Abstract
Background
During the COVID-19 pandemic, men who have sex with men (MSM) in the USA have reported similar or fewer sexual partners and reduced HIV testing and care access compared with before the pandemic. Pre-exposure prophylaxis (PrEP) use has also declined. We aimed to quantify the potential effect of COVID-19 on HIV incidence and HIV-related mortality among US MSM.
Methods
We used a calibrated, deterministic, compartmental HIV transmission model for MSM in Baltimore (MD, USA) and available data on COVID-19-related disruptions to HIV services to predict effects of reductions in sexual partners (0%, 25%, 50%), condom use (5%), HIV testing (20%), viral suppression (10%), PrEP initiations (72%), PrEP adherence (9%), and antiretroviral therapy (ART) initiations (50%). In our main analysis, we modelled disruptions due to COVID-19 starting Jan 1, 2020, and lasting 6 months. We estimated the median change in cumulative new HIV infections and HIV-related deaths among MSM over 1 and 5 years, compared with a base case scenario without COVID-19-related disruptions.
Findings
A 25% reduction in sexual partners for 6 months among MSM in Baltimore, without HIV service changes, could reduce new HIV infections by median 12·2% (95% credible interval 11·7 to 12·8) over 1 year and median 3·0% (2·6 to 3·4) over 5 years. In the absence of changes in sexual behaviour, the 6-month estimated reductions in condom use, HIV testing, viral suppression, PrEP initiations, PrEP adherence, and ART initiations combined are predicted to increase new HIV infections by median 10·5% (5·8 to 16·5) over 1 year, and by median 3·5% (2·1 to 5·4) over 5 years. Disruptions to ART initiations and viral suppression are estimated to substantially increase HIV-related deaths (ART initiations by median 1·7% [0·8 to 3·2], viral suppression by median 9·5% [5·2 to 15·9]) over 1 year, with smaller proportional increases over 5 years. The other individual disruptions (to HIV testing, PrEP and condom use, PrEP initiation, and partner numbers) were estimated to have little effect on HIV-related deaths (<1% change over 1 or 5 years). A 25% reduction in sexual partnerships is estimated to offset the effect of the combined service disruptions on new HIV infections (change over 1 year: median –3·9% [–7·4 to 1·0]; over 5 years: median 0·0% [–0·9 to 1·4]), but not on HIV deaths (change over 1 year: 11·0% [6·2 to 17·7]; over 5 years: 2·6% [1·5 to 4·3]).
Interpretation
Maintaining access to ART and adherence support is of the utmost importance to maintain viral suppression and minimise excess HIV-related mortality due to COVID-19 restrictions in the USA, even if disruptions to services are accompanied by reductions in sexual partnerships.
Funding
National Institutes of Health.
Introduction
The COVID-19 pandemic and responses to it have disrupted HIV prevention and treatment services around the world, and have influenced sexual behaviour, with consequences for HIV transmission and mortality.1, 2, 3, 4
Mathematical modelling can draw together information on HIV epidemiology and care, and disruptions caused by COVID-19, to estimate the potential effect of these disruptions on HIV transmission and mortality, and identify where efforts should be prioritised.5 Modelling for sub-Saharan Africa suggests that a 6-month interruption of antiretroviral therapy (ART) due to COVID-19 for 50% of people with HIV could lead to 39–87% more HIV-related deaths over the next year.6 Modelling for the USA projected that increases in HIV incidence due to disruptions to HIV screening, pre-exposure prophylaxis (PrEP), and ART could be offset by similar reductions in sexual activity.7, 8
In the USA, COVID-19 prevention and mitigation programmes included stay-at-home orders and venue closures that might limit access to in-person medical care and opportunities for sexual encounters with partners in other households.9, 10 Reduced provision of in-person medical care has been reported as health-care staff were diverted to the COVID-19 response and physical distancing was implemented;11, 12, 13, 14 decreased viral suppression was subsequently observed among people with HIV in San Francisco (CA, USA).15 In Baltimore (MD, USA), which has very high HIV prevalence (37% in 201716) among gay, bisexual, and other men who have sex with men (MSM), stay-at-home orders were in effect from March 30 to June 8, 2020. In late April, 2020, reduced testing capacity for HIV and sexually transmitted infections was reported in Baltimore, with many health department staff focusing on the COVID-19 response.11 In the USA, MSM—among whom, 70% of all new HIV infections occur nationally—reported fewer sexual partners because of COVID-19 in two national surveys,2, 10 but slightly increased or stable partner numbers in two other surveys,17, 18 all done in April–May, 2020. In these surveys, MSM also reported reduced access to HIV testing,2, 17 care,2 and PrEP2, 17 due to COVID-19. Although screening for HIV and bacterial sexually transmitted infections at a Boston (MA, USA) community health centre decreased by 81% early in the pandemic, the test positivity rate for gonorrhoea and chlamydia increased, suggesting ongoing condomless anal sex by a subset of the population.12
Research in context.
Evidence before this study
The COVID-19 pandemic and responses to it have disrupted HIV prevention and treatment services and led to changes in sexual behaviour in the USA, but the overall potential effect on HIV transmission and HIV-related mortality is not known. We searched PubMed for primary research articles documenting COVID-19-related disruptions to HIV prevention and treatment and changes in sexual behaviour in the USA, published Jan 1–Oct 7, 2020, with no language restrictions, using the terms “COVID*” AND (“HIV” OR “AIDS”) AND (“United States” OR “US”). We identified three cross-sectional surveys assessing changes in sexual partner numbers among men who have sex with men (MSM) in the USA, one finding a reduction, one a slight increase, and one no change in sexual partner numbers during COVID-19 restrictions. Two of these studies also found reductions in reported HIV testing, HIV care, or access to pre-exposure prophylaxis (PrEP) among MSM due to COVID-19. A separate study from a San Francisco (CA, USA) clinic found declines in viral suppression among its clients during lockdown. We searched PubMed for articles estimating the effect of COVID-19-related disruptions on HIV transmission and HIV-related mortality published Jan 1–Oct 12, 2020, with no language restrictions, using the following search terms: “COVID*” AND “model*” AND (“HIV” OR “AIDS”). We identified two published studies that had used mathematical modelling to estimate the effect of hypothetical COVID-19-related disruptions to HIV programmes on HIV-related deaths or new HIV infections in Africa, another published study using modelling to estimate the effect of COVID-19-related disruptions and linked HIV and SARS-CoV-2 testing on new HIV infections in six cities in the USA, and an unpublished study reporting modelling of the effect of COVID-19-related disruptions on HIV incidence among MSM in Atlanta (GA, USA). None of these studies were informed by data on the size of COVID-19-related disruptions. The two African studies and the Atlanta study assessed the effect of disruptions to different health-care services separately, and all found that the greatest negative effects on new HIV infections or deaths would arise from interruptions to antiretroviral therapy (ART) provision. All three studies found smaller effects on HIV-related mortality or incidence from other health-care disruptions, including HIV testing, PrEP initiation, and condom supplies. The US study assessing the effect of linked HIV and SARS-CoV-2 testing estimated that this could substantially reduce HIV incidence.
