Abstract
Arsenic (As) is a ubiquitous environmental and industrial toxin with known correlates of oxidative stress and cognitive deficits in the brain. Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcriptional factor that represents a central cellular antioxidant defense mechanism and transcribes many antioxidant genes. Peroxisome proliferator-activated receptor-gamma (PPARγ) is a well-known nuclear receptor to regulate lipid metabolism in many tissues, and it has been also associated with the control of oxidative stress, neuronal death, neurogenesis and differentiation. The role of Nrf2 and PPARγ in As-induced neurotoxicity is still debated. The present study was designed to investigate the neurobehavioral toxic effect of sub-chronic and middle-dose sodium arsenite exposure in mice hippocampus, as well as the response of Nrf2/PPARγ expression and influence on protein expression levels of their downstream antioxidant genes. Our results showed that mice treated with intraperitoneal injection of sodium arsenite (50 mg/kg body wt.) twice a week for 7 weeks resulted in increased generation of reactive oxygen species and impairment of spatial cognitive function. The present study also found a positive association between Nrf2/PPARγ expression in hippocampus of mice, and activation of antioxidant defenses by the evidently upregulated expression of their downstream genes, including superoxide dismutase, heme oxygenase-1 and glutathione peroxidase-3. Therefore, our findings were helpful for further understanding the role of Nrf2/PPARγ feedback loop in As-induced neurobehavioral toxicity.
Keywords: arsenic, oxidative stress, Nrf2, PPARγ, neurobehavioral alterations
Introduction
Arsenic (As) is a widespread environmental and industrial toxicant that found in groundwater, food, dust and ambient air. Exposure to As is associated with deleterious adverse effects that include different types of cancer, skin lesions, cardiovascular disorders, liver injury, renal damage and gastrointestinal effects. Moreover, neurobehavioral alterations are mainly noxious effects of As on the central nervous system [1, 2]. Epidemiological reports have revealed that early exposure to As from drinking water or industrial sources could result in several behavioral deficits, cognitive dysfunction and intellectual loss in preschool and school-age children [3]. Data from animal studies have been congruent and support epidemiological evidence of As-induced impaired cognition. Especially those behavioral studies evaluating hippocampal-dependent learning tasks in rodents have shown that chronic and acute exposure to As induces behavioral deficits such as impaired spatial, long- and short-term learning and memory [4]. Although there are many epidemiological and toxicological studies that indicate the neurobehavioral toxicity of As, this topic is still evolving.
Inorganic arsenic (iAs) can be easily transported cross the blood–brain barrier (BBB) and accumulate in the brain. Once inside the brain, several metabolic pathways of iAs could induce a number of free radicals, including superoxide anion, hydroxyl radical, hydrogen peroxide, singlet oxygen and peroxyl radicals [5]. Enzymatic and nonenzymatic antioxidants in body could balance the homeostatic condition by quenching these reactive oxygen species (ROS) [6, 7]. However, increasing iAs exposure with time, excess concentration of As could lead to ROS overproduction in the brain and break antioxidative defense systems in cells, thereby resulting in oxidative stress within subcellular compartments. Accordingly, it has been reported that the high-level of ROS could cause remarkably oxidative damage in hippocampus neurons, which is associated with deterioration of axons and myelin sheath, synapse formation loss and vacuolar degeneration [8]. Thus, oxidative stress seems to play key role in As-induced neuropathology damage and cognitive impairment [9].
The antioxidant transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) is considered as a protective factor by reducing oxidative stress. Under oxidative stress, Nrf2 gets separated from Kelch-like ECH-associated protein 1 (KEAP1) complex formation and transfers into nucleus, binding with small Maf protein and antioxidant response elements that can regulate many antioxidant genes, including NAD(P)H quinone oxidoreductase-1, heme oxygenase-1 (HO-1), glutathione S-transferase and superoxide dismutase (SOD), et al. [10]. In addition, peroxisome proliferator-activated receptor gamma (PPARγ), a well-known nuclear hormone receptor with central roles in carbohydrate metabolism, adipogenesis and cell differentiation, also has been responsible for antioxidative stress through transcriptional activation of several antioxidant defense enzymes such as HO-1, catalase (CAT), glutathione peroxidase-3 (GPx3), et al [11, 12].
Several studies have strongly suggested existence of reciprocal regulation of Nrf2 and PPARγ pathways to reinforce the expression of one another against oxidative stress. For example, Lijuan Zhan et al. reported that silencing KEAP1 lead to elevate induction of Nrf2 and PPARγ in response to As2O3 in non-small-cell lung carcinoma cells [13]. Knockdown of Nrf2 in human keratinocytes significantly increased the sensitivity to acute cytotoxicity of iAs [14]. Furthermore, PPARγ agonists exert potent antioxidant properties by increasing Nrf2 expression dependently from PPARγ [15, 16]. In this sense, Nrf2 and PPARγ pathways seem to be connected by a positive feedback loop against As toxicity. However, some adverse studies shown that a significant down-regulation was found in cardiac Nrf2 and PPAR-γ mRNA expression in As-treated Sprague–Dawley rats compared with control group after a sub-chronic exposure to a dose of orally 5 mg/kg sodium arsenite [17]. Similar phenomenon was found in the fetal cerebrum of pregnant Wistar rats administered with 5 and 10 mg/kg As2O3 for 20 days [18]. Accordingly, different response of Nrf2 and PPARγ feedback loop to As exposure is linked to the type of subjects and tissues, as well as exposure time and dose ofAs.
