Abstract
Background:
Bone marrow stromal/stem cells (BMSCs) remain a promising potential therapy for ischemic cardiomyopathy. The primary objective of this study was to evaluate the safety and feasibility of direct intramyocardial injection of autologous BMSCs in patients undergoing transmyocardial revascularization (TMR) or coronary artery bypass graft surgery (CABG).
Methods:
A phase I trial was conducted on adult patients suffering from ischemic heart disease with depressed left ventricular ejection fraction who were scheduled to undergo TMR or CABG. Autologous BMSCs were expanded for three weeks prior to scheduled surgery. Following completion of surgical revascularization, BMSCs were directly injected into ischemic myocardium. Safety and feasibility of therapy were assessed. Cardiac functional status and changes in quality of life were evaluated at one year.
Results:
Fourteen patients underwent simultaneous BMSC and surgical revascularization therapy (TMR+BMSCs = 10, CABG+BMSCs = 4). BMSCs were successfully expanded and no significant complications occurred as a result of the procedure. Regional contractility in the cell-treated areas demonstrated improvement at 12 months compared to baseline (TMR+BMSCs Δ strain: −4.6% ± 2.1%; P = 0.02. CABG+MSCs Δ strain: −4.2% ± 6.0%; P = 0.30). Quality of life was enhanced, with substantial reduction in angina scores at one year post-treatment (TMR+BMSCs: 1.3 ± 1.2. CABG+MSCs: 1.0 ± 1.4).
Conclusions:
In this phase 1 trial, direct intramyocardial injection of autologous BMSCs in conjunction with TMR or CABG was technically feasible and could be performed safely. Preliminary results demonstrate improved cardiac function and quality of life in patients at one year after treatment.
Keywords: Bone Marrow Stromal Mesenchymal Cells, Stem Cell Therapy, Ischemic Cardiomyopathy, Surgical Revascularization, Phase I Trial, Cell Transplantation, Clinical Trials, Coronary Artery Disease, Revascularization, Stem Cells
Recent studies have shown that bone marrow-derived stromal mesenchymal stem cells (BMSCs) may be recruited and incorporated into tissues undergoing neovascularization, including cardiac tissue. These cells have been pursued as an alternative therapeutic strategy for ischemic cardiovascular diseases that cannot be treated with traditional interventional revascularization techniques, such as percutaneous coronary interventions or coronary artery bypass graft surgery. Limitations with several types of multipotent stem cell types, such as time-consuming differentiation process, oncogenic risks, costs, and ethical implications have been cited.[1, 2] However, BMSCs have shown promise in multiple studies in which investigators have regenerated cardiomyocytes, encouraged angiogenesis, and improved left ventricular function following myocardial ischemia.[3–9] Furthermore, BMSCs can be produced in an economic and expedient manner, have a well-documented safety profile, and are less susceptible to immune rejection.[1, 10–12]
The primary therapeutic advantage of BMSCs is largely based on its ability to secrete a large number of paracrine-related growth factors and cytokines that ultimately enhance angiogenesis.[13–15] Therefore, in the context of cardiomyopathy, the hypothesis is that injection of BMSCs into damaged myocardium will encourage local repair and perfusion. Histological findings in animal models have demonstrated increased vascular cells, smooth muscle cells, and endothelial cells after injection of BMSCs.[24, 25] While BMSCs have been administered in a variety of clinical trials for subjects with left ventricular dysfunction after myocardial ischemia, variations in study reporting have made interpretation of their safety and efficacy challenging.[3, 7] Furthermore, there is limited data assessing the use of autologous BMSCs delivered by direct myocardial injection during simultaneous surgical revascularization procedure to treat ischemic myocardial regions, which is not amenable to traditional techniques alone.[11, 16]
This study was therefore performed to evaluate the safety and feasibility of direct intra-myocardial injection of autologous BMSCs in adult subjects concurrently undergoing transmyocardial revascularization (TMR) or coronary artery bypass graft surgery (CABG), and to determine whether this therapy improves cardiac function and quality of life.
MATERIAL AND METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request. This was a phase 1, non-randomized, open label treatment trial in which two parallel groups of subjects, undergoing either CABG or TMR, were treated with BMSCs to evaluate safety and feasibility. The study protocol was approved by the Institutional Review Board and the trial was registered with Clinicaltrials.gov (identification number NCT01557543). Recruitment and enrollment occurred from cardiology referrals and online through Clinicaltrials.gov. The investigational nature, research objectives of this study, the details of the procedure, and its attendant risks were explained and signed informed consent was obtained for all patients. Indications for enrollment criteria included adult subjects with three-vessel coronary artery disease, stable angina, depressed left ventricular ejection fraction (LVEF ≤ 50%), evidence of hypokinetic segments. All patients were scheduled for operative revascularization: CABG or, in cases of severe diffuse three-vessel coronary artery disease not amenable to bypass grafting, TMR. Subjects were excluded if they have had a recent myocardial infarction (within 30 days), bleeding disorder, active infection or HIV, or who were unable to wait the prerequisite 3 weeks for surgery while cells were being expanded prior to injection. Patients were enrolled in two groups based on the type of procedure scheduled: TMR+BMSCs or CABG+BMSCs.
