ABSTRACT
Tuberculosis (TB) contact investigation facilitates earlier TB diagnosis and initiation of preventive therapy, but little data exist about the quality of its implementation. We conducted a retrospective cohort study to evaluate processes of TB contact investigation for index TB patients diagnosed in Cali, Colombia, in 2017, including dropout at each stage and overall yield. We constructed multivariable models to identify predictors of completing 1) the baseline household visit and 2) a follow-up clinic visit for TB evaluation among referred contacts. Sixty-eight percent (759/1,120) of registered TB patients were eligible for contact investigation; 77% (582/759) received a household visit. Odds of completing a household visit were significantly lower among men (adjusted odds ratio [aOR]: 0.6; 95% CI: 0.4–0.9; P = 0.009) and patients living in Cali’s western zone (aOR: 0.5; 95% CI: 0.3–0.8; P = 0.008). Among 1880 screened contacts, 31% (n = 582) met the criteria for clinic referral, 47% (n = 271) completed a clinic visit, and 85% (231/271) completed testing. After adjusting for clustering by index patient, odds of completing referral were higher among contacts with cough (aOR: 22; 95% CI: 7.1–66; P < 0.001) and contacts living in the western zone (aOR: 4.1; 95% CI: 1.2–15; P = 0.03). The cumulative probability of a symptomatic contact from an eligible household completing TB evaluation was only 28%. The yield of active TB patients among contacts was only 0.3% (5/1880). Only 16% (17/103) of children aged < 5 years and none of the eight persons living with HIV, reported preventive therapy initiation. Routine monitoring of process indicators may facilitate quality improvement to close gaps in contact tracing and increase yield.
INTRODUCTION
Tuberculosis (TB) continues to be a major global public health problem, with an estimated two billion persons latently infected,1 and approximately 10 million patients with incident active TB disease in 2018.2 At least 30% of these patients are never diagnosed or reported to public health authorities, a situation that fosters transmission and hampers TB elimination efforts.2 The Pan-American Health Organization has recognized active case detection as a key element of the End TB Strategy in the Americas, to achieve the goals of reducing TB incidence to 20 cases per 100,000 inhabitants by 2025, and to 5.5 per 100,000 by 2030.3 Household TB contact investigation, also known as contact tracing, is an evidence-based intervention for screening household members of the index patient for active TB and linking them to evaluation, treatment, and prevention services. This approach has been shown to increase TB diagnosis relative to passive, facility-based screening of contacts.4 Home visits may also offer opportunities to provide social support to index patients during TB treatment and to offer TB preventive therapy (TPT) to household contacts,5 a new recommendation recently endorsed by the WHO.6–8
In Colombia, the Ministry of Health and Social Protection has adopted the End TB Strategy, prioritizing active TB case finding and contact tracing. However, contact investigation is a complex intervention, and its implementation has proven to be challenging in many countries, including Colombia, an intermediate TB burden country with substantial geographic heterogeneity in case notifications. Accelerated urbanization across the Latin American region fosters marginalization of the poor in overcrowded neighborhoods of large cities, where social inequities, violence linked to crime and drug and alcohol use disorders, and fragmentation of health systems make access to TB care and delivery of contact-tracing services more difficult.9
Recent operational data from Cali, one of the Colombia’s regional capitals, identified an unexpectedly low yield (< 1%) of active TB patients from household contact tracing, in contrast to the 3.1–4.5% yield reported in meta-analyses of contact investigation globally.4,10–12 When multicomponent interventions like contact tracing fail to deliver expected results,13 detailed evaluation of the individual processes can help pinpoint which components are not working well and why. Quantitative approaches such as cascade analysis start by estimating the number of prevalent TB cases in a target population and then carefully measure the proportions actually evaluated, diagnosed, treated, and restored to health to localize where patients were missed.14–16 A related technique, patient-pathway analysis, follows-up individuals undergoing evaluation for TB forward through their care journeys to achieve similar objectives.17,18 In this study, we applied a cascade analysis approach to Cali’s routinely collected TB contact-tracing data to determine where household TB contacts are lost to follow-up during TB screening, evaluation, treatment initiation, and prevention, and to identify individual- and household-level characteristics associated with dropout.
MATERIALS AND METHODS
Setting and investigators.
Cali, a city of 2.5 million inhabitants, is the largest metropolitan area in Colombia’s Pacific region. Tuberculosis is common, with an annual notification rate of 46 TB cases per 100,000 persons, 1.4 times the national average.19 We as members of Alianza-TB, an academic–public health partnership involving the Cali Secretary of Health and investigators at local universities and research institutes, were invited to lead this evaluation.
Study design and participants.
We performed a retrospective cohort study to assess the quality of contact investigation carried out by the TB Control Program of the Municipal Secretary of Health. We included data on all children and adults registered with the local TB program as new or retreatment patients with active TB disease between January and December 2017. We excluded index patients who reported no contacts, those who were incarcerated, and those later found to have nontuberculous mycobacterial disease. We included data on all contacts reported by index TB patients at diagnosis and encountered during household visits.
Procedures for household contact investigation.
