Abstract
Improved therapeutics and supportive care in hospitals have helped reduce mortality from COVID-19. However, there is limited evidence as to whether nursing home residents, who account for a disproportionate share of COVID-19 deaths and are often managed conservatively in the nursing home instead of being admitted to the hospital, have experienced similar mortality reductions. In this study we examined changes in thirty-day mortality rates between March and November 2020 among 12,271 nursing home residents with COVID-19. We found that adjusted mortality rates significantly declined from a high of 20.9 percent in early April to 11.2 percent in early November. Mortality risk declined for residents with both symptomatic and asymptomatic infections and for residents with both high and low clinical complexity. The mechanisms driving these trends are not entirely understood, but they may include improved clinical management within nursing homes, improved personal protective equipment supply and use, and genetic changes in the virus.
The COVID-19 pandemic has had devastating effects on nursing homes and other long-term care facilities, causing roughly 1.2 million infections and 147,000 deaths in this sector in the US as of early February 2021.1 The communal living environment combined with the overall frailty of residents, who generally must be in regular close proximity with staff for personal care, make this population extremely susceptible to adverse outcomes from COVID-19.2–4 Since the start of the COVID-19 pandemic, long-term care residents have consistently accounted for roughly 40 percent of total US deaths.1
COVID-19 mortality rates have declined in the general population as a result of factors that may have limited, if any, influence on mortality rates for nursing home residents. For example, more widespread testing has allowed for increased detection of SARS-CoV-2 in younger people, who are less susceptible to severe disease.5 Yet this phenomenon would not affect mortality rates in the nursing home population, which is primarily composed of frail older adults. Advances in therapeutics and supportive care have improved survival in hospitalized patients with severe infections. These include systemic corticosteroids, remdesivir, monoclonal antibodies, and prone positioning, as well as advanced non-invasive oxygen therapy.6–8 However, such therapies are generally not available outside of the hospital setting and have not been systematically tested or widely used in medically frail older adults.7,8 Because of the poor survival of this population after hypoxemic respiratory failure and prolonged intensive care unit admission, the mainstay of clinical management for nursing home residents with COVID-19 has been conservative care within the nursing home.8
Given these significant differences between the nursing home population and the general population, there is a need to examine changes in mortality risk over time for nursing home residents specifically. A state-level analysis by the Kaiser Family Foundation found that the average number of new COVID-19 deaths in long-term care facilities declined from April through August 2020.9 However, factors influencing underlying resident-level mortality risk cannot be measured in publicly reported, aggregate data.
To address this evidence gap, we used detailed, resident-level data from a large, multistate nursing home operator to examine the association of calendar time with short-term mortality after COVID-19 diagnosis. Our analysis tested two hypotheses that may explain changes in mortality risk over time in this population. First, we assessed whether increased detection of asymptomatic cases, which would add residents with less severe infection to the denominator, has led to reduced mortality rates for the population overall. Second, we examined whether differences in the case-mix of residents infected in earlier versus later months resulted in lower mortality. Findings from this study will improve understanding of mortality risk in this vulnerable population and help identify both intrinsic and extrinsic factors that may have contributed to changes in mortality risk over time.
Study Data And Methods
DATA SOURCES
Clinical data were obtained for 282 nursing homes in twenty-four states, owned by a large provider of postacute and long-term care services. All facilities had at least one COVID-19 resident case as of November 15, 2020. The main data sources for this analysis included electronic medical records (EMRs), facility infection tracking logs, and Minimum Data Set resident assessments. Auxiliary data sources used in secondary analyses are described in the online appendix.10 EMRs were the source for daily resident census data and relevant nursing documentation including vital sign records and structured notes documenting changes in condition. Infection tracking logs are maintained by each facility and include all SARS-CoV-2 testing dates and results. Minimum Data Set assessments are administered to all residents of Medicare- or Medicaid-certified nursing homes (including the studied facilities) both on admission and serially thereafter until discharge. The Minimum Data Set captures resident demographics and a variety of clinical elements including active diagnoses and validated measures of functional and cognitive status.
POPULATION
We identified all residents across the operator’s nursing homes who were infected with SARS-CoV-2, as confirmed by reverse transcriptase polymerase chain reaction (PCR) testing between March 16 and November 15, 2020. Reasons for testing included new-onset symptoms, potential exposure, and surveillance during facilitywide or unit-based point prevalence surveys.
OUTCOME
The primary outcome was all-cause mortality within thirty days of confirming SARS-CoV-2 infection. We identified both resident deaths occurring in the nursing home from Minimum Data Set discharge assessments and deaths occurring outside the facility (for example, in a hospital) from the EMR resident census.
