Abstract
Ag+ ions are a well-known antibacterial agent, and Ag nanoparticles act as a reservoir of these Ag+ ions for targeted therapy of bacterial infections. However, there are no tools to effectively trigger and monitor the release of Ag+ ions from Ag nanoparticles. Photoacoustic (PA) imaging is an emerging noninvasive imaging tool, and gold nanorods (AuNRs) are an excellent contrast agent for PA imaging. In this work, we developed Au/Ag hybrid nanoparticles by coating AuNRs with silver (Ag), which decreased their photoacoustic signal. The as-prepared, Ag-coated Au nanorods (Au/AgNRs) are stable under ambient conditions, but the addition of ferricyanide solution (1 mM) results in oxidative etching of the silver shell. The PA contrast is simultaneously recovered as the silver is released, and this PA signal offers noninvasive monitoring of localized release of Ag+ ions. The released Ag+ ions exhibit a strong bactericidal efficacy similar to equivalent free Ag+ ions (AgNO3), and the nanoparticles killed >99.99% of both (Gram-positive) methicillin-resistant Staphylococcus aureus (MRSA, 32 μM Ag+ equivalent) and (Gram-negative) Escherichia coli (8 μM Ag+ equivalent). The theranostic potential of these nanoparticles was demonstrated in a pilot in vivo study. Mice were inoculated with MRSA and Au/AgNRs were subcutaneously implanted followed by silver etching. There was a 730% increase in the PA signal (p < 0.01) pre- and post-etching, and the bacterial counts in infected tissues of the treated group were reduced by 1000-fold (log CFU/g = 4.15 vs 7.75) versus the untreated control; this treatment efficacy was confirmed with histology. We further showed that these hybrid nanoparticles could release Ag+ after stimulation by reactive oxygen species including hydrogen peroxide and peroxynitrite. These hybrid Au/Ag nanoparticles are a useful theranostic agent for the photoacoustic imaging and treatment of bacterial infections.
Keywords: antibacterial, gold nanorods, silver nanoparticles, photoacoustic, molecular imaging
Graphical Abstract

Bacterial infections are a leading cause of mortality in the world,1 especially with the emergence of multi-drug-resistant bacteria.2 Silver nanoparticles (AgNPs) are broad-spectrum antibacterial agents. The antibacterial activity of silver nanoparticles is based on the release of silver ions from the silver nanoparticles. These ions can denature microbial proteins, interfere with DNA replication, and consequently damage the bacterial cells.3-5 Therefore, there is a great need to generate therapeutically active Ag+ ions to treat bacterial infections.6 While silver ions have low toxicity to human cells relative to bacterial cells, silver ions at high concentration can affect the innate immune system and induce potential risks to human health.7,8 Therefore, it is necessary to effectively monitor silver release from silver nanoparticles and their subsequent antibacterial activities in vivo.
Silver nanoparticles are active against antibiotic-resistant bacteria9-11 and are included in commercially available wound dressings (e.g., Acticoat), antimicrobial creams, and silver-coated implantable devices for clinical use.12,13 While there are many FDA-approved dressings that use colloidal silver as an Ag+ source, these dressings remain controversial in their capacity to improve outcomes. While some trials showed that they increase healing rates,14,15 others showed no impact relative to traditional dressings.16 Importantly, while these trials use a variety of designs, none of them quantitated how much Ag+ was released from the Ag0 colloids into the wounds. This is the fundamental limitation of the existing technology. Tools to quantitate silver release in vivo would be useful to study the mechanism and correlate dose to outcomes.
Composite nanoparticle systems offer powerful antibacterial properties and can monitor the action of antibacterial silver and conventional antibiotics based on the fluorescence quenching/dequenching.17-21 However, there are no tools for noninvasive, real-time imaging of antibacterial therapy deep within the intact tissues.
Photoacoustic imaging is a perfect tool for in vivo imaging and therapeutic drug monitoring.22-25 Although fluorescence dyes,26,27 quantum dots,28 and carbon nanotubes (CNTs)29 are useful for optical molecular imaging of response to antibacterial therapy,30,31 conventional optical imaging suffers from low imaging depth.32 In contrast, photoacoustic imaging can substantially increase the signal penetration depth. In photoacoustic imaging, the signal can be generated by the ultrasonic pressure waves, which is emitted by the thermoelastic expansion under near-infrared (NIR) light illumination.33 Since ultrasonic waves have much longer wavelengths than light, the photoacoustic signals can penetrate deeper through the tissues.34 Moreover, photoacoustic imaging offers the high temporal and spatial resolution of ultrasound with concurrent anatomical data from ultrasound imaging.35
In photoacoustics, exogenous contrast agents can distinguish contrast from the endogenous tissues36-40 and also provide activatable signals upon detecting molecular targets or events.41-45 Gold nanorods (AuNRs) have strong light absorption in the NIR region and are an attractive photoacoustic contrast agent; they have been used extensively for photoacoustic molecular imaging of cells,46 proteins,47 and tumors48 and for photothermal therapy.49 Theranostic nanoparticle systems combine the features of diagnostic imaging and therapy and offer effective feedback of the controlled treatment.50-53 For theranostics, AuNRs can provide an easy surface to be functionalized with targeting agent54 or load drug molecules.55 Inorganic gold is very chemically inert and stable for fabrication of the hybrid composite materials with other inorganic species including silver.56 Most importantly, the optical absorption of gold is easily tuned for plasmonic sensors57-59 including reversible systems.
In this work, we developed Au/Ag hybrid nanoparticles and successfully demonstrated their use as a theranostic antibacterial/photoacoustic agent. The nanoparticles have a gold nanorod core coated with silver (Au/AgNRs). The characteristic strong NIR light absorption—and hence photoacoustic signal—of the AuNRs disappeared with the silver coating; however, it recovered when the silver shells are dissolved under mild oxidizing conditions. Photoacoustic (PA) contrast turns on and off during the cycle of silver deposition/oxidation on AuNRs. Thus, the recovered PA contrast reports antibacterial silver ion release and offers noninvasive and real-time dosimetry of Ag+ ions. The released silver ions can effectively kill Gram-positive methicillin-resistant Staphylococcus aureus (MRSA) in vitro and in vivo. Finally, we demonstrate the theranostic potentials of these hybrid nanoparticles for in vivo photoacoustic monitoring of the bactericidal action and the inhibition of wound infections.
RESULTS AND DISCUSSION
Synthesis and Characterization of Au/Ag Nanorods.
A schematic of the experiments and representative TEM images is shown in Figure 1. Ag-coated Au nanorods (Au/AgNRs) were successfully synthesized by the chemical reduction of silver nitrate using ascorbic acid as the reducing agent on AuNRs (Figure 1A).60 Silver ions can be reduced at elevated pH (>10) because the reducing power of ascorbic acid is increased (Figure 1B).61
Figure 1.
Schematic of the experiments and representative TEM images of AuNRs, Au/AgNRs, and etched Au/AgNRs. (A) AuNRs were synthesized with a seed-mediated growth method. They exhibit a strong photoacoustic contrast. (B) AuNRs are employed as seeds for the reductive deposition of silver ions and produced the isolated Ag-coated Au nanorods (Au/AgNRs). The photoacoustic contrast from AuNRs disappears upon Ag deposition. (C) When these nanoparticles are exposed to the mild oxidant ferricyanide ions ([Fe(CN)6]3−), the Ag shells are oxidized and release Ag+ ions to the aqueous solution. Here, AuNRs regenerated the strong photoacoustic contrast. The scale bar is 50 nm, respectively.
The color of the AuNR solution changed from purple to green to brown as more silver was coated on the nanorods. Transmission electron microscopy (TEM) showed a distinctive core/shell nanostructure of Au/Ag particles (width: 12–14 nm, length: 50–55 nm, shell thickness of Ag: 10 nm) (Figure 1B). The particles changed to short nanorods with a low aspect ratio upon silver coating. The electron density of silver is different from gold, and the boundary between Ag and Au can be easily distinguished by difference in contrast.
