Abstract
Background:
SPRINT found that treatment of systolic hypertension to a goal of <120 mmHg reduces risk of cardiovascular events and mortality, even in older adults. But concern remains that older adults will have excess serious adverse events (SAEs).
Objective:
To determine predictors of SAEs for syncope, hypotension, and falls, with particular attention to age, in the Systolic Blood Pressure Intervention Trial.
Design:
Randomized clinical trial.
Setting:
102 practices across the US and Puerto Rico.
Participants:
9361 adults aged ≥50 years with SBP 130–180 mmHg and increased risk for cardiovascular disease events, but without diabetes, history of stroke, symptomatic heart failure or ejection fraction <35%, dementia, or standing SBP <110 mmHg.
Intervention:
Treatment of SBP to a goal of <120 vs <140 mmHg.
Measurements:
Outcomes were SAEs for syncope, hypotension, and falls. Predictors were treatment assignment, demographics, comorbidities, baseline measurements, and baseline use of cardiovascular medications.
Results:
172 (1.8%) participants had SAEs for syncope, 155 (1.6%) for hypotension, and 203 (2.2%) for falls. Randomization to intensive SBP control was associated with greater risk of an SAE involving hypotension (HR 1.67 (1.21–2.32), p=.002), and possibly syncope (HR 1.32 (0.98–1.79), p=.07), but not falls (HR 0.98 (0.75–1.29), p=.90). Risk of all 3 outcomes was higher for participants with chronic kidney disease or frailty. Older age was also associated with greater risk of syncope, hypotension, and falls. However, there was no age by treatment interaction for any of the SAE outcomes.
Conclusions:
Compared to the standard group, participants randomized to intensive systolic BP control were at higher risk for hypotension and possibly syncope, but not falls. The increased risk of developing these events associated with intensive treatment did not vary by age.
Keywords: syncope, hypotension, falls, antihypertensive, elderly
INTRODUCTION:
Two-thirds of persons over age 65 have hypertension, yet older adults are less likely to have adequate blood pressure (BP) control1 despite multiple randomized clinical trials finding decreased cardiovascular morbidity and mortality among those treated.2 Epidemiologic studies have linked hypertension treatment with increased risk of adverse events, such as syncope and injurious falls.3–5 Conceivably because fall-related injuries, especially fractures, are costly and a significant source of disability and mortality in older adults,6–8 increased risk of falls has been a salient argument against intensive blood pressure control – despite conflicting data.3;4;9;10
The SPRINT study showed that randomization to a systolic blood pressure (SBP) treatment goal of <120 mmHg significantly reduced cardiovascular events and all-cause mortality compared to SBP treatment goal of <140 mmHg,11 even among those aged 75 and older.12 Although the overall number of serious adverse events (SAEs) was similar between the intensive and standard treatment groups, some have argued that the harms may have outweighed the benefits since there were more SAEs in the intensive arm for conditions expected to be related to intensive BP lowering, such as hypotension, electrolyte abnormalities, and acute kidney injury.13
Here, we more closely examine the risk of intensive BP treatment in SPRINT on SAEs for 3 conceptually related adverse events: syncope, hypotension, and falls, especially in participants ≥75 years old, since clinicians are most concerned about the potential risks in the aged. To assist clinicians in making more informed, individualized decisions with patients when contemplating whether to treat to a SBP goal of <120 mmHg, we addressed these questions:
What are the baseline risk factors for an SAE for syncope, hypotension, and falls?; and
Does the intensive BP goal result in greater risk of an SAE for syncope, hypotension, or fall for older adults, compared to those <age 75?
METHODS:
Participants and Intervention:
The SPRINT Trial enrolled 9,361 participants with hypertension at 102 sites across the United States and Puerto Rico between November 2010 and March 2013. Participants were randomly assigned to either intensive BP control (SBP goal <120 mmHg) or standard BP control (SBP <140 mmHg). Investigators chose from a formulary of study BP medications to achieve the targeted goal, and could prescribe other medications as needed or per participant preference. The main entry criteria for the trial were age 50 ≥years, SBP 130–180 mmHg, and an increased risk of cardiovascular events. Participants with diabetes, history of stroke, symptomatic congestive heart failure or ejection fraction <35%, dementia, or standing SBP below 110 mm Hg were excluded. Details of the inclusion criteria and intervention have been published.11;14 The intervention was stopped early, after a median follow-up of 3.26 years, due to benefit in the primary outcome (composite of myocardial infarction, acute coronary syndrome, stroke, acute decompensated heart failure, or death from cardiovascular causes) for the intensive treatment arm.11 Institutional Review Boards at all sites reviewed and approved the study protocol, and all participants provided written informed consent.
Collection of SAEs:
Sites queried participants about SAEs at quarterly study visits using a standardized data collection form. Sites may have also learned about SAEs at PRN visits or in other ways, such as participant-initiated contact, investigator involvement in participant care, or electronic medical record notifications. SAEs were defined as medical events that were fatal or life-threatening, resulted in significant or persistent disability, required hospitalization, or were judged by investigators to represent significant hazards or harm to the participant that might require intervention to prevent an event listed above. Syncope, hypotension, and injurious falls were pre-specified as safety events of interest. For SAEs related to syncope, hypotension, or fall, 95% involved hospitalization.
