS and 11C-MTO-PET, we found a false negative result in 11/47 (23%), contralateral side lateralization in 2/47 (4%), and false positive lateralization of bilateral disease in 10/47 (21%) of them. In the adrenalectomy and medical therapy subgroups, concordance for AVS and 11C-MTO-PET was 55 and 44%, respectively.
Figure 4.
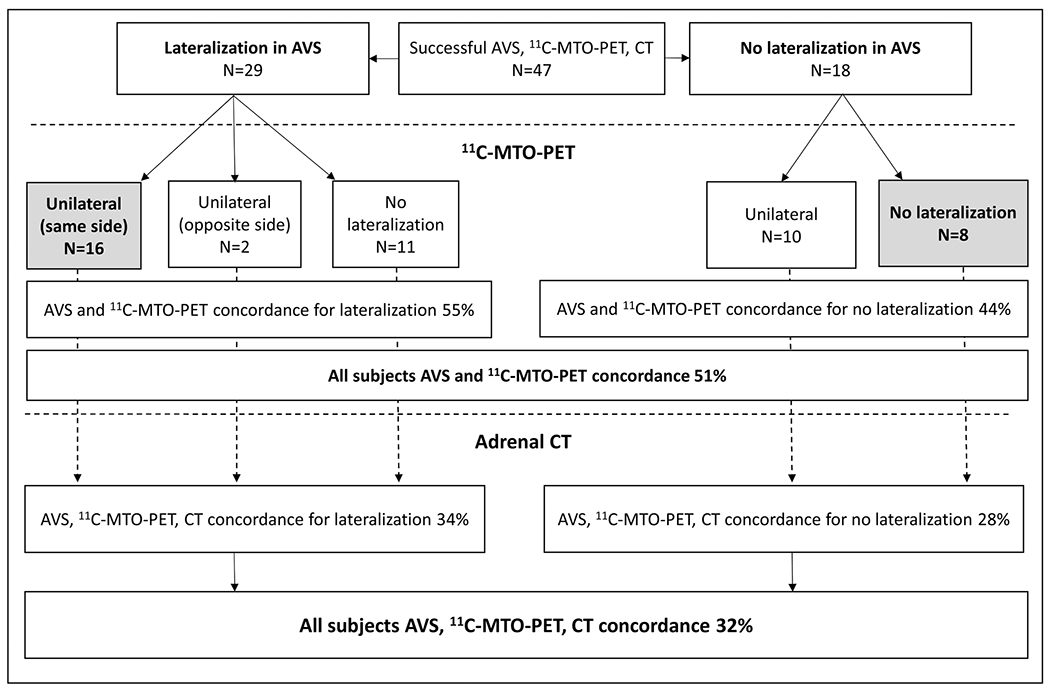
Study subjects divided into those with lateralizing (lateralization index, LI ≥ 4) or non-lateralizing adrenal venous sampling (AVS) result. The middle row shows the 11C-metomidate positron emission tomography (11C-MTO-PET) outcome and the lower row the adrenal CT outcome. Of all subjects, 15 demonstrated concordance uniformly in AVS, 11C-MTO-PET, and CT. Numbers or percentages are given for each possible outcome.
11C-MTO-PET concordance with adrenal venous sampling in the adrenalectomy group
As a secondary objective, we analyzed the concordance between AVS and 11C-MTO-PET in the adrenalectomy group. Among patients with CYP11B2-positive APA, the concordance between the AVS and 11C-MTO-PET studies was 53% (Table 2). Those with non-APA showed 60% concordance between AVS and 11C-MTO-PET. In two cases of APA, 11C-MTO-PET lateralized to the contralateral side and in 11 studies (7 APAs and 4 non-APAs) showed no lateralization. In these subjects with discordant lateralization, the decision for adrenalectomy was based on AVS lateralization, and all demonstrated either complete or partial clinical and biochemical improvement after adrenalectomy.
Adrenal CT concordance with 11C-MTO-PET
A total of 53 subjects had both 11C-MTO-PET and adrenal CT available. The overall concordance of this secondary outcome between the two methods was 55%. The statistical difference between concordance of 11C-MTO-PET with AVS or CT was not significant.