However, until now, few studies are available in the literature reporting the changes of Nrf2 and PPARγ expression associated with As-induced oxidative stress in hippocampus. Therefore, the aim of this study was planned to explore the neurobehavioral toxic effect of sub-chronic and middle-dose sodium arsenite exposure in mice hippocampus, as well as the response of Nrf2/PPARγ expression.
Materials and Methods
Materials and reagents
Sodium arsenite (NaAsO2) was purchased from Sigma-Aldrich Co. (San Francisco, USA). SOD and ROS kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Sodium hydroxide (NaOH), hydrochloric acid (HCl) and potassium borohydride (KBH4) used for determination of total As level in brain were purchased from Shanghai Chemical Co. (Shanghai, China) with As free (<0.01 mg/L). As standard solution in 5% HNO3 (100 μg/ml) was purchased from Guobiao (Beijing) Testing & Certification Co. (GNM-SAS-002-2013). Other chemicals and reagents of analytical grade were purchased from commercial supplies.
Animals and treatments
A total of 16 4-week-old male Kunming mice were obtained from the Animal Center of Gannan Medical University (Animals production license: SCXK [Gan] 2014-0001). The mice were allowed to acclimatize to the facilities for 1 week under constant conditions (12-hour light–dark cycle at 25 ± 1°C and 50-60% relative humidity) with water ad libitum and standard pelleted rodent chow. After acclimatization, all mice were randomly assigned to two groups by weight: the experimental group was administered NaAsO2 (50 mg/kg body wt.) twice a week by intraperitoneal route for 7 weeks, and the control group was given corresponding body weight dose of purified water at the same exposure time. After that, all mice were exposed to a training session in Morris water maze (MWM; 4 days) and probe trail for long-term memory assessment. General growth throughout the experiment was observed, and body weight was measured on every week. A total of 24 h later, the mice were decapitated under anesthesia, half of median sagittal sections of brain sample was taken and stored at −80°C for determination of As content. The hippocampus was immediately stripped from the rest of brain sample on ice for biochemical and pathological analyses. The schematic representation of the experimental procedure was presented in Fig. 1. All procedures involving animal were approved by the Gannan Medical University Institutional Animal Care and Use Committee, and the whole study was approved by the Ethnic Committee of Gannan Medical University.
Figure 1.
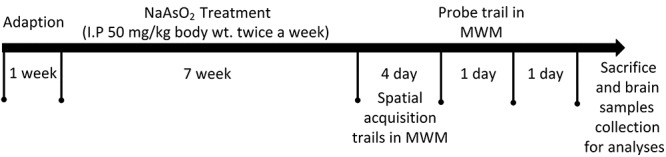
Schematic representation of the experimental procedure. IP, intraperitoneal injection.
Morris water maze test
The MWM test was used to assess learning and spatial memory abilities of mice after As exposure. The MWM consisted of a large circular pool (120 cm in diameter, 50 cm in height and filled to a depth of 28 cm with water at 23 ± 2°C) and a circular platform (12 cm in diameter and 0.5 cm above the water surface). The pool was randomly divided into four equal quadrants, and the platform was randomly placed in the center of a quadrant and remained stationary throughout the test. The water was made opaque with an innoxious black ink, and there were four different geometric figures sticked on the black curtain. As the mice gently putted into pool with its face facing the wall of the pool, the researcher left quietly and immediately. The MWM test comprised 4 days of training trial and a probe trial on the fifthday.
During training trial, cumulative distance, the time spent to find the hidden platform [escape latency (EL)], and their tracks in the platform for each mice were recorded by video tracking software EthoVision XT (Noldus, The Netherlands). Before the training trial, the mice were allowed to explore the pool for 120 seconds. If the mice found the platform within 60 seconds, allowed them to stay on it for 5 seconds; however, if it failed, the mice were guided gently to the platform and stayed for 20 seconds. From day 1 of the training trial, the platform was hidden 2 cm beneath the water surface and the cut-off time was set 60 seconds. The mice were given four trial sessions each day for four consecutive days entering the pool from four different quadrants, with an inter-trial interval of 20 minutes.
In a probe trial, platform was removed from the pool, and mice were exposed to the water maze by placing in the novel quadrant (opposite to the initial platform location). Each mouse was allowed to swim freely for 60s. The time spent in the target quadrant (where platform was earlier located) and the time annulus crossing the platform were recorded.
Total As contents in brain
The total As contents in brain of mice were tested using hydride generation atomic fluorescence spectrometry, operate according to a national standardized method in China (GB/T 5009.11-2014). Brain samples were dried at 60°C for 3 h after washed with PBS once, then weighed accurately and digested with 4 ml of concentrated nitric acid and 1 ml of H2O2 in the microwave digestion system (MARS 6, CEM Co., USA). Before measurement, 1.25 ml of 100 g/L mixture (thiourea and ascorbic acid) and 0.25 ml of concentrated hydrochloric acid was added to digested samples, then diluted to 25 ml and reacted for 30 minutes. A hydride atomic fluorescence spectrometer (AFS-8230, Beijing Titan Instruments, China) was used for detecting total As levels according to operating instructions.
