Abstract
The dipotassium phosphate (K2HPO4) is a source of phosphorus (P), which is an essential micronutrient for plant growth and reproduction and also acts as a stress alleviator against abiotic stresses. Therefore, it could also become a potential mineral to cope up with zinc oxide nanoparticles’ (ZnONPs) toxicity in crops. This study primarily includes synthesis, characterization and differential toxic impacts of ZnONPs on two crop plantsThis study includes synthesis, characterization and differential toxic impacts of ZnONPs on two crop plants, i.e. Triticum aestivum and Solanum lycopersicum, as well as assuage the toxic impacts of ZnONPs through nutrient management approach implied via supplementation of P. The growth and physiological changes under toxic doses of ZnONPs and ameliorative potential of P in crop plants were examined by analysing growth, intracellular Zn accumulation, photosynthetic pigment contents, the kinetics of photosystem II (PS II) photochemistry, root cell anatomy and cell viability via histochemical staining 4′,6-diamidino-2-phenylindole and propidium iodide. ZnONPs at 500 and 1000 μM concentrations significantly affected the growth, photosynthetic pigment and PS II photochemistry and cell death in both the plants. It also caused deformation in root anatomy of T. aestivum and S. lycopersicum. Whereas supplementation of P caused significant improvement against ZnONPs stress by causing remarkable enhancement in growth, photosynthetic pigments and activity of PS II photochemistry and decreased cell death. Moreover, the study also discloses the tolerant nature of S. lycopersicum comparing with T. aestivum seedlings. Thus, P is comparatively more effective in managing the ZnONPs toxicity in S. lycopersicum than in T. aestivum.
Keywords: zinc oxide nanoparticles, phosphorus, cell death, photosynthetic pigment, PS II photochemistry, root anatomy
Graphical Abstract
Graphical Abstract.

Introduction
Nanoparticles (NPs) research is gaining more attention due to intense scientific exploration, owing to potential applications in various industries relating to biomedical, optical and electronic fields. NPs are distinct as atomic and molecular aggregates characterized by size less than 100 nm [1]. They reformed from the bulk materials but differ from bulk form due to their unusual and fascinating properties [2]. Zinc oxide nanoparticles (ZnONPs) are extensively used in optical, cosmetics, pharmaceuticals and chemical sensing especially, gas sensors and biosensors due to astonishing properties [3]. It is also used in drug delivery system as well as in medical industries as an adhesive [3]. However, indiscriminate use and inappropriate handling leads to increasing concentration of ZnONPs that eventually cause environmental contamination [4].
Plants are an imperative system that serves as a potential pathway for the transportation and accumulation of NPs from the environment [5]. NPs easily penetrate the semipermeable membrane of the cell and are transported through the vascular system to the shoot [6]. The elevated concentration of ZnONPs significantly affects the plant growth, i.e. root and shoot length, fresh mass, photosynthetic pigment, i.e. chlorophyll (Chl a and b) and carotenoid (Car) content, that directly reduces the photosynthetic rate and impairs the photosystem II (PS II) photochemistry and leads to photo-oxidative damages and causes oxidative stress in plants [4, 7]. Numerous studies on the toxicity of ZnONPs in different plants, such as Phaseolus radiatus, Triticum aestivum, Lolium perenne, Fagopyrum esculentum, Cicer arietinum, Picochlorum sp. and Solanum lycopersicum, have been reported, which suggest that ZnONPs negatively affects the physiological and biochemical processes in plants [4, 7–12]. This alteration in the physiological and biochemical process leads to the improper growth and reduce biomass that eventually results in poor yield and susceptibility towards the diseases.
Triticum aestivum and S. lycopersicum were selected for the detail study, since these crops are cultivated all over the world. T. aestivum is the first important and strategic cereal crop that is considered as staple food for the majority of world populations. It is a rich source of carbohydrate, and the flour is used chiefly for making food items [4]. Similarly, S. lycopersicum is also one of the commercially grown crops, and its fruit is rich source of vitamins, antioxidants and organic acids, and besides this, it also serves as a model organism for the study of many physiological and biological processes [7]. Mitigation of toxicity imposed by various factor through nutrient management is an inventive approach inferred these days. The exogenous supplementation of nutrients, such as macronutrients: nitrogen, phosphorus, sulphur and calcium, and micronutrients: zinc, iron manganese and silicon exhibits an imperative role against the stress by enhancing growth, improving the status of photosynthetic pigment, increasing protein and carbohydrate content and augmenting the antioxidant enzyme as well as kinetics of PS II [13–15]. Furthermore, the pivotal role of these nutrients in lessening the toxicity imposed by NPs is less focused. Among these macro and micronutrients, phosphorus (P) is one of the essential macronutrients necessary for plant growth and development [16, 17]. It is involved in the photophosphorylation and carbon assimilation. P also affects the bioavailability of other elements by forming the metallophosphate mineral formation [18, 19]. Likewise, it is well documented that increasing P can bring or aggravate Zn deficiency in crop plants through decreasing the bioavailability of Zn due to precipitation of sparingly soluble Zn phosphates [20]. It decreases the metal solubility and reduces the transportation of heavy metal by forming the heavy metal phosphate precipitates [21, 22]. P interferes mainly with the zinc uptake, and several researchers concluded that excessive accumulation of P imbalance the Zn uptake in plants and leads to its deficiency, and it also reduces the transmission of Zn from root to shoot by own excessive concentration [19, 22–25]. Thus, we are hypothesizing that the P alone and in combination with ZnONPs displays stimulation in growth and growth promoting processes; therefore, the current study was undertaken to assess the variable impact of ZnONPs toxicity on crop plants via T. aestivum and S. lycopersicum and the mitigation approach through P supplementation by analysing growth, photosynthetic pigment and photochemistry of PS II as well cell viability and anatomical alteration.
Materials and Methods
Synthesis and characterization of ZnONPs
ZnONPs was synthesized using plant extract of Withania somnifera. Fresh leaves were plucked from the plant and washed several times with water to remove the dirt and debris. Leaves were finely chopped and soaked in 100 mL sterile double-distilled water (DDW). Then, the mixture was put on the magnetic stirrer for 2 h and the prepared mixture was filtered through the Whatman No. 1 filter paper to obtain a clear and pure extract. Furthermore, 0.02 M aqueous solution of zinc acetate dihydrate [Zn(CH3COO)2·H2O] was dissolved in 70 mL of distilled water and stirred continuously for 20 min, and the plant extract was mixed drop by drop in this solution followed by the constant adding of NaOH resulting in a white aqueous solution with pH = 12. After 2 h of continuous stirring, the formed precipitate was separated and washed thoroughly with DDW and sterilize via ethanol to eliminate the impurities. Washed precipitate was oven dried at 600 C for 24 h. The white coloured powder obtained was then characterized by UV–visible spectroscopy (UV–VIS), X-ray diffraction (XRD), particle size analyser (PSA) and Fourier transform infrared radiation (FTIR).