Concordance of all lateralization studies
Of 47 subjects with AVS, 11C-MTO-PET, and CT data available, only 32% displayed concordance for right, left, or no lateralization in all three investigations (Fig. 4). The success rates for AVS and 11C-MTO-PET were 88.0 and 94.8%, respectively. Figure 5 shows as an example two individual cases, one with discordant and one with concordant AVS and 11C-MTO-PET lateralization findings together with the immunohistochemical CYP11B2 staining after adrenalectomy.
Figure 5.
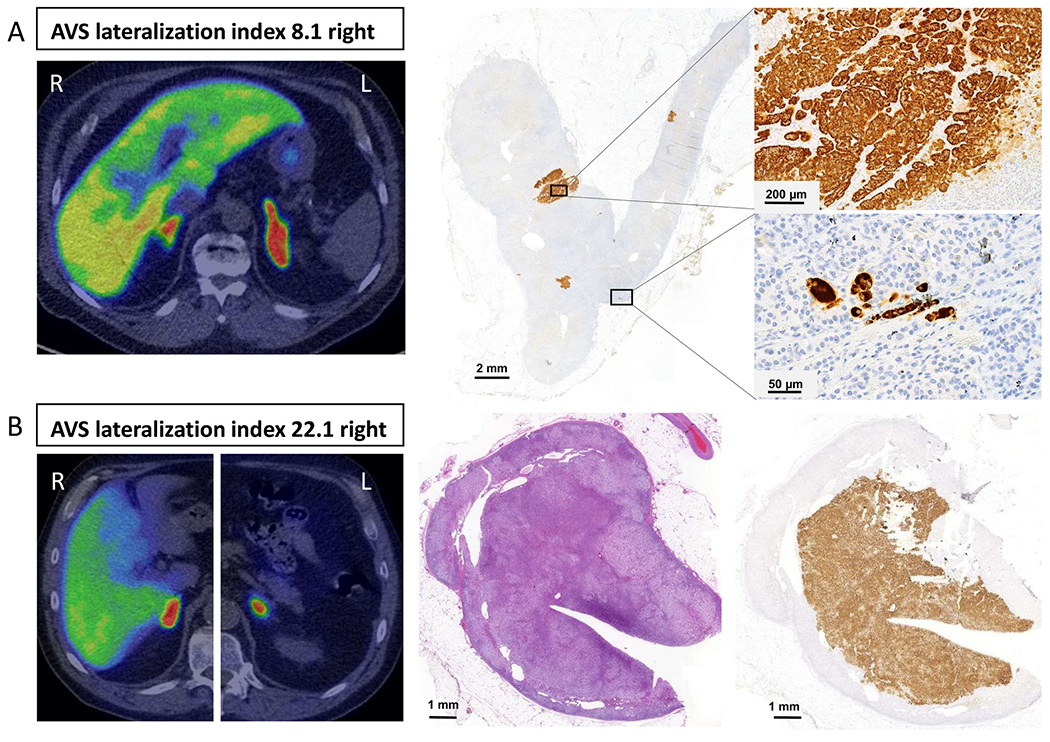
Two patients who underwent right adrenalectomy with complete biochemical cure. In both cases, the operation was based on adrenal venous sampling (AVS) lateralizing to the right. In the upper panel (Case A) the 11C-metomidate positron emission tomography (11C-MTO-PET) was discordant with AVS showing increased activity in the left (L) adrenal, whereas the AVS lateralized to the right. After adrenalectomy, immunohistochemistry revealed multiple small CYP11B2-positive focuses. In the lower panel (Case B), 11C-MTO-PET was concordant with AVS lateralization to the right (R) adrenal. In a histological examination of the right adrenal, the H&E stain revealed a cortical adenoma that was confirmed as an APA with CYP11B2-immunostain.
Reanalysis of 11C-MTO-PET with dexamethasone pretreatment
We performed a second 11C-MTO-PET study for five subjects with discordant and two subjects with concordant AVS and 11C-MTO-PET findings after DXM pretreatment of 0.5 mg every 6 h for 3 days before the scan. All seven subjects had a non-lateralizing lateralization index (LI) of 1.58 ± 0.33 (range, 1.16–2.14) in AVS and were in the medical treatment group. The 11C-MTO-PET outcome changed only in one subject after DXM in whom SUVmax-ratio decreased from 1.18 to a non-lateralizing value of 1.09. The mean SUVmax values on the right (15.1 vs 9.5 g/mL) and left (23.9 vs 19.3 g/mL) sides were statistically lower (P < 0.05) and the SUV-ratio higher (2.2 vs 5.4, P < 0.05) after DXM pretreatment. When we compared the CT findings in these seven subjects, three had discordant CT in both investigations, and two had consistent CT with AVS and two with 11C-MTO-PET.
