Abstract
Barley (Hordeum vulgare L.) Mla (Mildew resistance locus a) and its nucleotide-binding, leucine-rich-repeat receptor (NLR) orthologs protect many cereal crops from diseases caused by fungal pathogens. However, large segments of the Mla pathway and its mechanisms remain unknown. To further characterize the molecular interactions required for NLR-based immunity, we used fast-neutron mutagenesis to screen for plants compromised in MLA-mediated response to the powdery mildew fungus, Blumeria graminis f. sp. hordei. One variant, m11526, contained a novel mutation, designated rar3 (required for Mla6 resistance3), that abolishes race-specific resistance conditioned by the Mla6, Mla7, and Mla12 alleles, but does not compromise immunity mediated by Mla1, Mla9, Mla10, and Mla13. This is analogous to, but unique from, the differential requirement of Mla alleles for the co-chaperone Rar1 (required for Mla12 resistance1). We used bulked-segregant-exome capture and fine mapping to delineate the causal mutation to an in-frame Lys-Leu deletion within the SGS domain of SGT1 (Suppressor of G-two allele of Skp1, Sgt1ΔKL308–309), the structural region that interacts with MLA proteins. In nature, mutations to Sgt1 usually cause lethal phenotypes, but here we pinpoint a unique modification that delineates its requirement for some disease resistances, while unaffecting others as well as normal cell processes. Moreover, the data indicate that the requirement of SGT1 for resistance signaling by NLRs can be delimited to single sites on the protein. Further study could distinguish the regions by which pathogen effectors and host proteins interact with SGT1, facilitating precise editing of effector incompatible variants.
Keywords: barley, Blumeria graminis, resistance signaling, nucleotide binding, leucine-rich-repeat receptor (NLR), SGT1, in-frame deletion
Introduction
Blumeria graminis f. sp. hordei (Bgh), the causal agent of barley (Hordeum vulgare L.) powdery mildew, is an economically important biotrophic fungus (Murray and Brennan 2010). The association between Bgh and barley represents a principle model for interactions among Triticeae grain crops and biotrophic pathogens, which are constrained to colonize and gain their nutrients from living tissue (Draz et al. 2019). During infection, Bgh releases avirulence (AVR) effector proteins into the host via haustorial feeding structures (Kwaaitaal et al. 2017; Jaswal et al. 2020). These effectors function to subvert the host’s initial immune response to the presence of the pathogen and facilitate the absorption of nutrients (Krattinger and Keller 2016; Langin et al. 2020). In response, plants have evolved sets of resistance proteins that detect these effectors, initiating defense (Lo Presti et al. 2015). The most prevalent class of resistance proteins employed in agriculture are nucleotide-binding, leucine-rich-repeat receptors (NLRs) (Sun et al. 2020; van Wersch et al. 2020), with notable exceptions (Kourelis and van der Hoorn 2018).
Barley Mla (mildew resistance locus a) is a classic example of a complex resistance locus that encodes an NLR immune receptor; it is highly polymorphic with over 30 allelic variants conditioning race-specific immunity in diverse cultivars (Wei et al. 2002; Halterman and Wise 2004; Ridout et al. 2006; Seeholzer et al. 2010; Lu et al. 2016; Saur et al. 2019). To confer resistance, MLA proteins often require the assistance of the co-chaperones Heat Shock Protein 90 (HSP90) and Suppressor of G-two allele of Skp1 (SGT1) (Bieri et al. 2004). A third co-chaperone is also involved, the Zn2+ binding protein Required for Mla12 Resistance 1 (RAR1), which is essential for many, but not all MLA proteins (Shirasu et al. 1999; Shen et al. 2003; Bieri et al. 2004; Halterman and Wise 2004). Together HSP90, RAR1, and SGT1 form the HRS complex that acts to stabilize levels of MLA (Bieri et al. 2004; Shirasu 2009). Once MLA recognizes the presence of the appropriate effector, the MLA-HRS complex translocates to the nucleus where it initiates a signaling cascade, leading to immunity (Shen et al. 2007; Bai et al. 2012; Chang et al. 2013).
