Abstract
To regenerate, damaged tissue must heal the wound, regrow to the proper size, replace the correct cell types, and return to the normal gene-expression program. However, the mechanisms that temporally and spatially control the activation or repression of important genes during regeneration are not fully understood. To determine the role that chromatin modifiers play in regulating gene expression after tissue damage, we induced ablation in Drosophila melanogaster imaginal wing discs, and screened for chromatin regulators that are required for epithelial tissue regeneration. Here, we show that many of these genes are indeed important for promoting or constraining regeneration. Specifically, the two SWI/SNF chromatin-remodeling complexes play distinct roles in regulating different aspects of regeneration. The PBAP complex regulates regenerative growth and developmental timing, and is required for the expression of JNK signaling targets and the growth promoter Myc. By contrast, the BAP complex ensures correct patterning and cell fate by stabilizing the expression of the posterior gene engrailed. Thus, both SWI/SNF complexes are essential for proper gene expression during tissue regeneration, but they play distinct roles in regulating growth and cell fate.
Keywords: regeneration, chromatin, SWI/SNF complexes, Drosophila, wing imaginal disc
Introduction
Regeneration is a complex yet highly elegant process that some organisms can use to recognize, repair, and replace missing or damaged tissue. Imaginal disc repair in Drosophila is a good model system for understanding regeneration due to the high capacity of these tissues to regrow and restore complex patterning, as well as the genetic tools available in this model organism (Hariharan and Serras 2017). Regeneration requires the coordinated expression of genes that regulate the sensing of tissue damage, induction of regenerative growth, repatterning of the tissue, and coordination of regeneration with developmental timing. Initiation of regeneration in imaginal discs requires known signaling pathways such as the Reactive oxygen species (ROS), Jun N-terminal kinase (JNK), Wingless (Wg), p38, Janus kinase/signal transducer and activator of transcription (Jak/STAT), and Hippo pathways (Bosch et al. 2008; Smith-Bolton et al. 2009; Bergantinos et al. 2010; Schubiger et al. 2010; Grusche et al. 2011; Sun and Irvine 2011; Katsuyama et al. 2015; Santabárbara-Ruiz et al. 2015). These pathways activate many regeneration genes, such as the growth promoter Myc (Smith-Bolton et al. 2009) and the hormone-like peptide ilp8, which delays pupariation after imaginal disc damage (Colombani et al. 2012; Garelli et al. 2012). However, misregulation of these signals can impair regeneration. For example, elevated levels of JNK signaling can induce patterning defects in the posterior of the wing (Schuster and Smith-Bolton 2015), and elevated ROS levels can suppress JNK activity and regenerative growth (Brock et al. 2017). While the signals that initiate regeneration have been extensively studied, regulation of regeneration gene expression in response to tissue damage is not fully understood.
Such regulation could occur through chromatin modification. In Drosophila, chromatin modifiers include the Polycomb repressive complexes PRC1 and PRC2, which can be recruited to specific locations by the Pho repressor complex (PhoRC), the activating complexes Trithorax acetylation complex (TAC1), Complex of proteins associated with Set1 (COMPASS) and COMPASS-like, the nucleosome remodeling complex (NURF), and the switch/sucrose non-fermentable (SWI/SNF) chromatin remodelers Brahma-associated proteins (BAP) and Polybromo-associated proteins (PBAP) (Xiao et al. 2001; Kassis et al. 2017). PRC2 carries out trimethylation of histone H3 at lysine 27, recruiting PRC1 to repress transcription of nearby genes. COMPASS-like and COMPASS carry out histone H3 lysine 4 monomethylation and di- and trimethylation, respectively, thereby activating the expression of nearby genes. TAC1 acetylates histone H3 lysine 27, also supporting activation of gene transcription. NURF, BAP, and PBAP alter or move nucleosomes to facilitate binding of transcription factors and chromatin modifiers (Xiao et al. 2001; Kassis et al. 2017). Rapid changes in gene expression induced by these complexes may help facilitate a damaged tissue’s regenerative response.
A few chromatin modifiers and histone modifications have been reported to be important for regulating regeneration of Xenopus tadpole tails, mouse pancreas and liver, zebrafish fins, and Drosophila imaginal discs (Wang et al. 2008; Stewart et al. 2009; Blanco et al. 2010; Scimone et al. 2010; Tseng et al. 2011; Fukuda et al. 2012; Pfefferli et al. 2014; Jin et al. 2015; Skinner et al. 2015). Furthermore, components of Drosophila and mouse SWI/SNF complexes regulate regeneration in the Drosophila midgut and mouse skin, liver, and ear (Jin et al. 2013; Xiong et al. 2013; Sun et al. 2016). However, little is known about how these complexes alter gene expression, signaling, and cellular behavior to regulate regeneration. Importantly, genome-wide analysis of chromatin state after Drosophila imaginal disc damage revealed changes in chromatin around a large set of genes, including known regeneration genes (Vizcaya-Molina et al. 2018). Thus, chromatin modifiers likely play a key role in regulating activation of the regeneration program. However, it is unclear whether all regeneration genes are coordinately regulated in the same manner, or whether specific chromatin modification complexes target different subsets of genes that respond to tissue damage.
To probe the role of chromatin modifiers in tissue regeneration systematically, we assembled a collection of pre-existing Drosophila mutants and RNAi lines targeting components of these complexes as well as other genes that regulate chromatin, and screened these lines for regeneration defects using the Drosophila wing imaginal disc. We used a spatially and temporally controllable tissue-ablation method that uses transgenic tools to induce tissue damage only in the wing primordium (Smith-Bolton et al. 2009). This method ablates 94% of the wing primordium on average at the early third instar and allows the damaged wing discs to regenerate in situ. Previous genetic screens using this tissue ablation method have identified genes critical for regulating different aspects of regeneration, such as taranis, trithorax, and cap-n-collar, demonstrating its efficacy in finding regeneration genes (Schuster and Smith-Bolton 2015; Skinner et al. 2015; Brock et al. 2017).
Through this targeted genetic screen of chromatin regulators, we found that mutations in Drosophila SWI/SNF components caused striking regeneration defects. The SWI/SNF complexes are conserved multi-subunit protein complexes that activate or repress gene expression (Wilson and Roberts 2011) by using the energy from ATP hydrolysis to disrupt histone-DNA contacts and remodel nucleosome structure and position (Côté et al. 1994; Kwon et al. 1994). Brahma (Brm) is the only ATPase of the SWI/SNF complexes in Drosophila (Tamkun et al. 1992; Kassis et al. 2017). Moira (Mor) serves as the core scaffold of the complexes (Mashtalir et al. 2018). Other components contain domains involved in protein–protein interactions, protein–DNA interactions, or interactions with modified histones (Hargreaves and Crabtree 2011). There are two subtypes of SWI/SNF in Drosophila: the Brahma-associated proteins (BAP) and the Polybromo-associated BAP (PBAP) remodeling complexes (Collins and Treisman 2000; Mohrmann et al. 2004). They share common core components, including Brm, Snf5-related 1 (Snr1), Mor, Brahma-associated protein 55kD (Bap55), Brahma-associated protein 60kD (Bap60), Brahma-associated protein 111kD (Bap111), and Actin (Mohrmann et al. 2004), but contain different signature proteins. The PBAP complex is defined by the components Brahma-associated protein 170kD (Bap170), Polybromo, and Supporter of activation of yellow protein (Sayp) (Mohrmann et al. 2004; Chalkley et al. 2008). Osa defines the BAP complex (Collins et al. 1999; Vázquez et al. 1999).
Here, we show that the SWI/SNF complexes BAP and PBAP are required for regeneration, and that the two complexes play distinct roles. The PBAP complex is important for activation of JNK signaling targets such as ilp8 to delay metamorphosis and allow enough time for the damaged tissue to regrow, and for expression of Myc to drive regenerative growth. By contrast, the BAP complex functions to prevent changes in cell fate induced by tissue damage through stabilizing expression of the posterior identity gene engrailed. Thus, different aspects of the regeneration program are regulated independently by distinct chromatin regulators.
Materials and methods
Fly stocks
The following fly stocks were obtained for this study. In some cases, they were rebalanced before performing experiments: w1118;; rnGAL4, UAS-rpr, tubGAL80ts/TM6B, tubGAL80 (Smith-Bolton et al. 2009), w1118 (Wild type), w*; P{neoFRT}82B osa308/TM6B, Tb1 (Bloomington Drosophila stock center, BL#5949) (Treisman et al. 1997), w*; Bap170Δ135/T(2; 3)SM6a-TM6B, Tb1 was a gift from Jessica E. Treisman (Carrera et al. 2008), brm2 es ca1/TM6B, Sb1 Tb1 ca1 (BL#3619) (Kennison and Tamkun 1988), mor1/TM6B, Tb1 (BL#3615) (Kennison and Tamkun 1988), y1 w1; P{neoFRT}40A P{FRT(whs)}G13 cn1 PBac{SAstopDsRed}Bap55LL05955 bw1/CyO, bw1 (BL#34495) (Schuldiner et al. 2008), bap111 RNAi (Vienna Drosophila Resource Center, VDRC#104361), control RNAi background (VDRC#15293), bap60 RNAi (VDRC#12673), brm RNAi (VDRC#37721), P{PZ}tara03881 ry506/TM3, ryRK Sb1 Ser1 (BL#11613) (Gutierrez 2003), UAS-tara was a gift from Michael Cleary (Manansala et al. 2013), TRE-Red was a gift from Dirk Bohmann (Chatterjee and Bohmann 2012). mor2, mor11 and mor12 alleles were gifts from James Kennison (Kennison and Tamkun 1988), snr1E2 and snr1SR21 alleles were gifts from Andrew Dingwall (Zraly et al. 2003). Df(3R)RD31/Dp(3; 3)S462, In(3LR)EBL, In(3R)C, Sb1 ca1 (Hopmann et al. 1995) (BL#5127), w1118; Df(3R)BSC790, P+PBac{w[+mC]=XP3.WH3}BSC790/TM6C, Sb1 cu1 (Cook et al. 2012) (BL#27362), ry506 P {PZ}osa00090/TM3, ryRK Sb1 Ser1 (Spradling et al. 1995) (BL#11486).
