Abstract
Several metals have carcinogenic properties, but their associations with breast cancer are not established. We studied cadmium, a metalloestrogen, and 9 other metals—arsenic, cobalt, chromium, copper, mercury, molybdenum, lead, tin, and vanadium–—in relation to young-onset breast cancer (diagnosis age <50 years), which tends to be more aggressive than and have a different risk profile from later-onset disease. Recent metal exposure was measured by assessing element concentrations, via inductively coupled plasma mass spectrometry, in toenail clippings of 1,217 disease-discordant sister pairs in the US-based Sister (2003–2009) and Two Sister (2008–2010) studies. Conditional logistic regression was used to calculate odds ratios and 95% confidence intervals. After correcting for differential calendar time of sample collection, no statistically significant associations were observed between any metals and breast cancer. Vanadium had the largest odds ratio (for fourth vs. first quartile, odds ratio = 1.54, 95% confidence interval: 0.75, 3.16; P for trend = 0.21). The association between cadmium and young-onset breast cancer was near null, with no evidence of a dose-response relationship (for fourth vs. first quartile, odds ratio = 0.95, 95% confidence interval: 0.64, 1.43; P for trend = 0.64). Positive associations between urinary cadmium concentrations and breast cancer have been reported in case-control studies, but we observed no such association between young-onset breast cancer and toenail concentrations of any assessed metals.
Keywords: breast cancer, cadmium, metals, toenails, young-onset breast cancer
Humans are widely exposed to metals via contaminated air, water, and soil, and many metals have known negative affects on health. Arsenic, cadmium, and chromium, in particular, have been classified as carcinogenic to humans by the International Agency for Research on Cancer (1). For arsenic, the evidence is based on documented associations with cancers of the liver and bile duct, lung, skin (keratinocyte), prostate, kidney, and bladder. Cadmium has been linked to cancers of the lung, prostate, and kidney, and chromium to cancers of the lung and nasal cavity. In addition, cobalt, methylmercury, and lead are considered possibly carcinogenic to humans (2).
However, there are currently no established associations between metals and breast cancer, with few studies reporting on associations between metals and young-onset breast cancer, in particular. Although young-onset breast cancer is rare, it is an important area of public health research because it tends to be more aggressive than older-onset disease (3) and has some risk factors that are distinct from those for older-onset disease (4–6).
The most frequently studied metal in relation to breast cancer has been cadmium, which acts as a metalloestrogen in that it can mimic the effects of estradiol by binding to estrogen receptors (ERs) and modifying transcription of estrogen- and progesterone-related genes (7, 8). Because hormone levels naturally fluctuate with age and menopausal status, these cadmium-induced hormone-related changes could affect younger and older women differently. Cadmium may also contribute to carcinogenesis through stimulation of cell proliferation and inhibition of apoptosis and DNA repair mechanisms (9).
Several case-control studies have reported associations between elevated levels of urinary cadmium and increased risk of breast cancer (10–14). In a study in which specifically young-onset breast cancer was examined (11), an odds ratio of 2.3 (95% confidence interval (CI): 1.1, 5.0) was reported for the fourth (≥0.580 μg/g creatinine) versus first quartile (<0.263 μg/g creatinine) of urinary cadmium in women younger than 56 years. However, the urine specimens were collected from patients after diagnosis and these associations have not been replicated in prospective studies (15, 16). An inverse association between cadmium levels in stored erythrocytes (range of median concentrations, 0.55–0.71 μg/L) and breast cancer risk was found in an analysis of 3 prospective cohorts (17).
Because cadmium accumulates in the kidney, urinary cadmium concentrations are considered reasonable biomarkers of cumulative exposure and body burden (18). However, measured urine levels are highly dependent on urinary dilution at the time of sample collection, and it may be difficult to properly control for this variation, especially because cadmium (a nephrotoxin), breast cancer, and underlying kidney disorders may affect kidney function (18, 19). Blood and toenails can also be used to assess cadmium body burden. Both are thought to reflect more recent exposure than urine (17), but neither is subject to the same dilution-related fluctuations. Toenail clippings are especially easy to collect, ship, and store. Samples combining clippings from all 5 digits on 1 foot are thought to represent 4–6 months of exposure occurring approximately 6–12 months before collection (20, 21). Using mass spectrometry technology, it is possible to measure toenail concentrations of multiple metals simultaneously.
We previously used inductively coupled plasma mass spectrometry to conduct a reliability study of toenail trace-element levels over time by measuring metals in samples collected from the same women 4–10 years apart (22). We found that although concentrations of some elements decreased markedly over calendar time, most had modest within-individual correlations. We also reported that breast cancer status did not alter the magnitude of the decrease or the strength of the correlation. On the basis of these supporting data, we undertook additional investigations of the association between select trace elements and young-onset breast cancer, using toenail samples collected from 1,214 pairs of sisters discordant for young-onset breast cancer (diagnosed before age 50 years). Our main hypothesis was that cadmium and other potentially toxic metals, some of which may also act as metalloestrogens (23), would be positively associated with young-onset breast cancer. We also examined whether selenium, which counteracts the toxic effects of some metals (24, 25), modified any of the observed associations.