MAIN EXPLANATORY VARIABLE
The main explanatory variable was the date a resident first tested positive for SARS-CoV-2. To facilitate interpretation of adjusted analyses, we categorized diagnosis dates into sixteen semimonthly dummy variables capturing the set of diagnosis dates (from March 16 through November 15) in our study population.
ASYMPTOMATIC VERSUS SYMPTOMATIC CLASSIFICATION
Nurses conducted assessments including vital signs on all residents two to three times daily and documented any new symptoms in structured notes in the EMR documenting changes in condition. Relevant symptoms included cough, sore throat, nasal congestion, rhinorrhea, fever (temperature of 100 degrees F or higher), shortness of breath, chest congestion, nausea, vomiting, diarrhea, anosmia, malaise, confusion, tachycardia, and hypoxia (oxygen saturation below 92 percent or a 3-percentage-point decline from baseline). We classified residents infected with SARS-CoV-2 as asymptomatic if they exhibited none of these symptoms from the five days before their first positive PCR test up to fourteen days after that test. Residents with symptoms in this window were classified as symptomatic.
COVARIATES
We controlled for factors known to be associated with COVID-19-related mortality that may vary in prevalence over time with fluctuations in nursing home case-mix. These factors included age, comorbidities, cognitive and functional impairment, postacute versus long-stay resident status, and symptomatic versus asymptomatic infection status. To capture illness severity, we also controlled for whether the resident experienced new-onset fever, tachycardia, shortness of breath, or hypoxia.11 Additional demographic covariates included sex and race (classified as White, Black, and other).
We identified baseline chronic conditions from the Minimum Data Set assessment nearest to the initial positive SARS-CoV-2 test, including hypertension, diabetes mellitus, heart failure, coronary artery disease, chronic obstructive pulmonary disease or asthma, chronic kidney disease, and dementia. Functional and cognitive impairment were measured with the Morris activities of daily living (ADL) scale12 and the Cognitive Function Scale (CFS),13 respectively. The ADL score ranges from 0 to 28 (higher values indicate greater functional impairment) and was divided into quartiles to facilitate interpretation. The CFS ranges from 1 (intact cognitive function) to 4 (highly impaired) and was operationalized as four dummy variables.
STATISTICAL ANALYSIS
We illustrated the distribution of COVID-19-related thirty-day mortality rates over time by dividing residents into semimonthly diagnosis date periods, which included the second half of March through the first half of November. We also graphically assessed the relationship of thirty-day mortality with the entire distribution of diagnosis dates using locally weighted scatterplot smoothing estimates of trend. This method computes the probability of mortality separately on each diagnosis date, using a mean of nearby data points with greater weight on the nearest points. Because we expected asymptomatic infection rates to increase over time, we estimated separate trend lines for symptomatic and asymptomatic cases. As with the prevalence of asymptomatic infection, resident case-mix may also vary with time, particularly as residents die. Accordingly, we estimated separate trend lines for residents with high and low levels of functional and cognitive impairment—known risk factors for COVID-19-related mortality that indicate clinical complexity—based on ADL and CFS scores.14 ADL scores of 19 and higher (the upper two quartiles of scores) and CFS scores of 3 and 4 were used to indicate advanced functional and cognitive impairment, respectively.
We used Poisson regression with cluster-robust standard errors to calculate the adjusted risk of thirty-day mortality associated with COVID-19 diagnosis date.15 Instead of assuming linearity or a particular functional form for the relationship between diagnosis date and mortality, we calculated mortality risk for all semi-monthly periods relative to the first half of April, an early time point that includes a large number of residents. The regression model was estimated separately for the overall cohort, residents with symptomatic SARS-CoV-2 infection, and residents with asymptomatic infection.
All regression models included standard errors clustered at the nursing home level. In addition to the relative risks, we derived adjusted mortality rates per diagnosis period from the Poisson model, using the marginal standardization form of predictive margins. Analyses were conducted using Stata MP, version 16.0. Null hypotheses were tested assuming a two-sided type I error rate of 0.05.
SECONDARY ANALYSES AND ROBUSTNESS CHECKS
As a robustness check, we added nursing home fixed effects to our primary regression model to obtain the within-facility association between diagnosis date and thirty-day COVID-19-related mortality. This approach effectively compared mortality risk between residents living in the same nursing home who were diagnosed in early versus later months and thus controlled for important baseline facility attributes (for example, general level of quality). For this analysis we used a linear probability model because the inclusion of fixed effects may bias point estimates derived from a nonlinear model such as Poisson regression.16
We conducted two secondary analyses. First, we examined the relationship between nursing home–level and community-level case fatality rates (the number of deaths among the diagnosed population) over time. We described how we calculated the community case fatality rates in the appendix.10 Second, we assessed trends in hospitalization rates to gauge potential changes in the clinical care of residents with COVID-19. We used Minimum Data Set discharge records to indicate whether residents were hospitalized within thirty days of the COVID-19 diagnosis date, mirroring the mortality follow-up window. We then estimated the hospitalization rate for each period with the same risk-adjustment model used for the main mortality analysis.
