Abstract
Background and Aims
Patients with chronic hepatitis B (CHB) infection routinely undergo screening for hepatocellular carcinoma (HCC), but the efficacy of screening remains unclear. We aimed to evaluate the impact of screening with ultrasound (USS) and/or serum alpha-fetoprotein (AFP) on HCC-related mortality in patients with CHB.
Methods
We performed a matched case-control study of patients with CHB receiving care through the Veterans Affairs (VA) health administration. Cases were patients who died of HCC between 01/01/2004 and 12/31/2017, while controls were patients with CHB who did not die of HCC. Cases were matched to controls by CHB diagnosis date, age, sex, race/ethnicity, cirrhosis, antiviral therapy exposure, hepatitis B e antigen status, and viral load. We identified screening USS and AFPs obtained in the 4 years preceding HCC diagnosis in cases and the equivalent index date in controls. Using conditional logistic regression, we compared cases and controls with respect to receipt of screening. A lower likelihood of screening in cases corresponds to an association between screening and reduced risk of HCC-related mortality.
Results
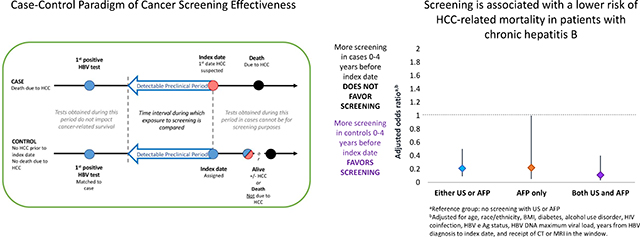
We identified 169 cases, matched to 169 controls. Fewer cases than controls underwent screening with either screening modality (33.7% versus 58.6%) or both modalities (19.5% versus 34.4%). In multivariable conditional logistic regression, screening with either modality was associated with a lower risk of HCC-related mortality (adjusted odds ratio [aOR] 0.21, 95% confidence interval [CI] 0.09–0.50), as was screening with both modalities (aOR of 0.13, 95% CI 0.04–0.43).
Conclusions
HCC screening was associated with a substantial reduction in HCC-related mortality in VA patients with CHB.
Keywords: Liver cancer, surveillance, ultrasound, alpha-fetoprotein, hepatitis B
LAY SUMMARY
Patients with hepatitis B infection have a high risk of developing liver cancer. It is therefore recommended that they undergo frequent screening for liver cancer, but whether this leads to a lower risk of dying from liver cancer is not clear. In this study, we show that liver cancer screening with is associated with a reduction in the mortality from liver cancer in patients with hepatitis B infection.
Graphical Abstract
INTRODUCTION
Patients with chronic hepatitis B virus infection (CHB) are at high risk of developing hepatocellular carcinoma (HCC). The American Association for the Study of Liver Disease (AASLD) recommends that patients with CHB-related cirrhosis and high-risk patients without cirrhosis undergo HCC screening every 6 months using ultrasound scanning (USS) with or without serum alpha-fetoprotein (AFP)[1–3]. The goal of screening is to improve survival by detecting tumors at an early stage when curative treatments are possible, such as ablation, surgical resection, and liver transplantation.
Although HCC screening is accepted as standard-of-care for many patients with CHB, the quality of evidence in support of screening is low[4–6]. Two randomized trials examined the impact of screening on HCC-related mortality among patients with CHB[7, 8]. One trial observed that screening was associated with lower mortality compared to no screening, but the validity of these findings has been questioned due to flaws in study design and analysis[5–7]. The other utilized AFP only and found no evidence of mortality benefit[8]. Observational studies are more abundant but are hampered by several limitations[9–12]. Most such studies compare survival between screen-detected and symptomatic cases, and are subject to lead-time, length-time, and selection bias, all of which exaggerate the potential benefit of screening. It is widely believed that conducting a randomized trial of screening in Western countries is not feasible due to ethical concerns and because potential participants are unlikely to consent to no screening[13].
Case-control studies are an alternative method for evaluating the impact of screening on cancer-related mortality[14]. In case-control studies of cancer screening, cases and controls are sampled from a population of patients at risk of developing the cancer of interest. Patients with fatal cancer (cases) are compared to persons sampled from the population from which the cases arose (controls) with respect to receipt of screening during the period of time preceding cancer diagnosis when a tumor is presumed to be detectable by the screening modality. If a screening test reduces cancer-related mortality, cases will have a lower likelihood of receipt of screening than controls. Case-control studies have been used to evaluate the efficacy of screening programs for colorectal[15], esophageal[16], and cervical cancers[17].
A recent case-control study observed no reduction in HCC-related mortality in patients with cirrhosis who underwent USS or AFP-based screening[18]. However, these results may not apply to patients with CHB, who were excluded from that study. The performance characteristics of both USS and serum AFP may be superior in patients with CHB because the nodular liver parenchyma in cirrhosis can impede detection of small tumors by USS[19, 20], and because serum AFP may be elevated in patients with cirrhosis in the absence of HCC[21, 22]. Furthermore, non-cirrhotic patients with CHB may have more curative treatment options for HCC than patients with cirrhosis, in whom portal hypertension and impaired liver function often preclude therapy.
Using a case-control design, our objective was to determine whether HCC screening with USS and AFP is associated with a reduction in HCC-related mortality among patients with CHB.
METHODS
Overall study design
We conducted a matched case-control study of patients with CHB receiving care through the United States Veterans Affairs (VA) healthcare system. We included patients with cirrhosis and male patients without cirrhosis who were ≥ age 40 during the screening window based on VA screening recommendations[23]. Current AASLD guidance statements provide no specific guidance for HCC screening in non-cirrhotic Caucasian patients [2]. Guidelines by the European Association for the Study of the Liver (EASL) recommend HCC screening in Caucasian patients with CHB and a PAGE-B score predicting intermediate-to-high risk of HCC (≥ 10)[24, 25]. Male patients ≥ age 40 with CHB have PAGE-B scores of at least 10, thus forming the basis of VA screening recommendations.
Cases were patients with fatal HCC, while controls were patients who were alive or died from non-HCC causes after the index date by the end of the study period. Additionally, controls could not have an HCC diagnosis at the time HCC was first suspected in their matched case (the index date) (Figure 1). Cases and controls were compared with respect to receipt of screening USS or AFP during the 4 years before the index date. Four years was chosen to approximate the detectable preclinical period (DPP), the period of time between when HCC is first detectable by screening and when it presents clinically in the absence of screening. The DPP was estimated to be ~ 3.2 years in prior studies that followed untreated HCC patients with serial ultrasounds and determined the time it took for a tumor to grow from 1 cm (minimum size potentially detectable by USS) to 10 cm (the size generally expected to cause symptoms) based on a median tumor doubling time of 117 days[26]. We analyzed screening tests performed up to 4 years before the index date because the maximal DPP provides the least biased estimate of the true association between screening and cancer mortality[27]. We excluded all patients with hepatitis C virus (HCV) co-infection, defined by a positive HCV viral load. This study was approved by the Institutional Review Board of the VA Puget Sound Healthcare System.