BMSC Manufacturing and Processing
The investigational product, BMSCs, was used under BB-IND 14758 for the protocol for which the FDA issued the “Safe to Proceed” letter. All donors met FDA criteria for autologous donor [21 CFR 640.3 and 640.12] prior to donation. Consented subjects meeting the inclusion criteria underwent bone marrow aspiration three weeks prior to scheduled CABG or TMR. The bone marrow aspiration (up to 15 ml) was performed using aseptic technique into a syringe containing preservative-free heparin. Autologous BMSCs were isolated from the marrow aspirate, cultured, and expanded in vitro for three weeks using the following process in an FDA-registered, AABB-accredited laboratory using current good manufacturing practices (cGMP). To produce BMSCs, peripheral RBCs were separated by gradient centrifugation using lymphocyte separation liquid, re-suspended in Minimum Essential Medium Eagle Alpha Modification (α-MEM media) (Invitrogen; Carlsbad, CA), supplemented with 20% fetal bovine serum and gentamicin sulfate, and cultured in T-flasks at a density of 2–3 × 105/cm2 at 37°C with 5% CO2. One day later, the non-attached, floating portion of the mononuclear cells were washed away by medium change and every three days thereafter. The attached colonies were continued in culture. When the attached cells reached confluence, they were split and expanded for a maximum of 3 passages in cell factories. The total ex-vivo expansion time of the cells was 21 days (± 3 days), and the targeted number of cells was 150 × 106 cells (minimum of 15 × 106 cells). BMSC surface makers were tested by BD FACSCalibur (BD Biosciences; San Jose, CA) via flow cytometry at three weeks after ex-vivo expansion and just prior to its administration, and showed strong positivity for CD90, CD44, and negative for CXCR4, CD34, C-kit, CD144, CD54, CD45, CD31. These results were cross-referenced with published literatures to confirm presence of BMSCs. All cells were tested for pathogens which included endotoxins, prior to its use in surgery for intramyocardial injections.
Surgical Procedure with Administration of BMSCs
On the day of surgery, the BMSCs were washed and re-suspended in 3.0 ml of 0.9% sodium chloride. CABG was performed in standard fashion through a midline sternotomy exposure, with cardiopulmonary bypass support. TMR was performed using a CO2 laser system (PLC Medical Systems; Franklin, MA) with channels fired one centimeter apart (20 joules/pulse) in the ischemic area with 20–30 channels per patient. After completion of CABG or TMR, BMSCs were injected directly into the ischemic area of the left ventricle. For patients who underwent CABG+BMSCs, the injections were made in the ischemic zone of the un-grafted anatomic territories with no distal targets for revascularization. Therefore, only partial revascularization by CABG was performed in these cases. For TMR+BMCSs patients, the injections were made between TMR channels. The cell injections were performed in a distribution of one injection per cm2 in the area of the ischemic zone, which was based on preoperative imaging of reversible ischemia. The BMSCs were injected in approximately 30 injections sites with 100 μl in each injection. A modified 22-gauge needle and syringe were used as a delivery device to deposit cells into the mid-myocardium of the ischemic zone at an injection depth which varied from 0.3 – 0.75 cm depending on the thickness of the left ventricle. To assure delivery of cells into the myocardium and not into the left ventricular cavity, myocardium of less than 0.5 cm in thickness was avoided.
Endpoints
The primary endpoint of the present study was to evaluate the safety and feasibility of direct intramyocardial injection of autologous BMSCs in adult subjects concurrently undergoing transmyocardial revascularization (TMR) or coronary artery bypass graft surgery (CABG). Serious safety events were defined as peri-operative life-threatening ventricular arrhythmia, myocardial infarction, worsening of cardiac function, pulmonary embolism, cardiac tamponade, and bleeding. Post-discharge events included re-hospitalization for heart failure, recurrent myocardial infarction, stroke, inflammatory responses, and/or other unexpected adverse events. Feasibility was defined as the capability to develop a cellular product to the stated minimum criteria in this patient population. Secondary endpoints were undertaken for quantitative assessment of therapeutic effects, specifically whether there were improvements to cardiac function and quality of life post-operatively. Cardiac functional improvements were assessed versus baseline measurements with transthoracic echocardiograms at three and 12 months after surgery, specifically evaluating global left ventricular ejection fraction and wall motion score index. Cardiac magnetic resonance imaging (CMR) strain tagging studies were additionally performed to evaluate myocardial regional functional change (HARP, Myocardial Solutions; Morrisville, NC). Quality of life was assessed using Short Form 36 Health Survey (SF-36), Seattle Angina Questionnaire (SAQ) and Canadian Cardiovascular Society (CCS) Angina Grading Scale at three and 12 months postoperatively. Continuous variables were presented as a mean and standard deviation and comparisons were made using Student’s t test; categorical variables were reported as percentages. All analyses were performed using SAS software (Version 9.2; Cary, NC).
