ABSTRACT
Knowledge of the clinical progress of severe fever with thrombocytopenia syndrome (SFTS) and the associated predictors of mortality is important for providing appropriate treatment in severe cases. A multihospital retrospective study was conducted in three SFTS-endemic cities, in 2018. Of the 208 SFTS-confirmed cases, there were 189 survivors and 19 deaths. The median age was 64 years; 104 (50.0%) patients were men, and 188 (90.4%) were farmers. Furthermore, 203 (97.6%) patients reported fever and 70 (33.7%) reported fatigue. Most fatal cases had complications including multiple-organ failure, central nervous syndrome (CNS) abnormalities, and disseminated intravascular coagulation. During the fever phase, alanine transaminase, aspartate aminotransferase (AST), blood urea nitrogen (BUN), creatinine, D-dimer, glucose, hydroxybutyrate dehydrogenase, lactate dehydrogenase (LDH), procalcitonin, prothrombin time, and uric acid levels were higher in fatal than in nonfatal cases (P < 0.05). Creatine kinase (CK), CK-MB (CKMB), AST, and LDH levels were significantly lower in nonfatal than in fatal cases (P < 0.05). Central nervous syndrome abnormalities (odds ratio [OR] = 20.9, 95% CI: 4.3, 100), body temperature ≥ 38.5°C (OR = 23.2, 95% CI: 3.4, 158), BUN levels ≥ 6.4 mmol/L (OR = 9.9, 95% CI: 2.2, 44), CKMB levels ≥ 100 U/L (OR = 33.2, 95% CI: 5.8, 192), and LDH levels ≥ 1,000 U/L (OR = 8.3, 95% CI: 1.9, 37) were predictors of mortality. Our findings reveal that the presence of specific complications and laboratory parameters may serve as predictors of mortality and aid in early identification of severe SFTS cases in clinical practice.
INTRODUCTION
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging tick-borne infectious disease caused by the SFTS virus (SFTSV) and transmitted via bites of the tick Haemaphysalis longicornis.1,2 Severe fever with thrombocytopenia syndrome virus belongs to the genus Bandavirus and family Phenuiviridae and was first isolated from the specimens of Chinese patients in 2009.3 This virus is related to, but distinctly different from, the Heartland virus.4 Following the confirmation of the presence of SFTSV, the associated infections have become a serious public health threat in Asia. Severe fever with thrombocytopenia syndrome virus infections have been reported in China, Japan, South Korea, and Vietnam.4–7 According to the China Information System for Disease Control and Prevention, China recorded the highest number of SFTS cases, with more than 23 provinces affected. The number of affected countries from 2011 to 2016 increased sharply from 98 to 167.8,9 Anhui Province is an SFTSV-endemic area; until December 31, 2017, 1,506 patients were diagnosed with SFTSV, and seven person-to-person transmissions were recorded.10
Severe fever with thrombocytopenia syndrome virus infections are characterized by a wide spectrum of clinical symptoms including fever, thrombocytopenia, leukocytopenia, and hemorrhagic symptoms.11,12 In addition, the commonly elevated laboratory parameters are serum aspartate aminotransferase (AST), alanine transaminase (ALT), blood urea nitrogen (BUN), uric acid (UA), creatine kinase (CK), and lactate dehydrogenase (LDH).13 Some patients with severe infection develop immediate clinical symptoms; the occurrence of shock, respiratory failure, acute respiratory distress syndrome (ARDS), disseminated intravascular coagulation (DIC), central nervous syndrome (CNS), or multiple-organ failures (MOFs) leads to a higher mortality rate in these patients from 11.2% to 30.0%.11,12
Several studies have focused on the risk factors for mortality among patients with SFTS. However, to the best of our knowledge, these studies did not conduct a comprehensive investigation of the associated factors and also lacked a considerable sample size. We conducted a hospital-based retrospective study to identify the risk factors for mortality including demographic features, clinical symptoms, and laboratory parameters in Anhui Province, China. Our findings can provide evidence to increase the understanding of SFTS and assist in early recognition of symptoms that might lead to death.
METHODS
Study design.
This hospital-based retrospective study conducted in 2018 included SFTS cases from four sentinel hospitals located in three SFTSV-endemic areas.
Study settings and patients.
Three cities, that is, Hefei, Lu’an, and Chaohu, in Anhui Province were chosen for the study. In these cities, the annual reported SFTS cases accounted for 70% of provincial patients. Of the four sentinel hospitals selected as study sites, two hospitals were from Hefei and one each from Lu’an and Chaohu.
The inclusion criteria were as follows: 1) hospitalization in one of the four hospitals between January 2015 and June 2018; 2) acute fevers ≥ 37.5°C, leukopenia, and/or thrombocytopenia, or other SFTS-like symptoms; and 3) laboratory confirmation of SFTSV infection through the detection of specific IgM using an ELISA and/or the positive result for SFTSV nucleic acid using PCR, as previously described.14,15 Patients diagnosed with any known blood system disease were excluded.
Definitions.