Classification of SAEs/Outcomes:
The Medical Dictionary for Regulatory Activities (MedDRA® Version 14.0) was used to classify the SAEs. SPRINT Safety Officers (KMS, MFL, DMF) at the coordinating center reviewed all SAEs of interest, including the site narrative of the event, the hospital admission history and physical, and the discharge summary. Coding was done at the preferred term level; up to 3 preferred terms were assigned for each SAE using all information available, but prioritizing hospital discharge diagnoses with a focus on reasons for admission. We then developed SPRINT specific Standardized MedDRA Queries (SMQs) for syncope, hypotension, and falls to capture various preferred terms under each heading. Hypotension, including orthostatic hypotension, was coded when symptomatic low BP (without specific BP cut-offs) was mentioned in the admission history and physical or discharge summary as a reason for admission. Since these were hospital admissions, SPRINT research site staff were not able to confirm hypotension with a study measured blood pressure. Incidentally noted low BP without symptoms was not coded as SAE for hypotension. Syncope was defined as a sudden temporary loss of consciousness. Pre-syncope or feeling faint or dizzy was not included as a syncopal event. A fall was defined as a sudden, unintentional change in position in which the participant came to rest on the ground, floor, or a lower level, not as the result of syncope or overwhelming external force. A fall due to syncope was not counted as a fall, since syncope was captured separately. For descriptive purposes only, we describe fall-related injuries in 5 groups: 1) major fracture, defined as large bone fracture or a fracture requiring surgical intervention; 2) minor fracture (digits, ribs, nose, non-operative vertebral compression fracture); 3) intracranial hemorrhage; 4) soft tissue injury, including hematomas, lacerations, sprains, ligamentous injuries, and joint dislocations; and 5) no identifiable injury, but nonetheless admitted to the hospital following a fall.
Covariates of Interest:
Baseline characteristics, assessed before any study-related treatment began, were considered as possible risk factors for syncope, hypotension, and falls. Demographics included self-reported age (modeled as <75, 75–84, and 85+ years old and separately as a continuous variable), education (≤12 years vs >12 years), sex, and race (white, black, and other). Health-related factors included known cardiovascular disease, chronic kidney disease (eGFR 20–59 ml/min), body mass index (<25, 25 to <30, and 30+), frailty (defined as frailty index >0.21),15 and alcohol use (non-drinker; light to moderate; and heavy, defined as ≥2 drinks per day). Baseline cardiovascular medications of interest were nitrates, diuretics, beta blockers, calcium channel blockers (dihydropyridine and non-dihydropyridine), ACE inhibitors, angiotensin receptor blockers, alpha blockers, digoxin, and number of BP medications before randomization (0, 1–2, or 3+). Participants self-reported their adherence to BP medications at baseline. Poor adherence was defined as a score of ≤6 on the 8-item Morisky Medication Adherence Scale.16–18
At the baseline visit, sitting and standing BP were measured. Orthostatic hypotension (yes/no) was defined as a drop in SBP of ≥20 mmHg or DBP ≥10 mmHg 1 minute after standing. Participants were asked during the standing BP measure if they felt dizzy (yes/no), regardless of whether BP had changed. Other baseline measures of interest included baseline BP, heart rate, and gait speed (usual walking speed over 4 meters, measured in participants ≥75 years old).
Statistical Methods:
All data presented involve contacts or events occurring on or before August 20, 2015 and are based on the January 31, 2016 data freeze. This data set excludes 18 syncope, 24 hypotension, and 19 fall SAEs reported previously11 which were reclassified after final review of clinical data. We also include 3 syncope, 3 hypotension, and 7 fall SAEs occurring before August 20, 2015 that were not previously reported.
Data are presented as counts and percentages or means ± standard deviations unless otherwise noted. We used cumulative incidence plots to examine time until first occurrence of an SAE involving syncope, hypotension, or fall by treatment group, and proportional hazards analyses to estimate effects of baseline characteristics on time to first SAE occurrence. Separate proportional hazard models were fit for the three SAEs of interest. For each type of SAE, initial analyses examined covariates one at a time. Categorical covariates were modeled relative to a reference category, using the Wald Chi-Square test to test for differences among categories. Continuous covariates were modeled per standard deviation unit to facilitate comparisons. We then fit a series of multivariate models for each type of SAE. For forest plots, we first fit a model containing only age category, randomized treatment group, and their interaction. Then we fit a sequence of three models sequentially, adding baseline covariates selected because of their known or likely association with either hypertension treatment or one of the 3 outcomes of interest. Model 1 included randomized treatment group, age category, sex, race, and education; model 2 included these factors plus health conditions (cardiovascular disease, chronic kidney disease, BMI category, alcohol use and frailty); and model 3 included all previous covariates plus baseline measurements (SBP, heart rate, measured orthostatic hypotension, dizziness, number of antihypertensive medications, and baseline use of nitrates, diuretics, beta blockers, calcium channel blockers (dihydropyridine and non-dihydropyridine), ACE inhibitors, angiotensin receptor blockers, alpha blockers, and digoxin). For each model, participants with missing data for any covariates used in that model were excluded. Models 1–3 were fit separately for each SAE type, and were fit with and without the two-way interaction between age category and randomized treatment. Age < or ≥75 was a pre-specified subgroup of the SPRINT Trial. However, we modeled age as <75, 75–84, and 85+ to inform readers about the risks in the oldest old. Additionally, we also examined age as a continuous variable, repeating each of the models 1–3 as described above.
