Abstract
Pomegranate (Punica granatum L.) is a fruit used extensively in traditional medicine by ancient and modern cultures. Different parts of the tree and fruit, such as leaf, peel, pericarp, aril, seed, and juice contain considerable amounts of phenolic compounds with high antioxidant activities. To improve its storability, pomegranate juice was microencapsulated by spray drying. The present study evaluated microencapsulated pomegranate juice (MPJ) for toxic effects in Wistar rats and CD-1 mice to determine if MPJ can be considered safe for human consumption and used as a nutraceutical. No deaths or deleterious effects occurred when high doses of 5000 mg/kg were orally administered in rats for 14 days, indicating an absence of subacute toxicity. Similarly, 3000 mg/kg MPJ administered to CD-1 mice for 90 days did not show subchronic toxicity. In fact, MPJ resulted in lowered weight gain in both rats and mice. Cytotoxic and microbiological analyses of MPJ were also performed. MPJ did not cause any cytotoxicity in epithelial cell culture as tested using the Alamar blue assay. Additionally, histopathological analysis of kidney and liver corroborated the absence of toxicity in CD-1 mice. The microbial load of the MPJ was low, and no pathogenic bacteria were present. In conclusion, the results reported here show that high doses of MPJ are apparently innocuous in rats and mice for the 14 and 90 days investigated, respectively. Although preliminary, our results suggest that MPJ may be safe to ingest and may even have beneficial effects in reducing weight gain.
Keywords: microencapsulated pomegranate juice, subacute toxicity, subchronic toxicity, nontoxic, safe
Graphical Abstract
Graphical Abstract.
Introduction
Pomegranate (Punica granatum L.), a native of Iran in the Middle East, is believed to be one of the first cultivated fruits because remains of leaves, branches, and seeds have been found there dating back to the early Bronze Age (3500–2000 BC) [1, 2]. Currently, the fruit attracts special interest for its health benefits due to its wide range of bioactivities such as antioxidant, antimicrobial, cardioprotective, antihypertensive, anticancer, anti-inflammatory, etc. [2–6].
Acute toxicity is defined as adverse effects occurring within a 24-h period after single, multiple, or continuous exposures of a test sample. Subchronic toxicity is defined as adverse effects occurring after the repeated or continuous administration of a test sample for up to 90 days or not exceeding 10% of the animal’s lifespan. Acute toxicity appears within hours or days of an exposure, whereas chronic toxicity can take many months or years to become a recognizable clinical disease [7]. Patel et al. [8] performed studies on the acute and subchronic toxicities of a standardized pomegranate fruit extract containing 30% punicalagins (polyphenols with antioxidant activity). Punicalagins are responsible for over 50% of the antioxidant potential of pomegranate juice. The acute oral LD50 of the punicalagin-rich extract in rats and mice was >5000 mg/kg body weight, the intraperitoneal LD50 in rats and mice was 217 and 187 mg/kg body weight, respectively, and the subchronic level was 600 mg/kg body weight/day. Based on the above report, pomegranate extract is safe at the concentrations tested, and there was no significant toxicity according to clinical observations, body weights, ophthalmic examinations, feed consumption, body weight gains, clinical pathology evaluation, and organ weights. Another study testing pomegranate juice punicalagin used Sprague–Dawley rats and repeated oral administration of a 6% punicalagin-containing diet for 37 days. The administration did not cause tissue alterations and the serum biochemical and hematological parameters were normal [9].
Xanthigen, a nutraceutical preparation used for weight management, is rich in punicic acid from pomegranate seed and was screened to determine its genotoxicity and its 90-day repeated oral toxicity in Sprague–Dawley rats. No genotoxicity was exhibited [10]. Subchronic toxicity was evaluated for daily oral administrations of 250, 500, and 1000 mg/kg/day body weight, doses over 90 days. No deaths and no deleterious effects were observed during the 90-day treatment, indicating an absence of subchronic toxicity. While pomegranate juice has been shown to be nontoxic, pomegranate root and bark have been found to be toxic because of their alkaloid content [10].
In order to extend the life of pomegranate juice and its bioactive compounds, the juice can be dried to a powder. Spray-drying is widely used for drying substances and is also used to entrap active compounds by using a protective matrix to encapsulate them, thereby preventing bioactive compounds in the food from oxidation. The process, known as microencapsulation, produces small capsules and is a good method to provide oral dietary supplements. Flavors, lipids, oils, carotenoids, vitamins, etc., are encapsulated within a matrix of stable, plant products [11]. The matrix materials employed are among the most used and innocuous materials in foods: carbohydrate polymers, plant extracts, marine extracts, microbial- and animal-derived polysaccharides, proteins, lipids, as well as some rarely used other materials [12]. We previously described the microencapsulation process of compounds from pomegranate juice using maltodextrin and gum arabic as encapsulating agents [13]. This work aims to investigate the subacute and subchronic toxicity of microencapsulated pomegranate juice (MPJ) in rats and mice.