Acute respiratory distress syndrome was defined as a patient having an acute onset of illness with a PaO2/FIO2 ≤ 200 mmHg, regardless of the positive end-expiratory pressure (PEEP), bilateral infiltrates observed in a frontal chest radiograph, and a pulmonary arterial wedge pressure ≤ 18 mmHg, with no clinical evidence of left atrial hypertension.16 Multiple-organ failure was defined by the existence of two or more of following conditions: 1) progressive ARDS requiring PEEP > 10 cm H2O and FIO2 > 0.5, 2) clinical jaundice with bilirubin ≥ 8–10 mg/dL, 3) requirement for renal dialysis, 4) the stress ulcers requiring transfusions, acalculous cholecystitis, 5) DIC, 6) progressive coma, and 7) a hypodynamic response refractory to inotropic support.13
Central nervous syndrome abnormalities included apathy, seizures, muscular tremors, and coma, as previously described.17 Disseminated intravascular coagulation was scored in accordance with the scoring system of the International Society on Thrombosis and Haemostasis.16 The scoring system was based on platelet (PLT) counts (> 100 × 109 cells/L, 0; < 100 × 109 cells/L but < 50 × 109 cells/L, one; and > 50 × 109 cells/L, two); elevated fibrin-related markers (no increase, 0; moderate increase, two; and strong increase, three) (D-dimer [DD] was used); prolonged prothrombin times (PTs) (< 3 seconds, 0; > 3 seconds but < 6 seconds, one; and > 6 seconds, two); and fibrinogen levels (> 1.0 g/L, 0; < 1.0 g/L, one). A total score of ≥ 5 was considered to indicate overt DIC.
Questionnaire and data collection.
A medical review method was used to retrospectively collect data; this method consisted of three broad categories: first, demographic features such as hospital admitted to, age, gender, and occupation; second, clinical symptoms related to the disease including fever, chills, diarrhea, nausea, headache, and abdominal pain; and third, laboratory parameters during the clinical course including routine blood tests, biochemical tests, and coagulation function. The review was conducted by two investigators who checked all the information against the medical records.
Statistical analysis.
EpiData software version 3.1 (The EpiData Association, Odense, Denmark) was used for data entry; both investigators independently entered the data twice and compared the data later. Data analysis was conducted using SPSS software version 11.0 (SPSS Inc, Chicago, IL). The categorical demographic features and early clinical symptoms are reported as frequencies and percentages. The differences between the nonfatal cases were assessed using the chi-square test or Fisher’s exact test. The continuous variables such as age, critical time intervals, and laboratory parameters are reported as median and interquartile range; the differences between the nonfatal and fatal cases were assessed using the Mann–Whitney U test. Furthermore, the risk factors for mortality were analyzed using the binary logistic regression method, and odds ratios (ORs) with 95% CIs were reported. The most abnormal clinical indices during the clinical course were collected and analyzed; univariate analysis followed by the stepwise method of “Enter” was used. Factors with P < 0.10 were further selected and analyzed using the forward stepwise method “Forward LR” multivariate logistic regression analysis. The significance level (α) was set to 0.05.
Ethical considerations.
This retrospective investigation was part of the routine SFTS surveillance program of 2018. Therefore, the ethics committee of the Anhui Provincial CDC waived the requirement of ethics approval.
RESULTS
Demographic features and critical time interval of patients.
The demographic features and critical time interval of the patients are shown in Table 1. A total of 208 patients with SFTS were included in this study, consisting of 189 nonfatal cases and 19 fatal cases. Of the 208 cases, 39 (18.8%) were from hospital A, 57 (27.4%) from hospital B, 34 (16.3%) from hospital C, and 78 (37.5%) were from hospital D. Of the total number of patients, 104 (50.0%) were men and 188 (90.4%) were farmers; the median age of the total cohort was 64 years. No significant difference in demographic features was observed between the nonfatal and fatal cases. The median time interval in all SFTS cases from the onset of symptoms to hospitalization was 5 days; the median duration of hospitalization was 10 days, and the median duration of the clinical course length was 16 days. The duration of hospitalization and clinical course in the nonfatal cases was significantly longer than in the fatal cases (P < 0.001).
Table 1.
Demographic features and critical time intervals of severe fever with thrombocytopenia syndrome cases
| Variable | All (n = 208) | Nonfatal case (n = 189) | Fatal case (n = 19) | P-value |
|---|---|---|---|---|
| Hospital, n (%) | 0.464 | |||
| A | 39 (18.8) | 34 (18.0) | 5 (26.3) | |
| B | 57 (27.4) | 51 (27.0) | 6 (31.6) | |
| C | 34 (16.3) | 30 (15.9) | 4 (21.1) | |
| D | 78 (37.5) | 74 (39.2) | 4 (21.1) | |
| Gender, n (%) | 0.470 | |||
| Male | 104 (50.0) | 96 (50.8) | 8 (42.1) | |
| Female | 104 (50.0) | 93 (49.2) | 11 (57.9) | |
| Occupation, n (%) | 1.000* | |||
| Farmer | 188 (90.4) | 171 (90.5) | 17 (89.5) | |
| Housewife/worker/retiree | 20 (9.6) | 18 (9.5) | 2 (10.5) | |
| Age, median (IQR), years | 64 (54, 72) | 64.0 (53, 71.5) | 70.0 (62, 75) | 0.056# |
| Critical time interval, median (IQR) | ||||
| Duration of hospitalization | 10.0 (8.0, 14.0) | 10.0 (8.0, 14.0) | 5.0 (3.0, 8.0) | < 0.001† |
| Duration of clinical course | 16.0 (12.0, 20.0) | 16.0 (13.0, 20.0) | 8.0 (6.0, 12.0) | < 0.001† |
IQR = interquartile range. Data are presented as frequency (percentage) or median (IQR).
Fisher exact test.
