Abstract
Background:
The aim of this study was to assess the discoloration of coronal tooth structure irrigated with different irrigation solutions and filled with calcium silicate-based materials containing bismuth oxide or calciumzirconia complex as radiopacifier.
Materials and Methods:
In this ex vivo study, 72 bovine enamel-dentin blocks were prepared and divided into three groups. The dentinal cavities in each group were irrigated with 5.25% sodium hypochlorite (NaOCl), 2% chlorhexidine (CHX), or normal saline for 30 min. After that, irrigation solutions were removed using a cotton pellet. Each group was then randomly divided into two subgroups according to the cavity-filling materials (ProRoot mineral trioxide aggregate [MTA] and RetroMTA). The color assessments were performed before filling the cavities and 1 month and 6 months after filling the cavities. Data were analyzed with two-way ANOVA. The level of statistical significance was set at P < 0.05.
Results:
The effect of irrigation solution on the color change of calcium silicate-based materials was not statistically significant at none of the time intervals (P = 0.334 and P= 0.252, respectively, for ProRoot MTA and RetroMTA). ProRoot MTA caused a significantly higher color change compared with RetroMTA exposed to different irrigation solutions at each time interval (P < 0.001). Color change of both materials exposed to each irrigation solution significantly increased over time (P < 0.05).
Conclusion:
Under the condition of this ex vivo study, irrigation of dentin with NaOCl and CHX and then removing the excess solution might be ineffective in increasing the tooth color change potential of either bismuth oxide or zirconium-containing calcium silicate-based materials. Furthermore, calcium silicate-based material, which contained bismuth oxide, caused higher tooth discoloration.
Key Words: Calcium silicate, chlorhexidine, mineral trioxide aggregate, root canal irrigant, sodium hypochlorite, tooth discoloration
INTRODUCTION
Several studies have reported unfavorable coronal tooth discoloration after the use of mineral trioxide aggregate (MTA) containing bismuth oxide as the radiopacifier.[1,2,3,4] Bismuth oxide is known as the main cause of discoloration.[3,5,6] Therefore, calcium silicate-based materials with alternative radiopacifiers have been introduced to overcome tooth discoloration induced by MTA in esthetic areas.
It has been shown that discoloration potential of materials deprived of bismuth oxide was significantly lower than that of materials which contain bismuth oxide in their composition.[7,8,9]
Sodium hypochlorite (NaOCl) and chlorhexidine (CHX) are more commonly used irrigation solutions in endodontics. Color alteration of bismuth oxide-containing calcium silicate-based materials following immersion in NaOCl and CHX has been shown by some investigations.[3,5,8,10] However, complete immersion of the materials in tested solutions does not mimic the exact clinical conditions. Therefore, this study was designed to investigate and compare discoloration of coronal tooth structure irrigated with 5.25% NaOCl, 2% CHX, or normal saline and filled with either ProRoot MTA as a bismuth oxide-containing material or RetroMTA which contained calcium-zirconia complex after removing the irrigation solutions and drying the cavities.
MATERIALS AND METHODS
In this ex vivo study, 72 bovine mandibular incisors were used. Extrinsic debris was removed using an ultrasonic scaler (Varios 970, NSK, Japan). The use of bovine teeth in this study was approved by a panel from the Tehran University of Medical Sciences Ethical Committee (Ethics code: 32011). The crowns were sectioned to obtain 7 mm × 7 mm enamel-dentin blocks. The thickness of each block was standardized at 3.5 mm ± 0.2 mm and checked with a gauge. A cavity with 2.5 mm width and 1.5 mm depth was prepared in the center of the dentinal surface of each block with a high-speed diamond bur.[3] The specimens were randomly divided into three groups (n = 24) based on the type of selected irrigating solution and were irrigated whether by 5.25% NaOCl (CHLORAXID, Cerkamed, Stalowa Wola, Poland), 2% CHX (Gluco-CHEX, Cerkamed, Stalowa Wola, Poland), or normal saline. The solutions were remained in the cavities for 30 min. After that, the cavities were dried with cotton pellets. Each group was then randomly divided into two subgroups (n = 12) according to the cavity-filling materials. ProRoot MTA (Dentsply Tulsa Dental Products, Tulsa, OK, USA) and RetroMTA (BioMTA, Seoul, Korea) were prepared according to the manufacturers' instructions. The materials were placed into the prepared cavities. The specimens were then incubated at 37°C in fully saturated humidity for 24 h. After the setting of the materials, surrounding dentin surface was etched with 35% phosphoric acid for 15 s (Vococid, Voco, Germany). The bonding agent (3M ESPE, St. Paul, MN, USA) was applied and light cured for 20 s. After that, a 2-mm thick composite material, A1 shade (Filtek Z350, 3M ESPE, St. Paul, MN, USA), was placed over conditioned dentin surface and calcium silicate-based materials and cured using a LED curing light (Valo; Ultradent Products Inc., South Jordan, UT, USA) for 40 s. The specimens were immersed in distilled water at 37°C throughout the study while being separately stored in Eppendorf tubes.