Conserved Mla or Mla locus orthologs display diversified recognition specificities to powdery mildew in transgenic Arabidopsis (Maekawa et al. 2012), wheat powdery mildew (Jordan et al. 2011), wheat and rye Ug99 stem rust (Periyannan et al. 2013; Mago et al. 2015; Cesari et al. 2016), wheat stripe rust (J. Bettgenhaeuser and M. Moscou, personal communication), as well as to the metabolite victorin, the cause of oat Victoria blight (Moscou et al. 2018), and rice blast (H. Brabham and M. Moscou, personal communication).
Herein, we present the molecular identification and phenotypic characterization of a 6-base-pair (bp) in-frame deletion in the SGS domain of Sgt1 that selectively compromises Mla-dependent disease resistance. Sgt1 has been historically difficult to investigate because silencing causes severe growth defects in most organisms and deletion is usually lethal (Azevedo et al. 2006; Bhattarai et al. 2007; Thao et al. 2007). Developmental and physiological assessments indicate that there are no significant phenotypic differences among plants with or without the Sgt1ΔKL308–309 mutation, other than compromised disease resistance. Considering the recent discoveries of pathogen effectors that target SGT1 (Redkar et al. 2015; Yu et al. 2020), and how Mla orthologues are critical sources of immunity for economically important crops, pursuit of this unique modification will likely yield breakthroughs for understanding a breadth of disease resistance interactions.
Materials and methods
Biological materials
The barley cereal introduction (CI) lines 16151 (Mla6), 16137 (Mla1), 16147 (Mla7), 16149 (Mla10), 16155 (Mla13), and 16153 (Mla15) were developed by introgression of Mla alleles into the universal susceptible cv Manchuria (Moseman 1972). Sultan-5 and rar1-m100 were gifts from J. Helms Jørgensen (Risø National Laboratory, Roskilde, Denmark; Torp and Jørgensen, 1986). The Pallas lines P03 (Mla6) and P04B (Mla7) were gifts from Lisa Munk (Kolster et al. 1986). Bgh isolates 5874 (AVRa1, AVRa6, and AVRa12) and CC148 (AVRa1, AVRa7, AVRa9, AVRa10, AVRa13, and AVRa15) were maintained on susceptible H. vulgare cv. Morex in separate growth chambers at 18 °C (16/8 h light/darkness).
Mutagenesis
Mutagenesis was performed as described in Meng et al. (2009) and Xi et al. (2009). Briefly, seeds of CI 16151 (Mla6) were treated with fast neutrons at 4 Gy Nf at the International Atomic Energy Agency (Vienna, Austria) and the resulting 34,800 M2 families were screened for mutant segregants 7 days after inoculation (DAI) with Bgh isolate 5874 (AVRa6) following the method of Wise and Ellingboe (1985). Chlorophyll mutations were used to evaluate the mutation rate of the population; 936 families segregated albino, 233 families segregated pale yellow, 63 families segregated pale green, and 6 families segregated green/white striped for a total of 3.56% observed chlorophyll mutations. Individuals that produced cell death symptoms or sporulating Bgh colonies were selected for rescue.
For this study, susceptible mutant m11506, containing Mla6 and Bln1 deletions, was backcrossed and selfed twice to CI 16151 to select homozygous mutants m18982 (mla6, Bln1, Rar3), m19089 (Mla6, bln1, Rar3), and m19028 (mla6, bln1, Rar3) (Xu et al. 2014). Similarly, the rar3 mutant, m11526 (Mla6, Bln1, rar3), was made homozygous following two backcrosses to CI 16151 with selection. F2 populations for exome capture and fine mapping were created by crossing m11526 to P03 (Pallas background, Mla6) or Sultan-5 (Mla12) and selfing the F1.
Phenotypic characterization
All plants were grown in a temperature-controlled greenhouse (16–20 °C; 16 h supplementary light). Physiological measurements were performed on seedlings that were grown for 14, 21 or 28 days. Stress tests were conducted by growing seedlings to 7 days old and then either moving to complete darkness, watering only with 100 mM NaCl, or removing watering completely. Height was then measured at 14, 21 and 28 days old if the plant survived. Analysis was performed as a pairwise t-test between each mutant and its wild-type progenitor for each trait. Significance was determined at P > 0.05.