Mann–Whitney U test.
Clinical symptoms and complications.
The clinical symptoms and complications of the study patients are presented in Table 2. Regarding clinical symptoms, 203 (97.6%) patients reported fever, 70 (33.7%) reported fatigue, and 60 (28.8%) reported chills. Regarding complications, 38 (18.3%) patients developed ARDS, 72 (34.6%) developed MOF, 60 (30.3%) developed CNS, and 77 (37.0%) exhibited DIC. The studied clinical symptoms were not significantly different between the nonfatal and fatal cases (Table 2). Of the studies’ complications, the development of MOF, CNS, and DIC was significantly higher among the fatal cases than among the nonfatal cases (P = 0.025, < 0.001, and 0.003, respectively).
Table 2.
Early clinical symptoms and complications of severe fever with thrombocytopenia syndrome cases
| Variable | All (n = 208) | Nonfatal case (n = 189) | Fatal case (n = 19) | P-value |
|---|---|---|---|---|
| Clinical symptoms, n (%) | ||||
| Fever | 203 (97.6) | 184 (97.4) | 19 (100.0) | 1.000* |
| Fatigue | 70 (33.7) | 63 (33.3) | 7 (36.8) | 0.758 |
| Chills | 60 (28.8) | 53 (28.0) | 7 (36.8) | 0.420 |
| Diarrhea | 49 (23.6) | 41 (21.7) | 8 (42.1) | 0.086 |
| Muscular soreness | 39 (18.8) | 36 (19.0) | 3 (15.8) | 0.969 |
| Nausea | 38 (18.3) | 34 (18.0) | 4 (21.1) | 0.986 |
| Vomiting | 28 (13.5) | 23 (12.2) | 5 (26.3) | 0.171 |
| Headache | 24 (11.5) | 24 (12.7) | 0 (0.0) | 0.099 |
| Abdominal pain | 20 (9.6) | 18 (9.5) | 2 (10.5) | 1.000 |
| Conjunctival hemorrhage | 1 (0.5) | 1 (0.5) | 0 (0.0) | 1.000* |
| Gingival bleeding | 4 (1.9) | 4 (2.1) | 0 (0.0) | 1.000* |
| Complications, n (%) | ||||
| Acute respiratory distress syndrome | 38 (18.3) | 32 (16.9) | 6 (31.6) | 0.206 |
| Multiple-organ failure | 72 (34.6) | 61 (32.3) | 11 (57.9) | 0.025 |
| Central nervous syndrome | 63 (30.3) | 50 (26.5) | 13 (68.4) | < 0.001 |
| Disseminated intravascular coagulation | 77 (37.0) | 64 (33.9) | 13 (68.4) | 0.003 |
Fisher exact test.
Laboratory parameters.
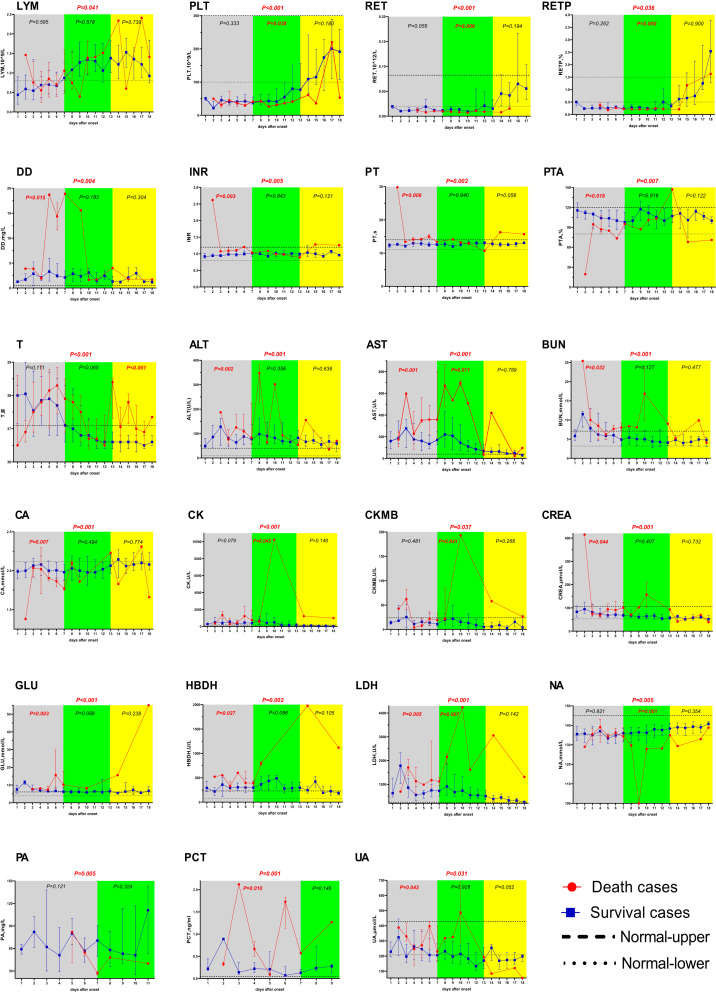
A total of 47 laboratory parameters were reviewed, and the most abnormal data during the clinical course were further compared; 22 parameters were significantly different between the two groups (Table 3). During the fever phase (0–7 days), ALT, AST, BUN, creatinine (CREA), DD, glucose (GLU), hydroxybutyrate dehydrogenase levels, international normalized ratio (INR), LDH, procalcitonin levels, PT, and UA levels among the fatal cases were significantly higher than those among the nonfatal cases (P = 0.001, < 0.001, < 0.001, 0.001, 0.004, < 0.001, 0.002, 0.005, < 0.001, 0.001, 0.002, and 0.031, respectively) (Figure 1); calcium levels and PT activity (PTA) among the fatal cases were significantly lower than those among the nonfatal cases (P = 0.001, P = 0.007, respectively). In the multi-organ dysfunction phase (8–13 days), the natrium PLT count and reticulocyte (RET) count among the fatal cases were significantly lower than those among the nonfatal cases (P < 0.001, P < 0.001, and P < 0.001, respectively); AST, CK, CK-MB (CKMB), and LDH levels among the fatal cases were significantly higher than those among the nonfatal cases (P < 0.001, P < 0.001, P = 0.037, and P < 0.001, respectively). During the convalescent phase, the body temperature recorded among the fatal cases was significantly higher than that recorded among the nonfatal cases (P < 0.001).