Discussion
The present prospective clinical trial evaluates the lateralization accuracy of 11C-MTO-PET in patients with PA based on AVS lateralization and outcome after adrenalectomy. Our main finding is that the 11C-MTO-PET is discrepant from AVS in half of the subjects with confirmed PA, and thus 11C-MTO-PET does not provide a non-inferior method to ascertain the subtype diagnosis in PA. Although the definite diagnosis remains uncertain in subjects with medical therapy and on those without an APA histology, we identified a prespecified subgroup of subjects with CYP11B2-positive APA, in whom 11C-MTO-PET identified only 53% of cases correctly. Therefore, the identification of subtypes in PA is not reliably facilitated with 11C-MTO-PET.
Our main result contradicts two previous publications (16, 17). In the study by Burton et al. (16), 35 subjects with PA of whom 22 were operated underwent 11C-MTO-PET. They found the SUVmax ratio cutoff of 1.25:1 to provide 87% specificity and 76% sensitivity in distinguishing unilateral and bilateral PA. O´Shea et al. (17) presented a case series of 15 patients of whom only 4 underwent adrenalectomy. Their study design did not allow comparison of the lateralization methods but concluded that 11C-MTO-PET provided useful information to aid clinical decision-making. In our ROC analysis (Fig. 3), the SUVmax ratio of dominant vs non-dominant adrenal could not significantly predict subject allocation to adrenalectomy vs medical therapy group. In contrast, the observed optimal cut point of 1.16:1 yielded a sensitivity of 55% and a specificity of 44% – test characteristics not suitable to support clinical treatment decision making.
The major differences between the present and the two previous investigations (16, 17) are in the study design. We present here the largest prospective multicenter study with prespecified primary and secondary endpoints, whereas Burton et al. (16) reported a single-center case series consisting of within-patient comparisons of diagnostic techniques, and O´Shea et al. (17) presented a case series based on clinical evaluation or retrospective audit. The Supplementary Table highlights other differences between these thee investigations in study design, patient selection, conduction of AVS and 11C-MTO-PET, and evaluation of outcome. Importantly, patient inclusion in our study was based on current guidelines (18), and spironolactone was discontinued for at least 6 weeks before all examinations. Burton et al. (16) presented the outcome of cases classified according to the prescan diagnosis, but the ten cases classified as bilateral PA did not undergo confirmatory testing, and spironolactone was not systematically discontinued before the examinations. We base the ROC evaluation on allocation to adrenalectomy or medical therapy because all subjects in the adrenalectomy group displayed significant CYP11B2 staining in immunohistochemical examination and at least partial clinical or biochemical cure. Furthermore, adrenalectomy was based on lateralization in AVS when successfully performed. Four patients were operated according to our prespecified study plan despite unsuccessful AVS because they had concordant 11C-MTO-PET and CT findings. Therefore, the risk of bias is probably lower than in many lateralization studies in the past.
Like AVS, 11C-MTO-PET is not without methodological challenges (please see the Supplementary material). DXM pretreatment has been used in previous studies (16, 17) to suppress background activity as 11C-metomidate traces also 11-hydroxylase (CYP11B1) activity in the adrenal cortex (14, 15, 24). We performed the studies without DXM pretreatment in subjects with confirmed PA because supraphysiological glucocorticoids may cause well-known side effects even with short-term use, and also simplify the protocol. After the report by Burton et al. (16), we repeated another 11C-MTO-PET scan during DXM pretreatment in seven subjects who were allocated to the medical therapy group. Although this group without lateralization in AVS presents the hard to classify patient population in all lateralization studies, the results of the PET scans without and with DXM were concordant in six of seven subjects. Although the absolute SUV values decreased with DXM pretreatment, the SUVmax difference or the SUVmax ratio between the adrenal glands or the report by the nuclear medicine specialist did not change the interpretation without or with DXM. Burton et al. (16) did not discontinue MRAs systematically, which may affect metomidate uptake of the adrenal cortex. They tested the impact of DXM pretreatment vs no pretreatment on six subjects and in agreement with our study found DXM to somewhat decrease the background adrenal activity compared with the APA activity (16). We instead reached the opposite conclusion that DXM pretreatment does not improve the diagnostic ability of 11C-MTO-PET in bilateral PA. However, we cannot exclude the possibility that the discrepant lateralization results in six subjects in the adrenalectomy group could have been more consistent with DXM pretreatment.
