Abstract
The black pepper, most commonly used in Indian cuisines for ages, is considered as “king of spices.” The present study evaluates the anticancer potential of black pepper and its main constituent, i.e. alkaloid piperine, against human leukemia cell line, K-562 cells. Gas chromatography–mass spectrometry (GC–MS) analysis confirmed the presence of piperine in black pepper extract. The methanolic extract of black pepper (BP-M) and pure piperine (PIP) showed a strong cytotoxic effect against this cell line. Both BP-M and PIP generated apoptotic bodies in K-562 cells and caused nuclear condensation as visualized by fluorescent microscopy, which was further confirmed by flow cytometry analysis. BP-M and PIP also generated reactive oxygen species in K-562 cells as established by flow cytometry. The translation of Bax, caspase-3 and caspase-9 genes was found to be upregulated with subsequent downregulation of Bcl-2 gene. The anti-proliferative effect of both BP-M and PIP was also observed by trypan blue staining and was further confirmed by the downregulated expression of proliferating cell nuclear antigen (PCNA). The molecular docking studies showed the binding of PIP with PCNA and Bcl-2 and supported the in vitro findings. The docking studies also proposed the binding of PIP to ADP binding pocket of Apaf-1 protein. Taken together, these findings signify the anticancer potential of both black pepper and PIP, thus proposing black pepper as a potent nutraceutical for preventing the progression of chronic myeloid leukemia.
Keywords: black pepper, piperine, chronic myeloid leukemia, intrinsic apoptosis pathway, reactive oxygen species
Graphical Abstract
Graphical Abstract.
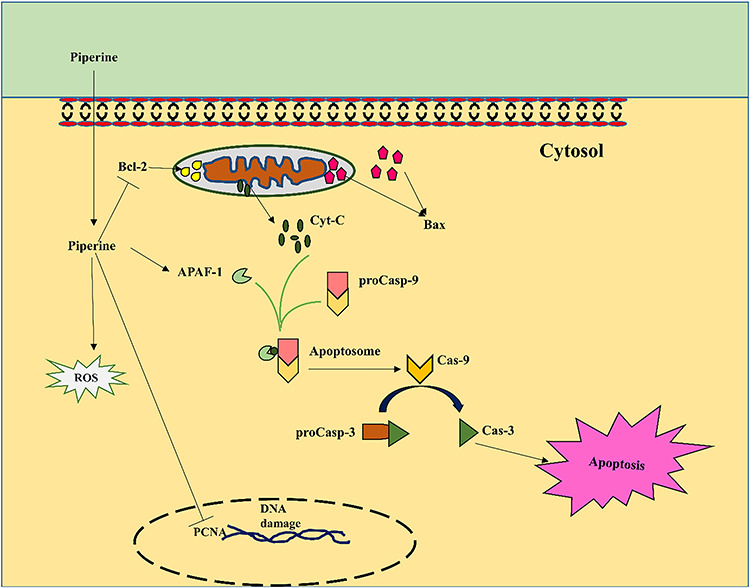
Introduction
Cancer has emerged as a significant health problem globally, affecting both developed and underdeveloped countries [1]. Cancer is the second leading cause of human death after heart disease. Among all other cancers, leukemia represents a fatal hematological malignancy, a cancer of the blood and bone marrow, which alone claims 0.62% death annually [2]. Human chronic myeloid leukemia (CML) is a common type of leukemia, generated due to t(9;22)(q34;11) chromosomal translocation [3]. This translocation generates oncogenic BCR-ABL tyrosine kinase fusion protein, which remains confined to cell cytoplasm, while the wild-type ABL protein shuttles between nucleus and cytoplasm [4]. This tyrosine kinase protein leads to aberrant phosphorylation of several substrates of different intracellular pathways [5].
The first-line treatment of CML involves tyrosine kinase inhibitor, namely imatinib, which was introduced 10 years back. Later dasatinib, nilotinib and bosutinib were also tested clinically. However, due to mutation in the ABL kinase domains, many patients developed resistance to these drugs [6]. The difficulty in the current time for CML therapy has led to the discovery and development of approaches based on traditional medicines, which are supposed to possess lower side effects than existing therapeutics.
Health care of ~80% of the world’s population relies mainly on traditional medicines [7]. Several medicinal plant products have been proven to aid in various ailments successfully. Black pepper plays a very conspicuous role in the Indian system of medicines—Ayurveda¸ Sidha and Unani, being a vital ingredient of different medicinal formulations used in these practices. Black pepper, considered as “king of spices,” is the most traded spice variety across the globe. The essential commercial part of black pepper plant is its dried and matured fruit, which is popular for its particular aroma and flavor because of the presence of volatile oils and major alkamide, i.e. piperine, which constitutes about 2.8–17.3 mg per gram of dried black pepper. The structure of piperine consists of piperidine linked to 3,4-methylenedioxyphenyl moiety by the C5 amide bridge (Fig. 1). Black pepper has been known to have bactericidal, insecticidal, antidiarrheal, antispasmodic and anticancer properties [8]. Piperine has also been reported to have immune-modulatory, antidepressant, anti-inflammatory and anti-diabetic and anticancer properties [9].
Figure 1.
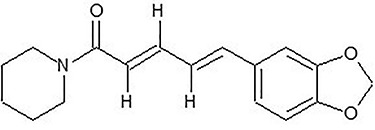
chemical structure of piperine
In previous studies, it has been reported that piperine, a major metabolite of Piper nigrum, responded as a prominent anticancer agent [10–13]. However, to the best of our knowledge, the potential of black pepper and piperine has not been investigated in the condition of leukemia to date. Therefore, the main objective of this study was to explore the anticancer potential of black pepper and piperine against leukemia cell line i.e., K-562 cells.
Materials and Methods
Plant material and extraction
The matured fruit (peppercorn) of black pepper was procured from local herbal product stores of Roorkee, Uttarakhand, India, who are engaged in making various traditional herbal formulations. The crushed powder of black pepper fruits was extracted with three different solvents: methanol (BP-M), ethanol (BP-E) and water (BP-Aq) (1:5, w/v) thrice independently. All the samples were stored at −80°C until used. The extract supernatant was evaporated under vacuum at room temperature to dryness as dark brown mass using a vacuum concentrator (Eppendorf Concentrator plus™, Eppendorf, Hamburg, Germany). The dried extract was then reconstituted in dimethyl sulfoxide (DMSO, Cat. No. TC185, Himedia, Mumbai, India) and filtered (0.22 μm filter, Axiva Sichem Biotech, Haryana, India) before use in cell culture analysis. Piperine (PIP) (Cat. No. P49007, Sigma, St. Louis, MO, USA) was also dissolved in DMSO and filtered before use [14]. Both were used at 0.1% v/v ratio in the analysis [15].
Estimation of total phenolic and total flavonoid contents
The total phenolic content of all three extracts (BP-M, BP-E and BP-Aq) was determined using the Folin–Ciocalteu method [16]. In brief, 200 μl of the extract was mixed with 500 μl of Folin–Ciocalteu reagent (1:9 dilutions with water) (Cat. No. 10822, Himedia, Mumbai, India) followed by 5-min incubation at room temperature. Then, 300 μl of sodium carbonate (5% solution) was added and the solution was incubated further for 20 min at room temperature in dark condition. The absorbance was measured at 765 nm using FLUOstar Omega (BMG Labtech, Ortenberg, Germany) microplate reader. Gallic acid (Cat. No. G7384, Sigma, St. Louis, MO, USA) was used as the internal standard and total phenolic content was expressed as micrograms of gallic acid equivalents per gram dry mass (GAE). The total flavonoid content of all three extracts was measured by AlCl3 method [16]. In brief, 500 μl of the extract was mixed with 500 μl of aluminum chloride (2%) solution (Cat. No. MB054, Himedia, Mumbai, India) in ethanol and incubated for 1 h at room temperature. Then, the absorbance was measured at 420 nm using FLUOstar Omega (BMG Labtech, Ortenberg, Germany) microplate reader. Quercitin (Cat. No. Q4951, Sigma, St. Louis, MO, US) was used as internal standard and the total flavonoid content was expressed as micrograms of quercetin equivalent per gram dry mass (QE).
Gas chromatography–mass spectrometry analysis
Gas chromatography–mass spectrometry (GC–MS) analysis was performed to separate and identify the metabolites present in three different extracts. For GC–MS analysis, 500 μl of extract was first mixed with 50 μl of internal standard, 2-phenylphenol (from 2 mg/ml in methanol stock) (Cat. No. P28263, Sigma, St. Louis, MO, USA). The mixture was then dried in a vacuum concentrator (Eppendorf Concentrator plusTM, Eppendorf, Hamburg, Germany). The dried mass was directly derivatized with 70 μl of N-Methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA) (Cat. No. M-132 Superlo, Sigma, St. Louis, MO, USA) at 37°C for 60 min, followed by centrifugation at 14 000 × g for 10 min [17]. The resulting supernatant was taken out in a GC glass vial and immediately analyzed by GC–MS. PIP was used as standard control and similar derivatization was performed. The GC–MS analysis was performed on Agilent 7890B gas chromatograph coupled with Agilent 8977C mass detector (Agilent Technologies, Santa Clara, CA, USA). HP-5 MS column [5% Phenyl Methyl Polysiloxane: 30 m × 0.25 m (I.D.) × 0.25 μm] was used for metabolite separation with helium as a carrier gas with a flow rate of 1 ml/min. The injection volume was 1 μl with split-less mode. The injector temperature was set at 280°C. The oven temperature was initially set at 70°C for 2 min, then ramped to 220°C at a rate of 10°C/min and then further increased to 310°C at the rate of 20°C/min and then hold for 14 min at 310°C. The mass spectrometer (MS) operation parameters were set as follows: MS quad temperature 150°C, ion source temperature 230°C, electron energy (70 eV) and mass spectra collection range of 40–1000 m/z. Each sample was replicated three times. The metabolites were identified by matching the mass spectra of target metabolites with library search (Wiley; National Institute of Standards & Technology library- NIST 17). PIP was quantified using an authentic standard.
Cell culture
The human bone marrow origin leukemia cell line, K-562 cells, was procured from the National Centre for Cell Science (Pune, India) and maintained in RPMI-1640 media (Cat. No. AT-162, Himedia, Mumbai, India). The normal human lung fibroblast cell line, MRC-5, was a kind gift from Dr. Hemant K. Gautam (Council of Scientific and Industrial Research-Institute of Genomics and Integrative Biology, New Delhi, India). The MRC-5 cell line was maintained in Eagle’s Minimum Essential Medium (Cat. No. AT-154, Himedia, Mumbai, India). The cell culture media was supplemented with 10% fetal bovine serum (Cat. No. 11573397) and 100-U/ml penicillin–streptomycin and 100-mg/ml ampicillin mixture (Cat. No. 15240062, both from Gibco, Thermo Fisher Scientific, Waltham, MA, USA), and the cells were cultured in a humidified incubator at 37°C with 5% CO2.