UV–Visible spectroscopy
UV–VIS spectra was obtained by diluting the synthesized ZnONPs with DDW, through the UV–visible double-beam spectrophotometer (UV–Vis 2202, Systronics, India) wavelength range between 200 and 700 nm operated at a resolution of 1 nm.
X-ray diffraction
The prepared ZnONPs were analysed for XRD by Rigaku d’max 2200 diffractometer with Cu Kα radiation (K = 1.5406 Å). Obtained data were recorded and plotted in the graph form.
Particle size analyser
Green synthesized ZnONPs were dispersed in DDW through the ultrasonication to check out the particle size distribution. The size of ZnONPs was recorded by the PSA (Nanotrac wave W3372).
Fourier transform infrared radiation
The pellets of synthesized ZnONPs were placed in the path of infrared (IR) beam of IR spectrophotometer (FT-IR Spectrum RX-1, Perkin Elmer) attached with detector (3800–500 cm−1 resolution); obtained data were recorded and plotted in the graph form.
Plant material and growth conditions
Triticum aestivum (wheat) and S. lycopersicum (tomato) seeds were procured from a certified seed agency of Prayagraj, Uttar Pradesh. Seeds were surface sterilized by 2% sodium hypochlorite solution for 10 minutes and washed thrice with distilled water. Then, the seeds were soaked in distilled water for 12 h under dark condition then wrapped in well-sterilized wet muslin cloth. After germination, seedlings were grown in growth chamber, with photosynthetically active radiation of 300 μmol photonsm−2 s−1 and light–dark regime of 16:8 h with a relative humidity of 60–70% at 26 ± 1°C during growth of the seedlings for 18 days.
Treatment designing
The matured seedlings with secondary leaves were uprooted and transferred into pots filled with 40 ml half strength Hoagland solution for 7 days for acclimatization in hydroponic culture. Subsequently, uniform size seedlings were carefully chosen and treated with ZnONPs and P alone and in combination.
After a series of screening experiment, two doses of ZnONPs, i.e. 500 and 1000 μM corresponding to LC 18 and 32% in T. aestivum and LC 12 and 28% in S. lycopersicum, were selected for the detail study. Similarly, screening experiment was also performed for phosphorus, K2HPO4 (dipotassium phosphate) with range from 0 to 10 mM from which 4 mM dose was selected for further study as it alone alleviated the growth by 12 and 18% in T. aestivum and S. lycopersicum, respectively, and under stress condition, with lower dose, it completely alleviated the toxic effect, and with higher dose, partial improvement was noticed.
The following setup was prepared control (without any treatment), 500 μM ZnONPs, 1000 μM ZnONPs, phosphorus (4 mM), 500 μM ZnONPs + P (4 mM) and 1000 μM ZnONPs + P (4 mM). There were three replicates for each treatment. After 7 days of treatment, seedlings were harvested and analysed for various physical, physiological and biochemical parameters.
Growth analysis
Growth in treated and untreated seedlings were analysed by determining the length and fresh weight of root and shoot.
Estimation of Zn and P content
For the estimation of Zn and P content in root and shoot, desired amount of samples were digested by triacid mixture (HNO3:HClO4:H2SO4 v/v). The volume of digested sample was maintained up to 50 ml by adding DDW, and the element was analysed by following the method of Allen et al. (1986) [26] using atomic absorption spectrometer (iCE 3000 Series, Thermo Fisher Scientific, UK).
Estimation of photosynthetic pigment contents
The photosynthetic pigment contents (Chl a, Chl b and Car) were estimated by following the method of Lichtenthaler (1987) [27]. Desired amount of leaves from treated and untreated seedlings were homogenized in 80% acetone and the absorbance was read out at 663.2, 646.5 and 470 nm, respectively.
Chlorophyll a fluorescence
To pinpoint the effect of ZnONPs on PS II, chlorophyll a fluorescence was performed in the 30 mint dark-adapted leaves by FluorPen FP 100 (Photon System Instruments, Czech Republic) (Strasser et al. 2000) [28].
Fluorescent analysis of dead cells (cell death)
The cell death in root tip of treated and untreated seedlings was seen via fluorescent dye propidium iodide (PI) and 4′,6-diamidino-2-phenylindole (DAPI) and visualized at 493/636 and 330–385 nm, respectively, by Olympus BX-01 spectrofluorometer (USA) by following the method of Ubeda-Tomas et al. (200) [29] and Culligan et al. (2004) [30], respectively.
Anatomical study of root
To visualize the anatomical effect of ZnONPs treatments, fine sections were cut from the control and treated seedlings and washed in distilled water to remove unwanted particles followed by staining in 50% safranin and mounted in glycerine. Sections were observed under the optical microscope linked to software image tool.
Statistical analysis
The experiments were performed in triplicates (n = 3) and results are presented as means ± SE. Results were statistically analysed by analysis of variance. Duncan multiple range test was used for the significant differences among treatments at P < 0.05 levels.
Experimental Findings
Characterization of green synthesis of ZnONPs
Spectroscopy (UV/Vis/IR) technique is mainly based on the light absorbed and scattered by the tested sample. NPs possess a unique optical property that depends on the agglomeration state, shape, size and refractive index near the particle surface. The UV–visible spectra of biologically synthesized NPs exhibited the highly shifted absorption maximum around 330–370 nm, which is characteristic peak for ZnONPs [31] (Fig. 1a).
Figure 1.
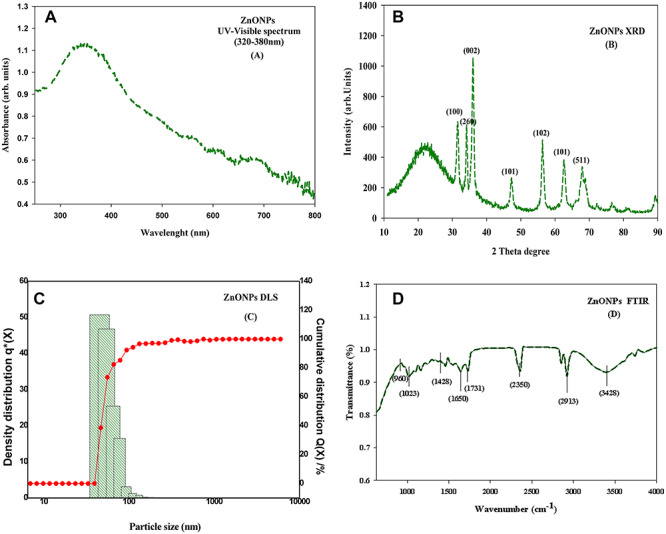
characterization of ZnONPs (a) UV spectra of ZnONPs (b) XRD pattern of the ZnONPs; (c) particle size distribution image of ZnONPs (d) FTIR spectrum of ZnONPs.
