Abstract
Background and Objectives:
Hypermucoviscoid Klebsiella(hvKP), a dreaded variant of Klebsiella, so far, fewer cases were reported from the community. This study was designed to evaluate the incidence of hvKP isolates, risk factors for hvKP infections, antibiotic sensitivity pattern and clinical outcome including morbidity and mortality.
Patients and Setting:
Patients who have got admitted under medical intensive care unit (MICU) and had positive culture of Klebsiella infections.
Materials and Methods:
This study was conducted at department of MICU at a tertiary care hospital between January 2018 and December 2018. A standardized proforma was prepared and data was collected, which includes basic demographics of the patients, co-morbidities, clinical details and mortality. This study was approved by the Institutional Review Board and Ethics Committee.
Results:
A total of 165 patients (males, 123; 74.5%) had Klebsiella pneumoniae infection during the study period, out of whom 32 was hvKP (19.4%). The mean age was 53.1 ± 16.8 years. Among the 32 hvKP patients, 22 (68.8%) were hospital acquired infection (HAI) and 10 were (31.2%) community acquired infection. The overall mortality rate of hvKP infection was 56.2% (18/32). The incidence of mortality rate was similar in patients having pan-drug sensitive and in patients with extreme drug-resistance (61.9% vs. 66.7%; P = 0.831). HAI is significantly associated with multi drug resistance of hvKP (odds ratio [OR], 7.917; P < 0.05) and diabetes is associated with increased risk of hvKP related mortality (OR, 5.250; P = 0.054).
Conclusions:
Our study results showed, increased incidence of HAI with hvKP predominantly associated with pneumonia and increase in trend of drug resistance with two cases being pan resistant. More number of studies are required to evaluate the existing antibiotics strategy and steps to curb the spread of this dreaded infection.
Keywords: Community acquired infection, hospital acquired infection, hvKP, hypermucoviscoid Klebsiella, Klebsiella
INTRODUCTION
Klebsiella species cause wide range of infections which includes pneumonia, urinary tract infections, blood stream infections and sepsis. The first Klebsiella pneumoniae (K. pneumoniae) bacterium was isolated by Carl Friedlander from the lungs of patients who had died from pneumonia in 1882.[1]Klebsiella infections are important cause for hospital acquired infections (HAIs), constituting 3%–42% of nosocomial infections with an attributable mortality risk of 20%–67% to Klebsiella.[2] In mid 1980s a new strain of Klebsiella called Hypermucoviscoid Klebsiella (hvKP) strain was detected in patients with pyogenic liver abscess.[3] This hvKP strain is supposed to be more virulent and unique in causing metastatic infections in younger healthy population,[4] however, the hvKP strain can be detected with simple string test.[5,6] The literature is limited and sparse regarding to the incidence, risk factors and attributable mortality to the hvKP strain. In a single observational study from India, the incidence of hvKP strains among Klebsiella isolates was more than 80% which were predominantly multidrug resistant (MDR).[7] The hvKP infections are most commonly community acquired infections (CAIs). In Asia, hvKP is the most common cause of pyogenic liver abscesses.[8,9] However, antibiotic-resistant hvKP isolates are increasingly being reported across the world.[10,11,12]
In this study, we have evaluated the incidence of hvKP isolates in community acquired and nosocomial infections, risk factors for hvKP infections, antibiotic sensitivity pattern and clinical outcome of patients including morbidity and mortality.
MATERIALS AND METHODS
Study design
The present study comprised of retrospective as well as prospective cases.
Study area
This study was conducted at department of medical intensive care unit (MICU) at a tertiary care hospital.
Study population
Patients who have got admitted under MICU and had positive culture of Klebsiella infections between January 2018 and December 2018, at Mazumdar Shaw Medical Center, Narayana Health City, Bengaluru.
Procedure
After obtaining the ethical committee approval, prospectively we have collected the data of mentioned study period. A standardized proforma was prepared, which includes basic demographics of the patients, co-morbidities, HAI or CAI details, severity of illness, antibiotic sensitivity and clinical response to antibiotics. We also collected other clinical parameters such as length of hospital/MICU stay, ventilation requirement, organ failures and mortality.
Statistical analysis
Data was analyzed by using SPPS statistical software for windows (version 11, SPSS Inc., Chicago, IL, USA). Continuous data are presented in terms of mean and standard deviation. Categorical data are presented in terms of number and percentages. Univariate logistic regression model was used to predict the risk factor for hvKP drug resistance and mortality.
