Abstract
Background
Certain types of human papillomavirus (HPV) in cervical samples are strongly associated with squamous intraepithelial lesions (SIL) and invasive cervical carcinoma. We determined and compared the test characteristics of testing for HPV with samples obtained by patients and with samples obtained by their physicians.
Methods
In a consecutive series of women referred to a colposcopy clinic at a teaching hospital because of abnormalities on cervical cytologic screening, 200 agreed to collect vulvar, vaginal and urine samples for HPV testing. The physician then collected cervical samples for HPV testing, and colposcopy, with biopsy as indicated, was performed. Presence of HPV was evaluated using the hybrid capture II assay (Digene Corp., Silver Spring, Md.) with a probe cocktail for 13 carcinogenic types. Cervical specimens were also tested for HPV by polymerase chain reaction and hybridization with type-specific probes. Cervical smears for cytologic examination were obtained from all women.
Results
High-grade lesions (high-grade squamous intraepithelial lesions [HSIL], equivalent to cervical intraepithelial neoplasia [CIN] grade 2 or 3, and adenocarcinoma) were found in 58 (29.0%) of the 200 women. Carcinogenic types of HPV were detected in the self-collected vaginal samples of 50 (86.2%) of these 58 women, in the self-collected vulvar samples of 36 (62.1%) and in the self-collected urine samples of 26 (44.8%). Carcinogenic types of HPV were detected in the cervical samples collected by physicians for 57 (98.3%) of these 58 women. The remaining 142 women (71.0%) had normal findings or low-grade squamous intraepithelial lesions (LSIL, CIN grade 1). Test results were negative or noncarcinogenic types of HPV were detected in the self-collected vaginal samples of 76 (53.5%) of these 142 women, in the self-collected vulvar samples of 89 (62.7%) and in the self-collected urine samples of 99 (69.7%). The sensitivity for self-collected samples ranged from 44.8% to 86.2%, and the specificity from 53.5% to 69.7%. For the samples collected by physicians, the sensitivity was 98.3% and the specificity 52.1%. The self-sampling methods were generally acceptable to the women: 98.4% of respondents (126/128) deemed urine sampling acceptable, 92.9% (118/127) found vulvar sampling acceptable, and 88.2% (112/127) found vaginal sampling acceptable.
Interpretation
Self-collection of samples for HPV testing was acceptable to women attending a colposcopy clinic for investigation of suspected cervical lesions and shows sufficient sensitivity to warrant further evaluation as a screening test for cervical cancer prevention program
Detection of squamous intraepithelial lesions by cervical cytologic screening can result in prevention of most cases of cervical cancer.1 It is agreed that high-grade squamous intraepithelial lesions (HSIL; equivalent to cervical intraepithelial neoplasia [CIN] grade 2 or 3) should be treated, because these lesions have the greatest risk of progressing to invasive cervical carcinoma.2 Since the introduction in developed countries of programs for preventing cervical cancer, the incidence of this form of cancer has declined appreciably; it now accounts for only 4.4% of new cancers in females, and the lifetime risk is only 1.1% in these countries.3 In contrast, cancer of the uterine cervix is still common in the developing world and is responsible for approximately 15% of cancers in females and a lifetime risk of about 3%.3 Strategies for preventing cervical cancer in these countries must overcome barriers such as inadequate medical infrastructure and poor rates of participation in screening programs.4
Certain human papillomavirus (HPV) genotypes are carcinogenic. One or more of these carcinogenic types are present in over 95% of cervical cancers and in the vast majority of cases of HSIL (CIN 2 or 3).5,6,7 Therefore, the clinical application of molecular tests for HPV has been of interest.8 A noninvasive method of sampling for carcinogenic types of HPV, if it could be incorporated into cervical screening strategies, has the potential advantage of increasing population coverage. Several studies have explored the use of self-collected material for HPV nucleic acid testing with various methods.9,10,11,12,13,14,15,16 The sensitivity for high-grade lesions in these studies, which have used urine, vaginal swab or tampon specimens, has ranged from 66% to 94%. Although these findings appear promising, the studies have had limitations because of test insensitivity, small sample sizes and methodological flaws, such as verification bias.
The primary objective of the study reported here was to determine the sensitivity and specificity of self-sampling for HPV in detecting HSIL (CIN 2 or 3). The secondary objectives were to compare results obtained from samples collected by women themselves with those obtained from samples collected by physicians and to evaluate the feasibility of asking women to collect their own samples.