To measure developmental characteristics, 10 seed from each genotype were planted in a randomized block design. Two individuals from each line were blocked and placed in five different locations within the same greenhouse room to account for microclimate variances. Plants were measured at regular intervals for plant height and number of tillers. Additional end-point data were collected on the total number of florets, seed fertility, and seed weight. Time series data were analyzed using a linear model with mixed effects with fixed factors as Genotype + time + Genotype*time + Position and the accession as the random effect. The end-point data were analyzed using a linear model with fixed effects using the design Trait ∼ Genotype + Position. A one-way ANOVA was performed and pairwise contrasts using the Tukey test. Genotypes were grouped using 0.01 significance level. All the analyses were run using the R software version 3.6 (R Core Team 2019) and the packages lsmeans (Lenth 2016) and multcompView (Graves et al. 2019).
For disease assessments, seedlings were grown in the greenhouse before being transferred to the relevant Bgh growth chamber for inoculation with fresh Bgh conidiospores. Phenotyping was performed at 7 DAI. A Chi-squared test was used to calculate the likelihood of phenotypic data matching expected genetic ratios.
Infection kinetics
Seedlings were grown in groups of six (one of each time point) by genotype in a randomized split plot design with genotype as the whole plot and time point as the sub plot before being inoculated with Bgh 5874. At each time point [16, 20, 24, 28, 32 and 48 h after inoculation (HAI)], five leaves of each group of six was collected and submerged in clearing solution (3:1, alcohol: acetic acid). Once all leaves were collected and had been submerged in clearing solution for at least 24 h, they were transferred to 70% ethanol for 24 h. Lastly, leaves were transferred to 20% ethanol and stored at 4 °C prior to scoring. Cleared leaves were treated with Coomassie blue for 2 min prior to scoring to visualize Bgh spores and trimmed to 5 cm in length from the tip. Each experiment was replicated three times.
Leaves were scored on both abaxial and adaxial sides to count the number of spores which were in each of five infection stages, attached spore, appressorium attachment, hyphal index 1, hyphal index 2, and hyphal index 3 (Supplementary Figure S1). After scoring, % Elongating Secondary Hyphae (ESH) was calculated by using the following calculation:
The analysis was performed using a mixed model as the fit for pairwise comparison between treatments with the response as percentage ESH using SAS software (SAS Institute Inc., Cary, North Carolina, USA). The fixed factors were set as treatment, leaf side, and treatment * leaf side. The random factors were leaf (treatment), replication and treatment * replication in a mixed model design. A false discovery rate of 0.05 was used.
Genetic crosses and F2 characterization
The rar3 mutant m11526 (Mla6, rar3) was crossed to multiple near-isogenic or diverse lines, and the F1s were selfed to generate segregating F2. F2 seed were planted in two 98-cell cone flats alongside both parents and F1, totaling 193 F2 seed sown. Each population was challenged with the appropriate Bgh isolate, 5874 or CC148, depending on Mla/AVR combinations. Parents F1 and F2 were phenotyped at 7 DAI and scored on a 0-4 scale for sporulation (successful growth of fungal spores), necrosis (plant cell death), and chlorosis (bleaching of leaf tissue). Subsequent to infection phenotyping, plants were grouped into four main categories. These were: resistant (R; no sporulation and no necrosis), resistant with necrosis (Rn; no sporulation but significant necrosis), susceptible with necrosis (Sn; significant sporulation and significant necrosis), and susceptible (S; significant sporulation but no necrosis).
The resistance or susceptible phenotype can be considered the major phenotype as it shows whether the plant is successfully defending against the pathogen, whereas the necrosis phenotype can be considered the minor phenotype as it may show whether defense is slow but successful (Rn) or too slow and failing (Sn). Additionally, it is possible that the necrosis phenotype is not associated with a Mla-based interaction at all, but rather due to other segregating loci (Yu et al. 2001).
CI 16151 reference genome
The CI 16151 genome sequence was based on the Morex genome (Assembly 082214v1, INSDC Assembly GCA_000326085.1) (Mayer et al. 2012), with high confidence variants (SNPs and short indels) introduced based on the input variant vcf files. Thirty seeds of CI 16151 were geminated on sterile 3M paper in each of four canisters. Eight-day-old etiolated seedlings (first leaf stage) were harvested, and DNA was isolated using a standard 2X CTAB prep. Samples were treated with RNAse A + T1, and their quality assessed on a 0.4% Tris-Phosphate-EDTA agarose gel. Samples were mechanically fragmented (∼500 bp) prior to library construction, and whole genome sequencing libraries were prepared using a Nextera DNA Sample Preparation Kit (Illumina, Inc., San Diego, CA, USA). Each sample was sequenced to 11.74x coverage (single-end read, 100 cycles) on one lane of an Illumina HiSeq 2000.