Figure 1.
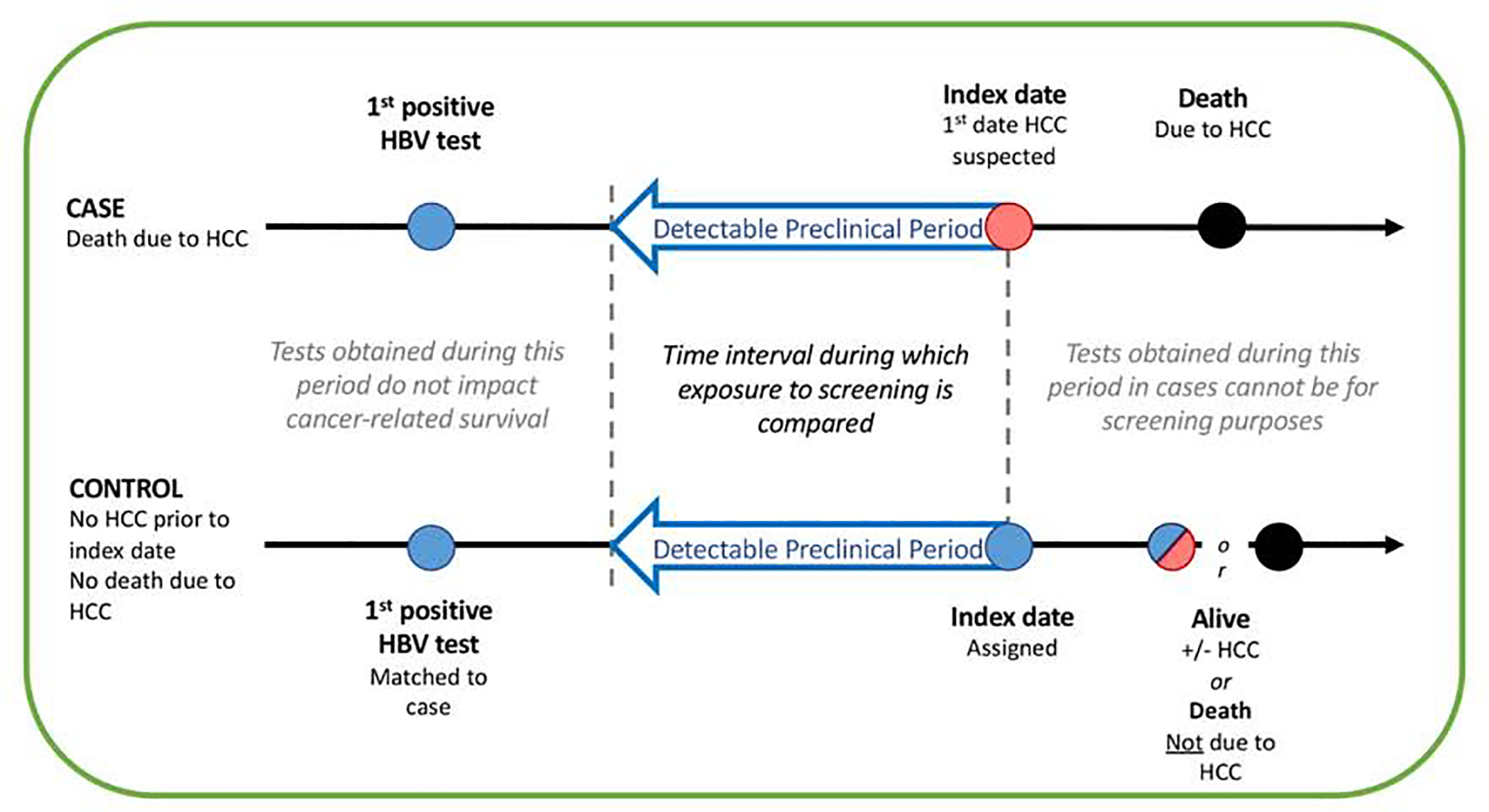
Schematic representation of the method used to match controls to cases, illustrating the Index Date and Detectable Preclinical Phase (DPP). The DPP comprised an identical period of calendar years for the case and control within each matched pair (e.g. 2010–2014), during which both case and control were in VA care.
Data source
The VA is the largest provider of healthcare in the United States and utilizes a nationally-integrated electronic medical record system. The VA Corporate Data Warehouse (CDW) is a repository of health information on all VA patients from October 1999 forward. Available data include demographic information, inpatient and outpatient encounters, International Classification of Diseases (ICD)-9 and −10 codes, diagnostic tests, procedures, and pharmacy records. The VA CDW was used to screen for potential cases and controls, whose medical records were then reviewed through the Compensation and Pension Record Interchange, an electronic interface providing online access to medical records from all VA facilities.
Cases
Using the VA CDW (Figure 2), we identified all patients with a positive hepatitis B surface antigen (HBsAg) or detectable hepatitis B viral load (HBV DNA), an ICD-9/10 code corresponding to a diagnosis of HCC (155.0, C22.0) recorded at least twice, and who died between 01/01/2004 and 12/31/2017. The first ICD-9/10 code for HCC must have been recorded at least 4 years after the first positive HBsAg or viral load in the VA system to ensure that cases had sufficient follow-up before their HCC diagnosis for screening to plausibly impact HCC-related mortality.
Figure 2.