The color assessments were performed before filling the cavities (T0), 1 month (T1), and 6 months (T6) after filling. For repeatable color assessment, a window measuring 3 mm × 3 mm was created on the enamel side of the cavities using a needle-shaped diamond bur. A spectrophotometer (Vita Easyshad; VITA Zahnfabrik, Bad Säckingen, Germany) was used to measure the L*, a*, and b* values. L* indicates the value of lightness-darkness, a* indicates greenness-redness, and b* indicates blueness-yellowness. The device was calibrated before use for each specimen. Color measurements were performed three times inside the marked window for every reading, and the mean value of three measurements was recorded. The color change (ΔE) comparing the baseline and other two intervals was measured using the following formula:
ΔE = ([ΔL]2+ [Δa]2+ [Δb]2)½
Data were analyzed using SPSS software (PASW Statistics 18; SPSS Inc, Chicago, IL, USA). Repeated measures two-way analysis of variance was used to evaluate the effects of two factors (type of calcium silicate-based material and type of irrigation solution) on color change at the end of 1-m and 6-m intervals compared to the baseline. If there was any significant interaction, appropriate analysis in each group (i.e., repeated measures one-way analysis of variance or an independent Student's t-test) was performed. The level of statistical significance was set at 0.20 and 0.05 for the evaluation of interactions and for the main effects, respectively.
RESULTS
The color change results obtained in this study are shown in Table 1 and Figure 1.
Table 1.
The color change results obtained in the study
| Group | Mean±SD | Median | Minimum | Maximum | 95% CI |
|||
|---|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||||
| T1 | CHX | ProRoot MTA | 6.04±2.54a | 5.49 | 3.01 | 12.75 | 4.43 | 7.66 |
| RetroMTA | 3.79±1.65b | 2.96 | 2.09 | 6.88 | 2.74 | 4.84 | ||
| T6 | ProRoot MTA | 7.8±2.35c | 7.17 | 4.65 | 12.00 | 6.31 | 9.3 | |
| RetroMTA | 4.34±1.39d | 4.44 | 2.58 | 6.16 | 3.46 | 5.22 | ||
| T1 | NaOCl | ProRoot MTA | 7.56±2.51a | 6.79 | 5.24 | 13.68 | 5.96 | 9.15 |
| RetroMTA | 4.29±1.7b | 3.46 | 2.42 | 7.61 | 3.21 | 5.38 | ||
| T6 | ProRoot MTA | 8.96±2.66c | 8.58 | 6.12 | 15.52 | 7.27 | 10.66 | |
| RetroMTA | 5.08±1.45d | 5.04 | 5.52 | 8.35 | 4.16 | 6.00 | ||
| T1 | Saline | ProRoot MTA | 6.43±1.51a | 6.11 | 4.1 | 8.7 | 5.47 | 7.4 |
| RetroMTA | 3.74±1.18b | 3.22 | 2.69 | 6.89 | 2.99 | 4.49 | ||
| T6 | ProRoot MTA | 8.1±2.4c | 7.4 | 5.1 | 13.1 | 6.58 | 9.63 | |
| RetroMTA | 4.18±1.47d | 3.88 | 2.54 | 7.25 | 3.24 | 5.11 | ||
T1: 1 month after filling; T6: 6 months after filling the dentinal cavities with tested calcium silicate-based materials. Groups displaying different superscript letters indicate a significant difference. SD: Standard deviation; CI: Confidence interval
Figure 1.
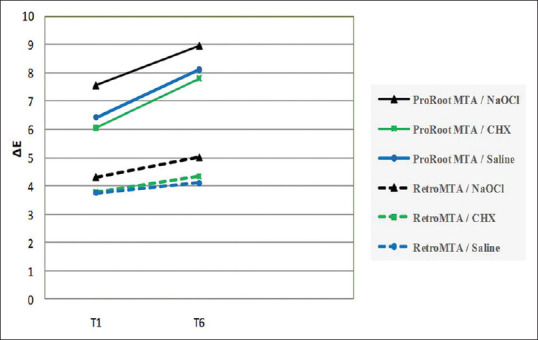
The mean ΔE value changes at each time interval. T1: 1 month after filling; T6: 6 months after filling the dentinal cavities with tested calcium silicate-based materials.