Materials and Methods
Microencapsulated pomegranate juice
We previously described the microencapsulation process of compounds from pomegranate arils [13], and in this work we followed the same procedure with slight modifications. Fruits were washed and peeled. The arils were removed and ground in a laboratory mill, then sieved to remove particles >0.5 mm. The resulting liquid was dried at 50°C. The dried material was ground again to obtain a fine powder and extracted with ethanol–water (1:1 v/v) for 2 h at room temperature. In order to remove small particles, the extract was then filtered through a glass microfiber filter paper. The filtered extract was evaporated using a rotary evaporator at 50°C to remove the ethanol. Maltodextrin–dextrose equivalent 16.5–19.5 (Amfher Foods, Mexico City, Mexico) and gum arabic (Sigma–Aldrich, St. Louis, MO) were used as coating materials, each was dispersed individually in water until reaching 10% solid content. The final solution of coating materials was prepared by combining the mixtures of maltodextrin and gum arabic at a 4:1 (v/v) ratio. The coating solution was combined with the aril extract and homogenized for 10 min at 60°C and 8000 rpm using a magnetic stirrer. Then, the homogenate was spray-dried in a B–191 Mini Spray-Dryer (Büchi, Flawil, Switzerland) and fed at room temperature with an inlet air temperature of 110°C and a pump flow of 600 ml/min. The microencapsulated powder was stored in darkness at room temperature until its use.
Proximate analyses of MPJ
The proximate composition of MPJ was determined in triplicate using standard methods [14]. The MPJ was dried in an oven at 100–105°C to determine moisture content. The crude protein content was determined by the micro-Kjeldahl method using 6.25 as the nitrogen conversion factor. The crude fat content of MPJ was determined by Soxhlet extraction. Ash content was determined by incinerating the samples at 600°C in a muffle furnace. Total carbohydrate content was calculated by difference after determining all other components of the proximate composition according to the following formula:
 |
Energy was calculated using the Atwater conversion factors of 4, 4, and 9 kcal/g for protein, carbohydrate, and lipid, respectively.
Microbial analysis of MPJ
Samples (25 g) of MPJ were dissolved in 225 ml of buffered sodium chloride–peptone broth pH 7.0 (Sigma–Aldrich, St. Louis, MO) or in physiological saline solution 0.85% (Sigma–Aldrich St. Louis, MO). The presence of the following microorganisms was determined: mesophilic aerobic bacteria [15], yeasts and molds [16], coagulase-positive staphylococci [17], Escherichia coli [18], Salmonella spp. [19].
Experimental animals and housing conditions
Animal management was supervised by a veterinarian in accordance with the principles set forth in the National Institutes of Health guide for the care and use of laboratory animals [20] and approved by the Animal Care Committee of the Institute of Health Sciences, Autonomous University of the State of Hidalgo (UAEH).
Male Wistar rats and male CD-1 mice were provided by the animal facility of UAEH. The rats were young, 6–7 weeks old. Body weight ranged from 120 to 180 g at the beginning of the test. The mice were also young, 9–10 weeks old, and body weights ranged from 32 to 44 g. All animals were acclimatized for 14 days and were housed in standard cages and under controlled conditions with a 12 h light/12 h dark cycle, constant temperature (22 ± 2 °C), relative humidity of 50%, and fed a standard rodent pellet diet (Harlan Teklad, Madison, WI) and provided water ad libitum throughout the period of the experiment.
Subacute oral toxicity study of MPJ in rats
Twelve young Wistar albino male rats were divided into four groups of three, so that weight differences did not exceed ±20% of the average body weight among groups. The procedures were consistent with Organisation for Economic Co-operation and Development (OECD) Test Guideline 425 [21]. Each group was administered treatments in 4 ml distilled water daily for 14 days by oral gavage: 4 ml of water (control), 1600 mg MPJ/kg body weight, 2900 mg MPJ/kg body weight, or 5000 mg MPJ/kg body weight. After administration, each animal was observed daily for mortality, clinical signs (diarrhea, loss of appetite, aggression, convulsions, tremors, restlessness, loss of hair, piloerection, hyperactivity), and gross lesions in organs (liver, kidney, heart, spleen, lung, stomach, pancreas, and digestive tract). Weight was recorded for each rat at 0, 7, and 14 days to the nearest 0.1 g. The assay was conducted in duplicate.