LIMITATIONS
This study had two primary limitations. First, our data came from a single, multifacility, long-term care provider with a particular concentration in the Northeast. Mortality trends in other samples of nursing home residents maybe dissimilar. Second, because of the observational study design, significant potential for residual confounding remains, and observed associations should not be interpreted as causal. The purpose of this analysis was to determine whether there were differences in COVID-19-related mortality rates over time and whether potential differences are accounted for by observable changes in resident case-mix and asymptomatic infection rates. However, it is possible that temporally different mortality risk may be due to unobserved changes in resident case-mix or survival bias. More generally, our analyses did not pinpoint potential mechanisms that may underlie temporal shifts in COVID-19-related mortality.
Study Results
The sample included 12,271 nursing home residents confirmed to have SARS-CoV-2 infection by PCR testing between March 16 and November 15, 2020. Residents’ median age was seventy-nine (interquartile range: 69–88), and 61 percent of residents were female. The racial composition of the sample was 70 percent White, 16 percent Black, and 14 percent other (exhibit 1). The mean baseline ADL score was 16.7 (standard deviation: 6.2), 38 percent had advanced cognitive impairment (CFS score of 3 or 4), and 13 percent were receiving postacute care. The prevalence of heart failure, coronary artery disease, chronic obstructive pulmonary disease or asthma, and chronic kidney disease ranged between 22 percent and 26 percent. Almost half of residents exhibited one or more COVID-19-related symptoms, including 24 percent with fever, 8 percent with tachycardia, 8 percent with hypoxia, and 5 percent with shortness of breath.
EXHIBIT 1.
Characteristics of residents with COVID-19 in a sample of US nursing homes, March 16-November 15, 2020
| Characteristics | Number/median/ meana |
Percent/ IQR/SDb |
|---|---|---|
| Median age, years (IQR) | 79 | 69–88 |
| Age, years | ||
| Less than 65 | 1,916 | 16 |
| 65–69 | 1,239 | 10 |
| 70–74 | 1,538 | 13 |
| 75–79 | 1,611 | 13 |
| 80–84 | 1,726 | 14 |
| 85–89 | 1,814 | 15 |
| 90 or older | 2,304 | 19 |
| Female sex | 7,538 | 61 |
| Race | ||
| White | 8,586 | 70 |
| Black | 1,978 | 16 |
| Other | 1,707 | 14 |
| Mean ADL score (SD)c | 16.7 | 6.2 |
| ADL dependency score quartile | ||
| 0–13 | 3,069 | 25 |
| 14–18 | 3,057 | 25 |
| 20–21 | 2,766 | 23 |
| 22–28 | 3,379 | 28 |
| CFS score | ||
| 1 | 4,761 | 39 |
| 2 | 2,796 | 23 |
| 3 | 3,558 | 29 |
| 4 | 1,064 | 9 |
| Postacute patient | 1,631 | 13 |
| Chronic conditions | ||
| Dementia | 5,599 | 46 |
| Heart failure | 2,691 | 22 |
| Coronary artery disease | 2,816 | 23 |
| COPD or asthmad | 2,987 | 24 |
| Chronic kidney diseasee | 3,155 | 26 |
| Hypertension | 9,526 | 78 |
| Diabetes | 4,775 | 39 |
| Symptoms at presentation | ||
| Any symptomf | 5,645 | 46 |
| Fever | 2,936 | 24 |
| Tachycardia | 959 | 8 |
| Hypoxia | 974 | 8 |
| Shortness of breath | 567 | 5 |
SOURCE Authors’ analysis of resident-level data from 282 nursing homes operated by a large long-term care provider.
NOTES N = 12,271 residents. IQR is interquartile range. SD is standard deviation. ADL is activities of daily living. CFS is Cognitive Function Scale. COPD is chronic obstructive pulmonary disease.
Number unless otherwise indicated.
Percent unless otherwise indicated.
Range: 0–28.
Classified in the Minimum Data Set as “asthma, chronic obstructive pulmonary disease, chronic bronchitis, and restrictive lung diseases.”
Classified in the Minimum Data Set as "renal insufficiency, renal disease, or end-stage renal disease."
Residents were classified as symptomatic if any of the following symptoms were present during the five days before their first positive test or during the fourteen days afterward: cough, sore throat, nasal congestion, rhinorrhea, fever, shortness of breath, chest congestion, nausea, vomiting, diarrhea, anosmia, malaise, confusion, tachycardia, and hypoxia. See the Study Data And Methods section for further details.