The local TB program requires all patients diagnosed with any form of active TB disease in the public or private sector to be offered contact investigation.20 Facility-based health workers interview the index patient at diagnosis and record patient and household information. Extramural teams are then dispatched to households by the local TB program or by private health insurers to carry out TB screening and referral for testing. Guidelines recommend up to three home visits for TB screening (baseline, month 6, and month 12), with the first visit expected to occur within 8 days after index patient diagnosis. At the household, teams screen household and other accessible close contacts for TB symptoms and other clinical features indicating a need for clinic referral (i.e., productive cough ≥ 2 weeks; age < 5 or ≥ 60 years; and living with HIV or diabetes).20 At clinics, TB diagnostic algorithms include sputum smear microscopy and culture, and depending on physician’s decision, chest radiography and/or molecular testing is provided. Tuberculosis preventive therapy is recommended for all household contacts younger than 5 years and all people living with HIV (PLHIV), after active TB disease has been excluded.21 Teams record all contact data on standardized paper forms, including individual demographic and clinical characteristics; household characteristics, including (for purposes of risk factor surveillance only) access to outside ventilation and sunlight (i.e., presence of exterior windows), and crowding (i.e., > 3 persons per room); and the results of clinic evaluation (although not the tests used). These are returned to the Secretary of Health for entry into a contact-tracing database.
Variables.
We extracted demographic and clinical variables from programmatic TB registers for index patients and contacts, including age, gender, health insurance, type of contact (household versus non-household close contact), productive cough (≥ 2 weeks), HIV status, microscopy results and culture results, tuberculin skin testing, and chest radiography, as well as whether treatment was initiated for latent TB infection.
Data analysis.
We described demographic and clinical variables using summary statistics. Then, we constructed a stepwise evaluation of contact investigation processes by estimating the following indicators: 1) proportion of index patients eligible for contact investigation, 2) proportion of eligible index patients receiving a baseline household visit, 3) proportion of contacts referred who completed TB evaluation by visiting the clinic (evidenced by results of sputum testing, chest radiography, or data on prescription of TPT), 4) proportion of referred contacts diagnosed with and treated for active TB disease, and 5) proportion of eligible contacts without active TB disease who initiated TPT. We estimated the cumulative probability of a household contact with active TB disease being visited, screened, referred to a clinic, and tested. We summarized the types of diagnostic testing performed for active TB disease using simple proportions. We estimated the cumulative probability of a symptomatic contact completing evaluation for active TB. To do so, we multiplied the probabilities of the following steps being completed, each conditioned on the previous step having been completed, for contacts residing in an eligible index household: 1) index patient completing the household visit, 2) contact encountered during the household visit, 3) referred contact completing referral (any evidence of a clinic visit by results of laboratory testing, chest radiography, or data on prescription of TPT), and 4) contact tested (results of sputum evaluation by microscopy, culture, and/or molecular testing). We performed bivariate and multivariable analyses examining associations between 1) demographic or clinical characteristics of index patients and completion of the initial household visit; and 2) demographic or clinical characteristics of contacts referred to clinics for evaluation and completion of a follow-up clinic visit. To identify independent predictors of completion of the baseline household visit, we constructed a model, including all variables with P-value ≤ 0.2 in bivariate analyses, as well as index patient age and smear results, which we forced into the model on grounds of face validity. Similarly, to identify independent predictors of completing the follow-up clinic visit, we constructed a multivariable, mixed-effects, logistic regression model, including variables with P-value ≤ 0.2 in bivariate analyses. To account for potential clustering of contact outcomes by association with index patient, we calculated the intra-class correlation coefficient (ICC) and planned to adjust for clustering using random intercepts and robust standard errors if the ICC exceeded 0.1. Finally, we ran a quadrature check to evaluate the accuracy of the regression model and ensure proper model fit. We used STATA 15 (StataCorp, College Station, TX) for all analyses. Sample size was based on convenience, and in lieu of power calculations, we reported exact binomial 95% CIs for all estimates. For multivariable analyses, we determined statistical significance at the P < 0.05 level.
Ethical considerations.
Institutional review boards at Universidad Icesi and the Centro Internacional de Entrenamiento e Investigaciones Médicas approved the study, waiving informed consent on grounds of minimal risk.
RESULTS
Household visit processes.
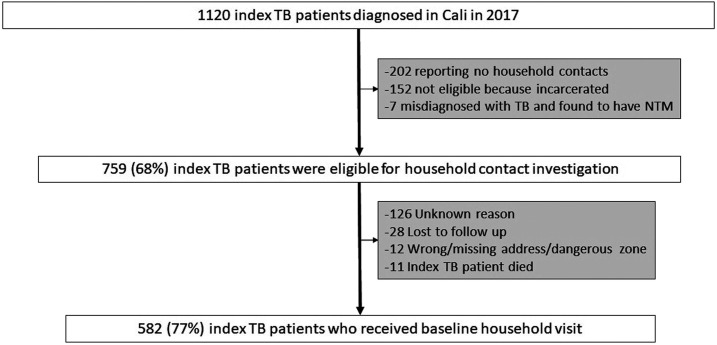
There were 1,120 patients diagnosed and registered as index TB patients in Cali in 2017 (Figure 1). Of these, 759 (68%) were eligible for household contact investigation. The remaining 361 (32%) index patients were ineligible because of incarceration (152/1,120, 14%), living alone (202/1,120, 18%), or being later found to have nontuberculous mycobacterial infection instead of TB (7/1,120, 0.6%). A baseline household visit was completed for 582 of 759 eligible index TB patients (77%, 95% CI: 76–82).
Figure 1.
Process evaluation of key steps in household TB contact investigation for index TB patients. NTM = nontuberculous mycobacteria; TB = tuberculosis.
Characteristics of index patients eligible for household visits.
A majority of index patients eligible for household visits were men (n = 444, 58%; Table 1). The median age of index patients was 45 years (interquartile range, IQR: 28–60), with 506 (67%) patients of working age (18–59 years). There were 73 (10%) eligible index TB patients who were also PLHIV. Most patients had bacteriologically confirmed pulmonary TB (n = 554, 73%), whereas 97 (13%) had clinically diagnosed pulmonary TB, and 108 (14%) had extrapulmonary TB. Most had private insurance (n = 406, 53%), a substantial proportion had public insurance (n = 310, 41%), and very few were uninsured (n = 43, 6%). The median duration of symptoms before diagnosis was 66 days (IQR: 33–140).