Table 3.
Comparison of clinical parameters between the nonfatal and fatal severe fever with thrombocytopenia syndrome cases
| Variable | All (n = 208) | Nonfatal case (n = 189) | Fatal case (n = 19) | P-value |
|---|---|---|---|---|
| Body temperature (°C) | 36.8 (36.6, 37.4) | 36.7 (36.5, 37.3) | 37.3 (36.8, 38.3) | < 0.001 |
| Lymphocyte (×109/L) | 1.2 (0.8, 1.6) | 1.1 (0.7, 1.6) | 0.8 (0.4, 1.5) | 0.041 |
| Platelet (×109/L) | 55.0 (39.0, 97.0) | 57.8 (35.0, 121.0) | 40.5 (29.8, 48.5) | < 0.001 |
| Reticulocyte (×1012/L) | 0.016 (0.010, 0.032) | 0.021 (0.012, 0.057) | 0.009 (0.008, 0.011) | < 0.001 |
| Reticulocyte count percentage (%) | 0.32 (0.23, 0.59) | 0.32 (0.23, 0.85) | 0.22 (0.21, 0.27) | 0.036 |
| D-dimer (mg/L) | 2.2 (1.2, 3.9) | 2.1 (1.1, 3.7) | 3.9 (1.7, 13.9) | 0.004 |
| International normalized ratio | 1.0 (0.9, 1.1) | 1.0 (0.9, 1.1) | 1.1 (1.0, 1.3) | 0.005 |
| PT (s) | 12.7 (12.2, 13.6) | 12.7 (12.0, 13.5) | 13.7 (13.1, 15.3) | 0.002 |
| PT activity (%) | 103.0 (93.0, 116.0) | 103.0 (92.9, 116.3) | 90.6 (72.9, 101.5) | 0.007 |
| Alanine transaminase (U/L) | 80.3 (51.0, 126.0) | 72.0 (47.0, 124.0) | 123.0 (73.0, 228.5) | 0.001 |
| Aspartate aminotransferase (U/L) | 118.0 (71.0, 207.0) | 110.5 (53.0, 224.0) | 365.0 (152.0, 623.0) | < 0.001 |
| Blood urea nitrogen (mmol/L) | 5.1 (3.7, 7.3) | 5.0 (3.5, 7.1) | 8.1 (5.3, 9.9) | < 0.001 |
| calcium (mmol/L) | 2.1 (1.9, 2.2) | 2.0 (1.9, 2.2) | 1.9 (1.8, 2.0) | 0.001 |
| CK (U/L) | 330.0 (161.9, 780.5) | 283.0 (108.0, 768.0) | 755.0 (388.0, 1,495.0) | < 0.001 |
| CKMB (U/L) | 12.8 (5.0, 26.0) | 12.5 (4.0, 24.0) | 21.5 (8.3, 46.6) | 0.037 |
| Creatinine (μmol/L) | 63.0 (55.0, 77.5) | 63.5 (52.0, 78.2) | 80.1 (59.8, 123.3) | 0.001 |
| Glucose (mmol/L) | 6.5 (5.5, 8.3) | 6.4 (5.4, 8.3) | 8.5 (6.8, 17.4) | < 0.001 |
| Hydroxybutyrate dehydrogenase (U/L) | 324.0 (221.5, 493.0) | 299.0 (206.5, 468.0) | 527.0 (297.0, 885.0) | 0.002 |
| Lactate dehydrogenase (U/L) | 591.8 (433.5, 1,050.8) | 555.5 (346.5, 972.3) | 1,459.0 (844.0, 2,407.0) | < 0.001 |
| Natrium (mmol/L) | 137.0 (133.8, 140.0) | 137.0 (133.4, 140.0) | 134.0 (130, 138.5) | 0.005 |
| Prealbumin (mg/L) | 101.5 (52.8, 165.3) | 94.0 (51.0, 180.5) | 47.0 (27.0, 72.0) | 0.005 |
| Procalcitonin (ng/L) | 0.2 (0.1, 0.5) | 0.2 (0.1, 0.4) | 0.6 (0.3, 1.7) | 0.001 |
| Uric acid (μmol/L) | 208.8 (159.6, 294.8) | 198.0 (147.0, 283.5) | 272.5 (155.8, 394.8) | 0.031 |
| Activated partial thromboplastin time (s) | 48.7 (39.8, 60.0) | 47.4 (38.0, 61.5) | 59.2 (42.2, 68.0) | 0.006 |
| Fibrinogen degradation product (mg/L) | 6.0 (3.9, 13.3) | 6.0 (3.7, 12.7) | 6.8 (4.8, 68.4) | 0.121 |
| Fibrinogen (g/L) | 2.7 (2.3, 3.2) | 2.7 (2.2, 3.3) | 2.3 (1.9, 3.0) | 0.068 |
| Thrombin time (s) | 22.0 (18.6, 28.3) | 21.5 (18.0, 28.5) | 29.3 (19.2, 47.9) | 0.053 |
| Granulocyte (%) | 61.8 (51.3, 70.0) | 61.7 (48.1, 72.8) | 62.8 (56.5, 79.4) | 0.122 |
| Hematocrit (L/L) | 0.4 (0.4, 36.1) | 0.5 (0.4, 36.0) | 27.7 (0.4, 38.8) | 0.059 |