RESULTS
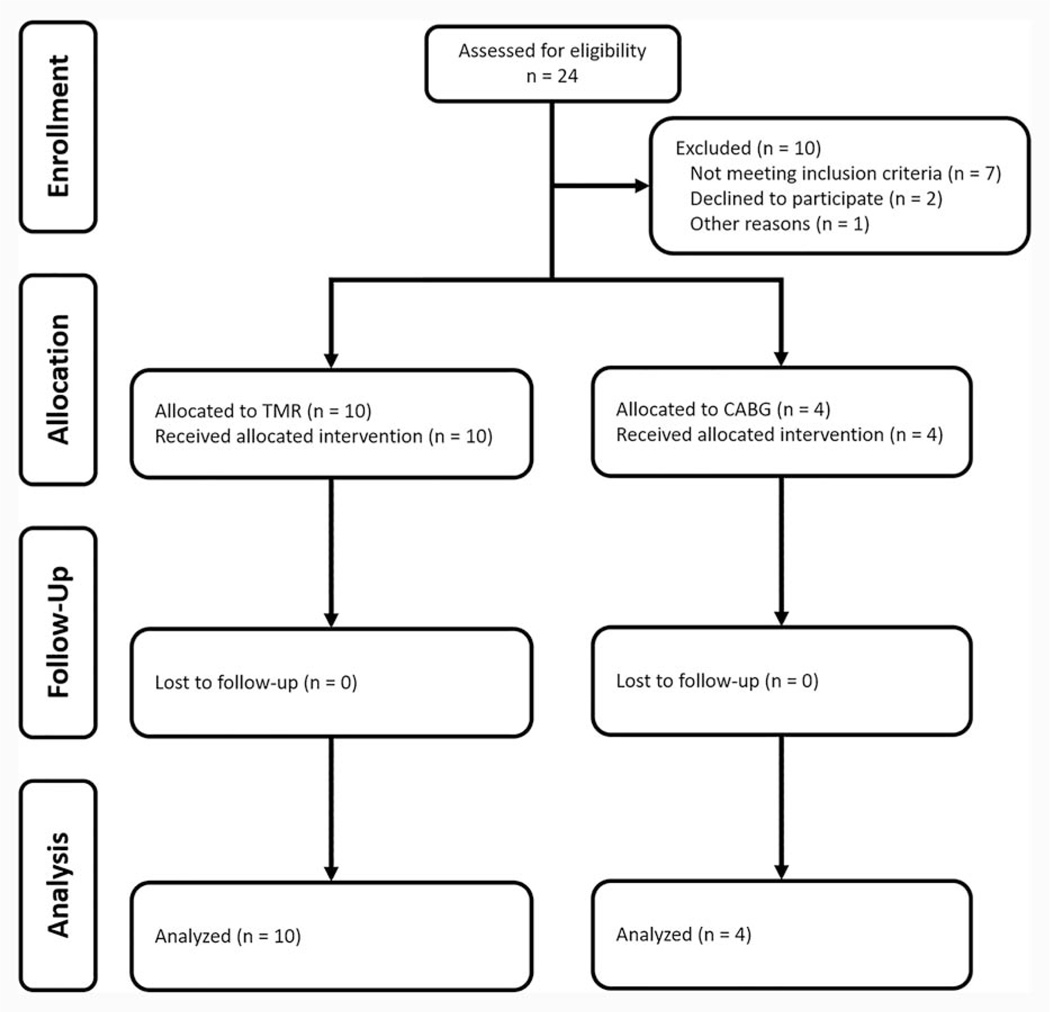
Over a three-year period, fourteen patients were enrolled and were followed in the trial, with ten patients undergoing TMR+BMSCs and four patients undergoing CABG+BMSCs (Figure 1). Baseline characteristics of patients are noted in Table 1. Many trial participants reported a history of myocardial infarction. Peri-operative characteristics, as shown in Table 2, reveal a total time of 105 ± 26 min and 215 ± 57 min to complete TMR+BMSCs and CABG+BMSCs procedures. In the ten TMR+BMSCs patients, 27 ± 4 TMR channels were created; a mean of 2 ± 1 bypass grafts were performed in the CABG+BMSCs cohort. The majority of patients (TMR+BMSCs: 0/10, CABG+BMSCs: 2/4) did not require blood product transfusion. Both cohorts were observed to have nominal ICU (TMR+BMSCs: 17 ± 5 hours, CABG+BMSCs: 20 ± 3 hours) and total hospital lengths of stay (TMR+BMSCs: 3 ± 1 days, CABG+BMSCs: 5 ± 2 days). The primary endpoint of this study was met: no patient was noted to experience a perioperative serious cardiac adverse event as defined as ventricular arrhythmia, myocardial infarction, worsening of cardiac function, pulmonary embolism, cardiac tamponade, and bleeding. There were no instances of renal failure, surgical site infections, or atrial fibrillation. None required hospital readmission within 30 days or expired within 90 days of procedure.
Figure 1.
CONSORT Flow Diagram.
Table 1.