Table 1.
Demographic and clinical characteristics of index patients eligible for household visits
| Patient characteristic | N (%) |
|---|---|
| (n = 759) | |
| Median age-group (IQR) (years) | 45 (28–60) |
| < 18 | 59 (8) |
| 18–40 | 281 (37) |
| 41–59 | 225 (30) |
| ≥ 60 | 194 (25) |
| Male gender | 444 (58) |
| Persons living with HIV | 73 (10) |
| Type of insurance | |
| Private | 406 (53) |
| Public | 310 (41) |
| No insurance | 43 (6) |
| Type of TB | |
| Pulmonary bacteriologically confirmed* | 554 (73) |
| Pulmonary clinically diagnosed | 97 (13) |
| Extrapulmonary | 108 (14) |
| Household characteristics† | |
| Lack of ventilation (lack of light and/or crowding) | 67 (11) |
| Lack of sunlight and/or crowding‡ | 33 (6) |
| Median duration of symptoms (IQR) (days)ɨ | 66 (33–140) |
IQR = interquartile range; TB = tuberculosis.
By sputum smear microscopy examination and/or sputum culture.
Available only for 582 visited households.
Crowding defined by the National Administrative Department of Statistics of Colombia as > 3 people living in the same room.
Predictors of index TB patient household visit.
In bivariate analysis, several variables were associated with completing the initial household visit, including gender, and city zone of residence (Table 2). In a multivariable model adjusted for age and smear results, the odds of household visit completion were lower among male index patients (adjusted odds ratio [aOR]: 0.6; 95% CI: 0.4–0.9; P = 0.009) and those living in the city’s western zone (aOR: 0.5; 95% CI: 0.3–0.8; P = 0.008) than among those in the city’s northern zone (the reference).
Table 2.
Predictors of completion of baseline household visit
| Household visit completed | ||||||
|---|---|---|---|---|---|---|
| Index patient characteristics | Yes (%) | No (%) | Bivariate analysis | Multivariable analysis | ||
| (n = 759) | (n = 582) | (n = 177) | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Age-group (years) | 0.36* | |||||
| < 18 | 46 (78) | 13 (22) | 1 | – | 1 | – |
| 18–40 | 220 (78) | 61 (22) | 1.0 (0.5–2.0) | 0.96 | 0.8 (0.3–1.8) | 0.56 |
| 41–59 | 163 (72) | 62 (28) | 0.7 (0.4–1.5) | 0.39 | 0.6 (0.3–1.5) | 0.29 |
| ≥ 60 | 153 (79) | 41 (21) | 1.0 (0.5–2.1) | 0.89 | 0.8 (0.3–1.9) | 0.61 |
| Gender | ||||||
| Female | 258 (82) | 57 (18) | 1 | – | – | – |
| Male | 324 (73) | 120 (27) | 0.6 (0.4–0.8) | 0.004 | 0.6 (0.4–0.9) | 0.009 |
| Person living with HIV | ||||||
| No | 526 (77) | 160 (23) | 1 | – | – | – |
| Yes | 56 (77) | 17 (23) | 1.0 (0.6–1.8) | 0.99 | – | – |
| Diabetes mellitus | ||||||
| No | 530 (77) | 161 (23) | 1 | – | – | – |
| Yes | 52 (76) | 16 (24) | 1.0 (0.5–1.8) | 0.97 | – | – |
| Type of TB | ||||||
| Pulmonary | 499 (77) | 152 (23) | 1.0 (0.6–1.6) | 0.96 | – | – |
| Extrapulmonary | 83 (78) | 25 (22) | 1 | – | – | |
| Type of insurance | ||||||
| Private | 307 (76) | 99 (24) | 0.9 (0.6–1.2) | 0.45 | – | – |
| Public/uninsured | 275 (78) | 88 (22) | 1 | – | – | – |
| Sputum acid-fast bacilli smear | ||||||
| Negative | 264 (74) | 92 (26) | 1 | – | – | 1 |
| Positive | 318 (79) | 85 (21) | 1.2 (0.8–1.7) | 0.29 | 1.3 (0.9–1.8) | 0.17 |
| Socioeconomic status† | ||||||
| Low | 534 (76) | 165 (24) | 1 | – | – | – |
| High | 48 (80) | 12 (20) | 1.2 (0.6–2.4) | 0.53 | – | – |
| City zone‡ | 0.002 | |||||
| Central | 107 (73) | 40 (27) | 0.5 (0.3–0.9) | 0.03 | 0.6 (0.3–1.2) | 0.16 |
| Southeast | 182 (82) | 39 (17) | 0.9 (0.5–1.6) | 0.72 | 0.9 (0.5–1.6) | 0.75 |
| Western | 182 (71) | 76 (29) | 0.5 (0.3–0.8) | 0.005 | 0.5 (0.3–0.8) | 0.008 |
| North | 109 (84) | 21 (16) | 1 | – | – | – |
OR = odds ratio; TB = tuberculosis. Statistically significant associations at the P < 0.05 level for multivariable analysis shown in bold.
Pearson chi square.
Based on the socioeconomic distribution of households per neighborhood reported by the National Administrative Department of Statistics of Colombia.
Divisions of the city, according to the Planning Department of Cali (n = 756).
Characteristics of contacts.
