ABSTRACT
Current chikungunya antibody prevalence and titers are likely to differ based on the exposure rates before the 2006 reemergence in India. For vaccine usage, such data are of immense importance. This study addresses age-stratified IgG titers and its subtypes in Pune, India, endemic for the disease. 170 age-stratified serum pools from 791 individuals with prior chikungunya exposure, and 15 samples from acute disease phase were analyzed. An indirect ELISA based on inactivated chikungunya virus was used to determine anti-CHIKV-IgG and its subtypes. Neutralizing antibody titers (plaque reduction neutralization test [PRNT]) were compared with binding antibody titers (ELISA). Anti–CHIKV-IgG titers along with IgG1 and IgG4 increased till the age-group of until 11–15 years and remained comparable thereafter till > 65 years. IgG1 was the predominant IgG subtype detected in all the pools, whereas IgG4 was present in 151/170 pools. Strong positive correlation of IgG1 was obtained with CHIKV–PRNT50 titers. None of the sample had anti–CHIKV-IgG2, whereas five pools had IgG3 antibody. In the acute-phase serum sample, IgG1 was present in all the samples, whereas IgG4 was present in 8/15 samples. IgG4 was predominant in four samples. During acute phase and at different times postinfection, IgG1 circulated in high titers followed by IgG4. Higher antibody titers in adults reflect reexposures. The data will prove useful in assessing immune response to CHIKV vaccine in relation to IgG subtype.
INTRODUCTION
Chikungunya virus (CHIKV) is well known to be associated with prolonged arthralgia.1,2 The acute febrile phase of the illness normally resolves within a few days, whereas the joint pain associated with CHIKV infection persists for weeks or months. Clinically, it is demonstrated that most of the CHIKV-positive patients develop severe arthralgia with higher pro-inflammatory cytokines in plasma or serum samples, suggesting the role of host immune response in pathogenesis.1
On the other hand, importance of antibodies that serve as critical barriers to viral infection is also demonstrated for CHIKV.3 Anti–CHIKV-IgM antibodies detected 4–20 days post-onset of disease in the patients during the acute phase of disease are shown to interfere with virus binding to the cells and may last up to 6 months,4 whereas anti–CHIKV-IgG are associated with viral clearance and shown to be induced around the same time but present for longer duration.4,5
Antibody phenotype testing revealed IgG3 to have neutralization potential.5–7 Reports from India have been controversial. Induction of anti–CHIKV-IgG3 was reported in samples collected during the second week after disease onset.8 Contrary to these finding, IgG1 and IgG2 antibodies were detected in acute-phase serum samples with negligible IgG3 and no IgG4.9
It is well reported that antiviral activity (virus neutralization and clearance) is associated with IgG1 and IgG3 subtypes that bind to all human Fc-gamma receptors (FcγR) classes and target antigen to phagocytosis.10 Both these antibodies are elicited against protein antigens and are outcome of Th1 response. On the contrary, IgG2 and IgG4 are the result of Th2 response and mainly associated with polysaccharide antigens, although they are shown to get induced after virus infection.11 IgG2 binds only to FcγRII and IgG4 only to FcγRII and III, although significantly weaker than the binding of IgG1. All antibody subtypes other than IgG4 bind to complement.
Virus exposure and immunological factors such as cytokines, chemokines, or molecular factors elicited post-CHIKV infection modulate IgG response qualitatively and quantitatively.12 Moreover, the degree of antibody modulation is shown to be age dependent.13,14 Age-dependent IgG response is not available from all age-groups that are presumably recovered from CHIKV infection. There is a possibility of difference in IgG or its subtype induction after CHIKV infection among age groups. Such information may be significant while assessing immune response following chikungunya vaccination.
