Abstract
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) such as gefitinib, erlotinib, and afatinib, are widely used in clinical practice and remarkably effective in treatment of advanced non-small cell lung cancer. However, there are some adverse effects while taking EGFR-TKIs, among which skin adverse reactions (SAR) are the most common events. At present, the poor outcome of SAR and insufficient research on SAR models need to be addressed. In this study we focused on the SAR models to lay a foundation for mechanism researches. Gefitinib, one of the EGFR-TKIs, was used as SAR inducing agents. We chose C57BL/6 and FVB/N mice as experimental model and they were divided into four groups. The weight and skin moisture of mice were detected every 7 days, itching behavior and abnormal eyelids were tested at 35th day after gavage, and survival rate was also recorded. The weight of unit area hair, length of whiskers and inflammatory cells were evaluated after mice sacrificed. C57BL/6 animals treated with gefitinib showed significant differences in survival rate, weight of unit area hair, skin moisture changes, skin dryness, itching behavior, whisker irregular growth, abnormal eyelids, and inflammatory cells; FVB/N animals treated with gefitinib only showed significant differences in survival rate, whisker irregular growth and abnormal eyelids, compared with the control group, respectively. In this study, we compared the similarities and differences of gefitinib-induced SAR between C57BL/6 and FVB/N mice, which illustrated different patients probably showing different symptoms clinically and provided experimental basis for researching mechanism of EGFR-TKIs induced SAR.
Keywords: EGFR-TKIs, skin adverse reactions, gefitinib, mice model
Introduction
Epidermal growth factor receptor (EGFR) belonging to the receptor tyrosine kinases (RTKs) of ErbB family, plays an important role in the physiological function of epithelial cells [1]. EGFR mutations are often detected in various epithelial tumors clinically [2] and the tumorigenic processes through activation of EGFR by regulating cell proliferation, cell survival, angiogenesis and metastasis worsen patient condition [3]. At present, there are two strategies for targeting inhibition of EGFR signaling transduction, monoclonal antibody and tyrosine kinase inhibitor (TKI), which have been approved for clinical application for anti-tumor therapy alone or combined with chemotherapy.
EGFR tyrosine kinase inhibitors (EGFR-TKIs) are widely used in the therapy of metastatic non-small cell lung cancer, colorectal cancer, squamous cell carcinoma, head and neck cancer, pancreatic cancer [4], which benefit from excessive activation of EGFR for their growth and survival. Along with EGFR-TKIs efficacy in the treatment of various cancers, serious adverse reactions lead a dose limited or discontinue therapy during the treatment process, affecting the quality of life of patients, which may due to EGFR-TKIs not only target to inhibit the mutant EGFR but also the expression of EGFR in normal tissue. Because EGFR is widespread in many areas of human body, such as skin and intestine etc., the most common adverse reaction is skin toxicity, with the incidence of 79–88% in EGFR-TKIs-treated patients, including skin rash, moisture skin, pruritus, eczema, pigmentation, hair changes and other symptoms [2].
However, the development and severity of the skin toxicity have been shown to be closely related to favorable antitumor responses [5, 6]. To understand the pathogenesis of skin toxicity caused by inhibition of EGFR, researchers constructed a mouse model of epidermal targeted ablation of EGFR, providing insights into the mechanisms underlying EGFRIs-induced skin inflammation [7, 8], which lacked process of induction of EGFRIs actually. Here, we compared the similarities and differences of gefitinib induced SAR between C57BL/6 and FVB/N mice and provided a suitable model for preclinical experimental basis for gefitinib induced SAR.
Materials and Methods
Animals and treatment groups
Female C57BL/6 mice (5 weeks old) and female FVB/N mice (5 weeks old) were purchased from SPF Biotechnology Co., Ltd (Beijing, China). All mice were allowed to eat and drink freely, and maintained on a 12 h light, 12 h dark cycle. All experimental procedures were approved by the Animal Ethics Committee of Beijing University of Chinese Medicine (BUCM-2016103101-1008) in accordance with the National Animal Welfare Law of China.