| Hemoglobin (g/L) | 120.3 (106.8, 132.0) | 118.0 (103.0, 132.0) | 124.0 (98.5, 135.3) | 0.293 |
| LYNP (%) | 26.6 (19.7, 35.6) | 26.2 (17.5, 37.6) | 28.7 (15.2, 37.3) | 0.857 |
| Red blood cell (×1012/L) | 4.1 (3.7, 4.4) | 4.0 (3.6, 4.5) | 4.1 (3.5, 4.5) | 0.540 |
| White blood cell (×109/L) | 4.1 (3.2, 5.8) | 4.3 (2.8, 6.2) | 3.4 (2.1, 7.4) | 0.271 |
| Albumin (g/L) | 32.2 (29.7, 35.5) | 31.7 (28.0, 34.9) | 31.8 (27.0, 35.7) | 0.632 |
| Amylase (U/L) | 150.0 (95.0, 212.0) | 150.0 (93.0, 228.0) | 121.0 (91.0, 331.0) | 0.845 |
| Chloride (mmol/L) | 101.0 (98.0, 103.6) | 101.0 (97.7, 104.0) | 100.0 (96.2, 103.6) | 0.272 |
| Carbon dioxide combining power (mmol/L) | 24.2 (20.6, 27.5) | 24.2 (21.2, 27.7) | 24.7 (20.7, 26.0) | 0.667 |
| CRP (mg/L) | 5.0 (1.5, 15.1) | 5.0 (1.3, 23.1) | 5.0 (0.8, 28.1) | 0.877 |
| Globulin (g/L) | 28.1 (25.6, 34.0) | 29.0 (25.5, 34.6) | 29.3 (24.4, 38.4) | 0.637 |
| Kalium (mmol/L) | 3.7 (3.4, 4.0) | 3.7 (3.4, 4.0) | 3.9 (3.2, 4.6) | 0.299 |
| Myoglobin (μg/L) | 137.0 (72.0, 406.0) | 150.5 (70.3, 402.5) | 92.0 (92.0, 92.0) | 0.447 |
| Magnesium (mmol/L) | 0.8 (0.7, 0.9) | 0.8 (0.7, 0.9) | 0.9 (0.6, 0.9) | 0.909 |
| Phosphate (mmol/L) | 0.9, (0.8, 1.1) | 0.9 (0.8, 1.1) | 0.9 (0.7, 1.3) | 0.816 |
| Total bilirubin (μmol/L) | 11.9 (8.9, 17.8) | 11.5 (8.2, 19.0) | 13.2 (8.8, 22.6) | 0.456 |
| Total biliary acid (μmol/L) | 4.4 (2.5, 7.9) | 4.0 (2.2, 7.5) | 5.9 (2.2, 18.8) | 0.193 |
| Total cholesterol (mmol/L) | 3.1 (2.4, 3.9) | 3.0 (2.3, 3.7) | 2.7 (2.4, 3.2) | 0.496 |
| Triglyceride (mmol/L) | 2.1 (1.5, 2.8) | 2.1 (1.5, 2.9) | 1.33 (0.69, 1.61) | 0.312 |
| Total protein (g/L) | 61.4 (57.1, 65.8) | 61.4 (56.5, 66.0) | 65.5 (56.6, 69.5) | 0.133 |
PT = prothrombin time. Data are presented as median (interquartile range).
Figure 1.
Data are expressed as median and interquartile range for LYM, PLT, RET, RETP, DD, INR, PT, PTA, T, ALT, AST, BUN, Ca, CK, CKMB, CREA, GLU, HBDH, lactate dehydrogenase, Na, PA, PCT, and uric acid. P values are the results of univariate analysis for comparison between the groups. The yellow and green square frames represent the early and middle stages of clinical progression, respectively. The fatal cases are represented by the red line; the nonfatal cases are represented by the blue line. Normal upper: the upper limit of the normal value range. Normal lower: the lower limit of the normal value range. ALT = alanine transaminase; AST = aspartate aminotransferase; BUN = blood urea nitrogen; Ca = calcium; CK = creatine kinase; CKMB = creatine kinase-MB; CREA = creatinine; GLU = glucose; HBDH = hydroxybutyrate dehydrogenase; LDH = lactate dehydrogenase; Na = Natrium; PA = prealbumin; PCT = procalcitonin; LYM = lymphocyte; PLT = platelet; RET = reticulocyte; RETP = reticulocyte count percentage; T = temperature; DD = D-dimer; INR = international normalized ratio; PT = prothrombin time; PTA = prothrombin time activity; UA = uric acid.