The 582 index patients who received a baseline household visit identified 2046 contacts. Baseline visits occurred at a median of 37 days (IQR: 15–80) after the index patient initiated TB treatment; only 62 (11%) occurred within eight days as guidelines specify. A total of 1880 contacts, 92% of those enumerated, were encountered during the baseline visit, with a median of four (IQR: 3–6) contacts per household. Most were household contacts (n = 1,514, 81%); the remainder were non-household close contacts. Of all contacts, 52% (n = 975) had private insurance, 43% (n = 815) had public insurance, or 4% (n = 77) were uninsured. The median age was 31 years (IQR: 16–52), and the majority visited were women (n = 1,051, 56%). Two hundred eighteen (12%) reported productive cough for ≥ 2 weeks (Table 3). Of the 582 homes visited, 100 (17%) had one or more characteristics believed to increase the risk of TB transmission, without access to sunlight, and/or without outside ventilation; and/or with crowding (Table 1).
Table 3.
Demographic and clinical characteristics of contacts encountered during the household visit
| Contact characteristic | N (%) |
|---|---|
| (n = 1880) | |
| Median age-group (interquartile range) (years) | 31 (16–52) |
| ≤ 18 | 526 (28) |
| 18–40 | 613 (33) |
| 41–59 | 428 (23) |
| ≥ 60 | 297 (16) |
| Male gender | 828 (44) |
| Type of insurance | |
| Private | 975 (52) |
| Public | 815 (43) |
| No insurance | 77 (4) |
| Type of contact | |
| Household | 1,514 (81) |
| Non-household | 365 (19) |
| Clinical characteristics | |
| Cough for two or more weeks | 218 (12) |
| Self-reported diabetes mellitus | 46 (2) |
| Self-reported HIV | 8 (0.4) |
Contact investigation processes.
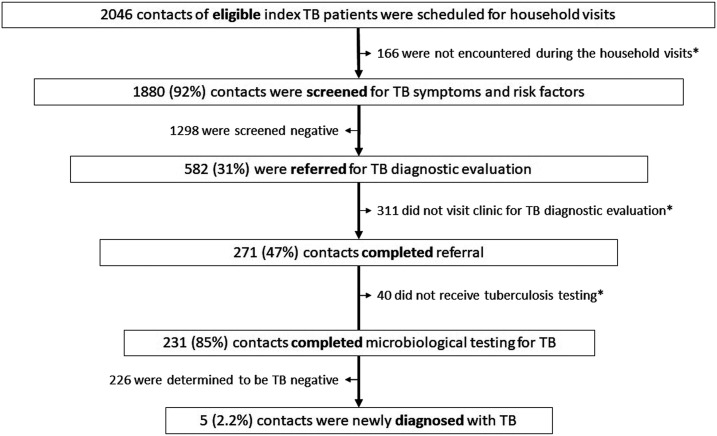
Figure 2 shows the flow of contacts through the investigation cascade. Among all contacts visited, 582 (31%) met one or more criteria for clinic referral for TB evaluation. Two hundred seventy-one (47%, 95% CI: 42–50) completed referral. Two hundred thirty-one (85%) contacts who completed referral underwent testing. All 231 had sputum smear microscopy; 54 (20%) had samples also tested by solid culture, and six others (3%) also underwent molecular testing by either GeneXpert MTB/RIF (Cepheid Inc., Sunnyvale, CA) or PCR-based reverse hybridization line probe assay. Sixty-five (24%) contacts underwent chest radiography, and 27 (41%) of these also underwent sputum testing. The cumulative probability that a symptomatic contact with indications for active TB evaluation would complete TB testing at a clinic at baseline was 28% (77% reached during the household visit, of whom 92% were found at home, of whom, 47% completed referral, of whom 85% were finally tested). The overall proportion of contacts bacteriologically confirmed to have active TB disease was 0.3% (5/1880). Of those eligible for preventive therapy, 16% (17/103) of children aged < 5 years, and none (0/8) of the contacts who were PLHIV initiated preventive treatment.
Figure 2.
Process evaluation of key steps in TB contact investigation for household and close contacts. *Contacts inappropriately lost from the contact investigation process. Those appropriately removed on completion of the contact evaluation process are shown to the left side of the figure.
Subsequent household visits.
One hundred and twenty-six (22%) households received a second visit, and 184 (32%) received all three visits. Second visits occurred at a median of 224 days (IQR: 192–276) after diagnosis, whereas third visits occurred at a median of 398 days (IQR: 367–482) after diagnosis. At the second visit, only 18 new contacts were referred for TB evaluation and 56% (10/18) completed evaluation. For the third visit, only five new contacts were referred and all completed TB evaluation, and no new TB patients were reported out of these subsequent visits.
Predictors of completion of clinic referral for the baseline visit.
Completion of clinic referrals was highly clustered by index patient (ICC: 0.68; 95% CI: 0.51–0.81). Bivariate analyses adjusted for clustering identified several variables associated with referral completion (P ≤ 0.20), including age, higher socioeconomic stratum, being a household rather than a non-household close contact, having the same insurer as the index patient, city zone of the household, productive cough ≥ 2 weeks, being a PLHIV, and having diabetes (Table 4. After adjusting for age, diabetes, type of contact, and socioeconomic stratum as fixed effects, and for household as a random effect, we found higher odds of referral completion among contacts with productive cough for ≥ 2 weeks (aOR: 21.6; 95% CI: 7.1–66; P < 0.001), those who were PLHIV (aOR: 68.1; 95% CI: 2.3–2008.7; P < 0.015), and those living in the western zone of the city (aOR: 4.1; 95% CI: 1.2–14.5; P = 0.03) than among those in the northern zone (Table 4). Quadrature checking confirmed the validity of our mixed-effects model (relative difference < 0.01 among 8, 12, and 16 integration points).