Earlier, we determined anti–CHIKV-IgG positivity in age-stratified population from Pune city, India.15 In view of the contradictory Indian reports and neutralization potential of IgG3 subtype, further analysis of anti–CHIKV-IgG–positive antibody samples was undertaken. A total of 791 anti–CHIKV-IgG–positive samples were distributed age wise in 170 pools and evaluated for antibody titers and subtypes using ELISA. Titers in ELISA were compared with neutralizing antibody titers (plaque reduction neutralization test [PRNT]). Patterns obtained are reported here.
MATERIALS AND METHODS
Ethics.
This study was approved by the human ethics committee of Institutional Ethics Committee of Bharati Medical College (IEC/2017/04, renewed IEC/2018/11). No prospective sample collection was carried out.
Serum samples.
Samples used to evaluate age-dependent antibody response was collected for during a cross-sectional, stratified, facility-based, multistage cluster sampling-based survey at Pune, India, for anti–DENV-IgG antibodies.16 Sampling was performed between May 4 and June 27, 2017 and stored as aliquots at −80°C until use. For titrations, 170 serum pools were prepared using 791 samples (Table 1), each pool consisting of 2–4 serum samples from the same age group and irrespective of gender. 127 pools consisted of 5 samples, 29 pools consisted of 4 samples, 12 pools consisted of 3 samples while 2 pools consist of 2 samples. The duration between exposure to CHIKV and sampling is not known. For testing acute-phase serum samples, 15 chikungunya patients were included. Of these, 11 were males (age 18–63 years) and 4 were females (age 20–55 years). The duration between onset of clinical symptoms and sampling was 2–7days (n = 12), 8 days (n = 2) and 15 days.
Table 1.
Details for sample pools
| Age-group (years) | No* of males/females | No. of pools tested by ELISA (total samples) | No. of pools tested by plaque reduction neutralization test |
|---|---|---|---|
| 0–5 | 8/11 | 4 (20) | 4 (20) |
| 6–10 | 18/17 | 7 (35) | 7 (35) |
| 11–15 | 16/7 | 5 (24) | 5 (24) |
| 16–25 | 87/62 | 31 (148) | 10 (50) |
| 26–35 | 79/102 | 39 (185) | 10 (50) |
| 36–45 | 54/95 | 32 (149) | 10 (50) |
| 46–55 | 43/51 | 23 (96) | 10 (50) |
| 56–65 | 40/44 | 18 (86) | 10 (50) |
| > 65 | 24/24 | 11 (48) | 10 (48) |
| Total | 369/413 | 170 (791) | 76 (377) |
Gender information not available for nine subjects.
ELISA.
One hundred and seventy pool were analyzed for anti-CHIKV-IgG, -IgG1, -IgG2, -IgG3 and -IgG4. Briefly, chikungunya virus grown on Vero cells was inactivated and purified as previously described.15 1.5 µg/mL purified inactivated CHIKV diluted in carbonate–bicarbonate buffer, pH 9.2, and 100 µL was added in ELISA wells for coating. Coated wells were incubated overnight at 4°C and washed five times using phosphate buffer saline (PBS) containing 0.1% tween 20 (Greiner Bio-One, Noida, India). Plates were blocked for 1 hour at 37°C using PBS containing 10% fetal bovine serum (FBS) (Gibco, Waltham, MA) and washed five times with 300 μl wash buffer. Washing protocol was kept constant for all further washing steps; Diluted samples were double diluted in sample diluent (PBS containing 10% FBS, 1% Vero cell supernatant, and 0.1% tween 20), 100 μl diluted sample was added to plates and incubated for 1 hour at 37°C. Samples were initially diluted initially diluted 1:10. This was followed by dilution to 1:1600 for anti-CHIKV-IgG, 1:800 for anti-CHIKV-IgG1 ELISA, 1:200 for anti-CHIKV-IgG4 and 1:100 for anti-CHIKV-IgG2 and -IgG3. After incubation, wells were washed, and 100 µL conjugate diluted in PBS with 10% FBS, 0.1% tween 20 (Sisco, India) was added to wells. The conjugates were added individually to the wells based on the type of antibody to be detected. The dilution of antihuman-IgG-horseradish peroxidase (HRP) (Sigma-Aldrich St. Louis, MO), was 1:20000, while dilution of antihuman-IgG1-HRP, antihuman-IgG2-HRP, antihuman-IgG3-HRP, or antihuman-IgG4-HRP (all from Southern Biotech, Birmingham, AL) was 1:5000, was added to wells. After incubation for 30 minutes at 37°C, 100 µL tetramethylbenzidine substrate containing hydrogen peroxide (Clinical Science Products Inc., Mansfield, MA) was added, and the reaction was stopped after 10 minutes using 50 µL 2N H2SO4. Plates were read at 450 nm with background subtraction filter of 655 nm. The cutoff value was determined by multiplying optical density (O.D.) value of negative control by three. Plasma samples scored negative or positive by Abcam ELISA and PRNT were used as negative and positive controls respectively.