Subchronic oral toxicity studies of MPJ in mice
The young–adult male CD-1 mice were divided into two groups consisting of six mice each, so that the weight differences between groups did not exceed ±20% of the average body weight. The control group and the experimental group were administered on a daily basis by oral gavage for 90 days as follows: the control group received 2 ml/kg/day of distilled water and the experimental group received 3000 mg/kg/day of MPJ dissolved in 2 ml of distilled water. After administration, each animal was observed daily for mortality, clinical signs, and gross lesions. Individual weights were obtained for all mice at 0, 60, and 120 days.
Histologic examination
To determine the effects of MPJ on the liver and kidney, histological examinations were conducted. The animals were sacrificed and organs from all animals were extracted, rinsed, weighed, and preserved in 10% formol–saline before they were completely dehydrated in absolute ethanol. The organs were then embedded in paraffin blocks and sliced into 5-μm-thick sections and stained with hematoxylin and eosin. The stained organs were observed for possible histological changes under a light microscope (Olympus, Center Valley, PA).
Cytotoxicity assay
The cytotoxicity of MPJ was evaluated by the microcolorimetric Alamar blue assay. Alamar blue redox dye (Sigma-Aldrich, Waltham, MA) is an indicator of viability and/or cellular growth. The oxidized form is blue and nonfluorescent and upon reduction, becomes pink and fluorescent [22]. The assay was performed with the epithelial cell line A549 obtained from the American Type Culture Collection (ATTC CCL-185), which was grown in F12 culture medium supplemented with 10% fetal bovine serum, gentamycin (25 mg/ml), and penicillin (50,000 U/ml) at 37°C in an atmosphere of 5% CO2. Monolayers of epithelial cells were prepared in a 48-well plate with 2 × 105 cells in each well. Monolayers were washed twice with Hank’s Balanced Saline Solution (Sigma–Aldrich, St. Louis, MO), and different amounts of MPJ were added to provide concentrations of 0, 6.25, 12.5, 25, 50, 100, and 200 μg/ml. The doses were selected based on its main biological activities described in the literature [23–25]. As a cytotoxicity control, a set of cells was treated with 80 μg/ml ursolic acid, which is toxic to the cells [26]. The monolayers were incubated for 24 h at 37°C in 5% CO2. Then, 50 μl of the Alamar blue solution (AbD Serotec, Oxford, UK) was added to each well and incubated until the cells of the viability control (0 MPJ) developed a fluorescent pink color. Fluorescence was then quantified using an excitation wavelength of 530 nm and measuring emission at 590 nm in a SpectraMax M3 microplate reader (Molecular Devices, San Jose, CA).
Data analysis
Data were expressed as means ± standard deviation (SD). Normality was verified with the Shapiro–Wilk test. Differences between mean among the groups were compared by one-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test with P < 0.05 significance level, using Prism version 8.4 (GraphPad Software, San Diego, CA).
Results and Discussion
Proximate composition of MPJ
The proximate composition of MPJ is shown in Table 1. The low moisture level reduces the possibility of decomposition of compounds allowing the contents a long lifespan. The high carbohydrate content is primarily due to the maltodextrin and gum arabic from the coating materials in the microencapsulation. The high caloric content (394.05 kcal/100 g) must be considered for certain patients. As expected, MPJ contains almost no fiber because the product was made from juice and not from peel or seed where fiber is mainly found [27].
Table 1.
proximate composition of MPJ per 100 g dry weight (DW/DW)
| Parameter | Composition (%) ± SD |
|---|---|
| Moisture | 1.85 ± 0.01 |
| Ash | 1.08 ± 0.04 |
| Protein | 1.29 ± 0.05 |
| Fat | 1.17 ± 0.02 |
| Fiber | 0.01 ± 0.01 |
| Carbohydrates | 94.59 ± 0.06 |
| Energy (kcal) | 394.05 |
Values are the mean ± SD of triplicate measurements.
Microbial analysis of MPJ
The microbial counts of MPJ are shown in Table 2. The results show that MPJ has a low total microbial load and, most importantly, an absence of the three pathogenic microorganisms that are commonly an issue in food preparation [28]. This demonstrates that the processing and storage conditions of MPJ make it a safe product in terms of not being a carrier of unwanted pathogenic microorganisms.