C57BL/6 and FVB/N mice were divided into four groups respectively: group 1, control (n = 6/6); group 2 as equivalent dosage of clinical dose, gefitinib 37.5 mg/kg/day (n = 6/6); group 3, gefitinib 75 mg/kg/day (n = 7/6); group 4, gefitinib 150 mg/kg/day (n = 8/6). Gefitinib was dissolved in distilled water for suspension at a stock concentration of 22.5 mg/ml and applied once daily for experimental group. Control group received the same volume of distilled water once daily. Gefitinib or the vehicle was administered orally to the mice for 49 days and body weight was measured once a week. The survival rates of mice were recorded before autopsy. All animals underwent dorsal hair removal before the experiment.
Measurement of skin moisture variation
Skin digital images and moisture variation were measured three times under avertin on the dorsal skin lesion per week using an ES-2600 Intelligent Skin Analysis System (Beijing Yilimei Technology Co., Ltd, Beijing, China), and the median value of three measurements at each time point was recorded.
Measurement of hair loss changes
A certain area of body hair was shaved with an electrical animal razor, then length and width of the specific area were measured. The shaved hair was collected and weighed (n = 3/group) and whiskers (n = 3/group) were collected after the mice sacrificed. Hair weight per unit area (mg/mm2) = Hair weight (mg)/area (mm2).
Measurement of scratching behavior
To measure scratching behavior, each mouse was placed in an observation glass container positioned with a camcorder to record the animals’ behavior. After an acclimation period of 0.5 h, the animals’ behavior was recorded on video for at least 1 hour with no experimenters present in the observation room. Scratching behavior was assessed by replaying each video and the number of bouts of scratching was scored for 1 h. An incidence of scratching behavior was defined as raising and lowering a hind limb.
Statistical analysis
Statistical analyses were performed using the Student’s t test and one-way or two-way analysis of variance using the Prism 5 software (GraphPad Software, La Jolla, CA). All data were presented as mean ± standard error of the mean (SEM) and P < 0.05 was considered statistically significant.
Results
Gefitinib-induced mouse body weight and survival rate changes
We established the mouse models of SAR induced by gefitinib in C57BL/6 and FVB/N mice. Firstly, we measured body weight and survival rate changes. As shown in Fig. 1A and B, gefitinib 150 mg/kg/day significantly reduced the weight of C57BL/6 mice on Day 7 (P < 0.05), and reduced the weight of FVB/N mice on Days 14 (P < 0.0001) and 21 (P < 0.0001). But compared with control group, the whole trends of weight changes had no differences. The death of C57BL/6 mice in 150 mg/kg (survival rate 87.5%) (Fig. 1C) occurred on Day 21 while FVB/N mice occurred on Day 7 in 150 mg/kg (survival rate 33.3%) (Fig. 1D), which all showed significant difference compared with control group. Not surprisingly, gefitinib 225 mg/kg/day in C57BL/6 mice showed serious toxicity on body weight and survival rate (Supplementary Fig. 1A and B).
Figure 1.

The effects of gefitinib on body weight changes and survival rate in C57BL/6 and FVB/N mice. The body weight (A, B) and survival rate (C, D) of C57BL/6 mice and FVB/N mice were recorded every 7 days, treated without (saline) or with gefitinib (37.5 mg/kg, 75 mg/kg and 150 mg/kg of body weight, n≥4, apart from the FVB/N in 150 mg/kg after 35 days, n=2). Data were analyzed using Two-way ANOVA and presented as the mean ± SEM. Compared with control, *p < 0.05, **p < 0.01, ***p < 0.001.
Effect of gefitinib on skin moisture variation, skin dryness and scratching behavior
To test gefitinib-induced dry skin, skin moisture variation was measured once a week. A significant decrease in the dermal water content by approximately 20% of C57BL/6 mice in 150 mg/kg was found (Fig. 2A) while there had no obvious difference between other groups and control group. Beyond that, we collected images of back skin in each group, which exhibited skin dryness on day 14 in all gefitinib-treated group but the phenotype was alleviative along with continuous administration, and dryness phenotype of C57BL/6 mice in 150 mg/kg group was most obvious (Fig. 3A).