Risk factors for mortality.
The results of binary logistic regression analysis are presented in Table 4. Body temperature, CNS manifestation, and levels of BUN, CKMB, GLU, and LDH were found to be the risk factors for mortality. In particular, SFTS patients with CNS manifestation (OR = 20.9, 95% CI: 4.3, 100), body temperatures ≥ 38.5°C (OR: 23.2; 95% CI: 3.4, 158), BUN levels ≥ 6.4 mmol/L (OR: 9.9; 95% CI: 2.2, 44), CKMB levels ≥ 100 U/L (OR: 33.2; 95% CI: 5.8, 192), and LDH levels ≥ 1,000 U/L (OR: 8.3; 95% CI: 1.9, 37) had an increased risk of death.
Table 4.
Predictors of fatal severe fever with thrombocytopenia syndrome cases
| Variable | Nonfatal case (n = 189), n (%) | Fatal case (n = 19), n (%) | Crude OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|---|---|
| MOF | ||||
| No | 128 (67.7) | 8 (42.1) | 1.0 | – |
| Yes | 61 (32.3) | 11 (57.9) | 2.9 (1.1, 7.5) | – |
| CNS | ||||
| No | 139 (73.5) | 6 (31.6) | 1.0 | 1.0 |
| Yes | 50 (26.5) | 13 (68.4) | 6.0 (2.2, 16.7) | 20.9 (4.3, 100) |
| DIC | ||||
| No | 125 (66.1) | 6 (31.6) | 1.0 | – |
| Yes | 64 (33.9) | 13 (68.4) | 4.2 (1.5, 11.7) | – |
| Temperature | ||||
| < 38.5°C | 176 (93.1) | 15 (78.9) | 1.0 | 1.0 |
| ≥ 38.5°C | 13 (6.9) | 4 (21.1) | 3.6 (1.0, 12.5) | 23.2 (3.4, 158) |
| BUN | ||||
| < 6.4 mmol/L | 138 (73.0) | 9 (47.4) | 1.0 | 1.0 |
| ≥ 6.4 mmol/L | 51 (27.0) | 10 (52.6) | 3.0 (1.2, 7.8) | 9.9 (2.2, 44) |
| CKMB | ||||
| < 100 U/L | 181 (95.8) | 13 (68.4) | 1.0 | 1.0 |
| ≥ 100 U/L | 8 (4.2) | 6 (31.6) | 10.4 (3.1, 34.6) | 33.2 (5.8, 192) |
| GLU | ||||
| ≤ 6.1 mmol/L | 55 (29.1) | 10 (52.6) | 1.0 | – |
| > 6.1 mmol/L | 134 (70.9) | 9 (47.4) | 0.4 (0.1, 0.9) | – |
| LDH | ||||
| < 1000 U/L | 145 (76.7) | 10 (52.6) | 1.0 | 1.0 |
| ≥ 1000 U/L | 44 (23.3) | 9 (47.4) | 3.0 (1.1, 7.8) | 8.3 (1.9, 37) |
BUN = blood urea nitrogen; CNS = Central nervous syndrome; CKMB = creatine kinase-MB; DIC = disseminated intravascular coagulation; GLU = glucose; LDH = lactate dehydrogenase; MOF = multiple-organ failure; OR = odds ratio. Data are presented as frequency (percentage) and odds ratio (95% CIs). The adjusted variables include age-group, vomiting, diarrhea, headache, MOF, CNS, DIC, body temperature, platelet, GLU, BUN, CKMB, and LDH.
DISCUSSION
As mentioned in the Guidelines and Recommendations for Preventing and Controlling SFTSV infection formulated by the Chinese CDC in 2010,18 early detection, identification, and treatment of SFTS are important measures to prevent disease severity. Therefore, early identification of risk factors associated with the severity of this disease would be beneficial. Our retrospective study, including 208 SFTS patients, systematically investigated the clinical characteristics of early symptoms, laboratory parameters, and risk factors associated with mortality. The results provide further insights into the clinical characteristics associated with SFTS, which in turn would facilitate immediate identification of the potential severe or fatal cases.
Our previous study reported that the medical history of SFTS cases included one time of outpatient and two times of inpatients.19 This study further demonstrated that fatal cases had shorter durations of hospitalization and clinical course than nonfatal cases. One of the reasons for this might be the rapid worsening of the clinical course in fatal cases, with most patients dying in the multi-organ dysfunction phase. It is known that viruses in the family Phenuiviridae can cause various tissue and cell injuries; the common symptoms that usually occur are fever, bleeding, and multi-organ injury.20 Severe fever with thrombocytopenia syndrome virus belongs to the Phenuiviridae family; However, the infection also causes leukocytopenia, thrombocytopenia, hemorrhage, gastrointestinal symptoms, elevated tissue enzymes, proteinuria, and hematuria.21 Our study also revealed that SFTS patients experienced fever, fatigue, diarrhea, conjunctival hemorrhage, gingival bleeding, and other early symptoms. However, we were unable to identify the potential severe for fatal cases based on early symptoms alone. These findings are consistent with those in a study by Gai et al.13 but contradictory to those in a study by Cui.22 Furthermore, the fatality rates of SFTSV infection appear to vary between different populations; the average rate recorded was 12%; however, the fatality rate in some populations was as high as 30%.23 The previously observed causes of death in patients with SFTS were shock, respiratory failure, ARDS, MOF, DIC, and other complications.24 Our results also found that fatal cases had more complications than nonfatal cases.