Ethical approval
This study was approved by the Narayana Health Medical Ethics Committee, Bengaluru.
Definitions
MDR: MDR was defined as acquired nonsusceptibility to at least 1 agent in 3 or more antimicrobial categories[13]
Extreme drug resistance (XDR): XDR was defined as nonsusceptibility to at least 1 agent in all, but two or fewer antimicrobial categories[13]
Pan drug resistance (PDR): PDR was defined as nonsusceptibility to all agents in all microbial categories[13]
Extended-spectrum beta-lactamase (ESBL) producing bacteria: ESBL bacteria are defined as nonsusceptibility to most beta-lactam antibiotics, including penicillins, cephalosporins, and the monobactam aztreonam.
RESULTS
A total of 3115 patients have admitted in MICU over the period of 1 year. Out of whom 5.5% (165 out of 3115) had K. pneumoniae infection and 1% (32 out of 3115) of patients had hvKP infections. Figure 1 is showing the depiction of classical K. pneumoniae and hvKP on the agar plate. The rate of hvKP incidence among Klebsiella culture positivity was 19.4% (32 out of 165). Seventy-five percent (123 out of 165) were males, the overall mean age was 53.1 ± 16.8 years. The mean age (53.1 ± 16.8 vs. 54.2 ± 15.6 years; P = 0.734), and gender (P = 1.000) were similar between Klebsiella infections and hvKP infections.
Figure 1.
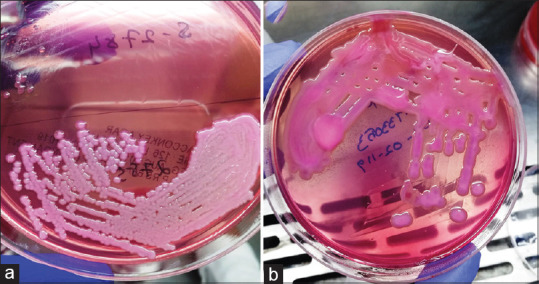
Depiction of (a) classical Klebsiella pneumoniae and (b) hypermucoviscoid Klebsiella on the agar plate
Among the 32 hvKP patients, 22 (68.8%) were HAIs and 10 were (31.2%) CAIs. The overall mortality rate in hvKP infection was 56.2% (18 out of 32). Ten patients (31.3%) were discharged in stable condition and 4 patients (12.5%) were discharged against medical advice for religious and financial reasons. The basic and clinical characteristics are presented in Table 1.
Table 1.
Basic and clinical characteristics of the study patents
| Characteristics | n (%) or mean±SD |
|---|---|
| Gender | |
| Male | 24 (75) |
| Female | 8 (25) |
| Age (years), range | 54.2±15.6 (20-77) |
| Diabetes | 19 (59.4) |
| Hypertension | 14 (43.8) |
| COPD | 3 (9.4) |
| Intubated | 23 (71.9) |
| Tracheostomy | 11 (34.4) |
| Central venous catheterization | 27 (84.4) |
| Foleys Cather use | 26 (81.3) |
| Infection type | |
| Hospital acquired | 22 (68.8) |
| Community acquired | 10 (31.2) |
| hvKP positive culture samples | |
| Blood | 9 (28.1) |
| Urine | 2 (6.1) |
| Tissue | 4 (12.5) |
| Tracheal secretion | 16 (50) |
| Bile | 1 (3.1) |
| PUS | 2 (6.3) |
| Patient outcome | |
| Discharged | 10 (31.3) |
| Dead | 18 (56.2) |
| Discharge against medical advice | 4 (12.5) |
COPD: Chronic obstructive pulmonary disease, SD: Standard deviation, hvKP: Hypermucoviscoid klebsiella
The predominant site of hvKP samples were obtained from the respiratory tract [Table 1]. The antibiotic sensitivity pattern of hvKP showed, 18 patients had pan-drug sensitive, 2 patients had pan-drug resistant, 11 patients were sensitive to only one drug (Colistin), 5 patients were sensitive to 2 drugs and 6 patients were sensitive to >2 drugs. Most of the (90.6%; n = 29) hvKP patient's samples were sensitive to colistin compared to other drugs used [Table 2].
Table 2.