Residents were disproportionately located in Northeastern states (appendix exhibit A1);10 about 65 percent of the sample resided in New Jersey, Pennsylvania, Massachusetts, Connecticut, or Maryland. The facilities of the nursing home operator were also disproportionately located in New England and Mideastern states compared with nursing homes nationally (appendix exhibit A2).10 In addition, the sample facilities were larger (120 versus 99 median beds), more heavily financed by Medicaid (73 percent versus 64 percent), and less likely to be located in rural counties (15 percent versus 29 percent).
Exhibit 2 lists trends in crude thirty-day mortality rates for the overall sample, stratified according to asymptomatic versus symptomatic infection status (locally weighted scatterplot smoothing estimated trend lines are in appendix exhibit A3).10 Also included in the exhibit is the proportion of residents who were asymptomatic across semimonthly diagnosis periods. For the overall sample, crude mortality rates fell from 26.4 percent in the second half of March to 10.0 percent during the first half of November. Concurrently, the proportion of residents with an asymptomatic infection rose from 24.9 percent in the second half of March to 69.2 percent in the first half of November. However, although mortality rates were higher among residents with symptomatic infections, for both groups, diagnosis date was inversely correlated with thirty-day mortality. Among residents with symptomatic infections, the mortality rate in the second half of March was 32.2 percent and fell to 10.2 percent in the first half of November. Among residents with asymptomatic infections, the mortality rate in the second half of March was 9.1 percent, rose to 16.7 percent in the first half of April, and fell to 9.9 percent in the first half of November.
EXHIBIT 2.
Trends in crude 30-day COVID-19-related mortality and asymptomatic infection rates among residents in a sample of US nursing homes, 2020
| Date of diagnosis (2020) | No. of residents |
30-day mortality rate (%) |
Infections that were asymptomatic (%) |
|||
|---|---|---|---|---|---|---|
| Asymptomatic | Symptomatic | Overall | ||||
| March 16–31 | 265 | 9.1 | 32.2 | 26.4 | 24.9 | |
| April 1–15 | 1,756 | 16.7 | 25.4 | 22.8 | 29.0 | |
| April 16–30 | 2,553 | 11.4 | 22.3 | 17.7 | 41.8 | |
| May 1–15 | 2,483 | 12.1 | 20.9 | 15.2 | 64.2 | |
| May 16–31 | 721 | 7.8 | 21.5 | 13.5 | 58.9 | |
| June 1–15 | 386 | 7.1 | 12.8 | 9.3 | 61.9 | |
| June 16–30 | 441 | 6.4 | 16.5 | 10.4 | 60.1 | |
| July 1–15 | 561 | 5.8 | 14.8 | 8.4 | 71.1 | |
| July 16–31 | 463 | 7.8 | 15.6 | 10.8 | 61.1 | |
| August 1–15 | 344 | 5.9 | 8.5 | 7.0 | 58.7 | |
| August 16–31 | 424 | 8.8 | 16.2 | 11.8 | 59.2 | |
| September 1–15 | 292 | 8.0 | 16.4 | 11.3 | 60.3 | |
| September 16–30 | 295 | 8.9 | 22.0 | 12.5 | 72.2 | |
| October 1–15 | 288 | 9.0 | 15.4 | 10.8 | 73.3 | |
| October 16–31 | 519 | 10.6 | 13.2 | 11.4 | 69.7 | |
| November 1–15 | 351 | 9.9 | 10.2 | 10.0 | 69.2 | |
SOURCE Authors’ analysis of resident-level data from 282 nursing homes operated by a large long-term care provider.
NOTE Symptomatic and asymptomatic classifications are described in the Study Data And Methods section.
Exhibit 3 illustrates trends in thirty-day COVID-19-related mortality among residents with and without advanced functional impairments (corresponding point estimates are in appendix exhibit A4).10 Although residents with greater cognitive and ADL impairment had higher mortality rates compared with residents who were less impaired, diagnosis date was inversely correlated with thirty-day mortality across all groups. Among the most impaired group of residents (CFS ≥3, ADL ≥19), the thirty-day mortality rate fell from 41.0 percent in the second half of March to 15.8 percent in the first half of November. Among the group of residents with the lowest levels of impairment (CFS <3, ADL <19), mortality rates fell from 18.4 percent in the second half of March to 8.1 percent in the first half of November.
Exhibit 3. Trends in daily and semimonthly crude 30-day COVID-19 mortality rates, by cognitive and functional status, among residents of a sample of US nursing homes, 2020.