Furthermore, the presence of elemental Au and Ag in the particles was confirmed by energy-dispersive X-ray (EDX) spectroscopy (Figure 2D). The ratio of Au and Ag was 1:3 by the quantitative analysis via inductively coupled plasma optical emission spectrometry (ICP-OES) measurement. There was no detectable silver peak for the AuNRs before Ag coating (Figure S1). The X-ray diffraction (XRD) pattern (Figure S2) confirmed that as-prepared NPs have characteristic peaks at 38.1°, 44.1°, 64.4°, and 77.3°, which correspond to (111), (200), (220), and (311) crystallographic planes of both Ag (JCPDS No. 04-0783) and Au nanoparticles (JCPDS No. 04-0784). The obtained XRD pattern is similar to segregated Ag–Au bimetallic nanoparticles.62
Figure 2.
Characterization of the nanoparticles. (A) Photographic images of AuNRs and Au/AgNRs with different Ag shell thicknesses. The silver shell gradually increased by adding increasing concentrations of 0.1 M AgNO3 solution (0, 5, 10, 20 μL) into 0.5 mL of an aqueous solution of AuNRs (100 μg Au/mL). The Ag shell thickness of Au/Ag1, Au/Ag2, and Au/Ag3 was 4, 7, and 10 nm, respectively. (B) UV–visible spectra of the corresponding Au/AgNRs with different Ag shell thicknesses from A. (C) Dynamic light scattering measurements of Au/AgNRs. The hydrodynamic diameter of Au/AgNRs was 104.9 nm (PDI: 0.235). (D) Energy-dispersive X-ray spectrum of Au/AgNRs. This confirmed the presence of a silver peak as well as gold in the Au/AgNRs. The approximate wt % of Ag and Au were 70.6 and 20.6, respectively. The ratio of Au and Ag (1:3.4) is similar to the relative concentrations of Au and Ag from the Au/Ag3 samples as confirmed by ICP measurement.
As shown in the photograph of nanoparticle solutions (Figure 2A) and their corresponding TEM images (Figure S3), the thickness of silver shell can be tuned by increasing the amount of silver nitrate. The Ag shell thickness of Au/Ag1, Au/Ag2, and Au/Ag3 was 4, 7, and 10 nm, respectively, as determined by line profile analysis in ImageJ. The light absorption of AuNR is strongly influenced by the presence of a Ag coating as shown in the UV–vis absorption spectrum (Figure 2B). AuNRs have two light absorption bands with transverse (~530 nm) and longitudinal plasmon resonances (~730 nm). The longitudinal plasmon light absorption band is blue-shifted when the silver shells are deposited on AuNRs. This dramatic band shift is attributed to the change of aspect ratio of AuNRs.63 The aspect ratio decreased from 4.2 to 2.2 after Ag coating. Additionally, as more Ag shells are deposited onto AuNR, the color of the solution changes from purple, to green, to brown (Figure 2A). When the Ag shells are completely coated on the surface of the AuNRs, they showed no more light absorption in the NIR region. Therefore, the absorption intensity of the Au/AgNRs has a peak at 530 nm with no absorption at 750 nm.
AuNRs have two peaks in the dynamic light scattering (DLS) size analysis.64 One is 1–5 nm (transverse peak) and the other is 40–80 nm (longitudinal peak) (black trace line in Figure S4). After silver coating, the hydrodynamic diameter of Au/AgNR was measured to be 104.9 m (PDI: 0.235) (Figure 2C). Here, the small peak (transverse peak) disappeared and only the large size peak (longitudinal) was observed. The zeta potentials of the Au/Ag nanorods were measured to be +29.4 ± 7.01 mV (Figure S5). These positively charged particles are well-dispersed in aqueous solution, and they showed excellent colloidal stability for at least 1 month.
Selective Silver Etching.
These Au/Ag nanorods are highly stable under ambient conditions, and there is not much premature release of Ag+ ions from these hybrid nanoparticles, as confirmed by ICP measurement. Silver shells are not prone to oxidation due to the continuous supply of electrons from the gold nanoparticles at the interface.65 However, these stable silver shells can be selectively etched upon exposure to the mild oxidant ferricyanide ions ([Fe(CN)6]3−) (1 mM). The ferricyanide ions ([Fe(CN)6]3−) are an ideal silver etchant for the selective dissolution of Ag since the standard reduction potential of the Fe3+/Fe2+ (E0 = 0.77 V) is close to that of Ag+/Ag (E0 = 0.79 V) but much lower than that of Au+/Au (E0 = 1.69 V). Moreover, the ferricyanide ions ([Fe(CN)6]3−) have been successfully demonstrated to be a biocompatible,66 cell membrane impermeable nanoparticle silver etchant for in vivo use.19,67 The rapid Ag ion release triggered with ferricyanide can improve their bioavailability. We also tested the ability of some reactive oxygen species (ROS) to leach silver off of the nanoparticles. Our candidate ROS were H2O2 and peroxynitrite because they have reduction potentials of 0.87 V (alkaline condition) and 1.76 V (acidic condition) for H2O261,68 and 1.2 V for peroxynitrite.69
Figure 1C and Figure 3A clearly show removal of the silver shell via TEM imaging. The silver shell could be completely etched within 5 min, and there was no longer a brighter silver shell on AuNRs. The nanostructure of the AuNR core was intact, and the color of the AuNR recovered to the original purple after the dissociation of the silver shells (Figure S6B). The size change was further confirmed with DLS. The mean diameter (longitudinal peak) decreased from 104.9 nm (red) to 86.5 nm (blue) after dissolution of the silver shells, and the transverse peak reappeared for the etched Au/AgNRs with increased aspect ratio (Figure S4).
Figure 3.
Photoacoustic properties of AuNRs, Au/AgNRs, and etched Au/AgNRs. (A) TEM image of Au/AgNRs before and after exposure to 1 mM Ag etchant of [Fe(CN)6]3− for 5 min. All the coated nanoparticle silver shells are selectively etched, and the distinctive rod shapes of Au particles reappeared. (B) UV/visible absorbance of AuNRs, Au/AgNRs, and etched Au/AgNRs. There is no light absorption at 750 nm for Au/AgNRs. After silver etching, the characteristic NIR light absorption of AuNR recovered, and the particles show strong absorption at 750 nm. (C) PA image and PA spectrum of cuvettes containing aqueous dispersions of AuNRs, Au/AgNRs, and etched Au/AgNRs. The addition of the Ag shell effectively quenches the PA contrast from AuNRs. However, the PA contrast is regenerated upon the selective etching of the Ag nanoparticle shell. Scale bar is 2 mm. (D) PA images of the etched Au/Ag with increasing H2O2 (0, 5, 10, 30 mM) and increasing ONOO− (0, 0.25, 0.5, 0.75, 1, 2.5 mM). The concentrations of the released Ag ions were 0, 10, 32, and 78 μM for H2O2 and 0, 4, 10, 25, 38, and 52 μM for ONOO−, as determined by ICP measurement. Scale bar is 2 mm. (E) Plot of the photoacoustic amplitude with the concentration of Ag ions from the samples in D. They showed a strong correlation between the PA contrast and the released silver ions for H2O2 (R2 = 0.93) and ONOO− (R2 = 0.96). Error bars represent the standard deviation.
Photoacoustic Quantitation of Silver Release from Au/Ag Nanorods.
AuNRs can generate a strong photoacoustic signal based on the conversion of absorbed light energy into heat and the successive production of pressure transients.70 However, as a thicker silver shell forms, the AuNRs have no more excitation of longitudinal plasmons and no NIR light absorption (Figure 2B and Figure 3B). Therefore, there was no detectable photoacoustic contrast from the Au/AgNRs (Figure 3C). However, the selective silver etching due to ferricyanide can regenerate the intact AuNRs and the characteristic NIR light absorption is recovered; the particles show strong absorption at 750 nm. Therefore, the PA contrast is simultaneously recovered, as shown in the PA image and spectrum (Figure 3C).
PA recovery from the hybrid Au/Ag nanoparticles could also be achieved by silver etching with biologically relevant ROS, which are commonly generated from the response of host tissues to pathogenic infections.71 The PA signal correlates with the amount of released silver. The Ag shell can be dissolved from the hybrid Au/Ag nanoparticles via H2O2 serving as an oxidant.68 We used H2O2 because it is an endogenously produced ROS and because ferricyanide quickly liberated all silver from the hybrid nanoparticles. However, with the ROS, we could control the etching rate and subsequent Ag+ doses via H2O2 concentration (see TEM images in Figure S7). We then plotted the PA intensity as a function of Ag doses. The PA contrast is recovered due to the strong NIR light absorption (Figure S8), and it showed a strong correlation between the PA contrast and the released silver ions as measured by inductively coupled plasma (R2 = 0.93). These nanoparticles can also be gradually etched by ONOO− ions and demonstrated that the PA intensity increased with Ag dose (R2 = 0.96) (Figure 3D,E).