Patient Demographics
| TMR+BMSCs (n = 10) | CABG+BMSCs (n = 4) | |
|---|---|---|
| Demographics | ||
| Age, years | 60 ± 11 | 58 ± 7 |
| Male | 90.0% (9/10) | 50% (2/4) |
| Body Mass Index, kg/m2 | 27.7 ± 4.0 | 26.3 ± 6.0 |
| Medical Comorbidities | ||
| Diabetes Mellitus | 40.0% (4/10) | 100.0% (4/4) |
| Insulin Dependence | 30.0% (3/10) | 50.0% (2/4) |
| Hypertension | 90.0% (9/10) | 100.0% (4/4) |
| Hyperlipidemia | 80.0% (8/10) | 75.0% (3/4) |
| Tobacco Use | 60.0% (6/10) | 25.0% (1/4) |
| Peripheral Vascular Disease | 10.0% (1/10) | 0.0% (0/4) |
| Cardiac Medical and Surgical History | ||
| Prior Myocardial Infarction | 90.0% (9/10) | 50.0% (2/4) |
| Prior Pacer/AICD | 40.0% (4/10) | 0.0% (0/4) |
| Prior PCI | 90.0% (9/10) | 25.0% (1/4) |
| Prior CABG | 80.0% (8/10) | 25.0% (1/4) |
Values represented as mean ± standard deviation or percentage (%)
AICD: Automatic implantable cardioverter/defibrillator; CABG: Coronary artery bypass graft surgery; BMSCs: Bone marrow stromal/stem cells; PCI: Percutaneous coronary intervention; TMR: Transmyocardial revascularization
Table 2.
Peri-Operative Characteristics
| TMR+BMSCs (n = 10) | CABG+BMSCs (n = 4) | |
|---|---|---|
| Total Procedure Time, min | 105 ± 26 | 215 ± 57 |
| Blood Products Transfused, units | 0 ± 0 | 1 ± 1 |
| Mechanical Ventilation, min | 219 ± 128 | 501 ± 160 |
| ICU Length of Stay, hours | 17 ± 5 | 20 ± 3 |
| Total Hospital Length of Stay, days | 3 ± 1 | 5 ± 2 |
| Serious Cardiac Adverse Event | 0% (0/10) | 0% (0/4) |
| Readmission Within 30 Days | 0% (0/10) | 0% (0/4) |
| Mortality Within 90 Days | 0% (0/10) | 0% (0/4) |
Values represented as mean ± standard deviation or percentage (%)
CABG: Coronary artery bypass graft surgery; ICU: Intensive care unit; BMSCs: Bone marrow stromal/stem cells; TMR: Transmyocardial revascularization
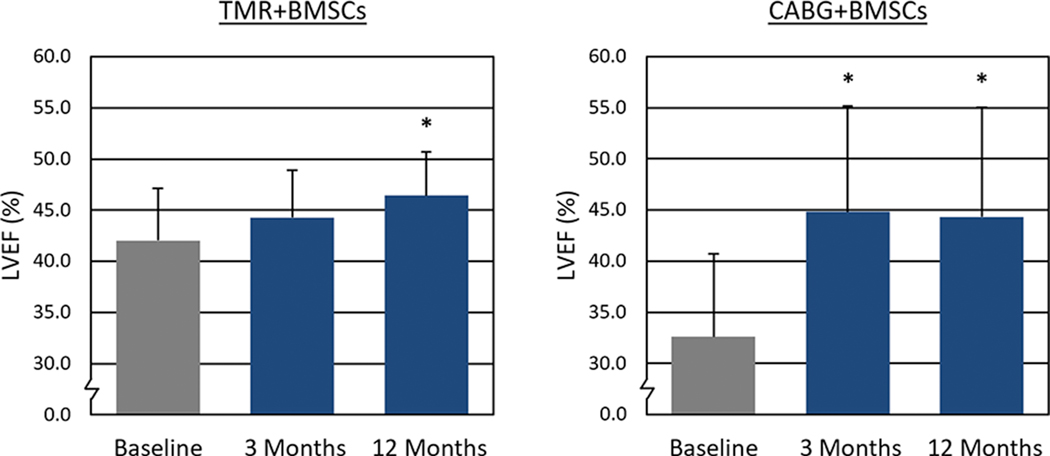
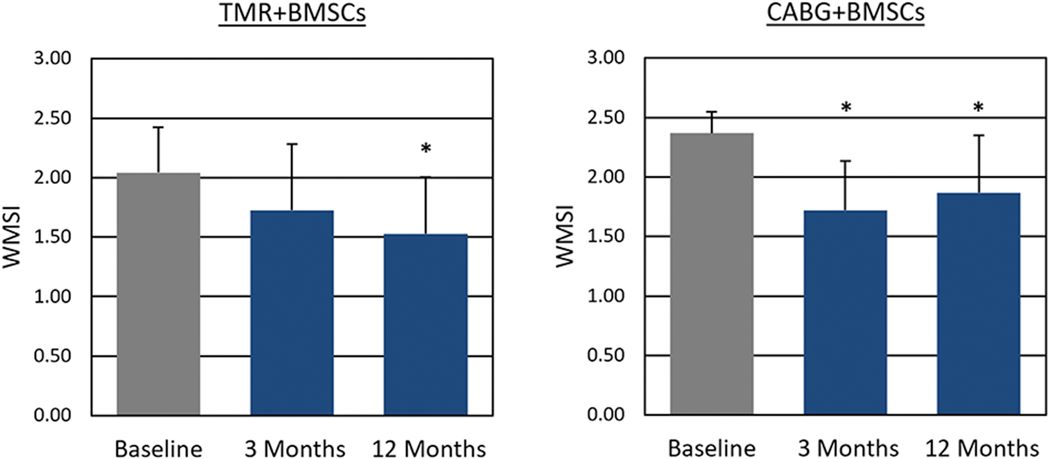
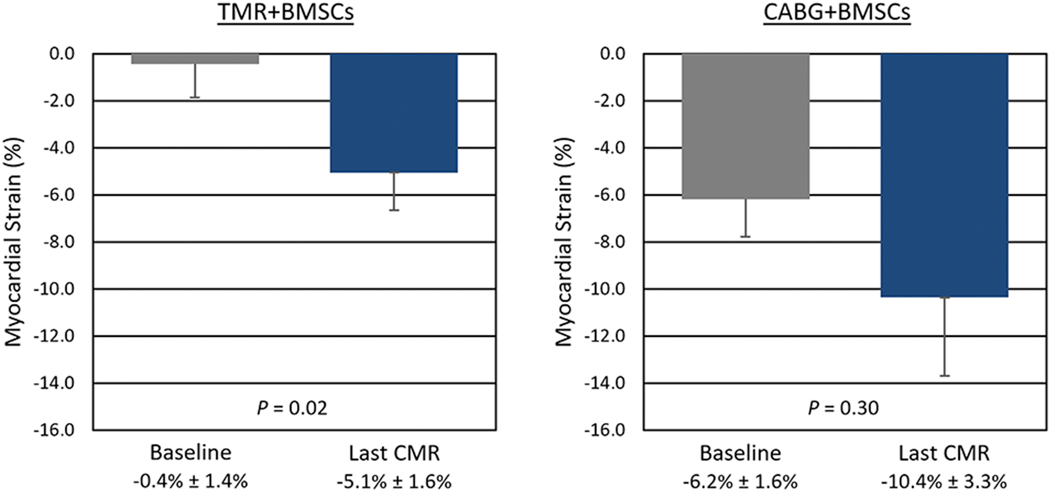