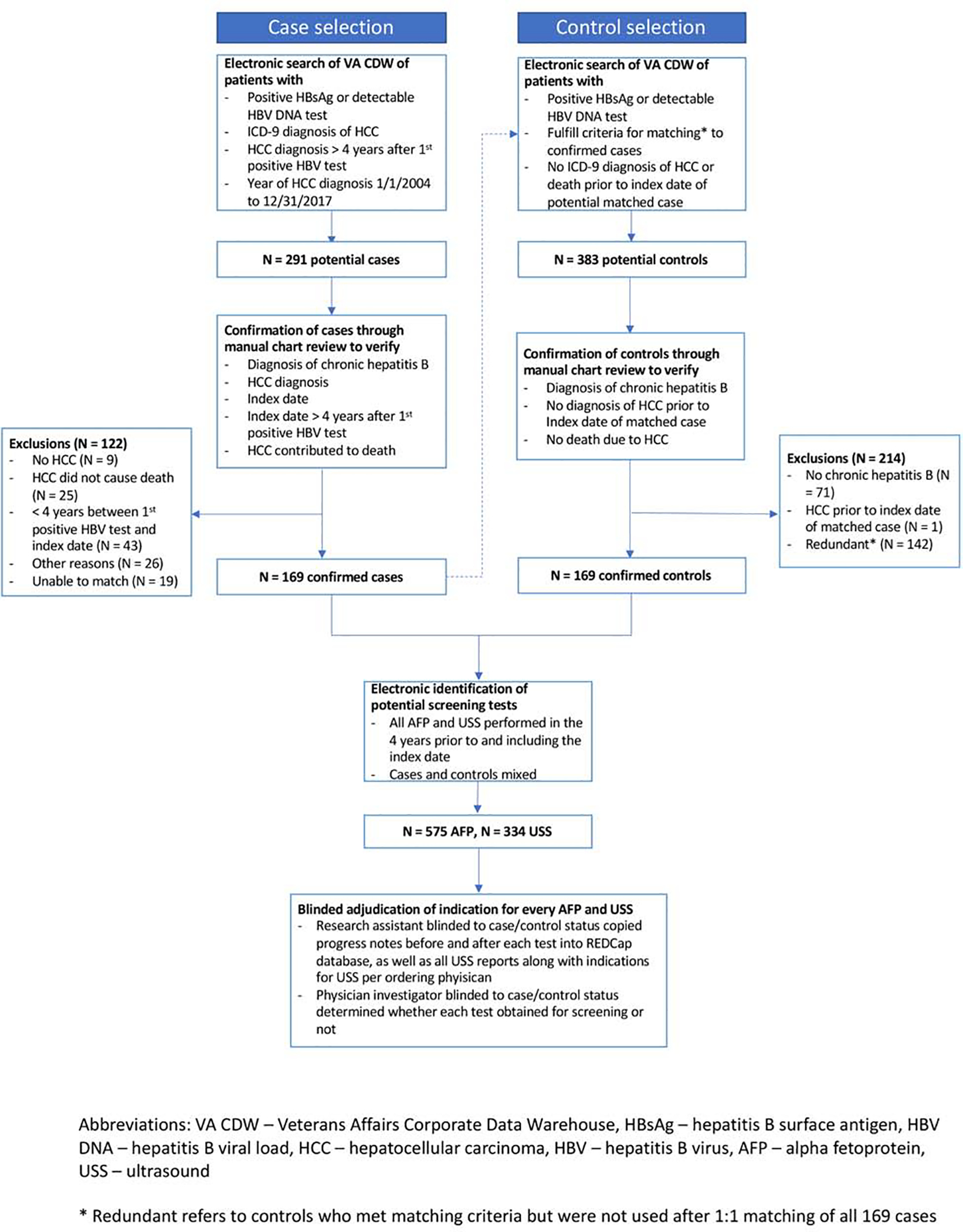
Flowchart illustrating the steps for identification and confirmation of cases and matched controls.
A physician investigator blinded to screening status reviewed the electronic medical records of all potential cases. Data was abstracted onto REDCap. Cases were included only if they fulfilled all of the following criteria: confirmed CHB, confirmed HCC by radiographic criteria or histology, fatal HCC defined as cancers that definitely or probably contributed to death, and at least 4 years of follow-up between their first positive HBsAg or HBV DNA and the index date. Details of these criteria are provided in supplemental material (Supplemental Tables 1–3). Criteria used to determine whether HCCs were fatal were validated by a pilot study of 50 cases reviewed independently by two authors that showed excellent inter-observer agreement (97.5% agreement, kappa = 0.94, p < 0.001).
Assignment of index date
For patients with confirmed HCC, we assigned an index date, which was the date of the earliest of the following: 1) first multiphasic imaging or histology diagnostic of HCC, 2) first symptoms of HCC, 3) first elevated AFP > 20 ng/mL, or 4) first suspicious imaging of any kind. Any USS or AFP obtained after the index date was not considered a screening test.
Controls
Using the VA CDW (Figure 2), we identified potential controls who were restricted to patients with a positive HBsAg or HBV DNA who fulfilled the matching criteria for their specified case, were in VA care, were not diagnosed with HCC as of the index date of their matched case, and did not later die from HCC. Controls were assigned an index date identical to that of their matched case. The medical records of controls were reviewed to confirm a diagnosis of CHB (Supplemental Table 1), the absence of HCC as of the index date, and – for controls who died – that HCC was not the cause of death.
Matching criteria
Controls were matched to cases in a 1:1 ratio by factors associated with both fatal HCC and the likelihood of screening: 1) Date of first positive HBsAg or HBV DNA in the VA CDW, 2) Race/Ethnicity (categorized as non-Hispanic White, non-Hispanic Black, Hispanic, Asian/Pacific Islander/Alaska Native/American Indian), 3) Age (within 2 years), 4) Gender, 5) Presence of cirrhosis (determined by ICD-9/10 codes) before the index date, 6) Receipt of hepatitis B antiviral medications before the index date, 7) hepatitis B e antigen (HBeAg) status, and 8) maximum viral load before the index date (<2000 or ≥2000 for HBeAg negative patients and <20,000 or ≥20,000 for HBeAg positive patients). Because cases and controls were matched for the date of the first positive HBsAg or HBV DNA, each case and control were compared with respect to receipt of screening during identical calendar years when they were both in VA care.
Determination of screening history in cases and controls
We abstracted the dates of all abdominal USS and serum AFPs performed during the 4 years preceding the index date for cases and controls. A research assistant abstracted USS reports onto a REDCap database along with progress notes before and after the date of each USS and AFP. A physician-investigator blinded to case-control status assigned the indication for each test after review of abstracted reports and progress notes. The indication was categorized as “definitely”, “probably”, “probably not”, or “definitely not” for screening, or “unable to determine” based on criteria in Supplemental Table 4. Patients who received tests “definitely” or “probably” for screening were categorized as having a history of screening.
Statistical Analysis
Cases and controls were compared with respect to receipt of screening during the DPP using conditional logistic regression. Models were adjusted for factors that are known or suspected to be associated with both the exposure (receipt of screening) and the outcome (HCC-related death): age, race/ethnicity, BMI, diabetes, alcohol use disorder, HIV coinfection, HBeAg status, maximum HBV DNA, years from hepatitis B diagnosis to index date, and receipt of CT or MRI in the screening window. The distribution of these characteristics in cases and controls is presented in Table 1, and in screened versus unscreened patients in Supplemental Table 5. Models were not adjusted for receipt of the other screening modality during the period of interest due to collinearity (almost all – 90.1% – patients who received screening USS also received screening AFP). We also did not adjust for frequency of medical care, because analytically forcing cases and controls to be similar with respect to this characteristic would force screening histories to be artificially similar, reducing our ability to identify a true association between screening and mortality from liver cancer.
Table 1.