We found that different ROS including 1O2, OCl−, •OH, and ONOO− can successfully oxidize the silver on AuNRs (Figure S9). 57 We also validated that endogenously generated ROS can oxidize the silver shells with detection by photoacoustic imaging using ovarian cancer cells (SKOV3) where high levels of free radicals are continuously generated (Figure S10).72-74 Although the current system establishes preinjection of particles and subsequent addition of etchant to accelerate the silver oxidations, the high levels of ROS accelerated the recovery of the PA contrast (Figure S9 and Figure S10). The elevated ROS can mimic the higher oxidative stress level in the wound areas where the immune cells such as neutrophils and macrophages invade.18,75 Therefore, the as-prepared hybrid Au/Ag nanoparticles could facilitate activatable release of Ag+ ions in response to an infection; however, other sources of ROS could reduce the specificity. Therefore, we envision using these materials with a dressing/bandage to immobilize the hybrid nanoparticles at the site of infection.
Antibacterial Efficacy of Au/Ag Nanorods in Vitro.
Next, we assessed the therapeutic potential of Au/Ag nanorods in vitro using the bacterial cultures of both (Gram-positive) MRSA and (Gram-negative) E. coli. First, we performed the bacterial assay using a culture of Gram-positive MRSA. When cotreated with the ferricyanide oxidant, the Au/AgNRs exhibited a strong bactericidal efficacy similar to equivalent free Ag+ ions (AgNO3) (Figure 4A,B). The addition of selective etchant induces fast and complete release of silver ions from the nanoparticles (as shown in TEM and DLS), and the released ions kill the bacteria. Therefore, there is a 1000-fold decrease in the bacterial CFUs (log10 CFU = 4.7 vs 7.2) compared to the untreated control group. In contrast, ferricyanide oxidant alone had no significant bacterial killing (log10 CFU = 7.0), and Au/Ag without oxidant showed substantially less bactericidal efficacy (log10 CFU = 6.5) (Figure 4B).
Figure 4.
Antibacterial efficacy of the Au/AgNRs system in vitro. (A) Representative images of the bacterial cultures by surface plating of MRSA treated with nanoparticles. MRSA (OD = 0.1) was treated with Au/Ag (2 μM Ag+), Au/Ag (2 μM Ag+) with 1 mM [Fe(CN)6]3−, 1 mM [Fe(CN)6]3− (Etchant only), and a AgNO3 solution (2 μM Ag+; free Ag+ ions), respectively. The serially diluted bacterial solutions (10 times for each row) were plated from top to bottom. The presence of MRSA is confirmed by the golden color of the colonies on the agar plate after overnight incubations. (B) Bar graph of bacterial colony counts from A, showing the bactericidal action of etched Au/AgNRs. The number of colonies was counted, and the CFU was calculated by multiplying counted colonies by the dilution ratio. Error bars represent standard deviations of the measurements (N = 3). The statistical significance was calculated with the Student’s t test; **, p < 0.01 versus the untreated control; n.s., not significant versus the untreated control. (C) Growth inhibition of Gram-positive MRSA and Gram-negative E. coli, evaluated by bacterial colony counts (log10 CFU vs concentration of Ag+). Inhibition concentration is based on the initial concentration of Ag+ ions in nanoparticle suspensions, as determined by ICP-OES. Error bars represent the standard deviations of the measurements (N = 3).
Silver has broad-spectrum antibacterial activity, and we studied if there was any difference in the utility of the hybrid nanoparticles with Gram-negative E. coli and Gram-positive MRSA with the etched Au/Ag. The etched Au/Ag killed >99.99% of both (Gram-positive) MRSA (32 μM Ag+ equivalent) and (Gram-negative) E. coli (8 μM Ag+ equivalent) (Figure 4C). The antibacterial activity of Au/Ag nanoparticles against (Gram-negative) E. coli is 4 times greater than against (Gram-positive) MRSA, which is consistent with previous literature studies that revealed the more active antibacterial activity of silver ions against Gram-negative bacteria such as E. coli and Pseudomonas aeruginosa.3,19,76 In particular, Gram-negative bacteria are difficult to treat due to the presence of an outer membrane wall, but silver ions can enhance the membrane permeability and have synergetic effects with many conventional antibiotics.3
While there is a prior blood chemistry analysis to indicate the general safety for this etchant with nanoparticle silver,67 we also validated the concentration of etchant and silver ions using the mammalian cell line SKOV3 (Figure S11). The MTT cell viability assay showed no obvious toxicity in vitro even at 10 mM ferricyanide ions, which is 10-fold higher than used here. Cytotoxicity of silver ions appeared at 500 μM (10 times higher than the maximum dose of the bacterial assay). This indicates that Ag+ ions have a lower background toxicity to mammalian cells while exerting significant bactericidal activities. Importantly, the photoacoustic technique allows us to measure how much silver is released (Figure 3D,E) to correlate dose to outcome.
Photoacoustic Monitoring of Silver Release in Vivo.
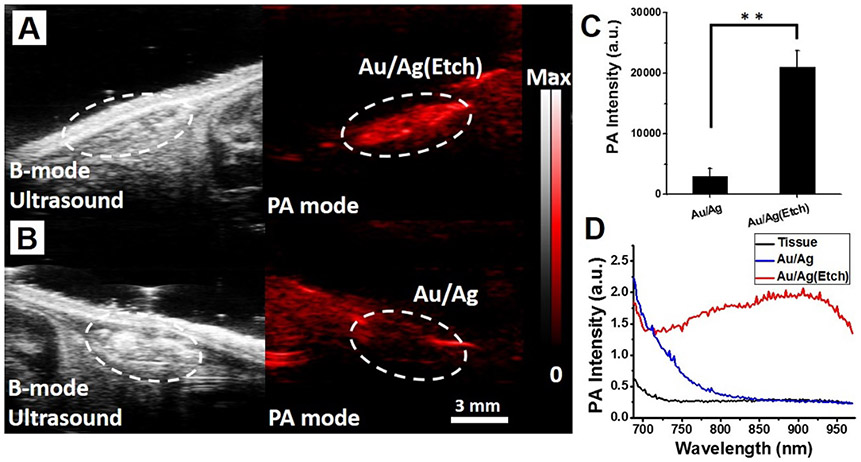
To evaluate the feasibility of our nanoprobe to monitor the released silver ions in vivo, we first implanted Au/Ag particles in 50% matrigel/phosphate-buffered saline (PBS) (200 μL) subcutaneously into wild-type male mice (n = 3) and imaged 4 h after intraperitoneal (i.p.) injection of 1 mM silver etchant (hexacyanoferrate(III) in PBS (500 μL)). Images were obtained with pulsed laser excitation at 800 nm at 30 MHz. We can clearly see the anatomical images from B-mode ultrasound images and confirm the injection and treatment efficacy of nanoparticles by using photoacoustic imaging. No photoacoustic contrast signal was detected on Au/AgNRs (Figure 5B); however, the strong photoacoustic contrast appeared upon the in vivo silver etching (Figure 5A). The recovered PA contrast indicates the localized release of silver from the hybrid nanoparticles, which can further provide important feedback on infection treatment. There was a 730% increase (Figure 5C) in the PA signal (p < 0.01) comparing pre-etching (PA intensity of 3046 ± 1323) and post-etching (PA intensity of 22085 ± 3695) (white dashed circle in Figure 5A relative to Figure 5B). Furthermore, the in vivo PA spectrum recovered as the silver dissociates. These spectral data can distinguish the area being treated from the surrounding tissues (Figure 5D). There was some red-shift of the photoacoustic spectrum compared to the original colloids of AuNRs (Figure 3C). This was likely due to aggregated AuNRs in vivo.48,77
Figure 5.