Identification and quantification of crystalline nature of produced particles is done by XRD method. Diffraction peaks were obtained by interaction between waves and a regular structure, whose repeat distance is similar to wavelength. The XRD peaks corresponding to the diffraction planes (100), (260), (002), (101), (102), (101) and (511) agree well with the JCPDS Card No. 89-0511, confirming the wurtzite structure of the ZnONPs [31] (Fig. 1b). A small amount of NPs was dispersed in DDW and the dispersed solution was then analysed under dynamic light scattering. It is based on Mie scattering theory. The particle size distribution curve for produced NPs revealed that the particle size in the solution was under the range of 35–100 nm (Fig. 1c).
FTIR technique was performed to identify the possible phytochemical functional groups present on plant extract, which might be responsible for production and stabilization of ZnONPs. The strong and broad IR bands at 3404 cm−1 correspond to amine group stretching of the hydroxyl group [32]. The sharp band at 2913 cm−1 corresponds to C-H vibration of aliphatic hydrocarbons. The bands at 2352 cm−1 correspond to the characteristics of the N-H stretching or C=O stretching vibration. The band at 1731 cm−1 is the characteristic of the C=O stretching mode of the carboxylic acid group [33]. The peak located at 1650 cm−1 could be assigned to the C=O stretching in the carboxyl or C=C stretching in aromatic ring [32]. The observed band at 1428 cm−1 corresponds to C-N stretching. The peak at 1023 cm−1 is the characteristics of C-O-C bending mode. The band at 960 cm−1 indicated the vibration of (NH)–C=O group (Fig. 1d).
Growth
Growth in both the tested seedlings was measured in terms of fresh mass [shoot fresh mass (SFM), root fresh mass (RFM)], root length (RL) and shoot length (SL) and depicted in Fig. 2a and b. Upon both the tested doses of ZnONPs, i.e. 500 and 1000 μM, a significant reduction in growth, that is SFM by 19 and 32%, RFM by 18 and 33%, RL by 20 and 34% and SL by 17 and 35%, respectively, was recorded in T. aestivum. Whereas, in S. lycopersicum, the fresh mass declined by 13 and 28% in shoot and 12 and 29% in root, similarly RL declined by 14 and 30% and SL by 11 and 27%, respectively, over control values. However, when growth medium was supplemented with P alone, it enhanced the SFM by 12 and 18%, RFM by 14 and 19%, RL by 10 and 16% and SL by 11 and 20% in T. aestivum and S. lycopersicum respective to control values. Moreover, P in combination with ZnONPs reduced the extent of damage, i.e. with lower dose, it exhibited only 7 and 9% reduction in SFM and RFM, respectively, similarly only 10 and 12% reduction in SL and RL, and with higher dose, it showed similar alleviating effect in T. aestivum, further similar values were also recorded for S. lycopersicum, but the improvement in values were more prominent in S. lycopersicum as compared to T. aestivum, indicating its resistant nature against ZnONPs toxicity.
Figure 2.

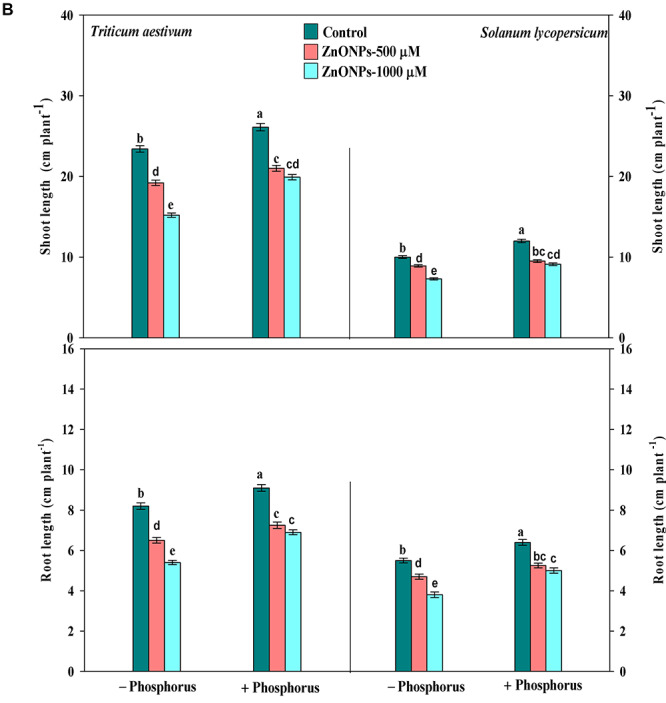
(a) growth parameters (fresh mass of shoot and root) of T. aestivum and S. lycopersicum seedlings grown in the presence of ZnONPs alone or in combination of P, and data are means ± standard error of three replicates; bars with different letters show significant differences at P < 0.05 according to the Duncan multiple range test; (b) Growth parameters (length of shoot and root) of T. aestivum and S. lycopersicum seedlings grown in the presence of ZnONPs alone or in combination of P, and data are means ± standard error of three replicates; bars with different letters show significant differences at P < 0.05 according to the Duncan multiple range test.
Zn and P content
The results pertaining to Zn and P accumulation analysed in root and shoot have been depicted in Fig. 3a and b. The untreated seedlings (control) showed 148 ± 2.56 and 210 ± 3.63 μg g−1 dry weights in shoot and root of T. aestivum and 151 ± 2.61 and 217 ± 3.75 μg g−1 dry weights in shoot and root, respectively, of S. lycopersicum. Furthermore, under both the concentration of ZnONPs, the content in shoot raised by 171 and 439% in T. aestivum and 157 and 383% in S. lycopersicum. Similar trend was also recorded in root in both the plants. However, the exogenous supplementation of P lessens the Zn accumulation, and as it competes at the binding site, the value recorded was 52 and 162% in T. aestivum and 37 and 133% in S. lycopersicum, respectively, in shoot. Similar results were also obtained in root, but the accumulation was greater in root compared to shoot in both the plants.
Figure 3.

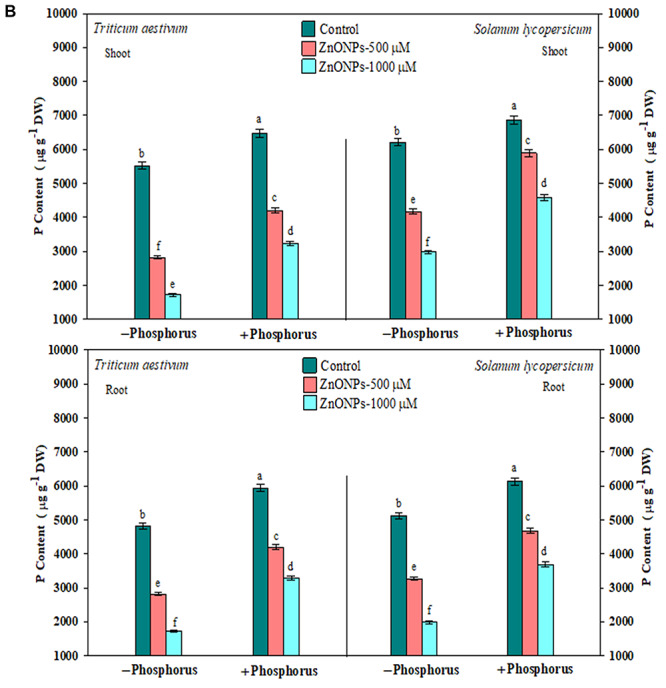
(a) zinc accumulation in root and shoot of T. aestivum and S. lycopersicum seedlings grown in the presence of ZnONPs alone or in combination of P, and data are means ± standard error of three replicates. Bars with different letters show significant differences at P < 0.05 according to the Duncan multiple range test; (b) Phosphorous accumulation in root and shoot of T. aestivum and S. lycopersicum seedlings grown in the presence of ZnONPs alone or in combination of P; and data are means ± standard error of three replicates; bars with different letters show significant differences at P < 0.05 according to the Duncan multiple range test.