Figure 2.

The effects of gefitinib on skin moisture variation in C57BL/6 and FVB/N mice. The body skin moisture of C57BL/6 mice (A)and FVB/N mice (B) were recorded every 7 days, treated without (saline) or with gefitinib (37.5 mg/kg, 75 mg/kg and 150 mg/kg of body weight, n≥4, apart from the FVB/N in 150 mg/kg after 35 days, n=2). Data were analyzed using One-way ANOVA and presented as the mean ± SEM. Compared with control, ***p < 0.001.
Figure 3.

The skin dryness induced by gefitinib. Images were collected from the back of C57BL/6 mice (A) and FVB/N mice (B) every 7 days, treated without (saline) or with gefitinib (37.5 mg/kg, 75 mg/kg and 150 mg/kg of body weight, n=3, apart from the FVB/N in 150 mg/kg after 35 days, n=2).
However, no difference of skin moisture variation in FVB/N mice was noted between gefitinib-treated and control groups (Fig. 2B) and only 150 mg/kg group exhibited mild skin dryness on Day 14 which also subsequently relieved along with continuous administration (Fig. 3B).
To test gefitinib-induced scratching behavior, the number of scratching bouts was measured on Day 35. We monitored the animals and noted the scratching frequency. The results showed that the scratching bouts were significantly higher in C57BL/6 mice with gefitinib 150 mg/kg than control mice (Fig. 4A), whereas no difference of scratching bouts in FVB/N mice was noted between gefitinib-treated and control groups, which was consistent with the results of skin moisture variation (Fig. 4B).
Figure 4.

Scratching and hair loss induced by gefitinib. The scratch bouts of C57BL/6 mice (A)and FVB/N mice (B) were recorded at 35th day, treated without (saline) or with gefitinib (37.5 mg/kg, 75 mg/kg and 150 mg/kg of body weight, n≥4, apart from the FVB/N in 150 mg/kg after 35 days, n=2). Mice hair weight per unit area were counted after mice sacrificed, n=3 (C, D). Data were analyzed using One-way ANOVA and presented as the mean ± SEM. Compared with control, **p < 0.01.
Gefitinib-induced mouse hair loss and whisker irregular growth
To test whether gefitinib could affect hair and whisker growth, we weighed mice hair per unit area and collected whisker. Promotion of hair loss in C57BL/6 mice 150 mg/kg group was observed demonstrated by hair weight per unit area (Fig. 4C) while there has no difference between gefitinib-treated groups and control group in FVB/N mice (Fig. 4D).
Different from the results of hair loss, whisker irregular growth (including amount and length of whisker) in a dose dependent manner demonstrated by the apparent observation was found in all gefitinib-treated groups of C57BL/6 and FVB/N mice (Fig. 5A and B).
Figure 5.
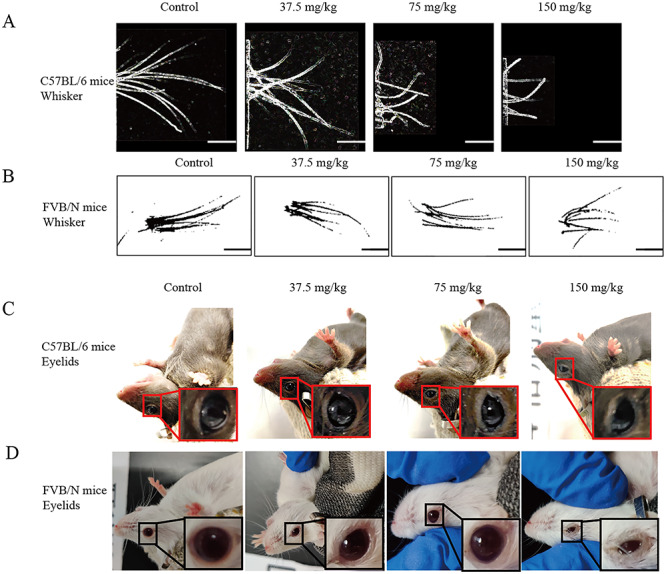
The irregular growth of whisker and abnormal eyelids induced by gefitinib. The whisker of C57BL/6 mice and FVB/N mice, treated without (saline) or with gefitinib (37.5 mg/kg, 75 mg/kg and 150 mg/kg of body weight, n=3, apart from the FVB/N in 150 mg/kg after 35 days, n=2), were appropriate cut out (A, B) and images of eyelids were collected (C, D) before sacrificed.
