Abstract
Elizabethkingia anophelis infections are emerging, especially among premature newborns, immunocompromised, and critically ill patients. The importance of correctly identifying this Gram-negative organism lies in the fact that it is associated with fatal complications such as meningitis, acute pulmonary edema, congestive cardiac failure, septic shock, and death. In addition, it is inherently resistant to multiple antibiotics which are used to treat Gram-negative bacilli. Here, we report a case of E. anophelis related meningitis and septicemia in a preterm neonate along with a brief review of literature.
Keywords: Elizabethkingia anophelis, meningitis, neonate, preterm, septicemia
INTRODUCTION
Elizabethkingia anophelis is an opportunistic pathogen that causes infection mainly in neonates, immunocompromised patients, and critically ill patients. Neonates, especially preterm, suffering from E. anophelis meningitis or septicemia, in the first two weeks of life have high chances of mortality.[1] Following meningitis, the patients may develop various neurological complications such as hydrocephalus, deafness, and developmental delay.[2] Prompt identification of the organism can help in early institution of the appropriate therapy.
CASE REPORT
One preterm male neonate (11 days old, 2.3 kg) born by cesarean section was referred to our hospital with fever and abnormal body movements (tonic posturing) since 3 days and up rolling of eyes for 2 days. The birth history was insignificant. On examination, the neonate had body temperature (T): 98.8°F, heart rate: 112/min, SpO2(percentage of oxygen saturation): 92% on oxygen supplementation, pupils bilaterally normal in size and reactive to light stimuli and bulging anterior fontanelle. Chest X-ray revealed patchy nonhomogeneous opacities in the right upper and mid zone suggesting bronchopneumonia. Due to progressive respiratory distress and hypoxia, the neonate was intubated and mechanically ventilated. A probable diagnosis of septicemia with acute bacterial meningitis was made and antibiotics namely injection gentamicin 16 mg intravenous (IV) once daily and injection ampicillin 300 mg IV 8 h were started empirically.
Initial blood investigations revealed high C-reactive protein (164.9 mg/L) and procalcitonin (7.18 ng/ml). Cerebrospinal fluid (CSF) analysis from lumbar puncture revealed cell count of >1000/μL of which 74% were neutrophils, protein - 216 mg/dl, lactate - 73.7 mg/dl, and glucose level below the measuring range. Gram stain of CSF showed numerous polymorphonuclear leucocytes and Gram-negative bacilli. Blood culture was performed. Urine culture was also performed and was sterile. Both blood and CSF samples were inoculated in Paeds Plus/F bottles and incubated in BD BACTEC FX40 (Becton Dickinson Diagnostics, USA). Both the bottles flagged positive signal after 12 h. The organism was Gram-negative bacillus which was nonfermentative and oxidase positive. The antibiotics were changed to injection meropenem and injection amikacin. Identification and antibiotic sensitivity testing was done using the BD Phoenix automated identification and susceptibility testing system (Becton Dickinson Diagnostics, USA). Both blood and CSF cultures revealed the growth of Elizabethkingia meningoseptica. Based on the organism's identification and a brief literature review, the antibiotics were changed to injection vancomycin 50 mg IV 6 h and injection piperacillin (1.125 g) - tazobactam (330 mg) IV 6 h. The Clinical and Laboratory Standards Institute criteria for Gram-negative bacteria were used to interpret the antimicrobial susceptibility as no recommendations regarding break-points are available for this rare pathogen and showed that the isolate was only sensitive to ciprofloxacin, levofloxacin, and co-trimoxazole and resistant to piperacillin-tazobactam. Vancomycin minimum inhibitory concentration (MIC) testing could not be performed due to unavailability of E-strips. MICs of the organism with respect to various antibiotics are shown in Table 1. After the antibiotic sensitivity report, ciprofloxacin was also added to the treatment regimen and piperacillin-tazobactam was continued though it had a higher MIC > 64/4 μg/ml. Appropriate infection control measures were taken to prevent the spread of the organism in the neonatal intensive care unit. The neonate recovered and was discharged after 25 days.
Table 1.