Photoacoustic imaging of the Au/AgNRs system in vivo. B-mode ultrasound (gray scale) and photoacoustic (red) images of subcutaneously injected Au/AgNRs (B) and Au/AgNRs with successive addition of silver etchant (i.p. route) (A) into mice (N = 3). The significant photoacoustic signal increased (730%) pre- and postinjection silver etching (p < 0.01) is shown in white dashed circles from A relative to B. The recovered PA contrast indicates the released silver from the hybrid nanoparticles. Scale bar is 3 mm and applies to all panels. (C) Quantification of the PA signal comparing pre- and post-Ag-etching (white dashed circle in A, B). The statistical significance was calculated with the Student’s t test; **, p < 0.01. Error bars represent the standard deviation of ROIs. (D) Photoacoustic spectrum of implanted Au/AgNRs before and after silver etching, as well as the background host tissues. The photoacoustic spectral properties of the etched Au/AgNRs are discernible from the surrounding tissues.
This result is consistent with the in vitro experiment (Figure 3C), and we can conclude that the Au/Ag nanoparticle system not only has efficient antibacterial activity via biochemically triggered silver release but also images this infection treatment using the recovered PA contrast.
Therapeutic Efficacy of Au/Ag Nanorods in Vivo against MRSA Skin Infections.
Finally, we evaluated the therapeutic efficacy of the hybrid Au/Ag nanoparticle system in vivo using the animal models of MRSA skin infection. Wild-type male mice (n = 3) were inoculated with MRSA (OD = 1) subcutaneously, and serious skin wounds developed overnight. The Au/AgNRs were applied to wound areas along with the ferricyanide etchant solution (via intraperitoneal injection); the size of the skin wounds was monitored serially for 1 week. Bacterial counts from the wound tissues were quantified.
Figure 6A shows macroscopic photographs of the wound at 1, 2, 4, and 6 days. After 6 days, wounds treated with the etched Ag/AuNRs showed prominent healing with a reduction of 41% and 25% relative to the nonetched Au/Ag and untreated controls, respectively (Figure 6B). Wounds without treatment became seriously infected and showed widespread edema by day 4. The bacteria counts in the treated group of mice (Au/Ag (Etch)) were reduced by 1000-fold (log10 CFU/g = 4.15 vs 7.75) when compared to the untreated control (Figure 6C). This is similar to the antibacterial activities of Au/Ag (Etch) to the bacterial solutions (>99.99% MRSA growth inhibition) in vitro.
Figure 6.
Antibacterial efficacy of the Au/AgNRs system in vivo. (A) Representative photograph of the MRSA (OD = 1)-infected wound from mice in three different treatment groups (Au/Ag (Etch), Au/Ag, and PBS) on days 1, 2, 4, and 6. (B) Corresponding areas of infected wound of the mice shown in A. (C) Bacterial counts showing the bactericidal action of etched Au/AgNRs. The quantity of viable bacteria in MRSA-infected wound tissue was measured with mean ± SD (N = 3) and calculated as log10 CFU/g. The statistical significance was calculated with the Student’s t test; *, p < 0.05 versus the untreated control.
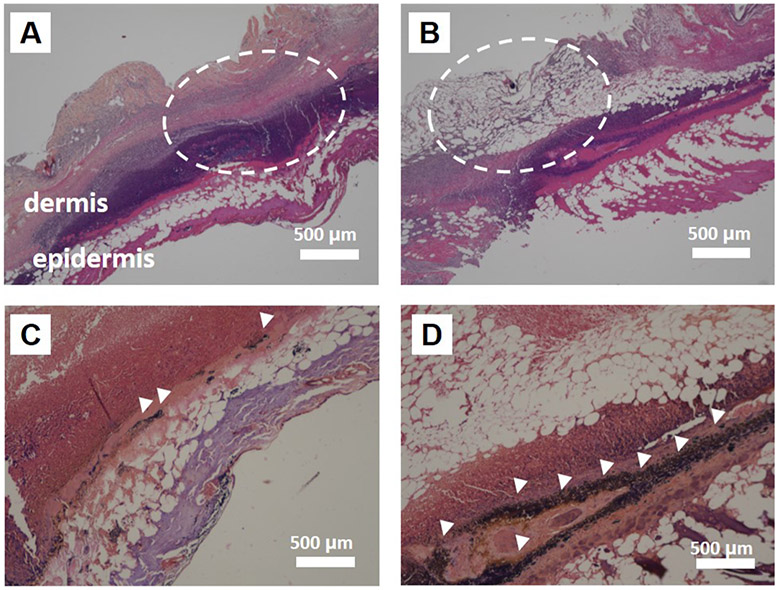
Additionally, histopathology (H&E staining and Gram-positive staining) was performed 1 week post-treatment (Figure 7). The histological outcome indicates superior therapeutic efficacy of Au/Ag nanorods in supporting wound healing. The H&E staining shows that the structures of the epidermis and dermis were maintained (white dashed circle in Figure 7A) in the treated group; a thin epidermis was observed, and the dermis structures were severely disrupted on untreated and infected tissues (white dashed circle in Figure 7B). Gram-positive bacteria, MRSA, are stained purple by crystal violet via the Gram staining method. There were much fewer cocci-shaped, Gram-positive MRSA in the treated group (white arrowhead in Figure 7C and Figure S12A). In contrast, more Gram-stained MRSA were seen from the tissue sections on the untreated group (white arrowhead in Figure 7D and Figure S12B).
Figure 7.
Histopathology. (A, B) Histology of the infected skin tissue of mice by H&E staining. Dermis and epidermis structure of treated group (Au/Ag (Etch)) were maintained (white dashed circle) in A. In contrast, serious damage was seen for the untreated control (white dashed circle) in B. Images were obtained 1 week after the treatment. Scale bar is 500 μm. (C, D) Gram-positive staining for MRSA. The purple-stained MRSA species on the infected tissue are indicated by white arrowheads in the images. There are dramatically fewer MRSA detected for the treated group (Au/Ag (Etch)) in C compared to the untreated control in D. Scale bar is 500 μm.
This hybrid nanoparticle is chemically modifiable and could be targeted after systemic administration. Many protocols exist for the conjugation of targeting moieties on nanoparticle gold,78 and the targeting agents can include antibacterial antibodies,29,79 cationic antimicrobial peptides,26,80,81 and small molecules (e.g., vancomycin, daptomycin).27,82 They could also be modified with glucose, which can rapidly internalize through the bacteria-specific maltodextrin transport pathway.83,84 The nanoparticles treated single bacterial infections; however, silver has also shown utility against biofilms when used with antibiotics for combination therapy.3 Therefore, the as-prepared nanoparticle tool could be used with antibiotics to treat complex biofilms; such a model will be the focus of future work.
This work shows an innovative theranostic approach and demonstrates photoacoustic image-guided localized therapy of wound infections. Future work can include wound dressings for a durable and efficient strategy for wound healing in conjunction with inorganic antibacterial agents (e.g., silver, gold, ZnO).85 Therefore, this hybrid nanoparticle can be embedded in cellulose-based hydrogels86 or loaded in chitosan wound dressing17 and potentially generate a stable therapeutic system for future topical applications against localized infections.
CONCLUSIONS
This study presented theranostic hybrid Au/Ag nanoparticles capable of imaging bacterial-induced infections and biochemically triggered antibacterial activity. These nanoparticles show a strong bactericidal activity that also facilitates noninvasive monitoring of localized release of Ag+ ions with photoacoustic imaging. Moreover, these nanoparticles have superior therapeutic efficacy in supporting wound healing for MRSA skin infections. Our nanosystem offers on-demand antimicrobial activity and self-reporting capabilities. It is useful for imaging and therapy of infectious diseases as an innovative tool to offer noninvasive and real-time dosimetry of Ag+ ions in vivo.
MATERIALS AND METHODS
Materials.
l-Ascorbic acid (Sigma-Aldrich, Cat. #A7506), potassium hexacyanoferrate(II) trihydrate (K4[Fe(CN)6]·3H2O, Cat. #P9387), sodium borohydride (Cat. #71320), sodium hydroxide (Catal. #221465), silver nitrate (≥99.0%, Cat. #209139), hexadecyltrimethylammonium bromide (CTAB; Cat. #H6269), iron(II) perchlorate hydrate (Cat. #334081), sodium nitrite (Cat. #237213), sodium hypochlorite solution (Cat. #239305), peroxynitrite (Cat. #20-107), 2′,7′-dichlorofluorescin diacetate (DCF-DA, Cat. #D6883), and aqueous hydrochloric acid (HCl) (36–38 wt %) were purchased from Sigma-Aldrich Chemicals (Atlanta, GA, USA). N-Acetyl-l-cysteine (NAC) (Cat. #02194603) was purchase from MP Biomedical (Santa Ana, CA, USA). Hydrogen peroxide (30 wt %, Cat. #H325) and aqueous PBS stock solutions were purchased from Thermo Scientific (Waltham, MA, USA). All chemicals were of analytical grade and used without further purification. All aqueous solutions were prepared with deionized water (18 MΩ).