Anti–CHIKV-IgM ELISA was performed as per the manufacturer protocol (Abcam, catalogue number: ab177835, Cambridge, United Kingdom).
Plaque reduction neutralization test (PRNT).
Chikungunya virus-specific PRNT50 was performed using n = 76 pools representing all age groups to correlate neutralizing antibody titers with anti–CHIKV-IgG, -IgG1, or IgG4 antibody titers. Plaque reduction neutralization test as described previously.15 In brief, 1 × 105 cells/mL Vero cells (CCL-81, The American Type Culture Collection [ATCC]) were seeded in 24-well plate (Corning, New York, United States) using MEM with 10% FBS (Gibco) with penicillin and streptomycin. Twenty four hours after seeding, heat-inactivated serum samples were diluted 4-fold in Minimum Essential Medium (MEM) with 2% FBS starting with 1:5 dilutions till 1:1,280. Equal volume of 500 plaque forming units (PFU)/mL CHIKV was mixed with diluted serum samples selected for the test and incubated for 1 hour at 37°C in an incubator with 5% CO2; 100 µL of serum–CHIKV mix was added to Vero cell monolayer and incubated for 1 hour at 37°C in an incubator with 5% CO2. The mixture was removed and MEM with 2% FBS, 1.2% Aquacide-II (Merck, Darmstadt, Germany), 0.2 unit/mL penicillin, and 0.2 µg/mL streptomycin was added to Vero cell monolayer. Plates were incubated for 48 hours at 37°C in an incubator with 5% CO2, the overlay medium was discarded, and cells were stained using 1% crystal violet. Plaques were counted after thorough washing and drying of crystal violet stained plates.
Statistical analysis.
Graphpad Prism v. 8.1.1 (Graphpad Software, Graphpad Holding, San Diego, CA) was used to perform statistical analysis. The two-tailed Mann–Whitney U test was used to compare IgG, -IgG1, -IgG4, and PRNT50 titers of pooled serum of various age-groups. *, **, ***, and **** represent P < 0.05, P < 0.01, P < 0.001, and P < 0.0001, respectively. Correlation coefficients between were quantified by the Spearman rank correlation coefficient, and significance was assessed by the nonparametric method. Simple linear regression with 95% CI profile likelihood was obtained to understand trend in correlation between antibody and PRNT50 titers. A nonlinear one-phase decay curve was obtained for plotting of binding antibody and neutralizing antibody titers.
RESULTS
Comparison of ELISA and PRNT for determining anti-CHIKV antibody titers.