Methods
From October 1996 to March 1997, consecutive women who were at least 18 years of age and who had been newly referred (because of abnormalities on cervical cytologic screening) to a colposcopy clinic at a teaching hospital in Hamilton, Ont., were invited to participate in the study (Fig. 1). A nurse explained the study, and written informed consent, as approved by the McMaster University Research Ethics Board, was obtained from each woman who agreed to participate.
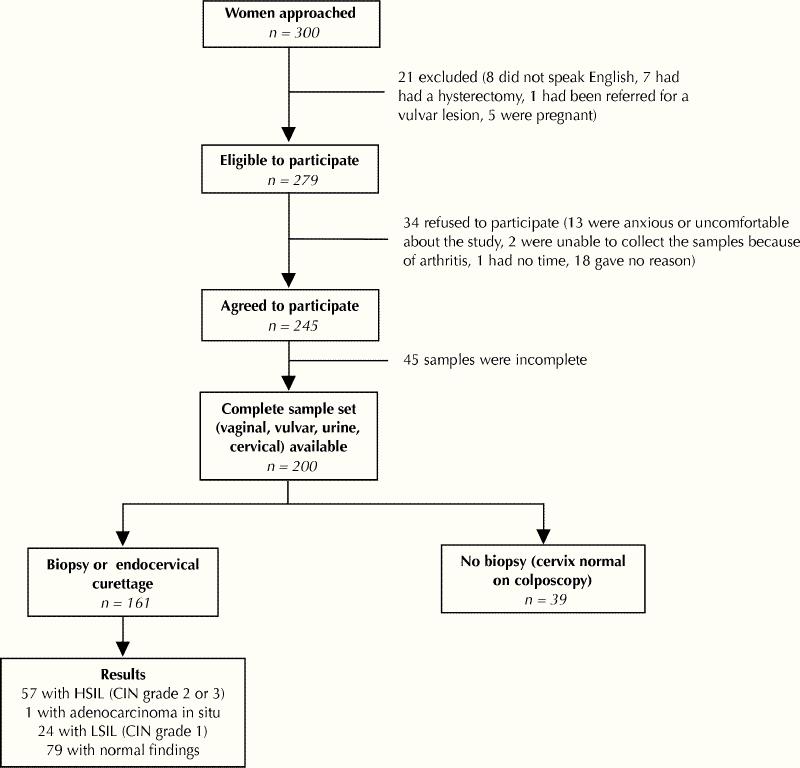
Fig. 1: Flow of 200 women through recruitment and screening. The following values were used as the “gold standard” in calculating sensitivity and specificity: 58 women with high-grade lesions (high-grade squamous intraepithelial lesion [HSIL; cervical intraepithelial neoplasia or CIN grade 2 or 3] or adenocarcinoma), 142 women without high-grade lesions (low-grade squamous intraepithelial lesion [LSIL; cervical intraepithelial neoplasia or CIN grade 1], normal findings, no biopsy performed).
After enrolment, the women were given written instructions and a diagram showing how to obtain the vulvar, vaginal and urine samples, in that order; self-sampling was performed in a clinic lavatory. After the self-sampling, each woman underwent a pelvic examination, which included sampling by cervical brush for HPV testing, sampling for Papanicolaou (Pap) smear and colposcopic examination. The results of the colposcopic examination and biopsy (as required) were used as the reference standard for determining the sensitivity and specificity of the various test methods.
To obtain the vulvar sample, each woman was asked to grasp a Dacron polyester swab (Medical Packaging Corp., Camarillo, Calif.) about halfway up the plastic shaft, to separate the labia and rub the swab tip across the introitus several times, and then to place the swab in a tube containing sample transport medium (Digene Corp., Silver Spring, Md.). To obtain the vaginal specimen, each woman was asked to grasp the shaft of a second Dacron polyester swab at its midpoint, to insert the tip of the swab into her vagina until her fingers touched the introitus, to rotate the swab by twirling it as she slowly removed it from the vagina and then to place the swab in a separate tube of sample transport medium. Finally, she was asked to collect a first-void urine specimen (the first 30 mL of urine voided) in a plastic bottle.