The mutants and RNA interference lines in Supplementary Table S1 used for the chromatin regulator screen were:
st1 in1 kniri-1 ScrW Pc3/TM3, Sb1 Ser1 (BL#3399),
cn1 Psc1 bw1 sp1/CyO (BL#4200),
y1 w*; P{neoFRT}42D Psce24/SM6b, P{eve-lacZ8.0}SB1 (BL#24155),
w*; P{neoFRT}82B Abd-BMcp-1 Sce1/TM6C, Sb1 Tb1 (BL#24618),
w*; P{neoFRT}82B ScmD1/TM6C, Sb1 Tb1 (BL#24158),
w*; E(z)731 P{1xFRT.G}2A/TM6C, Sb1 Tb1 (BL#24470),
w*; Su(z)122 P{FRT(whs)}2A/TM6C, Sb1 Tb1 (BL#24159),
esc21 b1 cn1/In(2LR)Gla, wgGla-1; ca1 awdK (BL#3623),
y1 w67c23; P{wHy}Caf1-55DG25308 (BL#21275),
w1118; P{XP}escld01514 (BL#19163),
y1 w*; phol81A/TM3, Ser1 y+ (BL#24164),
red1 e1 ash21/TM6B, Tb1 (BL#4584),
w1118; PBac{WH}Utxf01321/CyO (BL#18425),
w*; ash122 P{FRT(whs)}2A/TM6C, Sb1 Tb1 (BL#24161),
w1118; E(bx)Nurf301-3/TM3, P{ActGFP}JMR2, Ser1 (BL#9687),
y1 w67c23; P{lacW}Nurf-38k16102/CyO (BL#12206),
Mi-24 red1 e4/TM6B, Sb1 Tb1 ca1 (BL#26170),
mor RNAi (VDRC#6969),
psqE39/CyO; ry506 (BL#7321),
Rbf14 w1118/FM7c (BL#7435),
w1118 P{EP}Dsp1EP355 (BL#17270),
cn1 grhIM bw1/SM6a (BL#3270),
y1 w67c23; P{lacW}lolalk02512/CyO (BL#10515),
w*; P{neoFRT}42D Pcl5/CyO (BL#24157),
w*; HDAC1def24 P{FRT(whs)}2A P{neoFRT}82B/TM6B, Tb1 (BL#32239),
w1118; Sirt12A-7-11 (BL#8838),
Eip74EFv4 vtd4/TM3, st24 Sb1 (BL#5050),
sc1 z1 wis; Su(z)21.b7/CyO (BL#5572),
P{PZ}gpp03342 ry506/TM3, ryRK Sb1 Ser1 (BL#11585),
y1 w1118; P{lacW}mod(mdg4)L3101/TM3, Ser1 (BL#10312),
w1118; PBac{RB}su(Hw)e04061/TM6B, Tb1 (BL#18224),
cn1 P{PZ}lid10424/CyO; ry506 (BL#12367),
AsxXF23/CyO (BL#6041),
y1 w1; P{neoFRT}40A P{FRT(whs)}G13 cn1 PBac{SAstopDsRed}domLL05537 bw1/CyO, bw1 (BL#34496),
cn1 E(Pc)1 bw1/SM5 (BL#3056),
kis1 cn1 bw1 sp1/SM6a (BL#431),
kto1 ca1/TM6B, Tb1 (BL#3618),
skd2/TM6C, cu1 Sb1 ca1 (BL#5047).
Genetic screen
Mutants or RNAi lines were crossed to w1118;; rnGAL4, UAS-rpr, tubGAL80ts/TM6B, tubGAL80 flies. Controls were w1118 or the appropriate RNAi background line. Embryos were collected at room temperature on grape plates for 4 h in the dark, then kept at 18°C. Larvae were picked at 2 days after egg lay into standard Bloomington cornmeal media and kept at 18°C, 50 larvae in each vial, three vials per genotype per replicate. On day 7, tissue ablation was induced by placing the vials in a 30°C circulating water bath for 24 h. Then ablation was stopped by placing the vials in ice water for 60 s and returning them to 18 °C for regeneration. The regeneration index was calculated by summing the product of approximate wing size (0%, 25%, 50%, 75%, and 100%) and the corresponding percentage of wings for each wing size. The Δ Index was calculated by subtracting the regeneration index of the control from the regeneration index of the mutant or RNAi line.
To observe and quantify the patterning features and absolute wing size, adult wings that were 75% size or greater were mounted in Gary’s Magic Mount [Canada balsam (Sigma) dissolved in methyl salicylate (Sigma)]. The mounted adult wings were imaged with an Olympus SZX10 microscope using an Olympus DP21 camera, with the Olympus CellSens Dimension software. Wings were measured using ImageJ.
Immunostaining
Immunostaining was carried out as previously described (Smith-Bolton et al. 2009). Primary antibodies used in this study were rabbit anti-Myc (1:500; Santa Cruz Biotechnology), mouse anti-Nubbin (1:250; gift from Steve Cohen) (Ng et al. 1996), mouse anti-Engrailed/Invected [1:3; Developmental Studies Hybridoma Bank (DSHB)] (Patel et al. 1989), mouse anti-Patched (1:50; DSHB) (Capdevila et al. 1994), mouse anti-Achaete (1:10; DSHB) (Skeath and Carroll 1992), rabbit anti-PH3 (1:500; Millipore), mouse anti-Osa (1:1; DSHB) (Treisman et al. 1997), rat anti-Ci (1:10; DSHB) (Motzny and Holmgren 1995), rabbit anti-Dcp1 (1:250; Cell Signaling), mouse anti-βgal (1:100; DSHB), rabbit anti-phospho-Mad (1:100; Cell Signaling), mouse anti-Mmp1 (1:10 of 1:1:1 mixture of monoclonal antibodies 3B8D12, 5H7B11, and 3A6B4, DSHB) (Page-McCaw et al. 2003). The Developmental Studies Hybridoma Bank (DSHB) was created by the NICHD of the NIH and is maintained at the University of Iowa, Department of Biology, Iowa City, IA 52242, USA. Secondary antibodies used in this study were AlexaFluor secondary antibodies (Molecular Probes) (1:1000). TO-PRO-3 iodide (Molecular Probes) was used to detect DNA at 1:500.
Confocal images were collected with a Zeiss LSM700 Confocal Microscope using ZEN software (Zeiss). Images were processed with ImageJ (NIH) and Photoshop (Adobe). Average fluorescence intensity was measured by ImageJ. Quantification of fluorescence intensity and phospho-histone H3 positive cells was restricted to the wing pouch, as marked by anti-Nubbin immunostaining or morphology. The area of the regenerating wing primordium was quantified by measuring the anti-Nubbin immunostained area in ImageJ.
Quantitative RT-PCR
qPCR was conducted as previously described (Skinner et al. 2015). Each independent sample consisted of 50 wing discs. Three biological replicates were collected for each genotype and time point. Expression levels were normalized to the control Gapdh2. The fold changes compared to the w1118 undamaged wing discs are shown. Primers used in the study were:
Gapdh2 (Forward: 5′-GTGAAGCTGATCTCTTGGTACGAC-3′;
Reverse: 5′-CCGCGCCCTAATCTTTAACTTTTAC-3′),
ilp8 (Qiagen QT00510552),
mmp1 (Forward: 5′-TCGGCTGCAAGAACACGCCC-3′;
Reverse: 5′-CGCCCACGGCTGCGTCAAAG-3′),
moira (Forward: 5′-GATGAGGTGCCCGCTACAAT-3′;
Reverse: 5′-CTGCTGCGGTTTCGTCTTTT-3′),
brm (Forward: 5′-GCACCACCAGGGGATGATTT-3′;
Reverse: 5′-TTGTGTGGGTGCATTGGGT-3′),
Bap60 (Forward: 5′-AGACGAGGGATTTGAAGCTGA-3′;
Reverse: 5′-AGGTCTCTTGACGGTGGACT-3′)
Myc (Forward: 5′-CGATCGCAGACGACAGATAA-3′;
Reverse: 5′-GGGCGGTATTAAATGGACCT-3′)
Pupariation timing experiments
To quantify the pupariation rates, pupal cases on the side of each vial were counted at 24-h intervals starting from the end of tissue ablation until no new pupal cases formed. Three independent biological replicates, which consisted of three vials each with 50 animals per vial, were performed for each experiment. The median day is the day on which ≥50% of the animals had pupariated.
Data availability
All relevant data are available at https://doi.org/10.13012/B2IDB-1681718_V1 and upon request. Supplemental Material available at figshare: https://doi.org/10.25386/genetics.13260266.
Results
A genetic screen of chromatin modifier mutants and RNAi lines
To identify regeneration genes among Drosophila chromatin regulators, we conducted a genetic screen similar to our previously reported unbiased genetic screen for genes that regulate wing imaginal disc regeneration (Brock et al. 2017) (Figure 1A). To induce tissue ablation, rotund-GAL4 drove the expression of the pro-apoptotic gene reaper via UAS control in the imaginal wing pouch, and tubulin-GAL80ts provided temporal control, enabling us to turn ablation on and off by varying the temperature (Smith-Bolton et al. 2009). The ablation was carried out for 24 h during the early third instar. We characterized the quality of regeneration by assessing the adult wing size semi-quantitatively and (1) recording the numbers of wings that were 0%, 25%, 50%, 75%, or 100% the length of a normal adult wing (Figure 1, A and B), and (2) identifying patterning defects by scoring ectopic or missing features. This semi-quantitative evaluation method enabled a quick screen, at a rate of six genotypes per week including around 1400 adult wings, and identification of both enhancers and suppressors of regeneration (Figure 1, B–E). While control animals regenerated to varying degrees depending on the extent they delayed metamorphosis in response to damage (Smith-Bolton et al. 2009; Khan et al. 2017) as well as seasonal differences in humidity and food quality (Skinner et al. 2015), the differences between the regenerative capacity of mutants and controls were consistent (Smith-Bolton et al. 2009; Brock et al. 2017; Khan et al. 2017).
Figure 1.
A genetic screen of chromatin regulators identified important regeneration genes. (A) Method for screening mutants or RNAi lines using a genetic ablation system. Mutants or RNAi lines of genes involved in regulating chromatin were crossed to the ablation stock (w1118; +; rn-GAL4, UAS-rpr, tubGAL80ts/TM6B, tubGAL80). Animals were kept at 18°C until 7 days after egg lay (AEL), when they were moved to 30°C to induce tissue ablation for 24 h, then transferred back to 18°C to enable recovery (R). The size of the regenerated adult wings was assessed semi-quantitatively by counting the number of wings that were approximately 0%, 25%, 50%, 75%, or 100% of the length of a control adult wing that had not undergone damage during the larval phase. The regenerating discs were also examined at different times denoted by hours after the beginning of recovery, such as R0, R24, R48, and R72. (B) Conceptual model for the screen to identify mutants or RNAi lines showing enhanced (green) or reduced (purple) regeneration compared to control. (C) Summary of the screen of chromatin regulators, showing percent of lines tested that had a regeneration phenotype, as well as percent of those with a phenotype that regenerated better (Δ Index ≥ 10%) or worse (Δ Index ≤ −10%) compared to controls. (D) Comparison of the size of adult wings after imaginal disc damage and regeneration in phol81A/+ and wild-type (w1118) animals. n = 64 wings (phol81A/+) and 242 wings (w1118) from three independent experiments. Chi-square test P < 0.001 across all wing sizes. Error bars are SEM. (E) Comparison of the size of adult wings after imaginal disc damage and regeneration in E(bx)nurf301-3/+ and wild-type (w1118) animals. n = 219 wings (E(bx)nurf301-3/+) and 295 wings (w1118) from three independent experiments. Chi-square test P < 0.001 across all wing sizes. Error bars are SEM.