Antibiotic sensitivity pattern of hypermucoviscoid Klebsiella isolates in our study
| Antibiotic name | Sensitivity (n=32), n (%) |
|---|---|
| Colistin | 29 (90.6) |
| Tigecycline | 11 (24.4) |
| Aminoglycosides | 15 (46.9) |
| Carbapenems | 12 (37.5) |
| β-lactam-β-lactamase inhibitor | 9 (28.1) |
| Cephalosporin | 10 (31.3) |
| Chloramphenicol | 15 (46.9) |
| Co-trimoxazole | 11 (34.4) |
Seventeen (53.1%) of hvKP isolates were XDR, 8 (25%) were pan sensitive, 3 (9.4%) were ESBL producing type, 2 (6.3%) was pan resistance, 1 (3.1%) was MDR and 1 was colonizer.
The incidence of mortality rate in patients with pan-drug sensitive and in patients with extreme drug-resistance were 61.9% and 66.7% respectively (P = 0.831). The mortality rate did not differ between pan sensitive group and drug resistance group.
Univariate logistic regression analysis showed, Tracheostomy (odds ratio [OR], 5.385; P = 0.143) and HAI (OR, 7.317; P<0.05) are the risk factors for the resistance of hvKP to the antibiotics [Table 3]. Whereas, diabetes (OR, 5.250; P = 0.054) is the risk factor for mortality of the patients [Table 4]. The mortality rate between HAI and CAI were similar (OR, 0.813; P = 0.831).
Table 3.
Univariate logistic regression analysis to predict factors influencing hypermucoviscoid Klebsiella resistance to antibiotics
| Predictors | Logit (B) | SE | Wald | P | OR | 95.0% CI |
|---|---|---|---|---|---|---|
| Age (years) ≥55 | −2.208 | 1.149 | 3.695 | 0.055 | 0.110 | 0.012-1.044 |
| Female | 0.770 | 0.888 | 0.752 | 0.386 | 2.160 | 0.379-12.316 |
| Diabetes | −1.012 | 0.917 | 1.217 | 0.270 | 0.364 | 0.060-2.194 |
| Hypertension | 0.424 | 0.841 | 0.254 | 0.614 | 1.528 | 0.294-7.945 |
| Intubation | −0.272 | 0.934 | 0.085 | 0.771 | 0.762 | 0.122-4.751 |
| Tracheostomy | 1.684 | 1.149 | 2.148 | 0.143 | 5.385 | 0.567-51.172 |
| CVC | −0.388 | 1.202 | 0.104 | 0.747 | 0.679 | 0.064-7.161 |
| Foleys catheter | 0.460 | 0.985 | 0.218 | 0.641 | 1.583 | 0.230-10.904 |
| Dialysis | −1.012 | 0.917 | 1.217 | 0.270 | 0.364 | 0.060-2.194 |
| HAI | 2.069 | 0.914 | 5.121 | 0.024* | 7.917 | 1.319-47.512 |
*P value is significant at 0.05 level. CVC: Central venous catheter, HAI: Hospital acquired infection, SE: Standard error, OR: Odds ratio, CI: Confidence interval
Table 4.
Univariate logistic regression analysis to predict factors influencing mortality of hypermucoviscoid Klebsiella infection
| Predictors | Logit (B) | SE | Wald | P | OR | 95.0% CI |
|---|---|---|---|---|---|---|
| Age (years) ≥55 | 0.000 | 0.789 | 0.000 | 1.000 | 1.000 | 0.213-46.693 |
| Female | 0.405 | 0.893 | 0.206 | 0.650 | 1.500 | 0.261-8.636 |
| Diabetes | 1.658 | 0.859 | 3.725 | 0.054 | 5.250 | 0.975-28.278 |
| Hypertension | 1.070 | 0.837 | 1.634 | 0.201 | 2.917 | 0.565-15.054 |
| Intubation | 1.204 | 0.904 | 1.775 | 0.183 | 3.333 | 0.567-19.593 |
| Tracheostomy | 0.934 | 0.927 | 1.016 | 0.313 | 2.545 | 0.414-15.652 |
| CVC | 0.693 | 1.090 | 0.405 | 0.525 | 2.000 | 0.236-16.928 |
| Foleys catheter | 1.674 | 0.990 | 2.862 | 0.091 | 5.333 | 0.767-37.093 |
| Dialysis# | 20.866 | - | 0.000 | 0.999 | - | 0.000 |
| HAI | −0.431 | 0.950 | 0.206 | 0.650 | 0.650 | 0.101-4.181 |
| Drug resistance | −0.208 | 0.976 | 0.045 | 0.831 | 0.813 | 0.120-5.499 |
#All 4 patients were died in the dialysis group, hence no comparison happened. CVC: Central venous catheter, HAI: Hospital acquired infection, SE: Standard error, OR: Odds ratio, CI: Confidence interval
DISCUSSION
hvKP, is a dreaded variant of K. pneumoniae, newly emerged and clinically significant. This pathogen causes life-threatening infections in both healthy and immunocompromised patients.[14,15,16,17] So far, fewer cases were reported from the community. This study was designed to look at the type and characteristics of the infections.