Table 2.
microbial content of MPJ
| Microorganisms | cfu/g |
|---|---|
| Mesophilic aerobic bacteria | <10 |
| Yeasts and molds | <10 |
| Escherichia coli | ND |
| Coagulase-positive staphylococci | ND |
| Salmonella spp. | ND |
cfu: colony-forming units; ND: not detected.
Body weight gain
As expected for young, growing rats, body weight gain increased during the course of the test. Notably, these weight gains (Fig. 1A) were lower in the groups that received the higher doses (2900 and 5000 mg/kg) of MPJ. MPJ also caused a decrease in weight body gain in mice (Fig. 1B). We have previously reported that CD-1 mice supplemented daily with pomegranate juice showed lower body weights when compared with an unsupplemented group [4]. Pomegranate has been found to have antiobesity properties [29]. McFarlin et al., [30] demonstrated that pomegranate seed oil consumption during a period of high-fat feeding reduced weight gain in CD-1 mice. Similarly, Vroegrijk et al., [31] showed that male C57Bl/J6 mice fed with 1 g of pomegranate seed oil per 100 g of a high-fat diet for 12 weeks decreased body weight and fat mass. This corroboration that pomegranate components reduce weight gain could have potential value to human populations trying to lose weight. A potential explanation for the weight loss is the inhibition of pancreatic lipase activity by pomegranate juice constituents. The inhibition reduces the uptake of food energy from the intestine [32]. Also, it has been suggested that tannins (including ellagitannins) interact with food proteins and inhibit their digestion, thus resulting in weight loss [33]. In both cases, constituents of pomegranate juice caused more of the food that is ingested to remain unabsorbed into the body.
Figure 1.
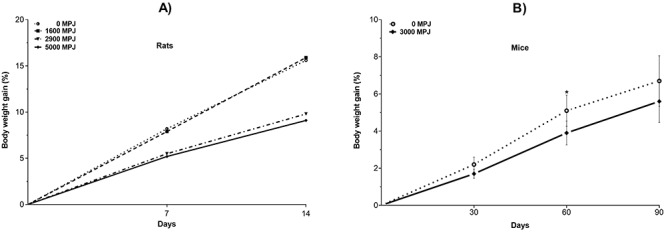
Depression of the mean body weight gain by administration of MPJ. (A) Effect of daily subacute oral administration of MPJ on body weight gain in Wistar rats. (B) Effect of daily subchronic oral administration of 3000 mg of MPJ on body weight gain in CD-1 mice. Body weight (BW) gain (%) = [(BW day N - BW day 0)/BW day 0] x 100. *significantly different at p < 0.05. Values are the mean ± standard deviation.
Subacute and subchronic oral toxicities in rats and mice, respectively
The subacute oral administration of three doses of MPJ, as much as 5000 mg/kg body weight, did not cause death in male Wistar rats, nor did it cause changes in clinical signs or gross lesions. Thus, MPJ can be considered to be nontoxic at this concentration by oral administration (Table 3). Likewise, subchronic oral administration with 3000 mg/kg/day of MPJ in CD-1 mice over the period of 90 days did not cause death, clinical changes, or organ damage (Table 4).
Table 3.
toxicity test of subacute oral administration over 14 day of different doses of MPJs in rats
| Dose of MPJ (mg/kg) | Survival | Mortality (%) | Clinical signsa | Gross lesionsb |
|---|---|---|---|---|
| 0 | 3/3 | 0 | None | None |
| 1600 | 3/3 | 0 | None | None |
| 2900 | 3/3 | 0 | None | None |
| 5000 | 3/3 | 0 | None | None |
aExamination was performed on (diarrhea, loss of appetite, aggression, convulsions, tremors, restlessness, loss of hair, piloerection, hyperactivity)
bThe gross lesion examination was performed on liver, kidney, heart, spleen, lung, stomach, pancreas and digestive tract
Table 4.
toxicity test of subchronic oral administration over 90 days of MPJ in mice
| MPJ (mg/kg) | Survival | Mortality (%) | Clinical signsa | Gross lesionsb |
|---|---|---|---|---|
| 0 | 6/6 | 0 | None | None |
| 3000 | 6/6 | 0 | None | None |
aClinical signal examination was performed on diarrhea, loss of appetite, aggression, convulsions, tremors, restlessness, loss of hair, piloerection, hyperactivity
bThe gross lesion examination was performed on liver, kidney, heart, spleen, lung, stomach, pancreas, and digestive tract
Several studies have looked for toxic effects of pomegranate. Punicalagin and punicalin are hydrolyzable tannins and the most abundant antioxidant in pomegranate juice as well as having the strongest antioxidant activities of this group of tannins. Patel et al., [8] reported no significant adverse effect in Wistar rats gavage administered with pomegranate extract containing 30% punicalagin at levels up to 600 mg/kg/day for 90 days. They evaluated several parameters, including biochemical analyses and histological examinations. Cerdá et al., [9] showed that a repeated oral administration of a 6% punicalagin-containing diet for 37 days was not toxic to Sprague–Dawley rats. This dose is about 25 times more than that used in the Patel study.