Using this system, we screened mutants and RNAi lines affecting chromatin regulators (Supplementary Table S1, Figure 1C, Supplementary Figure S1A). For each line, we calculated the Δ regeneration index, which is the difference between the regeneration indices of the line being tested and the control tested simultaneously (see Materials and Methods for regeneration index calculation). We set a cutoff Δ index of 10%, over which we considered the regenerative capacity to be affected. Seventy-eight percent of the mutants and RNAi lines tested had a change in regeneration index of 10% or more compared to controls (Supplementary Table S1, Figure 1C, Supplementary Figure S1A), consistent with the idea that changes in chromatin structure are required for the damaged tissue to execute the regeneration program. Twenty-two percent of the mutants and RNAi lines failed to meet our cutoff and were not pursued further (Supplementary Table S1, Figure 1C). Strikingly, 53% of the tested lines, such as phol81A/+, which affects the PhoRC complex, had larger adult wings after ablation and regeneration compared to control w1118 animals that had also regenerated (Figure 1D), indicating enhanced regeneration, although none were larger than a normal-sized wing. By contrast, 25% of the tested lines, such as E(bx)nurf301-3/+, which affects the NURF complex, had smaller wings (Figure 1E), indicating worse regeneration. Unexpectedly, mutations that affected the same complex did not have consistent phenotypes (Supplementary Table S1), suggesting that chromatin modification and remodeling likely regulate a delicate balance of genes that promote and constrain regeneration. Indeed, transcriptional profiling has identified a subset of genes that are upregulated after wing disc ablation (Khan et al. 2017), some of which promote regeneration, and some of which constrain regeneration, indicating that gene regulation after tissue damage is not as simple as turning on genes that promote regeneration and turning off genes that inhibit regeneration.
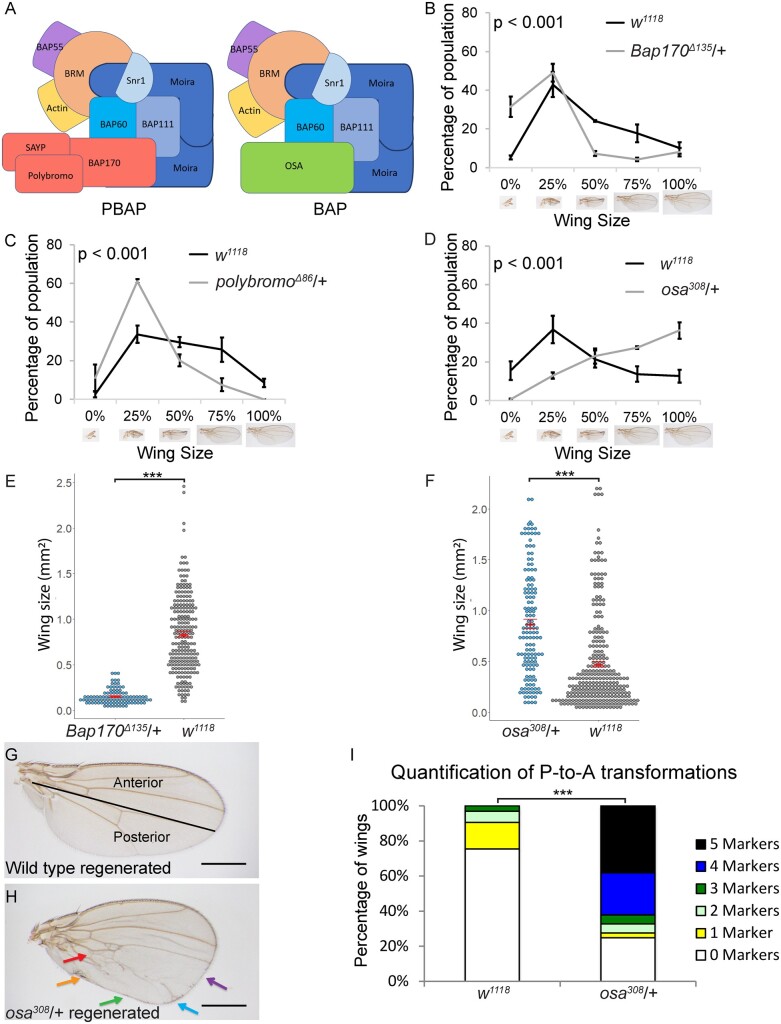
The SWI/SNF PBAP and BAP complexes have opposite phenotypes
To clarify the roles of one type of chromatin-regulating complex in regeneration, we focused on the SWI/SNF chromatin-remodeling complexes (Figure 2A). As shown in Supplementary Table S1, different components of the SWI/SNF complexes showed different phenotypes after ablation and regeneration of the wing pouches. Animals heterozygous mutant for the PBAP-specific components Bap170 (Bap170Δ135/+) and Polybromo (polybromoΔ86/+) had adult wings that were smaller after disc regeneration than w1118 adult wings after disc regeneration (Figure 2, B and C), suggesting that the PBAP complex is required for ablated wing discs to regrow. To confirm these semiquantitative results, we mounted adult wings and measured absolute wing sizes (N ≥ 100 wings for each genotype). The reduced regeneration of Bap170Δ135/+ wing discs was confirmed by measurement of the adult wings, while adult Bap170Δ135/+ wings without damage and regeneration in the discs were comparable to controls (Figure 2E, Supplementary Figure S1, B and D). By contrast, animals heterozygous mutant for the BAP-specific component Osa (osa308/+) had larger adult wings after disc regeneration compared to w1118 adult wings after disc regeneration (Figure 2D), suggesting that impairment of the BAP complex deregulates growth after tissue damage. Measurement of the adult wings of osa308/+ animals after disc regeneration confirmed the enhanced regeneration, while adult osa308/+ wings without damage and regeneration in the discs were only slightly larger than controls (Figure 2F, Supplementary Figure S1, C and D).
Figure 2.
SWI/SNF components Bap170, Polybromo and Osa are required for regeneration. (A) Schematics of the two Drosophila SWI/SNF chromatin-remodeling complexes: BAP and PBAP, drawn based on complex organization determined in (Mashtalir et al. 2018). (B) Comparison of the size of adult wings after imaginal disc damage and regeneration in Bap170Δ135/+ and wild-type (w1118) animals. n = 190 wings (Bap170Δ135/+) and 406 wings (w1118) from three independent experiments. Chi-square test P < 0.001 across all wing sizes. (C) Comparison of the size of adult wings after imaginal disc damage and regeneration in polybromoΔ86/+ and wild-type (w1118) animals. n = 180 wings (polybromoΔ86/+) and 396 wings (w1118) from three independent experiments. Chi-square test P < 0.001 across all wing sizes. (D) Comparison of the size of adult wings after imaginal disc damage and regeneration in osa308/+ and wild-type (w1118) animals. n = 146 wings (osa308/+) and 296 wings (w1118) from three independent experiments. Chi-square test P < 0.001 across all wing sizes. (E) Wings were mounted, imaged, and measured after imaginal disc damage and regeneration in Bap170Δ135/+ and wild-type (w1118) animals. n = 100 wings (Bap170Δ135/+) and 224 wings (w1118) from three independent experiments. Student’s t-test, P < 0.001. (F) Wings were mounted, imaged, and measured after imaginal disc damage and regeneration in osa308/+ and wild-type (w1118) animals. n = 142 wings (osa308/+) and 284 wings (w1118) from three independent experiments. (G) Wild-type (w1118) adult wing after disc regeneration. Anterior is up. (H) osa308/+ adult wing after disc regeneration. Arrows show five anterior-specific markers in the posterior compartment: anterior crossveins (red), alula-like costa bristles (orange), margin vein (green), socketed bristles (blue), and change of wing shape with wider distal portion of the wing, similar to the anterior compartment (purple). (I) Quantification of the number of Posterior-to-Anterior transformation markers described in (H) in each wing after damage and regeneration of the disc, using wings that were 75% normal size or larger, comparing osa308/+ wings to wild-type (w1118) wings, n = 51 wings (osa308/+) and 45 wings (w1118), from three independent experiments. Chi-square test P < 0.001. Error bars are SEM. Scale bars are 500 μm for all adult wings images. *P < 0.05, **P < 0.01, ***P < 0.001 Student’s t-test.
Interestingly, the osa308/+ adult wings also showed severe patterning defects after damage and regeneration of the disc (Figure 2, G–I). Specifically, the posterior compartment of the osa308/+ wings had anterior features after wing pouch ablation, but had normal wings when no tissue damage was induced (Supplementary Figure S1C). To quantify the extent of the posterior-to-anterior (P-to-A) transformations, we quantified the number of anterior features in the posterior of each wing, including socketed bristles and ectopic veins on the posterior margin, an ectopic anterior crossvein (ACV), costal bristles on the alula, and an altered shape that has a narrower proximal and wider distal P compartment (Schuster and Smith-Bolton 2015) (Figure 2I). While w1118 adult wings that had regenerated as discs had a low level of P-to-A transformations, 75% of the osa308/+ wings had P-to-A transformations, and 83% of these transformed wings had four or five anterior markers in the posterior of the wing. To confirm the phenotype, we tested an additional allele of osa and two deficiencies that remove the osa locus, all of which showed transformations of the posterior of the wing to anterior structures after damage and regeneration of the disc in heterozygous mutants (Supplementary Figure S1, E–H). Thus, Osa is required to preserve posterior cell fate during regeneration, suggesting that the BAP complex regulates cell fate after damage.
Reducing the core SWI/SNF components to varying levels produces either the BAP or PBAP phenotype
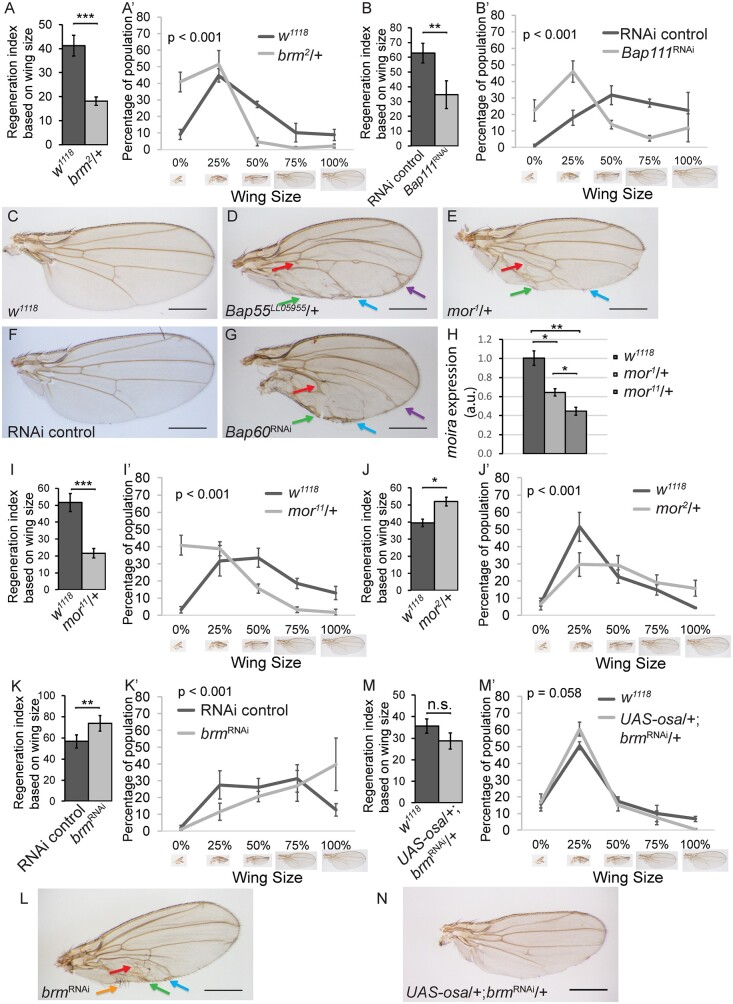
Because mutants of the BAP or PBAP complex-specific components showed distinct phenotypes, we also screened mutants of the core components for regeneration phenotypes. Interestingly, mutants or RNAi lines that reduced levels of the core components were split between the two phenotypes. For example, brm2/+ discs and discs expressing a Bap111 RNAi construct regenerated poorly, resulting in small wings (Figure 3, A and B), while Bap55LL05955/+ discs, mor1/+ discs, and discs expressing a Bap60 RNAi construct regenerated to produce larger wings overall that showed P-to-A transformations (Supplementary Table S1, Figure 3, C–G, , Supplementary Figure S1A).