The poor concordance of AVS and adrenal CT is well characterized (25). The SPARTACUS study and other studies have questioned whether AVS is required to recognize the optimal subgroup of patients with PA who benefit from adrenalectomy (26, 27). Their study is in contrast to our findings and many other studies showing AVS to be superior in predicting lateralization and operation outcome (11, 28, 29, 30, 31). Furthermore, in a long-term follow-up study, AVS-guided adrenalectomy cured biochemically 96% of patients with PA (28). In our analysis, the concordance between 11C-MTO-PET and adrenal CT was surprisingly low both in the lateralizing (55%) and non-lateralizing (44%) groups. When we compared all three localization methods together, the concordance of AVS, 11C-MTO-PET, and adrenal CT was 32%,that is, less than one would expect by chance. This highlights the fact that all these methods detect various aspects of either adrenal anatomy or function. A sufficiently powered trial where all patients would undergo both AVS and 11C-MTO-PET and would be randomly allocated to operation according to AVS or 11C-MTO-PET, could settle the discrepant results found so far.
Discordant lateralization studies present a situation not desired by patients or clinicians. In our study in two subjects, AVS and 11C-MTO-PET suggested lateralization to the opposite sides. After adrenalectomy, both subjects presented with CYP11B2-positive APA. Overall, our results suggest that AVS, despite its obstacles, should remain as the gold standard to guide subtype allocation in PA.
Immunohistochemical analysis of the steroidogenic enzyme aldosterone synthase (CYP11B2) agrees with the view that unilateral non-APA is more common than previously stated. Subjects with hyperplasia may have a somewhat lower cure rate after the operation and a risk of later recurrence of hyperaldosteronism. A large multicenter study investigated adrenals from patients with absent and partial biochemical success and demonstrated a higher prevalence of hyperplasia (49% vs 21%; P = 0.004) compared with those adrenals from matched patients with PA with the complete biochemical success (32). APCCs were a common finding in our study but whether they autonomously secrete aldosterone remains debated (33, 34, 35). Based on the cure rate of subjects with non-APAs, we speculate that hyperplasia and APCCs may represent a significant source of unilateral aldosterone excess. In such patients, AVS provides reliable lateralization, but the use of a radiolabel tracer in PET imaging does not reliably sort outpatients with bilateral asymmetrical PA who benefit from adrenalectomy.
Unilateral adrenalectomy may be more effective in preventing adverse outcomes when compared with lifelong MRA therapy to block the aldosterone excess (4, 5, 36, 37, 38). Whether surgical outcome in lateralizing non-APA is superior to medical therapy, in the long run, remains unclear but in this subtype of PA at least biochemical and clinical improvements are also seen (39, 40, 41, 42, 43, 44). Accordingly, in the present study, we detected a significantly better outcome in both systolic and diastolic blood pressure, DDD, and the quantity of antihypertensive medication used in the adrenalectomy vs medical therapy group despite less severe PA at baseline in the latter, which suggest a more advantageous change in the cardiovascular risk profile after adrenalectomy.
Our study has limitations that deserve discussion. The cure rate was evaluated once, and long-term follow-up data are not available. Some subjects in the adrenalectomy group did not show improvement in both biochemical and clinical outcomes. However, the strict PASO cure criteria (45) have been questioned because up to 70% of the subjects whose treatment was defined as ‘no clear success’ by these criteria demonstrated a blood pressure decrease that was considered significant in terms of reducing vascular risk (46). When evaluating clinically significant benefit, we were able to observe at least a partial benefit of adrenalectomy in all subjects.