In another study [34], subcutaneous injection of punicalagin at doses of 12.5 mg/kg of body weight protected male Wistar rats against liver damage induced by carbon tetrachloride administration. Nevertheless, a larger dose (25 mg/kg) of punicalin induced liver damage in the rats.
Nutritional formulations containing significant amounts of pomegranate components have also been found to be safe in subchronic toxicity studies. Fitnox, a product for physical endurance, containing 25–30% pomegranate peel extract, was orally administered to Wistar rats at 1000 mg/kg daily for 90 days [35], and Xhantigen, a product for weight management, containing pomegranate seed oil that is rich in punicic acid, was orally administered daily to Sprague–Dawley rats at 250, 500, and 1000 mg/kg for 90 days [10]. In both studies, no deaths and no deleterious effects were observed during the 90-day treatment.
The high carbohydrate content of MPJ may not be suitable for patients with certain physiological conditions, e.g. patients with type 2 diabetes. There were no significant (P > 0.05) differences in the glucose levels between groups throughout the test, as determined by one-way ANOVA (Fig. 2) and blood glucose levels were lower at each data point for the MPJ treated mice, but they did not reach statistical significance. Previously we reported that daily consumption of pomegranate juice for 4 months reduced blood glucose levels in streptozotocin-induced diabetic mice [36]. The results are particularly interesting because it appears that the sugars contained in pomegranate juice do not worsen overall blood sugar levels. Nevertheless, a peak postprandial glucose level could occur resulting in larger postprandial blood glucose excursions, and further studies should be conducted to examine this.
Figure 2.
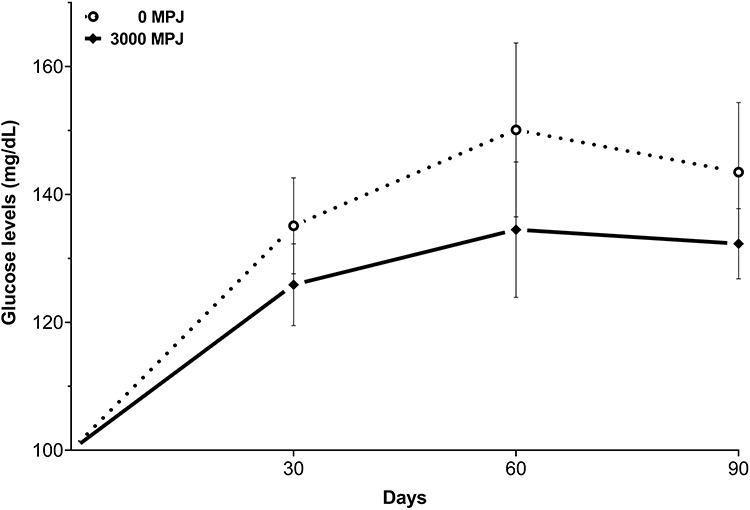
Effect of MPJ on blood glucose levels in mice over 90 days. Values are the mean ± standard deviation.
Histologic analysis
To corroborate the lack of toxicity in both the liver and kidney from mice, a microscopic examination of biological tissues was also carried out. Histopathological examination did not reveal any abnormalities of the liver or kidney in either the control group or the group administered with MPJ throughout the 120-day intervention period (Fig. 3).
Figure 3.
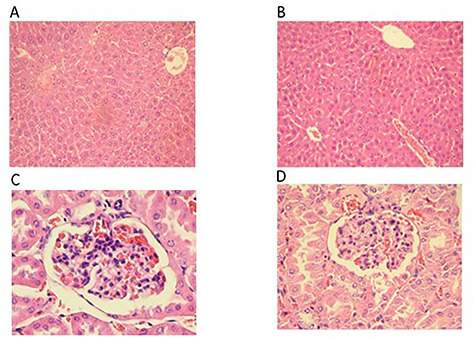
Histological sections of the liver and kidney of CD-1 mice administered orally with 3000 mg/kg MPJ daily for 120 days. Sections were stained with hematoxylin and eosin. (A) 10X liver section of control group (B) 10X liver section of group administered with 3000 mg/kg MPJ. Both sections show a normal histological structure of hepatocytes showing the central veins. (C) 40X kidney section of control group. (D) 40X kidney section of group administered with 3000 mg/kg MPJ. Both sections show normal structures for Bowmen's capsule, glomerulus, and proximal tubule.