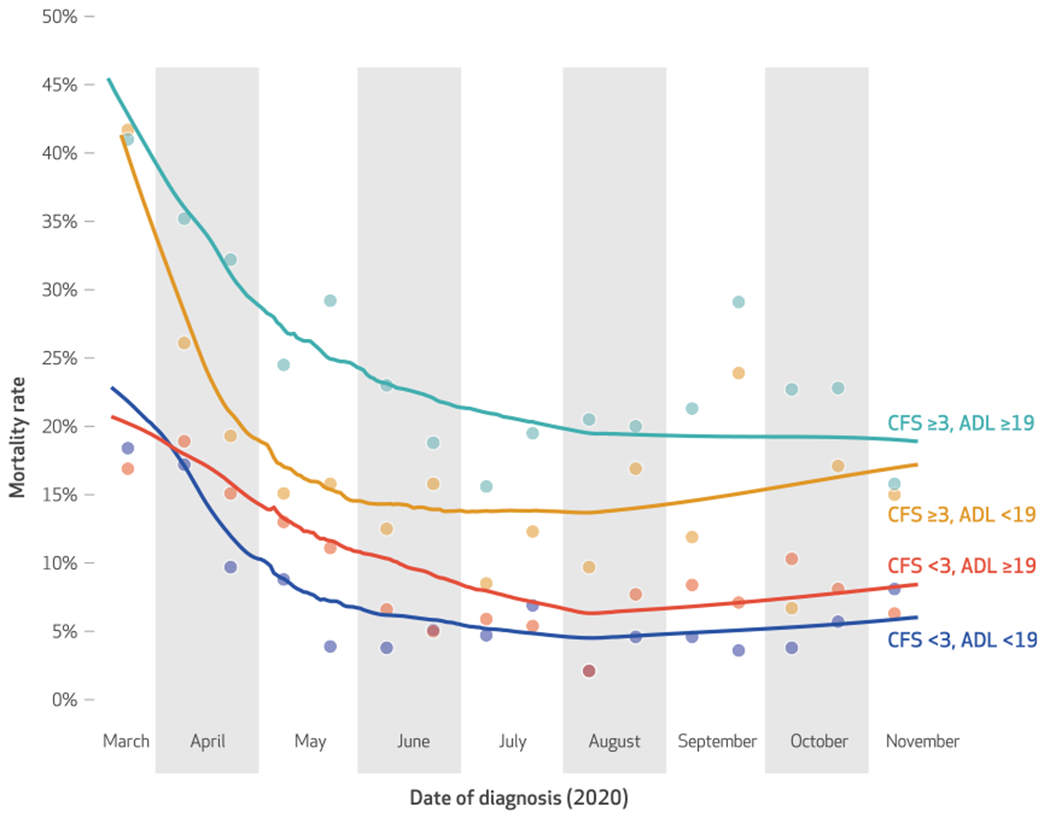
SOURCE Authors’ analysis of resident-level data from 282 nursing homes operated by a large, long-term care provider. NOTES Trend lines were estimated with locally weighted scatterplot smoothing, using all data points from the analytic sample (daily from March 16 through November 15). A scatterplot of unadjusted semimonthly mortality rates is superimposed. Cognitive Function Scale (CFS) scores range from 1 to 4; scores of 3 and 4 indicate advanced cognitive impairment. The Morris activities of daily living (ADL) score signifies the extent to which residents are dependent in seven activities of daily living, denoted by a higher score. An ADL score of 19 represents the seventy-fifth-percentile value of the analytic sample and reflects a substantial level of functional impairment.
exhibit 4 presents adjusted thirty-day mortality rates and the adjusted relative risk of mortality associated with semimonthly diagnosis date period. Point estimates for regression model covariates are in appendix exhibit A5.10 In the overall cohort, the mortality rate among residents diagnosed with COVID-19 in the second half of March was 19.3 percent. In the following period, the first half of April, the adjusted mortality rate (20.9 percent) did not significantly differ. However, COVID-19-related mortality risk was significantly lower in all subsequent semimonthly periods. Among residents diagnosed with COVID-19 in the first half of November, the adjusted thirty-day mortality rate was 11.2 percent, and their mortality risk was almost 50 percent lower than that of residents diagnosed in the first half of April (relative risk: 0.53). In stratified analyses, adjusted mortality rates also declined significantly for residents with either asymptomatic or symptomatic infections in almost all periods after the first half of April (exhibit 4). Trends in adjusted mortality rates were similar when derived from within-facility linear probability models with nursing home fixed effects, indicating that even within facilities, residents diagnosed in later versus earlier months had lower mortality risk (appendix exhibit A6).10
EXHIBIT 4.