Table 4.
Bivariate and multivariable predictors of completing clinic referral among referred contacts, adjusted for clustering by index patient
| Referral completed | Bivariate analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| Characteristic of referred contacts | Yes (%) | No (%) | OR* | P-value | AOR | P-value |
| (n = 582) | (n = 271) | (n = 311) | (95% CI) | (95% CI) | ||
| Age-group (years) | < 0.001 | |||||
| < 18 | 59 (42) | 82 (58) | – | – | 1 | – |
| 18–40 | 48 (72) | 19 (28) | 5.8 (1.9–16.9) | 0.001 | 0.9 (0.3–2.9) | 0.8 |
| 41–59 | 44 (61) | 28 (39) | 4.1 (1.4–11.7) | 0.008 | 1.1 (0.3–3.5) | 0.9 |
| ≥ 60 | 117 (41) | 169 (59) | 0.9 (0.4–1.9) | 0.7 | 1.6 (0.7–3.8) | 0.2 |
| Gender | ||||||
| Female | 155 (46) | 185 (54) | – | – | – | – |
| Male | 116 (48) | 126 (52) | 1.1 (0.6–1.9) | 0.8 | – | – |
| Persons living with HIV | ||||||
| No | 265 (46) | 309 (54) | – | – | – | – |
| Yes | 6 (75) | 2 (25) | 32.0 (1.4–749.5) | 0.03 | 68.1 (2.3–2009) | 0.015 |
| Diabetes mellitus: | ||||||
| No | 261 (49) | 275 (51) | – | – | – | – |
| Yes | 10 (22) | 36 (78) | 0.2 (0.05–0.6) | 0.008 | 0.3 (0.09–1.2) | 0.09 |
| Productive cough (≥ 2 weeks) | ||||||
| No | 113 (31) | 251 (69) | – | – | – | – |
| Yes | 158 (72) | 60 (28) | 19.9 (8.1–48.7) | < 0.001 | 21.6 (7.1–66) | < 0.001 |
| Household contact | ||||||
| No | 39 (36) | 69 (64) | – | – | 1 | – |
| Yes | 232 (49) | 242 (51) | 3.2 (1.1–9.1) | 0.03 | 1.8 (0.6–4.8) | 0.27 |
| Same insurer† | ||||||
| No | 239 (46) | 283 (54) | – | – | 1 | – |
| Yes | 32 (53) | 28 (47) | 2.4 (0.7–8.6) | 0.18 | 1.9 (0.6–6.5) | 0.29 |
| Socioeconomic status‡ | ||||||
| Lower | 246 (46) | 293 (54) | – | – | 1 | – |
| Higher | 25 (58) | 18 (42) | 3.3 (0.7–16.0) | 0.14 | 2.6 (0.5–13) | 0.24 |
| City zone§ | 0.03 | |||||
| Western | 86 (51) | 84 (49) | 4.1 (1.1–14.7) | 0.03 | 4.1 (1.2–15) | 0.03 |
| Central | 79 (59) | 56 (41) | 6.6 (1.6–26.7) | 0.008 | 3.7 (0.98–14) | 0.054 |
| Southeast | 65 (40) | 97 (60) | 1.5 (0.4–5.1) | 0.52 | 2.0 (0.6–6.7) | 0.27 |
| North | 40 (35) | 73 (65) | – | – | 1 | – |
aOR = adjusted odds ratio; OR = odds ratio; TB = tuberculosis. Statistically significant associations at the P < 0.05 level shown in bold.
Adjusted only for clustering by index patient.
When contact and index patient had the same insurer.
Socioeconomic status based on the socioeconomic distribution of households per neighborhood reported by the National Administrative Department of Statistics of Colombia.
Divisions of the city according to the planning department of Cali.
DISCUSSION
Household TB contact investigation is an evidence-based strategy to increase identification of undiagnosed TB and offers additional opportunities to provide preventive therapy to high-risk household members, while also supporting index TB patients during treatment.7 We were invited by the Secretary of Health of Cali to investigate why the yield of active TB patients from their contact investigation program has been unexpectedly low over several years. We identified numerous gaps in the contact-tracing cascade, suggesting that the process cumulatively reached less than a third of all contacts with active TB. There were three factors that likely contributed most to this low yield: 1) one-quarter of index patients never received a home visit, 2) TB evaluation at health facilities was not achieved for almost half of eligible contacts, and 3) about 80% of contacts who were evaluated at clinics did not receive a highly sensitive, molecular, or culture-based diagnostic test for TB.
First, we found that only 77% percent of index TB patients received a baseline household visit, although guidelines from the Colombian National Institute of Health recommend that all newly diagnosed TB patients be referred for contact tracing. We hypothesize that social conflicts such as poverty and violence could be hampering access of health teams to some areas of the city, a situation that could be potentiated by the fact that home visitors are predominantly female. For instance, contact tracing was less likely to occur in patients living in Cali’s western zone, an area with high rates of poverty (80% of households in lowest income sextile) and violent crime (88 homicides/100,000 inhabitants in 2019).22–24 Recent ecologic studies have shown a substantial impact of violence on arboviral surveillance activities conducted in the same zone of the city, highlighting the importance of integrating measures of social disparities and conflict into reports of case finding for infectious diseases.25,26 Another recent study, conducted in a violent, overcrowded urban slum in Brazil, demonstrated that training and hiring community members to deliver TB services increased treatment success and cure proportions among TB patients from that community.27 The Secretary of Health of Cali could consider a similar strategy of hiring (or involving volunteers) local residents to perform contact investigation activities in the western zone.