Severe fever with thrombocytopenia syndrome virus infection leads to a cascade of organ dysfunctions, reflected by abnormal parameters; abnormal values of PLT, lymphocytes (LYM), PT, INR, and DD indicate blood and coagulation disorders; abnormal ALT and AST levels indicate liver dysfunction; abnormal BUN, CREA, and UA levels represent kidney dysfunction;12 and abnormal CK, CKMB, and LDH levels indicated heart damage. Our study observed a series of abnormal laboratory parameters that revealed multi-organ damage; however, the severity of organ damage varied among different clinical phases. The clinical course of SFTSV infection is commonly divided into three phases (fever phase, multi-organ dysfunction, and convalescent phase), according to the different clinical symptoms and dynamic laboratory parameters.11 Furthermore, abnormal levels of white blood cells, PLT, LYM, AST, LDH, CK, and albumin, alkaline phosphatase, and gamma-glutamyl transferase distinguished fatal and nonfatal SFTS cases in different clinical phases.25 Our study further demonstrated that the laboratory parameters of SFTS cases varied throughout their clinical course. Moreover, these parameters, during each clinical phase, were significantly different d between the fatal and nonfatal cases of SFTS. Furthermore, an animal study including an SFTSV-infected mice model showed that the kidney and liver were the major target organs.26 In the fever phase, SFTSV infections caused multiple-organ damage, including the liver, kidney, and heart. The coagulation function impairment observed in our study was more severe in the fatal than in the nonfatal cases. During the multi-organ dysfunction phase, damage to the liver and heart persisted, but only the difference of those parameters due to infection was observed. During the convalescent phase, we found that the body temperature among the fatal cases was significantly higher than that among the nonfatal cases. Thus, the body temperature could be a predictor of mortality; therefore, device-related infections should be considered, and intensive care should be administered to these patients during the convalescent phase. In clinical practice, this information would be helpful in streamlining the treatment specific toward SFTS.
Severe fever with thrombocytopenia syndrome virus can cause a series of organ injuries, reflected in clinical symptoms and laboratory parameters. Liver damage was revealed via elevated ALT and AST levels. Blood urea nitrogen, CREA, and UA levels reflected the injured kidney, whereas elevated CK, CKMB, and LDH levels indicated heart damage.27,28 This study also investigated the risk factors for mortality. Five clinical parameters, that is, CNS manifestation; body temperature; and levels of BUN, CKMB, and LDH were identified as predictors of fatal SFTS. There is a discrepancy in the risk factors in previously published studies. According to Liu et al.,29 an older age and elevated levels of CK and LDH are useful predictors of mortality. A study by Gai et al.13 identified the period of 7–13 days, after the onset of illness, as a critical phase for SFTS patients; the risk factors reported were CNS symptoms, hemorrhagic tendencies, and DIC. Another study revealed that low PLT in the first stage, old age and elevated AST levels in the second stage, and a low LYM count and high LDH level were the predictors of mortality.25 A novel finding of this study is that the body temperatures ≥ 38.5°C, during the clinical course, was a risk factor for mortality. This result emphasizes the importance of monitoring the body temperature of SFTS patients. Furthermore, the level of BUN ≥ 6.4 mmol/L, CKMB ≥ 100 U/L, and LDH ≥ 1,000 U/L also indicated higher fatality risks, implying that the fatal cases had severe kidney and heart damage. Therefore, it is crucial to monitor these clinical parameters for timely treatment intervention. In this study, MOF and DIC were analyzed as the risk factors for mortality; however, the adjusted OR was excluded in regression output. Further studies with a considerable sample size are needed to confirm this finding and further explore the predictors of mortality comprehensively.
This study has several limitations. First, our study was based on SFTS infection cases from four sentinel hospitals, which may introduce Berkson’s bias. Second, the different treatment schemes in the four hospitals may have influenced the laboratory results. However, given the retrospective design of the study, it is not possible to avoid the aforementioned limitations. Third, although our study included 208 SFTS cases to determine the risk factors for mortality, some clinical characteristics such as body temperature and CKMB levels can be accurately assessed with a larger sample size. Despite these limitations, our findings reported a clear clinical progress of SFTS patients and the associated risk factors for mortality. We believe these findings would be beneficial for further clinical treatment; an accurate and timely identification of potential severe or fatal cases of SFTS would reduce the mortality rate.
ACKNOWLEDGMENTS
We thank the physicians and staff at the Chaohu Municipal CDC, Baohe District CDC, Shushan District CDC, and Jinan District CDC.