Cytotoxicity assay
The cytotoxicity of MPJ was evaluated by the Alamar blue assay in an epithelial cell model. The treatment with ursolic acid, used as a death control, decreased cell viability by >90%. On the other hand, MPJ did not decrease cell viability with viability over 80% at all concentrations (Fig. 4). Cytotoxicity can be expressed as a short-term loss of cell viability. According to the US National Cancer Institute criteria, MPJ can be considered to be inactive at the concentrations used in this work since no cytotoxicity half maximal inhibitory concentration (IC50) was identified [37].
Figure 4.
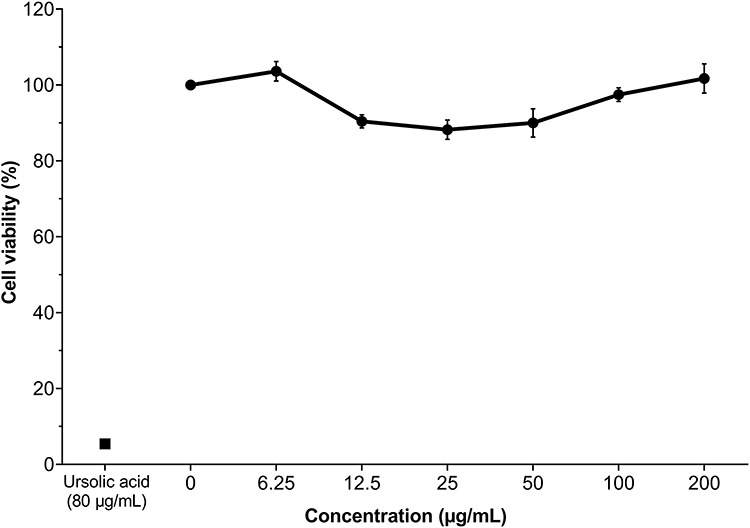
Viability (%) of epithelial cells treated with MPJ at different concentrations. Assay was by microcolorimetric Alamar blue after 24 h. All values are expressed as mean ± standard deviation (n=3).
Conclusion
Overall, the results provide evidence for the safety of MPJ for both nutraceutical and dietary supplements.
Acknowledgements
We thank the society of pomegranate producers, ‘El Oasis de Tasquillo’ and the Maguey Blanco community of Hidalgo for the collected pomegranates. We thank Héctor Enrique Fabela Illescas for the electronic. We also thank Dr. Pedro L. Flores-Chávez for your technical assistance.
Contributor Information
Pedro Álvarez-Cervantes, Área Académica de Nutrición, Instituto de Ciencias de la Salud, Universidad Autónoma del Estado de Hidalgo, Pachuca 42160, Mexico.
Jeannett A Izquierdo-Vega, Medicina, Instituto de Ciencias de la Salud, Universidad Autónoma del Estado de Hidalgo, Pachuca 42160, Mexico.
José Morán-León, Instituto de Artes, Universidad Autónoma del Estado de Hidalgo, Mineral del Monte, Hidalgo 42130, Mexico.
José A Guerrero-Solano, Área Académica de Enfermería, Instituto de Ciencias de la Salud, Universidad Autónoma del Estado de Hidalgo, Pachuca 42160, Mexico.
Blanca E García-Pérez, Departamento de Microbiología, Escuela Nacional de Ciencias Biológicas del Instituto Politécnico Nacional, Mexico City 11340, Mexico.
Juan C Cancino-Díaz, Departamento de Microbiología, Escuela Nacional de Ciencias Biológicas del Instituto Politécnico Nacional, Mexico City 11340, Mexico.
Helen Belefant-Miller, Dale Bumpers National Rice Research Center, Stuttgart, AR 72160, USA.
Gabriel Betanzos-Cabrera, Área Académica de Nutrición, Instituto de Ciencias de la Salud, Universidad Autónoma del Estado de Hidalgo, Pachuca 42160, Mexico; Medicina, Instituto de Ciencias de la Salud, Universidad Autónoma del Estado de Hidalgo, Pachuca 42160, Mexico.
Conflict of interest
The authors declare they have no conflict of interests regarding the publication of this paper.