Half of the patients with hvKP infections are young or do not have co-morbidities, most hvKP infections are CAI which is unusual in K. pneumoniae, which are generally nosocomial infections in patients with immunosuppression.[18] In our study, we observed that CAI was 32% and HAI was 68%, which is an alarming message that the rate of HAI is increasing with this variant.
hvKP infections are associated with a higher mortality rate, in our study, we found that the attributable mortality to hvKP infection was 56.2%, which is higher than the previous reports ranging from 3% to 42%[15,18,19,20,21,22] and this can be one of the indicators for independent predictor of mortality. A simple “string test” can be used to determine the hypermucoviscosity of the phenotype. The string test is positive when a viscous string >5 mm in length [Figure 2] is formed by stretching bacterial colonies on an agar plate.[6]
Figure 2.
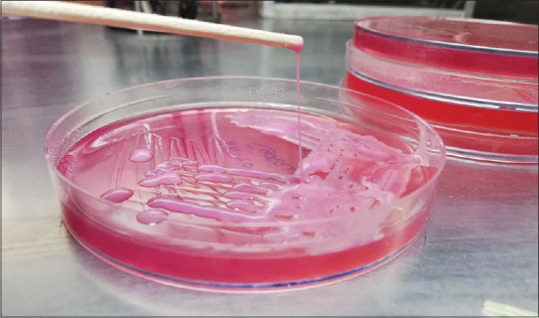
String test showing the length of string >5 mm in hypermucoviscoid klebsiella
The non-effectiveness of the therapy for nosocomial infections and sepsis is mainly due to antibiotic resistance. According to the WHO report, the antibiotic resistance of K. pneumoniae strains is associated mainly with the production of ESBL and it was included in the most dangerous superbugs.[23] But the antibiotic resistance of hvKP is mainly due to the hypermucoviscous, it is typically produced by the bacteria by overproduction of polysaccharide capsule.[17] Whereas, K. pneumoniae strains are hypervirulent, but not hypermucoviscous.[24] Unlike K. pneumoniae, hvKP can cause serious CAI even in healthy individuals.[25] However, in our study we found more HAI infections than CAI.
The high prevalence of antibiotic resistance is more common in conventional K. pneumoniae, whereas the prevalence of antibiotic resistance in hvKP isolates is rare.[16,17] But in our study, 75% (n = 24) had drug-resistance hvKP, out of whom 53.1% (n = 17) were XDR, 9.4% (n = 3) were ESBL producing type, 6.3% (n = 2) was pan resistance, 3.1% (n = 1) was MDR and 1 was colonizer. The antibiotic resistance pattern of all resistance isolates in our present study is described in Table 5 and the clinical profile of all resistant isolates are presented in Table 6. Lee et al.,[18] in their review, reported that the antibiotic-resistance are increasingly being reported for hvKP isolates across the world, most commonly in countries with an epidemic dissemination of hvKP, which correlates with our study.
Table 5.