Figure 3.
SWI/SNF core components are required for both growth and posterior fate during wing disc regeneration. (A) Comparison of the size of adult wings after imaginal disc damage and regeneration in brm2/+ and wild-type (w1118) animals. n = 142 wings (brm2/+) and 224 wings (w1118) from three independent experiments, student’s t-test P < 0.001. (Aʹ) Chi-square test P < 0.001 across all wing sizes. (B) Comparison of the size of adult wings after imaginal disc damage and regeneration in animals expressing Bap111 RNAi and control animals. n = 264 wings (Bap111 RNAi) and 291 wings (control) from three independent experiments. The control for RNAi lines is VDRC 15293 in all experiments, student’s t-test P < 0.01. (Bʹ) Chi-square test P < 0.001 across all wing sizes. (C–G) Adult wing after disc regeneration of wild-type (w1118) (C), Bap55LL05955/+ (D), mor1/+ (E), RNAi control (F) or Bap60 RNAi (G). Anterior is up for all adult wing images. Arrows point to anterior features identified in the posterior compartment. Arrows show five anterior-specific markers in the posterior compartment: anterior cross veins (red), alula-like costa bristles (orange), margin vein (green), socketed bristles (blue), and change of wing shape with wider distal portion of the wing, similar to the anterior compartment (purple). (H) moira expression determined by qPCR of mor1/+, mor11/+ and wild-type (w1118) undamaged wing discs at R24. The graph shows fold change relative to wild-type (w1118) discs. (I) Comparison of the size of adult wings after imaginal disc damage and regeneration in mor11/+ and wild-type (w1118) animals. n = 114 wings (mor11/+) and 328 wings (w1118) from three independent experiments, student’s t-test P < 0.001. (I’) Chi-square test P < 0.001 across all wing sizes. (J) Comparison of the size of adult wings after imaginal disc damage and regeneration in mor2/+ and wild-type (w1118) animals. n = 134 wings (mor2/+) and 414 wings (w1118) from three independent experiments, student’s t-test P < 0.05. (J’) Chi-square test P < 0.001 across all wing sizes. (K) Comparison of the size of adult wings after imaginal disc damage and regeneration in animals expressing brm RNAi and control animals. n = 234 wings (brm RNAi) and 281 wings (control) from three independent experiments, student’s t-test P < 0.01. (K’) Chi-square test P < 0.001 across all wing sizes. (L) Adult wing after disc regeneration while expressing brm RNAi. (M) Comparison of the size of adult wings after imaginal disc damage and regeneration in UAS-osa/+; brmRNAi/+ and wild-type (w1118) animals. n = 117 wings (UAS-osa/+; brmRNAi/+) and 348 wings (w1118) from three independent experiments, student’s t-test not significant. (M’) Chi-square test across all wing sizes P = 0.058, not significant at α = 0.05 level. (N) Adult wing after imaginal disc regeneration in UAS-osa/+; brmRNAi/+ animal. Error bars are SEM. Scale bars are 500 μm for all adult wing images. *P < 0.05, **P < 0.01, ***P < 0.001 Student’s t-test.
Given that the SWI/SNF complexes require the function of the scaffold Mor and the ATPase Brm (Moshkin et al. 2007; Mashtalir et al. 2018), it was surprising that reduction of Mor showed the BAP phenotype while reduction of Brm showed the PBAP phenotype. However, it is likely that some of the mutants and RNAi lines caused stronger loss of function than others, due to strength of the allele or the transient and localized nature of RNAi. A stronger reduction in function would result in malfunction of both BAP and PBAP, and show the reduced regeneration phenotype, masking any patterning defects. By contrast, a weaker or transient reduction in function could mainly affect the BAP complex. For example, Bap60 RNAi, which caused patterning defects after wing disc regeneration, only induced a moderate reduction in mRNA levels, suggesting that it causes a weak loss of function (Supplementary Figure S2A). Although it is unclear why a weaker reduction of function would mainly affect the BAP complex, it is possible that the BAP complex is less abundant than the PBAP complex, such that a slight reduction in a core component would have a greater effect on the amount of BAP in the tissue. Therefore, we hypothesized that stronger or weaker loss of function of the same core complex component might show different phenotypes.
To test this hypothesis, we used a strong loss-of-function mor mutant, mor11 (gift from J. Kennison, Supplementary Figure S2B), and two hypomorphic mor mutants mor1 and mor2 (Kennison and Tamkun 1988). Indeed, mor11/+ undamaged wing discs had significantly less mor transcript than mor1/+ or control undamaged wing discs (Figure 3H). Interestingly, mor11/+ animals showed the poor regeneration phenotype similar to the PBAP complex-specific Bap170Δ135/+ mutants (Figure 3I), while mor1/+ and mor2/+ showed the enhanced regeneration phenotype and the P-to-A transformation phenotype similar to the BAP complex-specific osa308/+ mutants (Figure 3, E and J, Supplementary Table S1). Importantly, both phenotypes were observed using mutant alleles, ruling out the possibility that one phenotype was the result of RNAi. To confirm these findings, we also used an amorphic allele of brm and an RNAi line that targets brm to reduce the levels of the core component brm. brm2 was generated through ethyl methanesulfonate mutagenesis and causes a loss of Brm protein without affecting transcript levels (Kennison and Tamkun 1988; Elfring et al. 1998). The brm RNAi causes a partial reduction in transcript, as rn>brmRNAi undamaged wing discs had less brm transcript than control undamaged wing discs (Supplementary Figure S2C). brm2/+ animals showed the small wing phenotype after disc damage, indicating poor regeneration (Figure 3A). By contrast, knockdown of brm by expressing the brm RNAi construct during tissue ablation induced larger wings and P-to-A transformations (Figure 3, K and L). Thus, slight reduction of the core SWI/SNF components, through mor1, brm RNAi, or Bap60 RNAi, produced the BAP phenotype, whereas stronger reduction of the core components, through mor11, produced the PBAP phenotype, suggesting that it is easier to compromise BAP function than to compromise PBAP function. If it is easier to compromise BAP function because there is less BAP complex in regenerating wing disc cells, overexpression of the BAP-specific component Osa would lead to an increase in the amount of BAP complex and rescue the brm RNAi phenotype. Indeed, overexpression of osa in regenerating tissue rescued the enhanced wing size and P-to-A transformations induced by brm RNAi (Figure 3, M and N).
The PBAP complex is required for Myc upregulation and cell proliferation during regrowth
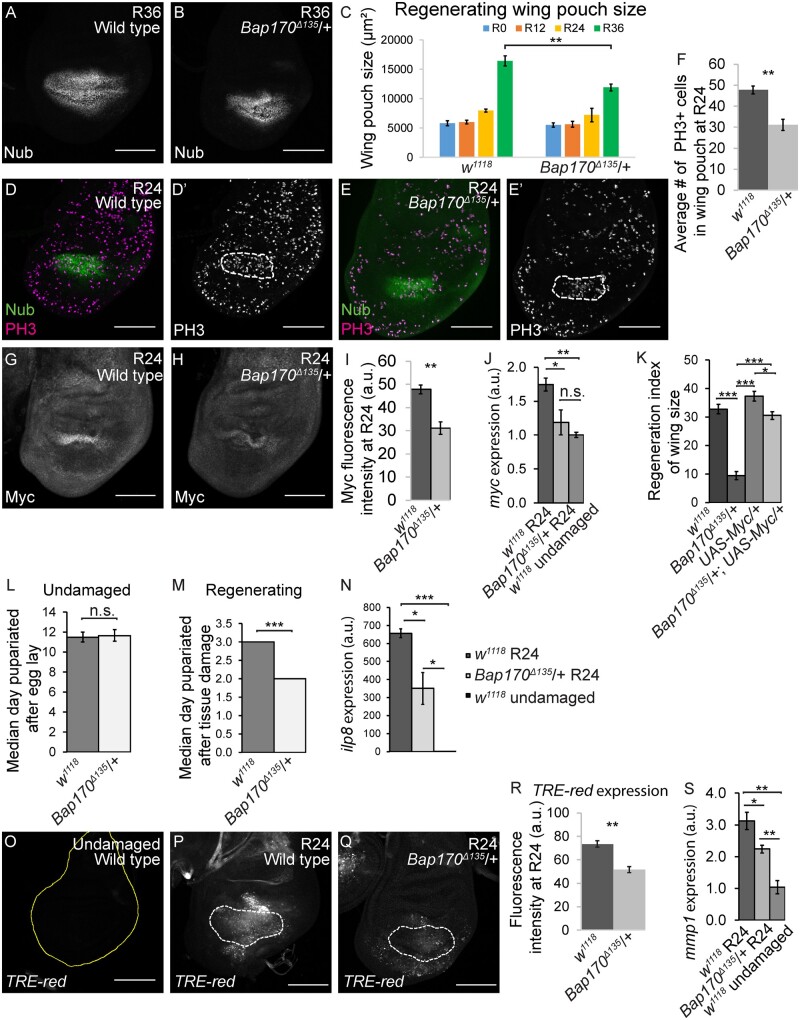
To identify when the defect in regrowth occurs in PBAP complex mutants, we measured the regenerating wing pouch using expression of the pouch marker nubbin in w1118 controls, Bap170Δ135/+ and brm2/+ mutants, as well as in the osa308/+ BAP mutant for comparison. The regenerating wing pouches of Bap170Δ135/+ mutant animals were not different in size compared to w1118 animals at 0, 12, or 24 h after tissue damage (R0, R12, or R24). However, the Bap170Δ135/+ regenerating wing pouches were smaller than w1118 by 36 hours after tissue damage (R36), shortly before the Bap170Δ135/+ mutant animals pupariated and entered metamorphosis (Figure 4, A–C). brm2/+ mutant animals also had smaller regenerating wing pouches by R24 (Supplementary Figure S3, A–C). By contrast, the regenerating osa308/+ wing pouches regrew at the same rate as controls (Supplementary Figure S3, D–H).