Preparation of Au Nanorods.
AuNRs were synthesized by the seed-mediated growth method with some modifications of the previous report.60 First, the gold seed solution was prepared by adding 0.6 mL of cold NaBH4 (0.01 M) to an aqueous solution of 5 mL of CTAB (0.2 M) and 5 mL of AuCl3 (0.005 M). The growth solution was prepared by adding 3.5 mL of ascorbic acid (0.089 M) to an aqueous solution containing 12 mL of AgNO3 (4 mM), 250 mL of CTAB (0.2 M), and 250 mL of AuCl3 (0.001 M). Next, 0.6 mL of the gold seed solution was poured into the growth solution, and the mixture solution became dark blue/purple/brown over 20–60 min. After additional reaction for 6 h, the mixture was then washed three times with distilled (DI) water by centrifugation (12 000 rpm, 20 min) to remove extra CTAB.
Preparation of Au/Ag Nanorods.
Ag-coated AuNRs were prepared by using the modified procedure from the previous report.63 Herein, AuNRs were employed as seeds to tailor the deposition of Ag shells. First, 200 μL of ascorbic acid (0.1 M) and 0.5 mL of AgNO3 (0.01 M) were mixed with 0.5 mL of as-prepared AuNRs (1 mg Au/mL) in 50 mL of DI water. Then 500 μL of NaOH (0.1 M) was added to increase the pH over 10 because the silver ions can be reduced by ascorbic acid only in basic solution. The mixture solution was then vigorously stirred for 1 h to ensure the complete coating of silver shells. The thickness of the silver shell is tuned by adding increasing amounts of AgNO3 or decreasing the amount of AuNRs stock.
Characterization of Au/Ag Nanorods.
TEM imaging was performed by using a FEI Tecnai Spirit G2 BioTWIN microscope operating at an accelerating voltage of 80 kV. TEM specimens were prepared to drop and dry a small amount of nanoparticle suspension in 2-propanol onto carbon-coated Cu grids. Powder XRD patterns were collected on a Bruker D8 Advance diffractometer operating at 40 kV and 40 mA using Cu Kα radiation (λ = 1.5418 Å) with a scan speed of 0.1 s, a step size of 0.04° in 22θ, and a 2θ range of 20–80°. The EDX spectral data were acquired with a Philips XL30 ESEM instrument operating at 20 keV. ICP-OES (PerkinElmer Optima 3000DV) was used to quantify the amount of Au and Ag. Standard curves for Au and Ag were obtained using a gold standard solution (Cat. #38168, Sigma-Aldrich) and silver standard solution (Cat. #12818, Sigma-Aldrich), respectively. The hydrodynamic diameter and zeta potentials of nanoparticles were measured by DLS (Zetasizer ZS 90, Malvern Instruments). The UV–visible absorption spectrum was measured with a microplate reader (SpectraMax; Molecular Devices).
Silver Ion Etching.
For the selective silver etching, tripotassium hexacyanoferrate (K3[Fe(CN)6], or HCF) was used. When hexacyanoferrate solution (1 mM) was introduced to the Au/Ag nanoparticle solutions, the immobilized silver shells were readily oxidized within a minute and the color of the particle solution also changed from brown to purple. Hydrogen peroxide (H2O2) was used for gradually dissolving the Ag shell. The working concentration of H2O2 was 5–50 mM. To examine the oxidation of silver shells by different ROS, various ROS were chemically generated. Superoxide (1O2) was generated by the addition of H2O2 to the aqueous solution of NaClO. Hydroxyl radicals (•OH) were generated by mixing H2O2 and ferrous perchlorate, and peroxynitrite (ONOO−) was yielded by H2O2 and nitrite. The working concentrations of ROS were 1–10 mM. To validate that endogenously generated ROS can oxidize the silver shell, we incubated Au/Ag nanoparticles in medium from ovarian cell cultures (SKOV3). Here, we detected ROS accumulations in SKOV3 cells using a ROS-responsive dye (DCF-DA). In detail, cells were incubated with ROS-responsive dye DCF-DA (10 μM) for 10 min, washed, and visualized with an epifluorescent microscope with FITC filter sets. In a separate experiment, we preincubated cells with the antioxidant NAC (30 mM) to scavenge ROS from SKOV3.
Antibacterial Assays.
Gram-negative E. coli bacteria and Gram-positive MRSA bacteria were cultured in an agar plate for 24 h at 37 °C to reach a stationary growth phase. E. coli and MRSA (OD = 0.1, respectively) were suspended in 1 mL of LB (lysogeny broth; Cat. #10855021, Thermo Fisher). Different doses of nanoparticles (Au/AgNRs; 0.5, 2, 8, 32, 64 μM Ag) were added to the bacteria solution. The stock solution of AuNRs contained the same Au concentration as for the stock of Au/Ag particles. For the silver oxidation, an HCF solution (5 μL, 1 mM) was added within 3 min, and the mixture incubated for 3 h at 37 °C. The number of bacterial species present can be determined by surface plating the samples on agar, growing, and counting. First, MRSA (OD = 0.1) were suspended in LB (1 mL) after the addition of Au/AgNRs (2 μL; 1 mM Ag+) and silver etchant (5 μL, 1 mM). Next, the bacterial solutions were serially diluted and plated on LB agar plates. After overnight incubation at 37 °C, the number of colonies was counted, and CFU was calculated by multiplying counted colonies by the dilution ratio. MRSA displayed a golden color on the plates. The minimum inhibitory concentration (MIC) values were presented by the initial Ag contents from the nanoparticle solutions.
Photoacoustic Imaging.
Photoacoustic imaging was performed using a Visualsonics LAZR PA scanner. A transducer with a center frequency of 30 MHz (LZ 400) was used. For in vivo imaging, Au/AgNRs (200 μg Ag/mL) in 50% matrigel/PBS (200 μL) were injected subcutaneously into wild-type male mice (n = 3) and imaged 4 h after i.p. injection of 1 mM silver etchant (hexacyanoferrate(III) in PBS (500 μL)). Mice were anesthetized with 1–2% isoflurane and positioned underneath the transducer with coupling gel. Images were obtained via 3D mode with pulsed laser excitation at 800 nm. The PA spectrum of the images can be achieved by using the features of the multispectral PA image scan.
In Vivo Bactericidal Action.
All animal experiments were performed in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of California, San Diego. Wild-type male C57BL/6 mice (6 weeks; ~20 g) were used. After 1 week of quarantine, inoculation was performed by subcutaneous injection of 100 μL of MRSA (OD = 1). One day after inoculation, 100 μL of Au/AgNRs (200 μg Ag/mL) was subcutaneously implanted in the wound site, and the silver etchant (HCF solution in PBS (500 μL, 1 mM); i.p. route) was subsequently introduced in 30 min. At 24 h after treatment, tissues of MRSA-infected wounds from etchant only (untreated group) or Au/AgNRs with etchant (treated group) were collected and homogenized for subsequent quantification of viable bacterial load. For the CFU measurements, the homogenized tissues were serially diluted in LB. A 100 μL portion of each dilution was plated in LB agar plates and incubated overnight at 37 °C. The colonies were counted, and CFU was calculated by multiplying counted colonies by the dilution ratio. Means ± SD (n = 3 for each) of MRSA were calculated as log10 CFU/g in homogenized infected tissues.
To evaluate the wound-healing efficacy of Au/Ag nanoparticles, the wound surface was monitored daily and the wounds were photographed on day 1, 2, 4, and 6. Also, the wound contraction was measured from all the images adjusted to the same size and resolution. At day 7, the tissue specimens containing the entire wound and surroundings normal skin were harvested, fixed in 10% formalin, paraffin embedded, and sectioned. Finally, the sectioned tissues were stained with hematoxylin and eosin (H&E). To detect the presence of bacteria, Gram-positive staining was performed on the tissue sections with infected wounds. All the samples were then examined under a bright field microscope (Keyence BZ-9000, Germany).
Statistical Analysis.