After self-sampling, a vaginal speculum was inserted, and the colposcopist used a modified Ayre spatula and cervical canal brush, as required, to obtain a cervical smear, which was immediately fixed. A modified soft, cone-shaped cervical brush (Cervical Sampler, Digene Corp.) was used to obtain samples from the transformation zone for the hybrid capture II HPV assay, and a Dacron polyester swab (Harwood Products Co., Guilford, Minn.) was used to obtain samples from this zone for polymerase chain reaction (PCR) testing. The brush was placed in sample transport medium and the swab in sterile phosphate-buffered saline. The colposcopist (J.W.S. and P.R., among others) used 5% acetic acid to wash the cervix before colposcopy. Directed biopsy and endocervical curettage were performed as required, and the colposcopic findings were recorded on a data form.
After the self-sampling and colposcopic examination, each participant completed a self-administered questionnaire, which included questions about sociodemographic characteristics and sexual behaviour. The last 128 women who were enrolled were asked to grade the acceptability of the sampling methods on a 5-point Likert scale, with 1 indicating that a method was totally acceptable, 3 indicating neutrality and 5 indicating that it was not at all acceptable. They were also asked to rank the 4 methods (vaginal, vulvar, urine and cervical) according to preference (from most preferred to least preferred).
Cervical cytologic results from all the women and histologic specimens from the 161 women who underwent cervical biopsy or endometrial curettage were processed in the standard fashion and reported by the hospital pathology department. A review of the cervical histologic specimens by a blinded external gynecological pathologist (W.C.) revealed excellent agreement (raw agreement 87.5%, kappa = 0.74) for the presence of high-grade lesions (HSIL [CIN 2 or 3] or adenocarcinoma in situ). Disagreements were resolved by consensus between the 2 expert gynecologic pathologists (W.C. and D.D.). For each woman, the highest-grade lesion diagnosed was used as the reference for assessing test performance.
HPV specimens were stored at 4∘C and shipped at room temperature within 2 weeks of collection. The cervical swab and 10 mL of the urine sample were sent to the McMaster University Regional Virology and Chlamydiology Laboratory for PCR testing. The cervical brush, vaginal swab, vulvar swab and the remaining 20 mL of urine (mixed with 20 mL of urine preparation buffer solution; Digene Corp.) were sent to the Digene Corp. laboratory (Silver Spring, Md.) for the hybrid capture II assay. The laboratories were blinded to the other data for each subject.
The hybrid capture II assay is a second-generation DNA probe test based on signal amplification, which uses a chemiluminescent readout to indicate the presence of one or more carcinogenic HPV types as a group (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68). The assay procedure has been described previously.17,18 The test was considered positive if the light emitted by a specimen was greater than the light emitted by the positive control.
Cervical swab samples (0.2 mL) and urine samples (0.5 mL) were processed by digesting the specimens with proteinase K and extracting DNA by means of the XTRAX kit (Gull Laboratories, Salt Lake City, Utah). Subsequently, all processed specimens were amplified using the consensus HPV L1 primers (highly conserved region of the viral genome), as well as human β-globin primers to assess specimen integrity. Specimens that were negative for β-globin were further processed by DNA extraction with phenol-chloroform and retested by PCR, as previously described.14,18 PCR detection and typing for carcinogenic HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, and 68) and for HPV types with low carcinogenic risk (6, 11, 42 and 53) were done according to protocols described by Bauer and colleagues.19
The sensitivity, specificity, positive and negative predictive values, and positive likelihood ratios of the hybrid capture II results for the 4 specimen types were calculated, with the results of colposcopic examination (with directed biopsy as required) as the reference standard. Women with HSIL (CIN 2 or 3) or adenocarcinoma in situ on histologic examination were regarded as having a ”positive” result, and all others (i.e., those with normal cervix on colposcopy or low-grade squamous intraepithelial lesion [LSIL; CIN 1] on biopsy) were regarded as having a ”negative” result. Exact methods were used to calculate confidence intervals (95% CI) for proportions and relative risks. Logistic regression was used to test for associations between risk factors and the presence of squamous intraepithelial lesions and HPV. The probability of a type I error (α) was set at 0.05 (2-tailed).
Results
Of the 300 women approached in the colposcopy clinic, 279 (93.0%) were eligible, and 245 (87.8%) of these agreed to participate. Complete specimen sets (cervical specimen obtained by the colposcopist, and vaginal, vulvar and urine specimens obtained by the woman) and completed questionnaires were available for 200 women, and the analysis was based on data for these subjects. Biopsies were performed for 161 (80.5%) of these women. HSIL (CIN 2 or 3) was identified in 57 (28.5%) of them, LSIL (CIN 1) in 24 (12.0%) and adenocarcinoma in 1 (0.5%); the adenocarcinoma was grouped with HSIL (CIN 2 or 3) for the analyses. The findings were normal in the remaining 79 women who underwent biopsy. Fig. 1 shows the flow of participants through the study and the reasons for ineligibility and refusal.