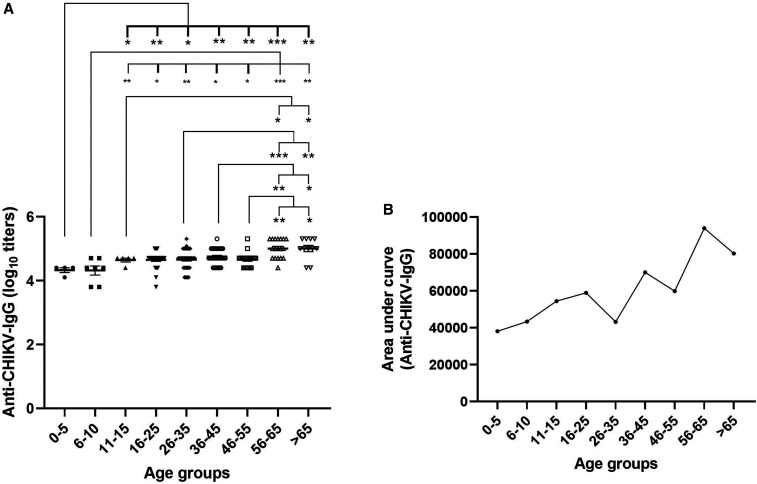
Earlier, we had used PRNT for the evaluation of performance of our ELISA and found excellent correlation between the two tests.15 For quantitation, PRNT-detecting neutralizing antibodies are most appropriate. However, for testing of large number of samples, especially in serosurveys, PRNT is of limited utility because of higher cost and longer time. Therefore, we first compared titers of 76 pools tested earlier by PRNT (neutralizing antibodies) with anti-CHIKV-IgG ELISA (binding antibodies) titers. The mean titers determined by ELISA (72,084, log10: 4.72) were ∼46-fold higher than PRNT (1,567). However, titers by both the tests showed similar pattern, i.e., antibody titers gradually increasing until the age of 11–15 years and remaining comparable during all the subsequent age groups. Encouraged by these findings and to generate additional data for ELISA we determined anti–CHIKV-IgG titers in ELISA for all the 170 pools representing different age-groups (Figure 1A). A trend toward increase in IgG titers with age was observed. Children in the age-groups of 0–5 and 6–10 years (mean IgG titer: 25,600, log10: 4.33) had significantly lower IgG titers than those in other age-groups. On the other hand, age-group 56–65 or > 65 years (mean IgG titer: 124,469, log10: 5.01) had significantly high antibody titers than all the age-groups, except 16- to 25-year age-group (mean IgG titer: 50,994, log10: 4.65).
Figure 1.
Antibody response after CHIKV infection. Serum pools (n = 170) of anti–CHIKV-IgG–positives samples from various age-groups were evaluated for (A) anti–CHIKV-IgG titers. Data are presented as average ± standard error of mean. (B) Area under the curve of serum dilutions for each age-group. Levels of significance are presented as *P < 0.05, ** = P < 0.01, and *** = P < 0.001. CHIKV = chikungunya virus.
Area under the curve (AUC, Figure 1B) for various age-groups was calculated between dilutions 1:1,600 and 1: 102,400 to understand affinity of IgG toward CHIKV. An increase in AUC was observed with increase in age. Age-group 0–5 years had the lowest AUC, whereas age-group 56–65 years had the highest AUC.
Phenotype of persisting anti–CHIKV-IgG antibodies after CHIKV infection.