Characteristics of cases and their matched controls
| Controls N = 169 | Cases N = 169 | |
|---|---|---|
| Male, % | 100 | 100 |
| Age at first positive HBV test, mean (yrs) | 52.0 | 52.2 |
| Age at index date, mean (yrs) | 59.9 | 60.3 |
| Year of first positive HBV test, % | ||
| 1999–2001 | 29.6 | 29.6 |
| 2001–2003 | 45.6 | 45.6 |
| 2004–2006 | 17.2 | 17.2 |
| 2007–2011 | 7.7 | 7.7 |
| Time interval between first positive HBV test and index date, yrs | 8.0 | 8.1 |
| Index Date Year, % | ||
| 2004–2007 | 18.3 | 18.3 |
| 2008–2010 | 33.1 | 33.1 |
| 2011–2013 | 34.9 | 34.9 |
| 2014–2017 | 13.6 | 13.6 |
| Race/Ethnicity, % | ||
| White, non-Hispanic | 46.2 | 44.4 |
| Black, non-Hispanic | 39.1 | 34.9 |
| Other | 14.8 | 20.7 |
| Cirrhosis, % | 36.7 | 36.7 |
| HBeAg positive, % | 36.7% | 40.1% |
| Maximum HBV DNA viral load 5 years prior to index date ≥ 2000 (e Ag negative) or ≥ 20,000 (e Ag positive) | 46.2 | 53.8 |
| HBV antiviral treatment, % | 46.2 | 46.2 |
| Facility complexity,% | ||
| Ambulatory (Basic or Advanced) | 3.7 | 5.1 |
| Inpatient Standard | 3.7 | 2.6 |
| Inpatient Intermediate | 13.5 | 14.1 |
| Inpatient Complex | 79.1 | 78.2 |
| BMI, mean (Kg/m2) | 27.9 | 27.1 |
| Diabetes, % | 23.7 | 28.4 |
| Alcohol Use Disorders, % | 36.7 | 42 |
| HIV Coinfection, % | 13.0 | 14.2 |
| CT or MRI before index date, % | ||
| 0–2 years | 29.6 | 21.3 |
| 0–3 years | 37.9 | 25.4 |
| 0–4 years | 44.4 | 27.2 |
Abbreviations: HBV – hepatitis B virus, HBeAg – hepatitis B e antigen, HBV DNA – hepatitis B viral load, BMI – body mass index, HIV – human immunodeficiency virus, CT – computed tomography, MRI – magnetic resonance imaging
We evaluated the following binary screening variables in different conditional logistic regression models to obtain odds ratios summarizing the association between screening and HCC-related mortality:
Screening with either USS or serum AFP versus no screening with either USS or serum AFP
Screening with both USS and serum AFP versus no screening with either USS or serum AFP
Screening with serum AFP only versus no screening with either USS or serum AFP
We were unable to evaluate the effect of screening with USS only because too few patients received USS screening alone without serum AFP.
In case-control studies of cancer screening effectiveness, receipt of screening during the DPP is modeled as a binary variable, i.e. whether a person had any screening. Cases and controls cannot be compared with respect to the number of screening tests (i.e. screening intensity) because this leads to a spurious association between low screening intensity and higher cancer mortality[28]. Cases are expected to have a lower intensity of screening because a case diagnosed with HCC at time t (the matched index date) is unlikely to have undergone multiple screening tests during the DPP before t because the first of these tests would likely have been positive (assuming the test is sensitive) and subsequent screening tests would not have been performed. In contrast, a control who does not have HCC would have been eligible to undergo repeated screening tests during the same period before t. Therefore, cases are likely to have fewer screening tests than controls even if HCC treatment is unavailable or ineffective.
We planned 2 sensitivity analyses. First, we compared cases and controls during the 1, 2, and 3 years before the index date to determine whether our results were robust to different estimates of the DPP. Second, we excluded patients who received abdominal CT or MRI during the DPP because some patients may have undergone CT/MRI screening in lieu of USS or AFP and so would appear to be “unscreened”. Lastly, we performed a subgroup analysis to explore whether the effectiveness of screening depends on the presence of underlying cirrhosis.
RESULTS
Identification of cases and controls
We identified 291 potential cases through the VA CDW. After medical record review, we excluded 9 patients who did not have HCC, 25 who did not have fatal HCC, 19 who did not have CHB, 43 who did not have at least four years of follow-up between a positive hepatitis B test and the index date, 7 for missing diagnosis information, and 19 who could not be matched to a control, leaving 169 cases in our analysis (Figure 2).
Baseline characteristics of cases and controls
Cases and controls were well matched with respect to age at first positive hepatitis B test (52 years), age at index date (60 years), sex, race/ethnicity, year of first positive hepatitis B test, cirrhosis, and receipt of antiviral therapy before the index date(Table 1). All patients were male. Approximately one third had cirrhosis. HBeAg was positive in 40.1% of cases and 36.7% of controls. Cases were slightly more likely than controls to have diabetes (28.4% versus 23.7%) or alcohol use disorder (42% versus 36.7%). Slightly more controls than cases received abdominal CT or MRI scans before the index date (44.4% versus 27.2%).
Outcomes in control patients
We reviewed our control patients for development of HCC and non-HCC deaths after the index date. Before the end of the study period, 1.8% of control patients were diagnosed with HCC and 22.5% died from non-HCC causes.
Characteristics of HCC in cases
The majority of fatal cases were diagnosed by imaging (94.7%) and nearly half had histologic confirmation of HCC (46.7%) (Table 2). At the time of diagnosis, 65.6% had HCC beyond Milan criteria, 27.8% had vascular invasion, and 14.2% had extrahepatic metastases. While the majority of the fatal cases received some type of HCC directed treatment (69.8%), only 1.2% underwent liver transplantation, 5.3% surgical resection, while 34.3% underwent transarterial chemoembolization, 11.2% radiofrequency ablation, and 4.1% Y-90 radioembolization. Since by definition this was the subset of HCC cases in VA care that were fatal, it is not surprising that they presented in advanced stages and generally did not receive curative treatments.
Table 2.
Characteristics of HCC among cases
| Cases N (%) | |
|---|---|
| Method of HCC diagnosis* | |
| Imaging (CT/MRI) | 160(94.7) |
| Histology | 79(46.7) |
| Treatment of HCC* | |
| Liver transplantation | 2(1.2) |
| Surgery (partial hepatectomy) | 9(5.3) |
| Systemic chemotherapy (sorafenib) | 72(42.6) |
| Trans-arterial chemoembolization | 58(34.3) |
| Radiofrequency ablation | 19(11.2) |
| Y-90 radioembolization | 7(4.1) |
| Percutaneous ethanol injection | 1(0.6) |
| Cryoablation | 0(0.0) |
| Other Treatment | 22(13.0) |
| Any one of the above treatments | 118(69.8) |
| Stage of HCC at Diagnosis | |
| Maximum dimension of largest tumor (cm), mean (SD) | 6.4(4.4) |
| Number of tumors, mean (SD) | 2.2(1.7) |
| Number of tumors (%) | |
| 1 | 86(57.0) |
| 2–3 | 28(18.5) |
| ≥4 | 37(24.5) |
| Size of largest tumor (%) | |
| 0–3 cm | 32(18.9) |
| 3 to <5 cm | 41(24.3) |
| 5 to <6 cm | 8(4.7) |
| 6 to <7 cm | 10(5.9) |
| ≥7 cm | 78(46.2) |
| Within Milan Criteria (%)† | 70(34.4) |
| Beyond Milan Criteria (%) | 117(65.6) |
| Vascular Invasion, % | 47(27.8) |
| Metastasis, % | 24(14.2) |
| HCC Contributed to patient’s death* | |
| Metastatic HCC | 58(34.3) |
| Multifocal HCC (>3 lesions) | 70(41.4) |
| Local or vascular invasion by HCC | 70(41.4) |
| Large Volume HCC (>6cm or AFP>1000) | 124(73.4) |
| Death due to complications of HCC treatment | 5(3.0) |
The categories for “method of HCC diagnosis”, “treatment of HCC” and “HCC contributed to patienťs death” are NOT mutually exclusive.