Photosynthetic pigment
In order to check the damage to the pigment system under stress condition, the photosynthetic pigment content (Chl a, Chl b and Car) was measured in both tested seedlings (Table 1). In T. aestivum, 500 and 1000 μM of ZnONPs declined Chl a by 21 and 36%, Chl b by 19 and 33% and Car content by 17 and 34%, respectively, while lesser damage was recorded in S. lycopersicum. Furthermore, exogenous supplementation of P alone raised the content, i.e. Chl a by 11 and 19%, Chl b by 13 and 20% and Car by 13 and 16% in T. aestivum and S. lycopersicum, respectively. However, P along with ZnONPs reduced the extent of toxicity in both the seedlings; it showed complete alleviation with lower dose of ZnONPs (500 μM), while with higher dose, it limits the reduction percentage and exhibited partial alleviation against ZnONPs stress.
Table 1.
effects of ZnONPs and P alone and in combination on chlorophyll a (Chl a), chlorophyll b (Chl b) and carotenoids (Car) in T. aestivum and S. lycopersicum
| Photosynthetic pigment contents (mg g −1 fresh weight) | ||||||
|---|---|---|---|---|---|---|
| Treatments | Chl a | Chl b | Car | |||
| Triticum aestivum | Solanum lycopersicum | Triticum aestivum | Solanum lycopersicum | Triticum aestivum | Solanum lycopersicum | |
| Control | 1.5900 ± 0.027b | 1.6230 ± 0.024b | 0.3760 ± 0.006b | 0.3990 ± 0.006b | 0.4890 ± 0.008b | 0.5120 ± 0.007b |
| ZnONPs—500 μM | 1.2500 ± 0.021c | 1.4150 ± 0.021c | 0.3040 ± 0.005c | 0.3590 ± 0.005c | 0.4010 ± 0.006d | 0.4480 ± 0.006c |
| ZnONPs—1000 μM | 1.0100 ± 0.017d | 1.1480 ± 0.017d | 0.2500 ± 0.004d | 0.2900 ± 0.004d | 0.3180 ± 0.005e | 0.3870 ± 0.005d |
| Phosphorus | 1.7800 ± 0.030a | 1.9390 ± 0.029a | 0.4270 ± 0.007a | 0.4800 ± 0.007a | 0.5550 ± 0.009a | 0.5990 ± 0.008a |
| ZnONPs—500 μM + phosphorus | 1.4700 ± 0.025b | 1.5680 ± 0.023b | 0.3390 ± 0.005b | 0.3800 ± 0.006b | 0.4370 ± 0.007c | 0.4860 ± 0.006b |
| ZnONPs—1000 μM + phosphorus | 1.3000 ± 0.022c | 1.4599 ± 0.021c | 0.3130 ± 0.005c | 0.3600 ± 0.005c | 0.4214 ± 0.007c,d | 0.4620 ± 0.006c |
The data are means ± standard error of three replicates. Different letters on values within same column show significant difference at P < 0.05.
Chlorophyll a fluorescence
Chlorophyll a fluorescence transient-JIP test was carried out to understand the kinetics of PS II under ZnONPs toxicity in T. aestivum and S. lycopersicum (Fig. 4). The chlorophyll a fluorescence kinetics parameter, quantum yield of primary photochemistry (Phi_P0), yield of electron transport per trapped excitation (Psi_0), the quantum yield of electron transport (Phi_E0) and performance index of PS II (PIABS), showed significant decline in values under ZnONPs (500 and 1000 μM) treatments. Contrary to the kinetics parameters, the energy flux parameters, i.e. the energy fluxes for absorption of photon per active reaction centre (ABS/RC), trapped energy flux per active reaction centre (TR0/RC), electron transport flux per active reaction centre (ET0/RC), energy dissipation flux per active reaction centre (DI0/RC), were increased in both seedlings as shown in radar plots (Fig. 4). Whereas with P supplementation, kinetics parameters were significantly increased as compared to control, whereas energy fluxes per reaction centre were reduced. The damage caused by ZnONPs in photochemistry of PS II is more evident in T. aestivum in comparison to S. lycopersicum pointing towards more sensitive nature of T. aestivum (Fig. 4).
Figure 4.
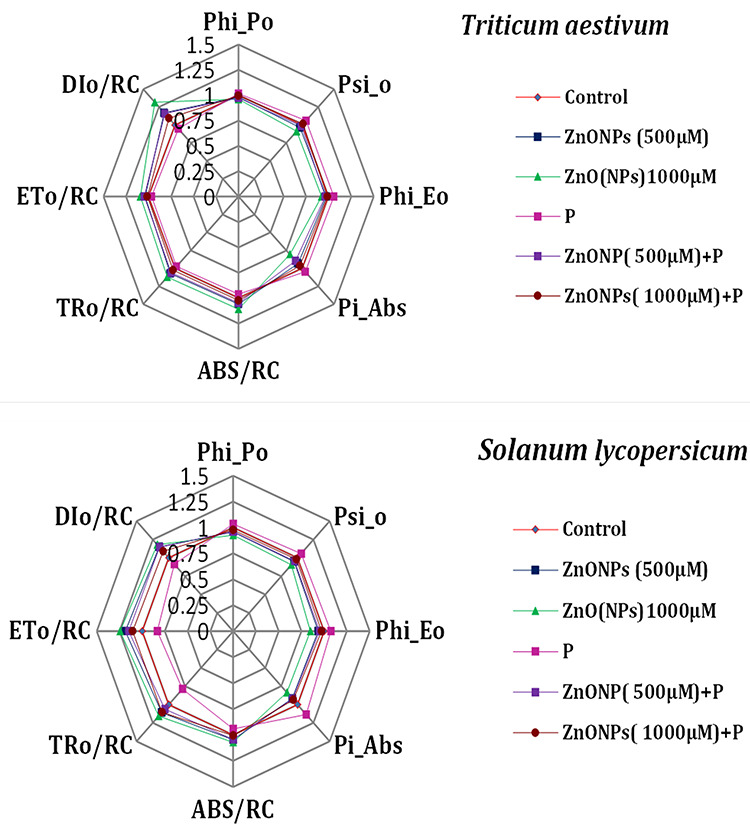
radar graph showing the altered kinetics of PS II in T. aestivum and S. lycopersicum seedlings in the presence of ZnONPs alone or in combination with P.