Antibiotics resistance pattern of extreme drug resistance, pan drug resistance, extended-spectrum beta-lactamase and multidrug resistance patients, and their corresponding minimal inhibitory concentration values as per CLSI
| n=23 | AMI (>32) | TOB (>8) | CFZ (>32) | NOR (>8) | CIP (>2) | GEN (>2) | COL (>4) | CHL (>18) | CFP (>16) | IMI (>16) | MER (>16) | CFT (>32) | ERT (>4) | TG (>2) | AMP (>16) | CXT (>32) | TR-SM (>8/152) | PC-TB (>64/4) | AM-CA (>16/8) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X-1 | S | R | R | R | R | R | S | R | R | R | R | R | R | R | R | R | R | R | R |
| X-2 | R | R | R | R | R | R | S | S | R | R | R | R | R | R | R | R | S | R | R |
| X-3 | R | R | R | R | R | R | R | S | R | R | R | R | R | R | R | R | R | R | R |
| X-4 | R | R | R | R | R | R | S | R | R | R | R | R | R | R | R | R | R | R | R |
| X-5 | R | R | R | R | R | R | S | R | R | R | R | R | R | R | R | R | R | R | R |
| X-6 | R | R | R | R | R | R | S | R | R | R | R | R | R | I | R | R | R | R | R |
| X-7 | R | R | R | R | R | R | S | R | R | R | R | R | R | R | R | R | R | R | R |
| X-8 | R | R | R | R | R | R | S | S | R | R | R | R | R | R | R | R | R | R | R |
| X-9 | R | R | R | R | R | R | S | R | R | R | R | R | R | R | R | R | S | R | R |
| X-10 | R | R | R | R | R | R | S | R | R | R | R | R | R | R | R | R | R | R | R |
| X-11 | R | R | R | R | R | R | S | R | R | R | R | R | R | R | R | R | R | R | R |
| X-12 | R | R | R | R | R | R | S | R | R | R | R | R | R | R | R | R | R | R | R |
| X-13 | R | R | R | R | R | R | S | R | R | R | R | R | R | R | R | R | R | R | R |
| X-14 | R | R | R | R | R | S | S | R | R | R | R | R | R | R | R | R | R | R | R |
| X-15 | R | R | R | R | R | R | S | R | R | R | R | R | R | S | R | R | R | R | R |
| X-16 | R | R | R | R | R | S | S | R | R | R | R | R | R | R | R | R | R | R | R |
| X-17 | R | R | R | R | R | R | S | R | R | R | R | R | R | R | R | R | R | R | R |
| E-1 | R | R | R | R | R | R | S | S | R | S | S | R | S | S | R | S | R | S | R |
| E-2 | S | R | R | R | S | S | S | S | I | S | S | R | S | NT | R | R | R | S | R |
| E-3 | S | S | R | R | S | S | S | S | R | S | S | R | S | S | R | S | S | S | R |
| P-1 | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| P-2 | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| M-1 | S | R | R | R | R | R | S | S | R | R | R | R | R | R | R | R | R | R | R |
MIC value for colistin sensitivity is <1 for all sensitive patients except X-7 (<0.5); MIC value for amikacin sensitivity is <4 for all sensitive patients; MIC value for imipenem sensitivity is <0.5, for meropenem is <0.125 and for ertapenem is <0.125 for all sensitive patients; MIC value for tigecycline sensitivity is <1 and for intermediate is 2. MIC value for trimethoprim-sulfamethoxazole is <1/19 for all sensitive patients; MIC value for piperacillin-tazobactam is 16/4 for E-1 and 4/4 for E-2 and E-3; MIC value for cefepime intermediate sensitivity is 8; MIC vale for chloramphenicol sensitivity is <8; MIC values for resistance is mentioned adjacent to antibiotics headers. X: Extreme drug resistance, P: Pan drug resistance, M: Multidrug resistance, I: Intermediate resistance, NT: Not tested, MIC: Minimal inhibitory concentration; AMI: Amikacin; TOB: Tobramycin; CFZ: Cefazolin; NOR: Norfloxacin, CIP: Ciprofloxacin, GEN: Gentamicin, COL: Colistin, CFP: Cefepime, IMI: Imipenem, MER: Meropenem, CFT: Ceftriaxone, ERT: Ertapenem, TG: Tigecycline, CXT: Cefoxitin, AMP: Ampicillin, TR/SM: Trimethoprim/sulfamethoxazole, PC/TB: piperacillin/tazobactam, AM/CA: Amoxicillin/clavulanic acid, CLSI: Clinical & Laboratory Standards Institute
Table 6.