In summary, our study presents prospective data that 11C-MTO-PET had lower sensitivity and specificity to detect lateralization when compared with AVS. The subgroups with CYP11B2-positive APA, CYP11B2-positive non-APA or no lateralization in AVS had low clinical benefit from the addition of 11C-MTO-PET. Furthermore, performing three lateralization tests decreases the likelihood of concordance to less than would be predicted by chance. Hopefully, future advances will lead to easier, more accessible, sensitive and specific diagnostic methods to complement or replace AVS in the subtype diagnosis of PA.
Supplementary Material
Acknowledgments
The authors thank Professor W Y Young, Mayo Clinic, Rochester, WY, for his valuable comments to the manuscript.
Funding
This work was supported by a research grant from the Jalmari and Rauha Ahokas Foundation (N M), Helsinki University Hospital research grants (VTR TYH2018111, N M and TYH2013243, M V), Competitive State Research Financing of the Expert Responsibility Area of Tampere University Hospital (VTR 9X046, I P), and Pirkanmaa Regional Fund of the Finnish Cultural Foundation (I P).
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this study.
Supplementary materials
This is linked to the online version of the paper at https://doi.org/10.1530/EJE-20-0532.
References
- 1.Young WFJ. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. Journal of Internal Medicine 2019. 285 126–148. ( 10.1111/joim.12831) [DOI] [PubMed] [Google Scholar]
- 2.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME & Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. Journal of the American College of Cardiology 2005. 45 1243–1248. ( 10.1016/j.jacc.2005.01.015) [DOI] [PubMed] [Google Scholar]
- 3.Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F & Mulatero P. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet: Diabetes and Endocrinology 2018. 6 41–50. ( 10.1016/S2213-8587(17)30319-4) [DOI] [PubMed] [Google Scholar]
- 4.Hundemer GL, Curhan GC, Yozamp N, Wang M & Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet: Diabetes and Endocrinology 2018. 6 51–59. ( 10.1016/S2213-8587(17)30367-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hundemer GL, Curhan GC, Yozamp N, Wang M & Vaidya A. Renal outcomes in medically and surgically treated primary aldosteronism. Hypertension 2018. 72 658–666. ( 10.1161/HYPERTENSIONAHA.118.11568) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hundemer GL. Primary aldosteronism: cardiovascular outcomes pre- and post-treatment. Current Cardiology Reports 2019. 21 93. ( 10.1007/s11886-019-1185-x) [DOI] [PubMed] [Google Scholar]
- 7.Velema MS, de Nooijer AH, Burgers VWG, Hermus ARMM, Timmers HJLM, Lenders JWM, Husson O & Deinum J. Health-related quality of life and mental health in primary aldosteronism: a systematic review. Hormone and Metabolic Research 2017. 49 943–950. ( 10.1055/s-0043-121706) [DOI] [PubMed] [Google Scholar]
- 8.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M & Young WFJ. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 2016. 101 1889–1916. ( 10.1210/jc.2015-4061) [DOI] [PubMed] [Google Scholar]
- 9.Nishimoto K, Tomlins SA, Kuick R, Cani AK, Giordano TJ, Hovelson DH, Liu CJ, Sanjanwala AR, Edwards MA, Gomez-Sanchez CE et al. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. PNAS 2015. 112 E4591–E4599. ( 10.1073/pnas.1505529112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanba K, Vaidya A, Williams GH, Zheng I, Else T & Rainey WE. Age-related autonomous aldosteronism. Circulation 2017. 136 347–355. ( 10.1161/CIRCULATIONAHA.117.028201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young WF, Stanson AW, Thompson GB, Grant CS, Farley DR & van Heerden JA. Role for adrenal venous sampling in primary aldosteronism. Surgery 2004. 136 1227–1235. ( 10.1016/j.surg.2004.06.051) [DOI] [PubMed] [Google Scholar]