Figure 4.
Decreased Bap170 expression limits regenerative growth and pupariation delay. (A) Wild-type (w1118) regenerating wing disc at R36 with wing pouch marked by anti-Nubbin (green) immunostaining. (B) Bap170Δ135/+ regenerating wing disc at R36 with wing pouch marked by anti-Nubbin (green) immunostaining. (C) Comparison of regenerating wing pouch size at 0, 12, 24, and 36 h after imaginal disc damage in Bap170Δ135/+ and wild-type (w1118) animals. (D, E) Regenerating wild-type (w1118) (D) and Bap170Δ135/+ (E) wing discs at R24 with Nubbin (green) and PH3 (magenta) immunostaining. Dashed white outline shows the regenerating wing primordium labeled with Nubbin. (F) Average number of mitotic cells (marked with PH3 immunostaining) in the wing primordium (marked by anti-Nubbin) at R24 in Bap170Δ135/+ and wild-type (w1118) animals. n = 8 wing discs (Bap170Δ135/+) and 10 wing discs (w1118). (G-H) Wild-type (w1118) (G) and Bap170Δ135/+ (H) regenerating wing discs at R24 with Myc immunostaining. (I) Quantification of anti-Myc immunostaining fluorescence intensity in the wing pouch in Bap170Δ135/+ and wild-type (w1118) regenerating wing discs at R24. n = 9 wing discs (Bap170Δ135/+) and 9 wing discs (w1118). (L) Median time to pupariation for animals during normal development at 18°C. n = 103 pupae (Bap170Δ135/+) and 227 pupae (w1118) from three independent experiments. Student’s t-test not significant. (M) Median time to pupariation for animals after tissue damage (30°C) and regeneration (18°C). n = 117 pupae (Bap170Δ135/+) and 231 pupae (w1118) from three independent experiments. Because the temperature shift to 30°C in the ablation protocol increases the developmental rate, the pupariation timing of regenerating animals (M) cannot be compared to the undamaged control animals (L). Student’s t-test P < 0.001. (N) ilp8 expression examined by qPCR of Bap170Δ135/+ and wild-type (w1118) regenerating wing discs at R24. The graph shows fold change relative to wild-type (w1118) undamaged discs. (O–Q) Expression of TRE-Red, a JNK signaling reporter, in wild-type (w1118) undamaged (O), as well as wild-type (w1118) (P) and Bap170Δ135/+ (Q) regenerating wing discs at R24. Yellow outline shows the wing disc in (O). White dashed lines show the wing pouch in (P) and (Q) as marked by anti-Nub. (R) Quantification of TRE-Red fluorescence intensity in Bap170Δ135/+ and wild-type (w1118) regenerating wing pouches at R24. n = 12 wing discs (Bap170Δ135/+) and 14 wing discs (w1118). (S) mmp1 expression examined by qPCR of wild-type (w1118) and Bap170Δ135/+ regenerating wing discs at R24, and wild-type (w1118) undamaged discs. The graph shows fold change relative to wild-type (w1118) regenerating discs at R24. Scale bars are 100μm for all wing discs images. *P < 0.05, **P < 0.01, ***P < 0.001, Student’s t-test.
To determine whether the Bap170Δ135/+ mutant animals had a slower rate of proliferation during regeneration, we quantified the number of mitotic cells by immunostaining for phospho-histone H3 (PH3) in the regenerating wing pouch. A 35% decrease in the number of PH3-positive cells was observed in Bap170Δ135/+ mutants (Figure 4, D–F, Supplementary Figure S3I). Interestingly, there was also a slight but significant decrease in PH3-positive cells outside of the regenerating wing pouch in Bap170Δ135/+ mutants (Supplementary Figure S3J), although there was no difference in pouch size or PH3-positive cells within or outside the pouch in undamaged discs (Supplementary Figure S3, K–N). While smaller adult wings could also be caused by increased cell death in the regenerating tissue, we did not find an increase in cell death in Bap170Δ135/+ regenerating wing discs as marked by immunostaining for cleaved caspase Dcp1 (Supplementary Figure S3, O and P).
To identify why proliferation was reduced in Bap170Δ135/+ mutants, we examined levels of Myc, an important growth regulator that is upregulated during Drosophila wing disc regeneration (Smith-Bolton et al. 2009). In mammals, c-myc is a direct target of the SWI/SNF BAF complex, which is similar to Drosophila BAP (Nagl et al. 2006), but a role for the PBAP complex in regulating the Drosophila Myc gene has not been established. Myc protein levels were significantly reduced in Bap170Δ135/+ and brm2/+ regenerating wing pouches compared to wild-type regenerating wing pouches (Figure 4, G-I and Supplementary Figure S4, A–D). Myc transcriptional levels were also significantly lower in Bap170Δ135/+ regenerating wing discs compared to wild-type regenerating discs (Figure 4J). By contrast, there was no change in Myc levels in osa308/+ mutants (Supplementary Figure S4, E–G), indicating that upregulation of Myc after tissue damage is sensitive to reduction of PBAP, but not BAP. To determine the extent to which reduction of Myc expression was responsible for the poor regeneration phenotype in BAP complex mutants, we overexpressed Myc in the Bap170Δ135/+ background during regeneration. Indeed, the Bap170Δ135/+, UAS-Myc/+ animals regenerated similar to the w1118 controls and significantly better than Bap170Δ135/+ animals, demonstrating partial rescue of the poor regeneration phenotype (Figure 4K and Supplementary Figure S4H).
The PBAP complex is required for the delay in pupariation induced by tissue damage
Damaged imaginal discs delay pupariation by expressing the peptide ILP8, which delays the production of ecdysone and onset of metamorphosis, providing more time for damaged tissue to regenerate (Colombani et al. 2012; Garelli et al. 2012). To determine whether the SWI/SNF complexes regulate the timing of metamorphosis, we quantified the pupariation rate in w1118 and Bap170Δ135/+ regenerating animals, and identified the day on which 50% of the larvae had pupariated. Without tissue damage, Bap170Δ135/+ mutants pupariated slightly later than w1118 animals (Figure 4L and Supplementary Figure S5A), but the difference is not significant. However, after wing disc damage, more than half of the Bap170Δ135/+ mutant animals had pupariated by 2 days after damage, whereas more than half of the w1118 animals had not pupariated until 3 days after damage, giving the mutants 1/3 less time to regenerate (Figure 4M and Supplementary Figure S5B). To uncover why Bap170Δ135/+ animals had less regeneration time, we quantified ilp8 transcript levels. Indeed, Bap170Δ135/+ animals had about 50% less ilp8 mRNA (Figure 4N), suggesting that the PBAP complex is required for ilp8 expression.
The PBAP complex regulates expression of JNK signaling targets
SWI/SNF complexes can be recruited by transcription factors to act as co-activators of gene expression (Becker and Workman 2013). Regenerative growth and the pupariation delay are regulated by JNK signaling (Bosch et al. 2008; Bergantinos et al. 2010; Colombani et al. 2012; Garelli et al. 2012; Skinner et al. 2015). Thus, it is possible that PBAP is recruited to JNK signaling targets like ilp8 by the AP-1 transcription factor, which acts downstream of JNK (Perkins et al. 1988), and that PBAP is required for full activation of these targets. To determine whether Bap170 is required for JNK-dependent transcription, we examined the activity of the TRE-Red reporter, which is comprised of four AP-1 binding sites (TREs) driving the expression of a DsRed.T4 reporter gene (Chatterjee and Bohmann 2012) in w1118 and Bap170Δ135/+ regenerating wing discs. The TRE-Red intensity was significantly decreased in the Bap170Δ135/+ regenerating tissue compared to the w1118 regenerating tissue (Figure 4, O–R), indicating that PBAP is required for full activation of this AP-1 transcriptional activity reporter, similar to its requirement for expression of ilp8. Furthermore, expression of the JNK signaling target matrix metalloproteinase 1 (mmp1) was significantly reduced in Bap170Δ135/+ regenerating wing discs at both the mRNA and protein levels (Figure 4S and Supplementary Figure S5, C–E). Thus, the PBAP complex plays a crucial role in the activation of JNK signaling targets.
The BAP complex maintains posterior cell fate during regeneration
After damage and regeneration of the disc, adult wings of osa308/+, Bap55LL05955/+, mor1/+, and mor2/+ discs, as well as discs expressing a brm RNAi construct or a Bap60 RNAi construct, had anterior bristles and veins in the posterior compartment (Figure 3, C–-G and K), but not after normal development (Supplementary Figures S1A and S2, D–F). To identify when the P-to-A transformations occurred, we examined the expression of anterior- and posterior-specific genes during tissue regeneration. engrailed (en) is essential for posterior cell fate both in development and regeneration (Kornberg et al. 1985; Schuster and Smith-Bolton 2015). To assess ability to maintain posterior cell fate, regenerating wing discs were dissected at different times during recovery (R) and immunostained for the posterior selector gene en. At 72 hours after damage (R72), in osa308/+ regenerating discs, en was expressed in some of the posterior compartment, but lost in patches (Figure 5, A–C). In addition, the proneural protein Acheate (Ac), which is expressed in sensory organ precursors in the anterior of wing discs (Skeath and Carroll 1991), was ectopically expressed in the posterior (Figure 5, D–F) marking precursors to the ectopic socketed bristles found in the posterior of the adult wings. The anterior genes cubitus interruptus (ci) (Eaton and Kornberg 1990) and patched (ptc) (Phillips et al. 1990) were also ectopically expressed in the posterior of the osa308/+ R72 regenerating wing discs but not in undamaged osa308/+ wing discs (Figure 6, A–C, Supplementary S5, F and G). The ectopic expression of these anterior genes was not observed at R48, suggesting that the P-to-A fate transformations happened late during regeneration (Supplementary Figure S5, H and I). Similarly, at R72, 80% of the brm RNAi wing discs had ectopic expression of the anterior genes ptc and ci in the posterior of the discs, while no expression of ptc or ci was observed in the posterior of control R72 discs (Figure 6, D and E).
Figure 5.
Reduction of Osa causes Posterior-to-Anterior transformations during wing disc regeneration. (A) Wild-type (w1118) undamaged wing disc with En (green) (Aʹ) and Ci (magenta) (Aʹʹ) immunostaining. DNA (blue) (Aʹʹʹ) was detected with Topro3 here and in subsequent panels. Anterior is left for all wing disc images. (B) Wild-type (w1118) regenerating wing disc at R72 with En (green) (Bʹ) and Ci (magenta) (Bʹʹ) immunostaining and DNA (blue) (Bʹʹʹ). (C) osa308/+ regenerating wing disc at R72 with En (green) (Cʹ) and Ci (magenta) (Cʹʹ) immunostaining, and DNA (blue) (Cʹʹʹ). Arrowhead points to the low En expression region in which Ci is expressed in the posterior compartment. (D) Wild-type (w1118) undamaged wing disc with Ac immunostaining. (E) Wild-type (w1118) regenerating wing disc at R72 with Ac immunostaining. (F) osa308/+ regenerating wing disc at R72 with Ac immunostaining. Arrowheads show Ac expression in the posterior compartment. Scale bars are 100μm for all wing discs images.