Baseline assessment of LVEF by echocardiography was conducted on all patients, and shown to be depressed. Following treatment, a statistically significant increase in LVEF (Figure 2) was observed at 12 months (TMR+BMSCs: 42.0% ± 5.1% vs. 46.4% ± 4.3%; P = 0.04. CABG+BMSCs: 36.6% ± 8.1% vs. 44.3% ± 10.7%; P = 0.04). Wall motion score index (WMSI) at 12 months also significantly improved (Figure 3) from 2.04 ± 0.38 to 1.52 ± 0.48 (P = 0.03) in TMR+BMSCs, with a similar significant improvement in CABG+BMSCs group (2.37 ± 0.18 to 1.87 ± 0.48; P = 0.02). CMR tagging studies evaluating myocardial shortening in the circumferential direction around the left ventricular short axis view showed quantitative evidence of improved regional myocardial function in segments with a baseline abnormal myocardial strain (Figure 4). When comparing myocardial strain at baseline to the last follow-up available (mean follow-up 0.9 years ± 0.25 years), treated segments exhibited significantly abnormal baseline regional strain (< −10%, with less negative values denoting impaired contractility) and were significantly improved in the TMR+BMSCs group from −0.4% ± 1.4% to −5.1% ± 1.6% (Δ strain: −4.6% ± 2.1%; P = 0.02). In the CABG+BMSCs group, a notable trend in strain improvement was also observed, from −6.2% ± 1.6% to −10.4% ± 3.3% (Δ strain: −4.2% ± 6.0%; P = 0.30).
Figure 2.
Left Ventricular Ejection Fraction as Assessed by Echocardiography.
* P < 0.05 compared to baseline
CABG: Coronary artery bypass graft surgery; LVEF: Left ventricular ejection fraction; BMSCs: Bone marrow stromal/stem cells; TMR: Transmyocardial revascularization
Figure 3.
Wall Motion Score Index as Assessed by Echocardiography.
* P < 0.05 compared to baseline
CABG: Coronary artery bypass graft surgery; WMSI: Wall motion score index; BMSCs: Bone marrow stromal/stem cells; TMR: Transmyocardial revascularization
Figure 4.
Regional Myocardial Contractility of Treated Segments as Assessed by Cine Cardiac Magnetic Resonance Imaging.