In addition, we found that almost 20% of all TB patients did not receive a household visit because they reported having no contacts. A study from Peru that also examined routine TB contact investigation data reported a similar proportion of index patients without contacts.28 Although some TB patients may be living alone, providers should consider that some patients may fail to report contacts because of fear of disclosing their TB status to relatives, friends, and neighbors and experiencing stigma as a result. Stigma is a well-described reason preventing TB patients from completing medical follow-up and treatment.29 Currently, we are conducting additional qualitative studies to better understand why some patients either directly or indirectly decline consent for contact tracing.
Second, there was also significant dropout of contacts who were referred for TB evaluation in clinics. Not surprisingly, contacts with productive cough were more likely to complete referrals; however, one-third of those still did not reach the clinic. A similar finding was reported from a study conducted in Uganda, where only 20% of all contacts who were possible TB patients visited a clinic to complete TB evaluation.12 Another study of routine contact investigation conducted in Peru showed that only 42% of contacts were evaluated in clinics.30 A recent scoping review reported lack of patient-centered approaches and poor accessibility to health services among the most important barriers to contact tracing.31 Some potential interventions for addressing the barriers identified in Cali and elsewhere include introducing index patients to home visitors at the time of diagnosis; equipping health workers to collect sputum at home; offering contacts transport vouchers to attend clinic when indicated; reinforcing the educational component during the visits on factors associated with TB, promoting the capacity for risk self-assessment; and increasing the sensitivity of diagnostic testing. In addition, telephone contact tracing is now being widely applied during the COVID-19 pandemic, and this strategy could be similarly used to improve TB contact tracing, by helping frontline health workers better access hard to reach areas of the city for baseline and follow-up screenings, and by enabling more referred contacts to complete some aspects of evaluation remotely by linking telephone contact tracing with home-based sputum collection.
With a number needed to screen of 376 to identify one new TB patient and very few patients initiated on preventive therapy, the public health value of contact tracing in Cali was minimal, especially compared with other similar settings.10,11 For example, the yield was 2-fold higher in Brazil32 and 6-fold higher in Peru.33 Several factors could explain this low yield. First, Colombia’s symptom screening protocol, by the time we conducted this study, included only cough for ≥ 2 weeks, which identifies only about one-third of culture-confirmed cases according to a WHO-commissioned systematic review of active case finding.34 Expanding this definition to cough of any duration and adding fever, night sweats, and weight loss doubles pooled sensitivity for bacteriologically confirmed pulmonary TB to 70%, although at a cost of decreased specificity (from 95% to 61%) and positive predictive value. The review also found that adding chest radiography could further increase pooled sensitivity up to 98%. Second, only one-fifth of the contacts who reached clinical evaluation had a highly sensitive culture-based test, and only a tiny proportion (3%) had a molecular test. As the End TB Strategy challenges countries to increase the proportion of all incident TB patients who are diagnosed and treated from 69% to ≥ 90% by 2025, the WHO’s recommendation that all countries adopt universal molecular testing is critical.35 Current Colombian guidelines recommend molecular testing and liquid culture on sputum samples of contacts with cough of any duration (National Regulation 00227 from 2020).
People sharing a household with a pulmonary TB patient are at higher risk of developing latent TB infection, with younger children (< 5 years) and PLHIV at highest risk for developing TB disease.8 Colombia’s national guidelines recommend that contacts aged < 5 years and PLHIV should be screened for TB, and if TB is excluded, TPT should be started, setting a TPT goal ≥ 90% for younger children (< 5 years) who are contacts of pulmonary TB patients by 2025.36 In our study, initiation of TPT was reported low, with less than one-fifth of children aged < 5 years and none of the eight PLHIV started on treatment. These figures are considerably lower than those observed in studies from Lima, Peru, where between 70% and 89% of contacts aged < 5 years started preventive therapy.28,30 This is important because younger children are at a higher risk of morbidity and mortality due to TB disease, especially in LMICs.37,38 Preventive therapy has been recognized by the WHO as a key element for TB elimination,35 particularly in low and intermediate TB-incidence countries such as Colombia. Future efforts should be focused on identifying and addressing barriers to prescribing, initiating, and completing TPT in children aged < 5 years and other high-risk groups living with TB patients.
Our study has some limitations. First, data abstraction was retrospective, which limited our analysis to variables that had already been collected. Whereas most of the important process measures were carefully and comprehensively recorded, the key outcome of referral completion was based on the results of laboratory testing, chest radiography, and data on prescription of TPT, as surrogate measures for confirmation of clinical evaluation. This may have led to some outcome misclassification, and it is uncertain how documentation of these services might differ according to the characteristics of individuals or their insurance coverage. Second, we lacked important information about the reasons why some participants did not receive or complete key steps in the contact-tracing process, including home and clinic visits. Ongoing qualitative studies with contact-tracing teams and participants sampled based on outcomes may help elucidate barriers and facilitators of these processes, and inform the design of targeted interventions to address these factors. Finally, we failed to identify significant individual or household predictors of household visits or clinical follow-up. Completion of clinic visits was highly clustered within households, suggesting that there may be predictive characteristics that either were not measured (there was not available information on familial relationship) or were not measured precisely enough (this characteristic was derived from the household location and data from the National Department of Statistics of Colombia).
Our study also has several strengths. First, we leveraged operational data from an entire year of contact tracing with a relatively low rate of missing data, while working directly with public health providers to define the research questions and map out choke points in implementation. Second, we conducted a formal process evaluation of the data, following a thorough cascade analysis approach. Finally, to the best of our knowledge, this is the first implementation research evaluation of TB contact tracing in Colombia, providing a relevant contribution to the understanding of this important intervention for TB elimination in the country.