The photoacoustic images were analyzed with ImageJ (Bethesda, MD, USA).87 The raw images were first converted to 8-bit images. Next, a region of interest (ROI) were drawn in at least three different fields of views for each sample. The mean gray values from these ROIs were measured, and the photoacoustic intensities were calculated. Mean values, standard deviations, and p-values were calculated in Microsoft Excel 2016. All error bars represent the standard deviations. The statistical significance was calculated with the two-tailed Student’s t-test; p-values of <0.05 were considered to be statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
T.K. would like to thank P. Castillo for ICP-OES analysis. J.V.J. acknowledges funding from the NIH R00 HL117048 and DP2 HL137187 and infrastructure from S10 OD021821. L.Z. acknowledges funding from the Defense Threat Reduction Agency Joint Science and Technology Office for Chemical and Biological Defense under Grant Number HDTRA1-16-1-0013. This work was performed in part at the San Diego Nanotechnology Infrastructure (SDNI) of UCSD, a member of the National Nanotechnology Coordinated Infrastructure, which is supported by the National Science Foundation (Grant ECCS-1542148).
Footnotes
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsnano.8b01362.
Additional TEM images, EDX spectroscopy, XRD measurement, DLS measurements, UV/vis absorption spectra, photoacoustic spectra, microscopy images, and MTT assay (PDF)
REFERENCES
- (1).Khabbaz RF; Moseley RR; Steiner RJ; Levitt AM; Bell BP Challenges of Infectious Diseases in the USA. Lancet 2014, 384, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Magiorakos AP; Srinivasan A; Carey RB; Carmeli Y; Falagas ME; Giske CG; Harbarth S; Hindler JF; Kahlmeter G; Olsson-Liljequist B; Paterson DL; Rice LB; Stelling J; Struelens MJ; Vatopoulos A; Weber JT; Monnet DL Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect 2012, 18, 268–281. [DOI] [PubMed] [Google Scholar]
- (3).Morones-Ramirez JR; Winkler JA; Spina CS; Collins JJ Silver Enhances Antibiotic Activity Against Gram-Negative Bacteria. Sci. Transl. Med 2013, 5, 190ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Rizzello L; Pompa PP Nanosilver-Based Antibacterial Drugs and Devices: Mechanisms, Methodological Drawbacks, and Guidelines. Chem. Soc. Rev 2014, 43, 1501–1518. [DOI] [PubMed] [Google Scholar]
- (5).Eckhardt S; Brunetto PS; Gagnon J; Priebe M; Giese B; Fromm KM Nanobio Silver: Its Interactions with Peptides and Bacteria, and Its Uses in Medicine. Chem. Rev 2013, 113, 4708–4754. [DOI] [PubMed] [Google Scholar]
- (6).Xiu ZM; Zhang QB; Puppala HL; Colvin VL; Alvarez PJJ Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Lett. 2012, 12, 4271–4275. [DOI] [PubMed] [Google Scholar]
- (7).Eisler R Silver Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review. In Contaminant Hazard Reviews: Biological Report 32; U.S. National Biological Service: Washington, DC, 1996; pp 1–44. [Google Scholar]
- (8).Setyawati MI; Yuan X; Xie JP; Leong DT The Influence of Lysosomal Stability of Silver Nanomaterials on Their Toxicity to Human Cells. Biomaterials 2014, 35, 6707–6715. [DOI] [PubMed] [Google Scholar]
- (9).Rai MK; Deshmukh SD; Ingle AP; Gade AK Silver Nanoparticles: The Powerful Nanoweapon against Multidrug-Resistant Bacteria. J. Appl. Microbiol 2012, 112, 841–852. [DOI] [PubMed] [Google Scholar]
- (10).Lara HH; Ayala-Nunez NV; Turrent LDI; Padilla CR Bactericidal Effect of Silver Nanoparticles against Multidrug-Resistant Bacteria. World J. Microbiol. Biotechnol 2010, 26, 615–621. [Google Scholar]
- (11).Kim T; Hyeon T Applications of Inorganic Nanoparticles as Therapeutic Agents. Nanotechnology 2014, 25, 012001. [DOI] [PubMed] [Google Scholar]
- (12).Rai M; Yadav A; Gade A Silver Nanoparticles as a New Generation of Antimicrobials. Biotechnol. Adv 2009, 27, 76–83. [DOI] [PubMed] [Google Scholar]
- (13).Chaloupka K; Malam Y; Seifalian AM Nanosilver as a New Generation of Nanoproduct in Biomedical Applications. Trends Biotechnol. 2010, 28, 580–588. [DOI] [PubMed] [Google Scholar]
- (14).Sibbald RG; Contreras-Ruiz J; Coutts P; Fierheller M; Rothman A; Woo K Bacteriology, Inflammation, and Healing: A Study of Nanocrystalline Silver Dressings in Chronic Venous Leg Ulcers. Adv. Skin & Wound care 2007, 20, 549–558. [DOI] [PubMed] [Google Scholar]
- (15).Strohal R; Schelling M; Takacs M; Jurecka W; Gruber U; Offner F Nanocrystalline Silver Dressings as an Efficient Anti-MRSA Barrier: A New Solution to an Increasing Problem. J. Hospital Infection 2005, 60, 226–230. [DOI] [PubMed] [Google Scholar]
- (16).Michaels J; Campbell B; King B; Palfreyman S; Shackley P; Stevenson M Randomized Controlled Trial and Cost-Effectiveness Analysis of Silver-Donating Antimicrobial Dressings for Venous Leg Ulcers (VULCAN trial). Br. J. Surg 2009, 96, 1147–1156. [DOI] [PubMed] [Google Scholar]
- (17).Li Q; Lu F; Zhou GF; Yu K; Lu BT; Xiao Y; Dai FY; Wu DY; Lan GQ Silver Inlaid with Gold Nanoparticle/Chitosan Wound Dressing Enhances Antibacterial Activity and Porosity, and Promotes Wound Healing. Biomacromolecules 2017, 18, 3766–3775. [DOI] [PubMed] [Google Scholar]
- (18).Chen XS; Wo FJ; Jin Y; Tan J; Lai Y; Wu JM Drug-Porous Silicon Dual Luminescent System for Monitoring and Inhibition of Wound Infection. ACS Nano 2017, 11, 7938–7949. [DOI] [PubMed] [Google Scholar]
- (19).Kim T; Braun GB; She ZG; Hussain S; Ruoslahti E; Sailor MJ Composite Porous Silicon-Silver Nanoparticles as Theranostic Antibacterial Agents. ACS Appl. Mater. Interfaces 2016, 8, 30449–30457. [DOI] [PubMed] [Google Scholar]
- (20).Yang Y; Liu T; Cheng L; Song GS; Liu Z; Chen MW MoS2-Based Nanoprobes for Detection of Silver Ions in Aqueous Solutions and Bacteria. ACS Appl. Mater. Interfaces 2015, 7, 7526–7533. [DOI] [PubMed] [Google Scholar]
- (21).Zhao ZW; Yan R; Yi X; Li JL; Rao JM; Guo ZQ; Yang YM; Li WF; Li YQ; Chen CY Bacteria-Activated Theranostic Nanoprobes against Methicillin-Resistant Staphylococcus aureus Infection. ACS Nano 2017, 11, 4428–4438. [DOI] [PubMed] [Google Scholar]
- (22).Cash KJ; Li CY; Xia J; Wang LHV; Clark HA Optical Drug Monitoring: Photoacoustic Imaging of Nanosensors to Monitor Therapeutic Lithium In Vivo. ACS Nano 2015, 9, 1692–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Wang JX; Chen F; Arconada-Alvarez SJ; Hartanto J; Yap LP; Park R; Wang F; Vorobyova I; Dagliyan G; Conti PS; Jokerst JV A Nano-scale Tool for Photoacoustic-Based Measurements of Clotting Time and Therapeutic Drug Monitoring of Heparin. Nano Lett. 2016, 16, 6265–6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Zhang YQ; Yu JC; Kahkoska AR; Gu Z Photoacoustic Drug Delivery. Sensors 2017, 17, 1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Xia J; Kim C; Lovell JF Opportunities for Photoacoustic-Guided Drug Delivery. Curr. Drug Targets 2015, 16, 571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Leevy WM; Gammon ST; Johnson JR; Lampkins AJ; Jiang H; Marquez M; Piwnica-Worms D; Suckow MA; Smith BD Noninvasive Optical Imaging of Staphylococcus Aureus Bacterial Infection in Living Mice Using a Bis-Dipicolylamine-Zinc(II) Affinity Group Conjugated to a Near-Infrared Fluorophore. Bioconjugate Chem. 2008, 19, 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).van Oosten M; Schafer T; Gazendam JAC; Ohlsen K; Tsompanidou E; de Goffau MC; Harmsen HJM; Crane LMA; Lim E; Francis KP; Cheung L; Olive M; Ntziachristos V; van Dijl JM; van Dam GM Real-Time In Vivo Imaging of Invasive- and Biomaterial-Associated Bacterial Infections Using Fluorescently Labelled Vancomycin. Nat. Commun 2013, 4, 2584. [DOI] [PubMed] [Google Scholar]