Cytotoxic activity
The cytotoxic effect of black pepper and PIP on K-562 cells were measured using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay [18]. All three black pepper extracts, i.e. BP-M, BP-E, BP-Aq and PIP, were tested three independent times. Briefly, 96-well plates were seeded with 200 μl of media containing 5000 cells with various concentrations of black pepper extracts (50, 100 and 200 μg/ml) or PIP (25, 50 and 100 μM). The DMSO-treated cells were considered as control. The cells were incubated for 24 and 48 h. After the incubation, 20 μl of MTT dye (Cat. No. TC191, Himedia, Mumbai, India) was supplemented to each well and the cells were incubated at 37°C for another 4 h. Then, after pipetting out the media, each well was replaced with 200 μl of DMSO to dissolve the formazan crystal formed. The absorbance of the lysate was then measured at 570 nm using FLUOstar Omega (BMG Labtech, Ortenberg, Germany) microplate reader. The inhibition percentage was calculated as follows:
100 − [(Mean absorbance of phytochemical treated cells/Mean absorbance of vehicle-treated cells) × 100].
The IC25 and IC50 values were evaluated on the basis of inhibition percentage values using Graph Pad Prism 6.01 software (San Diego, CA, USA).
Cellular staining
In order to visualize changes in nuclear status and formation of apoptotic bodies, the K-562 cells were visualized after performing dual Acridine Orange/Ethidium Bromide (AO/EB) staining [19]. Briefly, the cells were treated at IC50 concentration of both BP-M and PIP for 48 h. Then, the cells were harvested and washed twice with PBS and incubated with dye mixture (AO and EB, both 100 μg/ml in PBS, Cat. No. 318337 and E8751, respectively, Sigma, St. Louis, MO, USA) and observed under a fluorescence microscope (Zeiss, Axiovert 25, Germany).
The nuclear morphology of K-562 cells under similar treatment conditions was also visualized by performing Hoechst staining [20]. Briefly, the treated cells were harvested, washed twice with PBS and incubated for 15 min with 100 ng/ml of Hoechst 33342 dye (Cat. No. TC266, Himedia, Mumbai, India) in PBS. Then, the stained cells were observed under a fluorescent microscope (EVOS® FL Cell Imaging System, Thermo Fischer Scientific, Waltham, MA, USA). The observations were performed three independent times.
Trypan blue dye exclusion assay
The anti-proliferative effect of BP-M and PIP was examined by manual counting of cells after staining them with trypan blue [21]. Briefly, K-562 cells were treated with BP-M and PIP at IC25 and IC50 concentrations for 48 h, respectively. The K-562 cells treated with DMSO were considered as control. Then, the cells were stained with trypan blue dye (Cat. No. GRM263, Himedia, Mumbai, India) and counted manually using a hemocytometer.
Flow cytometry assay for measurement of intracellular reactive oxygen species levels
The intracellular accumulation of reactive oxygen species (ROS) was determined by performing flow cytometry analysis using a fluorescence-activated cell sorter (FACS Calibur, BD Biosciences, San Jose, CA, USA), as mentioned previously [22]. Briefly, K-562 cells were treated at IC25 and IC50 equivalent concentrations of BP-M and PIP, respectively, for 48 h. The H2O2 treated cells were considered as a positive control for this analysis. Then, the cells were harvested and washed twice with PBS followed by staining for 30 min with 2 μM concentration of 2′, 7′-dichlorodihydrofluorescein diacetate, acetyl ester (DCFDA) (Cat. No. D6883, Sigma, St. Louis, MO, USA) at room temperature in dark condition. Then, the fluorescence intensity of cell suspension was analyzed immediately at 530 nm (FL-1 channel).
Annexin-PI flow cytometry assay for induction of apoptosis
Black pepper and PIP-induced apoptosis in K-562 cells were determined quantitatively using Annexin V-conjugated Alexa Fluor 488 apoptosis assay kit (ThermoFischer Scientific, Waltham, MA, USA) following the manufacturer’s instructions. Briefly, after 48 h treatment of cells with BP-M, PIP (IC25 and IC50 values) and DMSO (control), the cells were harvested and washed twice with PBS. The cells were then incubated for staining with Annexin V AlexaFluor 488 and propidium iodide (PI) in binding buffer at room temperature for 15 min in dark. The cells were then analyzed on a fluorescence-activated cell sorter (FACS Calibur, BD Biosciences, San Jose, CA, USA), and the data were analyzed using software Cell Quest 3.3.
Gene expression analysis
Total RNA from K-562 cells treated at IC25 and IC50 equivalent concentrations of BP-M and PIP (48 h), respectively, or with DMSO (control) were isolated using RNA isolation reagent as per the instructions of manufacturers (RNA X-Press Reagent, Himedia, Mumbai, India). The RNA samples were then quantified, and the first-strand cDNA was synthesized using 2 μg RNA samples. The primers for caspase-3, caspase-9, Bax, Bcl-2, proliferating cell nuclear antigen (PCNA) and β-actin genes were designed using Primer3 software (Whitehead Institute for Biomedical Research, Cambridge, MA, USA) and the details of primer sequences and PCR conditions are depicted in Table 1. Finally, the PCR products were separated on 2% agarose gel and visualized in a gel documentation system (BioRad, Hercules, CA, USA). The intensity of the bands on gels was analyzed using ImageJ 1.52a software (NIH, Bethesda, MD, USA). The entire procedure was carried out three independent times.
Table 1.
details of oligonucleotide primer sequences used in this study for PCR
| Name of gene | Forward primer sequence (5′-3′) | Reverse primer sequence (5′-3′) | Product size (bp) | Annealing temp (°C) |
|---|---|---|---|---|
| Caspase-3 (>NM_004346.4) | GGTTCATCCAGTCGCTTTGTG | GCGTCAAAGGAAAAGGACTCA | 121 | 60.0 |
| Caspase-9 (>NM_001229.5) | AGGCCCCATATGATCGAGGA | GGCCTGTGTCCTCTAAGCAG | 139 | 60.3 |
| Bax (>NM_138763.4) | CCTGTGCACCAAGGTGCCGGAACT | CCACCCTGGTCTTGGATCCAGCCC | 99 | 68.2 |
| Bcl-2 (>NM_000657.3) | TTGTGGCCTTCTTTGAGTTCGGTG | GGTGCCGGTTCAGGTACTCAGTCA | 114 | 60.9 |
| PCNA (>NM_002592.2) | GTTACTGAGGGCGAGAAGCG | GGCGGGAAGGAGGAAAGTCT | 167 | 61.3 |
| β-Actin (>NM_001101.5) | TCACCCACACTGTGCCCCATCTACGA | CAGCGGAACCGCTCATTGCCAATGG | 296 | 65.0 |
| β-Actin (>NM_001101.5) (RT-qPCR) | TACGCCAACACAGTGCTGTCT | CTGCATCCTGTCGGCAATG | 63 | 60.0 |
The expression of caspase-3, caspase-9 and PCNA was also evaluated by quantitative real-time PCR (RT-qPCR) analysis. The primer sequence for the genes are listed in Table 1. RT-qPCR was performed using SYBR green master mix (PowerUp™ SYBR™ Green Master mix, Applied Biosystems, Thermo Fischer Scientific, Waltham, MA, USA, Cat. No. A25742) using an RT-qPCR equipment (Applied Biosystems™ QuantStudio™ 5, ThermoFischer Scientific, Waltham, MA, USA). The amount of PCR product for the gene of interest in different treatment conditions was compared with the amount of β–actin produced to estimate the variations. The entire procedure was carried out three independent times. The relative expression of mRNA of test genes were calculated using the comparative 2-ΔΔ method [23].
Western blotting analysis
Total protein of K-562 cells treated at IC25 and IC50 concentration of BP-M and PIP (48 h), respectively, or DMSO (control) was isolated with RIPA lysis buffer (Cat. No. TC131, Himedia, Mumbai, India) supplemented with 1X protease inhibitor cocktail (Cat. No. ML051, Himedia, Mumbai, India). The isolated protein was quantified with a BCA protein estimation kit (Cat. No. 71285-M, Sigma, St. Louis, MO, USA). The immunoblot analysis was performed, as mentioned elsewhere. Briefly, 40 μg of protein samples were analyzed on 10% polyacrylamide gels, then transferred to PVDF membrane and were blocked using 3% BSA in TBS-T buffer for 2 h. Then, the membrane was incubated (4°C, overnight) with primary anti-caspase-3, caspase-9, Bax, Bcl-2, PCNA and GAPDH antibodies (1:1000) (all from Elabsciences, Houston, TX, USA) in 3% BSA. After this step, the membranes were washed and incubated with horseradish peroxidase-conjugated secondary anti-rabbit or anti-mouse antibody (1:10,000) (Santa Cruz Biotechnology Inc., Dallas, TX, USA). The chemiluminescence was developed using ECL reagent (BioRad, CA, USA) and captured on X-ray films. The densitometric analyses of blots were performed by ImageJ 1.52a software (NIH, Bethesda, MD, USA) using GAPDH as an internal control.
Molecular docking study
The binding of PIP, the principal constituent of black pepper, with different proteins of apoptotic signaling pathway was assessed by performing molecular docking analysis using AutoDock version 4.2 software [24]. The target proteins selected for this study were (i) Bcl-2 family of protein, (ii) apoptotic protease-activating factor 1 (Apaf-1) and (iii) cellular proliferation factor, PCNA. Among the Bcl-2 family, the crystal structure of anti-apoptotic protein (human Bcl-2) in complex with N-heteroaryl sulfonamide inhibitor (PDB ID: 4IEH, Resolution 2.10 Å) was used for docking calculation with PIP [25]. For docking calculation of PIP with PCNA and Apaf-1, the crystal structure of PCNA in complex with T2AA (PDB ID: 3WGW, Resolution 2.80 Å) [26], and crystal structure of Apaf-1 with ADP (PDB ID: 1Z6T Resolution 2.21 Å) [27], were used, respectively. ADT tools was used for ligand and protein preparations. The grid maps were prepared using 60 × 60 × 60 grid points with 0.375 Å spacing in x, y and z directions centered at the active sites of individual macromolecules. Autogrid4 and autodock4 programs were used for calculating energy maps and docking of PIP, respectively. One hundred docked structures, i.e. 100 runs, were generated using Lamarckian Genetic Algorithm. All the generated binding poses of PIP with different proteins were ranked and clustered according to their root mean-square deviation (RMSD) values and binding affinities scores. Docked conformations were converted into PDBQT [Protein Data Bank, Partial Charge (Q) and Atom Type (T)] format and then analyzed. Chimera tool was used for visualization and figure generations [28].