CABG: Coronary artery bypass graft surgery; CMR: Cardiac magnetic resonance imaging; BMSCs: Bone marrow stromal/stem cells; TMR: Transmyocardial revascularization
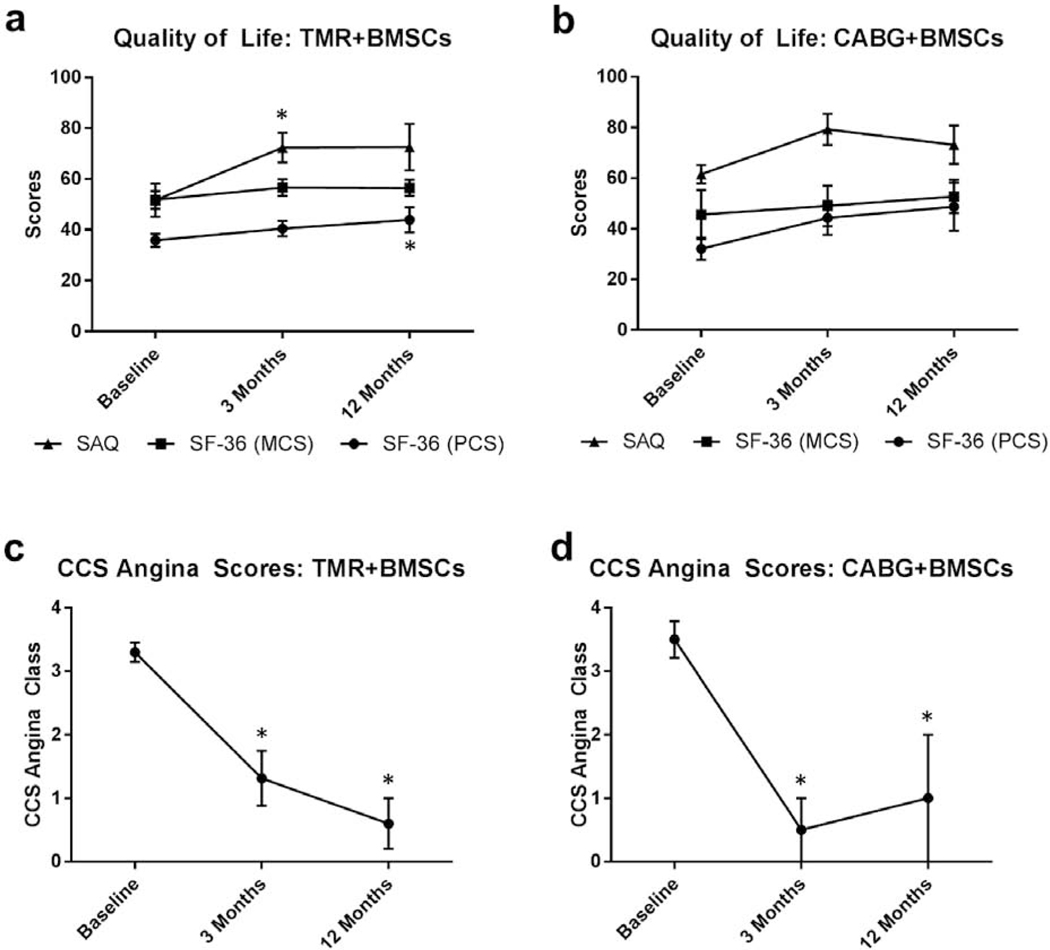
Quality of life assessments (SAQ and SF-36) documented significant angina relief, as well as enhanced physical and mental activity, at three and 12 months compared to baseline (Figure 5). SAQ values for TMR+BMSCs and CABG+BMSCs at baseline were 51.6 ± 20.5 and 61.6 ± 7.2, respectively. TMR+BMSCs patients had improved scores post-operatively at three months (70.6 ± 20.5; P = 0.03) and 12 months (69.8 ± 19.9; P = 0.07) compared to baseline. Following CABG+BMSCs, improvements of SAQ scores were also noted at three months (79.3 ± 7.2; P = 0.04) and 12 months (73.2 ± 15.1; P = 0.19). SF-36 evaluations were broken down by physical (PCS) and mental (MCS) component summaries. CABG+BMSCs had non-significant improvements with both PCS (32.1 ± 8.8 at baseline, 44.3 ± 13.3 at three months; P = 0.07, 48.7 ± 19.0 at 12 months; P = 0.12) and MCS (45.6 ± 19.4 at baseline, 49.1 ± 16.0 at three months; P = 0.12, 52.7 ± 13.0 at 12 months; P = 0.12). For TMR+BMSCs SF-36 scores, a significant improvement in PCS at 12 months compared to baseline was observed: PCS at baseline 35.8 ± 8.1, 40.5 ± 8.7 at three months; P = 0.09, 43.9 ± 12.0 at 12 months; P = 0.03, BMCS at baseline 51.8 ± 11.0, 56.6 ± 9.1 at three months; P = 0.16, 56.5 ± 7.7 at 12 months; P = 0.16.
Figure 5.
Postoperative Quality of Life and Symptom Improvement.
* P < 0.05
CABG: Coronary artery bypass graft surgery; CCS: Canadian Cardiovascular Society; BMCS: Mental component summary; BMSCs: Bone marrow stromal/stem cells; PCS: Physical component summary; SAQ: Seattle Angina Questionnaire; SF-36: Short Form 36 Health Survey; TMR: Transmyocardial revascularization
Using the CCS angina classification, the mean baseline value was 3.3 ± 0.5 for TMR+BMSCs and 3.5 ± 0.6 in CABG+BMSCs, correlating to considerable angina symptoms leading to moderate or severe limitation in activities of daily living. Overall, CCS angina scores were improved, with most patients reporting an angina class of 0 or 1 at three months (TMR+BMSCs: 1.5 ± 1.3; P = 0.01, CABG+BMSCs: 0.5 ± 1.0; P < 0.01) and 12 months (TMR+BMSCs: 1.3 ± 1.2; P < 0.01, CABG+BMSCs: 1.0 ± 1.4; P < 0.01).