Added value of this study
We used mathematical modelling to derive estimates of the potential effect of the COVID-19 pandemic and associated restrictions on HIV incidence and mortality among MSM in the USA, directly informed by data for the USA on disruptions to HIV testing, ART, and PrEP services and reported changes in sexual behaviour during the COVID-19 pandemic.
Implications of all the available evidence
In the USA, maintaining access to ART and adherence support for both existing and new users will be crucial to minimise excess HIV-related deaths arising from the COVID-19 pandemic among MSM. Although reductions in numbers of sexual partners might offset increases in new HIV infections arising from disruptions to HIV prevention and treatment services, this will not offset the additional HIV-related deaths that are also predicted to occur. There are mixed findings on the effect of an HIV testing campaign among US MSM during the COVID-19 lockdown. Together with previous studies, our study highlights the importance of maintaining effective HIV treatment provision during the COVID-19 pandemic.
In the UK, an HIV testing campaign (Test Now, Stop HIV) was launched in June, 2020, encouraging MSM and other groups at risk of HIV acquisition to test for HIV during lockdown using home-testing kits, taking advantage of temporary reductions in sex with partners outside the immediate household and hoping to break chains of HIV transmission. Modelling for the USA suggests that offering HIV testing alongside SARS-CoV-2 testing could reduce HIV incidence.8
We used mathematical modelling together with data from a clinic and surveys conducted among MSM to estimate the potential effect of COVID-19-related disruptions on new HIV infections and HIV-related deaths among MSM in Baltimore, and to identify where HIV prevention and treatment efforts should be focused to most effectively mitigate negative effects of COVID-19 on HIV transmission and survival for this population. We assessed the extent to which reductions in numbers of sexual partners might offset the effects of reduced access to HIV care and prevention. We also estimated the potential effect of implementing a hypothetical HIV testing campaign during lockdown in this setting.
Methods
Model
We adapted a previously published, deterministic, compartmental model of sexual HIV transmission and treatment among US MSM19 to include PrEP use. Briefly, the modelled population was divided into mutually exclusive compartments, stratified by age, race, PrEP use, HIV infection stage, set-point viral load, and HIV care engagement. In the model, HIV transmission occurs through main, casual, and commercial sexual partnerships, as a function of numbers of sexual partners, sex acts per partnership, circumcision rates, condom and PrEP use, and HIV infection and ART status of sexual partners. Model equations, schematics, and detailed descriptions are shown in the appendix (pp 2–11).
Model calibration
The model has previously been calibrated to data for MSM in Baltimore.19 Briefly, the model was parameterised with data (on demographics, sexual behaviour, and HIV testing) from the National HIV Behavioral Surveillance (NHBS) for MSM in Baltimore (2004–14), and fitted to data from the NHBS (for demographics, HIV prevalence, and ART coverage), and data from Maryland Department of Health (for HIV care access and viral suppression), for MSM in Baltimore using a Bayesian approach. From 1 million parameter combinations obtained using Latin hypercube sampling, we selected 169 unique parameter combinations (fits) giving outputs consistent with HIV prevalence, demographics, and ART coverage or viral suppression estimates from data up to 2014 (viral suppression validated to 2017). For the current analysis, we parameterised age-specific and race-specific PrEP adherence and dropout using US PrEP Demo Project data20 and calibrated the existing 169 model fits to NHBS PrEP coverage estimates (from 2014 and 2017) by adjusting PrEP uptake rates, keeping other non-PrEP-related parameters the same. Key parameters and care continuum levels in the model fits are shown in table 1 . Full parameter and fitting data tables and plots of model fits are in the appendix (pp 12–25).
Table 1.
Key sexual behaviour parameters, care continuum parameters, and care continuum levels in model fits for 2020 without COVID-19-related disruptions
| Range* | ||
|---|---|---|
| Sexual behaviour parameters | ||
| Mean new anal-sex partners per year for MSM | ||
| Black and aged 18–24 years | 2·35–4·21 | |
| Black and aged >24 years | 1·37–2·55 | |
| White and aged 18–24 years | 0·21–1·51 | |
| White and aged >24 years | 0·43–1·27 | |
| Proportion of sex acts in which condom used | ||
| Main partnerships in which both partners are Black, % | 47–67% | |
| Main partnerships in which either partner is White, % | 30–39% | |
| Casual partnerships with partners of any race, % | 63–72% | |
| Commercial partnerships with partners of any race, % | 21–78% | |
| Care continuum parameters | ||
| Proportion of undiagnosed MSM (not on PrEP) testing for HIV per year | ||
| Black and aged 18–24 years, % | 25·6–94·8% | |
| Black and aged >24 years, % | 20·4–70·1% | |
| White and aged 18–24 years, % | 14·2–81·7% | |
| White and aged >24 years, % | 13·8–69·5% | |
| Care continuum levels in model fits | ||
| Proportion of diagnosed MSM on ART, % | 43–87% | |
| Proportion of MSM on ART who are virally suppressed, % | 76–93% | |
| Proportion of HIV-negative MSM on PrEP, % | 10–21% | |
ART=antiretroviral therapy. MSM=men who have sex with men. PrEP=pre-exposure prophylaxis.
Range across 169 unique parameter combinations fitting to the data.
Scenarios modelled
The base case scenario—to which COVID-19 disruption scenarios were compared—represented the expected course of the HIV epidemic over time if the COVID-19 pandemic and related disruptions had not occurred. Rates of ART initiation, ART adherence, ART dropout, PrEP initiation, PrEP adherence, and PrEP dropout were all assumed to be maintained at their 2019 level from 2020 onwards. Levels of viral suppression and PrEP use increase over 2020–25 in the base case scenario (appendix pp 23–25) due to declines in HIV incidence and earlier increases in PrEP initiation rates.