Some of the possible facilitation strategies generated from this study may also be worth testing in the context of contact tracing for COVID-19, for which the WHO has issued thorough interim guidance.39 First, contact tracing for COVID-19 may benefit from active participation of community members, especially in neighborhoods with low rates of successful TB contact investigation, whether due to the “invisible barriers generated by areas controlled by illegal gangs” that make outsiders unwelcome, urban violence, and complex local geography, or other contextual factors. Specifically, special planning including public health messaging, two-way communications, and specific supports may be necessary to address social determinants including poverty and a loss of employment due to COVID-19 illness or exposure, a lack of housing or marginal housing characterized by crowding and poor ventilation, lack of education, and poor access to health services. Second, home-based testing may be critical to overcome barriers to visiting health facilities such as fear of the health and social consequences of a confirmed COVID-19 diagnosis, just as for TB. Finally, building rapport with the index patient during the initial interview may be important to obtain complete and accurate information that facilitates contact tracing, including a working phone number, email address, and alternative contact persons able to get in touch with contacts if needed.
In conclusion, our study showed that the three most important gaps in TB contact investigation in Cali occurred between diagnosis and completion of a household visit, between contact referral and completion of a clinic visit, and during TB testing. Systematic process analyses of TB programmatic data could be adapted for more efficient operational use to identify barriers and target solutions. Future efforts should focus on facilitating health worker visits to TB index households, designing home-based strategies to overcome barriers linked to evaluation, and increasing access to molecular testing.
ACKNOWLEDGMENTS
We want to thank the Secretary of Health of Cali, the staff at the Cali Tuberculosis Program, and all the other members of Alianza-TB.
REFERENCES
- 1.Houben RM, Dodd PJ, 2016. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med 13: e1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization , 2019. Global Tuberculosis Report, 2019. Available at: https://www.who.int/tb/publications/global_report/en/. Accessed March 5, 2020. [Google Scholar]
- 3.Pan American Health Organization , 2018. The End TB Strategy: Main Indicators in the Americas. Available at: https://www.paho.org/hq/index.php?option=com_docman&view=download&category_slug=factsheets-5632&alias=44188-the-end-tb-strategy-main-indicators-americas-2018-188&Itemid=270&lang=en. Accessed January 10, 2020. [Google Scholar]
- 4.Fox GJ, et al. 2018. Household-contact investigation for detection of tuberculosis in Vietnam. N Engl J Med 378: 221–229. [DOI] [PubMed] [Google Scholar]
- 5.Ayles H, et al. 2013. Effect of household and community interventions on the burden of tuberculosis in southern Africa: the ZAMSTAR community-randomised trial. Lancet 382: 1183–1194. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization , 2012. Recommendations for Investigating Contacts of Persons with Infectious Tuberculosis in Low- and Middle-Income Countries. Geneva, Switzerland: WHO. Available at: https://www.who.int/tb/publications/2012/contact_investigation2012/en/. Accessed November 26, 2019. [PubMed] [Google Scholar]
- 7.Fox GJ, Dodd PJ, Marais BJ, 2019, Household contact investigation to improve tuberculosis control. Lancet Infect Dis 19: 235–237. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization , 2018. Latent Tuberculosis Infection: Updated and Consolidated Guidelines for Programmatic Management. Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 9.Pan American Health Organization , World Health Organization , 2016. Framework for Tuberculosis Control in Large Cities of Latin America and the Caribbean. Geneva, Switzerland: WHO. Available at: https://www.paho.org/en/documents/framework-tuberculosis-control-large-cities-latin-america-and-caribbean-2016. Accessed April 15, 2020. [Google Scholar]
- 10.Fox GJ, Barry SE, Britton WJ, Marks GB, 2013. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J 41: 140–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison J, Pai M, Hopewell PC, 2008. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis 8: 359–368. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong-Hough M, et al. 2017. Drop-out from the tuberculosis contact investigation cascade in a routine public health setting in urban Uganda: a prospective, multi-center study. PLoS One 12: e0187145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore GF, et al. 2015. Process evaluation of complex interventions: medical research council guidance. BMJ 350: h1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization , Regional Office for the Eastern Mediterranean , 2017. HIV Test-Treat-Retain Cascade Analysis: Guide and Tools 2017, 2nd edition. Geneva, Switzerland: WHO. Available at: https://apps.who.int/iris/handle/10665/250533. Accessed October 12, 2019. [Google Scholar]