- 12.Young WF & Stanson AW. What are the keys to successful adrenal venous sampling (AVS) in patients with primary aldosteronism? Clinical Endocrinology 2009. 70 14–17. ( 10.1111/j.1365-2265.2008.03450.x) [DOI] [PubMed] [Google Scholar]
- 13.Rossi GP. Update in adrenal venous sampling for primary aldosteronism. Current Opinion in Endocrinology, Diabetes, and Obesity 2018. 25 160–171. ( 10.1097/MED.0000000000000407) [DOI] [PubMed] [Google Scholar]
- 14.Bergstrom M, Bonasera TA, Lu L, Bergstrom E, Backlin C, Juhlin C & Langstrom B. In vitro and in vivo primate evaluation of carbon-11-etomidate and carbon-11-metomidate as potential tracers for PET imaging of the adrenal cortex and its tumors. Journal of Nuclear Medicine 1998. 39 982–989. [PubMed] [Google Scholar]
- 15.Minn H, Salonen A, Friberg J, Roivainen A, Viljanen T, Langsjo J, Salmi J, Valimaki M, Nagren K & Nuutila P. Imaging of adrenal incidentalomas with PET using (11)C-metomidate and (18)F-FDG. Journal of Nuclear Medicine 2004. 45 972–979. [PubMed] [Google Scholar]
- 16.Burton TJ, Mackenzie IS, Balan K, Koo B, Bird N, Soloviev DV, Azizan EA, Aigbirhio F, Gurnell M & Brown MJ. Evaluation of the sensitivity and specificity of (11)C-metomidate positron emission tomography (PET)-CT for lateralizing aldosterone secretion by Conn’s adenomas. Journal of Clinical Endocrinology and Metabolism 2012. 97 100–109. ( 10.1210/jc.2011-1537) [DOI] [PubMed] [Google Scholar]
- 17.O’Shea PM, O’Donoghue D, Bashari W, Senanayake R, Joyce MB, Powlson AS, Browne D, O’Sullivan GJ, Cheow H, Mendichovszky I et al. 11C-Metomidate PET/CT is a useful adjunct for lateralization of primary aldosteronism in routine clinical practice. Clinical Endocrinology 2019. 90 670–679. ( 10.1111/cen.13942) [DOI] [PubMed] [Google Scholar]
- 18.Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, Young WF Jr, Montori VM & Endocrine Society. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 2008. 93 3266–3281. ( 10.1210/jc.2008-0104) [DOI] [PubMed] [Google Scholar]
- 19.Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, Satoh F, Amar L, Quinkler M, Deinum J et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet: Diabetes and Endocrinology 2017. 5 689–699. ( 10.1016/S2213-8587(17)30135-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez-Sanchez CE, Qi X, Velarde-Miranda C, Plonczynski MW, Parker CR, Rainey W, Satoh F, Maekawa T, Nakamura Y, Sasano H et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Molecular and Cellular Endocrinology 2014. 383 111–117. ( 10.1016/j.mce.2013.11.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura Y, Maekawa T, Felizola SJA, Satoh F, Qi X, Velarde-Miranda C, Plonczynski MW, Ise K, Kikuchi K, Rainey WE et al. Adrenal CYP11B1/2 expression in primary aldosteronism: immunohistochemical analysis using novel monoclonal antibodies. Molecular and Cellular Endocrinology 2014. 392 73–79. ( 10.1016/j.mce.2014.05.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nanba K, Tsuiki M, Sawai K, Mukai K, Nishimoto K, Usui T, Tagami T, Okuno H, Yamamoto T, Shimatsu A et al. Histopathological diagnosis of primary aldosteronism using CYP11B2 immunohistochemistry. Journal of Clinical Endocrinology and Metabolism 2013. 98 1567–1574. ( 10.1210/jc.2012-3726) [DOI] [PubMed] [Google Scholar]