Clinical profile of all resistant isolates
| ID | Age/sex | Culture | Clinical details | Outcome |
|---|---|---|---|---|
| X-1 | 51/male | Blood | Postcardiac arrest, secondary to STEMI with refractory septic shock | Dead |
| X-2 | 62/male | Trachea | Medullary hemangioblastoma, meningitis, VAP | Discharge |
| X-3 | 55/male | Trachea | DM, left lower limb diabetic foot with sepsis and septic shock, s/p debridement, respiratory failure | DAMA |
| X-4 | 20/male | Trachea | Severe necrotizing pancreatitis with refractory septic shock, multiorgan failure | Dead |
| X-5 | 51/male | Trachea | Advanced COPD, respiratory failure secondary to pneumonia | Dead |
| X-6 | 24/male | PUS | Necrotizing pancreatitis, right side empyema with pleura parenchymal fistula | Dead |
| X-7 | 47/male | Trachea | DM, HTN, CAD, CKD on maintenance HD, s/p cardiac arrest, pulmonary TB with respiratory failure | Discharge |
| X-8 | 70/male | Tissue | DM, HTN, malignancy MCA infarct, s/p cardiac arrest with anoxic encephalopathy, Grade IV bed sore, septic shock | DAMA |
| X-9 | 56/male | Tissue | CKD on maintenance HD, rhinocerebral mucormycosis | Dead |
| X-10 | 52/female | Trachea | Cardioembolic stroke and brain stem infarct with respiratory failure on mechanical ventilation | Dead |
| X-11 | 23/male | Trachea | Acute necrotizing pancreatitis status postpancreatic necrosectomy. | Discharge |
| X-12 | 77/male | Trachea | DM, HTN, old pulmonary koch’s with TB sequelae, hydropneumothorax s/p intercostal drain | Dead |
| X-13 | 57/female | Trachea | Alzheimer’s disease, recent H1N1 pneumonia, DVT with HCAP and gastroenteritis | Discharge |
| X-14 | 73/male | Tissue | Atypical Parkinson’s, Grade IV bed sore infected, septic shock | Dead |
| X-15 | 43/male | Blood | DM, acute myeloid leukemia-relapse with ischemic stroke and fungal pneumonia | Dead |
| X-16 | 22/male | Trachea | Autoimmune encephalitis and seizures. Intubated in view of seizures and extubated after 6 days of ventilator support | Discharge |
| X-17 | 52/male | Blood | Morbid obesity, severe ARDS secondary to H1N1 pneumonia and rhabdomyolysis, sudden cardiac arrest secondary to massive pulmonary embolism | Dead |
| E-1 | 54/female | Blood | Hepatitis-C, CKD on maintenance HD, catheter related blood stream infection | Discharge |
| E-2 | 46/male | Trachea | DCLD, bilateral lower limb cellulitis, septic shock, respiratory failure on ventilator | Dead |
| E-3 | 55/male | Tissue | Diabetic foot with sepsis, s/p debridement | Discharge |
| P-1 | 77/male | Blood | Parkinson’s, CKD on maintenance HD, recurrent UTI, catheter related blood stream infection | Dead |
| P-2 | 58/male | Blood | Old SAH, bed ridden status, pneumonia with Respiratory failure | Dead |
| M-1 | 62/female | Trachea | Massive intracerebral bleed, s/p decompression, Grade III bed sore | Discharge |
STEMI: ST-segment-elevation MI, CKD: Chronic kidney disease, HD: Hemodialysis, s/p: Status post, SAHL: Subarachnoid hemorrhage, DAMA: Discharge against medical advice, UTI: Urinary tract infection, ARDS: Acute respiratory distress syndrome, HCAP: Healthcare-associated pneumonia, DVT: Deep vein thrombosis, DM: Diabetes mellitus, HTN: Hypertension
Twenty-five percentage of patients had pan sensitive, out of whom 4 patients were dead (50%), which imply that in vitro sensitivity did not correlate with the in vivo scenario. This might be the indicator, that the existing antibiotic strategy might not be adequate enough to penetrate the thick mucoid layer.
We found, about 90% of hvKP isolates are sensitive to colistin in our study, however 2 (6.3%) isolates were pan resistant, which is a major concern. The antibiotic resistance is a multifactorial complex process.[26]
Studies from different regions reported lower rate of hvKP incidence than in Asia. A study in Spain reported 5.4% of hypermucoviscosity phenotype,[27] a Canadian study reported 8.2% of hvKP from patients with community-acquired bacteremia.[28] Similarly, 6.7% of isolates from Brazil[29] and 9.2% of isolates from Algeria were reported in the literature.[30] All the above results are supporting that the incidence and spread of hvKP are more common in the Asian region. Our study showed 19.4% hvKP among the Klebsiella isolates from the patients got admitted under MICU for different medical conditions.
Limitations of the study
This study did not compare the clinical characteristics and outcome of hvKp with conventional Klebsiella.