Gefitinib-induced mouse abnormal eyelids and inflammation in blood
During gefitinib administration, we also found abnormal eyelids in the treated mice and the change tendency is similar to whisker irregular growth. C57BL/6 mice in 75 mg/kg, 150 mg/kg, showed external morphological defects of the eyes (Fig. 5C). FVB/N mice in all gefitinib-treated groups remarkably developed into external morphological defects of the eyes as well and 150 mg/kg had almost closed eyelids (Fig. 5D).
The number of white blood cells and MONO% were detected by xs-800i automatic hematology analyzer (Sysmex Corporation, Kobe, Japan), markedly increased in C57BL/6 mice (Fig. 6A and B).
Figure 6.
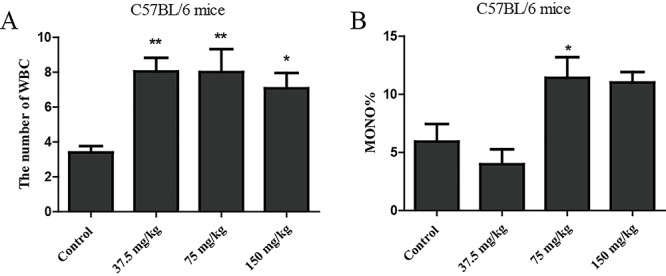
The inflammatory of C57BL/6 mice induced by gefitinib. The number of WBC (A) and MONO (B) percent levels in the whole blood of C57BL/6 mice were measured by the automatic biochemistry analyzer for inflammatory (n=3/group). Data were analyzed using One-way ANOVA and presented as the mean ± SEM. Compared with control, *p < 0.05, **p < 0.01.
Discussion
EGFR-TKIs, the molecular-targeted anti-tumor drugs, have been proved to be a promising therapeutic regimen for cancer patients with abnormal activation of EGFR, which has achieved favorable efficacy and extended survival rate of patients. However, clinical practice have discovered serious side effects under EGFR-TKIs treatment, among which skin toxicities are the most common event threatening patients’ physical and mental health [9].
Gefitinib, an EGFR-TKI, has shown efficacy for the treatment of advanced non–small-cell lung cancer (NSCLC) in patients who had received multiple prior treatments [10]. There has been reported that during phase I trials of gefitinib (50–1000 mg/day), rash, dry skin and pruritus appeared [11]. The adverse reactions of 34 patients received gefitinib were observed, such as itching, dryness, rash, diarrhea and mucositis with percentage of 58.8, 47.06, 26.5, 29.4 and 29.4%, respectively [12], which implied high incidence of gefitinib leading to dermatological toxicities. As reported, gefitinib-induced dry skin may be attributable to decreased AQP3 in the skin [13]. According to a meta-analysis of 20 151 patients treated with 14 distinct targeted agents, gefitinib has the highest incidence of high-grade pruritus [14], and an increasing number of dermal mast cells in skin rashes were found in EGFR-TKI-treated patients which play an important role in pruritus [15].