Statistical analysis
All data were represented as mean ± SEM. The difference among set conditions was evaluated by performing a one-way analysis of variance (ANOVA) test followed by the Bonferroni post hoc test using Graph Pad Prism 6.01 software (San Diego, CA, USA). The analyzed data with P-value <0.05 were considered statistically significant.
Results
Phytochemical evaluation of black pepper
The phytochemical composition of three black pepper extracts (BP-M, BP-E and BP-Aq) was evaluated. The total phenolics and flavonoids contents were estimated and are depicted in Table 2. As shown in Table 2, the total phenolic and flavonoid content in all three extracts were found to be around 50 GAE and 90 QE, respectively. The analysis confirmed that black pepper is a rich source of phenolic and flavonoid compounds. In the next phase, the GC–MS analysis revealed the presence of 44 different metabolites in all three extracts at varying concentrations. The metabolite contents were measured in terms of equivalent of internal standard (μg phenyl phenol/g dry weight) (Table 3). The representative total ion chromatogram of BP-M, BP-E, BP-Aq and PIP is shown in the supplementary figures (Figs S1 and S2). The presence of PIP in all three extracts was quantified by comparing the area of PIP in the extracts with the standard PIP obtained commercially (Sigma, St Louis, MO, USA). It was found that BP-M, BP-E and BP-Aq contained 0.78, 0.67 and 0.032 ng/mg dry weight of PIP, respectively (Table S1).
Table 2.
total phenolic and total flavonoid content in various black pepper extracts
| Black pepper extract | Total phenolic content (GAE) | Total flavonoid content (QE) |
|---|---|---|
| BP-M | 56.87 ± 2.01 | 92.14 ± 0.47 |
| BP-E | 57.64 ± 1.63 | 80.70 ± 1.45 |
| BP-Aq | 52.38 ± 1.99 | 98.81 ± 5.12 |
Data are the mean ± SEM of three independent experiments. BP-M, Black pepper methanol extract; BP-E, Black pepper ethanol extract; BP-Aq, Black pepper aqueous extract; GAE, μg gallic acid equivalent/g dry mass; QE, μg quercetin equivalent/g dry mass.
Table 3.
GC–MS based metabolite profile of three different black pepper extracts
| S. No. | Metabolites | NIST ID | Retention time (min) | Quantification ion | Relative metabolite content | ||
|---|---|---|---|---|---|---|---|
| BP-M | BP-E | BP-Aq | |||||
| 1 | D-Limonene | 79639 | 4.82 | 136, 121, 93, 68 | 89.55 | 131.23 | 3.51 |
| 2 | Glycine | 229287 | 7.296 | 147, 132, 114, 73 | 21.56 | 67.08 | 0.00 |
| 3 | 3-Methylcyclohexanol | 229453 | 7.441 | 186, 171, 143, 73 | 1.69 | 3.53 | 3.70 |
| 4 | Propanoic acid | 290898 | 7.919 | 146,131, 75, 73 | 7.27 | 1604.97 | 447.17 |
| 5 | Propanedioic acid | 229341 | 8.147 | 321, 292, 147, 73 | 5.85 | 10.88 | 0.00 |
| 6 | Terpinen-4-ol | 109117 | 8.253 | 154, 136,111, 71 | 17.54 | 11.72 | 0.00 |
| 7 | L-Valine | 26146 | 8.72 | 261, 218, 144, 73 | 7.24 | 2.58 | 52.64 |
| 8 | Acetic acid | 227635 | 9.365 | 132, 117, 75, 73 | 1.98 | 2.62 | 2.48 |
| 9 | 4-Aminobutanoic acid | 228101 | 9.871 | 247, 232, 147, 73 | 64.25 | 59.32 | 61.78 |
| 10 | Butanedioic acid | 229614 | 10.072 | 350, 335, 147, 73 | 159.70 | 145.74 | 167.69 |
| 11 | α-Terpineol | 114833 | 10.188 | 154, 136, 121, 59 | 13.37 | 7.31 | 0.00 |
| 12 | Glyceric acid | 150744 | 10.377 | 322, 292, 189, 73 | 138.77 | 155.09 | 32.70 |
| 13 | Copaene | 9240 | 10.572 | 204, 161, 119, 105 | 324.06 | 243.74 | 2.76 |
| 14 | 2-Methylglutaric acid | 242292 | 10.694 | 275, 247, 147, 73 | 38.53 | 39.97 | 0.00 |
| 15 | Serine | 228085 | 10.778 | 321, 306, 204, 73 | 17.65 | 15.71 | 21.18 |
| 16 | Phloroglucinol | 118676 | 11.312 | 342, 327, 147, 73 | 74.73 | 56.36 | 55.74 |
| 17 | Caryophyllene | 291486 | 11.668 | 204, 189, 133, 93 | 2665.39 | 1866.06 | 899.99 |
| 18 | cis-Carveol | 107006 | 11.829 | 224, 209, 181, 73 | 126.82 | 152.18 | 112.87 |
| 19 | β-Famesene | 141000 | 11.896 | 204, 189, 133,69 | 229.78 | 212.9 | 234.19 |
| 20 | Eugenol | 291519 | 12.146 | 236, 221, 179, 73 | 65.40 | 43.09 | 12.41 |
| 21 | Malic acid | 228569 | 12.43 | 350, 335, 237, 73 | 153.99 | 102.83 | 77.00 |
| 22 | β-Bisabolene | 9654 | 12.658 | 204, 189, 161, 69 | 931.72 | 857.51 | 0.29 |
| 23 | Erythritol | 292004 | 12.747 | 410, 395, 217, 73 | 560.21 | 462.10 | 378.12 |
| 24 | Catechol | 78898 | 12.93 | 254, 239, 166, 73 | 19.15 | 28.15 | 10.34 |
| 25 | Piperonylic acid | 134278 | 13.025 | 265, 223, 179, 73 | 136.97 | 108.21 | 45.79 |
| 26 | Pentanedioic acid | 290975 | 13.425 | 318, 303, 204, 73 | 28.03 | 30.31 | 26.34 |
| 27 | Asparagine | 229288 | 13.759 | 348, 333,231, 73 | 34.64 | 30.48 | 19.40 |
| 28 | D-Glucuronic acid | 79257 | 14.365 | 539,305,204, 73 | 42.04 | 49.62 | 45.97 |
| 29 | Arabinitol | 79245 | 15.26 | 512, 307, 217, 73 | 279.82 | 19.04 | 4.65 |
| 30 | Protocatechuic acid | 162416 | 15.455 | 370, 355, 193, 73 | 291.88 | 186.81 | 113.83 |
| 31 | Myristic acid | 290632 | 15.694 | 299, 285, 117, 73 | 117.86 | 98.64 | 85.48 |
| 32 | Fructose | 228156 | 16.667 | 525, 437, 217, 73 | 217.57 | 233.08 | 140.21 |
| 33 | D-Galactose | 76356 | 16.729 | 540, 435, 204, 73 | 61.12 | 54.40 | 26.48 |
| 34 | Syringic acid | 291989 | 16.962 | 342, 327, 141, 73 | 110.12 | 87.00 | 51.76 |
| 35 | Coumaric acid | 6103 | 17.885 | 308, 293, 147, 73 | 1474.78 | 1268.75 | 110.80 |
| 36 | Ferulic acid | 234120 | 18.041 | 338, 323, 249, 73 | 22.92 | 27.74 | 4.95 |
| 37 | Palmitic acid | 151973 | 18.18 | 328, 313, 117, 73 | 248.79 | 163.84 | 5.28 |
| 38 | Myo-inositol | 7496 | 18.72 | 612, 305, 147, 73 | 114.43 | 226.90 | 3.79 |
| 39 | Stearic acid | 290961 | 19.448 | 356, 341, 117, 73 | 50.58 | 41.69 | 11.94 |
| 40 | Pipecolic acid | 436786 | 20.36 | 273, 230, 156, 73 | 53.62 | 55.26 | 36.92 |
| 41 | Arachidic acid | 379431 | 20.477 | 384, 369, 117, 73 | 6.85 | 9.46 | 6.41 |
| 42 | Lignoceric acid | 231005 | 21.156 | 440, 425, 117, 73 | 16.68 | 119.93 | 28.89 |
| 43 | Piperine | 152960 | 23.169 | 285, 201, 115, 84 | 3482.95 | 3015.11 | 143.47 |
| 44 | Citric acid | 228892 | 31.417 | 408, 393, 201, 73 | 79.65 | 91.11 | 38.23 |
BP-M, black pepper methanol extract; BP-E, black pepper ethanol extract; BP-Aq, black pepper aqueous extract; NIST, National Institute of Standards and Technology; metabolite content was expressed as μg phenyl phenol equivalent/g dry mass.
Black pepper and piperine induce potent cytotoxic effect against K-562 cell line
The cytotoxic effect of black pepper was initially evaluated on K-562 cell line by MTT assay. Three different extracts of black pepper (BP-M, BP-E and BP-Aq) were investigated for their potential cytotoxic effects. The results confirmed the inhibition of cell viability by BP-M (Fig. 2a and e), BP-E (Fig. 2b and f) and BP-Aq (Fig. 2c and g) at both 24 and 48 h of treatment, albeit with high efficacy at a later hour. It is evident from the above data that at 200 μg/ml concentration, all the extracts showed less than 50% cell viability (P < 0.05). However, among all the tested extracts, BP-M showed the most significant cytotoxicity. This is based on the fact that 48 h of treatment with 200 μg/ml of BP-M resulted in almost 75% reduction in cell viability which in case of other extracts was close to only 50% (P < 0.05). These data were in line with PIP response as well, which showed a prominent dose-dependent inhibition of cell viability both at 24 h (Fig. 2d) and 48 h (Fig. 2h). Interestingly, the 48-h treatment of cells with PIP reduced the cell viability by ~80% with respect to vehicle-treated control cells (Fig. 2h) (P < 0.05). Based on these data, it could be conceived that among the tested extracts, BP-M is most effective in preventing K-562 cell viability, which was just next to PIP in its potency. The cytotoxic effect of both BP-M (Fig. 2i) and PIP (Fig. 2j) was also tested on normal cell line, MRC-5 cell. As shown in Fig. 2i and j, there was no significant cell death in MRC-5 cells in response to either of them (P < 0.05). We then determined the IC50 values of the test compounds in K-562 cell line. As shown in Table 4, BP-M was found to be most effective with an IC50 value of 193.6 and 116.6 μg/ml at 24 and 48 h, respectively. The major metabolite of black pepper, i.e. PIP, which showed a prominent cytotoxic effect earlier, demonstrated an IC50 value of 148.2 and 56.3 μM at 24 and 48 h, respectively. Based on these data among the tested extracts, only BP-M was selected for further analysis in the present study.