CONCLUSIONS
Our study results showed, increased incidence of HAI with hvKP predominantly associated with pneumonia and increase in trend of drug resistance with two cases being pan resistant. The mortality rate in hvKP was higher than Klebsiella and it was similar in pan sensitive and drug resistant hvKP. It requires a simple technique (string test) to detect hvKP, and advisable to include this technique in the routine clinical practice since hvKP has higher attributable mortality. More number of studies are required to evaluate the existing antibiotics strategy and steps to curb the spread of this dreaded infection.
Research quality and ethics statement
The authors of this manuscript declare that this scientific work complies with reporting quality, formatting and reproducibility guidelines set forth by the EQUATOR Network. The authors also attest that this clinical investigation was determined to require the Institutional Review Board/Ethics Committee review, and the corresponding protocol/approval number is NHH/MEC-CL-2018-501. We also certify that we have not plagiarized the contents in this submission and have done a Plagiarism Check.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors would like to acknowledge Anitha and Sandhya for their help in data collection.
REFERENCES
- 1.Friedlaender C. About the Schizomycetes in Acute Fibrous Pneumonia. Archives f. pathol. Anat. 1882;87:319–24. [Google Scholar]
- 2.Delle Rose D, Sordillo P, Gini S, Cerva C, Boros S, Rezza G, et al. Microbiologic characteristics and predictors of mortality in bloodstream infections in intensive care unit patients: A 1-year, large, prospective surveillance study in 5 Italian hospitals. Am J Infect Control. 2015;43:1178–83. doi: 10.1016/j.ajic.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 3.Cheng DL, Liu YC, Yen MY, Liu CY, Wang RS. Septic metastatic lesions of pyogenic liver abscess. Their association with Klebsiella pneumoniae bacteremia in diabetic patients. Arch Intern Med. 1991;151:1557–9. [PubMed] [Google Scholar]
- 4.Wu H, Li D, Zhou H, Sun Y, Guo L, Shen D. Bacteremia and other body site infection caused by hypervirulent and classic Klebsiella pneumoniae. Microb Pathog. 2017;104:254–62. doi: 10.1016/j.micpath.2017.01.049. [DOI] [PubMed] [Google Scholar]
- 5.Nadasy KA, Domiati-Saad R, Tribble MA. Invasive Klebsiella pneumoniae syndrome in North America. Clin Infect Dis. 2007;45:e25–8. doi: 10.1086/519424. [DOI] [PubMed] [Google Scholar]
- 6.Fang CT, Chuang YP, Shun CT, Chang SC, Wang JT. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J Exp Med. 2004;199:697–705. doi: 10.1084/jem.20030857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatterjee S, Adhikari A, Ghosh RR, Chatterjee N, Bhattacharyya K, Bhattacharya S. Evaluation of virulent multidrug resistant Klebsiella infection status in a tertiary care hospital in Eastern India. J Indian Med Assoc. 2012;110:815–8. [PubMed] [Google Scholar]
- 8.Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: A new invasive syndrome. Lancet Infect Dis. 2012;12:881–7. doi: 10.1016/S1473-3099(12)70205-0. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Yan Y, Xue X, Wang K, Shen D. Comparison of pyogenic liver abscesses caused by hypermucoviscous Klebsiella pneumoniae and non-Klebsiella pneumoniae pathogens in Beijing: A retrospective analysis. J Int Med Res. 2013;41:1088–97. doi: 10.1177/0300060513487645. [DOI] [PubMed] [Google Scholar]
- 10.Yao B, Xiao X, Wang F, Zhou L, Zhang X, Zhang J. Clinical and molecular characteristics of multi-clone carbapenem-resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae isolates in a tertiary hospital in Beijing, China. Int J Infect Dis. 2015;37:107–12. doi: 10.1016/j.ijid.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Zeng J, Liu W, Zhao F, Hu Z, Zhao C, et al. Emergence of a hypervirulent carbapenem-resistant Klebsiella pneumoniae isolate from clinical infections in China. J Infect. 2015;71:553–60. doi: 10.1016/j.jinf.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Zhan L, Wang S, Guo Y, Jin Y, Duan J, Hao Z, et al. Outbreak by Hypermucoviscous Klebsiella pneumoniae ST11 Isolates with Carbapenem Resistance in a Tertiary Hospital in China. Front Cell Infect Microbiol. 2017;7:182. doi: 10.3389/fcimb.2017.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 14.Prokesch BC, TeKippe M, Kim J, Raj P, TeKippe EM, Greenberg DE. Primary osteomyelitis caused by hypervirulent Klebsiella pneumoniae. Lancet Infect Dis. 2016;16:e190–5. doi: 10.1016/S1473-3099(16)30021-4. [DOI] [PubMed] [Google Scholar]