METHODS
Study sample
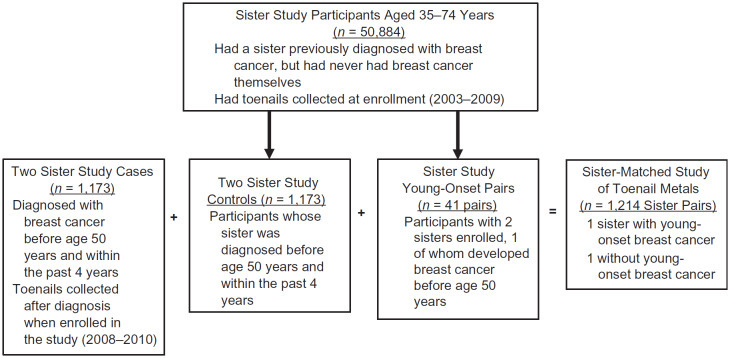
Our sister-matched case-control study included participants from the Sister Study (26) and Two Sister Study (Figure 1) (27). US women aged 35–74 years were recruited to the Sister Study between 2003 and 2009. To be eligible, participants had to have a sister who had been diagnosed with breast cancer but not have had breast cancer themselves at the time they enrolled. Baseline data were collected using computer-assisted telephone interviews and participants were asked to collect clippings from each toe using their own nail clippers, after first removing any nail polish. All participants provided written informed consent. Study approval and oversight were provided by the institutional review boards of the National Institute of Environmental Health Sciences and the Copernicus Group. Data were complete through August 2015 (data release 5.0.1).
Figure 1.
Flow chart describing selection of women with young-onset breast cancer and sister-matched control subjects from the Sister Study (2003–2009) and the Two Sister Study (2008–2010), United States.
The Two Sister Study (2008–2010) is a companion study to the Sister Study and focuses on young-onset breast cancer specifically. Sister Study participants whose affected sister had been diagnosed with invasive breast cancer or ductal carcinoma in situ before age 50 years and within the last 4 years were asked to participate in the Two Sister Study, along with their aforementioned affected sister (n = 1,173 sister pairs provided toenail samples). For these pairs, toenail clippings from the patient (hereafter, case sister) and the unaffected Sister Study participant (hereafter, control sister) were collected after the case sister had been diagnosed (i.e., “retrospectively”). Other consequences of the Two Sister Study’s design were that the case patient’s toenail samples were always collected after those of the control sister and that the control sister was usually older than her case sister.
For some families, more than 1 sister enrolled in the Sister Study. If 1 such sister was diagnosed with invasive breast cancer or ductal carcinoma in situ before age 50 years, the newly affected sister and 1 of her unaffected sisters were selected for inclusion in this sister-matched case-control study. For these 41 participating Sister Study pairs, the toenail samples were collected “prospectively” (i.e., before the case sister’s cancer was diagnosed).
Exposure assessment
We used the same panel as in our previous reliability study (22) to assess toenail concentrations of 16 elements (antimony, arsenic, cadmium, chromium, cobalt, copper, iron, lead, mercury, manganese, molybdenum, nickel, selenium, tin, vanadium, and zinc), using inductively coupled plasma mass spectrometry. Briefly, toenail clippings were washed and air dried before being digested in acid (9 parts nitric acid to 1 part hydrogen chloride) and diluted with deionized water. Inductively coupled plasma mass spectrometry analyses were conducted using an Agilent 8800 ICP-QQQ (Santa Clara, California). Data quality was monitored via continuous calibration verification, analysis of duplicates and spikes, within- and between-batch analyses of a laboratory-prepared toenail matrix digest, and comparison with standard reference materials (human hair; National Institute for Environmental Studies, Japan, Tsukuba-City, Ibaraki, Japan,). Laboratory staff were blinded to case status. We corrected for batch using random effects models. Nail clippings from sister pairs were analyzed in the same batch.
For this particular analysis, we focused on 10 putatively toxic metals, excluding iron, manganese, selenium, and zinc, though we considered selenium as a possible modifier. We also excluded nickel and antimony on the basis of our earlier finding that concentrations of these elements were not stable over time in women diagnosed with breast cancer between sample collections (22).
A small proportion of samples (n = 478 of 24,280) had concentrations less than or equal to 0 μg/g after quality-control–related corrections. We reassigned those values to 0.001 μg/g, a concentration lower than the smallest observed values for those elements. Some concentrations were below limits of quantification but were still assigned measured values, which we retained. All measured concentrations were log transformed.
Correction for calendar time
In our reliability study, we observed that toenail concentrations of the toxic metals had decreased markedly over time (22). The decreases were largest for lead, cadmium, and chromium, and appeared to reflect real declines in exposure over calendar time rather than changes due to increasing age. This observation is supported by nationwide trends (28, 29) and changes in environmental policies, including the removal of lead from gasoline (30) and public smoking bans (31). Even with these observed decreases, metal concentrations were correlated over time, and for the selected metals we saw no differences in levels among those in whom an intervening breast cancer developed versus those without breast cancer, suggesting that neither breast cancer nor its treatment were strongly influential.
As noted, most control sisters were enrolled before the enrollment of their case sister. Consequently, to the extent that exposure to toxic metals declined over calendar time, metal concentrations should be systematically lower for case sisters than for control sisters. To correct for this bias, we used prediction models to “time correct” all observed metal values for calendar year and age, thereby estimating the levels for the case and control sisters at the time the case sister’s cancer was diagnosed. We also accounted for smoking status in these correction models because case sisters were more likely to have quit smoking in the interim between case diagnosis (7% current smokers) and toenail-clipping collection (5% current smokers) than were control sisters (8% smoked at both time points).