Figure 2.
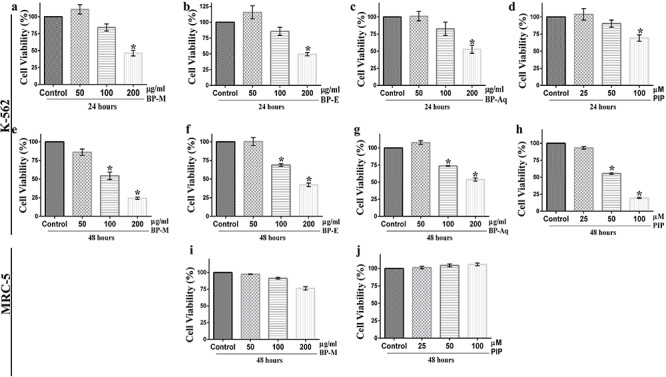
evaluation of the cytotoxic effect of black pepper extracts and piperine on K-562 cells. Viability of K-562 cells treated with black pepper extracts [methanol (a, e); ethanol (b, f) and aqueous (c, g)] and piperine (d, h) for varying periods of time, (a–d) 24 h and (e–h) 48 h, thereafter measured by MTT assay. The effect of (i) methanol extract and (j) piperine on the viability of MRC-5 cells after 48 h of treatment. All results are represented as mean ± SEM of three independent experiments. * indicates P < 0.05 as compared with the respective vehicle-treated control group, which was considered as 100 percent in all cases. BP-M, black pepper methanol extract; BP-E, black pepper ethanol extract; BP-Aq, black pepper aqueous extract; PIP, piperine.
Table 4.
inhibitory effects of various extracts of black pepper and its major constituent on the viability of K-562 cell line as determined by MTT assay
| Extract type/phytochemical | Time period (hours) | IC 25 value (Mean ± SEM) | IC 50 value (Mean ± SEM) |
|---|---|---|---|
| BP-M | 24 | 129.1 ± 7.6 μg/ml | 193.6 ± 8.3 μg/ml |
| 48 | 66.6 ± 11.7 μg/ml | 116.6 ± 14.7 μg/ml | |
| BP-E | 24 | 133.6 ± 6.5 μg/ml | 208.3 ± 3.5 μg/ml |
| 48 | 83.33 ± 13.3 μg/ml | 153.8 ± 11.1 μg/ml | |
| BP-Aq | 24 | 126.1 ± 8.9 μg/ml | 223.7 ± 3.5 μg/ml |
| 48 | 109.6 ± 9.7 μg/ml | 201.3 ± 6.4 μg/ml | |
| PIP | 24 | 93.8 ± 2.4 μM | 148.2 ± 6.2 μM |
| 48 | 37.5 ± 3.4 μM | 56.3 ± 3.5 μM |
BP-M, black pepper methanol extract; BP-E, black pepper ethanol extract; BP-Aq, black pepper aqueous extract; PIP, piperine. Data are Mean ± SEM of three independent experiments.
Black pepper and piperine induce apoptosis leading to nuclear condensation in K-562 cells
The inhibitory effect of BP-M and PIP on K-562 cell proliferation was further ascertained by performing cell staining experiments followed by microscopic visualization. Firstly, the stage of early and late apoptosis was distinguished by uptake of AO/EB dye mixtures. For this experiment, the K-562 cells were treated with BP-M and PIP with concentrations equivalent to their respective IC50 values as obtained earlier. It has already been known that AO pervades both live and dead cells making the nucleus appear green, while EB permeates only those cells whose cytoplasmic integrity is compromised and approaching apoptosis or necrosis. As shown in Fig. 3a, the vehicle-treated group (control) had a maximum number of viable cells (green) with regular cellular morphology and almost no penetration of EB. On the contrary, BP-M and PIP treated cells showed an increased count of EB stained cells, confirming the cells to be in early apoptotic and/or late apoptotic stage. The loss of cytoplasmic membrane integrity and nuclear condensation was further confirmed by Hoechst 33258 dye staining. Hoechst dye visualizes changes in cellular nuclei and the formation of the apoptotic body through the appearance of intense blue coloration after binding to cellular DNA. As shown in Fig. 3b, BP-M and PIP caused intense nuclear condensation in K-562 cells when treated at respective IC50 equivalent values of phytochemicals, while the vehicle-treated (control group) cells showed intact nuclear chromatin patterns.
Figure 3.
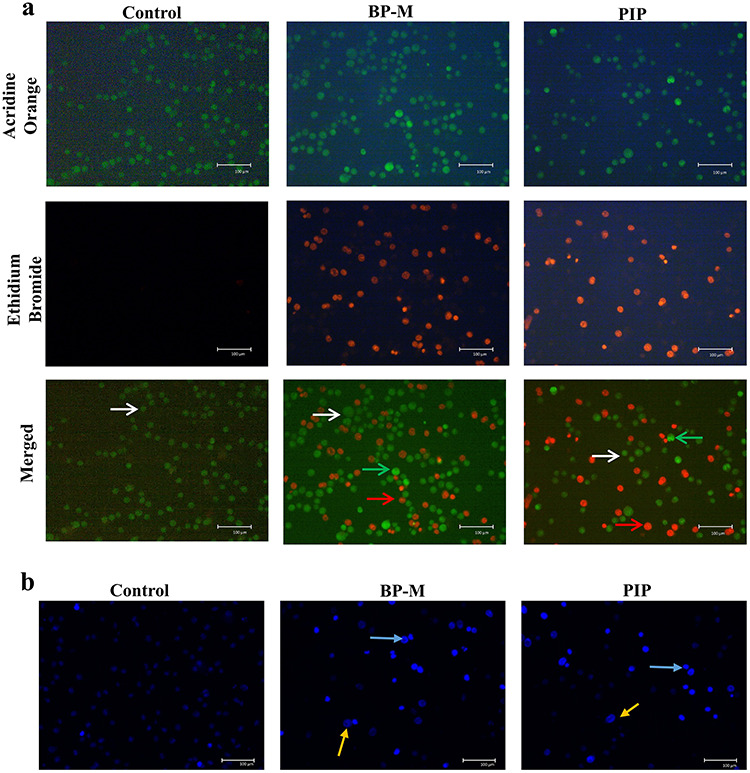
evaluation of apoptosis in response to black pepper. Representative (a) acridine orange/ethidium bromide dual staining and (b) Hoechst 33342 dye staining for nuclear condensation in apoptotic K-562 cells treated with methanol extract of black pepper and piperine at IC50 equivalent concentrations for 48 h. The images were visualized at 200× magnification using a fluorescent microscope. The experiment was performed three times independently. Arrows indicate (a) white, live cells, green, early apoptotic cells and red, late apoptotic cells; (b) blue, condensed chromatin; yellow, nuclear fragmentation. BP-M, black pepper methanol extract; PIP, piperine.
Furthermore, the morphological changes in K-562 cells after 48 h treatment with BP-M and PIP were examined microscopically. As shown in Fig. 4a, the characteristics shrinkage and loss of confluent cell population were observed in both BP-M and PIP treated conditions, while cells in the vehicle-treated control group showed regular spherical cellular morphology. The rate of cellular proliferation of K-562 cell lines was evaluated by trypan blue assay. The live-cell population of K-562 cells reduced by about 2- and 3-fold in response to IC50 equivalent concentrations of BP-M and PIP, respectively, indicative of inhibition of proliferation and/or viability of K-562 cells (P < 0.05) by these phytoconstituents (Fig. 4b).
Figure 4.
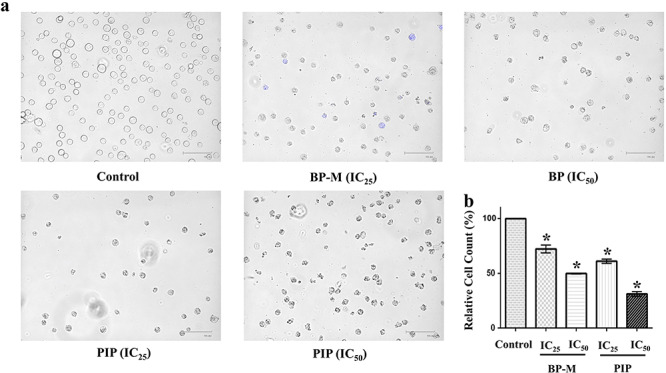
effect of black pepper methanol extract and piperine on the morphology and proliferation of K-562 cells. (a) Representative microscopic images of K-562 cells treated with respective phytochemicals at IC25 and IC50 equivalent concentrations for 48 h followed by trypan blue staining. (b) Bar graph representing the relative percentage count of K-562 cells as treated in (a) by trypan blue staining. Results are the mean ± SEM of three independent experiments. * represents P < 0.05 as compared with the control group. BP-M, black pepper methanol extract; PIP, piperine; IC25 and IC50, IC25 and IC50 equivalent concentrations, respectively.
Black pepper and piperine induce intracellular ROS generation in K-562 cells to initiate cell death
It has already been reported that prior to undergoing apoptosis, generally, there is an augmentation of intracellular ROS levels in cells. Therefore, in our experiment, the intracellular ROS level was measured to determine the probable cause of apoptosis in cancer cells after treatment with BP-M and PIP at IC25 and IC50 equivalent concentrations for 48 h. As shown in Fig. 5, when the phytochemicals (BP-M and PIP) and positive control (H2O2) treated cells were analyzed through FACS after stained with DCFDA, the levels of accumulated ROS increased dramatically (purple color zone). The most pronounced effect was observed in response to IC50 concentrations for both BP-M and PIP, although both BP-M and PP showed significant ROS generation even at IC25 equivalent levels as well (P < 0.05) (Fig. 5a). The results were further validated by quantifying the flow cytometry data where BP-M and PIP were found to increase the level of intracellular ROS in K-562 cells by 19.7- and 31.6-fold, respectively, in response to their respective IC50 equivalent concentrations (P < 0.05) (Fig. 5b).
Figure 5.