COMMENT
A variety of studies have been conducted in subjects with coronary artery disease using different cell/stem cell types and routes of administration.[3, 7] The present study, specifically evaluating the administration of autologous BMSCs using direct intramyocardial injection when combined with TMR or CABG, makes several unique observations. The manufacturing of BMSCs within the three-week period prior to scheduled surgery was found to be both feasible and practical. The target number of viable cells was successfully achieved in all cases, and were classified as BMSCs in accordance with the published guidelines for the nomenclature of BMSCs. Karyotyping of the cells prior to injection was also completed, and did not reveal any evidence of chromosomal mutations, which have been reported in other studies during ex-vivo expansion.[17] The primary endpoint was achieved with the demonstration that this experimental treatment could be performed safely. There was no evidence of contamination observed in this trial. With a one-year follow up, no safety issues from BMSC therapy have been observed, and no study participants suffered complications or mortality directly related to treatment.
While this study was not designed to demonstrate the individual impact of BMSC therapy, and definitive conclusion regarding treatment efficacy should be cautioned, these results suggest that the adjunctive use of BMSCs in conjunction with TMR or CABG may be beneficial. Based on assessment by transthoracic echocardiogram, patients were observed to have improvement of cardiac function in terms of ejection fraction and WMSI. In addition, regional myocardial shortening or strain has been recently used to evaluate systolic dysfunction.[18] This supplemental method was applied to assess myocardial function: recovery was noted in treated myocardial segments with abnormal strain at baseline. Quality of life surveys performed post-operatively were additionally encouraging and appear consistent with symptomatic relief leading to improvements in angina and functional capacity.
Autologous cells were chosen in this study to obviate the possibility of rejection in this phase I safety trial. There is increasing evidence that allogenic BMSCs can be used safely, as they do not express MHC II antigens and therefore are able to be transplanted without provoking an immune response.[10, 13] Only elective operative revascularization candidates were enrolled in this trial, which minimized concerns of the requisite three-week period for adequate cell expansion. However, the prospect of large scale allogenic BMSC manufacturing may provide greater flexibility and treatment availability as an off-the-shelf clinical therapy option.[1, 19] Based on our pre-clinical experiments, α-MEM media was used to culture BMSCs, and displayed characteristics of morphology and cell markers consistent with BMSCs.[20, 21] These cells maintained multi-potency differentiation abilities when appropriately stimulated. Previous studies have not demonstrated that injected BMSCs ultimately differentiate into functional or morphologically analogous cardiac myocytes.[12, 22, 23] Instead of cell transplant engraftment and differentiation, it is more likely that the primary therapeutic effect of BMSCs delivered into the ischemic area is its role as a paracrine source to release a set of growth factors to enhance angiogenesis. This is consistent with histological findings in prior pre-clinical models demonstrating local increase in vascular cells, smooth muscle cells, and endothelial cells, and may contribute to the cardiac functional improvement seen in these patients.[24, 25]
Several previous clinical trials, which used autologous bone marrow-derived cell therapy in cardiovascular disease, have utilized percutaneous methods with catheter devices to deliver cells through intracoronary injection.[3, 5, 8, 9, 26–28] However, the efficacy of this method is greatly diminished as a large fraction of the administered cells are ultimately not retained within the ischemic myocardium, but rather diffused into the bloodstream and frequently end up in the lung, liver or spleen.[29, 30] Furthermore, this administration method is generally limited to non- expanded cells due to the technical size constraints of catheter-based therapy, and consequentially are limited in efficacy, as a majority are hematopoietic lineage cells.[25] Our approach utilizes direct intramyocardial injection, which allows optimal delivery BMSCs, as this method overcomes the aforementioned obstacles. Other potential surgical approaches, such as thoracoscopic injection of BMSCs, may provide additional advantages. However, it remains unknown what the effects of repeated adjuvant administrations of cell therapies are, which remains outside the scope of this phase 1 trial but continues to be an area of future consideration.
There are several limitations in this current study. At present, we are unable to discern the individualized effects on stem cell therapy from the potential impact of simultaneous TMR or CABG procedures. However, because the primary objective of this FDA phase 1 trial was to assess the safety and feasibility of BMSC co-treatment with simultaneous surgical revascularization with either TMR or CABG, a control group was not utilized for comparison. This primary endpoint was achieved successfully, with no adverse effects attributed to this therapy noted. Patients appeared to experience post-treatment myocardial recovery based on echocardiographic and CMR imaging modalities as well as improved functional status. This was reflected in the quality of life survey scores, which further underscored its safety profile, if not additionally its beneficial effects. These findings advocate for the consideration of a phase 2 trial examining this experimental therapy’s efficacy. This may potentially be best accomplished through a multi-center, randomized control trial to answer the remaining questions regarding the discrete contribution of BMSC therapy as an adjunct during surgical revascularization.