REFERENCES
- 1.Zhang X, Liu Y, Zhao L, Li B, Yu H, Wen H, Yu XJ, 2013. An emerging hemorrhagic fever in China caused by a novel bunyavirus SFTSV. Sci China Life Sci 56: 697–700. [DOI] [PubMed] [Google Scholar]
- 2.Zhang SF, et al. 2019. Rickettsia typhi infection in severe fever with thrombocytopenia patients, China. Emerg Microbes Infect 8: 579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohl C. et al. , 2020. Zwiesel bat banyangvirus, a potentially zoonotic Huaiyangshan banyangvirus (formerly known as SFTS)–like banyangvirus in northern bats from Germany. Sci Rep 10: 1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu XJ, et al. 2011. Fever with thrombocytopenia associated with a novel bunyavirus in China. New Engl J Med 364: 1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin J, Kwon D, Youn SK, Park JH, 2015. Characteristics and factors associated with death among patients hospitalized for severe fever with thrombocytopenia syndrome, South Korea, 2013. Emerg Infect Dis 21: 1704–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tran XC, Yun Y, Van An L, Kim SH, Thao NTP, Man PKC, Yoo JR, Heo ST, Cho NH, Lee KH, 2019. Endemic severe fever with thrombocytopenia syndrome, Vietnam. Emerg Infect Dis 25: 1029–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi T, et al. 2014. The first identification and retrospective study of severe fever with thrombocytopenia syndrome in Japan. J Infect Dis 209: 816–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun J, Lu L, Wu H, Yang J, Ren J, Liu Q, 2017. The changing epidemiological characteristics of severe fever with thrombocytopenia syndrome in China, 2011–2016. Sci Rep 7: 9236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhan J, Wang Q, Cheng J, Hu B, Li J, Zhan F, Song Y, Guo D, 2017. Current status of severe fever with thrombocytopenia syndrome in China. Virol Sin 32: 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong L, et al. 2018. Human-to-human transmissions of severe fever with thrombocytopenia syndrome virus in Anhui province, 2010–2017. Clin Microbiol Infect 24: 920–922. [DOI] [PubMed] [Google Scholar]
- 11.Li DX, 2015. Severe fever with thrombocytopenia syndrome: a newly discovered emerging infectious disease. Clin Microbiol Infect 21: 614–620. [DOI] [PubMed] [Google Scholar]
- 12.Liu S, Chai C, Wang C, Amer S, Lv H, He H, Sun J, Lin J, 2014. Systematic review of severe fever with thrombocytopenia syndrome: virology, epidemiology, and clinical characteristics. Rev Med Virol 24: 90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gai ZT, et al. 2012. Clinical progress and risk factors for death in severe fever with thrombocytopenia syndrome patients. J Infect Dis 206: 1095–1102. [DOI] [PubMed] [Google Scholar]
- 14.Cui F, Cao HX, Wang L, Zhang SF, Ding SJ, Yu XJ, Yu H, 2013. Clinical and epidemiological study on severe fever with thrombocytopenia syndrome in Yiyuan county, Shandong province, China. Am J Trop Med Hyg 88: 510–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao L, et al. 2012. Severe fever with thrombocytopenia syndrome virus, Shandong province, China. Emerg Infect Dis 18: 963–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng B, et al. 2013. Clinical features and factors associated with severity and fatality among patients with severe fever with thrombocytopenia syndrome bunyavirus infection in Northeast China. PLoS One 8: e80802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Se Yoon P, Choi WY, Chong YP, Park SW, Wang EB, Lee WJ, Jee Y, Kwon SW, Kim SH, 2016. Use of plasma therapy for severe fever with thrombocytopenia syndrome encephalopathy. Emerg Infect Dis 22: 1306–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong L, et al. 2019. Knowledge, attitudes, and practices regarding severe fever with thrombocytopenia syndrome in endemic areas of Anhui province, eastern China. Am J Trop Med Hyg 100: 652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong L, et al. 2019. Socioeconomic burden of severe fever with thrombocytopenia syndrome in endemic areas of Anhui province, eastern China. Zoonoses Public Health 66: 879–885. [DOI] [PubMed] [Google Scholar]
- 20.Pepin M, Bouloy M, Bird BH, Kemp A, Paweska J, 2010. Rift Valley fever virus (Bunyaviridae: Phlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet Res 41: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robles NJC, Han HJ, Park SJ, Choi YK, 2018. Epidemiology of severe fever and thrombocytopenia syndrome virus infection and the need for therapeutics for the prevention. Clin Exp Vaccin Res 7: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui N, et al. 2015. Severe fever with thrombocytopenia syndrome bunyavirus-related human encephalitis. J Infect 70: 52–59. [DOI] [PubMed] [Google Scholar]
- 23.Liu K, et al. 2015. A national assessment of the epidemiology of severe fever with thrombocytopenia syndrome, China. Sci Rep 5: 9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, et al. 2019. A nomogram to predict mortality in patients with severe fever with thrombocytopenia syndrome at the early stage-A multicenter study in China. Plos Negl Trop Dis 13: e0007829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui N, et al. 2014. Clinical progression and predictors of death in patients with severe fever with thrombocytopenia syndrome in China. J Clin Virol 59: 12–17. [DOI] [PubMed] [Google Scholar]
- 26.Jin C, et al. 2012. Pathogenesis of emerging severe fever with thrombocytopenia syndrome virus in C57/BL6 mouse model. Proc Natl Acad Sci U S A 109: 10053–10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato H, Yamagishi T, Shimada T, Matsui T, Shimojima M, Saijo M, Oishi K; SFTS Epidemiological Research Group-Japan , 2016. Epidemiological and clinical features of severe fever with thrombocytopenia syndrome in Japan, 2013–2014. PLoS One 11: e0165207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Bae JM, 2019. Epidemiological and clinical characteristics of confirmed cases of severe fever with thrombocytopenia syndrome in Jeju province, Korea, 2014–2018. J Prev Med Public Health 52: 195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu W, et al. 2013. Case-fatality ratio and effectiveness of ribavirin therapy among hospitalized patients in China who had severe fever with thrombocytopenia syndrome. Clin Infect Dis 57: 1292–1299. [DOI] [PubMed] [Google Scholar]