Cell viability assessment
The results pertaining to the cell viability in T. aestivum and S. lycopersicum have been shown in Fig. 5a and b. The fluorescent stains DAPI (Fig. 5a) and PI (Fig. 5b) have been used for the detection of cell viability. These dyes exclude the membrane of living cells, whereas dead cells develop blue colour on reaction with DAPI and stain red fluorescent on reaction with PI. Tested seedlings under ZnONPs (500 and 1000 μM) showed more intense colour with respect to control indicating greater cell death under stress condition, which increases with increasing concentration of ZnONPs. Whereas P supplementation limits the toxicity and reduces the percentage of cell death showing an alleviating effect and thus improves the growth and shows lesser damage.
Figure 5.
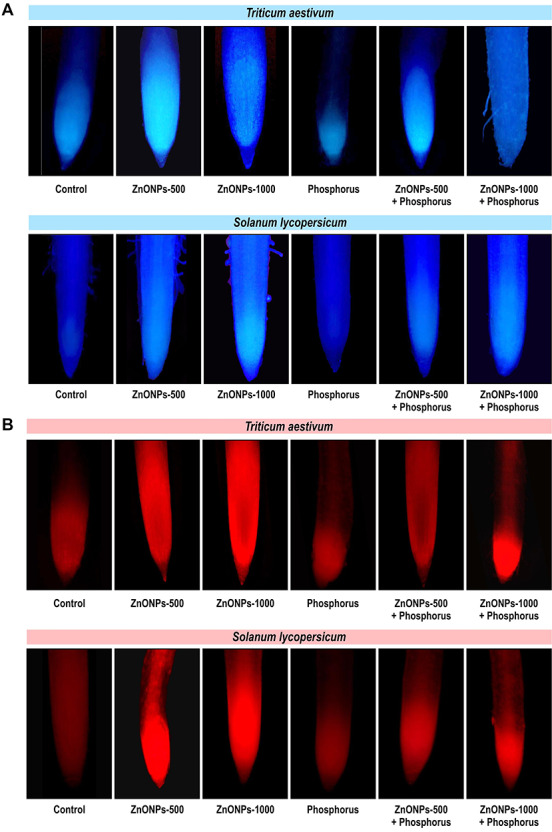
(a) representative images illustrating the in vivo detection of cell death through the fluorescence stain DAPI in root of T. aestivum and S. lycopersicum seedlings in the presence of ZnONPs alone or in combination with P, (b) Representative images illustrating the in vivo detection of cell death through the fluorescence stain PI in root of T. aestivum and S. lycopersicum seedlings in the presence of ZnONPs alone or in combination with P.
Anatomical behaviour
The changes in anatomical behaviour under ZnONPs in both the seedlings have been shown in Fig. 6. Untreated seedlings of T. aestivum showed single-layered epidermis; unicellular root hairs; well-developed cortex; barrel-shaped endodermis consisting of casparian strips at their inner tangential walls with passage cell, radial, exarch, polyarch and closed type vascular bundle and well-developed parenchymatous pith. Whereas P-treated seedlings showed fully developed cortical cell and suberized endodermal layer with well-developed metaxylem in comparison to control seedlings. While ZnONPs (500 μM) showed dissolve cortex and less metaxylem vessel in comparison to control seedlings. While raising the concentration of ZnONPs, it caused more alteration in root anatomy by disrupting the epidermis, dissolution and shrinkage of cortex cell as well as loss of endodermis and vascular bundles. Moreover, supplementation of P in combination with ZnONPs restores the damage by maintaining the epidermis integrity, increasing the suberization in endodermal layer and sustaining the number of vascular bundles, in respect to control seedlings (Fig. 5c).
Figure 6.
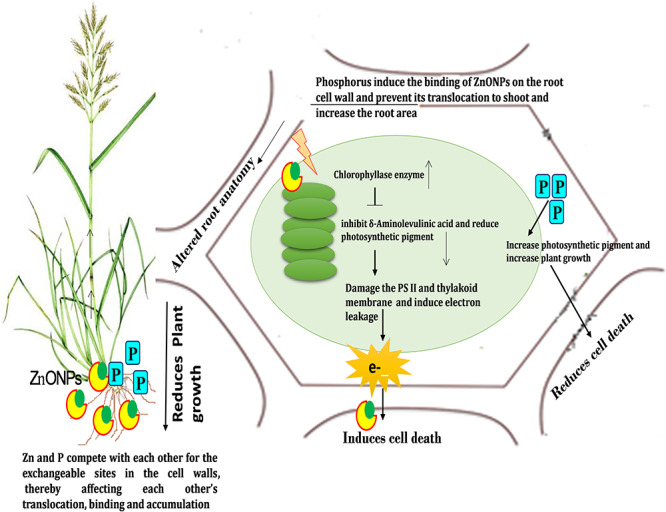
diagrammatic representation of toxicity induced by ZnONPs and mitigation by exogenous phosphorous.
Figure 5.
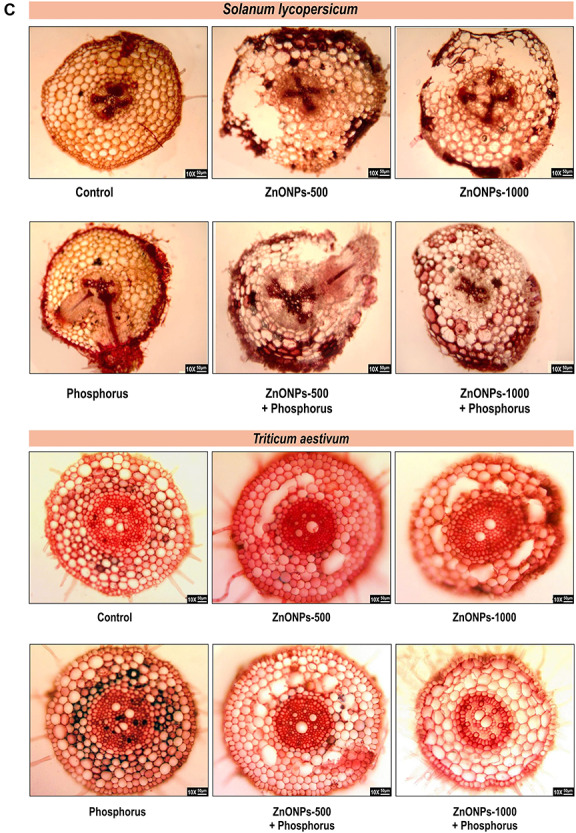
(c) Representative images illustrating the anatomical modification in root of T. aestivum and S. lycopersicum seedlings in the presence of ZnONPs alone or in combination with P.