Milan Criteria: One tumor <5 cm or 2–3 tumors each of which is < 3cm
Abbreviations: HCC – hepatocellular carcinoma, CT – computed tomography, MRI – magnetic resonance imaging, SD – standard deviation, AFP – alpha fetoprotein
Association between screening and HCC-related mortality
In the 4 years before the index date, cases received 133 USS exams (including 81 “definitely” and 2 “probably” obtained for screening) and 193 serum AFP tests (143 “definitely” and 6 “probably” for screening). Over the same interval, controls received 201 USS exams (148 “definitely” and 1 “probably” for screening) and 382 serum AFP tests (314 “definitely” and 2 “probably” for screening) (Table 3).
Table 3.
Distribution of categorization of USS scans and serum AFP tests during the 0–4 years prior to index date.
| Controls | Cases | |
|---|---|---|
| USS | ||
| All USS | 201 | 133 |
| Definitely screening | 148 (73.6%) | 81 (60.9%) |
| Probably screening | 1 (0.5%) | 2 (1.5%) |
| Probably not screening | 8 (4.0%) | 8 (6.02%) |
| Definitely not screening | 40 (19.9%) | 38 (28.6%) |
| Unable to determine | 4 (2.0%) | 4 (3.0%) |
| AFP | ||
| All AFP | 382 | 193 |
| Definitely screening | 314 (82.2%) | 143 (74.1) |
| Probably screening | 2 (0.5%) | 6 (3.1) |
| Probably not screening | 1 (0.3%) | 7 (3.6%) |
| Definitely not screening | 57 (15%) | 31 (16.1%) |
| Unable to determine | 8 (2.1%) | 6 (3.1%) |
Abbreviations: USS – ultrasound, AFP – alpha fetoprotein
Cases were less likely to have received screening with either USS or AFP (33.7%) than controls (58.6%). In multivariable analysis, screening with either USS or AFP was strongly associated with reduced HCC-related mortality (aOR 0.21, 95% CI 0.09–0.50) (Table 4).
Table 4.
Comparison of cases and controls with respect to occurrence of screening (defined as definitely or probably screening) prior to the index date.
| Controls N=169 | Cases N=169 | Unadjusted OR (95% CI) | Adjusteda OR (95% CI) | |
|---|---|---|---|---|
| 0–4 years prior to the index date | ||||
| No screening | 70 (41.4%) | 112 (66.3%) | 1 | 1 |
| Either USS or AFP | 99 (58.6%) | 57 (33.7%) | 0.19 (0.10–0.38) | 0.21 (0.09–0.50) |
| AFP only | 34 (20.1%) | 21 (12.4%) | 0.33 (0.12–0.92) | 0.22 (0.05–1.00) |
| USS onlyb | 7 (4.1%) | 3 (1.8%) | -- | -- |
| Both USS and AFP | 58 (34.3%) | 33 (19.5%) | 0.12 (0.04–0.34) | 0.11 (0.03–0.40) |
| 0–3 years prior to index date | ||||
| No screening | 76 (45.0%) | 117 (69.2%) | 1 | 1 |
| Either USS or AFP | 93 (55.0%) | 52 (30.8%) | 0.24 (0.13–0.44) | 0.24 (0.11–0.52) |
| AFP only | 32 (18.9%) | 21 (12.4%) | 0.40 (0.16–1.03) | 0.37 (0.10–1.38) |
| USS onlyb | 6 (3.6%) | 4 (2.4%) | -- | -- |
| Both USS and AFP | 55 (32.5%) | 27 (16.0%) | 0.17 (0.07–0.41) | 0.12 (0.04–0.38) |
| 0–2 years prior to index date | ||||
| No screening | 86 (50.9%) | 123 (72.8%) | 1 | 1 |
| Either USS or AFP | 83 (49.1%) | 46 (27.2%) | 0.26 (0.14–0.48) | 0.26 (0.13–0.55) |
| AFP only | 32 (18.9%) | 19 (11.2%) | 0.29 (0.11–0.80) | 0.31 (0.09–1.08) |
| USS onlyb | 8 (4.7%) | 4 (2.4%) | -- | -- |
| Both USS and AFP | 43 (25.4%) | 23 (13.6%) | 0.24 (0.11–0.55) | 0.15 (0.05–0.42) |
| 0–1 year prior to index date | ||||
| No screening | 103 (60.9%) | 129 (76.3%) | 1 | 1 |
| Either USS or AFP | 66 (39.1%) | 40 (23.7%) | 0.41 (0.24–0.71) | 0.42 (0.22–0.81) |
| AFP only | 33 (19.5%) | 18 (10.7%) | 0.45 (0.20–0.99) | 0.54 (0.21–1.35) |
| USS onlyb | 10 (5.9%) | 7 (4.1%) | -- | -- |
| Both USS and AFP | 23 (13.6%) | 15 (8.9%) | 0.31 (0.11–0.85) | 0.22 (0.06–0.73) |
Adjusted for age, race/ethnicity, BMI, diabetes, alcohol use disorder, HIV coinfection, HBV e Ag positive, HBV DNA maximum viral load, years from HBV diagnosis to index date, and receipt of CT or MRI in the window.
Unable to calculate odds ratio because too few patients received USS only.
Abbreviations: OR – odds ratio, CI – confidence interval, USS – ultrasound, AFP – alpha fetoprotein
Cases were also far less likely to have received screening with both USS and AFP (19.5%) than controls (34.3%). In multivariable analysis, screening with both tests was strongly associated with reduced HCC-related mortality (aOR 0.11, 95% CI 0.03–0.40) (Table 4).
With respect to screening with AFP only, cases were less likely to have received screening (12.4%) than controls (20.1%). In multivariable analysis, screening with AFP only was associated with reduced HCC-related mortality (aOR 0.22, 95% CI 0.05–1.00). While a smaller proportion of cases (1.8%) than controls (4.1%) received screening with USS only, there were too few patients to perform multivariable analysis.
In sensitivity analyses, screening with either USS or AFP was associated with a reduced risk of HCC-related mortality during the 3 years (aOR 0.24, 95% CI 0.11–0.52), 2 years (aOR 0.26, 95% CI 0.13–0.55), and 1 year (aOR 0.42, 95% CI 0.22–0.81) prior to the index date (Table 4).