On cervical cytologic examination, HSIL (CIN 2 or 3) was found in 71 (35.5%) of the 200 women, LSIL (CIN 1) in 54 (27.0%), atypical squamous cells of undetermined significance in 74 (37.0%) and adenocarcinoma in 1 (0.5%).
The mean age of the 200 women was 31.5 (standard deviation [SD] 9.4) years, the mean age at first intercourse 17.2 (SD 2.6) years and the median number of lifetime partners 5.0 (interquartile range 5.0). Most respondents (151/191 [79.0%]) had completed high school, and slightly more than half (106/195 [54.4%]) were living with a partner. Approximately one-third of respondents (70/191 [36.6%]) reported current use of oral contraceptives, and 134 of 192 respondents (70.0%) had previously been pregnant. Approximately half (88/191 [46.1%]) were current smokers and about one-fifth (40/191 [20.9%]) were past smokers. A history of a sexually transmitted infection was reported by 62 (36.9%) of 168 respondents.
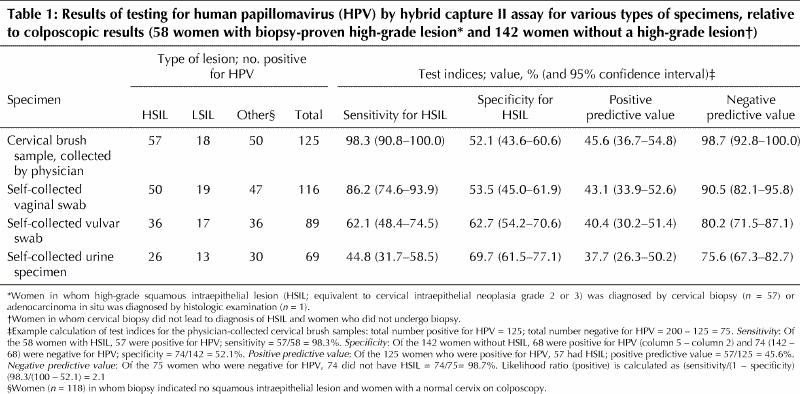
The prevalence of HPV in the cervical samples obtained by the physician was 62.5% (125/200). The accuracy of the various methods of specimen collection for detecting HSIL (CIN 2 or 3) is shown in Table 1. The sensitivity of cervical and vaginal specimens was 98.3% (57/58) and 86.2% (50/58) respectively, and the specificity was 52.1% (74/142) and 53.5% (76/142) respectively. The sensitivity of testing for HSIL (CIN 2 or 3) was progressively lower and the specificity progressively higher with increasing distance from the cervix (vagina, vulva and urine in that order). The likelihood ratios for a positive result with the hybrid capture II test for the cervical, vaginal, vulvar and urine samples were 2.1, 1.9, 1.7 and 1.5 respectively. Agreement (kappa statistic) between the cervical specimens and the vaginal, vulvar and urine specimens for the presence of HPV was 0.76, 0.55 and 0.41 respectively.
Table 1
Examination of associations between common risk factors and the presence of squamous intraepithelial lesions and HPV, after adjustment for age (less than 30 years and 30 years or older), revealed a significant association between current cigarette smoking and a histologic diagnosis of any grade of CIN (p = 0.04); there was also a significant association between positive HPV test result and current smoking (p < 0.01) and age at first intercourse (p = 0.03).