We modelled data-driven disruptions to HIV testing, condom use, viral suppression, PrEP adherence, and sexual partnerships due to COVID-19, informed by data from four online surveys of US MSM2, 10, 17, 18 and from a study at a Boston PrEP clinic12 in which there were observed reductions in PrEP initiations and in PrEP adherence and HIV testing among those on PrEP (table 2 ; appendix p 26). Where possible, we used age-stratified estimates, race-stratified estimates, or both, to inform age-specific and race-specific disruption estimates (table 2; appendix p 26). Because none of these studies assessed changes to ART initiations, we assumed that these were reduced by 50%, following qualitative reports of general (not specific to MSM) HIV care disruptions in Baltimore.11 Details about how these disruptions were represented in the model are shown in the appendix (p 13).
Table 2.
Summary of magnitudes of disruptions modelled, with sources
| Overall data-driven reduction used in main scenario | Age-specific or race-specific reductions used in main scenario | Overall reduction used in sensitivity analysis | Data sources | |
|---|---|---|---|---|
| HIV testing (off PrEP) | 20% | Age 18–24 years 25%; age ≥25 years 19% | 50%, 75%, 100% | Reported access to testing and difficulty getting an HIV test, overall and by age, national online survey of 1051 US MSM recruited through social media and websites in April–May, 2020;2 consistent with reported prevented access to HIV testing in another national online survey of 518 US MSM recruited through social-networking sites and hook-up apps in April–May, 202017 |
| ART initiations | 50% | .. | 50%, 75%, 100% | Assumption; qualitative reports in April, 2020, of HIV outreach disruptions in Baltimore11 |
| Viral suppression | 10% | White 9%; Black 15% | 10%, 25%, 50% | Reported proportion having fewer viral-load or other laboratory tests, reduced access to HIV medications, difficulty getting an ART prescription, taking medications daily less often—from national online survey of 1051 US MSM;2 race differences from reported ART access in a global online survey of 2732 MSM recruited through a gay social-networking app in April–May, 2020, stratified by whether MSM were members of a racial or ethnic minority group21 |
| PrEP initiations | 72% | .. | 50%, 75%, 100% | Numbers of PrEP initiations at a Boston PrEP clinic in April, 2020, vs January, 2020 (cohort of 3250 patients on PrEP)12 |
| PrEP adherence | 9% | Black, aged 18–24 years 13%; White, aged 18–24 years 11%; Black, aged ≥25 years 9%; White, aged ≥25 years 8% | 10%, 25%, 50% | Numbers of excess missed PrEP refills during lockdown out of the total number of patients on PrEP at a Boston PrEP clinic (cohort of 3250 patients on PrEP),12 reported difficulty getting a PrEP prescription or medication from two national online surveys of US MSM (n=1051 and n=518);2, 17 age and race differences from the proportion of missed PrEP refills by age and race in April, 2020, at a Boston PrEP clinic12 |
| HIV testing (on PrEP) | 85% | .. | 50%, 75%, 100% | From numbers of HIV tests done at a Boston PrEP clinic in April, 2020, vs January, 2020 (cohort of 3250 patients on PrEP)12 |
| Condom use | 5% | .. | 10%, 25%, 50% | From reported change in condom use in a national online survey of 1051 US MSM2 |
| Partner numbers | 0%, 25%, 50% | Age 18–24 years 0%, 22%, 44%; age ≥25 years 0%, 26%, 52% | 10%, 25%, 50% | 0% disruption from change in numbers of casual sex partners in national online MSM cohorts recruited through social-networking apps in May, 2020, vs November, 2017, to November, 2019,18 change in unprotected sexual partners reported in a national online survey of 518 US MSM;17 25% and 50% disruption from the proportion reporting fewer sexual partners in two national online surveys of US MSM (n=1051 and n=728) recruited through social-networking sites or hook up apps,2, 10 assuming these individuals reduced their number of partners by 50–100%; age differences from the proportion reporting fewer sexual partners in a national online survey of US MSM2 |
ART=antiretroviral therapy. MSM=men who have sex with men. PrEP=pre-exposure prophylaxis.
We modelled disruptions due to COVID-19 starting Jan 1, 2020. In our main analysis, we modelled the effect of a 6-month disruption, assuming all disruptions were reversed at the end of the period, with parameters reset instantaneously to their levels before the disruption. We assessed the effect of each disruption separately and in combination.
Sensitivity analysis
We tested the sensitivity of our findings to the magnitude of the different disruptions, comparing the individual effects of 10%, 25%, and 50% reductions in partner numbers, condom use, PrEP adherence, and viral suppression, and 50%, 75%, and 100% reductions in ART and PrEP initiation and HIV testing rates. We also explored the effect of 3-month and 12-month disruptions (starting Jan 1, 2020).
HIV testing campaign
We assessed the expected effect of an HIV testing campaign during lockdown, based on the UK Test Now, Stop HIV campaign. We modelled this testing campaign alongside plausible COVID-19-related disruptions to sexual partner numbers only or to sexual partner numbers, ART initiation, viral suppression, PrEP initiation, PrEP adherence, and condom use, for a 6-month period. We simulated HIV testing campaigns in which 90% of MSM tested at least once during the 6-month disruption period (to show the effect of a very effective campaign), after which HIV testing rates returned to levels observed before the disruption.