Figure 6.
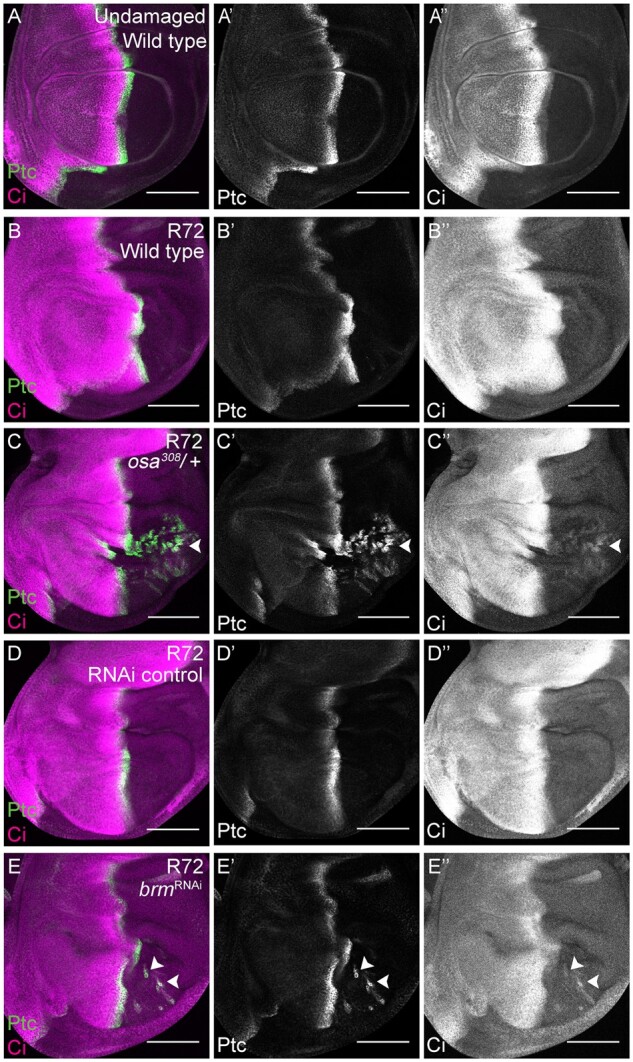
The BAP complex is required to maintain posterior cell fate during wing disc regeneration. (A) Wild-type (w1118) undamaged wing disc with Ptc (green) (Aʹ) and Ci (magenta) (Aʹʹ) immunostaining. (B) Wild-type (w1118) regenerating wing disc at R72 with Ptc (green) (Bʹ) and Ci (magenta) (Bʹʹ) immunostaining. (C) osa308/+ regenerating wing disc at R72 with Ptc (green) (Cʹ) and Ci (magenta) (Cʹʹ) immunostaining. Arrowhead shows Ptc and Ci co-expression in the posterior compartment. (D) RNAi control regenerating wing disc at R72 with Ptc (green) (Dʹ) and Ci (magenta) (Dʹʹ) immunostaining. (E) Regenerating wing disc of animals expressing brm RNAi at R72 with Ptc (green) (Eʹ) and Ci (magenta) (Eʹʹ) immunostaining. Arrowheads show Ptc and Ci co-expression in the posterior compartment. Scale bars are 100 μm for all wing disc images.
We previously showed that in Drosophila wing disc regeneration, elevated JNK increases expression of en, leading to PRC2-mediated silencing of the en locus in patches, and transformation of the en-silenced cells to anterior fate, and that Taranis (Tara) prevents this misregulation of en and resulting P-to-A cell fate transformations (Schuster and Smith-Bolton 2015). Thus, we wondered whether the BAP complex preserved en expression and posterior fate by reducing JNK signaling, or regulating tara expression, or working in parallel to Tara during the later stages of regeneration.
The BAP complex does not regulate JNK signaling
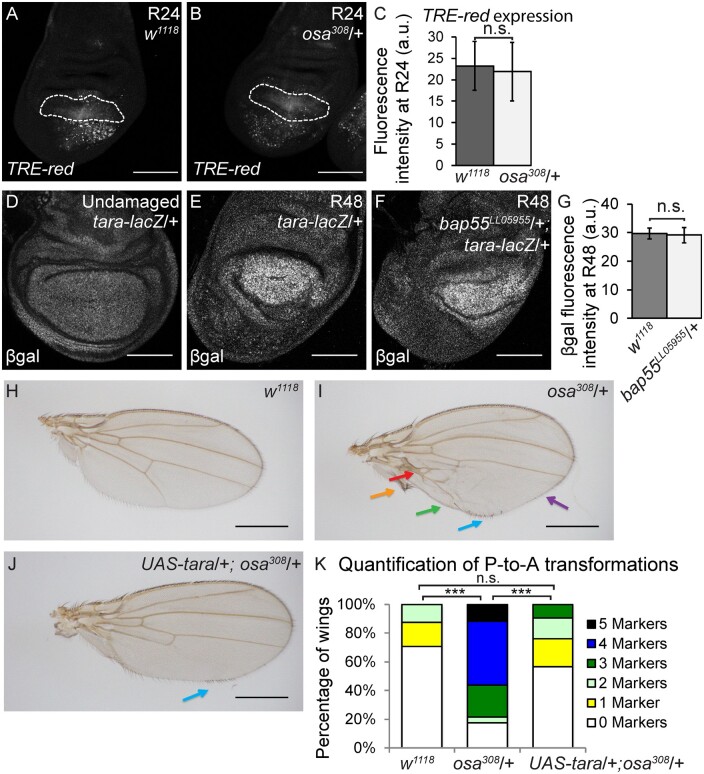
To determine whether the BAP complex regulates JNK signaling, we examined the JNK reporter TRE-Red in osa308/+ and w1118 regenerating wing discs. In contrast to Bap170Δ135/+ mutants (Figure 4, O–R), TRE-Red intensity was not different between osa308/+ and w1118 regenerating tissue (Figure 7, A–C). Thus, the BAP complex acts to protect posterior cell fate downstream of or in parallel to JNK signaling.
Figure 7.
The BAP complex functions in parallel to Tara to prevent P-to-A transformations. (A-B) Expression of TRE-Red, a JNK signaling reporter, in wild-type (w1118) (A) and osa308/+ (B) regenerating wing discs at R24. Dashed white outline shows the regenerating wing primordium as marked by anti-Nub and excluding the debris field. (C) Quantification of TRE-Red expression fluorescence intensity in osa308/+ and wild-type (w1118) regenerating wing pouches at R24. n = 26 wing discs (osa308/+) and 31 wing discs (w1118). Error bars are SEM. (D–F) tara expression detected with anti- β-gal immunostaining in tara-lacZ/+ undamaged (D), tara-lacZ/+ R48 (E) and Bap55LL05955/+; tara-lacZ/+ R48 (F) regenerating wing discs. (G) Quantification of β-gal expression via fluorescence intensity to determine levels of tara-lacZ expression in Bap55LL05955/+ and wild-type (w1118) regenerating wing pouches at R48. n = 8 wing discs (Bap55LL05955/+) and nine wing discs (w1118). Error bars are SEM. (H–J) Adult wings after disc regeneration in wild-type (w1118) (H), osa308/+ (I) and UAS-tara/+; osa308/+ (J) animals. Arrows show five anterior-specific markers in the posterior compartment: anterior crossveins (red), alula-like costa bristles (orange), margin vein (green), socketed bristles (blue), and change of wing shape with wider distal portion of the wing, similar to the anterior compartment (purple). Anterior is up for all adult wing images. (K) Quantification of the number of Posterior-to-Anterior transformation markers described above in each wing after damage and regeneration of the disc, comparing UAS-tara/+; osa308/+ wings to osa308/+ and wild-type (w1118) wings, n = 21 wings (UAS-tara/+; osa308/+), n = 16 wings (osa308/+) and n = 34 wings (w1118), from three independent experiments. ***P < 0.001, Chi-square test. Chi-square test measuring UAS-tara/+; osa308/+ against w1118, P = 0.86, is not significant. Scale bars are 100 μm for all wing discs images. Scale bars are 500 μm for all adult wings images. *P < 0.05, **P < 0.01, Student’s t-test for (C) and (G).
The BAP complex functions in parallel to Taranis to preserve cell fate
Because tara is regulated transcriptionally after tissue damage (Schuster and Smith-Bolton 2015), we examined whether the BAP complex is required for tara upregulation in the regenerating tissue. Using a tara-lacZ enhancer trap, we assessed expression in Bap55LL05955/+ regenerating wing discs, which had the same P-to-A transformations as the osa308/+ regenerating discs. We used the Bap55LL05955 allele instead of an osa allele for technical reasons, as our ablation system, tara, and osa are all on the third chromosome, and Bap55LL05955 and osa alleles gave the same phenotype. No change in tara-lacZ expression was identified in the regenerating wing pouches (Figure 7, D–G), indicating that the damage-dependent tara expression was not downstream of BAP activity.
To determine whether Tara can suppress the P-to-A transformations induced by the reduction of BAP, we overexpressed Tara using UAS-tara under control of rn-Gal4 in the osa308/+ mutant animals, generating elevated Tara levels in the rn-expressing cells that survived the tissue ablation. Indeed, the P-to-A transformation phenotype in osa308/+ mutant animals was rescued by Tara overexpression (Figure 7, H–K). To rule out the possibility that Tara regulates osa expression, we quantified Osa immunostaining in tara/+ mutant regenerating tissue. Osa protein levels did not change during regeneration, and were unchanged in tara1/+ mutant regenerating discs (Supplementary Figure S6, A–F). Taken together, these data indicate that the BAP complex likely functions in parallel to Tara to constrain en expression, preventing auto-regulation and silencing of en, thereby protecting cell fate from changes induced by JNK signaling during regeneration.
The enhanced growth in BAP mutants is caused by ectopic AP boundaries
The increased wing size after disc regeneration in tara/+ animals was due to loss of en in patches of cells, which generated aberrant juxtaposition of anterior and posterior tissue within the posterior compartment. These ectopic AP boundaries established ectopic Decapentaplegic (Dpp) morphogen gradients (Schuster and Smith-Bolton 2015), which can stimulate extra growth in the posterior compartment (Tanimoto et al. 2000). To determine whether the osa/+ regenerating discs also had ectopic AP boundaries and ectopic morphogen gradients, we immunostained for Ptc to mark AP boundaries and phospho-Smad (pSmad) to visualize gradients of Dpp signaling. Indeed, ectopic regions of Ptc expression were surrounded by ectopic pSmad gradients in osa308/+ regenerating discs (Figure 8, A–C). Thus, the enhanced regeneration in osa308/+ and other SWI/SNF mutant animals was likely a secondary result of the patterning defect. Furthermore, pupariation occurred later in osa308/+ regenerating animals compared to w1118 regenerating animals (Supplementary Figure S6, G and H), which provided more time for regeneration in the mutants. Such a delay in pupariation can be caused by aberrant proliferation (Colombani et al. 2012; Garelli et al. 2012) in addition to tissue damage, and the combination of the two likely led to the increase in delay in metamorphosis seen specifically in mutants with P-to-A transformations.