Funding
Research funded by Consejo Nacional de Ciencia y Tecnología CONACYT-PDC Problemas Nacionales PDCPN2013-01. Problemas Nacionales (PDCPN2013-01).
References
- 1. Haldane C. Direct evidence for organic cargoes in the late bronze age. World Archaeol 1993;24:348–60. [Google Scholar]
- 2. Kandylis P, Kokkinomagoulos E. Food applications and potential health benefits of pomegranate and its derivatives. Foods 2020;9:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Betanzos-Cabrera G, Montes-Rubio PY, Fabela-Illescas HEet al. Antibacterial activity of fresh pomegranate juice against clinical strains of staphylococcus Epidermidis. Food Nutr Res 2015;59:27620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Estrada-Luna D, Martínez-Hinojosa E, Cancino-Diaz JCet al. Daily supplementation with fresh pomegranate juice increases paraoxonase 1 expression and activity in mice fed a high-fat diet. Eur J Nutr 2018;57:383–9. [DOI] [PubMed] [Google Scholar]
- 5. Fahmy H, Hegazi N, El-Shamy Set al. Pomegranate juice as a functional food: a comprehensive review of its polyphenols, therapeutic merits, and recent patents. Food Funct 2020;11:5768–81. [DOI] [PubMed] [Google Scholar]
- 6. Guerrero-Solano JA, Jaramillo-Morales OA, Velázquez-González Cet al. Pomegranate as a potential alternative of pain management: a review. Plants (Basel) 2020;9:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chinedu E, Arome D, Solomon Ameh Fet al. An approach to acute, subacute, subchronic, and chronic toxicity assessment in animal models. Toxicology International 2015;22:83–7.26862266 [Google Scholar]
- 8. Patel C, Dadhaniya P, Hingorani Let al. Safety assessment of pomegranate fruit extract: acute and subchronic toxicity studies. Food Chem Toxicol 2008;46:2728–35. [DOI] [PubMed] [Google Scholar]
- 9. Cerdá B, Cerón JJ, Tomás-Barberán FAet al. Repeated oral Administration of High Doses of the pomegranate ellagitannin punicalagin to rats for 37 days is not toxic. J Agric Food Chem 2003;51:3493–501. [DOI] [PubMed] [Google Scholar]
- 10. López-Rios L, Vega T, Chirino Ret al. Toxicological assessment of xanthigen ® Nutraceutical extract combination: mutagenicity, Genotoxicity and oral toxicity. Toxicol Rep 2018;5:1021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gharsallaoui A, Roudaut G, Chambin Oet al. Applications of spray-drying in microencapsulation of food ingredients: an overview. Food Res Int 2007;40:1107–21. [Google Scholar]
- 12. Shahidi F, Han XQ. Encapsulation of food ingredients. Crit Rev Food Sci Nutr 1993;33:501–47. [DOI] [PubMed] [Google Scholar]
- 13. Estrada-Luna D, Carreón-Torres E, Bautista-Pérez Ret al. Microencapsulated pomegranate reverts high-density lipoprotein (HDL)-induced endothelial dysfunction and reduces postprandial triglyceridemia in women with acute coronary syndrome. Nutrients 2019;11:1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horwitz W, Latimer GW. Association of Official Analytical Chemists International. Gaithersburg, MD: Official Methods of Analysis of AOAC International, AOAC International, 2006. [Google Scholar]
- 15. ISO Microbiology . General guidance for the enumeration of microorganisms. Colony Count Technique at 30 degrees C. ISO 4833:1991. https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/01/08/10816.html (accessed on 18 November 2020).
- 16. ISO Microbiology of food and animal feeding stuffs . Horizontal method for the enumeration of yeasts and moulds — Part 2: Colony count technique in products with water activity less than or equal to 0,95. ISO 21527-2:2008https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/03/82/38276.html (accessed on 18 November 2020).
- 17. ISO Microbiology of food and animal feeding stuffs . Horizontal method for the enumeration of coagulase-positive staphylococci (Staphylococcus aureus and other species) — Part 2: Technique using rabbit plasma fibrinogen agar medium. ISO 6888-2:1999. https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/02/55/25571.html (accessed on 18 November 2020).
- 18. ISO Microbiology of food and animal feeding stuffs . Horizontal method for the detection and enumeration of presumptive Escherichia coli—Most probable number technique. ISO 7251:2005. https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/03/45/34568.html (accessed on 18 November 2020).
- 19. ISO Microbiology of food and animal feeding stuffs . Horizontal method for the detection of Salmonella spp. ISO 6579. 2002. https://www.iso.org/obp/ui/#iso:std:iso:6579 (accessed on 18 November 2020).