Outcome measures
We estimated short-term (1 year) and long-term (5 years) effects of COVID-19 on cumulative new HIV infections and HIV-related deaths measured from the start of COVID-19-related disruptions, overall and by race. We additionally estimated disability-adjusted life-years (DALYs) lost over 20 years (appendix p 11). Effects were calculated relative to the base case scenario and expressed as absolute or percentage change. The effect of the hypothetical HIV testing campaign was also expressed as the absolute difference in the percentage change in new HIV infections compared with scenarios without the testing campaign. The transmission model was coded and run in C++ and calculations were done using R version 3.6.2.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
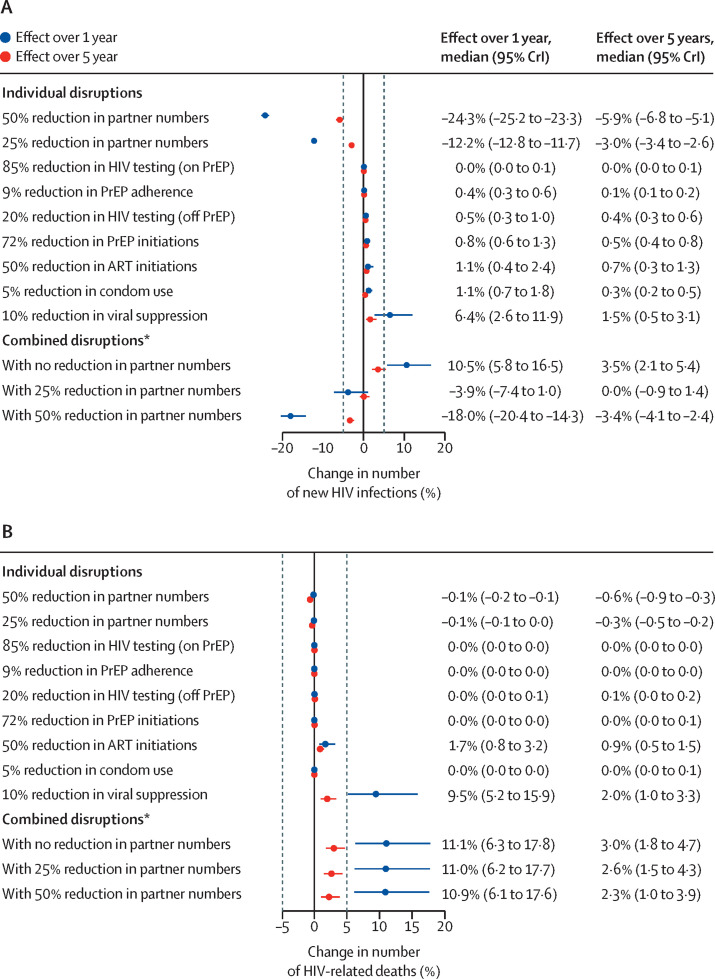
The results of the data-driven assessment of the effect of 6-month COVID-19-related disruptions on new HIV infections and HIV-related deaths are shown in figure 1 . Our model showed that reducing sexual partner numbers by 25% could substantially reduce new HIV infections among MSM, by median 12·2% (95% credible interval [CrI] 11·7–12·8) over the following 1 year and 3·0% (2·6–3·4) over the following 5 years, compared with if the COVID-19 pandemic and associated disruptions had not occurred (figure 1A). Twice that effect was seen for a 50% reduction in partnerships (figure 1A). Conversely, 10% reductions in levels of viral suppression among those on ART were predicted to increase new infections more than any other individual disruption, by median 6·4% (2·6–11·9) over 1 year and 1·5% (0·5–3·1) over 5 years (figure 1A). Our model also showed that a 50% reduction in ART initiations, 20% reduction in HIV testing for individuals not on PrEP, 72% reduction in PrEP initiations, 85% reduction in HIV testing for individuals on PrEP, 5% reduction in condom use, and 9% reduction in PrEP adherence each had a smaller negative effect than the reduction in viral suppression, increasing new infections by less than 2% each over 1 year (figure 1A). Predicted relative increases in new HIV infections decreased over 5 years for all disruptions, with larger reductions in relative effect over time for disruptions to condom use, viral suppression, and partner numbers (figure 1A).
Figure 1.
Effect over 1 year and 5 years of estimated 6-month disruptions due to COVID-19
(A) Effect of estimated 6-month disruptions on cumulative new HIV infections. (B) Effect of estimated 6-month disruptions on cumulative HIV-related deaths. Dashed vertical lines are at –5% and 5%. Estimated disruptions are based on available data (see table 2 for data sources and magnitude of the disruption to each service; see also appendix p 26). ART=antiretroviral therapy. CrI=credible interval. PrEP=pre-exposure prophylaxis. *Combination of all estimated individual disruptions.
Without any change in sexual behaviour, the 6-month estimated disruptions to HIV testing, ART initiations, viral suppression, PrEP initiations, PrEP adherence, and condom use combined were predicted to increase new HIV infections by median 10·5% (95% CrI 5·8 to 16·5) over 1 year, and by median 3·5% (2·1 to 5·4) over 5 years. The effect of these combined disruptions could be outweighed by a concurrent 6-month 25% reduction in partner numbers (overall change in new infections over 1 year: median –3·9% [–7·4 to 1·0]; over 5 years: median 0·0% [–0·9 to 1·4]; figure 1A).
We estimated substantial increases in HIV-related deaths from disruptions to ART initiations (median 1·7% [95% CrI 0·8 to 3·2] increase in HIV-related deaths) and viral suppression (median 9·5% [5·2 to 15·9] increase) over 1 year, with smaller proportional increases estimated over 5 years (figure 1B). The other individual disruptions (to HIV testing, PrEP and condom use, PrEP initiation, and partner numbers) were estimated to have little effect on HIV-related deaths (<1% change over 1 or 5 years). The predicted effect of disruptions to all HIV-related services combined, with no change in partner numbers, was median 11·1% (6·3 to 17·8) increase in HIV-related deaths over 1 year, equating to 4·8 (95% CrI 2·2–9·0) additional deaths in this population of around 2000 HIV-infected MSM. The proportional combined effect was almost 4 times smaller over 5 years than over 1 year, with an estimated 6·3 (2·8–11·5) additional deaths over 5 years. Unlike new infections, increases in HIV-related deaths over 1 or 5 years were not offset by reductions in the number of sexual partners (effect reduced by <1 percentage point when number of partners was reduced by 25% or by 50%; figure 1B).
Similar effects of COVID-19-related disruptions on new HIV infections and HIV-related deaths were estimated among Black and White MSM, except reduced partner numbers, which resulted in greater reductions in HIV incidence among White MSM compared with Black MSM (appendix pp 27–28).
Without changes in sexual behaviour, the combined 6-month disruptions to HIV-related services were predicted to lead to 133·9 (95% CrI 57·8–239·3) DALYs lost over 20 years across the population of MSM. This effect would be partially offset by a simultaneous 25% reduction in sexual partnerships, resulting in 64·3 (23·4–132·2) DALYs lost over 20 years (appendix p 29).