To obtain time-corrected metal levels, we first constructed prediction models using data from the sister pairs and the previously conducted reliability study. For each metal of interest, we modeled the effects of age, calendar year, and smoking status on the log of the metal concentration. Age and year were coded as restricted cubic splines with knots at the fifth, 35th, 65th, and 95th percentiles to allow flexibility. We extracted the parameter estimates for each metal model, which we used to define a multivariate normal distribution that captured all the estimated β coefficients and their covariances simultaneously. Then, to capture the uncertainties in the prediction models, we randomly drew from the multivariate normal distribution 20 times, each draw yielding β coefficients for each term in the prediction model. For each sampling iteration, we computed the difference in predicted metal concentrations for the true age, year, and smoking status at sample collection and the predicted metal concentration for the corrected age, year, and smoking status at sample collection, where the time-corrected values corresponded to the calendar time of the case sisters’ diagnosis. These estimated differences in predicted levels were then added to the observed metal levels to obtain time-corrected metal levels.
Statistical analysis
We provide descriptive tables comparing case and control sisters by baseline characteristics. Then, using conditional logistic regression to account for the matched-pairs design, we estimated odds ratios and 95% confidence intervals for the association between each metal and young-onset breast cancer. For the time-corrected analyses, we ran a conditional logistic regression model for each of the 20 iterations and then used multiple imputation software (PROC MIANALYZE in SAS, version 9.4; SAS Institute, Inc., Cary, North Carolina) to obtain odds ratios and 95% confidence intervals that accounted for correlations between the random samples. Metal levels were categorized into quartiles on the basis of the corrected distribution among the control sisters, using all the imputed data. All models were adjusted for age (i.e., age at the time the case sister’s cancer was diagnosed) and highest achieved education (i.e., high school or less, some college, college graduate, or graduate school). Trend tests were calculated by treating metal quartiles as ordinal variables. For some analyses, we limited the study sample to sister pairs in which the case sister had ER-positive (n = 961) or invasive breast cancer (n = 1,041). We did not conduct assessments of ER-negative breast cancer or ductal carcinoma in situ, because of the small sample size.
We also examined whether selenium levels modified any of the metal–breast cancer associations. For these models, we calculated odds ratios for metal–breast cancer associations within categories of time-corrected selenium concentrations (greater than the median of 0.941 μg/g vs. <0.941 μg/g). To conserve power, we also dichotomized time-corrected metal levels as greater than or less than the median. Heterogeneity P values were calculated by testing metal-by-selenium interaction terms.
RESULTS
Control sisters were usually older than their case sisters (59% of the time; mean difference = 1.4 years, standard deviation, 5.2 years). However, the average age at toenail-sample collection was older for case sisters than control sisters (47.7 vs. 47.1 years; Table 1). Distributions of body mass index, parity, and alcohol use were similar for the 2 groups at baseline, but case sisters were somewhat better educated than control sisters (26% with graduate degrees vs. 24%, respectively) and less likely to currently smoke (5% vs. 8%, respectively).
Table 1.
Characteristics at Time of Toenail Clipping Collection of Participants in a Sister-Matched, Case-Control Study of Young-Onset Breast Cancer (n = 1,217 Sister Pairs), Sister Study (2003–2009) and Two Sister Study (2008–2010), United States
| Characteristic | Control Sisters (n = 1,214) | Case Sisters (n = 1,214) | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, yearsa | 47.1 (6.2) | 47.7 (4.1) | ||
| Older sisterb | 719 | 59 | 518 | 43 |
| Education level | ||||
| High school or less | 133 | 11 | 137 | 11 |
| Some college | 385 | 32 | 342 | 28 |
| College graduate | 403 | 33 | 416 | 34 |
| Graduate school | 293 | 24 | 313 | 26 |
| Missing | 0 | 6 | ||
| Body mass indexc | ||||
| ≤25.00 | 580 | 48 | 549 | 46 |
| 25.01–29.99 | 354 | 29 | 365 | 30 |
| ≥30.00 | 276 | 23 | 287 | 24 |
| Missing | 4 | 13 | ||
| Parity | ||||
| Nulliparous | 275 | 23 | 253 | 21 |
| 1 | 184 | 15 | 202 | 17 |
| 2 | 443 | 37 | 464 | 39 |
| ≥3 | 311 | 26 | 284 | 24 |
| Missing | 1 | 11 | ||
| Alcohol use | ||||
| Never/social drinker | 184 | 15 | 166 | 14 |
| Regular former drinker | 82 | 7 | 87 | 7 |
| Regular current drinker | 948 | 78 | 955 | 79 |
| Missing | 0 | 6 | ||
| Smoking status | ||||
| Never | 803 | 66 | 782 | 65 |
| Former | 315 | 26 | 366 | 30 |
| Current | 96 | 8 | 61 | 5 |
| Missing | 0 | 5 | ||
| Estimated NO2 exposure in 2006, ppb | ||||
| 0.00–6.40 | 298 | 25 | 299 | 26 |
| 6.41–9.00 | 292 | 24 | 297 | 26 |
| 9.01–12.70 | 297 | 25 | 296 | 25 |
| ≥12.71 | 314 | 26 | 270 | 23 |
| Missing | 13 | 52 | ||
| Estimated PM2.5 exposure in 2006, μg/m3 | ||||
| 0.00–8.50 | 293 | 24 | 286 | 25 |
| 8.51–10.70 | 303 | 25 | 301 | 26 |
| 10.71–12.30 | 297 | 25 | 299 | 26 |
| ≥12.31 | 310 | 26 | 277 | 24 |
| Missing | 11 | 52 | ||
| % of households in census tract below the poverty line in 2000 | ||||
| 0–3 | 287 | 24 | 296 | 25 |
| 4–5 | 268 | 22 | 275 | 24 |
| 6–10 | 386 | 32 | 373 | 32 |
| ≥11 | 268 | 22 | 224 | 19 |
| Missing | 5 | 46 | ||
| >30% of population is in urban area | 292 | 24 | 310 | 27 |
| Missing | 5 | 46 | ||
| Well water as main source of drinking water | 198 | 16 | 187 | 16 |
| Missing | 1 | 5 | ||
| Residence within 2 miles of factory | 137 | 11 | 144 | 12 |
| Missing | 7 | 12 | ||
| Ever occupationally exposed to metal dust | 35 | 3 | 58 | 5 |
| Missing | 16 | 18 | ||
| Ever occupationally exposed to metals | 73 | 6 | 83 | 7 |
| Missing | 16 | 18 | ||
Abbreviations: NO2, nitrogen dioxide; PM2.5, exposure to particles <2.5 μm in diameter.