Figure 8.

Cell fate changes induce ectopic AP boundaries in the posterior compartment during wing disc regeneration. (A) Wild-type (w1118) undamaged wing disc with Ptc (green) (Aʹ) and pSMAD (magenta) (Aʹʹ) immunostaining. (B) Wild-type (w1118) regenerating wing disc at R48 with Ptc (green) (Bʹ) and pSMAD (magenta) (Bʹʹ) immunostaining. (C) osa308/+ regenerating wing disc at R48 with Ptc (green) (Cʹ) and Ci (magenta) (Cʹʹ) immunostaining. (D) Proposed working model for the functions of the PBAP and BAP complexes in regeneration.
Discussion
To address the question of how regeneration genes are regulated in response to tissue damage, we screened a collection of mutants and RNAi lines that affect a significant number of the chromatin regulators in Drosophila. Most of these mutants had regeneration phenotypes, confirming that these genes are important for both promoting and constraining regeneration and likely facilitate the shift from the normal developmental program to the regeneration program, and back again. The variation in regeneration phenotypes among different chromatin regulators and among components of the same multiunit complexes supports our previous finding that damage activates expression of genes that both promote and constrain regeneration (Khan et al. 2017). Such regulators of regeneration may be differentially affected by distinct mutations that affect the same chromatin-modifying complexes, resulting in different phenotypes.
We have demonstrated that both Drosophila SWI/SNF complexes play essential but distinct roles during epithelial regeneration, controlling multiple aspects of the process, including growth, developmental timing, and cell fate (Figure 8D). Furthermore, our work has identified multiple likely targets, including mmp1, Myc, ilp8, and en. Indeed, analysis of data from a recent study that identified regions of the genome that transition to open chromatin after imaginal disc damage showed such damage-responsive regions near Myc, mmp1, and ilp8 (Vizcaya-Molina et al. 2018). While previous work has suggested that chromatin modifiers can regulate regeneration (Wang et al. 2008; Stewart et al. 2009; Blanco et al. 2010; Scimone et al. 2010; Tseng et al. 2011; Fukuda et al. 2012; Jin et al. 2013, 2015; Xiong et al. 2013; Pfefferli et al. 2014; Skinner et al. 2015; Sun et al. 2016), and that the chromatin near Drosophila regeneration genes is modified after damage (Harris et al. 2016; Vizcaya-Molina et al. 2018), our results suggest that these damage-responsive loci are not all coordinately regulated in the same manner. The SWI/SNF complexes target different subsets of genes, and it will not be surprising if different cofactors or transcription factors recruit different complexes to other subsets of regeneration genes.
Is the requirement for the SWI/SNF complexes for growth and conservation of cell fate in the wing disc specific to regeneration? In contrast to tara, which is required for posterior wing fate only after damage and regeneration (Schuster and Smith-Bolton 2015), loss of mor in homozygous clones during wing disc development caused loss of en expression in the posterior compartment (Brizuela and Kennison 1997), although this result was interpreted to mean that mor promotes rather than constrains en expression, which is the opposite of our observations. Importantly, undamaged mor heterozygous mutant animals did not show patterning defects (Supplementary Figure S2, E and F), while damaged heterozygous mutant animals did (Figure 3E), indicating that regenerating tissue is more sensitive to reductions in SWI/SNF levels than normally developing tissue. Furthermore, osa is required for normal wing growth (Terriente-Félix and de Celis 2009), but reduction of osa levels did not compromise growth during regeneration (Supplementary Figure S3, D–H), and instead led to enhanced regeneration (Figure 2D). Thus, while some functions of SWI/SNF during regeneration may be the same as during development, other functions of SWI/SNF may be unique to regeneration.
SWI/SNF complexes help organisms respond rapidly to stressful conditions or changes in the environment. For example, SWI/SNF is recruited by the transcription factor DAF-16/FOXO to promote stress resistance in Caenorhabditis elegans (Riedel et al. 2013), and the Drosophila BAP complex is required for the activation of target genes of the NF-κB signaling transcription factor Relish in immune responses (Bonnay et al. 2014). Here we show that the Drosophila PBAP complex is similarly required after tissue damage for activation of target genes of the JNK signaling transcription factor AP-1 after tissue damage. Interestingly, the BAF60a subunit, a mammalian homolog of Drosophila BAP60, directly binds the AP-1 transcription factor and stimulates the DNA-binding activity of AP-1 (Ito et al. 2001), suggesting that this role may be conserved.
In summary, we have demonstrated that the two SWI/SNF complexes regulate different aspects of wing imaginal disc regeneration, implying that activation of the regeneration program is controlled by changes in chromatin, but that the mechanism of regulation is likely different for subsets of regeneration genes. Future identification of all genes targeted by BAP and PBAP after tissue damage, the factors that recruit these chromatin-remodeling complexes, and the changes they induce at these loci will deepen our understanding of how unexpected or stressful conditions lead to rapid activation of the appropriate genes.
Acknowledgments
The authors would like to thank A. Brock and K. Schuster for critical reading of the manuscript and helpful discussions; A. Dingwall, D. Bohmann, J. Kennison, J. Treisman, M Cleary, S. Cohen, the Bloomington Drosophila Stock Center (NIH P40OD018537), the Vienna Drosophila Resource Center, and the Developmental Studies Hybridoma Bank for fly stocks and reagents.
Funding
This work was supported by a Young Investigator Award from the Roy J. Carver Charitable Trust (#12-4041) (https://www.carvertrust.org) and a grant from the National Institutes of Health (NIGMS R01GM107140) (https://www.nigms.nih.gov).
Conflicts of interest
None declared.
Literature cited
- Becker PB, Workman JL.. 2013. Nucleosome remodeling and epigenetics. Cold Spring Harb Perspect Biol. 5:a017905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergantinos C, Corominas M, Serras F.. 2010. Cell death-induced regeneration in wing imaginal discs requires JNK signalling. Development. 137:1169–1179. [DOI] [PubMed] [Google Scholar]
- Blanco E, Ruiz-Romero M, Beltran S, Bosch M, Punset A, et al. 2010. Gene expression following induction of regeneration in Drosophila wing imaginal discs. Expression profile of regenerating wing discs. BMC Dev Biol. 10:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnay F, Nguyen X-H, Cohen-Berros E, Troxler L, Batsche E, et al. 2014. Akirin specifies NF-B selectivity of Drosophila innate immune response via chromatin remodeling. EMBO J. 33:2349–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Bagun J, Serras F.. 2008. Origin and proliferation of blastema cells during regeneration of Drosophila wing imaginal discs. Int J Dev Biol. 52:1043–1050. [DOI] [PubMed] [Google Scholar]
- Brizuela BJ, Kennison JA.. 1997. The Drosophila homeotic gene moira regulates expression of engrailed and HOM genes in imaginal tissues. Mech Dev. 65:209–220. [DOI] [PubMed] [Google Scholar]
- Brock AR, Seto M, Smith-Bolton RK.. 2017. Cap-n-collar promotes tissue regeneration by regulating ROS and JNK signaling in the Drosophila wing imaginal disc. Genetics. 206:1505–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila J, Estrada MP, Sánchez-Herrero E, Guerrero I.. 1994. The Drosophila segment polarity gene patched interacts with decapentaplegic in wing development. EMBO J. 13:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera I, Zavadil J, Treisman JE.. 2008. Two subunits specific to the PBAP chromatin remodeling complex have distinct and redundant functions during Drosophila development. Mol Cell Biol. 28:5238–5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalkley G EMoshkin Y MLangenberg KBezstarosti KBlastyak A, et al. . 2008. The Transcriptional Coactivator SAYP Is a Trithorax Group Signature Subunit of the PBAP Chromatin Remodeling Complex. MCB. 28:2920–2929. 10.1128/MCB.02217-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee N, Bohmann D.. 2012. A versatile ΦC31 based reporter system for measuring AP-1 and Nrf2 signaling in Drosophila and in tissue culture. PLoS One. 7:e34063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RT, Furukawa T, Tanese N, Treisman JE.. 1999. Osa associates with the Brahma chromatin remodeling complex and promotes the activation of some target genes. EMBO J. 18:7029–7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RT, Treisman JE.. 2000. Osa-containing Brahma chromatin remodeling complexes are required for the repression of wingless target genes. Genes Dev. 14:3140–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombani J, Andersen DS, Léopold P.. 2012. Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science. 336:582–585. [DOI] [PubMed] [Google Scholar]
- Cook RK, Christensen SJ, Deal JA, Coburn RA, Deal ME, et al. 2012. The generation of chromosomal deletions to provide extensive coverage and subdivision of the Drosophila melanogaster genome. Genome Biol. 13:R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté J, Quinn J, Workman JL, Peterson CL.. 1994. Stimulation of GAL4 derivative binding to nucleosomal DNA by the yeast SWI/SNF complex. Science. 265:53–60. [DOI] [PubMed] [Google Scholar]
- Eaton S, Kornberg TB.. 1990. Repression of ci-D in posterior compartments of Drosophila by engrailed. Genes Dev. 4:1068–1077. [DOI] [PubMed] [Google Scholar]
- Elfring LK, Daniel C, Papoulas O, Deuring R, Sarte M, et al. 1998. Genetic analysis of brahma: the Drosophila homolog of the yeast chromatin remodeling factor SWI2/SNF2. Genetics. 148:251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda A, Morris JP, Hebrok M.. 2012. Bmi1 is required for regeneration of the exocrine pancreas in mice. Gastroenterology. 143:821–831.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garelli A, Gontijo AM, Miguela V, Caparros E, Dominguez M.. 2012. Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science. 336:579–582. [DOI] [PubMed] [Google Scholar]
- Grusche FA, Degoutin JL, Richardson HE, Harvey KF.. 2011. The Salvador/Warts/Hippo pathway controls regenerative tissue growth in Drosophila melanogaster. Dev Biol. 350:255–266. [DOI] [PubMed] [Google Scholar]
- Gutierrez L. 2003. The Drosophila trithorax group gene tonalli(tna) interacts genetically with the Brahma remodeling complex and encodes an SP-RING finger protein. Development. 130:343–354. [DOI] [PubMed] [Google Scholar]