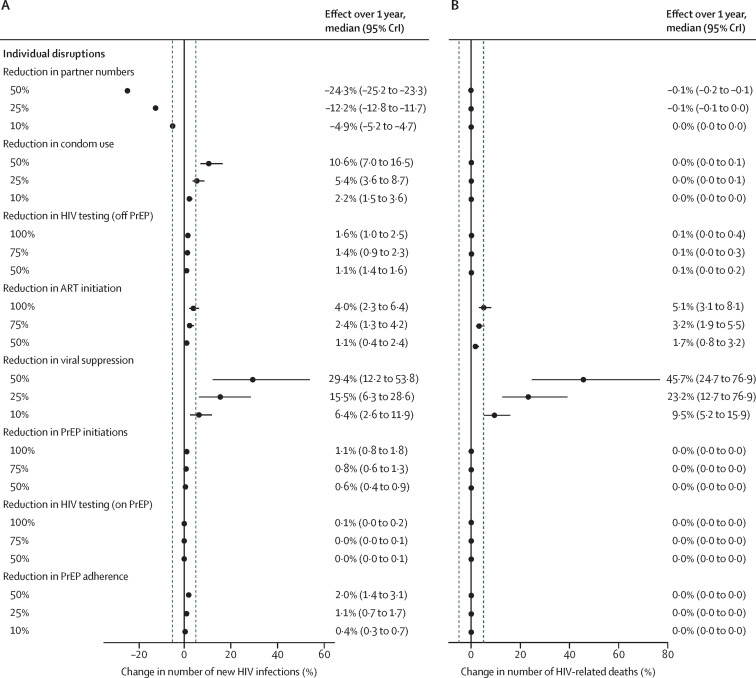
Our prespecified sensitivity analysis showed that for individual disruptions, a reduction in partner numbers of 10% or more, a reduction in condom use of 25% or more, or a reduction in viral suppression of 10% or more could alter the number of new HIV infections over 1 year by more than 5% (figure 2A ). By contrast, even complete cessation (100% reduction) of HIV testing, ART initiation, or PrEP initiation, or a 50% reduction in PrEP adherence individually would be insufficient to increase new HIV infections by more than 5% over 1 year. Complete cessation of ART initiations or a 10% or greater reduction in viral suppression was estimated to result in a 5% or greater increase in HIV-related deaths over 1 year (figure 2B). Effects over 5 years are shown in the appendix (p 30). The effects of prespecified 6-month disruptions on DALYs over 20 years are shown in the appendix (p 31).
Figure 2.
Sensitivity analysis of the effect over 1 year of prespecified magnitudes of individual disruptions due to COVID-19
(A) Estimated effect of prespecified 6-month disruptions on cumulative new HIV infections over 1 year. (B) Estimated effect of prespecified 6-month disruptions on cumulative HIV-related deaths over 1 year. Dashed vertical lines are at –5% and 5%. ART=antiretroviral therapy. CrI=credible interval. PrEP=pre-exposure prophylaxis.
Predicted effects on infections and deaths were proportional to the magnitude of reduction in sexual partnerships, condom use, viral suppression, and PrEP initiation (appendix pp 32–33). The effects of disruptions to ART initiations and HIV testing in individuals who are on PrEP increased more with larger magnitudes of disruption, whereas the effects of reduced PrEP adherence and HIV testing in individuals who are not on PrEP increased less with larger magnitudes of disruption (appendix pp 32–33).
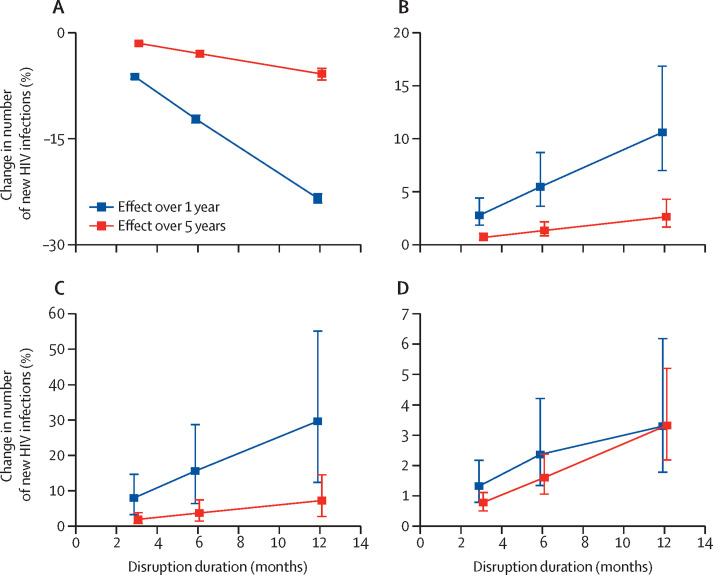
The predicted effects (measured over 1 and 5 years) of disruptions to partner numbers, condom use, viral suppression, and PrEP adherence on new HIV infections also increased proportionally with increased disruption duration (figure 3A–C ; appendix p 34). Effects of disruptions to PrEP and ART initiation and HIV testing varied proportionately with disruption duration when measured over 5 years but the effect over 1 year did not increase proportionally as duration increased (figure 3D; appendix p 34). Similar patterns were seen for HIV-related deaths (appendix p 35).
Figure 3.
Sensitivity analysis of the effect over 1 year and 5 years of disruption duration
(A) 25% reduction in partner numbers. (B) 25% reduction in condom use. (C) 25% reduction in viral suppression. (D) 75% reduction in ART initiations. Points are median and error bars are 95% credible intervals across all model fits. ART=antiretroviral therapy.
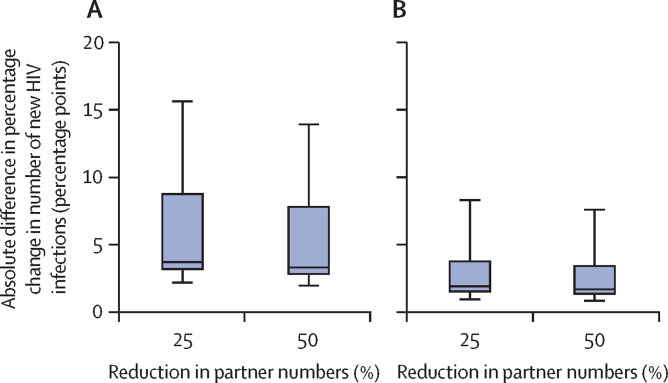
We estimated that if COVID-19 restrictions reduced the number of sexual partners by 25%, without disrupting HIV care and prevention services, then testing 90% of MSM at least once during the 6-month COVID-19-related disruption might reduce new HIV infections by 3·7 percentage points (95% CrI 2·3–13·4) over 1 year and 2·9 percentage points (1·7–14·7) over 5 years in addition to infections averted by reduced partner numbers (figure 4A ; appendix pp 36–37). However, taking into account all other likely disruptions due to COVID-19 alongside a 25% reduction in sexual partners, such an HIV testing campaign would be expected to have a more modest effect, reducing new infections by 1·9 percentage points (1·1–7·4) over 1 year and by 1·6 percentage points (0·9–10·4) over 5 years, compared with those averted without the testing campaign (figure 4B; appendix pp 36–37). The testing campaign was predicted to have a greater effect (new infections reduced by 3–11 percentage points more over 5 years) for scenarios in which fewer than 85% of MSM were aware of their HIV-positive status before COVID-19 (compared with scenarios in which ≥85% were aware of their HIV-positive status before COVID-19; appendix p 38).