Reads from the four replicates were aligned to the Morex genome sequence using BWA (commands mem –v 0 –M –t 2 –p) version 0.7.12 (Li and Durbin 2009). The output sam files were processed using the Genome Analysis Toolkit (GATK) program using described best practices (https://software.broadinstitute.org/gatk/best-practices/) (McKenna et al. 2010). Briefly the BWA output sam files were merged with their unmapped versions using MergeBamAlignment from Picard tools (http://broadinstitute.github.io/picard) and duplicates were marked with MarkDuplicates from Picard tools.
The raw SNPs and Indels were called by the HaplotypeCaller GATK program and the output g.vcf files were merged using the GenotypeGVCFs GATK program. The SelectVariants GATK program identified the raw SNPs and Indels from the merged vcf file and the high-quality SNPs and Indels were selected using the VariantFiltration GATK program (–filterExpression “QD < 2.0 ‖ FS > 60.0 ‖ MQ < 40.0 ‖ MQRankSum < −12.5 ‖ ReadPosRankSum < −8.0”). The resulting four vcf files were then used along with the Morex genome to create a Morex-based CI 16151 genome assembly using the FastaAlternateReferenceMaker GATK program (https://software.broadinstitute.org/gatk/documentation/tooldocs/3.8-0/org_broadinstitute_gatk_tools_walkers_fasta_FastaAlternateReferenceMaker.php).
Exome capture
Two-hundred 7-day old F2 seedlings from both the m11526 × P03 and m11526 × Sultan-5 populations (400 F2 in total) were inoculated with Bgh 5874, infection phenotyped 7 DAI, and then treated with fungicide. Tissue was harvested from third leaves at 21 days old, into groups of 10 F2 seedlings per standard 2X CTAB DNA extraction. The grouped gDNA was then further pooled for a total of four population pools; 150 resistant P03 derived, 50 susceptible P03 derived, 150 resistant Sultan-5 derived and 50 susceptible Sultan-5 derived. Samples were processed for exome capture according to the SeqCap EZ Library SR User Guide (Roche NimbleGen, Version 5.1, Barley Exome Custom Sequence Capture design, 06740278001) and using the KAPA HyperPrep Kit (KK8500) and sequenced on a single lane of an Illumina HiSeq 3000.
Exome capture reads from the four pools were aligned to a custom CI 16151 barley genome sequence (published herein) using BWA (commands mem –v 0 –M –t 2 –p) version 0.7.12 (Li and Durbin 2009). The output sam files were processed using the Genome Analysis Toolkit (GATK) program using described best practices (https://software.broadinstitute.org/gatk/best-practices/) (McKenna et al. 2010). Briefly the BWA output sam files were merged with their unmapped versions using MergeBamAlignment from Picard tools (http://broadinstitute.github.io/picard) and duplicates were marked with MarkDuplicates from Picard tools.
Raw SNPs were processed by HaplotypeCaller, SelectVariants, and VariantFiltration GATK programs and the output g.vcf files were merged using the GenotypeGVCFs GATK program as described above for the CI 16151 reference genome. The output filtered vcf files were then converted to table format using the VariantsToTable GATK program. High-quality SNPs were identified using the GQ quality metric (GQ > 99) using a custom bash script. Average SNP frequencies were calculated and graphed using custom bash and R scripts.
Fine mapping
As not all SNPs were similar in both P03 and Sultan-5 compared to m11526, we used the P03 × m11526 population for fine mapping so that we removed any potential affect the Mla12 allele may have. Four-thousand five hundred P03 × m11526 F2 individuals were phenotyped with Bgh 5874 and harvested for DNA extraction.
Molecular markers were generated from the exome capture data to delineate the causal mutation to Chr3HL 408.3 to 420.2 Mb. Markers were generated by examining the genes found in this region according to Ensembl (Assembly 082214v1, INSDC Assembly GCA_000326085.1) and looking for concurrent SNPs in the exome capture data. SNPs were examined for changes in restriction sites that would indicate a suitable Cleaved Amplified Polymorphic Sequence (CAPS) marker. CAPS markers that showed suitability for high-throughput genotyping were used on the F2 population (Supplementary Table S1). Genotyping results were used to identify individuals with recombination events within the candidate region.