Estimation of SOD activity and ROS generation in hippocampus
Oxidative stress was evaluated by assessing the amount of ROS generation in hippocampus. The level of ROS generation was assessed using 2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA) fluorescent probe kits. For the procedure, single-cell suspension was prepared from 1 mm3 hippocampus tissues by using 0.5% trypsin digestion procedure. The digested solution was filtered through 100-μm nylon mesh. Then, the cell suspension was resuspended with DCFH-DA (20 μM) solution and adjusted the number of cells to 1 × 106, and incubated in the dark at 37°C for 30 minutes. After incubation, the cells were washed twice with PBS and resuspended with 200 μL PBS solution. Then, transported into black 96-well microplate and the fluorescence was recorded by Multimode Microplate Reader (PerkinElmer, USA) at excitation/emission (488/525 nm). The ROS generation was expressed as fluorescence/cells and assessed in triplicate for each sample.
The total activity of SOD was assayed according to xanthine oxidase method provided by colorimetric assay kit. Some hippocampus tissues were homogenized in cold saline and centrifuged at 3000 rpm/minute for 15 minutes. Supernatant was collected for SOD measurement, and protein concentration was determined by a BCA protein assay kit (Solarbio, PC0020). The assay used the xanthine/xanthine oxidase system to produce O2− anions, which reacted with 2-(4-iodophenyl)-3-(4-nitrophenol-5-phenlyltetrazolium chloride) to form a red formazan dye, and the absorbance at 550 nm was determined, where one unit of SOD was defined as the amount of SOD inhibiting the rate of reaction by 50% at 25°C. The activity of SOD was expressed as units per milligrams of protein (U/mg protein). These procedures were performed according to the manufacturer’s instructions.
Nissl staining
The paraffin sections of hippocampus were dehydrated with xylene twice (10 minutes/time), absolute ethanol twice (5 minutes/time), 80% alcohol (5 minutes), 70% alcohol (5 minutes) and distilled water (3 minutes) successively. Then, the sections were stained with 1% toluidine blue for 30 minutes and washed with distilled water. After color separation with 70% alcohol, the sections were dehydrated with 80% alcohol and 95% alcohol for 0.5 minutes, respectively. The sections were dehydrated again with absolute alcohol twice (2 minutes/time) and xylene twice (2 minutes/time), and sealed with Neutral Balsam (Solarbio).
Western blotting analysis
The total protein from the cerebral cortex and hippocampus was extracted by RIPA lysis buffer (contained 0.1 mM PMSF, Beyotime Biotechnology), and centrifuged at 12 000 g for 10 minutes at 4°C. With bovine serum albumin as the standard, the protein concentration of each sample was determined using BCA protein assay kit (Solarbio, PC0020). 20 ~ 30 μg of protein was loaded and resolved by electrophoresis on 10% sodium dodecyl sulfate (SDS) polyacrylamide gels based on the molecular weight of the target protein. Then, the target strip was transferred to polyvinylidene fluoride (PVDF) membrane (Merck Millipore, 0.2 μm, ISEQ00010). The blots were blocked with 5% nonfat dried milk in Tris-buffered saline with 0.05% Tween 20 (TBST) for 2 h at room temperature. Blots were then incubated with the primary antibodies of β-actin (1:5000, Abcam, ab8226), Nrf2 (1:1000, Proteintech, 16396), PPARγ (1:1000, Proteintech, 16643), HO-1 (1:1000, Proteintech, 27282) and GPx3 (1:1000, Abcam, ab256470) in primary antibody dilution buffer overnight at 4°C. After three times washing with PBST for 10 minutes each, the membrane was incubated HRP-conjugated AffiniPure goat antirabbit IgG (H + L) or antimouse IgG (H + L) (1:5000 ~ 10000, Abcam, ab6721 or ab6789) in TBST buffer at RT for 1 h. After three times washing with PBST buffer, target protein bands were visualized with an enhanced chemiluminescence kit and optical density was calculated using ImageJ software (Version: 1.52a).
Statistical analysis
Data were represented as mean ± SEM of at least three independent preparations. Repeated measure-analysis of variance was applied to analyze the effect of treatment × day interaction on data of the training trial. Average values of two groups were compared using Student’s t-test (two-tailed). All data were analyzed using the Statistical Package for the Social Sciences 14.0 (SPSS 14.0) software. The P < 0.05 values were considered significant.
Results
General growth and as concentrations in brain
During the period of exposure, there was no difference in eating, drinking, defecation and hair glossy between two groups, and was no mortality in both groups. But the exposed group exhibited restlessness, irritability, weight stagnation and different degrees of hair loose. In comparison to control group, the exposed group exhibited slow increase of body weight and appeared weight stagnation since the fifth week (Fig. 2A). In order to detect the accumulation of As in brain, the total As levels were measured. The total As contents in brain after NaAsO2 exposure for 7 weeks and 6 days withdrawal period in MWM test were significantly elevated as compared to the control. The contents have already reached 397.38 ± 63.33 ng/g in the exposure group, above two times higher compared to 180.29 ± 26.62 ng/g in the control (P < 0.01, Fig. 2B). This result further strengthened the evidence that As can pass through the BBB and accumulate in the brain.
Figure 2.
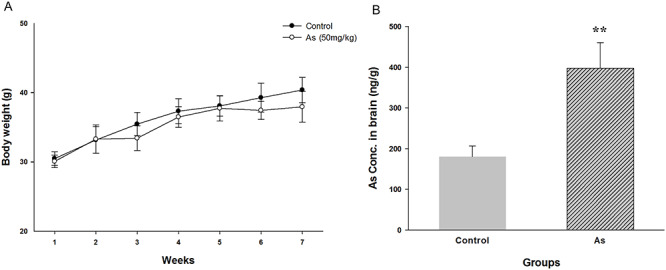
Changes of body weight throughout the experiment (A) and As concentrations in brain (B). Results are expressed as mean ± SEM (n = 8). **P < 0.01 when compared with control group.