Figure 4.
Effect of an HIV testing campaign on cumulative HIV infections over 1 year
(A) Additional effect of an HIV testing campaign reaching 90% of MSM for HIV testing alongside 6-month disruptions to numbers of sexual partners but no disruptions to HIV care and prevention services. (B) Additional effect of an HIV testing campaign reaching 90% of MSM for HIV testing alongside 6-month disruptions to HIV testing, ART initiation, viral suppression, PrEP initiation and adherence, and condom use, as well as number of sexual partners. Horizontal lines within the boxes are the median, boxes are IQRs, whiskers are full range across all model fits. ART=antiretroviral therapy. PrEP=pre-exposure prophylaxis. MSM=men who have sex with men.
Discussion
Our modelling results predict that modest reported COVID-19-related disruptions to HIV testing, PrEP adherence, condom use, and viral suppression, and larger disruptions to PrEP and ART initiations, without any change in the numbers of sexual partners (as suggested by some surveys), could lead to substantial short-term increases in new HIV infections (10·5% over 1 year for a 6-month disruption) and HIV-related deaths (11·1% over 1 year for a 6-month disruption) among MSM in Baltimore. Our results suggest that a 25% or 50% reduction in sexual partner numbers (consistent with other survey data) could offset increases in new HIV infections arising from COVID-19-related disruptions to HIV services but would not offset additional HIV-related deaths caused by these service disruptions. It is likely that this is because a reduced number of sexual partners only indirectly affects HIV-related deaths, through reducing new HIV infections. Together, the estimated 6-month service disruptions alongside a 25% reduction in partner numbers are predicted to give little change in new HIV infections (–3·9% over 1 year, 0·0% over 5 years). However, even with a 50% reduction in partner numbers, these same disruptions might still cause substantial short-term increases in HIV-related deaths (10·9% over 1 year, equivalent to 4·7 additional deaths per 2000 population).
Our sensitivity analysis suggests disruptions to viral suppression and condom use would have the greatest adverse effects on new HIV infections, whereas reductions in viral suppression and new ART initiations could lead to the greatest increase in HIV-related deaths. Even a 10% reduction in viral suppression among those on ART, lasting 6 months, would cause a 6·4% increase in new HIV infections and a 9·5% increase in HIV-related deaths over the following year. Increasing the duration of disruptions to viral suppression and condom use from 6 to 12 months could double their effect on both HIV infections and HIV-related deaths, as could doubling the size of each disruption. Finally, we find a widespread HIV testing campaign during lockdowns in this setting is likely to have only a modest incremental effect on HIV incidence, due to concurrent disruptions to ART, PrEP, and condom use.
Our results suggest that during COVID-19-related restrictions, HIV prevention and treatment efforts should focus on maintaining ART access and adherence support for both new and existing users, to minimise negative effects of COVID-19 on new HIV infections and, especially, HIV-related mortality. Multi-month ART dispensing and virtual or remote health services might be sufficient to help ART users who are already stably virally suppressed to maintain suppression during lockdown. Triaging to prioritise in-person care and viral load tests to new or less stably suppressed patients on ART will also be needed,13, 22 particularly for individuals who are homeless.15
There is uncertainty about the magnitude and duration of disruptions to HIV prevention and treatment services and how these might change during the COVID-19 pandemic. Service provision could improve as services adapt.12, 22 Conversely, service access in the USA could be hampered by loss of employer-provided health insurance as COVID-19 leads to increased unemployment.2 Losing health insurance might be particularly detrimental in US states that have not passed the Affordable Care Act. However, ART should be available through the Ryan White Care Program or AIDS Drug Assistance Programs in the USA.
There is also uncertainty about how long any reductions in numbers of sexual partners might last. A study among MSM in the southern USA who were using PrEP found numbers of partners and numbers of anal sex acts increased in June, 2020, following earlier reductions.23 Modelling for MSM in Atlanta (GA, USA) predicts increases in HIV incidence if service disruptions last longer than sexual behaviour changes.7
We find smaller but more persistent effects (still substantial after 5 years) following disruptions to HIV testing, ART initiations, and PrEP initiations compared with disruptions to partner numbers and viral suppression. Post-disruption catch-up campaigns could reduce the longer term effects of COVID-19-related disruptions to HIV testing, ART initiations, and PrEP initiations.
Several modelling studies have estimated the effect of COVID-19-related disruptions on HIV in sub-Saharan Africa.6, 24, 25, 26 In agreement with our results, these studies all suggest the largest detrimental effect is from disruptions to ART provision.6, 24, 25, 26 In an analysis using five different models applied in African settings, a 6-month interruption to ART for 50% of the population was predicted to increase HIV-related deaths by 63% (range across models 39–87%) over the following year.6 Our US estimate of the effect of a 50% reduction in viral suppression (45·7% more deaths) falls within this range. The effects on HIV-related deaths estimated for Africa and the USA are probably similar because the models for each setting have similar levels of viral suppression among all people living with HIV (approximately 53% of HIV-positive MSM are virally suppressed in our USA model, 54% of all people living with HIV are virally suppressed across Africa in the HIV Synthesis model6). A previous modelling study estimated the effect of COVID-19-related disruptions on HIV incidence among MSM in Atlanta.7 Similar to our study, they found that reductions in sexual partnerships could offset the effect of service disruptions on new HIV infections, and PrEP reductions had less effect than ART reductions, partly due to low PrEP coverage before the disruptions (15% in Atlanta).7 Another modelling study assessing effects of disruptions to HIV services on HIV incidence in six US cities, including Baltimore, similarly found that reductions in sexual partnerships could offset health service reductions.8 However, in contrast to our findings, they projected that substantial reductions in HIV incidence would result from expanded HIV testing in Baltimore during COVID-19-related disruptions. This larger effect might arise from testing heterosexual populations, who have lower HIV testing rates than MSM, or because lower levels of awareness of HIV-positive status were assumed (eg, 78% among MSM vs 74–95% in our study).8
This analysis only considered effects among MSM because data on COVID-19-related disruptions to HIV care and treatment for other US populations were scarce, but we expect reductions in ART initiations and viral suppression to similarly increase HIV incidence and mortality in other populations.