Standard diagnostic procedures were run to examine model assumptions; no important violations were identified. All p-values were two-sided; no adjustments were made for multiple comparisons. All analyses were performed using SAS version 9.4 (SAS Institute, Inc.).
RESULTS:
We identified SAEs involving syncope in 172 (1.8%), hypotension in 155 (1.7%), and falls in 203 (2.2%) participants during the trial. Of these, three participants experienced all 3 types of events during the trial, while 7 participants experienced both syncope and fall, 8 experienced both hypotension and fall, and 35 experienced both hypotension and syncope.
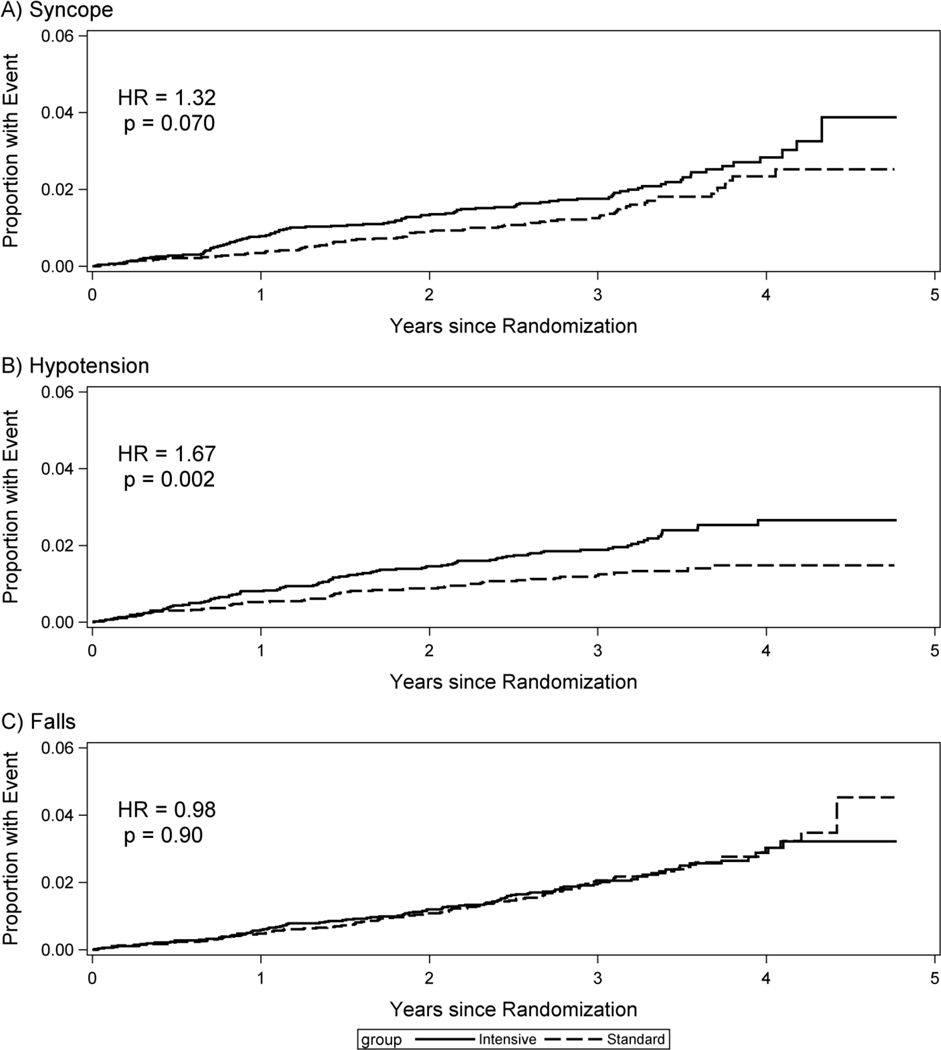
The cumulative incidence of SAEs for syncope, hypotension, and falls over time by treatment group is shown in Figure 1. The largest difference between treatment groups was for participants with hypotension events, where the two curves began to diverge at about 6 months and continued to separate through at least 4 years (HR=1.67, p=0.002). Differences were similar, but smaller and not statistically significant, for syncope (HR=1.32, p=0.07). There was no difference between groups in cumulative incidence of SAEs for falls (HR=0.98, p=0.90).