The MWM test
Considering the significantly higher As concentration in brain, it might influence learning and memory abilities. Therefore, the MWM was conducted to investigate the ability of spatial learning and memory of mice. Throughout 4 days of training, the As-treated group had significant shorter cumulative distance (P < 0.05; Fig. 3A) and longer EL (P < 0.01; Fig. 3B). According to observation, the NaAsO2 exposed mice presented nonstrong will to swim and tended to linger on in the pool wall area at the start of the tests, which spent more time than that of control group before actually activated and started to swim around. These abnormal performances also explained the shorter cumulative distance and longer EL in exposure group. Moreover, significant effect of treatment × day interaction (F = 3.44, P < 0.05) was found, which revealed that the middle-dose of NaAsO2 treatment could significantly affect learning and memory abilities. The decline of EL in the control group signified normal learning and memory formation process. Short-term spatial reference memory data from the probe trial test shown that the time to first find the initial platform in exposed mice was much longer than that in the control group (P < 0.01, Fig. 3C). Compared with the control group, the exposed group showed less annulus crossing (P < 0.01, Fig. 3D) and spent less time in the target quadrant (P < 0.01, Fig. 3E). The mean heatmap of swimming tracks in each group also indicated that arsenite-exposed mice spent less time in the target quadrant and less annulus crossing platform than that of control group (Fig. 3F).
Figure 3.
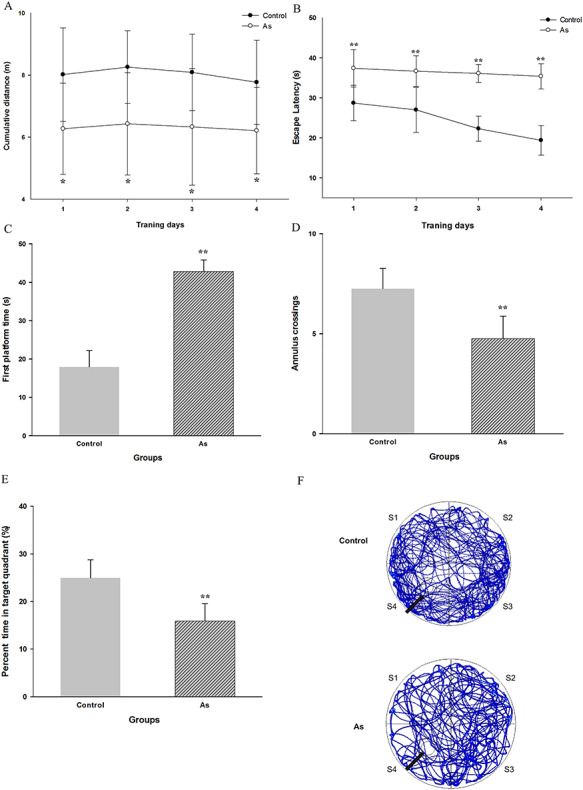
Date from Morris water maze test. This figure shows (A) cumulative distance, (B) EL during the training trial, (C) first touched platform time, (D) annulus crossing, (E) percent time in quadrant and (F) heatmap of swimming track in each group from the probe trial. S1–S4 represents four different quadrants, respectively. S4 is target quadrant. The black arrow indicates the position of removed previous platform. Results are expressed as mean ± SEM (n = 8). *P < 0.05, **P < 0.01 when compared with control group.
Effect of As exposure on oxidative level in hippocampus
The result of our MWM test suggested that NaAsO2 exposure reduced the spatial learning and memory ability. The hippocampus, a part of the limbic system that is responsible for spatial learning and memory [19]. Furthermore, numerous evidences indicated that the neurotoxicity of As is its ability to cause oxidative stress [20, 21]. So, the level of ROS generation was measured to evaluate the extent of oxidative stress imposed on the mice hippocampus by NaAsO2 exposure. As shown in Fig. 4A, the arsenite-induced oxidative stress was evident by the significant increased ROS measured in hippocampus of NaAsO2 exposed mice. Moreover, the enzyme SOD is considered to be the first line of cellular antioxidant defense. We detected that the SOD activity in hippocampus remarkably increased in response to the ROS production (P < 0.01; Fig. 4B).
Figure 4.
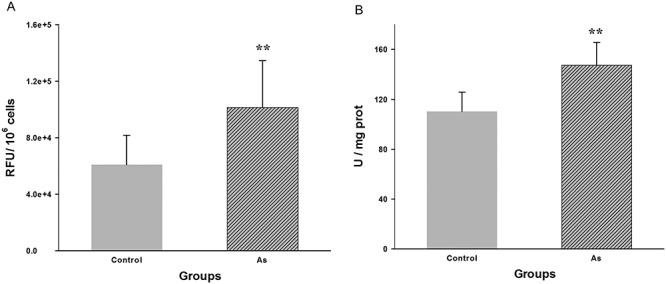
Comparison of mean level of ROS generation (A) and SOD activity (B) in hippocampus between NaAsO2-exposed and control group. Values are expressed as mean ± SEM (n = 5). **P < 0.01 when compared with control group.