The effect of irrigation solution on the color change of calcium silicate-based materials was not significant at none of the time intervals (P = 0.334 and P = 0.252, respectively, for ProRoot MTA and RetroMTA). After adjustment for irrigation solution and time, a comparison of calcium silicate-based materials revealed that the ΔE was significantly different (P < 0.001). Considering the type of calcium silicate-based material, ProRoot MTA caused a significantly higher color change compared with RetroMTA exposed to different irrigation solutions at each time interval (P < 0.02 and P < 0.001, respectively, for 1-m specimens exposed to CHX and the other groups). There was a significant interaction between the type of calcium silicate-based material and time interval. The results showed that irrespective of the type of calcium silicate-based material and irrigation solution, the correlation of time and color change was statistically significant (P < 0.001). Color change of both materials exposed to each irrigation solution significantly increased over time (P < 0.001).
DISCUSSION
Bovine incisors were used to evaluate the discoloration, as reported in previous studies.[6,11,12] Although the use of human teeth is more reasonable to extrapolate the results of ex vivo studies to the clinical situation, the use of human teeth in ex vivo studies is partially limited due to ethical issues and insufficient flat labial surface for color assessment.[6]
This study showed that dental discoloration following the use of RetroMTA was significantly lower than ProRoot MTA. This finding was consistent with the previous study which showed less tooth discoloration with zirconium oxide-containing materials (RetroMTA and ENDOCEM Zr) compared to materials containing bismuth oxide (ProRoot MTA and MTA Angelus).[7] Several studies have revealed that ProRoot MTA and other bismuth oxide-containing calcium silicate-based materials were associated with a strong potential for dentinal discoloration.[4] It has been shown that materials containing alternative radiopacifiers such as zirconium oxide and tantalum oxide and reduced amount of heavy metals could cause less extent of tooth color alteration.[7,9,13,14] It has been noted that the destabilization of bismuth oxide due to exposure to strong oxidizing agents,[5] ultraviolet and visible light irradiation in an oxygen-free environment,[15] and interaction with amino acids present in collagen fibers of adjacent dentin might be correlated with the discoloration of calcium silicate-based material and the cement/dentin interface.[6] The color change is presumably due to the conversion of yellow bismuth oxide to dark metallic bismuth or bismuth carbonate, which could eventually lead to darkening of material.[5,16] Therefore, changes in the color of bismuth oxide-containing materials and their adjacent tooth structure are more likely than that of bismuth oxide-free substances.
The current study evaluated the effect of routinely used endodontic irrigation solutions on calcium silicate-based materials. In the present study, irrigation of dentinal cavities with 5.25% NaOCl or 2% CHX before filling the cavities with either ProRoot MTA or RetroMTA did not result in further tooth discoloration compared with normal saline. Nevertheless, in some studies, strong discoloration of bismuth oxide and bismuth oxide-containing calcium silicate-based materials was observed following immersion in NaOCl for 24 h[5] and 28 days[8] due to its oxidizing property. It has been noted that overoxidation of bismuth oxide by strong oxidizers could result in discoloration.[5] On the contrary, in our study, NaOCl and normal saline had similarly affected tooth color change induced by ProRoot MTA, which might be attributed to the difference in the methodology of the present study in comparison with other mentioned investigations.[5,8,10]
There is not enough information regarding the effect of CHX on the color change of calcium silicate-based materials. In the present study, irrigation of cavities with 2% CHX had the same effect as saline on tooth color change following the use of ProRoot MTA and RetroMTA. However, Keskin et al. showed a negative effect of 5% NaOCl and 2% CHX compared with distilled water on color change of calcium silicate-based materials which were cured in ring molds and immersed into the irrigation solutions for 24 h after setting.[10] In the current study, removing the excess of irrigation solution by the use of cotton pellets, after irrigating the cavities for 30 min, minimized the direct exposure of materials to irrigation solutions. Immersion of calcium silicate-based materials in irrigation solutions in previous studies[5,8,10] made the environment completely different from what was created in this study.
The results of this study showed that the tooth discoloration induced by both materials exposed to different irrigation solutions increased over time. These findings are in agreement with the results of the study showed an increase in color change of different calcium silicate materials from 1 to 6 months.[17]
It is worth mentioning that in clinical situations, discoloration potential of calcium silicate-based materials might be impacted by variation in dentin thickness in different teeth.
CONCLUSION
According to the results of this ex vivo study, the use of material containing radiopacifier alternative to bismuth oxide resulted in the lower coronal color change. Furthermore, irrigation of dentin with NaOCl and CHX and then removing the excess solution before the placement of calcium silicate-based materials might be ineffective in increasing the tooth color change potential of either bismuth oxide or zirconium-containing calcium silicate-based materials. However, extrapolation of the results of this ex vivo study performed on standardized enamel-dentin blocks of bovine teeth to various clinical settings requires further clinical trials.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
Acknowledgment
This study was supported by Tehran University of Medical Sciences (grant no: 32011).