Figure 1:
Cumulative incidence of syncope, hypotension, and falls by randomized group
Of the 203 SAEs for fall, 74% were injurious falls: 84 (41.4%) resulted in a major fracture, 18 (8.9%) in a minor fracture, and 34 (16.7%) in a soft tissue injury. Intracranial hemorrhage occurred in 15 falls (7.4%), 3 of which also had a major fracture. 52 (25.6%) falls had no identifiable injury, but nonetheless resulted in a hospital admission and thus met criteria for an SAE. Two thirds of the falls without injury had medical reasons for admission that may have caused or contributed to the fall such as infections, cancer, and altered mental status. Similarly, in 46.5% of SAEs involving hypotension, something other than hypotension was the primary reason for admission (e.g. gastroenteritis, other infections, and sepsis). In the remaining 84 cases of SAE involving hypotension (54.2%), where hypotension was listed as the primary reason for admission, dehydration or other medical event (e.g. atrial flutter) likely caused the hypotension in 14.5%.
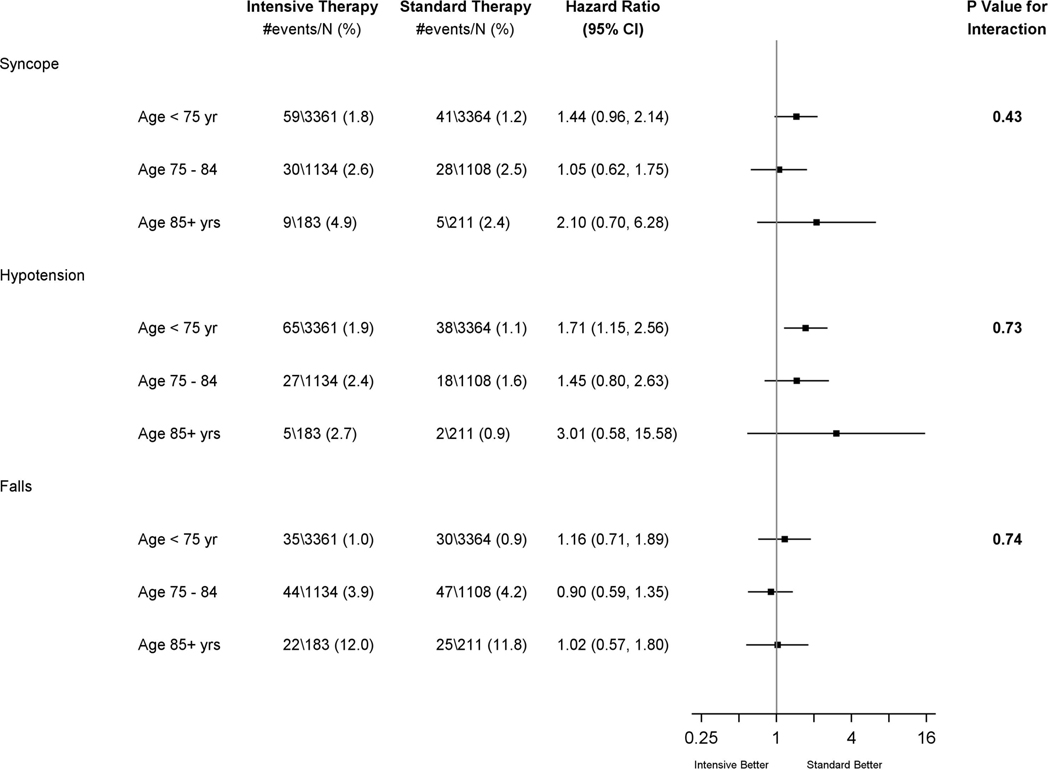
Univariate relationships between baseline characteristics and the 3 types of SAE events are shown in Table 1, with additional details in the supplemental table. Risk for the three SAE types was higher for participants with stage 3 or 4 chronic kidney disease or frailty and lower for those who self-reported poor BP medication adherence at baseline. Measured orthostatic hypotension at baseline was a risk factor for future SAEs involving a fall (HR 1.77, 95% CI 1.17–2.70; p= 0.007), but not syncope (HR 1.2, 95% CI 0.70–2.03, p=0.51) or hypotension (HR 1.58, 95% CI 0.96–2.62, p=0.08). However, report of being dizzy upon standing was not associated with SAEs for fall (HR 0.97, p=0.93), syncope (HR 1.01, p=0.99), or hypotension (HR 1.62, p=0.14). Older age was associated with greater risk of syncope, hypotension, and falls. However, there was no evidence of an age-by-randomized group interaction for any of the 3 SAE types (Figure 2).
Table 1.