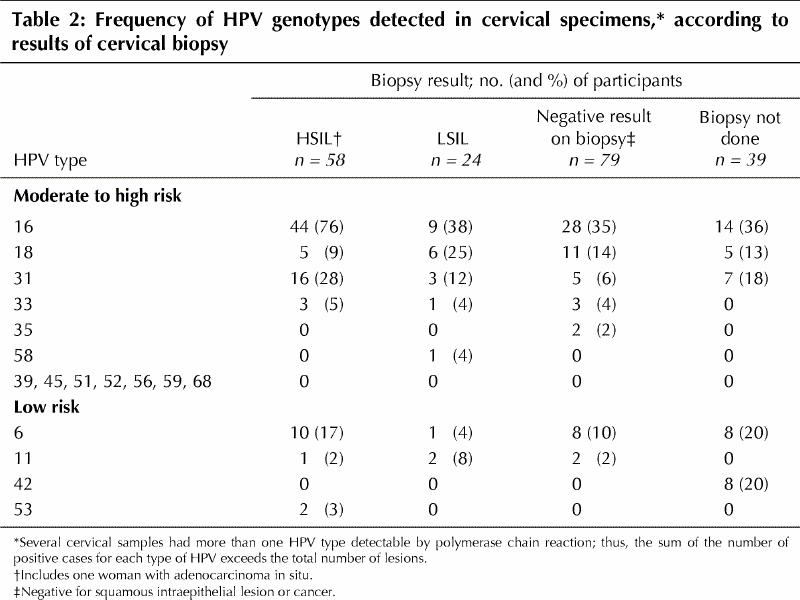
The distribution of each HPV type at the cervix, categorized by histologic results, is shown in Table 2. Women with HPV types 16 (relative risk [RR] 3.47, 95% CI 2.02-6.33) and 31 (RR 2.08, 95% CI 1.23-3.12) were significantly more likely to have HSIL (CIN 2 or 3) than women who did not have either of these 2 HPV types. At least 1 of 6 carcinogenic types of HPV (16, 18, 31, 33, 35 or 58) was detected in the cervical or urine specimen of 138 (69.0%) of the 200 women; 103 (51.5% of the entire sample) had positive results for both specimens, 31 (15.5%) had a positive result only for the cervical specimen and 4 (2.0%) had a positive result only for the urine specimen. Multiple HPV types were present in 16 (27.6%) of the 58 women with HSIL (CIN 2 or 3), 4 (16.7%) of the 24 women with LSIL (CIN 1) and 11 (28.2) of the 39 women whose result was negative.
The sensitivity, specificity, and positive and negative predictive values of cervical cytologic examination for detecting HSIL (CIN 2 or 3) were 77.6% (45/58), 81.0% (115/142), 62.5% (45/72) and 89.8% (115/128) respectively.
The prevalence of HPV was 90.3% (65/72) in women with HSIL (CIN 2 or 3) on cervical cytology, 77.8% (42/54) in women with LSIL (CIN 1) and 37.8% (28/74) in women with atypical squamous cells of undetermined significance.
The self-sampling methods were generally more acceptable: 126 (98.4%) of 128 women found the urine sampling acceptable, 118 (92.9%) of 127 found the vulvar sampling acceptable, and 112 (88.2%) of 127 found the vaginal sampling acceptable, whereas only 98 (79.0%) of 124 women found cervical sampling by the physician acceptable. The preference rankings indicated that the urine sampling method was the most preferred (ranked first by 105 [89.7%] of 117 women), followed by the vulvar (ranked second by 89 [76.7%] of 116 women), vaginal (ranked third by 89 [77.4%] of 115 women) and cervical (ranked fourth by 88 [77.2%] of 114 women) sampling methods.
Interpretation
In this survey of 200 women referred to a colposcopy clinic, a positive HPV test result on vaginal swabs obtained by the women themselves had 86.2% sensitivity for HSIL (CIN 2 or 3), whereas the sensitivity of cervical brush samples obtained by physicians was 98.3%. Although the sensitivity declined for specimens obtained progressively further from the cervix, women‚s perceptions of acceptability of the method increased. A recent study using a previously unscreened population of South African women 35 years of age and older demonstrated the feasibility of clinic-based self-sampling for vaginal HPV tests to predict high-grade cervical lesions.16 The sensitivity and specificity for high-grade lesions were 66.1% and 82.9% respectively. These test indices may be misleading because of verification bias, since women who had negative HPV test results were not followed up for colposcopic assessment with the same intensity as those who had positive HPV test results.