In a second sensitivity analysis, screening with either USS or AFP remained strongly associated with reduced HCC-related mortality (aOR 0.20, 95% CI 0.05–0.78) after excluding patients who received abdominal CT or MRI in the 4 years before the index date (Supplemental Table 6).
Lastly, screening with USS or AFP was associated with reduced HCC-related mortality in persons without cirrhosis (aOR 0.07, 95% CI 0.02–0.29) and in persons with cirrhosis (aOR 0.17, 95% CI 0.03–0.91) (Table 5).
Table 5.
Subgroup analysis of patients with and without cirrhosis. Cases and controls compared with respect to occurrence of screening with either USS or AFP at given time intervals prior to the index date.
| Cirrhosis | ||||
| Controls N = 62 | Cases N = 62 | Unadjusted OR (95% CI) | Adjusted ORa (95% CI) | |
| 0–4 years prior to index date | ||||
| Either USS or AFP | 46 (74.2%) | 25 (40.3%) | 0.16 (0.06–0.46) | 0.17 (0.03–0.91) |
| 0–3 years prior to index date | ||||
| Either USS or AFP | 45 (72.6%) | 24 (38.7%) | 0.19 (0.07–0.50) | 0.24 (0.06–1.00) |
| 0–2 years prior to index date | ||||
| Either USS or AFP | 41 (66.1%) | 22 (35.5%) | 0.24 (0.10–0.59) | 10.30 (0.08–1.15) |
| 0–1 year prior to index date | ||||
| Either USS or AFP | 31 (50.0%) | 22 (35.5%) | 0.53 (0.24–1.13) | 0.76 (0.24–2.39) |
| No cirrhosis | ||||
| Controls N = 107 | Cases N = 107 | Unadjusted OR (95% CI) | Adjusted OR* (95% CI) | |
| 0–4 years prior to index date | ||||
| Either USS or AFP | 53 (49.5) | 32 (29.9) | 0.22 (0.09–0.54) | 0.07 (0.02–0.29) |
| 0–3 years prior to index date | ||||
| Either USS or AFP | 48 (44.9) | 28 (26.2) | 0.29 (0.13–0.63) | 0.12 (0.04–0.38) |
| 0–2 years prior to index date | ||||
| Either USS or AFP | 42 (39.3) | 24 (22.4) | 0.28 (0.12–0.65) | 0.12 (0.04–0.41) |
| 0–1 year prior to index date | ||||
| Either USS or AFP | 35 (32.7) | 18 (16.8) | 0.32 (0.14–0.71) | 0.16 (0.06–0.46) |
Adjusted for age, race/ethnicity, BMI, diabetes, alcohol use disorder, HIV coinfection, HBV e Ag positive, HBV DNA maximum viral load, years from HBV diagnosis to index date, and receipt of CT or MRI in the window.
Abbreviations: OR – odds ratio, CI – confidence interval, USS – ultrasound, AFP – alpha fetoprotein
DISCUSSION
In this matched case-control study, HCC screening with USS and serum AFP was associated with reduced HCC-related mortality among patients with CHB in VA care in the United States. Our results provide strong support for efforts to increase screening uptake in patients with CHB.
Although HCC screening is considered standard-of-care for many patients with CHB, professional organizations have not reached consensus regarding the benefits of screening. The three major liver societies all recommend HCC screening in patients with cirrhosis and in high-risk patients with CHB[2, 24, 29]. In contrast, the National Cancer Institute states that available evidence does not suggest a mortality benefit from screening whereas there is potential harm[30]. Neither the United States Preventative Services Task Force nor the American Cancer Society have formal positions on HCC screening.
The lack of consensus is primarily because available evidence on HCC screening is of low quality[4–6]. Current AASLD guidelines cite 2 randomized controlled trials to support screening recommendations. However, closer scrutiny of these trials reveals limitations that threaten their validity. In one study, patients with CHB who were assigned to receive semi-annual USS and AFP were found to have a lower risk of HCC-related mortality compared to persons not so assigned (RR 0.62, 95% CI 0.41 – 0.98)[7]. However, the method of allocation of study participants was not clearly described, and persons not assigned to the screening arm were not actively followed in the same way as those in the intervention arm. In the second study, screening with serum AFP did not appreciably reduce mortality (RR 0.83, 95% CI 0.68 – 1.03)[8], but the study used an AFP assay (reverse passive hemagglutination) that is less sensitive than assays currently in use (immunoassay)[5].
In the absence of high quality randomized controlled trials, many observational studies have attempted to evaluate the efficacy of HCC screening[12]. Most compare survival between cases with screen-detected versus symptomatically diagnosed HCC. However, these study designs are highly susceptible to lead-time, length-time, and selection bias[31]. Some studies corrected for lead-time bias[9–12, 32–36] but only a small number of patients with CHB were included in these studies[9–11, 34–36]. Moreover, results of studies that correct for lead-time bias vary widely depending on assumptions of the tumor growth rate and the duration of follow-up[10, 32, 37], and they remain susceptible to length-time bias.
In case-control studies of cancer screening, the screening history of cases is compared to that of controls who did not die of the relevant cancer, selected from the population from which the cases arose. The rationale for selecting as controls patients who did not die of HCC is to avoid lead-time bias. It may seem more intuitive for controls to be patients with HCC who had not (yet) succumbed to HCC by the end of the study period. However, choosing “HCC survivors” as controls is inappropriate because some number of survivors at any point in time will later die from HCC but are alive now only because screening pushed their diagnosis forward in time. Consequently, choosing such patients as controls overestimates the proportion of controls who have undergone screening. While we avoided specifically selecting “HCC survivors” as controls, we did not prohibit including as controls patients who developed HCC after the index date but who did not die of HCC by the end of the study period (Figure 1). It should also be emphasized that cases in our study are selected from the population of patients with CHB and fatal HCC. As such, cases tended to have more advanced tumors and were less likely to be candidates for curative treatment than the average VA patient with CHB and HCC (Table 2).
The main finding of our study is that receipt of either screening AFP or USS – or both – was associated with a substantially lower risk of HCC-related mortality (Table 4). We were unable to examine USS screening alone because very few patients received USS only, which is an important limitation as USS is the main screening modality recommended by professional societies. This pattern of low USS utilization, however, is consistent with prior studies of HCC screening in the VA[32]. A greater number of patients underwent screening with AFP only, which is also consistent with prior studies[32]. Although the association between AFP only and HCC mortality suggests a possible benefit to AFP-based screening, too few patients were in this group to evaluate the independent effect of this screening modality with any statistical precision.
The proportion of patients who underwent screening in our study is consistent with previously reported screening utilization rates in the VA and in other healthcare systems in the United States. HCC screening rates in patients with CHB are generally low in most real-world populations[32, 38, 39]. However, low screening utilization in our study does not mean that an evaluation of screening effectiveness is not possible. In order to compare the benefit of screening versus no screening, a substantial proportion of patients under study must fall into the “no screening” group. Indeed, power to detect an association between screening and mortality is optimized when screening rates approach 50%. The magnitude and statistical precision of the associations in our study clearly demonstrate that it is possible to detect a benefit to screening if one truly exists even in the context of low screening utilization.