Nissl staining results
Changes in neuron structure of hippocampus will cause learning and memory ability. So, we evaluated the morphological changes of pyramidal neurons in mice hippocampus by way of Nissl staining. As shown in Fig. 5A, pyramidal cells in the CA1 region of the control group were arranged orderly, intact with normal cytoarchitecture. However, the pyramidal cell layer was loosely distributed with certain amount of misalignment of the pyramidal cells in NaAsO2 exposed group of mice, whereas showed more degenerating neurons with smaller cell bodies and pyknotic nuclei (Fig. 5B).
Figure 5.
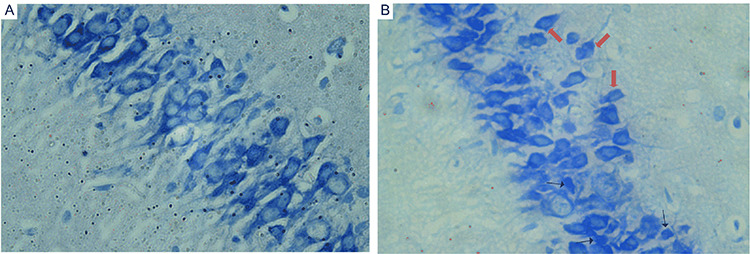
The morphological changes of coronal sections of CA1 in hippocampus were observed with light microscope after Nissl staining (magnification of ×400). (A) Control group and (B) NaAsO2 exposed group. Increased number of degenerating neurons with smaller cell bodies or pyknotic nuclei (black arrow) and ectopic pyramidal cells (red arrow) can be found in hippocampus of NaAsO2 exposedmice.
Influence of NaAsO2 exposure on protein expression levels of oxidative stress markers
Studies have reported that Nrf2 and PPARγ pathways are connected by a positive feedback loop, which play important roles in antioxidant defensive responses [22]. In this study, we found that sub-chronic and middle-dose NaAsO2 exposure could induce co-activate the expression of Nrf2 and PPARγ in hippocampus (Fig. 6). Furthermore, the level of HO-1, a response biomarker for As exposure in various types of cells, was induced higher in hippocampus of NaAsO2 treated mice. The inducible HO-1 could exhibit antioxidative property and thus be a neroprotectant in neurons [23]. In addition, we also observed the higher level of GPx3 expression in hippocampus of mice in exposed group (Fig. 6). GPx3 protect cells from oxidative stress, which can be induced by activation of PPARγ [24].
Figure 6.
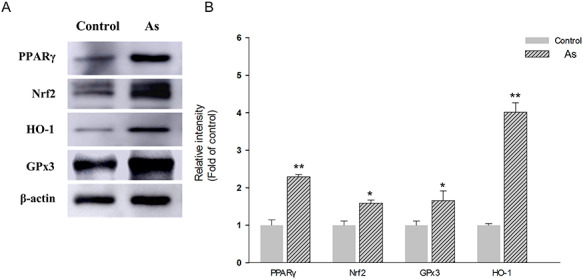
Western blot analysis showing the expression of oxidative stress markers in hippocampus of control and NaAsO2 exposed mice. Representative images of immunoblottings (A), and relative protein levels of PPARγ, Nrf2, HO-1 and GPx3 (B). For internal control, β-actin was used. Results were expressed as mean ± SEM (n = 3). *P < 0.05, **P < 0.01 when compared with control group.
Discussion
As is a widespread poisonous element and a global environmental pollutant. Acute or chronic As exposure affects almost every organ system in the body including the brain. Previous animal studies have shown that As could pass through the BBB, enter into brain and influence learning and memory abilities [25–27]. In the present study, significantly higher As concentration was observed in brain in the exposed group, confirming that As entered into brain to exert its neurotoxicity. In the MWM test, the performance of animal in a spatial navigation test reflects spatial learning ability, whereas the spatial probe test is a measure of spatial reference memory [28]. In our study, the control mice exhibited gradual decline of EL during training trial, presenting normal learning ability. On the contrary, the exposed mice spent more EL to find the hidden platform and displayed a decline in learning ability. In the probe trial test, the less dwell time in the target quadrant and less platform crossings in exposed group strongly suggested that NaAsO2 exposed mice could not remember the location of previous platform the training days later and their spatial reference memory was impaired notably. These significant differences indicated that the impairment of spatial cognitive function was attributed to NaAsO2 exposure.
Furthermore, the performance of animal in water maze was involved in cognitive processes in which the hippocampus played a crucial role [29]. Meanwhile, a number of literatures have proposed that As at high concentration induced ROS overproduction in brain, resulting in oxidative stress associated with neurotoxic effects in central nervous system. Oxidative stress is considered as the key factor mediating the behavioral impairments and memory deficits. It was proposed that oxidative stress conduced to increased neuronal injury through DNA damage, protein oxidation and peroxidation of membrane lipids [30]. As-induced oxidative damage is thought to play a crucial role in the pathogenesis of cognitive dysfunction and memory impairment in hippocampus [4]. As a reliable index of oxidative stress, the generation of ROS is applied to reflect a state of imbalance between the antioxidant defense system levels and the production of free radicals. SOD is considered to reflect the property of scavenging free oxygen radicals. The present study showed a significantly increase in ROS generation in hippocampus after middle-dose of NaAsO2 exposure, along with activated antioxidative defense to elevate SOD activity.