Adjusted relative risks (RRs) and adjusted semimonthly rates of 30-day COVID-19-related mortality among residents in a sample of US nursing homes, 2020
| Full sample |
Symptomatic residents |
Asymptomatic residents |
||||
|---|---|---|---|---|---|---|
| Date of diagnosis (2020) | 30-day mortality rate | RR | 30-day mortality rate | RR | 30-day mortality rate | RR |
| March 16–31 | 19.3 | 0.92 | 27.3 | 1.01 | 8.3 | 0.49 |
| April 1–15 | 20.9 | Ref | 27.0 | Ref | 16.8 | Ref |
| April 16–30 | 16.5 | 0.79** | 22.6 | 0.84** | 11.2 | 0.67** |
| May 1–15 | 15.5 | 0.74** | 20.9 | 0.77** | 10.9 | 0.65 |
| May 16–31 | 13.3 | 0.64** | 19.5 | 0.72** | 8.2 | 0.49** |
| June 1–15 | 10.2 | 0.49** | 14.2 | 0.53** | 6.9 | 0.41** |
| June 16–30 | 10.9 | 0.52** | 16.2 | 0.60** | 7.0 | 0.42** |
| July 1-15 | 10.3 | 0.49** | 16.7 | 0.62** | 6.3 | 0.38** |
| July 16–31 | 11.7 | 0.56** | 15.7 | 0.58** | 8.4 | 0.50** |
| August 1–15 | 7.2 | 0.35** | 7.4 | 0.27** | 7.0 | 0.42** |
| August 16–31 | 10.7 | 0.51** | 13.5 | 0.5** | 7.8 | 0.46** |
| September 1–15 | 11.4 | 0.54** | 15.4 | 0.57** | 8.1 | 0.48** |
| September 16–30 | 13.3 | 0.64** | 18.4 | 0.68** | 9.0 | 0.53** |
| October 1–15 | 11.4 | 0.55** | 12.7 | 0.47 | 8.5 | 0.51** |
| October 16–31 | 14.0 | 0.67** | 13.6 | 0.50** | 11.4 | 0.68 |
| November 1–15 | 11.2 | 0.53** | 9.3 | 0.34** | 10.4 | 0.62** |
SOURCE Authors’ analysis of resident-level data from 282 nursing homes operated by a large long-term care provider.
NOTES Adjusted mortality rates and relative risks were derived from a robust Poisson regression model. Covariates were age, sex, race (categorized as White, Black, and other), Morris activities of daily living score, Cognitive Function Scale score, baseline comorbidities, postacute versus long-stay status, and presence of COVID-19-related symptoms (see the Study Data And Methods for details). Referent = 1.00.
p < 0.05
Case fatality rates of the surrounding communities were substantially lower than the case fatality rate of the sample (appendix exhibit A7).10 However, the community and sample case fatality rates were highly correlated during the study period. Analyses of thirty-day hospitalization revealed no significant changes in adjusted hospitalization rates in the majority of semimonthly periods compared with the first half of April (appendix exhibit A8).10 This finding was consistent in analyses stratified by asymptomatic infection status.
Discussion
In this study of more than 12,000 US nursing home residents with COVID-19, we found that both unadjusted and adjusted thirty-day mortality rates declined from late March to early November 2020. Concurrently, the proportion of COVID-19 cases that were asymptomatic increased over time as a result of improvements in surveillance testing. However, even after adjusting for whether residents were symptomatic and whether they had symptoms of more severe infection, we still found that residents infected in later months had a significantly lower probability of dying within thirty days of diagnosis compared with those infected in earlier months. In addition, stratified analyses of symptomatic versus asymptomatic residents revealed that mortality risk declined for both groups over the course of the pandemic. This suggests that the overall decline in mortality rates was not driven by the increased detection of asymptomatic, less severe cases.
Residents with significant cognitive and functional impairment had higher mortality rates than those with less impairment across all months. However, mortality rates declined both for residents who were significantly impaired and for those who were not. Had we seen larger differences between the most and least clinically complex groups in earlier versus later months, it would have suggested that more vulnerable residents were more likely to have died during the early months of the pandemic, leaving a less at risk population in later months. We did not find this to be the case. In analyses adjusted for multiple resident characteristics capturing clinical complexity, a later diagnosis date was associated with a 21–65 percent reduction in thirty-day mortality risk in all semimonthly periods from mid-April onward.
A number of other factors could explain temporal changes in mortality rates. Early COVID-19 outbreaks overwhelmed the long-term care sector and health care systems overall, potentially leading to excessively high mortality at the beginning of the pandemic. After initial outbreaks, the freeing up of resources, increased knowledge of and experience with COVID-19, and reduced burden on nursing homes may have led to better outcomes for residents. In some areas of the country not well represented in our sample, such as the Rocky Mountain and Plains states, severe COVID-19 outbreaks first occurred during the late summer and early fall. There is recent evidence demonstrating high initial nursing home resident death rates corresponding to outbreak timing in these areas.17
There is also the potential for temporal variation in infection severity and case fatality due to different SARS-CoV-2 variants.18–20 A study examining genomic variation across the US found a positive correlation between fatality rates and circulating levels of two variants that were highly prevalent in the Northeastern states—the predominant location of residents of the present study—and less prevalent in Southern, Central, and Western states.20 Community COVID-19 prevalence highly correlates with the probability and severity of nursing home outbreak;18,21 thus, higher prevalence of a more lethal variant in the community would increase the likelihood of that variant being introduced into the nursing homes in that area. Relatedly, we found that the case fatality rates of our nursing home sample corresponded with the case fatality rates of the surrounding communities over time.