Results of this study contrast with those of our prior study showing no association between screening and reduced HCC-related mortality in patients with cirrhosis[18]. A potential explanation for the discordant results is that a minority of patients in this study had cirrhosis (36.7%). The presence of cirrhosis may diminish the benefits of HCC screening for 2 reasons: 1) USS and AFP have lower sensitivity in cirrhotic patients, particularly for detection of early stage HCC; and 2) patients with cirrhosis have fewer curative options because portal hypertension and liver dysfunction often preclude treatment. It is also possible that, among patients with cirrhosis, screening efficacy differs depending on the cause of cirrhosis. Patients with CHB-related cirrhosis may have less advanced pathology than those with cirrhosis from other etiologies due to the availability of highly effective antiviral therapy, which can stabilize liver function for many years. A study of patients with Child’s-Turcot-Pugh (CTP) Class A cirrhosis due to CHB or HCV (in the pre-direct acting antiviral era) found that patients with CHB were less likely to develop primary liver cancer, experience decompensation, and had better survival than those with HCV[40]. A key difference between the two populations was that substantially more patients with CHB were on antiviral therapy with full suppression of viremia. Patients with CHB-related cirrhosis may be more likely than patients with other etiologies of cirrhosis to benefit from screening because they have better preserved liver function and are more likely to be candidates for curative treatment. Therefore, although screening may be associated with a reduced risk of fatal HCC in CHB patients with cirrhosis (as suggested by our subgroup analysis), this benefit may not extend to all patients with cirrhosis.
We acknowledge several limitations of our study. First, as previously discussed, it is not possible to evaluate screening intensity in case-control studies of cancer screening. Second, some tests could have been misclassified as screening because the indication was not explicit in medical records. While the indication for ultrasounds was recorded in each report, provider progress notes were the only source of information for determining the indication for AFPs. The impact of such misclassification, however, would be to underestimate the benefit of screening. Third, we were not always able to match cases and controls by date of CHB diagnosis because laboratory tests obtained before October 1999 – the date the VA CDW was created – were not available. The next best choice was to match by the date of the first positive HBV test after October 1999. Fourth, while we accounted for antiviral treatment before the index date, it is conceivable that antivirals started after the index date could have impacted survival. Overall, 15.4% of cases (18.7% of cases without cirrhosis) initiated antiviral therapy after the index date versus 1.2% of controls (1.9% of controls without cirrhosis). This may be because detection of HCC in cases led some providers to start antivirals in previously untreated patients. If this had any effect on HCC-related mortality, it would have been to underestimate the difference in mortality between cases and controls attributable to screening. However, we did not adjust for antiviral initiation after the index date in multivariable analysis because it is not a true confounder (i.e. a variable associated with both the outcome and the exposure). While it may impact HCC-related mortality, it cannot possibly impact whether a patient received screening before the index date. Fifth, all patients were male potentially limiting generalizability, however we are not aware of evidence that the effect of screening on HCC-related mortality differs by sex. Sixth, we were unable to match cases and controls by CTP or Model for End Stage Liver Disease (MELD) scores. The CTP score requires subjective assessment of ascites and encephalopathy which is difficult to determine retrospectively, and a large proportion of patients were missing laboratory values required for MELD calculation. Seventh, while we carefully reviewed medical records to ensure cases died of HCC, cause of death can be challenging to ascertain in an observational study, leading to potential misclassification. Lastly, in an observational study it is difficult to completely exclude the possibility of unmeasured confounders. For example, patients engaged in HCC screening may practice positive health behaviors or possess other determinants of health that could have contributed to their decreased risk of fatal HCC.
In summary, in a matched case-control study, HCC screening was associated with a reduced risk of HCC-related mortality among patients with CHB. Our results suggest that currently recommended screening approaches for patients with CHB are appropriate.
Supplementary Material
HIGHLIGHTS.
We used a case-control paradigm to investigate HCC screening effectiveness in HBV-infected patients
We identified 169 cases who died of HCC, matched to 169 controls who did not die of HCC
HCC screening by ultrasound and/or serum AFP was associated with a significant reduction in HCC-related mortality
Acknowledgments
Role of Funding Source
The funding source played no role in study design, collection, analysis or interpretation of data.
Declaration of Funding Sources
The study was funded by a NIH/NCI grant R01CA196692 and VA CSR&D grant I01CX001156 to GNI and NSW, and a NIH T32 grant DK007742–2 to FS.
Abbreviations
- AASLD
American Association for the Study of Liver Disease
- AFP
alpha-fetoprotein
- aOR
adjusted odds ratio
- BMI
body mass index
- CDW
Corporate Data Warehouse
- CHB
chronic hepatitis B
- CI
confidence interval
- CT
computed tomograp
- DPP
detectable preclinical period
- HBeAg
hepatitis B e antigen
- HBsAg
hepatitis B surface antigen
- HBV DNA
hepatitis B viral load
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- ICD
International Classification of Diseases
- LIRADS
Liver Imaging and Reporting Data System
- MRI
magnetic resonance imaging
- USS
ultrasound
- VA
Veterans Affairs
Footnotes
Declaration of Personal Interests
None
Disclaimer
The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Data availability:
The data that support the findings of this study are available upon reasonable request from the corresponding author, GNI.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- [3].Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358–380. [DOI] [PubMed] [Google Scholar]
- [4].Kansagara D, Papak J, Pasha AS, O’Neil M, Freeman M, Relevo R, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med 2014;161:261–269. [DOI] [PubMed] [Google Scholar]
- [5].Aghoram R, Cai P, Dickinson JA. Alpha-foetoprotein and/or liver ultrasonography for screening of hepatocellular carcinoma in patients with chronic hepatitis B. Cochrane Database Syst Rev 2012:CD002799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lederle FA, Pocha C. Screening for liver cancer: the rush to judgment. Ann Intern Med 2012;156:387–389. [DOI] [PubMed] [Google Scholar]