Although our analysis maximised the use of available data on the effects of COVID-19 on HIV prevention and care and sexual behaviour for US MSM, it has some limitations, partly due to uncertainty about the exact magnitude and duration of these disruptions. Many of the data informing disruption sizes were collected at a single timepoint, and do not capture changes as the COVID-19 pandemic progressed. Some estimates were only semi-quantitative, for example, 50% of MSM reporting fewer partners does not detail how many fewer partners they had. We found no data on reductions in ART initiations. However, our sensitivity analysis explored the effect of different disruption levels and durations. In the absence of specific data on main, casual, or commercial sexual partnerships,2, 10 we assumed COVID-19 equally affected all partnership types. Because most sex acts in our model occur in main partnerships, this led to large reductions in total sex acts, consistent with many MSM reporting fewer sex acts due to COVID-19.2, 10 If larger reductions actually occurred in casual or commercial sexual partnerships than in main sexual partnerships, we would expect smaller reductions in HIV incidence, due to the shorter duration and higher condom use in casual and commercial sexual partnerships. Our HIV transmission model was calibrated to Baltimore, but estimates of disruption magnitude were based on national surveys2, 10 and a Boston study;12 disruptions might differ between US states due to differences in COVID-19 prevalence, COVID-19 response measures, and health funding. Therefore, we might not have captured the true effect of COVID-19 among MSM in Baltimore.
Data informing behavioural parameters and HIV prevalence validation were from 2014 and earlier, meaning we might not have fully captured recent Baltimore prevalence trends. However, the model was validated against viral suppression and PrEP data from 2017, so both baseline levels and the effect of COVID-19-related reductions in viral suppression and PrEP should be well reflected. Although our model calibration approach and credible intervals captured parameter uncertainty, structural uncertainty was not assessed. Our effect estimates might not be directly applicable to other US locations with different HIV epidemics and services, although we expect qualitative insights from our model to still be useful.
Our predicted small effect of an HIV testing campaign during lockdown might partly be due to high modelled HIV testing rates at baseline (based on self-reported data); we predict larger effects for populations with lower HIV testing rates. Our small predicted effects of reduced PrEP adherence might be partly due to low PrEP use among MSM in Baltimore (12% in 2017), and larger effects might be seen in places with greater PrEP use, such as Boston (MA, USA) or San Francisco (CA, USA).27 In our study, we assumed reductions in PrEP adherence and reductions in partner numbers occur independently, whereas MSM might cease PrEP use while abstaining from sex and those apparently lapsing in PrEP use might be switching to non-daily use.28 In this case, our analysis might overestimate negative effects of PrEP disruptions. We modelled COVID-19-related disruptions starting Jan 1, 2020, although Baltimore stay-at-home orders began March 30, 2020; however, because we project that HIV prevalence and ART coverage would have stayed almost constant during these 3 months in the absence of disruptions, this is unlikely to influence our results. We used a deterministic model with memoryless transitions between compartments, meaning that individuals leave compartments at fixed rates rather than spending fixed durations in age groups or viral suppression compartments. Finally, we did not account for mortality that is directly caused by COVID-19; we might have underestimated effects on mortality because COVID-19 outcomes are worse among patients with comorbidities or low CD4 counts than among patients with no comorbidities.29
In conclusion, maintaining access to ART and adherence support for those on treatment and those newly diagnosed with HIV is crucial to minimise excess HIV-related mortality due to COVID-19-related restrictions in the USA. A reduction in viral suppression of 10% could have negative consequences for both HIV incidence and HIV-related mortality and any reductions in numbers of sexual partners would offset increases in incidence, but not mortality. It will be important to collect quantitative data on COVID-19-related effects from multiple timepoints and settings, to improve modelling estimates and inform public health decisions. Modelling that assesses the effects of post-COVID-19 catch-up campaigns can inform health-care priorities as restrictions are lifted.
Data sharing
All of the data used for model parameterisation and calibration can be found in the appendix (pp 14–22, 26). The model code is available from the authors on request (kate.mitchell@imperial.ac.uk).
Acknowledgments
Acknowledgments
KMM, DD, MM, and M-CB are supported by the HIV Prevention Trial Network (HPTN) Modelling Centre, which is funded by the US National Institutes of Health (grant number UM1 AI068617) through the HPTN Statistical and Data Management Center. KMM, RS, LG, and M-CB have received funding from the Medical Research Council (MRC) Centre for Global Infectious Disease Analysis (grant number MR/R015600/1), which is jointly funded by the UK MRC and the UK Foreign, Commonwealth & Development Office (FCDO), under the MRC/FCDO Concordat agreement and is also part of the EDCTP2 programme supported by the EU, and acknowledge funding by Community Jameel. LG is funded by an MRC Doctoral Training Partnership scholarship.
Contributors
KMM, DD, RS, LG, and M-CB contributed to the conception and design of the modelling analyses. KMM, RS, AL, CB, KHM, and SB identified and interpreted the data sources to inform the disruption effects. KMM and RS verified the underlying data. KMM did the modelling analysis. RS, LG, and M-CB provided advice on technical aspects of modelling analysis. All authors contributed to interpreting the modelling results. KMM wrote the first draft of the manuscript. All authors contributed to manuscript revision and critical review. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Declaration of interests
M-CB and KMM have received grants from the National Institutes of Health, during the conduct of the study. KMM has received an honorarium for speaking from Gilead, outside the submitted work. AL has received funding for investigator sponsored research from Gilead and ViiV and has led studies in which Gilead has donated study drug, outside the submitted work. All other authors declare no competing interests.