Given the geographic concentration of the sample population in the Northeast, lower mortality risk among residents with later exposure may suggest some influence of advances in the management of COVID-19 in nursing homes. Yet current evidence regarding the influence of changes in clinical and operational management on mortality in this population remains limited. Therapeutics such as remdesivir, monoclonal antibodies, and systemic intravenous corticosteroids are primarily administered to hospitalized patients and have not been systematically tested or used in nursing home or other frail older adult populations.7,8 Clinical management of nursing home residents with COVID-19 has primarily focused on supportive treatment within the facility,22 and no available evidence suggests that such care substantially changed between March and November 2020. In our sample we found that risk-adjusted hospitalization rates did not significantly change over the course of the study period.
Personal protective equipment (PPE) supply, another potential explanatory factor, has improved since March. During the early months of the pandemic, PPE shortages were widespread, and most nursing home providers were forced to rely on federal crisis standard guidelines for extended use and reuse of gowns and masks.23 Reuse of PPE could relate to the viral load at time of infection, a metric shown to correlate with mortality,24 but further work is needed to examine this hypothesis. Similarly, the evidence regarding staffing levels and resident COVID-19 outcomes is also evolving. A recent cross-sectional analysis provided some evidence that higher nurse staffing levels were associated with fewer cases and deaths once a case was already confirmed in a facility,25 but there have been no studies to date examining the impact of longitudinal changes in staffing on resident outcomes.
Conclusion
In this large, multistate sample of nursing home residents with COVID-19, we observed significant declines in thirty-day mortality rates between March and November 2020. Neither improved detection of asymptomatic cases nor changes in observed case-mix explained these trends. Understanding the dynamic risk for mortality from COVID-19 among nursing home residents is critical for identifying the mechanisms affecting outcomes in this vulnerable population. Future research is needed to explore the impact of other extrinsic factors affecting COVID-19 mortality in nursing homes, including improvements in PPE supply, staff adoption of and skill with PPE, specific changes in the clinical management of COVID-19, and potential SARS-CoV-2 genetic variants. Furthermore, it will be critical to monitor infection and mortality rates with the rollout of the SARS-CoV-2 vaccines, which are the most urgent and effective interventions available to protect this vulnerable population. ▪
Supplementary Material
Acknowledgments
This study was funded by a grant from the National Institute on Aging (Grant No. 3P01AG027296-11S1; principal investigator: Vincent Mor). Cyrus Kosar and Elizabeth White contributed equally as co-first authors. Stefan Gravenstein, Orestis Panagiotou, Kevin McConeghy, and Mor are affiliated with the Center on Innovation in Long-Term Services and Supports, Providence Veterans Affairs Medical Center, in Providence, Rhode Island. Panagiotou reports receiving personal fees from International Consulting Associates, Inc., outside the submitted work. Mor is chair of the Scientific Advisory Board at NaviHealth, Inc.; former chair of the Independent Quality Committee at HCR ManorCare; and a former director on the board of PointRight, Inc., where he holds less than 1 percent equity. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government. [Published online March 11, 2021.]
Footnotes
Residents infected in later months had a significantly lower probability of dying within thirty days of diagnosis compared with those infected in earlier months.
Contributor Information
Cyrus M. Kosar, doctoral candidate in the Department of Health Services, Policy, and Practice, Brown University School of Public Health, in Providence, Rhode Island
Elizabeth M. White, investigator in the Center for Gerontology and Healthcare Research, Brown University School of Public Health
Richard A. Feifer, chief medical officer of Genesis Physician Services at Genesis HealthCare, in Kennett Square, Pennsylvania
Carolyn Blackman, Northeast Region vice president for medical affairs of Genesis Physician Services at Genesis HealthCare.
Stefan Gravenstein, director of the Division of Geriatrics and Palliative Medicine, Department of Medicine, Warren Alpert Medical School, Brown University, in Providence.
Orestis A. Panagiotou, assistant professor in the Department of Health Services, Policy, and Practice and the Center for Gerontology and Healthcare Research, Brown University School of Public Health
Kevin McConeghy, doctoral student in the Department of Health Services, Policy, and Practice, Brown University School of Public Health.
Vincent Mor, Florence Pirce Grant University Professor in the Department of Health Services, Policy, and Practice and the Center for Gerontology and Healthcare Research, Brown University School of Public Health, and a research health scientist at the Providence Veterans Affairs Medical Center.