- 15.Shon AS, Bajwa RP, Russo TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: A new and dangerous breed. Virulence. 2013;4:107–18. doi: 10.4161/viru.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Struve C, Roe CC, Stegger M, Stahlhut SG, Hansen DS, Engelthaler DM, et al. Mapping the evolution of hypervirulent Klebsiella pneumoniae. mBio. 2015;6:e00630. doi: 10.1128/mBio.00630-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paczosa MK, Mecsas J. Klebsiella pneumoniae: Going on the offense with a strong defense. Microbiol Mol Biol Rev. 2016;80:629–61. doi: 10.1128/MMBR.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee CR, Lee JH, Park KS, Jeon JH, Kim YB, Cha CJ, et al. Antimicrobial resistance of hypervirulent Klebsiella pneumoniae epidemiology, hypervirulence-associated determinants, and resistance mechanisms. Front Cell Infect Microbiol. 2017;7:483. doi: 10.3389/fcimb.2017.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko WC, Paterson DL, Sagnimeni AJ, Hansen DS, Von Gottberg A, Mohapatra S, et al. Community-acquired Klebsiella pneumoniae bacteremia: Global differences in clinical patterns. Emerg Infect Dis. 2002;8:160–6. doi: 10.3201/eid0802.010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL, Chang SC. Klebsiella pneumoniae genotype K1: An emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis. 2007;45:284–93. doi: 10.1086/519262. [DOI] [PubMed] [Google Scholar]
- 21.Ku YH, Chuang YC, Yu WL. Clinical spectrum and molecular characteristics of Klebsiella pneumoniae causing community-acquired extrahepatic abscess. J Microbiol Immunol Infect. 2008;41:311–7. [PubMed] [Google Scholar]
- 22.Decré D, Verdet C, Emirian A, Le Gourrierec T, Petit JC, Offenstadt G, et al. Emerging severe and fatal infections due to Klebsiella pneumoniae in two university hospitals in France. J Clin Microbiol. 2011;49:3012–4. doi: 10.1128/JCM.00676-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Branswell H. WHO Releases List of World's Most Dangerous Superbugs. [Last accessed on 2019 Sep 11]. Available from: https://www.scientificamerican.com/article/who-releases-list-of-worlds-most-dangerous-superbugs/
- 24.Catalán-Nájera JC, Garza-Ramos U, Barrios-Camacho H. Hypervirulence and hypermucoviscosity: Two different but complementary Klebsiella spp. phenotypes? Virulence. 2017;8:1111–23. doi: 10.1080/21505594.2017.1317412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel PK, Russo TA, Karchmer AW. Hypervirulent Klebsiella pneumoniae. Open Forum Infect Dis. 2014;1:ofu028. doi: 10.1093/ofid/ofu028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watkins RR, Bonomo RA. Overview: Global and local impact of antibiotic resistance. Infect Dis Clin North Am. 2016;30:313–22. doi: 10.1016/j.idc.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Cubero M, Grau I, Tubau F, Pallarés R, Dominguez MA, Liñares J, et al. Hypervirulent Klebsiella pneumoniae clones causing bacteraemia in adults in a teaching hospital in Barcelona, Spain (2007-2013) Clin Microbiol Infect. 2016;22:154–60. doi: 10.1016/j.cmi.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 28.Peirano G, Pitout JD, Laupland KB, Meatherall B, Gregson DB. Population-based surveillance for hypermucoviscosity Klebsiella pneumoniae causing community-acquired bacteremia in Calgary, Alberta. Can J Infect Dis Med Microbiol. 2013;24:e61–4. doi: 10.1155/2013/828741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pereira SC, Vanetti MC. Potential virulence of Klebsiella sp. isolates from enteral diets. Braz J Med Biol Res. 2015;48:782–9. doi: 10.1590/1414-431X20154316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El Fertas-Aissani R, Messai Y, Alouache S, Bakour R. Virulence profiles and antibiotic susceptibility patterns of Klebsiella pneumoniae strains isolated from different clinical specimens. Pathol Biol (Paris) 2013;61:209–16. doi: 10.1016/j.patbio.2012.10.004. [DOI] [PubMed] [Google Scholar]