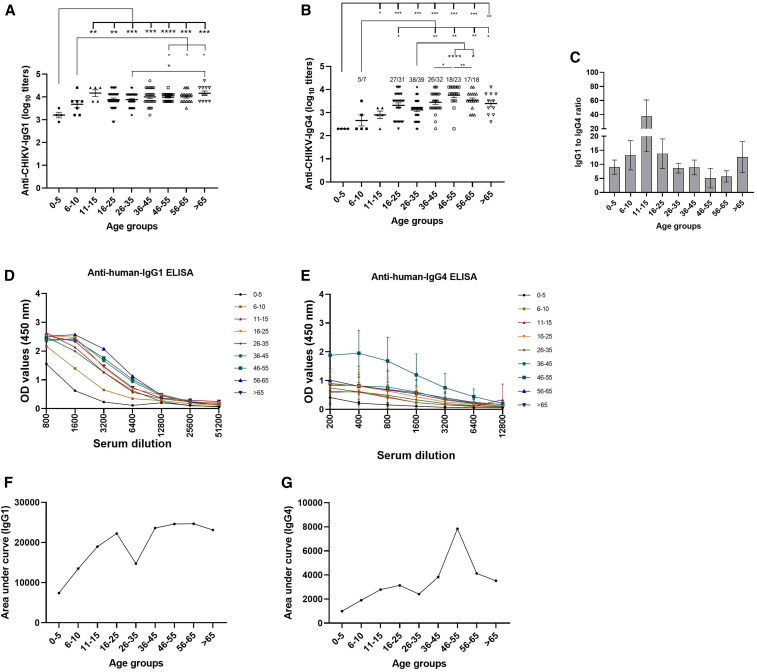
Next, we carried out IgG subtype analysis using ELISA. None of the 170 pools were reactive for IgG2. IgG3 antibodies were detected in five pools (two pool from the age-group of 56–65 or > 65 years and one from the age-group of 26–35 years ). IgG1 was present in all the pools and IgG4 in 151/170 (88.8%) pools. Age-group 11–15 years exhibited highest IgG1/IgG4 ratio, whereas 46- to 55-year age-group had the lowest ratio. The pattern of IgG and IgG1 titers (Figure 2A) was similar, with titers gradually increasing till age-group 11–15 years and remaining comparable in the subsequent age-groups (P = 0.02–0.95). Dilution of serum samples revealed difference in dilution patterns for both IgG1 and IgG4 (Figure 2D and E), resulting in high AUC for IgG1 and low for IgG4 (Figure 2F and G).
Figure 2.
Phenotype of antibody response after CHIKV infection. Pooled serum (N = 170) positive for anti–CHIKV-IgG was used to quantify (A) anti–CHIKV-IgG1 and (B) anti–CHIKV-IgG4. Obtained titers were used to calculate (C) age-wise CHIKV-specific IgG1/IgG4 ratio. Dilution curve for (D) anti–CHIKV-IgG1 or (E) anti–CHIKV-IgG4 was obtained by plotting OD values against serum dilutions. Area under the curve for (F) IgG1 and (G) IgG4. Data are presented as average ± standard error of mean. Levels of significance are presented as *P < 0.05, ** = P < 0.01, *** = P < 0.001, and **** = P < 0.0001. CHIKV = chikungunya virus. This figure appears in color at www.ajtmh.org.
Correlation between neutralizing and binding antibody titers.
Correlation analyses (Figure 3A–C) using 76 sample pools revealed a strong positive correlation of binding IgG antibody titers (ELISA) with neutralizing antibody titers (PRNT50, r = 0.582, P = < 0.001, 95% CI of slope: 0.013–0.017). Similarly, a positive correlation of PRNT50 titers with anti–CHIKV-IgG1 titers was observed (r = 0.557, P < 0.001, 95% CI of slope: 0.050–0.073). However, anti–CHIKV-IgG4 titers showed poor correlation (r = 0.317, P = 0.006, 95% CI of slope: 0.155–0.259).
Figure 3.
Correlation between PRNT50 and ELISA titers. Neutralizing antibody titers obtained by PRNT50 assay and binding antibody titers obtained by ELISA were plotted to understand the role of (A) IgG (B) IgG1 or (C) IgG4 in chikungunya virus neutralization. The correlation is demonstrated as Spearman’s r value. Each dot represents an outcome from individual pool. PRNT = plaque reduction neutralization test.
Antibody response among chikungunya patients in the acute phase.