- 23.Yamazaki Y, Nakamura Y, Omata K, Ise K, Tezuka Y, Ono Y, Morimoto R, Nozawa Y, Gomez-Sanchez CE, Tomlins SA et al. Histopathological classification of cross-sectional image-negative hyperaldosteronism. Journal of Clinical Endocrinology and Metabolism 2017. 102 1182–1192. ( 10.1210/jc.2016-2986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hennings J, Lindhe O, Bergstrom M, Langstrom B, Sundin A & Hellman P. [11C]metomidate positron emission tomography of adrenocortical tumors in correlation with histopathological findings. Journal of Clinical Endocrinology and Metabolism 2006. 91 1410–1414. ( 10.1210/jc.2005-2273) [DOI] [PubMed] [Google Scholar]
- 25.Kempers MJ, Lenders JW, van Outheusden L, van der Wilt GJ, Schultze Kool LJ, Hermus AR & Deinum J. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Annals of Internal Medicine 2009. 151 329–337. ( 10.7326/0003-4819-151-5-200909010-00007) [DOI] [PubMed] [Google Scholar]
- 26.Dekkers T, Prejbisz A, Kool LJS, Groenewoud HJMM, Velema M, Spiering W, Kolodziejczyk-Kruk S, Arntz M, Kadziela J, Langenhuijsen JF et al. Adrenal vein sampling versus CT scan to determine treatment in primary aldosteronism: an outcome-based randomised diagnostic trial. Lancet: Diabetes and Endocrinology 2016. 4 739–746. ( 10.1016/S2213-8587(16)30100-0) [DOI] [PubMed] [Google Scholar]
- 27.Umakoshi H, Tsuiki M, Takeda Y, Kurihara I, Itoh H, Katabami T, Ichijo T, Wada N, Yoshimoto T, Ogawa Y et al. Significance of computed tomography and serum potassium in predicting subtype diagnosis of primary aldosteronism. Journal of Clinical Endocrinology and Metabolism 2018. 103 900–908. ( 10.1210/jc.2017-01774) [DOI] [PubMed] [Google Scholar]
- 28.Lim V, Guo Q, Grant CS, Thompson GB, Richards ML, Farley DR & Young WFJ. Accuracy of adrenal imaging and adrenal venous sampling in predicting surgical cure of primary aldosteronism. Journal of Clinical Endocrinology and Metabolism 2014. 99 2712–2719. ( 10.1210/jc.2013-4146) [DOI] [PubMed] [Google Scholar]
- 29.Nanba AT, Nanba K, Byrd JB, Shields JJ, Giordano TJ, Miller BS, Rainey WE, Auchus RJ & Turcu AF. Discordance between imaging and immunohistochemistry in unilateral primary aldosteronism. Clinical Endocrinology 2017. 87 665–672. ( 10.1111/cen.13442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ladurner R, Sommerey S, Buechner S, Dietz A, Degenhart C, Hallfeldt K & Gallwas J. Accuracy of adrenal imaging and adrenal venous sampling in diagnosing unilateral primary aldosteronism. European Journal of Clinical Investigation 2017. 47 372–377. ( 10.1111/eci.12746) [DOI] [PubMed] [Google Scholar]
- 31.Rossi GP, Mulatero P & Satoh F. 10 good reasons why adrenal vein sampling is the preferred method for referring primary aldosteronism patients for adrenalectomy. Journal of Hypertension 2019. 37 603–611. ( 10.1097/HJH.0000000000001939) [DOI] [PubMed] [Google Scholar]
- 32.Meyer LS, Wang X, Susnik E, Burrello J, Burrello A, Castellano I, Eisenhofer G, Fallo F, Kline GA, Knosel T et al. Immunohistopathology and steroid profiles associated with biochemical outcomes after adrenalectomy for unilateral primary aldosteronism. Hypertension 2018. 72 650–657. ( 10.1161/HYPERTENSIONAHA.118.11465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, Shibata H, Itoh H, Mitani F, Yamazaki T et al. Adrenocortical zonation in humans under normal and pathological conditions. Journal of Clinical Endocrinology and Metabolism 2010. 95 2296–2305. ( 10.1210/jc.2009-2010) [DOI] [PubMed] [Google Scholar]
- 34.Dekkers T, ter Meer M, Lenders JWM, Hermus ARM, Schultze Kool L, Langenhuijsen JF, Nishimoto K, Ogishima T, Mukai K, Azizan EAB et al. Adrenal nodularity and somatic mutations in primary aldosteronism: one node is the culprit? Journal of Clinical Endocrinology and Metabolism 2014. 99 E1341–E1351. ( 10.1210/jc.2013-4255) [DOI] [PubMed] [Google Scholar]