Of note, neurons are particularly susceptible to oxidative damage because of high contents of polyunsaturated lipid-rich neuronal parenchyma and acting as substrates for free radicals [31]. ROS mediated oxidative stress has been intimately linked with neuronal cell morphological injuries and such changes subsequently resulted in the impairments of synaptic plasticity and behavior. In the present study, we also observed that the NaAsO2 exposure caused severe structural changes, like alteration of the pyramidal cell layer arrangements and increase of pyknotic neurons and ectopic pyramidal cells, in the hippocampal CA1area.
Nrf2 is a critical transcription factor accounting for prompting the expression of various antioxidant genes in response to oxidative stress. As has been considered as an activator of Nrf2 pathway resulted in upregulation of antioxidant defenses [8, 32, 33]. Conversely, several studies demonstrated that As could reduce the expression of Nrf2 and its downstream genes [34, 35]. The reduction of antioxidant enzymes induced by As was more obvious in Nrf2-suppressed cells, indicating that As may inhibit the Nrf2 pathway [36]. Furthermore, PPARγ is emerging as an important regulator oxidative stress, acting synergically with Nrf2 in the promotion of antioxidant genes expression [15, 37]. However, the role of collaborative action of both transcription factors against As-induced oxidative stress is still debated. Taken together, the positive feedback loop of both Nrf2 and PPARγ, and the expression of antioxidant genes may depend on the dose and exposure time of As, as well as the type of subjects and tissues [38, 39]. Our results confirmed that middle-dose of NaAsO2 caused evidently dual activation of Nrf2 and PPARγ expression in hippocampus of mice after sub-chronic exposure.
HO-1, as one of Nrf2 and PPARγ target genes, can catalyze the degradation of heme into bilirubin, which possesses radical scavenging properties [40]. Enzymatic activity of HO-1 can be strongly induced in many tissues in response to cellular stress caused by ROS [23, 41]. Given that HO-1 also has been identified as a response biomarker for As exposure in various types of cells, we observed that HO-1 protein was induced in hippocampus of NaAsO2-treated mice. The finding was consistent with the studies that promotion of HO-1 can be mediated by activation of Nrf2 and PPARγ pathway [42].
GPx3 is the most important selenoenzyme involved in deactivating ROS, catalyzing the reduction of hydroperoxides, whereas protecting the organism from oxidative damage [43, 44]. It has been demonstrated to be a direct transcriptional target of PPARγ and expressed in many tissues, including kidney, lung, heart, liver, eyes, and brain [24, 45, 46]. In the present study, we showed a promotion in GPx3 expression in hippocampus of NaAsO2-treated mice, which could be mediated by activation of PPARγ.
Altogether, we found a positive association between Nrf2 and PPARγ expression in hippocampus of mice administered with 50 mg/kg NaAsO2 in sub-chronic exposure, and activation of antioxidant defenses by the evidently upregulated expression of their downstream genes, including SOD, HO-1 and GPx3. In conclusion, the results of this study have further confirmed that As exposure could lead to oxidative stress in hippocampus of mice and reduce the spatial learning and memory ability. Our finding is helpful for further understanding the role of Nrf2/PPARγ feedback loop in As-induced neurobehavioral toxicity.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81460504 and 81960599), the Open Project of Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases, Ministry of Education (No. XN201901), the Project of Scientific and Technological Innovation Team of Gannan Medical University (TD201801), the Science and Technology Project funded by the Education Department of Jiangxi Province (GJJ201525), and the Doctor Initial Funding for Gannan Medical University (QD201503 and QD201701).
Contributor Information
Liang Xiong, Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases of Ministry of Education, Gannan Medical University, Number 1 Yixueyuan Road, Ganzhou 341000, China; School of Public Health and Health Management, Gannan Medical University, Number 1 Yixueyuan Road, Ganzhou 341000, Jiangxi, China.
Jinyu Huang, Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases of Ministry of Education, Gannan Medical University, Number 1 Yixueyuan Road, Ganzhou 341000, China; Department of Anatomy, School of Basic Medicine Sciences, Gannan Medical University, Number 1 Yixueyuan Road, Ganzhou 341000, Jiangxi, China.
Ying Gao, Department of rehabilitation medicine, School of Rehabilitation, Gannan Medical University, Number 1 Yixueyuan Road, Ganzhou 341000, Jiangxi, China.
Yanfang Gao, Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases of Ministry of Education, Gannan Medical University, Number 1 Yixueyuan Road, Ganzhou 341000, China; School of Public Health and Health Management, Gannan Medical University, Number 1 Yixueyuan Road, Ganzhou 341000, Jiangxi, China.
Chunmei Wu, Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases of Ministry of Education, Gannan Medical University, Number 1 Yixueyuan Road, Ganzhou 341000, China; School of Public Health and Health Management, Gannan Medical University, Number 1 Yixueyuan Road, Ganzhou 341000, Jiangxi, China.
Shengfa He, School of Public Health and Health Management, Gannan Medical University, Number 1 Yixueyuan Road, Ganzhou 341000, Jiangxi, China.
Lijun Zou, School of Public Health and Health Management, Gannan Medical University, Number 1 Yixueyuan Road, Ganzhou 341000, Jiangxi, China.
Dongmei Yang, School of Public Health and Health Management, Gannan Medical University, Number 1 Yixueyuan Road, Ganzhou 341000, Jiangxi, China.
Yuhao Han, School of Public Health and Health Management, Gannan Medical University, Number 1 Yixueyuan Road, Ganzhou 341000, Jiangxi, China.