- 20. National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals . Guide for the Care and Use of Laboratory Animals, 8th ed., The National Academies Collection: Reports funded by National Institutes of Health, National Academies Press (US): Washington (DC), 2011. [Google Scholar]
- 21. OECD Test Guideline 425: Acute Oral Toxicity-Up-and-Down-Procedure. 2008, 27. https://www.oecd-ilibrary.org/environment/test-no-425-acute-oral-toxicity-up-and-down-procedure_9789264071049-en (accessed on 2 December 2020).
- 22. Ahmed SA, Gogal RM, Walsh JE. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J Immunol Methods 1994;170:211–24. [DOI] [PubMed] [Google Scholar]
- 23. BenSaad LA, Kim KH, Quah CCet al. Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum. BMC Complement Altern Med 2017;17:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Navarro M, Amigo-Benavent M, Mesias Met al. An aqueous pomegranate seed extract ameliorates oxidative stress of human hepatoma HepG2 cells. J Sci Food Agric 2014;94:1622–7. [DOI] [PubMed] [Google Scholar]
- 25. Seeram NP, Adams LS, Henning SMet al. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem 2005;16:360–7. [DOI] [PubMed] [Google Scholar]
- 26. Castrejón-Jiménez NS, Leyva-Paredes K, Baltierra-Uribe SLet al. Ursolic and oleanolic acids induce Mitophagy in A549 human lung cancer cells. Molecules 2019;24:3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rowayshed G, Salama A, Abul-Fad Met al. Nutritional and chemical evaluation for pomegranate (Punica granatum L.) fruit peel and seeds powders by products. Middle East J Appl 2013;2:169–79. [Google Scholar]
- 28. Kolaček S, Hojsak I, Berni Canani Ret al. Commercial probiotic products: a call for improved quality control. A position paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr 2017;65:117–24. [DOI] [PubMed] [Google Scholar]
- 29. Al-Muammar MN, Khan F. Obesity: the preventive role of the pomegranate (Punica granatum). Nutrition 2012;28:595–604. [DOI] [PubMed] [Google Scholar]
- 30. McFarlin BK, Strohacker KA, Kueht ML. Pomegranate seed oil consumption during a period of high-fat feeding reduces weight gain and reduces type 2 diabetes risk in CD-1 mice. Br J Nutr 2009;102:54–9. [DOI] [PubMed] [Google Scholar]
- 31. Vroegrijk IOCM, Diepen JA, Berg Set al. Pomegranate seed oil, a rich source of Punicic acid, prevents diet-induced obesity and insulin resistance in mice. Food Chem Toxicol 2011;49:1426–30. [DOI] [PubMed] [Google Scholar]
- 32. Lei F, Zhang XN, Wang Wet al. Evidence of anti-obesity effects of the pomegranate leaf extract in high-fat diet induced obese mice. Int J Obes (Lond) 2007;31:1023–9. [DOI] [PubMed] [Google Scholar]
- 33. Butler, L. G., Rogler, J. C.. Biochemical mechanisms of the antinutritional effects of tannins. In Phenolic Compounds in Food and Their Effects on Health I. ACS Symposium Series, 1992, Vol. 506, Washington DC, USA: American Chemical Society, pp. 298–304. doi: 10.1021AQ12/bk-1992-0506.ch023. [DOI] [Google Scholar]
- 34. Lin CC, Hsu YF, Lin TCet al. Antioxidant and hepatoprotective activity of punicalagin and punicalin on carbon tetrachloride-induced liver damage in rats. J Pharm Pharmacol 1998;50:789–94. [DOI] [PubMed] [Google Scholar]
- 35. Jacob J, Amalraj A, Divya Cet al. Oral toxicity study of sports nutritional powder in Wistar rats: a 90 day repeated dose study. Toxicol Rep 2018;5:497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Betanzos-Cabrera G, Guerrero-Solano JA, Martínez-Pérez MMet al. Pomegranate juice increases levels of Paraoxonase1 (PON1) expression and enzymatic activity in streptozotocin-induced diabetic mice fed with a high-fat diet. Food Res Int 2011;44:1381–5. [Google Scholar]
- 37. Nordin NL, Kadir AA, Zakaria ZAet al. In vitro investigation of cytotoxic and antioxidative activities of Ardisia crispa against breast cancer cell lines, MCF-7 and MDA-MB-231. BMC Complement Altern Med 2018;18:87. [DOI] [PMC free article] [PubMed] [Google Scholar]