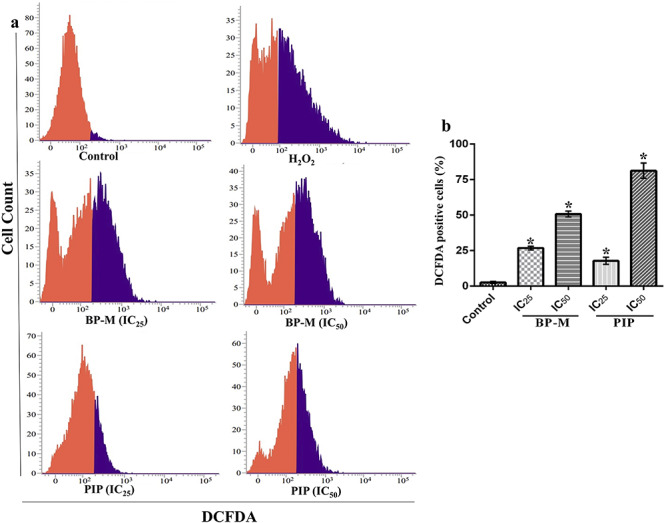
treatment of black pepper methanol extract and piperine (IC25 and IC50, 48 h) induces the generation of ROS in K-562 cells. (a) Representative FACS analysis of cells using DCFDA staining. Red and purple zone represents unstained and stained cells, respectively. (b) Bar graph indicating the percentage of DCFDA stained K-562 cell count determined by FACS analysis as treated in (a). Results are the representation of three independent experiments with mean ± SEM. * indicates P < 0.05 as compared with control group. BP-M, black pepper methanol extract; PIP, piperine; IC25 and IC50, IC25 and IC50 equivalent concentrations, respectively.
Black pepper and piperine induce apoptosis in K-562 cells
In a milieu of cytotoxicity and microscopic observations, flow cytometry analysis was performed to examine the intensity with which the K-562 cells were undergoing apoptosis. The images as shown by FACS analysis divide the cell population into four quadrants with live cells in the lower left (LL) and dead cells in the upper left (UL). In contrast, the lower right (LR) and upper right (UR) quadrant represent cells in early and late apoptosis, respectively. When K-562 cells were treated at IC25 and IC50 equivalent concentrations of BP-M and PIP for 48 h, apoptosis was distinctly observed as depicted by the distribution of cells in LR and UR quadrants. As shown in bar diagram of Fig. 6, the total apoptotic cells percentage (both early and late apoptotic cells) were found to be 67.17 and 79.58% (for BP-M) and 25.41 and 69.82% (for PIP) for individual phytochemical treated cells at IC25 and IC50 equivalent concentrations, respectively, as compared with vehicle-treated cells (P < 0.05).
Figure 6.
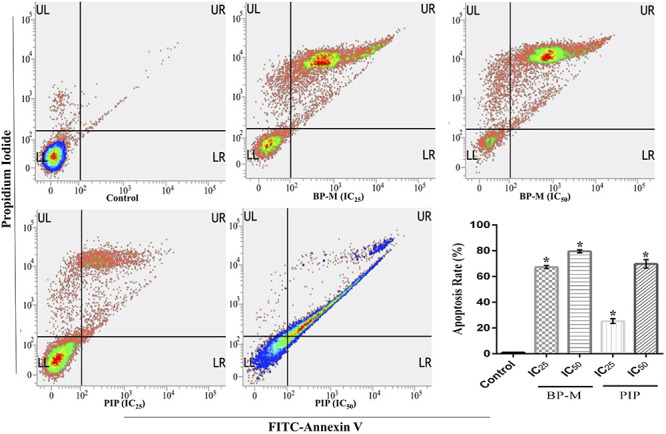
effect of black pepper methanol extract and piperine (IC25 and IC50, 48 h) on apoptosis in K-562 cells. Representative FACS analysis of the cells performed using Annexin V as a marker. Bar graph indicating the sum of cells in the UR and LR quadrants of respective treatment groups. Each bar indicates mean ± SEM of three independent experiments and expressed as percent apoptosis rate. * represents P < 0.05 as compared with the control group. UL, upper left; UR, upper right; LL, lower left; LR, lower right; BP-M, black pepper methanol extract; PIP, piperine; IC25 and IC50, IC25 and IC50 equivalent concentrations, respectively.
Black pepper and piperine induce caspase-dependent apoptosis involving intrinsic pathway and downregulate PCNA expression
As it has already been reported that apoptosis is triggered by multi-signal pathways, transcriptional analysis of caspases and its activator genes was performed by semi-quantitative RT-PCR and quantitative RT-qPCR. In K-562 cells treated with IC25 and IC50 equivalent concentrations for 48 h, the expression of caspase-3 was found to increase by about 1.4- and 1.6-fold in BP-M treated cells (Fig. 7a), while 1.7- and 1.9-fold in PIP-treated cells (Fig. 7b) at two dosages, respectively (P < 0.05). In a similar pattern, the expression of caspase-9 increased by about 1.5- and 1.6-fold, respectively, in response to IC50 equivalent concentrations of BP-M (Fig. 7a) and PIP (Fig. 7b) (P < 0.05). The expression of apoptosis regulator gene, Bax, in treated cells were found to increase by about 1.4- and 1.6-fold in response to BP-M (Fig. 7a) and PIP (Fig. 7b), respectively, at their maximum concentrations. However, the level of Bcl-2 expression did not change under any treatment conditions (Fig. 7a and b). On the contrary, the level of transcription of PCNA was found to be downregulated by about 1.4- and 1.6-fold in BP-M (Fig. 7a) and PIP (Fig. 7b) treated cells, respectively, at IC50 equivalent concentrations of both the phytochemicals (P < 0.05). The expression patterns for caspase-9, caspase-3 and PCNA were also evaluated through quantitative RT-qPCR. A 2.2- and 1.5-fold increase in caspase-9 expression was observed in response to IC50 equivalent concentrations of BP-M and PIP treatments, respectively (Fig. 7c) (P < 0.05). In similar treatment conditions, the expression of caspase-3 was increased by 3.2- and 2.2-fold in response to IC50 equivalent concentrations of BP-M and PIP, respectively (Fig. 7c) (P < 0.05). As expected, the expression of PCNA was downregulated by 6.7- and 1.6-fold in the presence of IC50 equivalent concentrations of BP-M and PIP, respectively (Fig. 7c) (P < 0.05).
Figure 7.
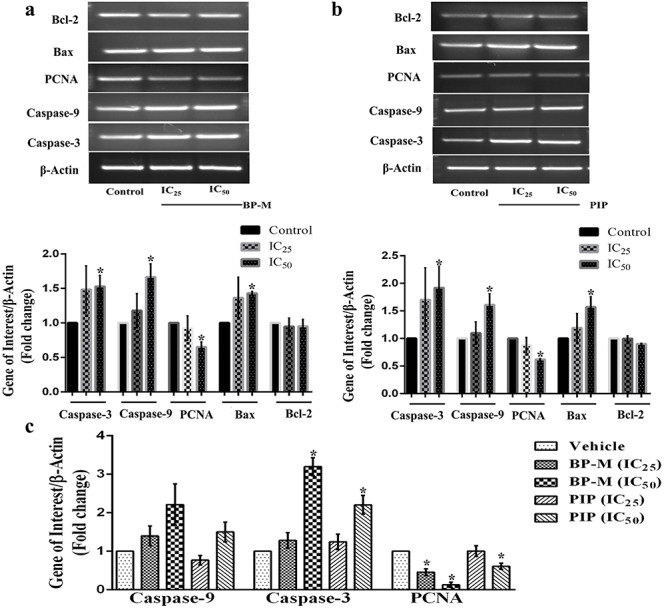
effect of black pepper methanol extract and piperine (IC25 and IC50, 48 h) in regulating the transcription of some (anti)apoptotic genes in K-562 cells. Representative semi quantitative PCR images indicating the transcriptional patterns of various caspase, pro- and anti-apoptotic genes in response to (a) black pepper methanol extract and (b) piperine. β-actin was used as a gel loading control. Bar graph below respective gel images represent fold change in expression as compared to the respective vehicle-treated control group. (c) The relative quantification of mRNA expression of caspase-9, caspase-3 and PCNA genes using RT-qPCR in K-562 cells after treatment with black pepper methanol extract and piperine. Results are the mean ± SEM of three independent experiments. * indicates P < 0.05 as compared with the respective control groups for each genes. BP-M, black pepper methanol extract; PIP, piperine; IC25 and IC50, IC25 and IC50 equivalent concentrations, respectively, at 48 h.
To assess if the impact of test phytochemicals is linked to both, transcription as well as translation of target genes, in the next phase, we determined changes in the level of proteins of the same genes as discussed above by immunoblot analysis. For this experiment, K-562 cells were treated similarly at IC25 and IC50 equivalent concentrations of both BP-M and PIP for 48 h. As shown in Fig. 8, the expression of cleaved caspase-3 was found to be upregulated by about 6.1- and 7.6-fold, respectively, in response to IC50 equivalent concentration of BP-M (Fig. 8a) and PIP (Fig. 8b) (P < 0.05). The expression of caspase-9 and Bax was also found to be upregulated by about 3.5- and 6.5-fold, respectively, in response to IC50 equivalent concentration of BP-M (Fig. 8a) (P < 0.05). A similar trend of upregulation was also observed in repose to PIP for caspase-9 and Bax (2.1- and 3.7-fold, respectively) at IC50 equivalent concentrations (Fig. 8b) (P < 0.05). Interestingly, in accordance to our transcription data (Fig. 7a and b), the level of translation of Bcl-2 also did not show any significant change in response to PIP (Fig. 8b) with respect to the vehicle-treated groups (P < 0.05), whereas a 2-fold reduction was observed in the presence of BP-M (Fig. 8a). This aspect of differential transcription and translation patterns for Bcl-2 in response to BP-M needs further validation involving detailed studies. When tested for PCNA, the expression profile showed a decrease by about 2.7- and 1.5-fold in response to BP-M (Fig. 8a) and PIP (Fig. 8b), respectively, in response to IC50 equivalent concentrations of phytochemicals with respect to vehicle-treated cells (P < 0.05). Thus, taken together, it can be speculated that both BP-M and PIP induce the intrinsic pathway of apoptosis in K-562 cells while regulating its proliferation and inducing cytotoxicity.
Figure 8.
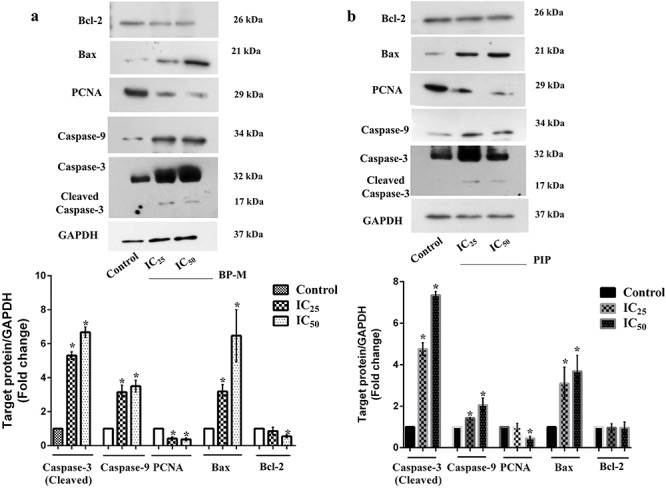
effect of black pepper methanol extract and piperine (IC25 and IC50, 48 h) in regulating the translation of (anti)apoptotic genes in K-562 cells. Representative protein expression patterns of various caspase, pro- and anti-apoptotic genes as determined by immunoblot analysis in response to (a) black pepper methanol extract and (b) piperine. GAPDH was used as a gel loading control for immunoblot analysis. Bar graph below respective gel images represent fold change in expression as compared with the respective vehicle-treated control group. Results are the mean ± SEM of three independent experiments. * indicates P < 0.05 as compared with the respective control group in each protein. BP-M, black pepper methanol extract; PIP, piperine; IC25 and IC50, IC25 and IC50 equivalent concentration, respectively, at 48 h.