Qiong Yuan, School of Public Health and Health Management, Gannan Medical University, Number 1 Yixueyuan Road, Ganzhou 341000, Jiangxi, China.
Zuobing Zheng, Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases of Ministry of Education, Gannan Medical University, Number 1 Yixueyuan Road, Ganzhou 341000, China.
Gonghua Hu, Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases of Ministry of Education, Gannan Medical University, Number 1 Yixueyuan Road, Ganzhou 341000, China; School of Public Health and Health Management, Gannan Medical University, Number 1 Yixueyuan Road, Ganzhou 341000, Jiangxi, China.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1. Gong G, Hargrave KA, Hobson Vet al. Low-level groundwater arsenic exposure impacts cognition: a project FRONTIER study. Environ Health 2011;74:16–22. [PubMed] [Google Scholar]
- 2. Edwards M, Johnson L, Mauer Cet al. Regional specific groundwater arsenic levels and neuropsychological functioning: a cross-sectional study. Int J Environ Health Res 2014;24:546–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rodriguez-Barranco M, Lacasana M, Aguilar-Garduno Cet al. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysis. Sci Total Environ 2013;454-455:562–77. [DOI] [PubMed] [Google Scholar]
- 4. Tyler CR, Allan AM. The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review. Curr Environ Health Rep 2014;1:132–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Flora SJ, Bhadauria S, Pant SCet al. Arsenic induced blood and brain oxidative stress and its response to some thiol chelators in rats. Life Sci 2005;77:2324–37. [DOI] [PubMed] [Google Scholar]
- 6. Olsen LF, Issinger OG, Guerra B. The Yin and Yang of redox regulation. Redox Rep 2013;18:245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Finkel T. Signal transduction by reactive oxygen species. J Cell Biol 2011;194:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang Y, Duan X, Li Jet al. Inorganic arsenic induces Nrf2-regulated antioxidant defenses in both cerebral cortex and hippocampus in vivo. Neurochem Res 2016;41:2119–28. [DOI] [PubMed] [Google Scholar]
- 9. Dwivedi N, Flora SJ. Concomitant exposure to arsenic and organophosphates on tissue oxidative stress in rats. Food Chem Toxicol 2011;49:1152–9. [DOI] [PubMed] [Google Scholar]
- 10. Das BN, Kim YW, Keum YS. Mechanisms of Nrf2/Keap1-dependent phase II cytoprotective and detoxifying gene expression and potential cellular targets of chemopreventive isothiocyanates. Oxid Med Cell Longev 2013;2013:839409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim JH, Song J, Park KW. The multifaceted factor peroxisome proliferator-activated receptor gamma (PPARgamma) in metabolism, immunity, and cancer. Arch Pharm Res 2015;38:302–12. [DOI] [PubMed] [Google Scholar]
- 12. Polvani S, Tarocchi M, Galli A. PPARγ and oxidative stress: con(β) catenating NRF2 and FOXO. PPAR Res 2012;2012:641087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhan L, Zhang H, Zhang Qet al. Regulatory role of KEAP1 and NRF2 in PPARγ expression and chemoresistance in human non-small-cell lung carcinoma cells. Free Radic Biol Med 2012;53:758–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao R, Hou Y, Zhang Qet al. Cross-regulations among NRFs and KEAP1 and effects of their silencing on arsenic-induced antioxidant response and cytotoxicity in human keratinocytes. Environ Health Perspect 2012;120:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park EY, Cho IJ, Kim SG. Transactivation of the PPAR-responsive enhancer module in chemopreventive glutathione S-transferase gene by the peroxisome proliferator-activated receptor-gamma and retinoid X receptor heterodimer. Cancer Res 2004;64:3701–13. [DOI] [PubMed] [Google Scholar]
- 16. Faine LA, Rudnicki M, César FAet al. Anti-inflammatory and antioxidant properties of a new arylidene-thiazolidinedione in macrophages. Curr Med Chem 2011;18:3351–60. [DOI] [PubMed] [Google Scholar]
- 17. Mukherjee AA, Kandhare AD, Bodhankar SL. Elucidation of protective efficacy of Pentahydroxy flavone isolated from Madhuca indica against arsenite-induced cardiomyopathy: role of Nrf-2, PPAR-gamma, c-fos and c-jun. Environ Toxicol Pharmacol 2017;56:172–85. [DOI] [PubMed] [Google Scholar]
- 18. Ahmed RG, El-Gareib AW. Gestational arsenic trioxide exposure acts as a developing neuroendocrine-disruptor by downregulating Nrf2/PPARγ and upregulating Caspase-3/NF-ĸB/Cox2/BAX/iNOS/ROS. Dose Response 2019;17:1559325819858266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron 2002;35:625–41. [DOI] [PubMed] [Google Scholar]
- 20. Mochizuki H. Arsenic neurotoxicity in humans. Int J Mol Sci 2019;20:3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prakash C, Soni M, Kumar V. Mitochondrial oxidative stress and dysfunction in arsenic neurotoxicity: a review. J Appl Toxicol 2016;36:179–88. [DOI] [PubMed] [Google Scholar]