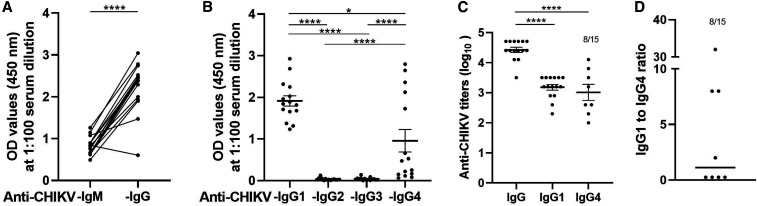
Absence of IgG2 and IgG3 antibodies observed by us was in contrast to previous studies.5–8 To understand if the negativity was possibly related to longer and variable duration between CHIKV exposure and sample collection during serosurvey, we analyzed acute-phase serum samples (Figure 4A). As samples collected during serosurvey represent prior exposure to CHIKV before variable period, we determined subtype status during the acute phase. For this, 15 acute-phase samples were used. IgG antibody subtype ELISA revealed induction of IgG1 in all the samples, whereas 8/15 (53.3%) were positive for IgG4 (Figure 4B). IgG2 and IgG3 antibodies were undetectable. The mean log10 titers of anti–CHIKV-IgG1 and IgG4 were 3.1 and 3.0, respectively (Figure 4C). To our surprise, 4/8 IgG4-positive samples had higher titer than corresponding IgG1 titers. The IgG1/IgG4 ratio for these four samples was 0.25 (Figure 4D). For the other four samples with predominant IgG1, the ratio varied between two and 32.
Figure 4.
Antibody response in acute samples. N = 15 samples were evaluated for presence of both (A) anti–CHIKV-IgM and anti–CHIKV-IgG antibody or (B) IgG subtypes raised against CHIKV. The data are presented as O.D. values at 450 nm at serum dilution 1:100. These samples were used to determine (C) anti–CHIKV-IgG, -IgG1, and -IgG4 titers. (D) IgG1/IgG4 titer ratio was calculated for serum samples that were positive for both anti–CHIKV-IgG1 or -IgG4. CHIKV = chikungunya virus.
DISCUSSION
Chikungunya reemerged in India in 2005–2006 after a gap of 32 years with a change in infecting genotype from Asian to East/Central/South/Africa (ECSA).17 In 2017, when the blood samples were collected from age-stratified population for the present study, antibody titers were likely to differ depending on whether previous exposure was pre-1972 and/or post-2006. Lowest titers in children up to the age of 10 years suggest first exposure, whereas higher titers among individuals aged > 55 years indicate prior exposure pre-1972 and reinfection/boosting post-2006. Importantly, antibody tiers in PRNT and ELISA correlated well (r = 0.582, P < 0.001) suggestive of utility of quantitative ELISA described here.
Our anti-CHIKV-IgG subtype data representing 791 samples from age-stratified population highlighted predominant induction of IgG1 followed by IgG4 in individuals exposed earlier at different time points before testing during the current study. In contrast, 2–3 months follow up of 30 chikungunya patients from Singapore identified IgG3 antibodies to be the predominant subtype using whole CHIKV5 or a single linear epitope located at the N-terminus of the E2 glycoprotein6 for coating ELISA wells. Possibility of subtype shift over time was considered first as the subjects included by us may have been infected before a longer duration. Lack of IgG3 antibodies during acute phase as well confirmed that our results were at variance from other studies.5–7 Importantly, IgG3 antibodies were neutralizing, and increased risk of disease was associated with the absence of early induction of these antibodies. We, on the other hand, found better correlation of IgG1, the predominant subtype, with neutralizing antibodies. IgG1 predominance was documented for other RNA viruses such as West Nile and Measles.18,19
Host genetic factors may not be responsible for these striking differences in subtype response as two other Indian studies reported contradictory findings. IgG3 was identified as the predominant subtype when peptides corresponding to the full length of E1, E2, and E3 proteins of S27 strain of CHIKV were used for ELISA.8 On the contrary, induction of IgG1 and IgG2 in acute-phase plasma samples with negligible IgG3 and no IgG4 was found when screened with CHIKV.9 Taken together, when CHIKV was used for ELISA, both Indian studies identified IgG1 (Nayak et al.9 and present study), although IgG2 and IgG4 subtype detection rates did vary; IgG3 was the predominant serotype with envelope protein peptides.8 The possibility of unavailability of IgG3 reactive peptide epitopes on the surface of the virus cannot be ruled out. Nonetheless, despite the use of CHIKV, differential subtype response in patients from Singapore and Malaysia (Kam et al.5–7) and India remains unclear. Testing of our samples against the peptide suggested by Kam et al.5–7 may provide important information and clarification. The role of IgG4 detected in 88% of the samples surveyed and in chikungunya patients during the acute phase needs to be investigated.