Baseline Characteristics of Participants with a Serious Adverse Event for Syncope, Hypotension, or Fall
| Baseline Characteristica | Syncope | Hypotension | Falls | |||
|---|---|---|---|---|---|---|
| HRb (95% CI) | P-valuec | HRb (95% CI) | P-valuec | HRb (95% CI) | P-valuec | |
| Age, years | 1.51 (1.30,1.76) | <0.01 | 1.26 (1.07,1.47) | 0.004 | 3.00 (2.57,3.50) | <0.001 |
| Age Class, years | ||||||
| 50 to 74 | REF | <0.001 | REF | 0.2 | REF | <0.001 |
| 75 to 84 | 1.92 (1.39,2.66) | 1.37 (0.96,1.94) | 4.59 (3.34,6.32) | |||
| 85 or older | 2.88 (1.65,5.05) | 1.31 (0.61,2.81) | 15.53 (10.66,22.63) | |||
| Intensive Treatment Group | 1.32 (0.98,1.79) | 0.07 | 1.67 (1.21,2.32) | 0.002 | 0.98 (0.75,1.29) | 0.9 |
| Women | 1.13 (0.83,1.53) | 0.45 | 0.75 (0.53,1.07) | 0.11 | 1.56 (1.18,2.05) | 0.002 |
| Race/Ethnicity | ||||||
| White | REF | 0.073 | REF | 0.017 | REF | <0.001 |
| Black | 0.89 (0.64,1.25) | 0.83 (0.59,1.19) | 0.35 (0.24,0.52) | |||
| Other | 0.50 (0.28,0.91) | 0.36 (0.18,0.74) | 0.37 (0.21,0.65) | |||
| CVD History | 1.29 (0.91,1.83) | 0.16 | 2.01 (1.44,2.81) | <0.001 | 1.58 (1.16,2.15) | 0.004 |
| CKD - Stage 3 or 4 | 1.49 (1.09,2.03) | 0.012 | 2.03 (1.48,2.79) | <0.001 | 2.13 (1.61,2.81) | <0.001 |
| Orthostatic Hypotension | 1.20 (0.7,2.03) | 0.51 | 1.58 (0.96,2.62) | 0.075 | 1.77 (1.17,2.7) | 0.007 |
| Dizzy on Standing | 1.01 (0.47,2.14) | 0.99 | 1.62 (0.85,3.08) | 0.14 | 0.97 (0.48,1.97) | 0.93 |
| BMI Categories | ||||||
| Normal weight | REF | 0.003 | REF | 0.19 | REF | <0.001 |
| Over weight | 0.60 (0.41,0.87) | 0.7 (0.46,1.06) | 0.57 (0.41,0.79) | |||
| Obese | 0.54 (0.37,0.79) | 0.72 (0.48,1.08) | 0.37 (0.26,0.52) | |||
| # of BP Meds | ||||||
| none | REF | 0.15 | REF | 0.007 | REF | 0.41 |
| 1 or 2 | 1.73 (0.87,3.4) | 1.42 (0.71,2.81) | 1.3 (0.75,2.26) | |||
| 3 or more | 2.01 (0.99,4.08) | 2.26 (1.12,4.57) | 1.47 (0.82,2.63) | |||
| Poor BP Med Adherence | 0.60 (0.39,0.95) | 0.028 | 0.48 (0.28,0.8) | 0.005 | 0.58 (0.38,0.89) | 0.012 |
| Beta-blocker Use | 1.30 (0.96,1.78) | 0.093 | 1.5 (1.09,2.07) | 0.013 | 1.6 (1.21,2.11) | 0.001 |
| Nitrate Use | 2.40 (1.33,4.31) | 0.004 | 3.92 (2.37,6.48) | <0.001 | 1.66 (0.88,3.13) | 0.12 |
| Alpha blocker Use | 1.77 (1.01,3.12) | 0.048 | 1.31 (0.67,2.58) | 0.43 | 1.99 (1.21,3.27) | 0.007 |
| Frail | 1.45 (1.06,1.98) | 0.021 | 2.47 (1.8,3.39) | <0.001 | 2.39 (1.81,3.16) | <0.001 |
| Systolic Blood Pressure, mmHg | 1.03 (0.88,1.19) | 0.73 | 0.97 (0.82,1.13) | 0.68 | 1.15 (1,1.31) | 0.043 |
| Gait speedd, m/s | 1.13 (0.9,1.42) | 0.3 | 0.91 (0.68,1.21) | 0.53 | 0.72 (0.61,0.87) | <0.001 |
CVD= cardiovascular disease, CKD= chronic kidney disease, BMI= body mass index, BP= blood pressure
for additional baseline characteristics, see supplemental table online
Hazard Ratios are relative to reference category for categorical variables and per standard deviation unit for continuous variables
P-values are for tests of any difference among categories
Assessed only for participants ≥75 years of age
Figure 2:
Forest Plot of SAE outcomes by treatment arm and age subgroups
Table 2 shows adjusted results for age-by-treatment arm interactions in successively more comprehensive models. Older age remained an important predictor of SAE for syncope and fall, but not hypotension, even after adjustment for baseline potential confounders and in spite of increasing loss of sample size due to missing covariates. In all models, treatment assignment remains an important predictor for hypotension. The intervention effect on syncope is equivocal in model 1 and is further attenuated after controlling for health conditions and baseline factors. For falls, we found no intervention effect in any model. There was no evidence that the effect of treatment differed by age for any of the 3 conditions whether age was treated as a categorical or continuous variable (Table 2 treatment by age interaction). While not a focus of this paper, we also examined whether baseline orthostatic hypotension or frailty modified the relationship between treatment assignment and the 3 SAE outcomes. There was no evidence of a two-way interaction between treatment group and either orthostatic hypotension or frailty for any of the 3 outcomes (p>0.7 in all cases). Additionally, because we did not count an SAE for a fall that resulted from syncope as a fall (but rather as syncope since nearly all cases of syncope will result in a fall), some may be interested in the results of a composite outcome that combines SAEs for both fall and syncope. These results are presented in Supplemental Table 2 and don’t substantively change our results.