a Values are expressed as the mean (standard deviation).
b There were 23 sets of twins.
c Weight (kg)/height (m)2.
Race/ethnicity was self-reported as non-Hispanic white by 90% of the sister pairs, and most case sisters (66%) were premenopausal at diagnosis. Case sisters were somewhat more likely to have been occupationally exposed to metal dust or metals, but overall exposure prevalence was low (5% and 7%, respectively, in the case group vs. 3% and 6%, respectively, in the control group; for any occupational metal exposure and breast cancer, odds ratio (OR) = 1.26, 95% confidence interval (CI): 0.91, 1.73, adjusting for age and education).
Observed and time-corrected metal levels are listed in Table 2. Because of the design, control sisters contributed toenail samples earlier than did case sisters, on average (median year of collection, 2007.8 for the control group, 2009.6 for the case group). Toenail clipping collection usually occurred after the case sister was diagnosed (median diagnosis year, 2006.8). When we applied the time corrections, we observed modest increases in the concentrations of most metals, with larger discrepancies seen for case sisters than control sisters, due to the more substantial time gap between their true and desired sample-collection time points (median gap for case sisters, 3.0 years, interquartile range, 2.4–3.8 years; median gap for control sisters, 0.82 years, interquartile range, 0.5–1.4 years).
Table 2.
Toenail Metal Levels in Women With Young-Onset Breast Cancer and Their Unaffected Sisters (n = 1,217 Sister Pairs), Sister Study (2003–2009) and Two Sister Study (2008–2010), United States
| Characteristic | Observed, median (IQR) | Corrected Back to Calendar Time of Case Diagnosis, With Corresponding Age Change, median (IQR) | ||
|---|---|---|---|---|
| Control Sisters (n = 1,214) | Case Sisters (n = 1,214) | Control Sisters (n = 1,214) | Case Sisters (n = 1,214) | |
| Year of collection | 2007.8 (2007.2–2008.3) | 2009.6 (2009.4–2010.0) | 2006.8 (2006.1–2007.4) | 2006.8 (2006.1–2007.4) |
| Age at collection | 46.9 (42.7–51.6) | 48.7 (45.3–50.9) | 45.9 (41.8–50.6) | 45.8 (42.6–48.0) |
| Baseline metal levels, μg/ga | ||||
| Arsenic | 0.049 (0.037–0.070) | 0.048 (0.034–0.069) | 0.048 (0.036–0.070) | 0.051 (0.036–0.073) |
| Cadmium | 0.006 (0.003–0.011) | 0.004 (0.003–0.008) | 0.006 (0.003–0.011) | 0.006 (0.003–0.011) |
| Cobalt | 0.008 (0.005–0.013) | 0.007 (0.004–0.013) | 0.007 (0.005–0.013) | 0.008 (0.005–0.014) |
| Chromium | 0.222 (0.116–0.487) | 0.208 (0.106–0.464) | 0.231 (0.119–0.504) | 0.240 (0.125–0.539) |
| Copper | 3.600 (3.090–4.382) | 3.704 (3.122–4.538) | 3.609 (3.081–4.397) | 3.754 (3.164–4.617) |
| Mercury | 0.099 (0.044–0.194) | 0.096 (0.042–0.200) | 0.100 (0.044–0.194) | 0.100 (0.043–0.202) |
| Molybdenum | 0.007 (0.005–0.012) | 0.007 (0.005–0.011) | 0.007 (0.005–0.011) | 0.008 (0.005–0.012) |
| Lead | 0.104 (0.057–0.188) | 0.087 (0.046–0.179) | 0.111 (0.060–0.205) | 0.117 (0.060–0.236) |
| Tin | 0.072 (0.043–0.132) | 0.071 (0.040–0.127) | 0.072 (0.043–0.135) | 0.079 (0.045–0.142) |
| Vanadium | 0.013 (0.006–0.026) | 0.013 (0.006–0.026) | 0.013 (0.006–0.026) | 0.015 (0.007–0.029) |
Abbreviation: IQR, interquartile range.
a Includes participants with levels below the limit of quantification (data reported as number of control sisters and case sisters, respectively, for each metal): arsenic (5; 9); cadmium (55; 75); cobalt (25; 22); chromium (114; 136); copper (1 case sister); mercury (119; 117); molybdenum (441; 487); lead (24; 33); tin (27; 37); vanadium (533; 552).
