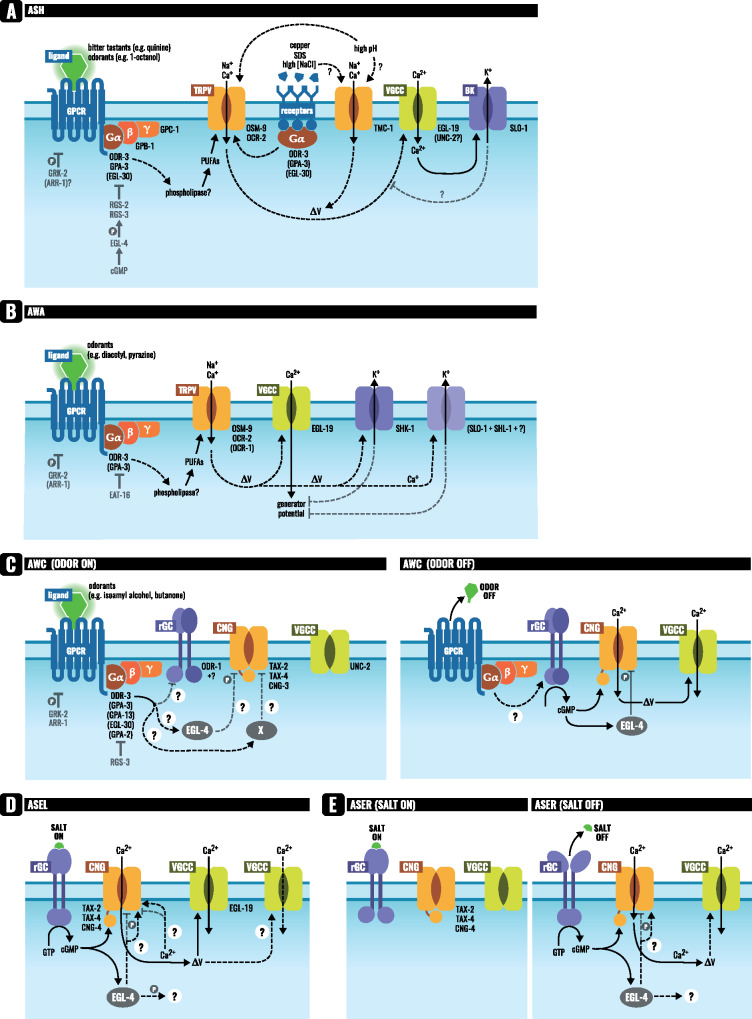
Figure 4.
Signal transduction pathways in the ASH, AWA, AWC, and ASE sensory neurons. Simplified models of the potential signal transduction pathways for these representative neurons are shown. See text within the Signal Transduction section for additional details. (A) ASH: Odorant or tastant binding to a GPCR initiates G protein-coupled signaling that likely leads to the generation of PUFAs that activate TRPV channels. Stimuli may also activate other classes of receptors or channels directly. The resulting membrane depolarization activates voltage-gated calcium channels (VGCCs). In a regulatory feedback loop, ASH excitability may be dampened by a calcium-activated potassium channel. Signaling can also be downregulated at the level of GPCRs (via phosphorylation by GRK-2) or at the level of G proteins (by RGS proteins). (B) AWA: AWA signaling is initiated by odorant binding to a GPCR that initiates G protein-coupled signaling that likely leads to the generation of PUFAs that activate TRPV channels. The resulting membrane depolarization can trigger an all or none feed-forward action potential that is generated by opening of the VGCC EGL-19. The amplified voltage change opens voltage-gated potassium channels that subsequently dampen signaling. Signaling is also downregulated by GRK-2 and arrestin, and by an RGS protein. (C) AWC: In the presence of odorant, AWC is silenced. Odorant binding to a GPCR might activate a Gα that inhibits cGMP formation by guanylyl cyclases. The CNG channels may also be inhibited by EGL-4 and possibly by an unidentified protein “X.” Once odor is removed, opening of the CNG channels leads to membrane depolarization that activates VGCCs. Negative regulation of the AWC response occurs via GRK-2 and arrestin, and by an RGS protein. The cGMP-dependent protein kinase EGL-4 likely phosphorylates CNG channels during the adaptation response. (D) ASEL: Signaling is initiated when salt binds to the extracellular domain of the rGC and the intracellular cyclase domains dimerize to cyclize GTP into cGMP. The cGMP produced binds to and opens CNG channels. Membrane depolarization activates VGCCs. EGL-4 is required for calcium signals in response to salt, but its targets (besides TAX-2), and role are unknown. (E) ASER: Salt binding to the extracellular domain of the rGC inhibits cyclase activity and signaling is silenced. Signaling is initiated when salt is removed and the rGC cyclase domains dimerize to cyclize GTP into cGMP, which opens the CNG channel. Membrane depolarization activates a VGCC. Via an unknown mechanism, EGL-4 is required for the calcium flux in ASER.