- 15.Subbaraman R, Nathavitharana RR, Satyanarayana S, Pai M, Thomas BE, Chadha VK, Rade K, Swaminathan S, Mayer KH, 2016. The tuberculosis cascade of care in India’s public sector: a systematic review and meta-analysis. PLoS Med 13: e1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subbaraman R, Nathavitharana RR, Mayer KH, Satyanarayana S, Chadha VK, Arinaminpathy N, Pai M, 2019. Constructing care cascades for active tuberculosis: a strategy for program monitoring and identifying gaps in quality of care. PLoS Med 16: e1002754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chin DP, Hanson CL, 2017. Finding the missing tuberculosis patients. J Infect Dis 216 (Suppl 7): S675–S678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanson CL, Osberg M, Brown J, Durham G, Chin DP, 2017. Conducting patient-pathway analysis to inform programming of tuberculosis services: methods. J Infect Dis 216 (Suppl 7): S679–s685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Secretaría de Salud Pública Municipal de Cali , Alcaldia de Santiago de Cali , 2019. Informe de Vigilancia en Salud Pública de Tuberculosis (evento 813), semana epidemiológicas 1–52, AÑO, 2019. Cali, Colombia: Alcaldía de Santiago de Cali. (Document in Spanish). [Google Scholar]
- 20.Ministerio de Salud y Protección Social de Colombia. Instituto Nacional de Salud , 2018. Protocolo de vigililancia en Salud Publica. Bogotá, Colombia: INS. (Document in Spanish). [Google Scholar]
- 21.Ministerio de Protección Social de Colombia , Universidad Nacional de Colombia , 2007. Guia de promocion de salud y prevencion de enfermedades en la salud pública. Bogotá-Colombia: Scripto Ltda, Vol. 2 (Document in Spanish). [Google Scholar]
- 22.Departamento Administrativo de Planeación , Alcaldia de Santiago de Cali , 2016. Cali en Cifras. Cali, Colombia: Alcaldía de Santiago de Cali, 184 (Document in Spanish). [Google Scholar]
- 23.Observatorio de Seguridad , Secretaría de Seguridad y Justicia , Alcaldia de Santiago de Cali , 2019. Informe Homicidios por Comuna Enero 01 Dec 18 de 2019. Cali, Colombia: Alcaldía de Santiago de Cali. [Google Scholar]
- 24.Poveda AC, 2011. Economic development, inequality and poverty: an analysis of urban violence in Colombia. Oxford Development Stud 39: 453–468. [Google Scholar]
- 25.Krystosik AR, Curtis A, Buritica P, Ajayakumar J, Squires R, Dávalos D, Pacheco R, Bhatta MP, James MA, 2017. Community context and sub-neighborhood scale detail to explain dengue, chikungunya and Zika patterns in Cali, Colombia. PLoS One 12: e0181208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krystosik AR, Curtis A, LaBeaud AD, Dávalos DM, Pacheco R, Buritica P, Álvarez Á, Bhatta MP, Rojas Palacios JH, James MA, 2018. Neighborhood violence impacts disease control and surveillance: case study of cali, Colombia from 2014 to 2016. Int J Environ Res Public Health 15: 2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soares EC, et al. 2013. Tuberculosis control in a socially vulnerable area: a community intervention beyond DOT in a Brazilian favela. Int J Tuberc Lung Dis 17: 1581–1586. [DOI] [PubMed] [Google Scholar]
- 28.Otero L, Battaglioli T, Ríos J, De la Torre Z, Trocones N, Ordoñez C, Seas C, Van der Stuyft P, 2020. Contact evaluation and isoniazid preventive therapy among close and household contacts of tuberculosis patients in Lima, Peru: an analysis of routine data. Trop Med Int Health 25: 346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Courtwright A, Turner AN, 2010. Tuberculosis and stigmatization: pathways and interventions. Public Health Rep 125 (Suppl 4): 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuen CM, Millones AK, Contreras CC, Lecca L, Becerra MC, Keshavjee S, 2019. Tuberculosis household accompaniment to improve the contact management cascade: a prospective cohort study. PLoS One 14: e0217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biermann O, Lönnroth K, Caws M, Viney K, 2019. Factors influencing active tuberculosis case-finding policy development and implementation: a scoping review. BMJ Open 9: e031284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gazetta CE, Ruffino-Netto A, Pinto Neto JM, Santos MeL, Cury MR, Vendramini SH, Villa TC, 2006. Investigation of tuberculosis contacts in the tuberculosis control program of a medium-sized municipality in the southeast of Brazil in 2002. J Bras Pneumol 32: 559–565. [DOI] [PubMed] [Google Scholar]
- 33.Otero L, Shah L, Verdonck K, Battaglioli T, Brewer T, Gotuzzo E, Seas C, Van der Stuyft P, 2016. A prospective longitudinal study of tuberculosis among household contacts of smear-positive tuberculosis cases in Lima, Peru. BMC Infect Dis 16: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van’t Hoog AH, Langendam MW, Mitchell ZE, Cobelens FG, Sinclair D, Leeflang MMG, Lonnroth K, 2013. A Systematic Review of the Sensitivity and Specificity of Symptom- and Chest-Radiography Screening for Active Pulmonary Tuberculosis in HIV-Negative Persons and Persons with Unknown HIV Status . Geneva, Switzerland: World Health Organization. [Google Scholar]
- 35.World Health Organization , 2014. The End TB Strategy: Global Strategy and Targets for Tuberculosis Prevention, Care and Control after 2015 . Geneva, Switzerland: WHO. Available at: https://who.int/tb/strategy/End_TB_Strategy.pdf. Accessed December 12, 2019. [Google Scholar]
- 36.Ministerio de Salud y Protección Social de Colombia , 2016. Plan estratégico: “Hacia el Fin de la Tuberculosis”. Convenio 519 de 2015 Bogotá, Colombia: MSPS. (Document in Spanish). [Google Scholar]
- 37.Gomes VF, Andersen A, Wejse C, Oliveira I, Vieira FJ, Joaquim LC, Vieira CS, Aaby P, Gustafson P, 2011. Impact of tuberculosis exposure at home on mortality in children under 5 years of age in Guinea-Bissau. Thorax 66: 163–167. [DOI] [PubMed] [Google Scholar]
- 38.Perez-Velez CM, Marais BJ, 2012. Tuberculosis in children. N Engl J Med 367: 348–361. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization , 2020. Contact Tracing in Context of COVID-19. Interim Guidence . Geneva, Switzerland: WHO. WHO/2019-nCoV/Contact_Tracing/2020.1. Available at: https://www.who.int/publications/i/item/contact-tracing-in-the-context-of-covid-19. Accessed August 15, 2020. [Google Scholar]