Our results showed that in accordance with IgG titers, both IgG1 and IgG4 levels were low in children and high in adults. Though the subtype patterns were different, the IgG subtype titers against F protein of RSV20 and following influenza immunization were higher in adults.21 Repeated virus exposures (RSV, Influenza and chikungunya) of adults could be a possible reason for the observed rise in adults. Whether these the age-dependent differences in antibody levels are substantial enough to make an impact on CHIKV reinfection is presently not known. If > 10 PRNT50 titers are considered protective,3 almost all the exposed individuals including children had circulated protective antibody levels and should be protected. Considering that IgG1 has the longest half-life among IgG subtypes is an efficient mediator of antibody-dependent cellular cytotoxicity along with complement, its role in CHIKV neutralization cannot be ruled out10,22 and deserves immediate attention. In addition, induction of IgG subtype might vary depending on the virus and age. It is reported that the immunity in children mature with age, remains constant in adults and declines in elderly.20 According, serum RSV F specific IgG1 and IgG2 were higher in adults than in children while IgG3 response was lower in adults.23 In our study, we found predominant induction of IgG1 followed by IgG4. In addition, CHIKV-specific subtype titers were lower in children as compared to adults. These findings might be because of repeated exposure of adults to CHIKV. However, after influenza immunization, antibody titers were predominated by IgG1 and IgG3, but titers in adults were higher than in children,21 suggesting age might play an import role in induction of quantitatively and qualitatively superior immune response.
In conclusion, IgG subtype response in Indian patients and age-stratified general population is dominated by IgG1 followed by IgG4. IgG and IgG1 titers correlated with neutralizing antibodies. This information will prove useful while assessing immune response of Indian population to chikungunya vaccines, especially if induction of appropriate IgG subtype is considered as one of the criteria for protection. Whether this response will vary when whole virus or envelop protein-based vaccines are used is another important concern. The role of IgG1 in protection after CHIKV infection/immunization needs to be elucidated.
ACKNOWLEDGMENT
We thank Interactive Research School for Health Affairs, Bharati Vidyapeeth (deemed to be University), Pune, for funding the study.