Table 2.
Age and treatment group vs. time until first syncope, hypotension and fall SAE.
| Syncope | Hypotension | Falls | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Categorical Age | ||||||
| Model 1a | ||||||
| Age <75 | REF | <.001 | REF | .36 | REF | <.001 |
| Age 75 – 84 | 1.85 (1.33,2.58) | 1.29 (0.90,1.84) | 3.95 (2.85,5.47) | |||
| Age 85+ | 2.78 (1.58,4.91) | 1.27 (0.59,2.74) | 12.88 (8.76,18.95) | |||
| Standard Therapy | REF | .061 | REF | .002 | REF | .98 |
| Intensive Therapy | 1.33 (0.99,1.80) | 1.68 (1.22,2.33) | 1.00 (0.76,1.31) | |||
| Rx by Age Interaction | .35 | .72 | .75 | |||
| Model 2b | ||||||
| Age <75 | REF | .002 | REF | .42 | REF | <.001 |
| Age 75 – 84 | 1.74 (1.22,2.47) | 1.00 (0.68,1.47) | 3.32 (2.33,4.72) | |||
| Age 85+ | 2.34 (1.27,4.33) | 0.51 (0.18,1.41) | 9.04 (5.81,14.07) | |||
| Standard Therapy | REF | .21 | REF | .005 | REF | .73 |
| Intensive Therapy | 1.22 (0.89,1.66) | 1.63 (1.16,2.28) | 0.95 (0.71,1.27) | |||
| Rx by Age Interaction | .43 | .53 | .45 | |||
| Model 3c | ||||||
| Age <75 | REF | .003 | REF | .62 | REF | <.001 |
| Age 75 – 84 | 1.72 (1.19,2.48) | 1.00 (0.67,1.49) | 3.12 (2.17,4.50) | |||
| Age 85+ | 2.32 (1.22,4.43) | 0.60 (0.22,1.69) | 8.44 (5.28,13.49) | |||
| Standard Therapy | REF | .44 | REF | .011 | REF | .76 |
| Intensive Therapy | 1.14 (0.82,1.56) | 1.57 (1.11,2.23) | 1.05 (0.77,1.42) | |||
| Rx by Age Interaction | .48 | .66 | .57 | |||
| Continuous Age | ||||||
| Model 1a | ||||||
| Aged | 1.50 (1.28,1.76) | <0.001 | 1.24 (1.05,1.46) | 0.011 | 2.83 (2.41,3.3) | <0.001 |
| Standard Therapy | REF | 0.050 | REF | 0.002 | REF | 0.98 |
| Intensive Therapy | 1.34 (0.99,1.81) | 1.68 (1.22,2.33) | 1.00 (0.76,1.31) | |||
| Rx by Age Interaction | 0.80 | 0.62 | 0.29 | |||
| Model 2b | ||||||
| Aged | 1.45 (1.22,1.72) | <0.001 | 1.05 (0.87,1.26) | 0.64 | 2.46 (2.05,2.94) | <0.001 |
| Standard Therapy | REF | 0.21 | REF | 0.005 | REF | |
| Intensive Therapy | 1.22 (0.90,1.66) | 1.63 (1.16,2.28) | 0.95 (0.71,1.27) | 0.74 | ||
| Rx by Age Interaction | 0.72 | 0.90 | 0.091 | |||
| Model 3c | ||||||
| Aged | 1.43 (1.19,1.72) | <0.001 | 1.04 (0.86,1.28) | 0.67 | 2.36 (1.96,2.85) | <0.001 |
| Standard Therapy | REF | 0.44 | REF | 0.012 | REF | 0.74 |
| Intensive Therapy | 1.13 (0.82,1.56) | 1.57 (1.10,2.22) | 1.05 (0.78,1.42) | |||
| Rx by Age Interaction | 0.62 | 0.52 | 0.13 | |||
Model 1 (N=9344) includes age, treatment group and demographic factors (sex, race and education).
Model 2 (N=8778) includes age, treatment group, demographic factors and health conditions (cardiovascular disease history, chronic kidney disease, BMI category, alcohol use, being frail).
Model 3 (N=7872) includes age, treatment group, demographic factors, health conditions and baseline measurements (systolic blood pressure, heart rate, orthostatic hypotension, dizziness, number of BP medications and use of beta blockers, diuretics, ace inhibitors, angiotensin II receptor blockers, calcium channel blockers, alpha blockers, nitrates and digoxin).