Identification of the Sgt1 mutation
The Sgt1 gene was amplified from m11526 gDNA and cDNA using the primers Sgt1_3ʹ_F1 (GCTCCCCAAAGTCTTCGTCT) and Sgt1_5ʹ_R2 (TGTAAGCTGTTTGCGTGGCAGG) with Phusion High-Fidelity DNA polymerase (Thermo Scientific). A nested PCR was performed on the Sgt1 cDNA product using Sgt1_Start (ATGGCCGCCGCCGCC) and Sgt1_5ʹ_R2 to make an in-frame transcript which was cloned into the pCR8 backbone using the pCR™8/GW/TOPO® TA Cloning Kit (ThermoFisher, K250020). Sequences were determined by Sanger sequencing.
Hydrogen peroxide accumulation and hypersensitive reactions
The observation of hypersensitive reactions (HR) was performed as described by Xu et al.(2014). Seven-day-old barley first leaves (PO: 0007094) were inoculated with Bgh 5874, harvested at 24 HAI, fixed and decolorized for 48 h in ethanol: acetic acid (3:1, v/v) with one change with 70% ethanol (v/v). Following fixation, leaves were cleared in lacto:glycerol:water solution (1:1:1, v/v). Hypersensitive responses were examined using a fluorescence microscope (Leitz Fluovert, Wetzlar, Germany) with a fluorescein filter set H3 (excitation filter BP420–490, suppression filter LP 520).
Hydrogen peroxide (H2O2), a reactive oxygen species (ROS), was assayed by diaminobenzidine tetrahydrochloride (DAB) staining. First leaves inoculated with Bgh 5874 were cut at 16 HAI and moved to DAB solution (1 mg/ml). After 8 h (total 24 HAI), the stained tissues were fixed and cleared. ROS accumulation was examined under a Leitz Fluovert microscope.
Differential transcript accumulation
RNA-Seq data were extracted from a time course of barley CI 16151 and derived fast-neutron mutants covering key stages of Bgh infection. First leaves were inoculated with fresh Bgh-conidiospores (Caldo et al. 2004) and sampled from a split-plot design at 0, 16, 20, 24, 32, and 48 HAI (5 genotypes × 6 time points × 3 biological replications) [NCBI-GEO GSE101304 (Hunt et al. 2019)].
Samples were grouped by time point and analyzed using R package DESeq2 in Bioconductor (Love et al. 2014). A model was fit with read counts as response, and time point and genotype terms as explanatory variables. Taxon-specific normalization was performed as described by Klingenberg and Meinicke (2017): count matrices were separated for barley and Bgh and size factors for DESeq2 were calculated, then those were combined to calculate the final normalized counts matrix. Differential expression analysis was done after normalization, adjusting the P-values controlling for multiple testing, using methods described by Benjamini and Hochberg (1995). Genes that had a log2 fold change of at least one, and were differentially expressed at a q-value of >0.001 were considered significant.
Data availability
Strains and plasmids are available upon request. Supplementary files available at FigShare: https://doi.org/10.25386/genetics.12935036. Supplementary Figure S1 contains categorization of Bgh spore growth stages. Supplementary Figure S2 contains phenotypes of m9450 and m9455 at 3, 5, and 7 DPI. Supplementary Figure S3 phenotypes of crosses between m11526 and CI 16147 or CI 16155. Supplementary Table S1 contains molecular markers used for fine mapping of Rar3. Supplementary Table S2 contains a summary of fast-neutron mutants, phenotypes, and genes identified by them. Supplementary Table S3 contains morphology data of mutant and wild-type barley under normal growing conditions. Supplementary Table S4 contains measurements and analysis of mla6, bln1, and rar3 mutants from seed-to-seed during development. Supplementary Table S5 contains morphology data of mutant and wild-type barley under stressed growing conditions. Supplementary Table S6 contains F2 infection-type results from crosses among m11526 and other lines. Supplementary Table S7 contains expected ratios of F2 from cross m11526 (Mla6, rar3) and CI 16147 (Mla7, Rar3). Supplementary Table S8 contains acronyms used.