Similarly, untreated seedlings of S. lycopersicum showed well-developed epidermis with several thin walled cells in which some cells with unicellular hairs were present. Below epidermis a large well-developed parenchymatous cortex filled with starch grains were present. Pericycle was present closed to endodermis with open, exarch vascular bundles that are radially arranged. Pith is reduced. Phosphorus-treated seedlings showed well-developed cortex region and fully define vascular bundles with the formation of lateral roots in comparison to control seedlings. While ZnONPs with lower dose showed distortion in the epidermal layer and deformed cortex cell, at higher concentration, suberization of endodermal layer was affected; beside this, it also caused the breakage of endodermal layer and loosening of vascular bundles. Moreover, supplementation of P with the ZnONPs limits the extent of damage to root structure by repairing the cortex cells and maintaining the vascular bundle in comparison to ZnONPs-treated seedlings (Fig. 5c).
Discussion
The present study elucidates the alleviating role of mineral nutrient approach primarily (phosphorus) against the toxicity induced by ZnONPs (500 and 1000 μM) in two crop plants viz. Triticum aestivum and Solanum lycopersicum by analysing growth, intracellular Zn accumulation, photosynthetic pigment contents, kinetics of PS II photochemistry and alteration in anatomy of root cell and cell viability. Growth (SL, RL, SFM and RFM) (Fig. 2a and b) was significantly decreased under both the toxic doses of ZnONPs, and this reduction is correlated with increased Zn accumulation (Fig. 3) associated with reduced pigment content (Table 1) and reduced efficiency of PS II photochemistry. Similar to our finding, Dimkpa et al. (2012) [4], Stewart et al. (2015) [34] and Watson et al. (2015) [35] also reported the declining growth in T. aestivum exposed to ZnONPs. Furthermore, Mukherjee et al. (2016) [36] and Zafar et al. (2016) [37] also showed similar reduction in growth in Pisum sativum and Brassica sp. exposed to ZnONPs. The increased Zn accumulation in current study is primarily due to its easy transportation inside the cell and its toxicity is totally depended on the potential dissolution of metal ions from NPs, and ZnONPs have higher solubility compared to other metallic NPs [38]. Concurrent to our study, Du et al. (2011) [39] also reported increased Zn accumulation in T. aestivum exposed to ZnONPs. Photosynthetic pigments (Chl a and Chl b) that are the essential component of photosystem while carotenoids as an accessory pigments were also found to increase in concentration-dependent manner in both the plants; however, greater reduction was observed in T. aestivum compared to S. lycopersicum; the reasons are (i) the replacement of inorganic phosphate (iP), needed for Chl biosynthesis [40]; (ii) alteration of enzyme involved in pigment biosynthesis; (iii) inhibition of chlorophyll biosynthesis precursor [40] and (iv) oxidation of photochemical machinery that impair the photosynthetic pigments [41]. Similarly, Wang et al. (2016) [42] also reported reduction in pigment content under ZnONPs stress due to reduced expression level of chlorophyll synthesis genes and photosystem structure gene in Arabidopsis. Parallelly, similar declining trend was also observed with Car content that was found to decrease under stress condition, which might be due to disturbance in the thylakoid membrane as reported by Singh et al. (2018) [43] under arsenic stress. Reduced pigment contents apparently affect the efficiency of PS II photochemistry; thus, to pinpoint the target sites of ZnONPs on photosynthetic machinery, Chl a fluorescence (JIP-test) was performed. The values of the quantum yield of primary photochemistry (Phi_Po), the yield of electron transport per trapped excitation (Psi_o), the quantum yield of electron transport (Phi_Eo) and performance index of PS II (Pi_ABS) under ZnONPs were decreased under ZnONPs treatment. The decreased Phi_Po might be due to decrease in the primary charge separation or by disconnection of some minor antenna from PS II. The impairment of PS II in turn decreased the Phi_Eo and Psi_o. These findings corroborate a previous report on ZnONPs toxicity by Wang et al. (2018) [7] in S. lycopersicum. Moreover, lowering of photosynthetic performance under ZnONPs due to inactiveness of reaction centre leads to enhancement in the energy flux ratios: ETo/RC and TRo/RC, DIo/RC (Fig. 3), these values were found to enhance due to reduction in number and size of the active reaction centre [15]. Furthermore, the overall performance as indicated by PIABS was also found to be decreased in both the seedlings over the respective control; however, exogenous supplementation of phosphorus attenuated the ZnONPs-induced toxicity, which could be analysed by the enhancement in plant growth correlated with the lesser accumulation of Zn content.
Lesser intracellular Zn content might be due to (i) competitive binding affinity of these ions, as high concentrations of phosphorus reduces the Zn uptake; (ii) P increases the absorption of Zn but reduces the translocation and (iii) P fixes the active Zn on root cell wall thereby acting as a barrier against active uptake of Zn from the medium. Similar reason of reduced Zn accumulation was also stated by Jiang et al. (2007) [19], Salimpour et al. (2010) [23], Khorgamy and Farnis (2009) [24] and Das et al. (2005) [25]. Furthermore, P-induced decline in Zn uptake also stimulates the photosynthetic pigment contents as it inhibits the activity of chlorophyllase enzyme (chlorophyll degrading enzyme) and stimulates the synthesis of enzymes involved in Chl biosynthesis [19]. Alternatively, P also protect the central metal ion (Mg) thus suppressing its replacement by the Zn ion [19]. Exogenic P enhanced the photosynthetic pigment content, which in turn improves the efficiency of PS II photochemistry, the JIP parameters that was found to decrease under stress condition due to reduction in number of active reaction centre was substantially improved due to increase number of active RC [15, 44]. (Fig. 3) Unlike JIP parameters, the energy flux parameters that were found to increase under stress condition were relieved by exogenous P thus improving overall performance of PS II photochemistry. Therefore, P mediates lowering in the Zn accumulation and improves photosynthetic pigment and photochemistry of PS II that may be the reason of mitigation of ZnONPs phytotoxicity in T. aestivum and S. lycopersicum. However, the recorded value suggests more sensitive nature of T. aestivum compare to S. lycopersicum. Beside affecting physiological and biochemical parameters, ZnONPs also altered the ultrastructure of tested plants as visualized by root anatomy. The ZnONPs at both the doses induces deformation in epidermis, shrinkage in cortical cells as well as loss of endodermis and vascular bundles by affecting the metaxylem vessel of root in both tested plants. Similar results were also visualized by Lin and Xing (2008) [10] in Lolium perenne, Kumari et al. (2011) [45] in Allium cepa and Kouhi et al. (2015) [46] in Brassica napus due to ZnONPs. Root is the primary organ that directly contacts with the ZnONPs, it also prevents Zn translocation from root to shoot by binding it to root cell wall. Moreover, ZnONPs disturb the elasticity and uniformity of root cell (Pokhrel and Dubey 2013) [47] and leads to wavy, irregular and loosed edge of cell wall [48]. Whereas exogenous supplementation of P maintains the loss of root anatomical structure induced by ZnONPs, by increasing the size and number of xylem vessel, which ultimately expands the root area [49]. Phosphorus also acts as a barrier against active uptake of Zn 20 from the medium, which might be a reason of revamping cell integrity and structure of root. The toxicity of ZnONPs leads to impairment in photochemistry of PS II, and the leakage of electron results in excessive accumulation of reactive oxygen species (SOR, H2O2), which thereby causes membrane damage and leads to cell death. In current study, in vivo visualization through PI and DAPI shows the greater percentage of cell death in ZnONPs, treated seedlings shows more intense colour, because these dyes can cross only damage membrane; it is impermeable to living cell. The cell death is more pronounced in T. aestivum indicating more sensitive nature over S. lycopersicum, whereas exogenous addition of P reduces the cell death as evident by less intense colour by increasing the efficiency of PS II. The S. lycopersicum showed better ameliorative response in comparison to T. aestivum. Thus, enhanced growth and photosynthetic pigment and improved PS II photochemistry, and cell viability under exogenic P suggests towards the potential application of mineral nutrient approach under adverse environmental conditions.