- [7].Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen JG, Parkin DM, Chen QG, Lu JH, Shen QJ, Zhang BC, et al. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. Journal of medical screening 2003;10:204–209. [DOI] [PubMed] [Google Scholar]
- [9].Costentin CE, Layese R, Bourcier V, Cagnot C, Marcellin P, Guyader D, et al. Compliance With Hepatocellular Carcinoma Surveillance Guidelines Associated With Increased Lead-Time Adjusted Survival of Patients With Compensated Viral Cirrhosis: A Multi-Center Cohort Study. Gastroenterology 2018;155:431–442.e410. [DOI] [PubMed] [Google Scholar]
- [10].Wong GL, Wong VW, Tan GM, Ip KI, Lai WK, Li YW, et al. Surveillance programme for hepatocellular carcinoma improves the survival of patients with chronic viral hepatitis. Liver Int 2008;28:79–87. [DOI] [PubMed] [Google Scholar]
- [11].Tong MJ, Sun HE, Hsien C, Lu DS. Surveillance for hepatocellular carcinoma improves survival in Asian-American patients with hepatitis B: results from a community-based clinic. Dig Dis Sci 2010;55:826–835. [DOI] [PubMed] [Google Scholar]
- [12].Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med 2014;11:e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Poustchi H, Farrell GC, Strasser SI, Lee AU, McCaughan GW, George J. Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology 2011;54:1998–2004. [DOI] [PubMed] [Google Scholar]
- [14].Weiss NS. Application of the case-control method in the evaluation of screening. Epidemiol Rev 1994;16:102–108. [DOI] [PubMed] [Google Scholar]
- [15].Selby JV, Friedman GD, Quesenberry CP, Weiss NS. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med 1992;326:653–657. [DOI] [PubMed] [Google Scholar]
- [16].Corley DA, Mehtani K, Quesenberry C, Zhao W, de Boer J, Weiss NS. Impact of endoscopic surveillance on mortality from Barrett’s esophagus-associated esophageal adenocarcinomas. Gastroenterology 2013;145:312–319 e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rustagi AS, Kamineni A, Weinmann S, Reed SD, Newcomb P, Weiss NS. Cervical screening and cervical cancer death among older women: a population-based, case-control study. Am J Epidemiol 2014;179:1107–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Moon AM, Weiss NS, Beste LA, Su F, Ho SB, Jin GY, et al. No Association Between Screening for Hepatocellular Carcinoma and Reduced Cancer-Related Mortality in Patients With Cirrhosis. Gastroenterology 2018;155:1128–1139.e1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Son JH, Choi SH, Kim SY, Jang HY, Byun JH, Won HJ, et al. Validation of US Liver Imaging Reporting and Data System Version 2017 in Patients at High Risk for Hepatocellular Carcinoma. Radiology 2019;292:390–397. [DOI] [PubMed] [Google Scholar]
- [20].Morgan TA, Maturen KE, Dahiya N, Sun MRM, Kamaya A, Group ACoRULIaRDSUL-RW. US LI-RADS: ultrasound liver imaging reporting and data system for screening and surveillance of hepatocellular carcinoma. Abdom Radiol (NY) 2018;43:41–55. [DOI] [PubMed] [Google Scholar]
- [21].Di Bisceglie AM, Sterling RK, Chung RT, Everhart JE, Dienstag JL, Bonkovsky HL, et al. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol 2005;43:434–441. [DOI] [PubMed] [Google Scholar]
- [22].Richardson P, Duan Z, Kramer J, Davila JA, Tyson GL, El-Serag HB. Determinants of serum alpha-fetoprotein levels in hepatitis C-infected patients. Clin Gastroenterol Hepatol 2012;10:428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hepatocellular Carcinoma Surveillance. [cited 12/31/2019]; Available from: https://www.hepatitis.va.gov/cirrhosis/complications/hcc-surveillance.asp
- [24].EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- [25].Papatheodoridis G, Dalekos G, Sypsa V, Yurdaydin C, Buti M, Goulis J, et al. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol 2016;64:800–806. [DOI] [PubMed] [Google Scholar]
- [26].Sheu JC, Sung JL, Chen DS, Yang PM, Lai MY, Lee CS, et al. Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications. Gastroenterology 1985;89:259–266. [DOI] [PubMed] [Google Scholar]
- [27].Etzioni RD, Weiss NS. Analysis of case-control studies of screening: impact of misspecifying the duration of detectable preclinical pathologic changes. Am J Epidemiol 1998;148:292–297. [DOI] [PubMed] [Google Scholar]
- [28].Weiss NS, Etzioni R. Estimating the influence of rescreening interval on the benefits associated with cancer screening: approaches and limitations. Epidemiology 2002;13:713–717. [DOI] [PubMed] [Google Scholar]
- [29].Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].PDQ Liver (Hepatocellular) Cancer and Screening. [cited 10/12/2019]; Available from: https://www.cancer.gov/types/liver/hp/liver-screening-pdq
- [31].IARC. Colorectal cancer screening. IARC Handbooks of Cancer Prevention 2019. [cited 9/23/2019]; Volume 17:[Available from: http://publications.iarc.fr/573] [Google Scholar]
- [32].El-Serag HB, Kramer JR, Chen GJ, Duan Z, Richardson PA, Davila JA. Effectiveness of AFP and ultrasound tests on hepatocellular carcinoma mortality in HCV-infected patients in the USA. Gut 2011;60:992–997. [DOI] [PubMed] [Google Scholar]
- [33].Tanaka H, Nouso K, Kobashi H, Kobayashi Y, Nakamura S, Miyake Y, et al. Surveillance of hepatocellular carcinoma in patients with hepatitis C virus infection may improve patient survival. Liver Int 2006;26:543–551. [DOI] [PubMed] [Google Scholar]
- [34].Yu EW, Chie WC, Chen TH. Does screening or surveillance for primary hepatocellular carcinoma with ultrasonography improve the prognosis of patients? Cancer J 2004;10:317–325. [DOI] [PubMed] [Google Scholar]
- [35].Trevisani F, De Notariis S, Rapaccini G, Farinati F, Benvegnù L, Zoli M, et al. Semiannual and annual surveillance of cirrhotic patients for hepatocellular carcinoma: effects on cancer stage and patient survival (Italian experience). Am J Gastroenterol 2002;97:734–744. [DOI] [PubMed] [Google Scholar]
- [36].Singal AG, Mittal S, Yerokun OA, Ahn C, Marrero JA, Yopp AC, et al. Hepatocellular Carcinoma Screening Associated with Early Tumor Detection and Improved Survival Among Patients with Cirrhosis in the US. Am J Med 2017;130:1099–1106.e1091. [DOI] [PubMed] [Google Scholar]
- [37].Cucchetti A, Trevisani F, Pecorelli A, Erroi V, Farinati F, Ciccarese F, et al. Estimation of lead-time bias and its impact on the outcome of surveillance for the early diagnosis of hepatocellular carcinoma. J Hepatol 2014;61:333–341. [DOI] [PubMed] [Google Scholar]
- [38].Singal AG, Yopp A, S Skinner C, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med 2012;27:861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Goldberg DS, Valderrama A, Kamalakar R, Sansgiry SS, Babajanyan S, Lewis JD. Hepatocellular carcinoma surveillance rates in commercially insured patients with noncirrhotic chronic hepatitis B. J Viral Hepat 2015;22:727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Trinchet JC, Bourcier V, Chaffaut C, Ait Ahmed M, Allam S, Marcellin P, et al. Complications and competing risks of death in compensated viral cirrhosis (ANRS CO12 CirVir prospective cohort). Hepatology 2015;62:737–750. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