In silico investigation of piperine confirms the activation of Apaf-1 and inhibition of Bcl-2 and PCNA
The binding patterns of PIP with possible target proteins (Bcl-2, PCNA and Apaf-1) were investigated using computational-based docking studies. Following our earlier experimental data where we showed the regulation of most of the (anti)apoptotic genes by BP-M and PIP, our docking study also substantiated the same where PIP showed binding with all these proteins. The predicted binding energy and affinity of PIP against these three proteins are presented in Table 5.
Table 5.
predicted binding parameters and interactions of piperine with respective protein targets
| Target protein | Binding constant (μM) | Binding energy (kcal/mol) | Residues involved in binding | Residues involved in H- bonding |
|---|---|---|---|---|
| Apaf-1 | 0.456 | -8.65 | ASP244, SER161, HIS438, PRO123, VAL125, SER325, LEU322, PRO321, GLY159, GLY157, CYS158, LYS160, MET155, ARG265 | SER325, GLY159, LYS160 |
| PCNA | 3.03 | -7.53 | ASP29, CYS27, GLU25, ASN24, ILE23, SER42, ASP41, HIS44, MET40, GLU124, LEU126, GLN38, VAL123 | GLU25, LEU126 |
| Bcl-2 | 41.14 | -5.98 | TYR67, ARG66, PHE63, ASP62, ALA59, ALA108, LEU96, ARG105, GLY104, ASN102 | ARG105 |
PIP was involved in binding with Apaf-1 through polar as well as with non-polar interactions (Fig. 9), and the binding site of PIP was found to be the same as in the complex structure of Apaf-1 with its activator ADP. Binding energy and binding constants were calculated using Autodock scoring function, and they were found to be −8.65 kcal/mol and 0.456 μM, respectively. The overall structure of Apaf-1 with docked PIP in the active site, and deep buried active site are shown in Fig. 9a and b, respectively. PIP was found to interact with SER325, GLY159 and LYS160 residues of Apaf-1 using hydrogen bond mediated binding (Fig. 9c and d), while CYS158, MET155, PRO123, SER325, VAL125, LEU322, SER161, HIS438, GLY157, LYS160, GLY159 and PRO321 residues of Apaf-1 interacted with PIP through hydrophobic interactions (Fig. 9c and d).
Figure 9.
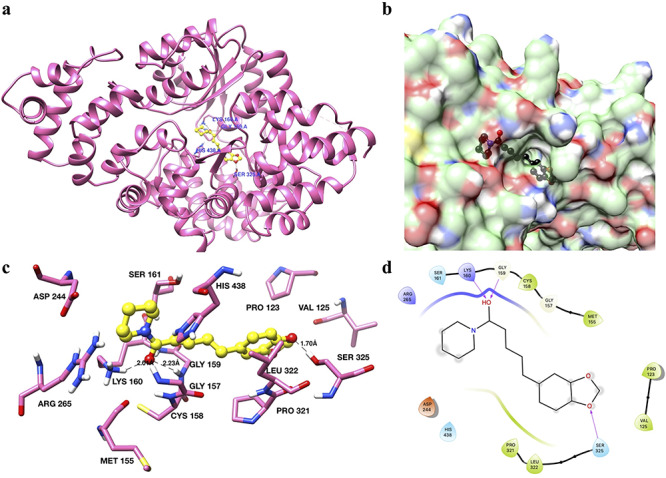
binding analysis of piperine with Apaf-1. (a) The overall structure of Apaf-1 (ribbon diagram) with piperine docked in the active site shown in yellow color and ball and stick representation. Interacting residues are shown in sticks and labeled blue. (b) Surface diagram of Apaf-1 protein showing the binding pockets. Piperine is shown in black color. (c) Interactions of piperine with the active site residues of Apaf-1. Interacting residues are shown as sticks and in hot pink color. Piperine is shown in yellow color with a ball and stick representation. Hydrogen bonds are labeled and shown as black dash lines. (d) 2D interaction diagram of piperine with Apaf-1.
When we analyzed the interaction of PIP with the next protein target, i.e. PCNA, it showed that both hydrophobic as well as hydrophilic interactions play a significant role in the binding of PIP with PCNA (Fig. 10). The binding energy and binding constant of PIP with PCNA were found to be −7.53 kcal/mol and 3.03 μM, respectively (Table 5). The PIP bound docked PCNA structure is shown in Fig. 10a and surface diagram indicating the deep cavity that interacts with PIP- box (PCNA interacting protein box) is shown in Fig. 10b. The binding patterns showed that GLU25 and LEU126 residues of PCNA were involved in the binding with PIP through hydrogen bonding (Fig. 10c and d). Other residues that seemed to be involved in the binding through hydrophobic interactions were ASP29, CYS27, GLU25, ASN24, ILE23, SER42, ASP41, HIS44, MET40, GLU124, LEU126, GLN38 and VAL123 (Fig. 10c and d and Table 5).
Figure 10.
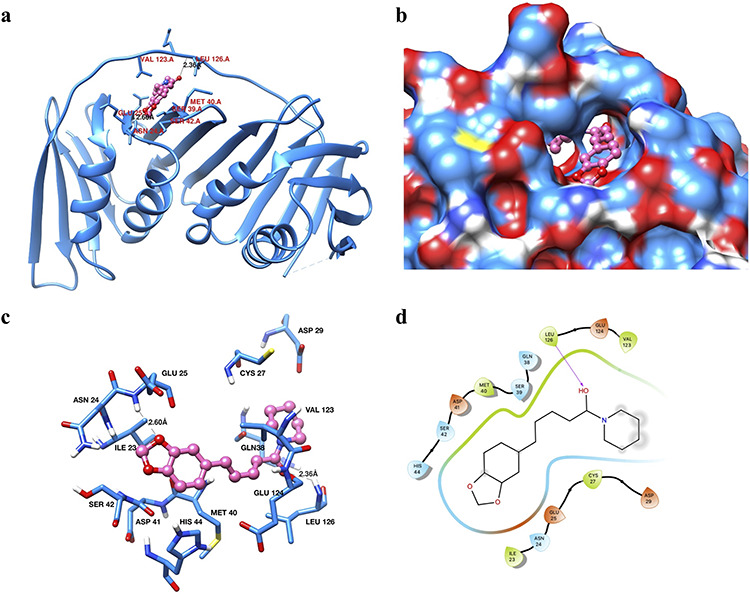
binding analysis of piperine with PCNA. (a) Ribbon diagram of PCNA protein showing the different secondary structure elements and PIP in the binding site pocket (shown in hot pink color and ball and stick representation). Interacting residues are shown in dodger blue color as sticks. (b) Surface diagram of PCNA showing the active site pocket with piperine (hot pink color and ball-stick representation). (c) Interactions of piperine with the active site residues of PCNA. Interacting residues are in dodger blue color and shown in sticks representation. Piperine is shown in hot pink color as a ball and stick. Hydrogen bonds are labeled and shown as black dashed lines. (d) 2D interaction diagram of piperine with PCNA.
Like that of Apaf-1 and PCNA, PIP was also found to interact and bind to Bcl-2 through both hydrophobic and hydrophilic interactions. Figure 11 shows the different aspects of PIP binding with Bcl-2. The overall structure of Bcl-2 with docked PIP is shown in Fig. 11a. The active site pocket of Bcl-2 with docked PIP is shown in the surface diagram (Fig. 11b). Based upon the analysis of best docked pose, it has been found that ARG105 interacted with PIP through hydrogen bonding (Fig. 11c and d), while residues that contributed to binding through hydrophobic interactions were found to be LEU96, ALA108, GLY104, ARG66, ASP62, PHE63 and ARG105 (Fig. 11c and d).
Figure 11.
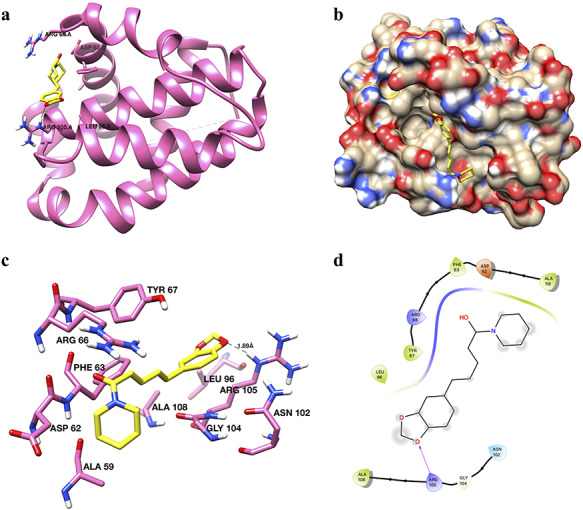
binding of piperine with Bcl-2. (a) Ribbon diagram of Bcl-2 with piperine in the active site. Piperine has been shown in yellow color and stick representation. Interacting residues are shown in hot pink color as sticks. (b) Surface diagram of Bcl-2 protein with piperine (yellow color and stick representation) showing the active site pocket. (c) Interaction of piperine with the critical residues of Bcl-2. Interacting residues are shown in hot pink color as sticks. Piperine is shown in yellow color as sticks. Hydrogen bonds are labeled and shown as black dash lines. (d) 2D interaction diagram of piperine with Bcl-2.
Discussion
It is since ancient times that black pepper has been used popularly in culinary practices across the globe. Black pepper, along with its major bioactive component, i.e. PIP, has been evaluated for its medicinal properties widely. Black pepper improves the functioning of the digestive system and also imparts antioxidant, antidiarrheal, antidepressant, anti-inflammatory, anti-diabetic and anti-tumor activity along with various other healthcare benefits [29]. In vitro investigation has confirmed the role of black pepper and PIP in the inhibition of proliferation of colon cancer cells [12] and androgen-dependent and independent prostate cancer cells [11]. According to some recent reports, black pepper has also been shown to improve breast cancer [10] and lung adenocarcinoma [13] conditions, while Brazilian Piper species has been found to be cytotoxic towards oral carcinoma cells [30].