Illumina sequence data to construct the CI 16151 reference genome are available in NCBI’s Gene Expression Omnibus (GEO) under the accession number PRJNA630064. Illumina sequence data supporting the Exome capture is available under GEO accession number PRJNA645935. HvSgt1 (HORVU3Hr1G055920) from CI 16151 and rar3-m11526 are available under GenBank Accessions AF439974 and MT787218, respectively. RNA-Seq datasets are available in NCBI-GEO under the accession number GSE101304 (https://www.ncbi.nlm.nih.gov/gds/? term=GSE101304).
Results
m11526 (rar3) is developmentally equivalent to its resistant progenitor, CI 16151 (Mla6), and Bgh infection kinetics are indistinguishable from the susceptible m18982 (mla6)
To identify new factors in the Mla signaling pathway, we performed fast-neutron mutagenesis on CI 16151, which carries the Mla6 resistance allele (Moseman 1972), and challenged the resultant 34,800 M2 families with the avirulent Bgh isolate 5874 (AVRa6) (Moscou et al. 2011; Xu et al. 2014). As shown in Figure 1A, we identified both localized (e.g., m9463, m9467, and m11542) and systemic (e.g., m9450 and m9455) cell-death mutants (Supplementary Figure S2).
Figure 1.
Barley line CI 16151 and its fast neutron-derived mutants. (A) Phenotypes of resistant mutants displaying cell death phenotypes, m9450, m9455, m9463, m9467, and m11542. The causal mutation for m11542 was previously cloned as rrp46 (Xi et al. 2009). (B) Phenotypes of CI 16151 and the mutants displaying sporulation phenotypes, m18982, m19029, m11526, and m19089. The causal mutation present in m18982 and m19089 were previously characterized as Mla6 (Halterman et al. 2001) and Bln1 (Meng et al. 2009), respectively. Gene names indicate the presence of normal (black) or mutated (red) alleles of either Mla6, Rar3, Bln1, or RRP46. Pictures show phenotypes at 7 DAI with Bgh isolate 5874 (AVRa6), with their designated macroscopic phenotype and score for sporulation. An infection type of 0 is resistant (no sporulation), 1–2 is considered resistant, but with minor Bgh colonization, and an infection type of 3–4 is susceptible (abundant sporulation). 1n, few small necrotic flecks (0.5 mm); 1 – 2n, significant small necrotic flecks (1 mm); 2N, abundant cell death (>2 mm); c, limited chlorosis; C, abundant chlorosis. Earlier time points for m9450 and m9455 are in Supplementary Figure S2.
Of particular interest to this report, however, were five M2 families that segregated three resistant (R; no Bgh colonization) to one susceptible (S; abundant Bgh colonization) (Figure 1B). We rescued the susceptible individuals, confirmed that they were homozygous, and established a diallel to determine complementation groups (Supplementary Table S2). Sequencing of the Mla6 allele in all identified mutants revealed that four of them, m9472, m9480, m11538, and m18982, possessed Mla6 deletions (Xu et al. 2014), but m11526 had no changes to Mla6. In addition, m11526-mediated susceptibility segregated independently from the four mla6 mutations. Therefore, m11526 possesses a mutation in a gene other than Mla6, but is required for Mla6-specified resistance. This unknown gene was designated Rar3 (Required for Mla6 resistance 3).
Comparisons were made between m11526 and the wild-type progenitor CI 16151 for multiple traits, as differences in these could suggest modes of action for Rar3. To examine developmental traits, plant height, root number and morphology, number of stem nodes, internodal distance, leaf size, and leaf number were measured up to 4 weeks old (Supplementary Table S3). In addition, plant height and number of tillers were also recorded every 2 weeks from seed-to-seed in the plant life cycle culminating in final number of seeds per spike, fertility, and seed weight (see Methods, Supplementary Table S4). Lastly, plants were grown under standard greenhouse conditions for 7 days and then put under dark (no light), salt (only watered with 100 Mm NaCl2) and drought (no watering) stress conditions (Supplementary Table S5). Height was measured for these individuals and overall phenotypes examined to see whether their macroscopic responses to stress differed. No significant differences were observed between CI 16151 and m11526 for any measured physiological characteristic, under normal or stress conditions.