Supplementary Material
References
- 1.Jiang H, Zhou Y, Tang W. Maintaining HIV care during the COVID-19 pandemic. Lancet HIV. 2020;7:e308–e309. doi: 10.1016/S2352-3018(20)30105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanchez TH, Zlotorzynska M, Rai M, Baral SD. Characterizing the impact of COVID-19 on men who have sex with men across the United States in April, 2020. AIDS Behav. 2020;24:2024–2032. doi: 10.1007/s10461-020-02894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luis H, Fridayantara WD, Mahariski P, Wignall FS, Irwanto I, Gedela K. Evolving ART crisis for people living with HIV in Indonesia. Lancet HIV. 2020;7:e384–e385. doi: 10.1016/S2352-3018(20)30138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao A, Rucinski K, Jarrett B, et al. Potential interruptions in HIV prevention and treatment services for gay, bisexual, and other men who have sex with men associated with COVID-19. medRxiv. 2020 doi: 10.1101/2020.08.19.20178285. published online Aug 22. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sands P. HIV, tuberculosis, and malaria: how can the impact of COVID-19 be minimised? Lancet Glob Health. 2020;8:e1102–e1103. doi: 10.1016/S2214-109X(20)30317-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jewell BL, Mudimu E, Stover J, et al. Potential effects of disruption to HIV programmes in sub-Saharan Africa caused by COVID-19: results from multiple mathematical models. Lancet HIV. 2020;7:e629–e640. doi: 10.1016/S2352-3018(20)30211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenness SM, Le Guillou A, Chandra C, et al. Projected HIV and bacterial STI incidence following COVID-related sexual distancing and clinical service interruption. J Infect Dis. 2021 doi: 10.1093/infdis/jiab051. published online Jan 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zang X, Krebs E, Chen S, et al. The potential epidemiological impact of COVID-19 on the HIV/AIDS epidemic and the cost-effectiveness of linked, opt-out HIV testing: a modeling study in six US cities. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1547. published online Oct 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bramer CA, Kimmins LM, Swanson R, et al. Decline in child vaccination coverage during the COVID-19 pandemic—Michigan care improvement registry, May 2016–May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:630–631. doi: 10.15585/mmwr.mm6920e1. [DOI] [PubMed] [Google Scholar]
- 10.McKay T, Henne J, Gonzales G, Quarles R, Gavulic KA, Garcia Gallegos S. The COVID-19 pandemic and sexual behavior among gay and bisexual men in the United States. SSRN. 2020 doi: 10.2139/ssrn.3614113. published online May 31. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellmann J. The Hill; April 23, 2020. Pandemic sparks concerns about surging STD, HIV rates.https://thehill.com/policy/healthcare/494236-pandemic-sparks-concerns-about-surging-std-hiv-rates [Google Scholar]
- 12.Krakower DS, Solleveld P, Levine K, Mayer KH. Impact of COVID-19 on HIV preexposure prophylaxis care at a Boston community health center. 23rd International AIDS Conference; virtual; July 6–10, 2020 (abstr OACLB0104).
- 13.Ridgway JP, Schmitt J, Friedman E, et al. HIV care continuum and COVID-19 outcomes among people living with HIV during the COVID-19 pandemic, Chicago, IL. AIDS Behav. 2020;24:2770–2772. doi: 10.1007/s10461-020-02905-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagendra G, Carnevale C, Neu N, Cohall A, Zucker J. The potential impact and availability of sexual health services during the COVID-19 pandemic. Sex Transm Dis. 2020;47:434–436. doi: 10.1097/OLQ.0000000000001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spinelli MA, Hickey MD, Glidden DV, et al. Viral suppression rates in a safety-net HIV clinic in San Francisco destabilized during COVID-19. AIDS. 2020;34:2328–2331. doi: 10.1097/QAD.0000000000002677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention HIV infection risk, prevention, and testing behaviors among men who have sex with men—national HIV behavioral surveillance, 23 US cities, 2017. HIV Surveillance Special Report 22. Feb, 2019. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-special-report-number-22.pdf
- 17.Stephenson R, Chavanduka TMD, Rosso MT, et al. Sex in the time of COVID-19: results of an online survey of gay, bisexual and other men who have sex with men's experience of sex and HIV prevention during the US COVID-19 epidemic. AIDS Behav. 2021;25:40–48. doi: 10.1007/s10461-020-03024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starks TJ, Jones SS, Sauermilch D, et al. Evaluating the impact of COVID-19: a cohort comparison study of drug use and risky sexual behavior among sexual minority men in the USA. Drug Alcohol Depend. 2020;216 doi: 10.1016/j.drugalcdep.2020.108260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell KM, Hoots B, Dimitrov D, et al. Improvements in the HIV care continuum needed to meaningfully reduce HIV incidence among men who have sex with men in Baltimore, US: a modelling study for HPTN 078. J Int AIDS Soc. 2019;22 doi: 10.1002/jia2.25246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu AY, Cohen SE, Vittinghoff E, et al. Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med. 2016;176:75–84. doi: 10.1001/jamainternmed.2015.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santos G-M, Ackerman B, Rao A, et al. Economic, mental health, HIV prevention and HIV treatment impacts of COVID-19 and the COVID-19 response on a global sample of cisgender gay men and other men who have sex with men. AIDS Behav. 2021;25:311–321. doi: 10.1007/s10461-020-02969-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shoptaw S, Goodman-Meza D, Landovitz RJ. Collective call to action for HIV/AIDS community-based collaborative science in the era of COVID-19. AIDS Behav. 2020;24:2013–2016. doi: 10.1007/s10461-020-02860-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pampati S, Emrick K, Siegler AJ, Jones J. Changes in sexual behavior, PrEP adherence, and access to sexual health services due to the COVID-19 pandemic among a cohort of PrEP-using MSM in the South. medRxiv. 2020 doi: 10.1101/2020.11.09.20228494. published online Nov 12. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jewell B, Smith J, Hallett T. The potential impact of interruptions to HIV services: a modelling case study for South Africa. medRxiv. 2020 doi: 10.1101/2020.04.22.20075861. published online April 27. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stover J, Chagoma N, Taramusi I, Teng Y, Glaubius R, Mahiane G. Estimation of the potential impact of COVID-19 responses on the HIV epidemic: analysis using the Goals model. medRxiv. 2020 doi: 10.1101/2020.05.04.20090399. published online May 8. (preprint). [DOI] [Google Scholar]
- 26.Hogan AB, Jewell BL, Sherrard-Smith E, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8:e1132–e1141. doi: 10.1016/S2214-109X(20)30288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finlayson T, Cha S, Xia M, et al. Changes in HIV preexposure prophylaxis awareness and use among men who have sex with men—20 urban areas, 2014 and 2017. MMWR Morb Mortal Wkly Rep. 2019;68:597–603. doi: 10.15585/mmwr.mm6827a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow EPF, Hocking JS, Ong JJ, et al. Changing the use of HIV pre-exposure prophylaxis among men who have sex with men during the COVID-19 pandemic in Melbourne, Australia. Open Forum Infect Dis. 2020;7:a275. doi: 10.1093/ofid/ofaa275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dandachi D, Geiger G, Montgomery MW, et al. Characteristics, comorbidities, and outcomes in a multicenter registry of patients with HIV and coronavirus disease-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1339. published online Sept 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All of the data used for model parameterisation and calibration can be found in the appendix (pp 14–22, 26). The model code is available from the authors on request (kate.mitchell@imperial.ac.uk).