From the body weight and survival rate changes data, it appears that gefitinib is safety for cancer treatment in a safe dosage (Fig. 1, Supplementary Fig. 1A and B). Consistent with a previous report [16], we focused on the phenotype of gefitinib-induced dry skin model. Compared with control mice, a significant decrease in the dermal water content of C57BL/6 mice in high-dose group, dryness phenotype of C57BL/6 mice in gefitinib-treated groups and the increasing scratching bouts in C57BL/6 mice with gefitinib 150 mg/kg showed that C57BL/6 mice may be a useful model for researching the mechanism of EGFR-TKIs induced itching and dryness. Beyond that, based on the dryness phenotype of both two models, we observed a remission tendency of dryness symptom in line with the clinical practice [9, 17, 18]. As shown in Fig. 5B, the cutaneous toxicity from gefitinib was also manifested as abnormal eyelids which progressively worsened with exposure dosage. Clinical data shows that conjunctivitis, blepharitis, xerotic, keratitis and lacrimation of eyes also occurred as EGFR-TKIs-associated skin toxicity possibly [19]. We detected gefitinib 225 mg/kg in C57BL/6 and FVB/N mice as well, the skin moisture had a significant decrease in C57BL/6 mice (Supplementary Fig. 2). In addition, skin dryness (Supplementary Fig. 3), whisker irregular growth (Supplementary Fig. 4A and B) and abnormal eyelids (Supplementary Fig. 4C and D) emerged with no exception in these two models.
Although the mouse models of epidermal targeted ablation of EGFR have been reported leading to a characteristic cutaneous inflammatory phenotype in mice [7, 8], they are different from EGFR-TKIs induced skin disorder in pathogenesis. The same dosage of gefitinib induced different SAR in C57BL/6 and FVB/N mice implying that SAR might occur varying with different individuals in clinical practice, which suggested giving more care on patients’ prognosis. Moreover, the data presented here also provided substantial evidence that C57BL/6 mice of gefitinib 150 mg/kg would be a useful model for mechanism research of EGFR-TKIs-induced SAR. Management of cutaneous side effects of targeted cancer therapies should be highly valued and provided more aggressive treatment regimens, which could improve patients’ quality of life. In our research, SAR phenotypes similar to patients, skin dryness, itching behavior, whisker irregular growth, abnormal eyelids and inflammatory cells infiltrating, were found in mice models. And the above fundamental research data could support a significant role of the development of SAR models, which may provide a suitable model for preclinical drug research and development for the treatment of EGFR-TKIs induced SAR.
Funding
This work was supported in part by the National Natural Science Foundation of China (81973667) and grants from the Regional Collaborative Innovation Center of Tibetan Medicine (2018XTCX014).
Conflict of interest statement
There are no conflicts of interest to declare.
Supplementary Material
Contributor Information
Yali Zhang, Beijing University of Chinese Medicine, Sunshine South street, Fangshan district, Beijing 102488, China.
Yalei Wang, Beijing University of Chinese Medicine, Sunshine South street, Fangshan district, Beijing 102488, China.
Ziwei Chen, Beijing University of Chinese Medicine, Sunshine South street, Fangshan district, Beijing 102488, China.
Shuo Cheng, Beijing University of Chinese Medicine, Sunshine South street, Fangshan district, Beijing 102488, China.
Chengcheng Ding, Beijing University of Chinese Medicine, Sunshine South street, Fangshan district, Beijing 102488, China.
Jiani Zhang, Beijing University of Chinese Medicine, Sunshine South street, Fangshan district, Beijing 102488, China.
Tiantian Peng, Beijing University of Chinese Medicine, Sunshine South street, Fangshan district, Beijing 102488, China.
Weihang Chen, Beijing University of Chinese Medicine, Sunshine South street, Fangshan district, Beijing 102488, China.
Dingyang Zhang, Beijing University of Chinese Medicine, Sunshine South street, Fangshan district, Beijing 102488, China.
Yan Tan, Beijing University of Chinese Medicine, Sunshine South street, Fangshan district, Beijing 102488, China.
Xu Wang, Beijing University of Chinese Medicine, Sunshine South street, Fangshan district, Beijing 102488, China.
Ruijuan Dong, Beijing University of Chinese Medicine, Sunshine South street, Fangshan district, Beijing 102488, China.
Miao Jiang, Beijing University of Chinese Medicine, Sunshine South street, Fangshan district, Beijing 102488, China.
Qian Hua, Beijing University of Chinese Medicine, Sunshine South street, Fangshan district, Beijing 102488, China.