Age as a continuous variable. HRs for age are for each 1 SD of age (9.4 years)
DISCUSSION:
In the SPRINT trial, randomization to intensive BP control was associated with a greater risk of an SAE involving hypotension and possibly syncope, but not falls. These topline results are qualitatively similar to the primary results of SPRINT, even when combining SAEs with emergency department visits (syncope (HR 1.44, p = 0.003), hypotension (HR 1.70, p = < 0.001, and falls (HR 1.00, p = 0.97),11 but are no longer statistically significant for syncope. The current analyses add depth and detail, with a focus on risks of the intervention for older adults. Importantly, while older age was associated with increased risk of an SAE for syncope, hypotension, and fall, there was no evidence of differential risk associated with intensive treatment between older and younger adults. These results should reassure clinicians that treating patients ≥75 years old to a SBP goal of <120 mmHg does not result in excess risk of SAEs for syncope, hypotension, or falls versus patients aged 50–74. However, the few cases of syncope (14) and hypotension (7) in those ≥85 years make those results less certain. We also identified baseline risk factors for SAEs due to syncope, hypotension, and fall, most of which are biologically plausible. Importantly, self-reported dizziness upon standing was not associated with any of these 3 SAE types, though confirmed orthostatic hypotension at baseline was a risk factor for future SAEs involving falls. Even though orthostatic hypotension was a risk factor for a future SAE for fall, the relative risk of intensive BP treatment on falls, syncope, and hypotension events was not greater for participants with baseline orthostatic hypotension than without.
An SAE involving syncope, hypotension, or fall occurred in about 2% of participants in the intensive arm. Comparing our results to other hypertension treatment trials is difficult due to differences in what or how SAEs were reported, but rates and risks for the intensive intervention appear similar to reports from other hypertension trials. For example, in the Systolic Hypertension in the Elderly Program (SHEP), syncope occurred in 2.2% of participants treated to SBP <150 mg Hg, versus 1.3% in the placebo group.19 In the Secondary Prevention of Small Subcortical Strokes (SPS3) trial, SAEs related to hypotension occurred in 1.5% of participants in the intensive arm (SBP goal <130 mm Hg) vs 1.0% of the standard arm participants (SBP goal 130–149 mm Hg) (HR 1.53, 95% CI 0.8–2.93, p 0.20).20 The confidence interval overlaps the SPRINT confidence interval for SAE related to hypotension. Finally, the ACCORD-BP trial also found no increased risk of falls (or fractures) in the intensive arm (SBP goal <120 mm Hg) versus the standard BP group (RR 0.84, 95% CI 0.54–1.29, p = 0.43).9
The SPRINT study design provided several important strengths for this analysis. We rigorously collected, classified, and reported SAEs associated with syncope, hypotension, or a fall, since these events are of particular interest to clinicians and patients. We included SAEs that involved one of these even if the syncope, hypotension, or fall was not the primary reason for the event; in many cases, other medical illnesses were the primary reason for the SAE. The rich clinical data in SPRINT allowed us to control for factors, such as frailty, that may have confounded the relationship between BP treatment and syncope, hypotension, or falls in prior observational studies. Moreover, over 2, 600 persons aged 75 years old or older participated in SPRINT, providing substantial power to detect important differences in treatment effects compared to younger participants. That said, because the trial was stopped early, the statistical power to examine some interactions and/or outcomes may be diminished.
Nonetheless, we acknowledge several methodologic considerations that affect interpretation of our results, the most important of which may be ascertainment bias. SAEs for syncope, hypotension, and falls may have been over-reported in the intensive treatment group. While other SPRINT outcomes were only collected at quarterly visits to reduce the potential for reporting bias, SAEs could be reported at any visit or ascertained in other ways. Since the intensive treatment group had approximately 30% more PRN study visits than the control group, they had more opportunities to report adverse events, and may have been motivated to do so since they were not blinded to treatment assignment and risks of these events were specifically highlighted in the consent form. Another methodologic consideration is that participants were monitored closely, including annual orthostatic BP measurements, and medications were adjusted as needed per site clinician judgment. In addition, while participants with orthostatic hypotension were permitted, those with standing SBP <110 mm Hg at baseline were excluded. Thus, risk of SAEs may be greater among patients less carefully screened and closely monitored. Finally, we did not have every possible baseline characteristic that may be of interest to clinicians and the baseline predictors we identified may be hard to use clinically. Although they identify individuals at higher risk of these SAEs, they also identify individuals at higher risk of cardiovascular events who may also benefit more from the intervention.12
Conclusion:
Among three conceptually related, common concerns about risks of intensive BP treatment – syncope, hypotension, and falls – only hypotension was more common in participants randomized to intensive treatment. While older age was associated with higher absolute risk of SAE for syncope, hypotension, and falls, the relative risks of the SPRINT intervention for older participants were not different than those for younger participants, nor for those with frailty or orthostatic hypotension compared to those without.
Thus, our results suggest that treating patients ≥75 years old to a SBP goal of <120 mmHg does not increase risk for SAEs for syncope, hypotension, or falls versus patients aged 50–74, although results are less certain for those ≥85 due to fewer events.
Supplementary Material
Supplemental Table 1: Extended Characteristics of Participants with a Serious Adverse Event for Syncope, Hypotension, or Fall
Supplemental Table 2: Age and treatment group vs. time until first syncope or fall SAE (composite outcome).
Acknowledgments:
Funding:
The Systolic Blood Pressure Intervention Trial is funded with Federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13–002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc.