NOTES
- 1.Henry J Kaiser Family Foundation. State COVID-19 data and policy actions [Internet]. San Francisco (CA): KFF; 2020. [cited 2021 Feb 17]. Available from: https://www.kff.org/coronavirus-covid-19/issue-brief/state-covid-19-data-and-policy-actions/ [Google Scholar]
- 2.Hägg S, Jylhävä J, Wang Y, Xu H, Metzner C, Annetorp M, et al. Age, frailty, and comorbidity as prognostic factors for short-term outcomes in patients with coronavirus disease 2019 in geriatric care. J Am Med Dir Assoc. 2020;21(11):1555–9.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hewitt J, Carter B, Vilches-Moraga A, Quinn TJ, Braude P, Verduri A, et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5(8):e444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi SM, Bakaev I, Chen H, Travison TG, Berry SD. Risk factors, presentation, and course of coronavirus disease 2019 in a large, academic long-term care facility. J Am Med Dir Assoc. 2020;21(10):1378–1383.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boehmer TK,DeVies J,Caruso E,van Santen KL, Tang S, Black CL, et al. Changing age distribution of the COVID-19 pandemic—United States, May-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(39): 1404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weatherald J, Solverson K, Zuege DJ, Loroff N, Fiest KM, Parhar KKS. Awake prone positioning for COVID-19 hypoxemic respiratory failure: a rapid review. J Crit Care. 2021;61: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC. Remdesivir for the treatment of Covid-19—Final Report. N Engl J Med. 2020;383(19):1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chidambaram P Key questions about the impact of coronavirus on long-term care facilities over time [Internet]. San Francisco (CA): Henry J. Kaiser Family Foundation; 2020. Sep 1 [cited 2021 Feb 9]. Available from: https://www.kff.org/coronavirus-covid-19/issue-brief/key-questions-about-the-impact-of-coronavirus-on-long-term-care-facilities-over-time/ [Google Scholar]
- 10.To access the appendix, click on the Details tab of the article online. [Google Scholar]
- 11.Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19) [Internet]. Atlanta (GA): CDC; 2020. Dec 8 [cited 2021 Feb 9].Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html [Google Scholar]
- 12.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci. 1999; 54(11):M546–53. [DOI] [PubMed] [Google Scholar]
- 13.Thomas KS,Dosa D,Wysocki A, Mor V. The Minimum Data Set 3.0 Cognitive Function Scale. Med Care. 2017;55(9):e68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panagiotou OA, Kosar CM, White EM, Bantis LE, Yang X, Santostefano CM, et al. Risk factors associated with all-cause 30-day mortality in nursing home residents with COVID-19. JAMA Intern Med. 2021. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou G A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. [DOI] [PubMed] [Google Scholar]
- 16.Greene W The behaviour of the maximum likelihood estimator of limited dependent variable models in the presence of fixed effects. Econom J. 2004;7(1):98–119. [Google Scholar]
- 17.Konetzka RT, Gorges RJ. Nothing much has changed: COVID-19 nursing home cases and deaths follow fall surges. J Am Geriatr Soc. 2021; 69(1):46–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becerra-Flores M, Cardozo T. SARS-CoV-2 viral spike G614 mutation exhibits higher case fatality rate. Int J Clin Pract. 2020;74(8):e13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toyoshima Y,Nemoto K,Matsumoto S, Nakamura Y,Kiyotani K. SARS-CoV-2 genomic variations associated with mortality rate of COVID-19. J Hum Genet. 2020;65(12):1075–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young BE, Fong SW, Chan YH, Mak TM, Ang LW, Anderson DE, et al. Effects of a major deletion in the SARS-CoV-2 genome on the severity of infection and the inflammatory response: an observational cohort study. Lancet. 2020;396(10251): 603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White EM, Kosar CM, Feifer RA, Blackman C, Gravenstein S, Ouslander J, et al. Variation in SARS-CoV-2 prevalence in U.S. skilled nursing facilities. J Am Geriatr Soc. 2020;68(10):2167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lester PE, Holahan T, Siskind D, Healy E. Policy recommendations regarding skilled nursing facility management of coronavirus 19 (COVID-19): lessons from New York State. J Am Med Dir Assoc. 2020; 21(7):888–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berklan JM. McKnight’s COVID survey reveals vast PPE staffing shortages. McKnight’s Long-Term Care News [serial on the Internet]. 2020. Mar 30 [cited 2021 Feb 9]. Available from: https://www.mcknights.com/news/mcknights-covid-survey-reveals-vast-ppe-staffing-shortages/
- 24.Pujadas E, Chaudhry F, McBride R, Richter F, Zhao S, Wajnberg A, et al. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir Med. 2020;8(9):e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorges RJ, Konetzka RT. Staffing levels and COVID-19 cases and outbreaks in U.S. nursing homes. J Am Geriatr Soc. 2020;68(11):2462–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