- 35.Boulkroun S, Samson-Couterie B, Dzib JF, Lefebvre H, Louiset E, Amar L, Plouin PF, Lalli E, Jeunemaitre X, Benecke A et al. Adrenal cortex remodeling and functional zona glomerulosa hyperplasia in primary aldosteronism. Hypertension 2010. 56 885–892. ( 10.1161/HYPERTENSIONAHA.110.158543) [DOI] [PubMed] [Google Scholar]
- 36.Hundemer GL, Curhan GC, Yozamp N, Wang M & Vaidya A. Incidence of atrial fibrillation and mineralocorticoid receptor activity in patients with medically and surgically treated primary aldosteronism. JAMA Cardiology 2018. 3 768–774. ( 10.1001/jamacardio.2018.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossi GP, Rossitto G, Amar L, Azizi M, Riester A, Reincke M, Degenhart C, Widimsky JJ, Naruse M, Deinum J et al. Clinical outcomes of 1625 patients with primary aldosteronism subtyped with adrenal vein sampling. Hypertension 2019. 74 800–808. ( 10.1161/HYPERTENSIONAHA.119.13463) [DOI] [PubMed] [Google Scholar]
- 38.Lechner B, Lechner K, Heinrich D, Adolf C, Holler F, Schneider H, Beuschlein F & Reincke M. Therapy of endocrine disease: medical treatment of primary aldosteronism. European Journal of Endocrinology 2019. 181 R147–R153. ( 10.1530/EJE-19-0215) [DOI] [PubMed] [Google Scholar]
- 39.Novitsky YW, Kercher KW, Rosen MJ, Cobb WS, Jyothinagaram S & Heniford BT. Clinical outcomes of laparoscopic adrenalectomy for lateralizing nodular hyperplasia. Surgery 2005. 138 1009–1016; discussion 1016. ( 10.1016/j.surg.2005.09.027) [DOI] [PubMed] [Google Scholar]
- 40.Hennings J, Andreasson S, Botling J, Hagg A, Sundin A & Hellman P. Long-term effects of surgical correction of adrenal hyperplasia and adenoma causing primary aldosteronism. Langenbeck’s Archives of Surgery 2010. 395 133–137. ( 10.1007/s00423-009-0498-4) [DOI] [PubMed] [Google Scholar]
- 41.Quillo AR, Grant CS, Thompson GB, Farley DR, Richards ML & Young WF. Primary aldosteronism: results of adrenalectomy for nonsingle adenoma. Journal of the American College of Surgeons 2011. 213 106–112; discussion 112. ( 10.1016/j.jamcollsurg.2011.03.007) [DOI] [PubMed] [Google Scholar]
- 42.Iacobone M, Citton M, Viel G, Boetto R, Bonadio I, Tropea S, Mantero F, Rossi GP, Fassina A, Nitti D et al. Unilateral adrenal hyperplasia: a novel cause of surgically correctable primary hyperaldosteronism. Surgery 2012. 152 1248–1255. ( 10.1016/j.surg.2012.08.042) [DOI] [PubMed] [Google Scholar]
- 43.Volpe C, Hamberger B, Höög A, Mukai K, Calissendorff J, Wahrenberg H, Zedenius J & Thorén M. Primary aldosteronism: functional histopathology and long-term follow-up after unilateral adrenalectomy. Clinical Endocrinology 2015. 82 639–647. ( 10.1111/cen.12645) [DOI] [PubMed] [Google Scholar]
- 44.Shariq OA, Mehta K, Thompson GB, Lyden ML, Farley DR, Bancos I, Dy BM, Young WF & McKenzie TJ. Primary aldosteronism: does underlying pathology impact clinical presentation and outcomes following unilateral adrenalectomy? World Journal of Surgery 2019. 43 2469–2476. ( 10.1007/s00268-019-05059-y) [DOI] [PubMed] [Google Scholar]
- 45.Williams TA, Burrello J, Sechi LA, Fardella CE, Matrozova J, Adolf C, Baudrand R, Bernardi S, Beuschlein F, Catena C et al. Computed tomography and adrenal venous sampling in the diagnosis of unilateral primary aldosteronism. Hypertension 2018. 72 641–649. ( 10.1161/HYPERTENSIONAHA.118.11382) [DOI] [PubMed] [Google Scholar]
- 46.Vorselaars WMCM, Nell S, Postma EL, Zarnegar R, Drake FT, Duh QY, Talutis SD, McAneny DB, McManus C, Lee JA et al. Clinical outcomes after unilateral adrenalectomy for primary aldosteronism. JAMA Surgery 2019. 154 e185842. ( 10.1001/jamasurg.2018.5842) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