Minimum inhibitory concentration of antibiotics determined by the BD phoenix automated system or broth Microdilution Method
| Name of the antibiotic | MIC (µg/mL) |
|---|---|
| Amikacin | >32 |
| Gentamicin | >8 |
| Imipenem | >8 |
| Meropenem | >8 |
| Cefazolin | >16 |
| Ceftazidime | >16 |
| Cefotaxime | >32 |
| Cefepime | >16 |
| Cefoperazone-sulbactam | >16/8 |
| Cefoxitin | 8 |
| Aztreonam | >16 |
| Ampicillin | >16 |
| Piperacillin | >64 |
| Amoxicillin-Clavulanic acid | >16/8 |
| Piperacillin-tazobactam | >64/4 |
| Colistin | >2 |
| Co-trimoxazole | 2/38 |
| Chloramphenicol | >16 |
| Ciprofloxacin | 1 |
| Levofloxacin | ≤1 |
MIC: Minimum inhibitory concentration
Elizabethkingia isolates from both CSF and blood culture were subjected to 16S ribosomal RNA (rRNA) gene sequencing for species confirmation. The Elizabethkingia isolates which were identified as E. meningospeticum based on biochemical phenotyping in the BD Phoenix system were found to be E. anophelis based on 16S rRNA gene sequencing report. The 16S rRNA gene sequence of E. anophelis isolated from CSF and blood have been deposited in the GenBank sequence database under accession no. MK811253 and MK811254, respectively [Figure 1].
Figure 1.
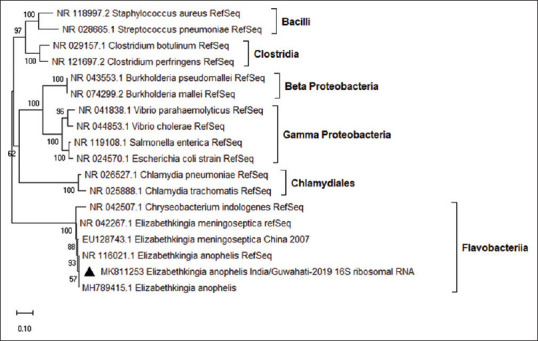
Elizabethkingia anophelis partial 16S ribosomal RNA (>872 bp) was analyzed for evolutionary history (phylogenetic tree) using the maximum likelihood method and Kimura 2-parameter model with gamma distribution for evolutionary rate, based on best-fit model testing. A 100 nos. of bootstrap replicate was performed to choose the best tree. This analysis involved 18 nucleotide sequences including representative reference sequences of different families of bacteria. Evolutionary analyses were conducted in MEGA X software
DISCUSSION
Elizabethkingia species are nonmotile, nonfastidious, and glucose nonfermentative Gram-negative bacilli under the family Flavobacteriaceae, with three species E. meningoseptica, Elizabethkingia miricola, and E. anophelis, known to cause human clinical infections.[1] E. anophelis which was initially isolated from the mid-gut of the mosquito Anopheles gambiae, was soon reported to be a clinically significant pathogen causing septicemia.[3,4,5] E. anophelis is the dominant species of the genus Elizabethkingia causing septicemia and is associated with fulminant complications such as acute pulmonary edema, congestive cardiac failure, septic shock, and death. Infection due to E. anophelis should always be considered as clinically significant unless proven otherwise.[3] E. anophelis is ubiquitously found in hospital environments and has been found to be associated with severe nosocomial infections related to contaminated medical devices. In our case, since the neonate was referred to us from a different hospital, so the exact source of infection could not be ascertained.
There are several reports of mis-identification of E. anophelis as E. meningoseptica by Automated systems namely Vitek-2, API.[5,6,7] Lau et al. in 2015 reported the failure of Matrix-Assisted Laser Desorption/Ionization-Time Of Flight Mass Spectrometry (MALDI-TOF MS) to correctly identify E. anophelis,[8] while Chew et al. in 2018 reported that 78/79 (98.7%) of the isolates which were identified as either E. meningosepticum (96.2% isolates) or E. miricola (3.8%) by the Bruker MALDI Biotyper (bioMérieux), were actually identified as E. anophelis by 16S rRNA gene sequencing.[9] In this case too, BD Phoenix could not correctly identify the species and may require a data base upgradation to include E. anophelis and other species as well.