REFERENCES
- 1.Chow A, et al. 2011. Persistent arthralgia induced by chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. J Infect Dis 203: 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ninla-Aesong P, Mitarnun W, Noipha K, 2020. Long-term persistence of chikungunya virus-associated manifestations and anti-chikungunya virus antibody in southern Thailand: 5 years after an outbreak in 2008–2009. Viral Immunol 33: 86–93. [DOI] [PubMed] [Google Scholar]
- 3.Yoon IK, et al. 2015. High rate of subclinical chikungunya virus infection and association of neutralizing antibody with protection in a prospective cohort in the Philippines. PLoS Negl Trop Dis 9: e0003764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chua CL, Sam IC, Chiam CW, Chan YF, 2017. The neutralizing role of IgM during early chikungunya virus infection. PLoS One 12: e0171989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kam YW, Simarmata D, Chow A, Her Z, Teng TS, Ong EKS, Rénia L, Leo YS, Ng LFP, 2012. Early appearance of neutralizing immunoglobulin G3 antibodies is associated with chikungunya virus clearance and long-term clinical protection. J Infect Dis 205: 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kam YW, et al. 2012. Early neutralizing IgG response to chikungunya virus in infected patients targets a dominant linear epitope on the E2 glycoprotein. EMBO Mol Med 4: 330–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kam YW, et al. 2012. Longitudinal analysis of the human antibody response to chikungunya virus infection: implications for serodiagnosis and vaccine development. J Virol 86: 13005–13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verma P, Bhatnagar S, Kumar P, Chattree V, Parida MM, Hoti SL, Ali S, Rao DN, 2014. Analysis of antibody response (IgM, IgG, IgG3) to chikungunya virus using a panel of peptides derived from envelope protein for serodiagnosis. Clin Chem Lab Med 52: 297–307. [DOI] [PubMed] [Google Scholar]
- 9.Nayak K, et al. 2020. Antibody response patterns in chikungunya febrile phase predict protection versus progression to chronic arthritis. JCI Insight 5: e130509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daëron M, 2009. Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood 113: 3716–3725. [DOI] [PubMed] [Google Scholar]
- 11.Isa MB, Martínez L, Giordano M, Zapata M, Passeggi C, De Wolff MC, Nates S, 2001. Measles virus-specific immunoglobulin G isotype immune response in early and late infections. J Clin Microbiol 39: 170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McHeyzer-Williams M, Okitsu S, Wang N, McHeyzer-Williams L, 2012. Molecular programming of B cell memory. Nat Rev Immunol 12: 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aranburu A, et al. 2017. Human B-cell memory is shaped by age- and tissue-specific T-independent and GC-dependent events. Eur J Immunol 47: 327–344. [DOI] [PubMed] [Google Scholar]
- 14.Pichichero ME, 2014. Challenges in vaccination of neonates, infants and young children. Vaccine 32: 3886–3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patil HP, Rane PS, Gosavi M, Mishra AC, Arankalle VA, 2020. Standardization of ELISA for anti-chikungunya-IgG antibodies and age-stratified prevalence of anti-chikungunya-IgG antibodies in Pune, India. Eur J Clin Microbiol Infect Dis 39: 1925–1932. [DOI] [PubMed] [Google Scholar]
- 16.Mishra AC, Arankalle VA, Gadhave S, Mahadik P, Shrivastava S, Bhutkar M, Vaidya VM, 2018. Stratified sero-prevalence revealed overall high disease burden of dengue but suboptimal immunity in younger age groups in Pune, India. PLoS Negl Trop Dis 12: e0006657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yergolkar PN, Tandale BV, Arankalle VA, Sathe PS, Sudeep AB, Gandhe SS, Gokhle MD, Jacob GP, Hundekar SL, Mishra AC, 2006. Chikungunya outbreaks caused by African genotype, India. Emerg Infect Dis 12: 1580–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofmeister Y, Planitzer CB, Farcet MR, Teschner W, Butterweck HA, Weber A, Holzer GW, Kreil TR, 2011. Human IgG subclasses: in vitro neutralization of and in vivo protection against West Nile virus. J Virol 85: 1896–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isa MB, Martínez L, Giordano M, Passeggi C, De Wolff MC, Nates S, 2002. Comparison of immunoglobulin G subclass profiles induced by measles virus in vaccinated and naturally infected individuals. Clin Diagn Lab Immunol 9: 693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simon AK, Hollander GA, Mcmichael A, Mcmichael A, 2015. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci 282: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nachbagauer R, Choi A, Izikson R, Cox MM, Palese P, Krammer F, 2016. Age dependence and isotype specificity of influenza virus hemagglutinin stalk-reactive antibodies in humans. MBio 7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vidarsson G, Dekkers G, Rispens T, 2014. IgG subclasses and allotypes: from structure to effector functions. Front Immunol 5(OCT): 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jounai N, et al. 2017. Age-specific profiles of antibody responses against respiratory syncytial virus infection. EBioMedicine 16: 124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]