The number of participants with metal levels below the limit of quantification also is listed in Table 2, with vanadium (n = 533 control sisters, n = 552 case sisters) and molybdenum (n = 441 control sisters, n = 487 case sisters) having the most values below the specified limits. The highest between-metal correlations were observed for lead and cadmium (Spearman R = 0.55, P < 0.001; Web Table 1).
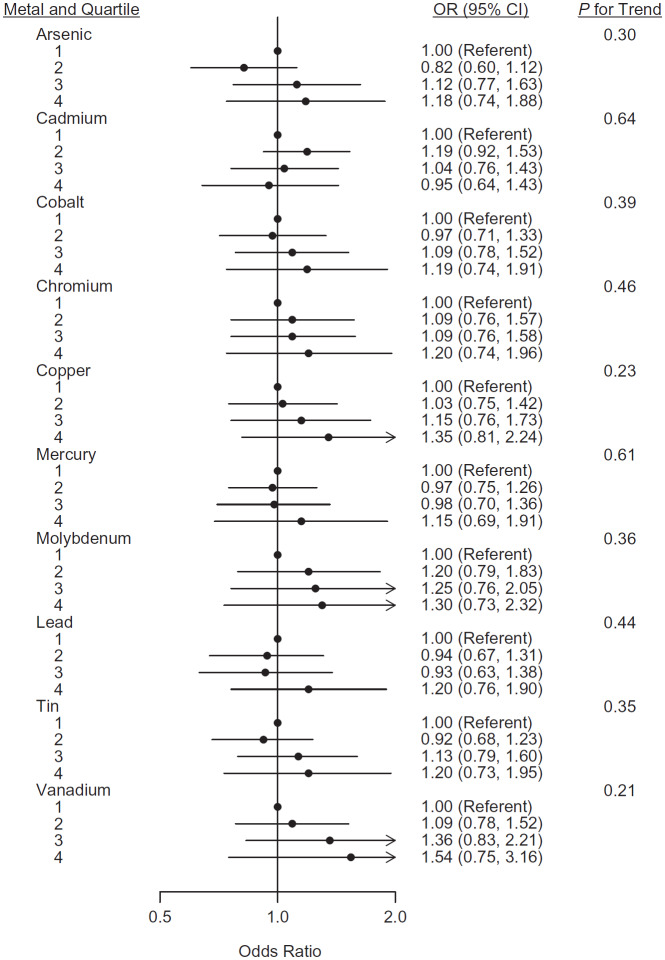
In the time-corrected analyses, we observed no statistically significant associations between any of the 10 metals and young-onset breast cancer (Figure 2; Web Table 2). The strongest odds ratio was for vanadium (for fourth vs. first quartile, OR = 1.54, 95% CI: 0.75, 3.16; P for trend = 0.21). The second quartile of cadmium was associated with increased risk (OR = 1.19, 95% CI: 0.92, 1.53) relative to the first quartile, but there was no evidence of a dose-response trend (P for trend = 0.64). The odds ratios for the association between cadmium and young-onset breast cancer were similar when we limited our analysis to never smokers (data not shown).
Figure 2.
Forest plot showing the association between time-corrected metal levels and young-onset breast cancer in participants of the Sister Study (2003–2009) and the Two Sister Study (2008–2010), United States. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using conditional logistic regression and adjusted for age and education. These results also are presented in Web Table 2.
Given the large proportion of values below the limit of quantification, we calculated odds ratios comparing the top 3 quartiles to the first quartile for both molybdenum and vanadium. These results were consistent with the previously reported findings (for molybdenum, OR = 1.25, 95% CI: 0.78, 1.99; for vanadium, OR = 1.29, 95% CI: 0.83, 2.00).
Results for the time-uncorrected analysis are listed in Web Table 2, as are the results from the analysis restricted to ER-positive or invasive breast cancers. The odds ratios from the uncorrected models indicated statistically significant inverse associations between these elements and young-onset breast cancer. This distortion is expected, given that case samples were usually collected later than control samples, and metal concentrations, particularly those of cadmium, chromium, and lead, decreased over the follow-up period. Also as expected, given that most cases of breast cancer were invasive and ER-positive, the results from the time-corrected analysis of these subtypes were similar to those of our overall analysis, with some elevated odds ratios but little evidence of dose-response trends.
Selenium did not modify the association between any of the metals and young-onset breast cancer (Table 3). The largest observed discrepancy was for copper, which was not associated with breast cancer among women with selenium levels no greater than 0.941 μg/g (OR = 1.02, 95% CI: 0.71, 1.46); contrary to expectation, however, copper was positively associated with breast cancer among women with selenium levels greater than 0.941 μg/g (OR = 1.49, 95% CI: 1.05, 2.13; P for trend = 0.04).
Table 3.