A brief literature review showed the successful use of vancomycin, piperacillin-tazobactam, ciprofloxacin, etc., in E. meningitis and septicemia cases.[10,11,12] In our case also, combination regimen of vancomycin, piperacillin-tazobactam, and ciprofloxacin administered to the patient yielded favorable outcome. Fluoroquinolones especially ciprofloxacin should be preferred due to their better penetration through the blood–brain barrier. Moreover, these drugs are not significantly affected by the variation of volume distribution during sepsis.[13] In a previous study by Lau et al., all the 17 isolates of E. anophelis were sensitive to cefoperazone-sulbactam along with ciprofloxacin and vancomycin.[3] However, in this case, the isolate was resistant to cefoperazone-sulbactam. As E. meningoseptica and E. miricola have similar antibiotic susceptibility profiles as E. anophelis, so for better clinical outcomes, a combination of two or three drugs namely vancomycin, ciprofloxacin, piperacillin-tazobactam, and cefoperazone-sulbactam can be administered empirically as soon as the identification of Elizabethkingia is known and can be modified later as per the sensitivity report. The importance of correctly identifying Elizabethkingia spp. isolates lies in the fact that it has a peculiar antibiotic sensitivity pattern. Hence, in this era of automated identification systems, the microbiologist should be alert and the organism's identification should be made known to the clinicians as soon as possible to facilitate the timely administration of the specific antibiotics.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Jian MJ, Cheng YH, Perng CL, Shang HS. Molecular typing and profiling of topoisomerase mutations causing resistance to ciprofloxacin and levofloxacin in Elizabethkingia species. PeerJ. 2018;6:e5608. doi: 10.7717/peerj.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhat KS, Priya R, Krishnan L, Kanungo R. Elizabethkingia meningoseptica bacteremia in a neonate: A case report and mini-review of the literature. J Curr Res Sci Med. 2016;2:42–5. [Google Scholar]
- 3.Lau SK, Chow WN, Foo CH, Curreem SO, Lo GC, Teng JL, et al. Elizabethkingia anophelis bacteremia is associated with clinically significant infections and high mortality. Sci Rep. 2016;6:26045. doi: 10.1038/srep26045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perrin A, Larsonneur E, Nicholson AC, Edwards DJ, Gundlach KM, Whitney AM, et al. Evolutionary dynamics and genomic features of the Elizabethkingia anophelis 2015 to 2016 Wisconsin outbreak strain. Nat Commun. 2017;8:15483. doi: 10.1038/ncomms15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teo J, Tan SY, Tay M, Ding Y, Kjelleberg S, Givskov M, et al. First case of E anophelis outbreak in an intensive-care unit. Lancet. 2013;382:855–6. doi: 10.1016/S0140-6736(13)61858-9. [DOI] [PubMed] [Google Scholar]
- 6.Lin JN, Lai CH, Yang CH, Huang YH, Lin HF, Lin HH. Comparison of four automated microbiology systems with 16S rRNA gene sequencing for identification of Chryseobacterium and Elizabethkingia species. Sci Rep. 2017;7:13824. doi: 10.1038/s41598-017-14244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank T, Gody JC, Nguyen LB, Berthet N, Le Fleche-Mateos A, Bata P, et al. First case of Elizabethkingia anophelis meningitis in the Central African Republic. Lancet. 2013;381:1876. doi: 10.1016/S0140-6736(13)60318-9. [DOI] [PubMed] [Google Scholar]
- 8.Lau SK, Wu AK, Teng JL, Tse H, Curreem SO, Tsui SK, et al. Evidence for Elizabethkingia anophelis transmission from mother to infant, Hong Kong. Emerg Infect Dis. 2015;21:232–41. doi: 10.3201/eid2102.140623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chew KL, Cheng B, Lin RT, Teo JW. Elizabethkingia anophelis is the dominant Elizabethkingia species found in blood cultures in Singapore. J Clin Microbiol. 2018;56(3):1445–17. doi: 10.1128/JCM.01445-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin PY, Chen HL, Huang CT, Su LH, Chiu CH. Biofilm production, use of intravascular indwelling catheters and inappropriate antimicrobial therapy as predictors of fatality in Chryseobacterium meningosepticum bacteraemia. Int J Antimicrob Agents. 2010;36:436–40. doi: 10.1016/j.ijantimicag.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 11.Issack MI, Neetoo Y. An outbreak of Elizabethkingia meningoseptica neonatal meningitis in Mauritius. J Infect Dev Ctries. 2011;5:834–9. doi: 10.3855/jidc.1885. [DOI] [PubMed] [Google Scholar]
- 12.Arbune M, Fotea S, Nechita A, Stefanescu V. Emerging infection with Elizabethkingia meningoseptica in neonate. A case report. J Crit Care Med (Targu Mures) 2018;4:96–100. doi: 10.2478/jccm-2018-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang YC, Lin YT, Wang FD. Comparison of the therapeutic efficacy of fluoroquinolone and non-fluoroquinolone treatment in patients with Elizabethkingia meningoseptica bacteraemia. Int J Antimicrob Agents. 2018;51:47–51. doi: 10.1016/j.ijantimicag.2017.05.018. [DOI] [PubMed] [Google Scholar]