High sensitivity for HSIL (CIN 2 or 3) is only one of the requirements of a putative screening tool such as HPV testing of self-obtained vaginal swabs. To use colposcopy services efficiently, the positive predictive value must also be adequate. Positive predictive value depends greatly on the specificity of the screening test and the prevalences of HPV and HSIL (CIN 2 or 3). The prevalence of HPV at the cervix (62.5% by the hybrid capture II assay) was much higher in our sample than in the general population, where HPV is detected in 20% to 25% of younger women (35 years of age or younger) and 5% to 10% of older women.18,20,21,22 HSIL (CIN 2 or 3) is estimated to be present in about 1% of women in the general population,23 a much lower prevalence than that of our sample (29.0%). Given the high prevalence of HPV in our cohort, the specificity of HPV testing was poor and the test generated at least one false-positive result for each true positive in all types of specimens. Research is required on the predictive values of HPV testing for self-obtained specimens in a general population if conclusions are to be drawn about the value of HPV testing of self-obtained or physician-obtained specimens for screening. The accuracy of repeat cervical cytologic examination at colposcopy was higher than would be expected in a screening situation24 and was probably a reflection of selection bias, since all of the women in the study had been referred because of abnormal cytologic results.
In our sample, HPV types 16 and 31 were the most prevalent and conferred the highest risk for HSIL (CIN 2 or 3). This result is consistent with the results of other studies showing that these types are associated with high risk for HSIL (CIN 2 or 3).25 Koutsky and colleagues26 found that the relative risk for HPV type 16 or 18 (compared with women who had no HPV) was 11.
A global effort to improve prevention of cervical cancer, especially in developing countries, is worthwhile because a large proportion of deaths and disability occur during the most productive years of life and these adverse consequences are associated with substantial personal, social and economic losses.4 Given that the accuracy of the Pap smear is far from perfect24 and that a cytology screening program is not feasible in many developing countries, there is a need to rigorously evaluate promising alternatives to cytologic screening. For example, a comparison of HPV testing of self-collected specimens with visual inspection of the cervix after application of acetic acid4 could be expected to reveal potential advantages and disadvantages to both approaches, such as test cost, predictive values, accessibility, acceptability, need for equipment and need for highly trained personnel. In our study, self-sampling for HPV testing detected most cases of HSIL (CIN 2 or 3), but it was impossible to make any inferences about predictive values in a screening population. Further research is needed on whether HPV testing of self-obtained specimens could form the basis of cervical cancer prevention programs in developing countries and in other locations where access to cervical cytology services is limited.
Table 2
Footnotes
Presented in part at the 16th International Human Papillomavirus Conference, Sept. 5-11, 1997, Siena, Italy
This article has been peer reviewed.
This research was funded by the Father Sean O‚Sullivan Research Foundation, St. Joseph‚s Hospital, Hamilton, Ont. Hybrid capture II assays for human papillomavirus were provided free of charge by Digene Corporation, Silver Spring, Md.
Reprint requests to: Dr. John W. Sellors, Department of Family Medicine, HSC-2V10, McMaster University, 1200 Main St. W, Hamilton ON L8N 3Z5; fax 905 521-5594; sellors@fhs.mcmaster.ca
References
- 1.Clarke EA, Anderson TW. Does screening by ”Pap” smears help prevent cervical cancer? A case-control study. Lancet 1979;2:1-4. [DOI] [PubMed]
- 2.Bishop A, Sherris J, Tsu VD. Cervical dysplasia treatment in developing countries: a situation analysis. Washington: Program for Appropriate Technology in Health, The World Bank; 1995.
- 3.Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin 1999;49:33-64. [DOI] [PubMed]
- 4.Sankaranarayanan R, Pisani P. Prevention measures in the third world: Are they practical? In: Franco E, Monsonego J, editors. New developments in cervical cancer screening and prevention. Oxford: Blackwell Science; 1997. pp. 70-83.
- 5.Franco EL. Cancer causes revisited: human papillomavirus and cervical neoplasia. J Natl Cancer Inst 1995;87:779-80. [DOI] [PubMed]
- 6.Schiffman MH. New epidemiology of human papillomavirus infection and cervical neoplasia. J Natl Cancer Inst 1995;87:1345-7. [DOI] [PubMed]
- 7.Pisani P, Parkin M, Muñoz N, Ferlay J. Cancer and infection: estimates of the attributable fraction in 1990. Cancer Epidemiol Biomarkers Prev 1997;6: 387-400. [PubMed]
- 8.Meijer CJL, Rozendaal L, van der Linden JC, Helmerhorst TJM, Voorhorst FJ, Walboomers JMM. Human papillomavirus testing for primary cervical cancer screening. In: Franco E, Monsonego J, editors. New developments in cervical cancer screening and prevention. Oxford: Blackwell Science; 1997 pp. 338-47.