REFERENCES
- 1.Araghi S, Khavid A, Godiny M, Saeidipour M. In vitro evaluation of coronal discoloration following the application of calcium-enriched mixture cement, Biodentine, and mineral trioxide aggregate in endodontically treated teeth. Dent Res J (Isfahan) 2019;16:53–9. [PMC free article] [PubMed] [Google Scholar]
- 2.Vallés M, Roig M, Duran-Sindreu F, Martínez S, Mercadé M. Color stability of teeth restored with biodentine: A 6-month in vitro study. J Endod. 2015;41:1157–60. doi: 10.1016/j.joen.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Marciano MA, Duarte MA, Camilleri J. Dental discoloration caused by bismuth oxide in MTA in the presence of sodium hypochlorite. Clin Oral Investig. 2015;19:2201–9. doi: 10.1007/s00784-015-1466-8. [DOI] [PubMed] [Google Scholar]
- 4.Możyńska J, Metlerski M, Lipski M, Nowicka A. Tooth discoloration induced by different calcium silicate-based cements: A systematic review of In vitro studies. J Endod. 2017;43:1593–601. doi: 10.1016/j.joen.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Camilleri J. Color stability of white mineral trioxide aggregate in contact with hypochlorite solution. J Endod. 2014;40:436–40. doi: 10.1016/j.joen.2013.09.040. [DOI] [PubMed] [Google Scholar]
- 6.Marciano MA, Costa RM, Camilleri J, Mondelli RF, Guimarães BM, Duarte MA. Assessment of color stability of white mineral trioxide aggregate angelus and bismuth oxide in contact with tooth structure. J Endod. 2014;40:1235–40. doi: 10.1016/j.joen.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 7.Kang SH, Shin YS, Lee HS, Kim SO, Shin Y, Jung IY, et al. Color changes of teeth after treatment with various mineral trioxide aggregate-based materials: An ex vivo study. J Endod. 2015;41:737–41. doi: 10.1016/j.joen.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Camilleri J. Staining potential of neo MTA plus, MTA plus, and biodentine used for pulpotomy procedures. J Endod. 2015;41:1139–45. doi: 10.1016/j.joen.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 9.Kohli MR, Yamaguchi M, Setzer FC, Karabucak B. Spectrophotometric analysis of coronal tooth discoloration induced by various bioceramic cements and other endodontic materials. J Endod. 2015;41:1862–6. doi: 10.1016/j.joen.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Keskin C, Demiryurek EO, Ozyurek T. Color stabilities of calcium silicate-based materials in contact with different irrigation solutions. J Endod. 2015;41:409–11. doi: 10.1016/j.joen.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Lenherr P, Allgayer N, Weiger R, Filippi A, Attin T, Krastl G. Tooth discoloration induced by endodontic materials: A laboratory study. Int Endod J. 2012;45:942–9. doi: 10.1111/j.1365-2591.2012.02053.x. [DOI] [PubMed] [Google Scholar]
- 12.Akcay M, Arslan H, Yasa B, Kavrık F, Yasa E. Spectrophotometric analysis of crown discoloration induced by various antibiotic pastes used in revascularization. J Endod. 2014;40:845–8. doi: 10.1016/j.joen.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Marconyak LJ, Jr, Kirkpatrick TC, Roberts HW, Roberts MD, Aparicio A, Himel VT, et al. A comparison of coronal tooth discoloration elicited by various endodontic reparative materials. J Endod. 2016;42:470–3. doi: 10.1016/j.joen.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Ramos JC, Palma PJ, Nascimento R, Caramelo F, Messias A, Vinagre A, et al. 1-year in vitro evaluation of tooth discoloration induced by 2 calcium silicate-based cements. J Endod. 2016;42:1403–7. doi: 10.1016/j.joen.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Vallés M, Mercadé M, Duran-Sindreu F, Bourdelande JL, Roig M. Color stability of white mineral trioxide aggregate. Clin Oral Investig. 2013;17:1155–9. doi: 10.1007/s00784-012-0794-1. [DOI] [PubMed] [Google Scholar]
- 16.Vallés M, Mercadé M, Duran-Sindreu F, Bourdelande JL, Roig M. Influence of light and oxygen on the color stability of five calcium silicate-based materials. J Endod. 2013;39:525–8. doi: 10.1016/j.joen.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Shokouhinejad N, Nekoofar MH, Pirmoazen S, Shamshiri AR, Dummer PM. Evaluation and comparison of occurrence of tooth discoloration after the application of various calcium silicate-based cements: An ex vivo study. J Endod. 2016;42:140–4. doi: 10.1016/j.joen.2015.08.034. [DOI] [PubMed] [Google Scholar]