Conclusion
The present study concludes that the ZnONPs (500 and 1000 μM) significantly declined the growth of both the crop plants due to enhanced accumulation of Zn that results in reduction of pigment content and alteration in photochemistry of PS II that leads to cell death. ZnONPs binds to cell wall and also induces the alteration in the root anatomy. Study also elucidates the more sensitive nature of T. aestivum against the ZnONPs in comparison to S. lycopersicum. Exogenous supplementation of P shielded both the plants against the ZnONPs by reducing Zn accumulation and maintaining the photosynthetic pigment and PS II photochemistry. Phosphorus also repairs the internal injuries in root structure caused by ZnONPs by expanding the root area. Phosphorus is comparatively more effective in managing the ZnONPs toxicity in S. lycopersicum than in T. aestivum. Therefore, study recommends the alleviation of ZnONPs toxicity through the nutrient approach in order to increase the plant growth and productivity (Fig. 6).
Acknowledgement
V.Y. is thankful to CSIR-JRF, File No. 09/001(0427)/2019-EMR-1, and N.A. is thankful to the University Grants Commission, New Delhi, for providing financial support (as UGC—AU Research Scholar) to carry out present work. D.K.C. is gratifying to the Department of Science and Technology, New Delhi, for providing research facilities in the department under DST-FIST. Authors are grateful to the MNNIT, department of biotechnology, for providing experimental facility related to fluorescence microscopy.
Contributor Information
Vaishali Yadav, D. D. Pant Interdisciplinary Research Laboratory, Department of Botany, University of Allahabad, Prayagraj 211002, India.
Namira Arif, D. D. Pant Interdisciplinary Research Laboratory, Department of Botany, University of Allahabad, Prayagraj 211002, India.
Devendra Kumar Chauhan, D. D. Pant Interdisciplinary Research Laboratory, Department of Botany, University of Allahabad, Prayagraj 211002, India.
Conflicts of interest statement
Authors declare no conflict of interest.
References
- 1. Rico CM, Majumdar S, Duarte-Gardea M, et al. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J Agric Food Chem 2011;59:3485–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jeevanandam J, Barhoum A, Chan YS, et al. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol. 2018;9:1050–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jin SE, Jin HE. Synthesis, characterization, and three-dimensional structure generation of zinc oxide-based nanomedicine for biomedical applications. Pharmaceutics 2019;11:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dimkpa CO, McLean JE, Latta DE, et al. CuO and ZnO nanoparticles: phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J Nanopart Res 2012;14:1125. [Google Scholar]
- 5. Wang Z, Xie X, Zhao J, et al. Xylem-and phloem-based transport of CuO nanoparticles in maize (Zea mays L.). Environ Sci Technol 2012;46:4434–41. [DOI] [PubMed] [Google Scholar]
- 6. Lv J, Christie P, Zhang S. Uptake, translocation, and transformation of metal-based nanoparticles in plants: recent advances and methodological challenges. Environ Sci Nano 2019;6:41–59. [Google Scholar]
- 7. Wang XP, Li QQ, Pei ZM, et al. Effects of zinc oxide nanoparticles on the growth, photosynthetic traits, and antioxidative enzymes in tomato plants. Biol Plant 2018;62:801–8. [Google Scholar]
- 8. Lee S, Kim S, Kim S, et al. Assessment of phytotoxicity of ZnO NPs on a medicinal plant, Fagopyrum esculentum. Environ Sci Pollut Res 2013;20:848–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee WM, An YJ, Yoon H, et al. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): plant agar test for water-insoluble nanoparticles. Environ Toxicol Chem 2008;27:1915–21. [DOI] [PubMed] [Google Scholar]
- 10. Lin D, Xing B. Root uptake and phytotoxicity of ZnO nanoparticles. Environ Sci Technol 2008;42:5580–5. [DOI] [PubMed] [Google Scholar]
- 11. Burman U, Saini M, Kumar P. Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol Environ Chem 2013;95:605–12. [Google Scholar]
- 12. Hazeem LJ, Bououdina M, Rashdan S, et al. Cumulative effect of zinc oxide and titanium oxide nanoparticles on growth and chlorophyll a content of Picochlorum sp. Environ Sci Pollut Res 2016;23:2821–30. [DOI] [PubMed] [Google Scholar]
- 13. Sarwar N, Malhi SS, Zia MH, et al. Role of mineral nutrition in minimizing cadmium accumulation by plants. J Sci Food Agric 2010;90:925–37. [DOI] [PubMed] [Google Scholar]
- 14. Singh S, Parihar P, Singh R, et al. Heavy metal tolerance in plants: role of transcriptomics, proteomics, metabolomics, and ionomics. Front Plant Sci 2016;6:1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Singh S, Prasad SM. Management of chromium (VI) toxicity by calcium and sulfur in tomato and brinjal: implication of nitric oxide. J Hazard Mater 2019;373:212–23. [DOI] [PubMed] [Google Scholar]
- 16. Lambers H, Yuan L, Liu X. Highlights of special issue on" sustainable phosphorus use in agri-food system". Front Agric Sci Eng 2019;6:311–2. [Google Scholar]
- 17. Mo Q, Li ZA, Sayer EJ, et al. Foliar phosphorus fractions reveal how tropical plants maintain photosynthetic rates despite low soil phosphorus availability. Funct Ecol 2019;33:503–13. [Google Scholar]
- 18. Cooper EM, Strawn DG, Sims JT, et al. Effect of Chemical Stabilization by Phosphate Amendment on the Desorption of P and Pb from a Contaminated Soil. 1998 Agronomy Abstracts. Madison, WI: ASA, 1998, 343. [Google Scholar]
- 19. Jiang HM, Yang JC, Zhang JF. Effects of external phosphorus on the cell ultrastructure and the chlorophyll content of maize under cadmium and zinc stress. Environ Pollut 2007;147:750–6. [DOI] [PubMed] [Google Scholar]
- 20. Mousavi SR. Zinc in crop production and interaction with phosphorus. Aust J Basic Appl Sci 2011;5:1503–9. [Google Scholar]