In the present study, all three black pepper extracts (methanol, ethanol and aqueous) were found to be a rich source of phenolic and flavonoid compounds, which suggested for its pharmacological potential. GC–MS analysis confirmed that PIP is the major metabolite present in black pepper extract. The other metabolites present in the black pepper extract in considerable amounts were caryophyllene, a terpenoid with anticancer property [31] and P-coumaric acid, a phenolic acid which has cytotoxic activity against various tumor conditions and also has been reported to show antioxidant, antimicrobial and antiviral properties [32]. PIP has been widely studied for its potent role in different cancer conditions, bio-enhancement of various other drugs and prevention of various chronic disease conditions [33]. Based on these facts, PIP is considered as one of the most bioactive metabolites of the pepper family. Altogether, these studies and phytochemical investigations led us to analyse the anticancer potential of black pepper and its major constituent PIP against unexplored CML conditions. Initial cellular proliferation studies showed the potential of both black pepper extracts and PIP to be effective cytotoxic phytochemicals against CML. Among three extracts tested by us, the methanol extract was found to be most potent one. This could be attributed to comparatively high proportion of PIP in this extract as compared with other two extracts. At the same time, methanol extract was also found to be marginally more effective than PIP alone, atleast in some of the parametrs as tested by us. This actually indicates a pattern of combined effect by various phytoconstituents present in the extract which at times may be more than the isolated compounds, as claimed by various traditional healthcare systems. However, all these aspects need further detailed studies in order to establish the concentration dependent effects of various metabolites in black pepper.
The calcium-dependent phospholipid flip flop movement along with the loss of aminophospholipid translocase activity leads to the appearance of phosphatidylserine in outer leaflets of the plasma membrane of apoptotic cells. Annexin V selectively binds with the exposed phosphatidylserine protein moiety and distinguishes the apoptotic cells from non-apoptotic ones [34]. Earlier, it has been reported that PIP induces late apoptosis in triple-negative breast cancer cells [35]. In our study, the flow cytometry analysis by Annexin V assay elucidated a dose-dependent increase in Annexin V positive cells in the presence of both black pepper and PIP, confirming the occurrence of apoptotic cell death. These results are in accordance with the progression of cancer prevention conditions, as confirmed earlier [35].
Cysteinylaspartateprotease (caspase) enzymes family is the executioner of programmed cell death called apoptosis. The progression of apoptosis is regulated by two distinctively interconnected pathways, namely extrinsic and intrinsic pathways. Caspase-8 and -9 are two initiator caspases which exist as inactive procaspase monomers. Caspase-8 leads to the extrinsic pathway involving various death receptors such as tumor necrosis factor receptor 1, Apo-1, Apo-2, Apo-3 and nerve growth factor receptor [36]. Caspase-9 initiates mitochondria-assisted intrinsic pathway involving the Bcl-2 protein family. Bcl-2 proteins family involves different pro- and anti-apoptotic proteins. The generation of stress signals within cell inhibits the anti-apoptotic proteins (Bcl-2, Bcl-xL) and promotes the assembly of pro-apoptotic protein (Bak-Bax) oligomers within the outer mitochondrial membrane and thus leading to the formation of apoptosomes. The activation of pro-initiator caspases further activates executioner caspases (−3, −6 and −7) [37]. PIP has a structural similarity with piperidine, which also has been reported to have anticancer properties. Piperidine and its various structural analogs have been reported to demonstrate cytotoxic effects against K-562 cells [38] and gliomas [39]. PIP has been reported to induce caspase activation leading to the inhibition of human colon cancer growth [12, 40]. In the current study, the situation of apoptosis in K-562 cells was found to be associated with an increase in the level of Bax protein, which leads to its hetero-dimerization with Bcl-2 protein and inhibiting its expression. In our study, surprisingly, the transcription of Bcl-2 was not altered either by BP-M or PIP, although the latter was able to inhibit the translation of this protein and also showed prominent binding to Bcl-2 protein in docking studies. This aspect needs further detailed validation in future studies. This increased ratio of Bax/Bcl-2 in cells lead to the release of cytochrome c in the cytosol from its mitochondrial compartments, which bind to the Apaf-1 factor of cells and activates caspase-9 [18, 41].
Every aerobic cell maintains various biochemical antioxidants and ROS in its milieu. The generation of oxidative stress leads to an imbalance between ROS and antioxidant moieties. It has been already reported that various therapeutic agents generate oxidative stress and are selectively toxic to tumors [42, 43]. The generation of ROS leads to the activation of pro-apoptotic protein such as Bax and another pathway such as the MAPK pathway, which trigger changes in mitochondrial membrane permeability, subsequently leading to mitochondria-dependent cell death pathway. In the present study, both black pepper and PIP generated ROS in K-562 cells and also increased the Bax/Bcl-2 ratio, which might have led to the release of cytochrome C from mitochondria. This in turn leads to the formation of apoptosomes with Apaf-1, thus initiating intrinsic pathway and finally causing the activation of caspase-9/-3 cascade [44, 45]. However, the level of caspase-8 remained unchanged in response to both black pepper and PIP (data not shown).
PCNA is a non-histone, cell nuclear protein that performs the crucial cell survival functions by regulating DNA replication and DNA repair mechanisms. The regulation of PCNA is considered as a hallmark of proliferating cells. The activation of executioner caspase-3 leads to the degradation of PCNA interacting peptides into short PCNA binding peptides. These peptides functionally inactivate PCNA, making it unavailable for functioning [46]. The expression of PCNA has been shown to be downregulated both in the presence of black pepper and PIP at transcription as well as translational level, which may be considered as the final impetus for cells to enter apoptosis.
Molecular docking is considered as an indispensable computational tool in structural biology and drug design studies [47]. In the current study, the preliminary in silico analysis supported in vitro data obtained earlier. The macromolecule–ligand interaction study by molecular docking showed that PIP interacts through hydrophobic and hydrophilic interactions, which might be responsible for the inhibition of Bcl-2 and PCNA. By comparing the predicted binding pose of PIP docked PIP-Bcl-2 complex with the N-heteroaryl sulfonamide-bound Bcl-2 (PDB ID:4IEH), it was found that PIP binds with Bcl-2 in a similar fashion interacting with the key residues (TYR67, ARG66, ASP62 and ALA59). Similarly, PIP-PCNA docked conformation compared with the ligand-bound crystal structure of PCNA (PDB ID: 3WGW) confirmed the binding of PIP with the PIP-box cavity of PCNA, which might be responsible for its inhibition [26]. Apaf-1 is an essential protein that leads to the formation of apoptosomes, which in turn, leads to the activation of caspase-9. The active conformation of Apaf-1 confirms ATP/ADP hydrolysis [27]. The strong binding of PIP to the ADP binding site of Apaf-1, as observed in molecular docking analysis, confirmed that it might also lead to Apaf-1 activation, which ultimately causes the activation of caspase-9 and the initiation of the intrinsic apoptotic pathway to prevent the proliferation of cancerous cells. Altogether, these observations indicate that both black pepper and PIP has the potentiality to prevent the progression of CML.
In conclusion, the findings reported in this study preliminarily affirm that both black pepper and its main metabolite PIP are potent impediment of cellular proliferation in leukemic cells. These phytochemicals initiate the persuasion of apoptosis in human CML cells by upregulating genes involved in intrinsic pathway, i.e. caspase-9, -3 and Bax and subsequent downregulation of PCNA expressions. The cytotoxic potential of both black pepper and PIP increases with the progression of time and dose of treatments. Based on these data, it could be accepted that the involvement of black pepper in regular dietary practice may prevent the development of CML. Therefore, further in detailed studies involving more precise and specific in vitro and in vivo experimental models are warranted to appreciate the highly effective therapeutic potential of black pepper and its major metabolite, i.e. PIP, for which these data sets as reported in this study would provide a strong supportive base.
Funding
This work was supported by a research fellowship (JRF/SRF) assisted in pursuing doctoral research to S.B. and P.K. (CSIR, India).
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Authors’contribution
S.B. and P.R. designed the study. S.B. completed the experiment process, literature search and generation of figures. S.B. and P.R. wrote the manuscript, while P.K., V.K., S.S.S., R.V., V.D.K. and D.S. edited the manuscript. All authors read and approved the final manuscript.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Supplementary Material
Contributor Information
Somesh Banerjee, Molecular Endocrinology Laboratory, Department of Biotechnology, Indian Institute of Technology Roorkee, Roorkee 247667, Uttarakhand, India.
Parul Katiyar, Molecular Endocrinology Laboratory, Department of Biotechnology, Indian Institute of Technology Roorkee, Roorkee 247667, Uttarakhand, India.
Vijay Kumar, Laboratory of Structural Microbiology, Regional Centre for Biotechnology, Faridabad 121001, Haryana, India.
Shashank Sagar Saini, Plant Molecular Biology Laboratory, Department of Biotechnology, Indian Institute of Technology Roorkee, Roorkee 247667, Uttarakhand, India.
Ritu Varshney, Molecular Endocrinology Laboratory, Department of Biotechnology, Indian Institute of Technology Roorkee, Roorkee 247667, Uttarakhand, India.
Vengadesan Krishnan, Laboratory of Structural Microbiology, Regional Centre for Biotechnology, Faridabad 121001, Haryana, India.
Debabrata Sircar, Plant Molecular Biology Laboratory, Department of Biotechnology, Indian Institute of Technology Roorkee, Roorkee 247667, Uttarakhand, India.
Partha Roy, Molecular Endocrinology Laboratory, Department of Biotechnology, Indian Institute of Technology Roorkee, Roorkee 247667, Uttarakhand, India.