Modification by Selenium: Association Between Each Metal and Breast Cancer Within Categories of Selenium (Time-Corrected Metal Levels) (n = 1,217 Sister Pairs), Sister Study (2003–2009) and Two Sister Study (2008–2010), United States
| Trace Elementa | Overall | Selenium Level ≤ 0.941 μg/gb | Selenium Level > 0.941 μg/gb | P Value for Heterogeneity by Selenium Level | |||
|---|---|---|---|---|---|---|---|
| ORc | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Arsenic | 1.27 | 0.95, 1.70 | 1.24 | 0.91, 1.69 | 1.31 | 0.91, 1.89 | 0.75 |
| Cadmium | 0.90 | 0.67, 1.21 | 0.95 | 0.69, 1.30 | 0.85 | 0.59, 1.23 | 0.54 |
| Cobalt | 1.15 | 0.88, 1.52 | 1.15 | 0.78, 1.70 | 1.15 | 0.88, 1.52 | 1.00 |
| Chromium | 1.09 | 0.84, 1.42 | 1.03 | 0.77, 1.39 | 1.16 | 0.84, 1.59 | 0.53 |
| Copper | 1.22 | 0.89, 1.68 | 1.02 | 0.71, 1.46 | 1.49 | 1.05, 2.13 | 0.04 |
| Mercury | 1.07 | 0.77, 1.48 | 0.95 | 0.64, 1.41 | 1.19 | 0.84, 1.68 | 0.21 |
| Molybdenum | 1.16 | 0.83, 1.61 | 1.19 | 0.84, 1.67 | 1.12 | 0.75, 1.68 | 0.76 |
| Lead | 1.09 | 0.83, 1.44 | 1.15 | 0.81, 1.64 | 1.04 | 0.77, 1.39 | 0.55 |
| Tin | 1.21 | 0.90, 1.63 | 1.19 | 0.83, 1.72 | 1.23 | 0.89, 1.71 | 0.87 |
| Vanadium | 1.36 | 0.85, 2.17 | 1.43 | 0.86, 2.37 | 1.29 | 0.79, 2.12 | 0.58 |
Abbreviations: CI, confidence interval; OR, odds ratio.
a Time-corrected trace element levels. All models were adjusted for age and educational level. Data are shown for those with element levels greater than the median (0.944 μg/g); the reference group were those with element levels less than or equal to the median.
b The median value was 0.944 μg/g.
c Adjusted for selenium.
DISCUSSION
In this toenail biomarker study, we found little evidence to support a positive association between young-onset breast cancer and exposure to cadmium or any other metal. Results were similar for analyses limited to ER+ or invasive breast cancer. With the possible exception of copper, which went in the opposite direction to what we hypothesized, selenium did not strongly modify any of the observed associations.
Positive associations between urinary cadmium and breast cancer have been reported in several case-control studies (10–14), with 1 reporting strong associations with young-onset breast cancer in particular (11). However, those findings were not replicated in 2 prospective studies of urinary cadmium (15, 16) and 1 of circulating cadmium (17), leading us to speculate that the positive case-control study findings could be due to reverse causation, with patients with breast cancer having higher cadmium levels because of the effects of the disease, its treatment, or breast cancer–related lifestyle changes. Alternatively, the existing case-control and cohort studies could be capturing different time periods of exposure, with the urine-based case-control studies reflecting the risk associated with recent exposure, the urine-based cohort studies instead reflecting the risk associated with more long-term or lagged effects, and some mix of time periods being measured in the blood-based cohort study.
The results of our recent reliability study (22) did not support the idea that reverse causation could explain the discrepancy, because intervening breast cancer diagnoses had no measureable effect on the levels in samples from the same individuals years apart. However, in another reliability study, cadmium concentrations from urine and toenails collected at the same time were not correlated, indicating that the 2 measures may represent different time windows or types of exposure (32). Therefore, we cannot rule out reverse causation effects on urinary levels, particularly if breast cancer or cadmium affect kidney function, nor can we establish the most relevent time window and biomarker for assessing the effect of cadmium exposure on breast cancer risk.
With our time-corrected analysis, we predicted what metal concentrations would have been for both sisters just before the case sister’s cancer was diagnosed. The primary purpose of this correction was to remove the bias that was present because case sisters’ toenail clippings were collected after those of control sisters, and metal levels decreased systematically over time. Although it is possible that metals affect breast cancer risk by further influencing cells that are already damaged by carcinogenic processes begun years before, we also consider the time-corrected metal concentrations to be proxies for more long-term concentrations, which we previously demonstrated to be correlated over 4–10 years (22).
Despite a biological rationale supporting a relationship between metal exposure and breast cancer, exposure history is hard to characterize and the epidemiologic evidence to date is inconclusive. Because of its estrogenic properties demonstrated in laboratory assays (9), cadmium is the most studied metal in relation to breast cancer risk, but other metals have been examined. Many prior studies of metals and breast cancer have been ecologically (33–35) or occupation based (36, 37), but some have used exposure biomarkers. For example, Garland et al. (38) measured toenail arsenic, copper, and chromium levels in a nested case-control sample within the Nurses’ Health Study and found no association between any of the measured elements and breast cancer risk (mean concentrations of 0.12, 5.68, and 1.69 μg/g for arsenic, copper, and chromium, respectively, among control subjects). McElroy et al. (39) observed a positive association between urinary lead levels (median concentration, 0.64 μg/L in control subjects) and breast cancer risk in a population-based, case-control study, though this association was attenuated when women taking nonsteroidol aromatase inhibitors (and thus more prone to bone loss) were excluded. There was no association between blood lead level and breast cancer risk in any of the 3 prospective cohorts analyzed together (range of median concentrations, 25–88 μg/L).
All our models were adjusted for age and education only. Although we collected data on other covariates, we did not consider any of them to be important confounders, because they either were not likely to be related to toenail metal levels (e.g., parity), not strongly related to breast cancer risk (e.g., smoking), or were potentially only related to breast cancer because they were a source of metal exposures (e.g., occupational metal exposure, estimated exposure to particles <2.5 μm in diameter).