- 21. Cotter-Howells J, Caporn S.. Remediation of contaminated land by formation of heavy metal phosphates. Appl Geochem 1996;11:335–42. [Google Scholar]
- 22. Wang H, Wang PF, Zhang H. Use of phosphorus to alleviate stress induced by cadmium and zinc in two submerged macrophytes. Afr J Biotechnol 2009;8:2176–83. [Google Scholar]
- 23. Salimpour SI, Khavazi K, Nadian H, et al. Enhancing phosphorous availability to canola ('Brassica napus' L.) using P solubilizing and sulfur oxidizing bacteria. Aust J Crop Sci 2010;4:330. [Google Scholar]
- 24. Khorgamy A, Farnia A. Effect of phosphorus and zinc fertilisation on yield and yield components of chick pea cultivars. In: 9th African Crop Science, Conference Proceedings, Cape Town, South Africa, 28 September–2 October 2009 (pp. 205–8). Kampala, Uganda: African Crop Science Society. [Google Scholar]
- 25. Das K, Dang R, Shivananda TN, et al. Interaction between phosphorus and zinc on the biomass yield and yield attributes of the medicinal plant stevia (Stevia rebaudiana). Sci World J 2005;5:390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Allen SE, Grimshaw HM, Rowland AP. Chemical analysis. In: Moore PD, Chapman SB (eds).. Methods in Plant Ecology. Oxford London: Backwell Scientific Publications, 1986. [Google Scholar]
- 27. Lichtenthaler H K. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:351–83. [Google Scholar]
- 28. Strasser RJ, Srivastava A, Tsimilli-Michael M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Mohammad Yunus, Uday Pathre, Prasanna Mohanty (eds).. Probing Photosynthesis: Mechanisms, Regulation and Adaptation 2000, 445–83.
- 29. Ubeda-Tomás S, Federici F, Casimiro I, et al. Gibberellin signaling in the endodermis controls Arabidopsis root meristem size. Curr Biol 2009;19:1194–9. [DOI] [PubMed] [Google Scholar]
- 30. Culligan K, Tissier A, Britt A. ATR regulates a G2-phase cell cycle checkpoint in Arabidopsis thaliana. Pl Cell 2004;16:1091–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Singh RP, Shukla VK, Yadav RS, et al. Biological approach of zinc oxide nanoparticles formation and its characterization. Adv Mater Lett 2011;2:313–7. [Google Scholar]
- 32. Kathiravan V, Ravi S, Ashokkumar S, et al. Green synthesis of silver nanoparticles using Croton sparsiflorus morong leaf extract and their antibacterial and antifungal activities. Spectrochim Acta A Mol Biomol Spectrosc 2015;139:200–5. [DOI] [PubMed] [Google Scholar]
- 33. Philip D. Biosynthesis of au, ag and au–ag nanoparticles using edible mushroom extract. Spectrochim Acta A Mol Biomol Spectrosc 2009;73:374–81. [DOI] [PubMed] [Google Scholar]
- 34. Stewart J, Hansen T, McLean JE, et al. Salts affect the interaction of ZnO or CuO nanoparticles with wheat. Environ Toxicol Chem 2015;34:2116–25. [DOI] [PubMed] [Google Scholar]
- 35. Watson JL, Fang T, Dimkpa CO, et al. The phytotoxicity of ZnO nanoparticles on wheat varies with soil properties. Biometals 2015;28:101–12. [DOI] [PubMed] [Google Scholar]
- 36. Mukherjee A, Sun Y, Morelius E, et al. Differential toxicity of bare and hybrid ZnO nanoparticles in green pea (Pisum sativum L.): a life cycle study. Front Plant Sci 2016;6:1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zafar H, Ali A, Ali JS, et al. Effect of ZnO nanoparticles on Brassica nigra seedlings and stem explants: growth dynamics and antioxidative response. Front Plant Sci 2016;7:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ma X, Geiser-Lee J, Deng Y, et al. Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci Total Environ. 2010;408:3053–61. [DOI] [PubMed] [Google Scholar]
- 39. Du W, Sun Y, Ji R, et al. TiO 2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J Environ Monit 2011;13:822–8. [DOI] [PubMed] [Google Scholar]
- 40. Mishra S, Alfeld M, Sobotka R, et al. Analysis of sublethal arsenic toxicity to Ceratophyllum demersum: subcellular distribution of arsenic and inhibition of chlorophyll biosynthesis. J Exp Bot 2016;67:4639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stoeva N, Berova M, Zlatev Z.. Effect of arsenic on some physiological parameters in bean plants. Biol Plant 2005;49:293–6. [Google Scholar]
- 42. Wang X, Yang X, Chen S, et al. Zinc oxide nanoparticles affect biomass accumulation and photosynthesis in Arabidopsis. Front Plant Sci 2016;6:1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Singh R, Parihar P, Prasad SM. Simultaneous exposure of sulphur and calcium hinder as toxicity: up-regulation of growth, mineral nutrients uptake and antioxidants system. Ecotoxicol Environ Saf 2018;161:318–31. [DOI] [PubMed] [Google Scholar]
- 44. Bashri G, Prasad SM. Indole acetic acid modulates changes in growth, chlorophyll a fluorescence and antioxidant potential of Trigonella foenum-graecum L. grown under cadmium stress. Acta Physiol Plant 2015;37:49. [Google Scholar]
- 45. Kumari M, Khan SS, Pakrashi S, et al. Cytogenetic and genotoxic effects of zinc oxide nanoparticles on root cells of Allium cepa. J Hazard Mater 2011;190:613–21. [DOI] [PubMed] [Google Scholar]
- 46. Kouhi SM, Lahouti M, Ganjeali A, et al. Long-term exposure of rapeseed (Brassica napus L.) to ZnO nanoparticles: anatomical and ultrastructural responses. Environ Sci Pollut Res 2015;22:10733–43. [DOI] [PubMed] [Google Scholar]
- 47. Pokhrel LR, Dubey B. Evaluation of developmental responses of two crop plants exposed to silver and zinc oxide nanoparticles. Sci Total Environ 2013;452:321–32. [DOI] [PubMed] [Google Scholar]
- 48. Todeschini V, Lingua G, D’agostino Get al. Effects of high zinc concentration on poplar leaves: a morphological and biochemical study. Environ Exp Bot 2011;71:50–6. [Google Scholar]
- 49. Schnappinger MG Jr, Bandel VA, Kresge CB. Effect of phosphorus and potassium on alfalfa root anatomy 1. Agron J 1969;61:805–8. [Google Scholar]