In conclusion, this phase 1 clinical trial assessed the use of autologous BMSCs delivered by direct intramyocardial injection in combination with TMR or CABG surgical revascularization in patients with ischemic heart disease. The primary endpoint of this preliminary study demonstrates that this treatment modality is feasible and can be performed safely. Moreover, secondary endpoints were met, including improvement in cardiac function and quality of life after one-year post-treatment. These findings encourage further examination of this regenerative cell therapy strategy for the management of advanced coronary artery disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hao M, Wang R, Wang W. Cell therapies in cardiomyopathy: Current status of clinical trials. Anal Cell Pathol (Amst) 2017;2017:9404057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freund C, Mummery CL. Prospects for pluripotent stem cell-derived cardiomyocytes in cardiac cell therapy and as disease models. J Cell Biochem 2009;107(4):592–599. [DOI] [PubMed] [Google Scholar]
- 3.Britten MB, Abolmaali ND, Assmus B et al. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (topcare-ami): Mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation 2003;108(18):2212–2218. [DOI] [PubMed] [Google Scholar]
- 4.Schachinger V, Assmus B, Britten MB et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: Final one-year results of the topcare-ami trial. J Am Coll Cardiol 2004;44(8):1690–1699. [DOI] [PubMed] [Google Scholar]
- 5.Strauer BE, Brehm M, Zeus T et al. Regeneration of human infarcted heart muscle by intracoronary autologous bone marrow cell transplantation in chronic coronary artery disease: The iact study. J Am Coll Cardiol 2005;46(9):1651–1658. [DOI] [PubMed] [Google Scholar]
- 6.Strauer BE, Brehm M, Zeus T et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 2002;106(15):1913–1918. [DOI] [PubMed] [Google Scholar]
- 7.Perin EC, Dohmann HF, Borojevic R et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation 2003;107(18):2294–2302. [DOI] [PubMed] [Google Scholar]
- 8.Chen SL, Fang WW, Ye F et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol 2004;94(1):92–95. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs S, Satler LF, Kornowski R et al. Catheter-based autologous bone marrow myocardial injection in no-option patients with advanced coronary artery disease: A feasibility study. J Am Coll Cardiol 2003;41(10):1721–1724. [DOI] [PubMed] [Google Scholar]
- 10.Hare JM, Traverse JH, Henry TD et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 2009;54(24):2277–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karantalis V, DiFede DL, Gerstenblith G et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The prospective randomized study of mesenchymal stem cell therapy in patients undergoing cardiac surgery (prometheus) trial. Circ Res 2014;114(8):1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheng CC, Zhou L, Hao J. Current stem cell delivery methods for myocardial repair. Biomed Res Int 2013;2013:547902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosen MR, Myerburg RJ, Francis DP, Cole GD, Marban E. Translating stem cell research to cardiac disease therapies: Pitfalls and prospects for improvement. J Am Coll Cardiol 2014;64(9):922–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ 2014;21(2):216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao Y, Huang J, Geng Y et al. Paracrine action of mesenchymal stem cells revealed by single cell gene profiling in infarcted murine hearts. PLoS One 2015;10(6):e0129164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viswanathan C, Davidson Y, Cooper K, Tipnis S, Pujari G, Kurian VM. Tansplantation of autologous bone marrow derived mesenchymal stem cells trans-epicardially in patients undergoing coronary bypass surgery. Indian Heart J 2010;62(1):43–48. [PubMed] [Google Scholar]
- 17.Zhou YF, Bosch-Marce M, Okuyama H et al. Spontaneous transformation of cultured mouse bone marrow-derived stromal cells. Cancer Res 2006;66(22):10849–10854. [DOI] [PubMed] [Google Scholar]
- 18.Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S. Myocardial strain imaging: How useful is it in clinical decision making? Eur Heart J 2016;37(15):1196–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michler RE. Stem cell therapy for heart failure. Methodist Debakey Cardiovasc J 2013;9(4):187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pittenger MF, Mackay AM, Beck SC et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284(5411):143–147. [DOI] [PubMed] [Google Scholar]
- 21.Asahara T, Murohara T, Sullivan A et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275(5302):964–967. [DOI] [PubMed] [Google Scholar]
- 22.Amado LC, Saliaris AP, Schuleri KH et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A 2005;102(32):11474–11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer GP, Wollert KC, Drexler H. The role of stem cells in the post-mi patient. Curr Heart Fail Rep 2007;4(4):198–203. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y, Wang S, Yu Z, Hoyt RF Jr., Qu X, Horvath KA. Marrow stromal cells differentiate into vasculature after allogeneic transplantation into ischemic myocardium. Ann Thorac Surg 2011;91(4):1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y, Wang S, Yu Z et al. Direct injection of autologous mesenchymal stromal cells improves myocardial function. Biochem Biophys Res Commun 2009;390(3):902–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer GP, Wollert KC, Lotz J et al. Intracoronary bone marrow cell transfer after myocardial infarction: 5-year follow-up from the randomized-controlled boost trial. Eur Heart J 2009;30(24):2978–2984. [DOI] [PubMed] [Google Scholar]
- 27.Schachinger V, Aicher A, Dobert N et al. Pilot trial on determinants of progenitor cell recruitment to the infarcted human myocardium. Circulation 2008;118(14):1425–1432. [DOI] [PubMed] [Google Scholar]
- 28.Menasche P, Alfieri O, Janssens S et al. The myoblast autologous grafting in ischemic cardiomyopathy (magic) trial: First randomized placebo-controlled study of myoblast transplantation. Circulation 2008;117(9):1189–1200. [DOI] [PubMed] [Google Scholar]
- 29.Tongers J, Losordo DW, Landmesser U. Stem and progenitor cell-based therapy in ischaemic heart disease: Promise, uncertainties, and challenges. Eur Heart J 2011;32(10):1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terrovitis JV, Smith RR, Marban E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ Res 2010;106(3):479–494. [DOI] [PMC free article] [PubMed] [Google Scholar]