Metallic toenail clippers could have contaminated the clippings. We attempted to minimize potential contamination by washing samples before analysis, but some exposure misclassification may still be present. Exposure misclassification would be nondifferential by case status and thus likely to produce bias toward the null. Lack of data on metal speciation (40) is also a source of misclassification that is likely to bias our results toward the null. We were only able to measure all metal species combined, despite evidence that some metal species are known carcinogens (e.g., chromium VI) and others are not (e.g., chromium III) (1, 2).
We applied time-correction methods in an attempt to recapture a more relevant time period and address discrepancies in the timing of sample collections between case and control sisters. Although the results of our reliability study document the need for such correction, given the reductions in levels of toxic metals over calendar time, we cannot quantify the accuracy of our predictive models. Misclassification, therefore, is likely and could be differential by case status because the time gap between true and desired sample collection time points was larger for case sisters than control sisters because of the study design. The magnitude and direction of this potential bias are uncertain. Despite these limitations, our time-corrected analysis is a strength of this study and we emphasize that reliability studies such as the 1 we conducted (22) are crucial to understanding time trends in environmental factors. This level of understanding is particularly important for biomarker studies conducted using retrospective study designs in which biological samples are collected after patients have received a diagnosis and have been treated.
Young-onset breast cancer deserves to be studied separately from older-onset breast cancer because of its distinct tumor characteristics and risk-factor profile. Element concentrations in toenails are useful as biomarkers for environmental research, because of their temporal stability, their ability to capture recent yet stable exposure of many metals simultaneously, and the ease with which toenail clippings can be collected, shipped, and stored. Although we did not observe associations between levels of any metals and young-onset breast cancer in this analysis, we believe there is sufficient epidemiologic evidence to support additional studies of this topic, particularly if they assess possible genetic and epigenetic modifiers or can measure multiple exposure biomarkers prospectively and at several time points.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Epidemiology Branch, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina (Katie M. O’Brien, Alexandra J. White, Dale P. Sandler); Department of Earth Sciences, Dartmouth College, Hanover, New Hampshire (Brian P. Jackson); Department of Epidemiology and Children’s Environmental Health and Disease Prevention Research Center at Dartmouth, Geisel School of Medicine at Dartmouth, Lebanon, New Hampshire (Margaret R. Karagas); and Biostatistics and Computational Biology Branch, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina (Katie M. O’Brien, Clarice R. Weinberg).
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (grants Z01-ES044005 to D.P.S. and Z01-ES102245 to C.R.W.), Susan G. Komen for the Cure (grant FAS0703856 to C.R.W.), Superfund (grant P42ES007373 to M.R.K. and B.P.J.), and the National Cancer Center Support (grant 5P30CA023108 to M.R.K. and B.P.J.).
We thank Dr. Jacob Kresovich and Dr. Stephani Kim for their comments on an early draft of this paper.
Conflict of interest: none declared.
Abbreviations
- CI
confidence interval
- ER
estrogen receptor
- OR
odds ratio
REFERENCES
- 1. International Agency for Research on Cancer . Arsenic, Metals, Fibres, and Dusts. Vol 100 C. Lyon, France: International Agency for Research on Cancer; 2012.
- 2. International Agency for Research on Cancer . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. http://monographs.iarc.fr/ENG/Classification/. Published 2018. Accessed April 2, 2018.
- 3. Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26(20):3324–3330. [DOI] [PubMed] [Google Scholar]
- 4. Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. [DOI] [PubMed] [Google Scholar]
- 5. Anderson WF, Matsuno RK, Sherman ME, et al. Estimating age-specific breast cancer risks: a descriptive tool to identify age interactions. Cancer Causes Control. 2007;18(4):439–447. [DOI] [PubMed] [Google Scholar]
- 6. Bertrand KA, Bethea TN, Adams-Campbell LL, et al. Differential patterns of risk factors for early-onset breast cancer by ER status in African American women. Cancer Epidemiol Biomarkers Prev. 2016;26(2):270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garcia-Morales P, Saceda M, Kenney N, et al. Effect of cadmium on estrogen receptor levels and estrogen-induced responses in human breast cancer cells. J Biol Chem. 1994;269(24):16896–16901. [PubMed] [Google Scholar]
- 8. Johnson MD, Kenney N, Stoica A, et al. Cadmium mimics the in vivo effects of estrogen in the uterus and mammary gland. Nat Med. 2003;9(8):1081–1084. [DOI] [PubMed] [Google Scholar]
- 9. Luevano J, Damodaran C. A review of molecular events of cadmium-induced carcinogenesis. J Environ Pathol Toxicol Oncol. 2014;33(3):183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gallagher CM, Chen JJ, Kovach JS. Environmental cadmium and breast cancer risk. Aging (Albany NY). 2010;2(11):804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McElroy JA, Shafer MM, Trentham-Dietz A, et al. Cadmium exposure and breast cancer risk. J Natl Cancer Inst. 2006;98(12):869–873. [DOI] [PubMed] [Google Scholar]
- 12. Nagata C, Nagao Y, Nakamura K, et al. Cadmium exposure and the risk of breast cancer in Japanese women. Breast Cancer Res Treat. 2013;138(1):235–239. [DOI] [PubMed] [Google Scholar]