- (28).Leevy WM; Lambert TN; Johnson JR; Morris J; Smith BD Quantum Dot Probes for Bacteria Distinguish Escherichia Coli Mutants and Permit In Vivo Imaging. Chem. Commun 2008, 2331–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Bardhan NM; Ghosh D; Belcher AM Carbon Nanotubes as In Vivo Bacterial Probes. Nat. Commun 2014, 5, 4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Signore A; Mather SJ; Piaggio G; Malviya G; Dierckx RA Molecular Imaging of Inflammation/Infection: Nuclear Medicine and Optical Imaging Agents and Methods. Chem. Rev 2010, 110, 3112–3145. [DOI] [PubMed] [Google Scholar]
- (31).Bunschoten A; Welling MM; Tennaat MF; Sathekge M; van Leeuwen FWB Development and Prospects of Dedicated Tracers for the Molecular Imaging of Bacterial Infections. Bioconjugate Chem. 2013, 24, 1971–1989. [DOI] [PubMed] [Google Scholar]
- (32).Ntziachristos V Going Deeper Than Microscopy: The Optical Imaging Frontier in Biology. Nat. Methods 2010, 7, 603–614. [DOI] [PubMed] [Google Scholar]
- (33).Wang LV Multiscale Photoacoustic Microscopy and Computed Tomography. Nat. Photonics 2009, 3, 503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Kim C; Erpelding TN; Jankovic L; Pashley MD; Wang LHV Deeply Penetrating In Vivo Photoacoustic Imaging Using a Clinical Ultrasound Array System. Biomed. Opt. Express 2010, 1, 278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Zhang HF; Maslov K; Stoica G; Wang LHV Functional Photoacoustic Microscopy for High-Resolution and Noninvasive In Vivo Imaging. Nat. Biotechnol 2006, 24, 848–851. [DOI] [PubMed] [Google Scholar]
- (36).Weber J; Beard PC; Bohndiek SE Contrast Agents for Molecular Photoacoustic Imaging. Nat. Methods 2016, 13, 639–650. [DOI] [PubMed] [Google Scholar]
- (37).Kim T; Lemaster JE; Chen F; Li J; Jokerst JV Photoacoustic Imaging of Human Mesenchymal Stem Cells Labeled with Prussian Blue-Poly(L-lysine) Nanocomplexes. ACS Nano 2017, 11, 9022–9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Lemaster JE; Jokerst JV What Is New In Nanoparticle-Based Photoacoustic Imaging? Wiley Interdiscip. Rev.-Nanomed. Nanobiotechnol 2017, 9, e1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Lyu Y; Fang Y; Miao QQ; Zhen X; Ding D; Pu KY Intraparticle Molecular Orbital Engineering of Semiconducting Polymer Nanoparticles as Amplified Theranostics for In Vivo Photoacoustic Imaging and Photothermal Therapy. ACS Nano 2016, 10, 4472–4481. [DOI] [PubMed] [Google Scholar]
- (40).Ho IT; Sessler JL; Gambhir SS; Jokerst JV Parts Per Billion Detection of Uranium with a Porphyrinoid-Containing Nanoparticle and In Vivo Photoacoustic Imaging. Analyst 2015, 140, 3731–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Xie C; Zhen X; Lyu Y; Pu KY Nanoparticle Regrowth Enhances Photoacoustic Signals of Semiconducting Macromolecular Probe for In Vivo Imaging. Adv. Mater 2017, 29, 1703693. [DOI] [PubMed] [Google Scholar]
- (42).Pu KY; Shuhendler AJ; Jokerst JV; Mei JG; Gambhir SS; Bao ZN; Rao JH Semiconducting Polymer Nanoparticles as Photoacoustic Molecular Imaging Probes in Living Mice. Nat. Nanotechnol 2014, 9, 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Zhang JJ; Zhen X; Upputuri PK; Pramanik M; Chen P; Pu KY Activatable Photoacoustic Nanoprobes for In Vivo Ratiometric Imaging of Peroxynitrite. Adv. Mater 2017, 29, 1604764. [DOI] [PubMed] [Google Scholar]
- (44).Jiang YY; Pu KY Advanced Photoacoustic Imaging Applications of Near-Infrared Absorbing Organic Nanoparticles. Small 2017, 13, 1700710. [DOI] [PubMed] [Google Scholar]
- (45).Yang K; Zhu L; Nie LM; Sun XL; Cheng L; Wu CX; Niu G; Chen XY; Liu Z Visualization of Protease Activity In Vivo Using an Activatable Photo-Acoustic Imaging Probe Based on CuS Nanoparticles. Theranostics 2014, 4, 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Jokerst JV; Thangaraj M; Kempen PJ; Sinclair R; Gambhir SS Photoacoustic Imaging of Mesenchymal Stem Cells in Living Mice via Silica-Coated Gold Nanorods. ACS Nano 2012, 6, 5920–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Bayer CL; Chen YS; Kim S; Mallidi S; Sokolov K; Emelianov S Multiplex Photoacoustic Molecular Imaging Using Targeted Silica-Coated Gold Nanorods. Biomed. Opt. Express 2011, 2, 1828–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Jokerst JV; Cole AJ; Van de Sompel D; Gambhir SS Gold Nanorods for Ovarian Cancer Detection with Photoacoustic Imaging and Resection Guidance via Raman Imaging in Living Mice. ACS Nano 2012, 6, 10366–10377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Huang XH; El-Sayed IH; Qian W; El-Sayed MA Cancer Cell Imaging and Photothermal Therapy in The Near-Infrared Region by Using Gold Nanorods. J. Am. Chem. Soc 2006, 128, 2115–2120. [DOI] [PubMed] [Google Scholar]
- (50).Xie J; Lee S; Chen XY Nanoparticle-Based Theranostic Agents. Adv. Drug Delivery Rev 2010, 62, 1064–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Kim T; Lee N; Park YI; Kim J; Lee EY; Yi M; Kim BG; Hyeon T; Yu T; Na HB Mesoporous Silica-Coated Luminescent Eu3+ Doped GdVO4 Nanoparticles for Multimodal Imaging and Drug Delivery. RSC Adv. 2014, 4, 45687–45695. [Google Scholar]
- (52).Jokerst JV; Gambhir SS Molecular Imaging with Theranostic Nanoparticles. Acc. Chem. Res 2011, 44, 1050–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Huang HY; Lovell JF Advanced Functional Nanomaterials for Theranostics. Adv. Funct. Mater 2017, 27, 1603524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Huang XH; Peng XH; Wang YQ; Wang YX; Shin DM; El-Sayed MA; Nie SM A Reexamination of Active and Passive Tumor Targeting by Using Rod-Shaped Gold Nanocrystals and Covalently Conjugated Peptide Ligands. ACS Nano 2010, 4, 5887–5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Alkilany AM; Thompson LB; Boulos SP; Sisco PN; Murphy CJ Gold Nanorods: Their Potential for Photothermal Therapeutics and Drug Delivery, Tempered by the Complexity of Their Biological Interactions. Adv. Drug Delivery Rev 2012, 64, 190–199. [DOI] [PubMed] [Google Scholar]
- (56).Sugikawa K; Nagata S; Furukawa Y; Kokado K; Sada K Stable and Functional Gold Nanorod Composites with a Metal-Organic Framework Crystalline Shell. Chem. Mater 2013, 25, 2565–2570. [Google Scholar]