We also acknowledge the support from the following CTSAs funded by NCATS: CWRU: UL1TR000439, OSU: UL1RR025755, U Penn: UL1RR024134& UL1TR000003, Boston: UL1RR025771, Stanford: UL1TR000093, Tufts: UL1RR025752, UL1TR000073 & UL1TR001064, University of Illinois: UL1TR000050, University of Pittsburgh: UL1TR000005, UT Southwestern: 9U54TR000017–06, University of Utah: UL1TR000105–05, Vanderbilt University: UL1 TR000445, George Washington University: UL1TR000075, University of CA, Davis: UL1 TR000002, University of Florida: UL1 TR000064, University of Michigan: UL1TR000433, Wake Forest: UL1TR001420, Tulane University: P30GM103337 COBRE Award NIGMS.
Sponsor’s Role: All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the U.S. Department of Veterans Affairs, or the United States Government.
Funding: This trial was funded by the NIH (NHLBI, NIA, NIDDK, and NINDS). Complete funding information appears in the acknowledgment section.
We also acknowledge the editorial assistance of Karen Klein, MA, in the Wake Forest Clinical and Translational Science Institute (UL1 TR001420; PI: McClain).
Footnotes
Conflicts of Interest: Greg Evans reports receiving salary support on an institutional grant from AstraZeneca Investments (China) Co. All other authors declare they have no conflicts to report. Kaycee Sink was employed by Wake Forest School of Medicine at the time of submission of this manuscript. However, at the time of publication she is employed by Genentech, a Member of the Roche Group. Genentech had no involvement in the study design, data collection, interpretation of data, or preparation of this manuscript.
Other:
Use of the ©MMAS is protected by US copyright laws. Permission for use is required. A license agreement is available from: Donald E. Morisky, 294 Lindura Court, Las Vegas, NV 89138–4632; dmorisky@gmail.com.
Trial Registration: Clinicaltrials.gov identifier: NCT01206062
Reference List
- 1.Yoon SS, Gu Q, Nwankwo T et al. Trends in blood pressure among adults with hypertension: United States, 2003 to 2012. Hypertension 2015;65:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Musini VM, Tejani AM, Bassett K et al. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev 2009;CD000028. [DOI] [PubMed] [Google Scholar]
- 3.Corrao G, Mazzola P, Monzio CM et al. Antihypertensive Medications, Loop Diuretics, and Risk of Hip Fracture in the Elderly: A Population-Based Cohort Study of 81,617 Italian Patients Newly Treated Between 2005 and 2009. Drugs Aging 2015;32:927–936. [DOI] [PubMed] [Google Scholar]
- 4.Tinetti ME, Han L, Lee DS et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med 2014;174:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Chen MH, Larson MG et al. Risk factors for syncope in a community-based sample (the Framingham Heart Study). Am J Cardiol 2000;85:1189–1193. [DOI] [PubMed] [Google Scholar]
- 6.Shumway-Cook A, Ciol MA, Hoffman J et al. Falls in the Medicare population: incidence, associated factors, and impact on health care. Phys Ther 2009;89:324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tinetti ME, Williams CS. The effect of falls and fall injuries on functioning in community-dwelling older persons. J Gerontol A Biol Sci Med Sci 1998;53:M112–M119. [DOI] [PubMed] [Google Scholar]
- 8.Tajeu GS, Delzell E, Smith W et al. Death, debility, and destitution following hip fracture. J Gerontol A Biol Sci Med Sci 2014;69:346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Margolis KL, Palermo L, Vittinghoff E et al. Intensive blood pressure control, falls, and fractures in patients with type 2 diabetes: the ACCORD trial. J Gen Intern Med 2014;29:1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipsitz LA, Habtemariam D, Gagnon M et al. Reexamining the Effect of Antihypertensive Medications on Falls in Old Age. Hypertension 2015;66:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright JT Jr., Williamson JD, Whelton PK et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williamson JD, Supiano MA, Applegate WB et al. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged >/=75 Years: A Randomized Clinical Trial. JAMA 2016;315:2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ortiz E, James PA. Let’s Not SPRINT to Judgment About New Blood Pressure Goals. Ann Intern Med 2016;164:692–693. [DOI] [PubMed] [Google Scholar]
- 14.Ambrosius WT, Sink KM, Foy CG et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: The Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pajewski NM, Williamson JD, Applegate WB et al. Characterizing Frailty Status in the Systolic Blood Pressure Intervention Trial. J Gerontol A Biol Sci Med Sci 2016;71:649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morisky DE, Ang A, Krousel-Wood M et al. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich ) 2008;10:348–354. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Morisky DE, DiMatteo MR. Improving the measurement of self-reported medication nonadherence: response to authors. J Clin Epidemiol 2011;64:255–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krousel-Wood M, Islam T, Webber LS et al. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. Am J Manag Care 2009;15:59–66. [PMC free article] [PubMed] [Google Scholar]
- 19.SHEP Cooperative Research Group. Prevention of Stroke by Antihypertensive Drug Treatment in Older Persons With Isolated Systolic Hypertension. JAMA 1991;265:3255–3264. [PubMed] [Google Scholar]
- 20.Benavente OR, Coffey CS, Conwit R et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013;382:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Extended Characteristics of Participants with a Serious Adverse Event for Syncope, Hypotension, or Fall
Supplemental Table 2: Age and treatment group vs. time until first syncope or fall SAE (composite outcome).