- 13. Strumylaite L, Kregzdyte R, Bogusevicius A, et al. Association between cadmium and breast cancer risk according to estrogen receptor and human epidermal growth factor receptor 2: epidemiological evidence. Breast Cancer Res Treat. 2014;145(1):225–232. [DOI] [PubMed] [Google Scholar]
- 14. Larsson SC, Orsini N, Wolk A. Urinary cadmium concentration and risk of breast cancer: a systematic review and dose-response meta-analysis. Am J Epidemiol. 2015;182(5):375–380. [DOI] [PubMed] [Google Scholar]
- 15. Adams SV, Shafer MM, Bonner MR, et al. Urinary cadmium and risk of invasive breast cancer in the Women’s Health Initiative. Am J Epidemiol. 2016;183(9):815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eriksen KT, McElroy JA, Harrington JM, et al. Urinary cadmium and breast cancer: a prospective Danish cohort study. J Natl Cancer Inst. 2017;109(2):djw204. [DOI] [PubMed] [Google Scholar]
- 17. Gaudet MM, Deubler EL, Kelly RS, et al. Blood levels of cadmium and lead in relation to breast cancer risk in three prospective cohorts. Int J Cancer. 2019;144(5):1010–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Järup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 2009;238(3):201–208. [DOI] [PubMed] [Google Scholar]
- 19. O’Brien KM, Upson K, Buckley JP. Lipid and creatinine adjustment to evaluate health effects of environmental exposures. Curr Environ Health Rep. 2017;4(1):44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grashow R, Zhang J, Fang SC, et al. Toenail metal concentration as a biomarker of occupational welding fume exposure. J Occup Environ Hyg. 2014;11(6):397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yaemsiri S, Hou N, Slining MM, et al. Growth rate of human fingernails and toenails in healthy American young adults. J Eur Acad Dermatol Venereol. 2010;24(4):420–423. [DOI] [PubMed] [Google Scholar]
- 22. O’Brien KM, White AJ, Sandler DP, et al. Do post-breast cancer diagnosis toenail trace element concentrations reflect pre-diagnostic concentrations? Epidemiology. 2019;30(1):112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Darbre PD. Metalloestrogens: an emerging class of inorganic xenoestrogens with potential to add to the oestrogenic burden of the human breast. J Appl Toxicol. 2006;26(3):191–197. [DOI] [PubMed] [Google Scholar]
- 24. Wei XL, He JR, Cen YL, et al. Modified effect of urinary cadmium on breast cancer risk by selenium. Clin Chim Acta. 2015;438:80–85. [DOI] [PubMed] [Google Scholar]
- 25. Jan AT, Azam M, Siddiqui K, et al. Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. Int J Mol Sci. 2015;16(12):29592–29630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sandler DP, Hodgson ME, Deming-Halverson SL, et al. The Sister Study: baseline methods and participant characteristics. Environ Health Perspect. 2017;125(12):127003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fei C, Deroo LA, Sandler DP, et al. Fertility drugs and young-onset breast cancer: results from the Two Sister Study. J Natl Cancer Inst. 2012;104(13):1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsoi MF, Cheung CL, Cheung TT, et al. Continual decrease in blood lead level in Americans: United States National Health Nutrition and Examination Survey 1999–2014. Am J Med. 2016;129(11):1213–1218. [DOI] [PubMed] [Google Scholar]
- 29. Ruiz-Hernandez A, Navas-Acien A, Pastor-Barriuso R, et al. Declining exposures to lead and cadmium contribute to explaining the reduction of cardiovascular mortality in the US population, 1988–2004. Int J Epidemiol. 2017;46(6):1903–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Environmental Protection Agency . Ambient concentrations of lead. Washington, DC: Environmental Protection Agency; 2017. https://cfpub.epa.gov/roe/indicator.cfm?i=5. Accessed March 1, 2018.
- 31. Sánchez-Rodríguez JE, Bartolomé M, Cañas AI, et al. Anti-smoking legislation and its effects on urinary cotinine and cadmium levels. Environ Res. 2015;136:227–233. [DOI] [PubMed] [Google Scholar]
- 32. White AJ, O’Brien KM, Jackson BP, et al. Urine and toenail cadmium levels in pregnant women: a reliability study. Environ Int. 2018;118:86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Núñez O, Fernández-Navarro P, Martín-Méndez I, et al. Arsenic and chromium topsoil levels and cancer mortality in Spain. Environ Sci Pollut Res Int. 2016;23(17):17664–17675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Coyle YM, Hynan LS, Euhus DM, et al. An ecological study of the association of environmental chemicals on breast cancer incidence in Texas. Breast Cancer Res Treat. 2005;92(2):107–114. [DOI] [PubMed] [Google Scholar]
- 35. Pan SY, Morrison H, Gibbons L, et al. Breast cancer risk associated with residential proximity to industrial plants in Canada. J Occup Environ Med. 2011;53(5):522–529. [DOI] [PubMed] [Google Scholar]
- 36. Loomis DP, Wolf SH. Mortality of workers at a nuclear materials production plant at Oak Ridge, Tennessee, 1947–1990. Am J Ind Med. 1996;29(2):131–141. [DOI] [PubMed] [Google Scholar]
- 37. Boice JD Jr, Mumma MT, Blot WJ. Cancer and noncancer mortality in populations living near uranium and vanadium mining and milling operations in Montrose County, Colorado, 1950–2000. Radiat Res. 2007;167(6):711–726. [DOI] [PubMed] [Google Scholar]
- 38. Garland M, Morris JS, Colditz GA, et al. Toenail trace element levels and breast cancer: a prospective study. Am J Epidemiol. 1996;144(7):653–660. [DOI] [PubMed] [Google Scholar]
- 39. McElroy JA, Shafer MM, Gangnon RE, et al. Urinary lead exposure and breast cancer risk in a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2008;17(9):2311–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Templeton DM. Speciation in metal toxicity and metal-based therapeutics. Toxics. 2015;3(2):170–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.