- 9.Fairley CK, Chen S, Tabrizi SN, Quinn MA, NcNeil JJ, Garland SM. Tampons: a novel patient-administered method for the assessment of genital human papillomavirus infection. J Infect Dis 1992;165:1103-6. [DOI] [PubMed]
- 10.Morrison EAB, Goldberg GL, Hagan RJ, Kadish AS, Burk RD. Self-administered home cervicovaginal lavage: a novel tool for the clinical-epidemiologic investigation of genital human papillomavirus infections. Am J Obstet Gynecol 1992;167:104-7. [DOI] [PubMed]
- 11.Forslund O, Hansson BG, Rymark P, Bjerre B. Human papillomavirus DNA in urine samples compared with that in simultaneously collected urethra and cervix samples. J Clin Microbiol 1993;31:1975-9. [DOI] [PMC free article] [PubMed]
- 12.Moscicki AB. Comparison between methods for human papillomavirus DNA testing: a model for self-testing in young women. J Infect Dis 1993;167: 723-5. [DOI] [PubMed]
- 13.Vossler JL, Forbes BA, Adelson MD. Evaluation of the polymerase chain reaction for the detection of human papillomavirus from urine. J Med Virol 1995;45:354-60. [DOI] [PubMed]
- 14.Coutlee F, Hankins C, Lapointe N, and the Canadian Women‚s HIV Study Group. Comparison between vaginal tampon and cervicovaginal lavage specimen collection for detection of human papillomavirus DNA with the polymerase chain reaction. J Med Virol 1997;51:42-7. [DOI] [PubMed]
- 15.Hillemanns P, Kimmig R, Hüttemann U, Dannecker C, Thaler CJ. Screening for cervical neoplasia by self-assessment for human papillomavirus DNA. Lancet 1999;354:1970. [DOI] [PubMed]
- 16.Wright TC, Denny L, Kuhn L, Pollack A, Lorincz A. HPV DNA testing of self-collected vaginal samples compared with cytologic screening to detect cervical cancer. JAMA 2000;283:81-6. [DOI] [PubMed]
- 17.Lorincz A. Molecular methods for the detection of human papillomavirus infection. Obstet Gynecol Clin North Am 1996;23:707-30. [PubMed]
- 18.Sellors JW, Mahony JB, Kaczorowski J, Lytwyn A, Bangura H, Chong S, et al. Prevalence and predictors of human papillomavirus infection in women in Ontario, Canada. CMAJ 2000;163(5):503-8. Available: www.cma.ca/cmaj/vol-163/issue-5/503.htm [PMC free article] [PubMed]
- 19.Bauer HM, Greer CE, Manos MM. Determination of genital human papillomavirus infection using consensus PCR. In: Herrington CS, McGee JOD, editors. Diagnostic molecular pathology: a practical approach. Oxford: Oxford University Press; 1992. pp. 132-52.
- 20.Melkert PWJ, Hopman E, van den Brule AJC, Risse EKJ, van Diest PJ, Bleker OP, et al. Prevalence of HPV in cytomorphologically normal cervical smears, as determined by the polymerase chain reaction, is age-dependent. Int J Cancer 1993;53:919-23. [DOI] [PubMed]
- 21.Muñoz N, Kato I, Bosch FX, Eluf-Neto J, de Sanjose S, Asunce N, et al. Risk factors for HPV detection in middle-aged women. Sex Transm Dis 1996; 23:504-10. [DOI] [PubMed]
- 22.Herrero R, Schiffman MH, Bratti C, Hildesheim A, Balmaceda I, Sherman ME, et al. Design and methods of a population-based natural history study of cervical neoplasia in a rural province of Costa Rica: the Guanacaste Project. Pan Am J Public Health 1997;1:362-75. [DOI] [PubMed]
- 23.Kiviat N, Koutsky LA, Paavonen J. Cervical neoplasia and other STD-related genital tract neoplasias. In: Holmes KK, Mardh PA, Sparling PF, Lemon SM, Stamm WE, Piot P, et al, editors. Sexually transmitted diseases. 3rd ed. New York: McGraw-Hill; 1999. pp. 811-31.
- 24.Fahey MT, Irwig L, Macaskill P. Meta-analysis of Pap test accuracy. Am J Epidemiol 1995;141:680-9. [DOI] [PubMed]
- 25.Ho GYF, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998;338:423-8. [DOI] [PubMed]
- 26.Koutsky LA, Holmes KK, Critchlow CW, Stevens CE, Paavonen J, Beckmann AM, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med 1992;327: 1272-8. [DOI] [PubMed]