- Hargreaves DC, Crabtree GR.. 2011. ATP-dependent chromatin remodeling: genetics, genomics and mechanisms. Cell Res. 21:396–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan IK, Serras F.. 2017. Imaginal disc regeneration takes flight. Curr Opin Cell Biol. 48:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RE, Setiawan L, Saul J, Hariharan IK.. 2016. Localized epigenetic silencing of a damage-activated WNT enhancer limits regeneration in mature Drosophila imaginal discs. Elife. 5:e11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopmann R, Duncan D, Duncan I.. 1995. Transvection in the Iab-5,6,7 region of the bithorax complex of Drosophila: homology independent interactions in trans. Genetics. 139:815–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Yamauchi M, Nishina M, Yamamichi N, Mizutani T, et al. 2001. Identification of SWI·SNF complex subunit BAF60a as a determinant of the transactivation potential of Fos/Jun Dimers. J Biol Chem. 276:2852–2857. [DOI] [PubMed] [Google Scholar]
- Jin J, Hong I-H, Lewis K, Iakova P, Breaux M, et al. 2015. Cooperation of C/EBP family proteins and chromatin remodeling proteins is essential for termination of liver regeneration. Hepatology. 61:315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Xu J, Yin M-X, Lu Y, Hu L, et al. 2013. Brahma is essential for Drosophila intestinal stem cell proliferation and regulated by Hippo signaling. Elife. 2:e00999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis JA, Kennison JA, Tamkun JW.. 2017. Polycomb and trithorax group genes in Drosophila. Genetics. 206:1699–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuyama T, Comoglio F, Seimiya M, Cabuy E, Paro R.. 2015. During Drosophila disc regeneration, JAK/STAT coordinates cell proliferation with Dilp8-mediated developmental delay. Proc Natl Acad Sci USA. 112:E2327–E2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennison JA, Tamkun JW.. 1988. Dosage-dependent modifiers of polycomb and antennapedia mutations in Drosophila. Proc Natl Acad Sci USA. 85:8136–8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SJ, Abidi SNF, Skinner A, Tian Y, Smith-Bolton RK.. 2017. The Duox maturation factor is a key component of a positive feedback loop that sustains regeneration signaling. PLoS Genet. 13:e1006937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg T, Sidén I, O'Farrell P, Simon M.. 1985. The engrailed locus of drosophila: In situ localization of transcripts reveals compartment-specific expression. Cell. 40:45–53. [DOI] [PubMed] [Google Scholar]
- Kwon H, Imbalzano AN, Khavari PA, Kingston RE, Green MR.. 1994. Nucleosome disruption and enhancement of activator binding by a human SW1/SNF complex. Nature. 370:477–481. [DOI] [PubMed] [Google Scholar]
- Manansala MC, Min S, Cleary MD.. 2013. The Drosophila SERTAD protein Taranis determines lineage-specific neural progenitor proliferation patterns. Dev Biol. 376:150–162. [DOI] [PubMed] [Google Scholar]
- Mashtalir N, D’Avino AR, Michel BC, Luo J, Pan J, et al. 2018. Modular organization and assembly of SWI/SNF family chromatin remodeling complexes. Cell. 175:1272–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrmann L, Langenberg K, Krijgsveld J, Kal AJ, Heck AJR, et al. 2004. Differential targeting of two distinct SWI/SNF-related Drosophila chromatin-remodeling complexes. Mol Cell Biol. 24:3077–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshkin YM, Mohrmann L, van Ijcken WFJ, Verrijzer CP.. 2007. Functional differentiation of SWI/SNF remodelers in transcription and cell cycle control. Mol Cell Biol. 27:651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzny CK, Holmgren R.. 1995. The Drosophila cubitus interruptus protein and its role in the wingless and hedgehog signal transduction pathways. Mech Dev. 52:137–150. [DOI] [PubMed] [Google Scholar]
- Nagl NG, Zweitzig DR, Thimmapaya B, Beck GR, Moran E.. 2006. The c-myc gene is a direct target of mammalian SWI/SNF–related complexes during differentiation-associated cell cycle arrest. Cancer Res. 66:1289–1293. [DOI] [PubMed] [Google Scholar]
- Ng M, Diaz-Benjumea FJ, Vincent J-P, Wu J, Cohen SM.. 1996. Specification of the wing by localized expression of wingless protein. Nature. 381:316–318. [DOI] [PubMed] [Google Scholar]
- Page-McCaw A, Serano J, Santé JM, Rubin GM.. 2003. Drosophila matrix metalloproteinases are required for tissue remodeling, but not embryonic development. Dev Cell. 4:95–106. [DOI] [PubMed] [Google Scholar]
- Patel NH, Martin-Blanco E, Coleman KG, Poole SJ, Ellis MC, et al. 1989. Expression of engrailed proteins in arthropods, annelids, and chordates. Cell. 58:955–968. [DOI] [PubMed] [Google Scholar]
- Perkins KK, Dailey GM, Tjian R.. 1988. Novel Jun-and Fos-related proteins in Drosophila are functionally homologous to enhancer factor AP-1. EMBO J. 7:4265–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferli C, Müller F, Jaźwińska A, Wicky C.. 2014. Specific NuRD components are required for fin regeneration in zebrafish. BMC Biol. 12:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, Roberts IJ, Ingham PW, Whittle JR.. 1990. The Drosophila segment polarity gene patched is involved in a position-signalling mechanism in imaginal discs. Development. 110:105–114. [DOI] [PubMed] [Google Scholar]
- Riedel CG, Dowen RH, Lourenco GF, Kirienko NV, Heimbucher T, et al. 2013. DAF-16 employs the chromatin remodeller SWI/SNF to promote stress resistance and longevity. Nat Cell Biol. 15:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santabárbara-Ruiz P, López-Santillán M, Martínez-Rodríguez I, Binagui-Casas A, Pérez L, et al. 2015. ROS-induced JNK and p38 signaling is required for unpaired cytokine activation during Drosophila regeneration. PLoS Genet. 11:e1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubiger M, Sustar A, Schubiger G.. 2010. Regeneration and transdetermination: the role of wingless and its regulation. Dev Biol. 347:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner O, Berdnik D, Levy JM, Wu JS, Luginbuhl D, et al. 2008. piggyBac-based mosaic screen identifies a postmitotic function for cohesin in regulating developmental axon pruning. Dev Cell. 14:227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster KJ, Smith-Bolton RK.. 2015. Taranis protects regenerating tissue from fate changes induced by the wound response in Drosophila. Dev Cell. 34:119–128. [DOI] [PubMed] [Google Scholar]
- Scimone ML, Meisel J, Reddien PW.. 2010. The Mi-2-like Smed-CHD4 gene is required for stem cell differentiation in the planarian Schmidtea mediterranea. Development. 137:1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeath JB, Carroll SB.. 1991. Regulation of achaete-scute gene expression and sensory organ pattern formation in the Drosophila wing. Genes Dev. 5:984–995. [DOI] [PubMed] [Google Scholar]
- Skeath JB, Carroll SB.. 1992. Regulation of proneural gene expression and cell fate during neuroblast segregation in the Drosophila embryo. Dev Camb Engl. 114:939–946. [DOI] [PubMed] [Google Scholar]
- Skinner A, Khan SJ, Smith-Bolton RK.. 2015. Trithorax regulates systemic signaling during Drosophila imaginal disc regeneration. Development. 142:3500–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Bolton RK, Worley MI, Kanda H, Hariharan IK.. 2009. Regenerative growth in Drosophila imaginal discs is regulated by Wingless and Myc. Dev Cell. 16:797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling AC, Stern DM, Kiss I, Roote J, Laverty T, et al. 1995. Gene disruptions using P transposable elements: an integral component of the Drosophila genome project. Proc Natl Acad Sci USA. 92:10824–10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S, Tsun Z-Y, Belmonte JCI.. 2009. A histone demethylase is necessary for regeneration in zebrafish. Proc Natl Acad Sci USA. 106:19889–19894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Irvine KD.. 2011. Regulation of Hippo signaling by Jun kinase signaling during compensatory cell proliferation and regeneration, and in neoplastic tumors. Dev Biol. 350:139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Chuang J-C, Kanchwala M, Wu L, Celen C, et al. 2016. Suppression of the SWI/SNF component Arid1a promotes mammalian regeneration. Cell Stem Cell. 18:456–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamkun JW, Deuring R, Scott MP, Kissinger M, Pattatucci AM, et al. 1992. brahma: a regulator of Drosophila homeotic genes structurally related to the yeast transcriptional activator SNF2SWI2. Cell. 68:561–572. [DOI] [PubMed] [Google Scholar]
- Tanimoto H, Itoh S, ten Dijke P, Tabata T.. 2000. Hedgehog creates a gradient of DPP activity in Drosophila wing imaginal discs. Mol Cell. 5:59–71. [DOI] [PubMed] [Google Scholar]
- Terriente-Félix A, de Celis JF.. 2009. Osa, a subunit of the BAP chromatin-remodelling complex, participates in the regulation of gene expression in response to EGFR signalling in the Drosophila wing. Dev Biol. 329:350–361. [DOI] [PubMed] [Google Scholar]
- Treisman JE, Luk A, Rubin GM, Heberlein U.. 1997. eyelid antagonizes wingless signaling during Drosophila development and has homology to the Bright family of DNA-binding proteins. Genes Dev. 11:1949–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng A-S, Carneiro K, Lemire JM, Levin M.. 2011. HDAC activity is required during Xenopus tail regeneration. PLoS One. 6:e26382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez M, Moore L, Kennison JA.. 1999. The trithorax group gene osa encodes an ARID-domain protein that genetically interacts with the brahma chromatin-remodeling factor to regulate transcription. Development. 126:733–742. [DOI] [PubMed] [Google Scholar]
- Vizcaya-Molina E, Klein CC, Serras F, Mishra RK, Guigó R, et al. 2018. Damage-responsive elements in Drosophila regeneration. Genome Res. 28:1852–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G-L, Salisbury E, Shi X, Timchenko L, Medrano EE, et al. 2008. HDAC1 cooperates with C/EBPalpha in the inhibition of liver proliferation in old mice. J Biol Chem. 283:26169–26178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BG, Roberts CWM.. 2011. SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer. 11:481–492. [DOI] [PubMed] [Google Scholar]
- Xiao H, Sandaltzopoulos R, Wang HM, Hamiche A, Ranallo R, et al. 2001. Dual functions of largest NURF subunit NURF301 in nucleosome sliding and transcription factor interactions. Mol Cell. 8:531–543. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Li W, Shang C, Chen RM, Han P, et al. 2013. Brg1 governs a positive feedback circuit in the hair follicle for tissue regeneration and repair. Dev Cell. 25:169–181. [DOI] [PubMed] [Google Scholar]
- Zraly CB, Marenda DR, Nanchal R, Cavalli G, Muchardt C, et al. 2003. SNR1 is an essential subunit in a subset of Drosophila brm complexes, targeting specific functions during development. Dev Biol. 253:291–308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are available at https://doi.org/10.13012/B2IDB-1681718_V1 and upon request. Supplemental Material available at figshare: https://doi.org/10.25386/genetics.13260266.