References
- 1. Eaton L. World cancer rates set to double by 2020. BMJ 2003;326:728. doi: 10.1136/bmj.326.7392.728/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. IHME (2020). Global burden of diseases. Lancet. https://www.thelancet.com/lancet/visualisations/gbd-compare(4 January 2020, date last accessed). [Google Scholar]
- 3. Ureshino H, Shindo T, Kimura S. Role of cancer immunology in chronic myelogenous leukemia. Leuk Res 2020;88:106273. doi: 10.1016/j.leukres.2019.106273. [DOI] [PubMed] [Google Scholar]
- 4. Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am J Hematol 2020;95:691–709. doi: 10.1002/ajh.25792. [DOI] [PubMed] [Google Scholar]
- 5. Massimino M, Stella S, Tirrò Eet al. ABL1-directed inhibitors for CML: efficacy, resistance and future perspectives. Anticancer Res 2020;40:2457–65. doi: 10.21873/anticanres.14215. [DOI] [PubMed] [Google Scholar]
- 6. Deininger M, O’Brien SG, Guilhot Fet al. International randomized study of interferon Vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with Imatinib. Blood 2009;114:1126. doi: 10.1182/blood.V114.22.1126.1126. [DOI] [Google Scholar]
- 7. Ramawat KG, Goyal S. The Indian herbal drugs scenario in global perspectives. In: Ramawat KG, Merillon JM (eds). Bioactive Molecules and Medicinal Plants. Berlin, Heidelberg: Springer Berlin Heidelberg, 2008, 325–47. doi: 10.1007/978-3-540-74603-4_18 [DOI] [Google Scholar]
- 8. Ahmad N, Fazal H, Abbasi BHet al. Biological role of Piper nigrum L. (black pepper): a review. Asian Pac J Trop Biomed 2012;2:S1945–53. doi: 10.1016/S2221-1691(12)60524-3 [DOI] [Google Scholar]
- 9. Smilkov K, Ackova DG, Cvetkovski Aet al. Piperine: old spice and new nutraceutical? Curr Pharm Des 2019;25:1729–39. doi: 10.2174/1381612825666190701150803. [DOI] [PubMed] [Google Scholar]
- 10. Deng Y, Sriwiriyajan S, Tedasen Aet al. Anti-cancer effects of Piper nigrum via inducing multiple molecular signaling in vivo and in vitro. J Ethnopharmacol 2016;188:87–95. doi: 10.1016/j.jep.2016.04.047. [DOI] [PubMed] [Google Scholar]
- 11. Ouyang D, Zeng L, Pan Het al. Piperine inhibits the proliferation of human prostate cancer cells via induction of cell cycle arrest and autophagy. Food Chem Toxicol 2013;60:424–30. doi: 10.1016/j.fct.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 12. Yaffe PB, Power Coombs MR, Doucette CDet al. Piperine, an alkaloid from black pepper, inhibits growth of human colon cancer cells via G1 arrest and apoptosis triggered by endoplasmic reticulum stress. Mol Carcinog 2015;54:1070–85. doi: 10.1002/mc.22176. [DOI] [PubMed] [Google Scholar]
- 13. Marques da Fonseca L, Jacques da Silva LR, Santos dos Reis Jet al. Piperine inhibits TGF-β Signaling pathways and disrupts EMT-related events in human lung adenocarcinoma cells. Medicines 2020;7:19. doi: 10.3390/medicines7040019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lang SJ, Schmiech M, Hafner Set al. Antitumor activity of an Artemisia annua herbal preparation and identification of active ingredients. Phytomedicine 2019;62:152962. doi: 10.1016/j.phymed.2019.152962. [DOI] [PubMed] [Google Scholar]
- 15. Srivastava JK, Gupta S. Antiproliferative and apoptotic effects of chamomile extract in various human cancer cells. J Agric Food Chem 2007;55:9470–8. doi: 10.1021/jf071953k. [DOI] [PubMed] [Google Scholar]
- 16. Sarkate A, Banerjee S, Mir JIet al. Antioxidant and cytotoxic activity of bioactive phenolic metabolites isolated from the yeast-extract treated cell culture of apple. Plant Cell Tiss Org Cult 2017;130:641–9. doi: 10.1007/s11240-017-1253-0. [DOI] [Google Scholar]
- 17. Lisec J, Schauer N, Kopka Jet al. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat Protoc 2006;1:387–96. doi: 10.1038/nprot.2006.59. [DOI] [PubMed] [Google Scholar]
- 18. Nikhil K, Sharan S, Chakraborty Aet al. Role of isothiocyanate conjugate of pterostilbene on the inhibition of MCF-7 cell proliferation and tumor growth in Ehrlich ascitic cell induced tumor bearing mice. Exp Cell Res 2014;320:311–28. doi: 10.1016/j.yexcr.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 19. Kasibhatla S, Amarante-Mendes GP, Finucane D et al. Acridine Orange/Ethidium bromide (AO/EB) staining to detect apoptosis. Cold Spring Harb Protoc 2006;3:pdb.prot4493-pdb.prot4493. doi: 10.1101/pdb.prot4493. [DOI] [Google Scholar]
- 20. Crowley LC, Marfell BJ, Waterhouse NJ. Analyzing cell death by nuclear staining with Hoechst 33342. Cold Spring Harb Protoc 2016;9:778–81. doi: 10.1101/pdb.prot087205. [DOI] [PubMed] [Google Scholar]
- 21. Crowley LC, Marfell BJ, Christensen MEet al. Measuring cell death by trypan blue uptake and light microscopy. Cold Spring Harb Protoc 2016;7:643–6. doi: 10.1101/pdb.prot087155. [DOI] [PubMed] [Google Scholar]
- 22. Nishio S, Teshima Y, Takahashi Net al. Activation of CaMKII as a key regulator of reactive oxygen species production in diabetic rat heart. J Mol Cell Cardiol 2012;52:1103–11. doi: 10.1016/j.yjmcc.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 23. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24. Morris GM, Huey R, Lindstrom Wet al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 2009;30:2785–91. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Touré BB, Miller-Moslin K, Yusuff Net al. The role of the acidity of N-heteroaryl sulfonamides as inhibitors of Bcl-2 family protein-protein interactions. ACS Med Chem Lett 2013;4:186–90. doi: 10.1021/ml300321d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Inoue A, Kikuchi S, Hishiki Aet al. A small molecule inhibitor of monoubiquitinated proliferating cell nuclear antigen (PCNA) inhibits repair of interstrand DNA cross-link, enhances DNA double strand break, and sensitizes cancer cells to cisplatin. J Biol Chem 2014;289:7109–20. doi: 10.1074/jbc.M113.520429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Riedl SJ, Li W, Chao Yet al. Structure of the apoptotic protease-activating factor 1 bound to ADP. Nature 2005;434:926–33. doi: 10.1038/nature03465. [DOI] [PubMed] [Google Scholar]
- 28. Pettersen EF, Goddard TD, Huang CCet al. UCSF chimera - a visualization system for exploratory research and analysis. J Comput Chem 2004;25:1605–12. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 29. Sanlier N, Gencer F. Role of spices in the treatment of diabetes mellitus: a minireview. Trends Food Sci Technol 2020;99:441–9. doi: 10.1016/j.tifs.2020.03.018. [DOI] [Google Scholar]
- 30. Macedo AL, Silva DPD, Moreira DLet al. Cytotoxicity and selectiveness of Brazilian Piper species towards oral carcinoma cells. Biomed Pharmacother 2019;110:342–52. doi: 10.1016/j.biopha.2018.11.129. [DOI] [PubMed] [Google Scholar]
- 31. Fidyt K, Fiedorowicz A, Strządała Let al. β-Caryophyllene and β-Caryophyllene oxide—natural compounds of anticancer and analgesic properties. Cancer Med 2016;5:3007–17. doi: 10.1002/cam4.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pei K, Ou J, Huang Jet al. P-Coumaric acid and its conjugates: dietary sources, pharmacokinetic properties and biological activities. J Sci Food Agric 2016;96:2952–62. doi: 10.1002/jsfa.7578. [DOI] [PubMed] [Google Scholar]
- 33. Derosa G, Maffioli P, Sahebkar A. Piperine and its role in chronic diseases. In: Gupta S, Prasad S, Aggarwal B. (eds). Anti-inflammatory Nutraceuticals and chronic diseases. Advances in Experimental Medicine and Biology. 928:173–84, 2016. doi: 10.1007/978-3-319-41334-1_8 [DOI] [PubMed] [Google Scholar]
- 34. Arur S, Uche UE, Rezaul Ket al. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev Cell 2003;4:587–98. doi: 10.1016/S1534-5807(03)00090-X. [DOI] [PubMed] [Google Scholar]
- 35. Greenshields AL, Doucette CD, Sutton KMet al. Piperine inhibits the growth and motility of triple-negative breast cancer cells. Cancer Lett 2015;357:129–40. doi: 10.1016/j.canlet.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 36. Lavrik I, Golks A, Krammer PH. Death receptor signaling. J Cell Sci 2005;118:265–7. doi: 10.1242/jcs.01610 [DOI] [PubMed] [Google Scholar]
- 37. Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene 2008;27:6194–06. doi: 10.1038/onc.2008.297. [DOI] [PubMed] [Google Scholar]
- 38. Vinaya K, Kavitha CV, Chandrappa Set al. Synthesis and antileukemic activity of novel 4-(3-(Piperidin-4-yl) propyl)Piperidine derivatives. Chem Biol Drug Des 2011;78:622–30. doi: 10.1111/j.1747-0285.2011.01184.x. [DOI] [PubMed] [Google Scholar]
- 39. Gariboldi MB, Ravizza R, Petterino Cet al. Study of in vitro and in vivo effects of the piperidine nitroxide Tempol - a potential new therapeutic agent for gliomas. Eur J Cancer 2003;39:829–37. doi: 10.1016/S0959-8049(02)00742-6. [DOI] [PubMed] [Google Scholar]
- 40. Zhao Y, Luo Q, Mo Jet al. Metformin in combination with JS-K inhibits growth of renal cell carcinoma cells via reactive oxygen species activation and inducing DNA breaks. J Cancer 2002;11:3701–12. doi: 10.7150/jca.36372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dasari S, Bakthavachalam V, Chinnapaka S, Samy ALPAet al. Neferine, an alkaloid from lotus seed embryo targets HeLa and SiHa cervical cancer cells via pro-oxidant anticancer mechanism. Phytother Res 2020;34:1–19. doi: 10.1002/ptr.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee WYW, Liu KWK, Yeung JHK. Reactive oxygen species-mediated kinase activation by dihydrotanshinone in tanshinones-induced apoptosis in HepG2 cells. Cancer Lett 2009;285:46–57. doi: 10.1016/j.canlet.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 43. Moon D-O, Kim M-O, Kang S-Het al. Sulforaphane suppresses TNF-α-mediated activation of NF-κB and induces apoptosis through activation of reactive oxygen species-dependent caspase-3. Cancer Lett 2009;274:132–42. doi: 10.1016/j.canlet.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 44. Liang JR, Yang H. Ginkgolic acid (GA) suppresses gastric cancer growth by inducing apoptosis and suppressing STAT3/JAK2 signaling regulated by ROS. Biomed Pharmacother 2020;125:109585. doi: 10.1016/j.biopha.2019.109585. [DOI] [PubMed] [Google Scholar]
- 45. Liu ZH, Yang CX, Zhang Let al. Baicalein, as a prooxidant, triggers mitochondrial apoptosis in MCF-7 human breast cancer cells through mobilization of intracellular copper and reactive oxygen species generation. Onco Targets Ther 2019;12:10749–61. doi: 10.2147/OTT.S222819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Paunesku T, Mittal S, Protić Met al. Proliferating cell nuclear antigen (PCNA): ringmaster of the genome. Int J Radiat Biol 2001;77:1007–21. doi: 10.1080/09553000110069335. [DOI] [PubMed] [Google Scholar]
- 47. Guedes IA, Magalhães CS, Dardenne LE. Receptor-ligand molecular docking. Biophys Rev 2014;6:75–87. doi: 10.1007/s12551-013-0130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