References
- 1. Schlessinger J. Receptor tyrosine kinases: legacy of the first two decades. Cold Spring Harb Perspect Biol 2014;6:pii: a008912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer 2012;12:553–63. [DOI] [PubMed] [Google Scholar]
- 3. Huang SM, Harari PM. Epidermal growth factor receptor inhibition in cancer therapy: biology, ratio- nale and preliminary clinical results. Invest New Drugs 1999;17:259–69. [DOI] [PubMed] [Google Scholar]
- 4. Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med 2008;358:1160–74. [DOI] [PubMed] [Google Scholar]
- 5. Stintzing S, Kapaun C, Laubender RPet al. Prognostic value of cetuximabrelated skin toxicity in metastatic colorectal cancer patients and its correlation with parameters of the epidermal growth factor receptor signal transduction pathway: results from a randomized trial of the German AIO CRC study group. Int J Cancer 2013;132:236–45. [DOI] [PubMed] [Google Scholar]
- 6. Liu HB, Wu Y, Lv TFet al. Skin rash could predict the response to EGFR tyrosine kinase inhibitor and the prognosis for patients with non-small cell lung cancer: a systematic review and meta-analysis. PLoS One 2013;8:e55128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lichtenberger BM, Gerber PA, Holcmann Met al. Epidermal EGFR controls cutaneous host defense and prevents inflammation. Sci Transl Med 2013;5:199ra111. [DOI] [PubMed] [Google Scholar]
- 8. Mascia F, Lam G, Keith Cet al. Genetic ablation of epidermal EGFR reveals the dynamic origin of adverse effects of anti-EGFR therapy. Sci Transl Med 2013;21:199ra110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mitchell EP, Perez-Soler R, Van Cutsem Eet al. Lacouture, clinical presentation and pathophysiology of EGFRI dermatologic toxicities. Oncology (Williston Park) 2007;21:4–9. [PubMed] [Google Scholar]
- 10. Herbst RS, Kies MS. ZD1839 (Iressa™) in non-small cell lung cancer. Oncologist 2002;7:9–15. [DOI] [PubMed] [Google Scholar]
- 11. Herbst RS, LoRusso PM, Purdom Met al. Dermatologic side effects associated with Gefitinib therapy: clinical experience and management. Clin Lung Cancer 2003;4:366–9. [DOI] [PubMed] [Google Scholar]
- 12. Thomas P, Vincent B, George Cet al. A comparative study on erlotinib & gefitinib therapy in non-small cell lung carcinoma patients. Indian J Med Res 2019;150:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ikarashi N, Kaneko M, Watanabe Tet al. Epidermal growth factor receptor tyrosine kinase inhibitor erlotinib induces dry skin via decreased in aquaporin-3 expression. Biomolecules 2020;10:pii: E545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Santoni M, Conti A, Andrikou Ket al. Risk of pruritus in cancer patients treated with biological therapies: a systematic review and meta-analysis of clinical trials. Crit Rev Oncol Hematol 2015;96:206–19. [DOI] [PubMed] [Google Scholar]
- 15. Gerber PA, Buhren BA, Cevikbas Fet al. Preliminary evidence for a role of mast cells in epidermal growth factor receptor inhibitor-induced pruritus. J Am Acad Dermatol 2010;63:163–5. [DOI] [PubMed] [Google Scholar]
- 16. Chandra F, Sandiono D, Sugiri Uet al. Cutaneous side effects and transepidermal water loss to gefitinib: a study of 11 patients. Dermatol Ther (Heidelb) 2017;7:133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hidalgo M, Siu LL, Nemunaitis Jet al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol 2001;19:3267–79. [DOI] [PubMed] [Google Scholar]
- 18. Boone SL, Rademaker A, Liu Det al. Impact and management of skin toxicity associated with anti-epidermal growth factor receptor therapy: survey results. Oncology 2007;72:152–9. [DOI] [PubMed] [Google Scholar]
- 19. Kozuki T. Skin problems and EGFR-tyrosine kinase inhibitor. Jpn J Clin Oncol 2016;46:291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