- (57).Chen ZX; Li JJ; Chen XQ; Cao JT; Zhang JR; Min QH; Zhu JJ Single Gold@Silver Nanoprobes for Real-Time Tracing the Entire Autophagy Process at Single-Cell Level. J. Am. Chem. Soc 2015, 137, 1903–1908. [DOI] [PubMed] [Google Scholar]
- (58).Gu Y; Song J; Li MX; Zhang TT; Zhao W; Xu JJ; Liu M; Chen HY Ultrasensitive MicroRNA Assay via Surface Plasmon Resonance Responses of Au@Ag Nanorods Etching. Anal. Chem 2017, 89, 10585–10591. [DOI] [PubMed] [Google Scholar]
- (59).Yu CX; Irudayaraj J Multiplex Biosensor Using Gold Nanorods. Anal. Chem 2007, 79, 572–579. [DOI] [PubMed] [Google Scholar]
- (60).Sau TK; Murphy CJ Seeded High Yield Synthesis of Short Au Nanorods in Aqueous Solution. Langmuir 2004, 20, 6414–6420. [DOI] [PubMed] [Google Scholar]
- (61).Choi E; Kwak M; Jang B; Piao Y Highly Monodisperse Rattle-Structured Nanomaterials with Gold Nanorod Core-Mesoporous Silica Shell as Drug Delivery Vehicles and Nanoreactors. Nanoscale 2013, 5, 151–154. [DOI] [PubMed] [Google Scholar]
- (62).Goudeli E; Pratsinis SE Surface Composition and Crystallinity of Coalescing Silver-Gold Nanoparticles. ACS Nano 2017, 11, 11653–11660. [DOI] [PubMed] [Google Scholar]
- (63).Xiang YU; Wu XC; Liu DF; Li ZY; Chu WG; Feng LL; Zhang K; Zhou WY; Xie SS Gold Nanorod-Seeded Growth of Silver Nanostructures: From Homogeneous Coating to Anisotropic Coating. Langmuir 2008, 24, 3465–3470. [DOI] [PubMed] [Google Scholar]
- (64).Liu HL; Pierre-Pierre N; Huo Q Dynamic Light Scattering for Gold Nanorod Size Characterization and Study of Nanorod-Protein Interactions. Gold Bull. 2012, 45, 187–195. [Google Scholar]
- (65).Mott DM; Dao TNA; Singh P; Shankar C; Maenosono S Electronic Transfer as a Route to Increase the Chemical Stability in Gold and Silver Core-Shell Nanoparticles. Adv. Colloid Interface Sci 2012, 185, 14–33. [DOI] [PubMed] [Google Scholar]
- (66).Pearce J Studies of Any Toxicological Effects of Prussian-Blue Compounds in Mammals - A Review. Food Chem. Toxicol 1994, 32, 577–582. [DOI] [PubMed] [Google Scholar]
- (67).Braun GB; Friman T; Pang HB; Pallaoro A; de Mendoza TH; Willmore AMA; Kotamraju VR; Mann AP; She ZG; Sugahara KN; Reich NO; Teesalu T; Ruoslahti E Etchable Plasmonic Nanoparticle Probes to Image and Quantify Cellular Internalization. Nat. Mater 2014, 13, 904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Zhang Q; Cobley CM; Zeng J; Wen LP; Chen JY; Xia YN Dissolving Ag from Au-Ag Alloy Nanoboxes with H2O2: A Method for Both Tailoring the Optical Properties and Measuring the H2O2 Concentration. J. Phys. Chem. C 2010, 114, 6396–6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Radi R Peroxynitrite, a Stealthy Biological Oxidant. J. Biol. Chem 2013, 288, 26464–26472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Kim C; Favazza C; Wang LHV In Vivo Photoacoustic Tomography of Chemicals: High-Resolution Functional and Molecular Optical Imaging at New Depths. Chem. Rev 2010, 110, 2756–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Paiva CN; Bozza MT Are Reactive Oxygen Species Always Detrimental to Pathogens? Antioxid. Redox Signaling 2014, 20, 1000–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Saunders JA; Rogers LC; Klomsiri C; Poole LB; Daniel LW Reactive Oxygen Species Mediate Lysophosphatidic Acid Induced Signaling in Ovarian Cancer Cells. Free Radical Biol. Med 2010, 49, 2058–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Hileman EO; Liu JS; Albitar M; Keating MJ; Huang P Intrinsic Oxidative Stress in Cancer Cells: A Biochemical Basis for Therapeutic Selectivity. Cancer Chemother. Pharmacol 2004, 53, 209–219. [DOI] [PubMed] [Google Scholar]
- (74).Fahrenholtz CD; Swanner J; Ramirez-Perez M; Singh RN Heterogeneous Responses of Ovarian Cancer Cells to Silver Nanoparticles as a Single Agent and in Combination with Cisplatin. J. Nanomater 2017, 2017, 5107485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Droge W Free Radicals in The Physiological Control of Cell Function. Physiol. Rev 2002, 82, 47–95. [DOI] [PubMed] [Google Scholar]
- (76).Song J; Kim H; Jang Y; Jang J Enhanced Antibacterial Activity of Silver/Polyrhodanine-Composite-Decorated Silica Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 11563–11568. [DOI] [PubMed] [Google Scholar]
- (77).Comenge J; Fragueiro O; Sharkey J; Taylor A; Held M; Burton NC; Park BK; Wilm B; Murray P; Brust M; Levy R Preventing Plasmon Coupling between Gold Nanorods Improves the Sensitivity of Photoacoustic Detection of Labeled Stem Cells In Vivo. ACS Nano 2016, 10, 7106–7116. [DOI] [PubMed] [Google Scholar]
- (78).Burrows ND; Lin W; Hinman JG; Dennison JM; Vartanian AM; Abadeer NS; Grzincic EM; Jacob LM; Li J; Murphy CJ Surface Chemistry of Gold Nanorods. Langmuir 2016, 32, 9905–9921. [DOI] [PubMed] [Google Scholar]
- (79).Liong M; Fernandez-Suarez M; Issadore D; Min C; Tassa C; Reiner T; Fortune SM; Toner M; Lee H; Weissleder R Specific Pathogen Detection Using Bioorthogonal Chemistry and Diagnostic Magnetic Resonance. Bioconjugate Chem. 2011, 22, 2390–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Lupetti A; Welling MM; Mazzi U; Nibbering PH; Pauwels EKJ Technetium-99m Labelled Fluconazole and Antimicrobial Peptides for Imaging of Candida Albicans and Aspergillus Fumigatus Infections. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 674–679. [DOI] [PubMed] [Google Scholar]
- (81).Bandyopadhyay A; McCarthy KA; Kelly MA; Gao JM Targeting Bacteria via Iminoboronate Chemistry of Amine-Presenting Lipids. Nat. Commun 2015, 6, 6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Kell AJ; Stewart G; Ryan S; Peytavi R; Boissinot M; Huletsky A; Bergeron MG; Simard B Vancomycin-Modified Nanoparticles for Efficient Targeting and Preconcentration of Gram-Positive and Gram-Negative Bacteria. ACS Nano 2008, 2, 1777–1788. [DOI] [PubMed] [Google Scholar]
- (83).Ning XH; Lee S; Wang ZR; Kim D; Stubblefield B; Gilbert E; Murthy N Maltodextrin-Based Imaging Probes Detect Bacteria In Vivo with High Sensitivity and Specificity. Nat. Mater 2011, 10, 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Gowrishankar G; Hardy J; Wardak M; Namavari M; Reeves RE; Neofytou E; Srinivasan A; Wu JC; Contag CH; Gambhir SS Specific Imaging of Bacterial Infection Using 6 ″-F-18-Fluoromaltotriose: A Second-Generation PET Tracer Targeting the Maltodextrin Transporter in Bacteria. J. Nucl. Med 2017, 58, 1679–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Hamdan S; Pastar I; Drakulich S; Dikici E; Tomic-Canic M; Deo S; Daunert S Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Cent. Sci 2017, 3, 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Mao CY; Xiang YM; Liu XM; Cui ZD; Yang XJ; Yeung KWK; Pan HB; Wang XB; Chu PK; Wu SL Photo-Inspired Antibacterial Activity and Wound Healing Acceleration by Hydrogel Embedded with Ag/Ag@AgCl/ZnO Nanostructures. ACS Nano 2017, 11, 9010–9021. [DOI] [PubMed] [Google Scholar]
- (87).Schneider CA; Rasband WS; Eliceiri KW NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.