- 22. Lee C. Collaborative power of Nrf2 and PPARγ activators against metabolic and drug-induced oxidative injury. Oxid Med Cell Longev 2017;2017:1378175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hung SY, Liou HC, Kang KHet al. Overexpression of heme oxygenase-1 protects dopaminergic neurons against 1-methyl-4-phenylpyridinium-induced neurotoxicity. Mol Pharmacol 2008;74:1564–75. [DOI] [PubMed] [Google Scholar]
- 24. Chung SS, Kim M, Youn BSet al. Glutathione peroxidase 3 mediates the antioxidant effect of peroxisome proliferator-activated receptor gamma in human skeletal muscle cells. Mol Cell Biol 2009;29:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luo JH, Qiu ZQ, Shu WQet al. Effects of arsenic exposure from drinking water on spatial memory, ultra-structures and NMDAR gene expression of hippocampus in rats. Toxicol Lett 2009;184:121–5. [DOI] [PubMed] [Google Scholar]
- 26. Tolins M, Ruchirawat M, Landrigan P. The developmental neurotoxicity of arsenic: cognitive and behavioral consequences of early life exposure. Ann Glob Health 2014;80:303–14. [DOI] [PubMed] [Google Scholar]
- 27. Jing J, Zheng G, Liu Met al. Changes in the synaptic structure of hippocampal neurons and impairment of spatial memory in a rat model caused by chronic arsenite exposure. Neurotoxicology 2012;33:1230–8. [DOI] [PubMed] [Google Scholar]
- 28. Baldi E, Efoudebe M, Lorenzini CAet al. Spatial navigation in the Morris water maze: working and long lasting reference memories. Neurosci Lett 2005;378:176–80. [DOI] [PubMed] [Google Scholar]
- 29. D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev 2001;36:60–90. [DOI] [PubMed] [Google Scholar]
- 30. Medda NPR, Ghosh TK, Maiti S. Neurotoxic mechanism of arsenic: synergistic effect of mitochondrial instability, oxidative stress, and hormonal-neurotransmitter impairment. Biol Trace Elem Res 2020;198:8–15. [DOI] [PubMed] [Google Scholar]
- 31. Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993;262:689–95. [DOI] [PubMed] [Google Scholar]
- 32. Aono J, Yanagawa T, Itoh Ket al. Activation of Nrf2 and accumulation of ubiquitinated A170 by arsenic in osteoblasts. Biochem Biophys Res Commun 2003;305:271–7. [DOI] [PubMed] [Google Scholar]
- 33. Jiang J, Tam LM, Wang Pet al. Arsenite targets the RING finger domain of Rbx1 E3 ubiquitin ligase to inhibit proteasome-mediated degradation of Nrf2. Chem Res Toxicol 2018;31:380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prabu SM, Muthumani M. Silibinin ameliorates arsenic induced nephrotoxicity by abrogation of oxidative stress, inflammation and apoptosis in rats. Mol Biol Rep 2012;39:11201–16. [DOI] [PubMed] [Google Scholar]
- 35. Vineetha RC, Archana V, Binu Pet al. L-ascorbic acid and alpha-tocopherol reduces hepatotoxicity associated with arsenic trioxide chemotherapy by modulating Nrf2 and Bcl2 transcription factors in Chang liver cells. Nutr Cancer 2018;70:684–96. [DOI] [PubMed] [Google Scholar]
- 36. Shafik NM, El Batsh MM. Protective effects of combined selenium and Punica granatum treatment on some inflammatory and oxidative stress markers in arsenic-induced hepatotoxicity in rats. Biol Trace Elem Res 2016;169:121–8. [DOI] [PubMed] [Google Scholar]
- 37. Ishii T, Itoh K, Ruiz Eet al. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ Res 2004;94:609–16. [DOI] [PubMed] [Google Scholar]
- 38. Hu Y, Li J, Lou Bet al. The role of reactive oxygen species in arsenic toxicity. Biomolecules 2020;10:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang C, Niu Q, Ma Ret al. The variable regulatory effect of arsenic on Nrf2 signaling pathway in mouse: a systematic review and meta-analysis. Biol Trace Elem Res 2019;190:362–83. [DOI] [PubMed] [Google Scholar]
- 40. Hung SY, Liou HC, Fu WM. The mechanism of heme oxygenase-1 action involved in the enhancement of neurotrophic factor expression. Neuropharmacology 2010;58:321–9. [DOI] [PubMed] [Google Scholar]
- 41. Habtemariam S. The Nrf2/HO-1 Axis as targets for Flavanones: neuroprotection by pinocembrin, naringenin, and eriodictyol. Oxid Med Cell Longev 2019;2019:4724920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chorley BN, Campbell MR, Wang Xet al. Identification of novel NRF2-regulated genes by ChIP-Seq: influence on retinoid X receptor alpha. Nucleic Acids Res 2012;40:7416–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brigelius-Flohé R. Glutathione peroxidases and redox-regulated transcription factors. Biol Chem 2006;387:1329–35. [DOI] [PubMed] [Google Scholar]
- 44. Lee YS, Kim AY, Choi JWet al. Dysregulation of adipose glutathione peroxidase 3 in obesity contributes to local and systemic oxidative stress. Mol Endocrinol 2008;22:2176–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Noworyta-Sokołowska K, Kamińska K, Rzemieniec Jet al. Effects of exposure to 5-MeO-DIPT during adolescence on brain neurotransmission and neurotoxicity in adult rats. Forensic Toxicol 2019;37:45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reddy AT, Lakshmi SP, Banno Aet al. Role of GPx3 in PPARγ-induced protection against COPD-associated oxidative stress. Free Radic Biol Med 2018;126:350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