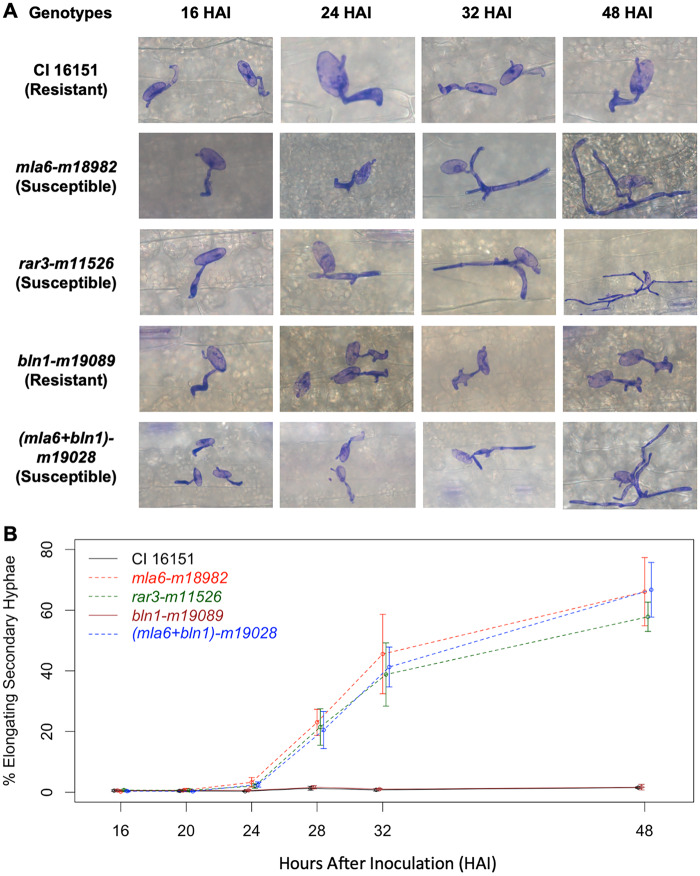
To determine quantitative differences in development of Bgh on the different host lines, the infection kinetics of Bgh 5874 was scored microscopically on m11526 (Mla6, Bln1, rar3), m18982 (mla6, Bln1, Rar3) and CI 16151 leaves across a time course of primary infection. As shown in Figure 2A, we used elongating secondary hyphae (ESH) as an indicator of successful colonization, as it is a necessity to absorb nutrients from the host via functional haustoria during development (Ellingboe 1972). Additionally, two other mutants from the same fast-neutron screen, m19089 (Mla6, bln1, Rar3) and m19028 (mla6, bln1, Rar3) were examined as resistant and susceptible controls, respectively (Figure 1B). For each genotype, 5 first leaves were harvested at 16, 20, 24, 28, 32, and 48 HAI. Leaves were cleared, stained to highlight spore development and examined under a light microscope at x5 to x20 magnification (Supplementary Figure S1). These data indicate a significant difference between the number of Bgh conidiospores able to successfully colonize epidermal cells when the resistant lines, CI 16151 or m19089, were compared to the susceptible lines m11526, m18982, or m19028. However, there was not a significant difference among the susceptible lines (Figure 2B).
Figure 2.
Percentage elongating secondary hyphae (ESH) from Bgh isolate 5874 measured at six time points on CI 16151 and its derived mutant genotypes. (A) Microscopic images at 20× magnification of four time points (16, 24, 32, and 48 HAI) for each genotype. (B) Time-course graphs representing infection kinetics of Bgh on resistant and susceptible genotypes. The x-axis represents the time points at which measurements were taken (hours after inoculation, HAI) and the y-axis represents the percentage elongating secondary hyphae [which indicate successful colonization events (Ellingboe 1972)]. The percentage elongating secondary hyphae was calculated as 100× (sum of three hyphal indices/total). Total is sum of spore, appressorium, and the three hyphal indices. The dashed and solid lines represent the susceptible and resistant genotypes, respectively. Error bars indicate one standard deviation.
Mla6-, Mla7-, and Mla12-, but not Mla1-, Mla9-, Mla10-, and Mla13-mediated immunity are compromised by the rar3 mutation
Most, but not all, Mla-encoded NLR variants require Rar1 (HORVU2Hr1G097800) as part of the conserved HRS complex that influences resistance signaling (Torp and Jørgensen 1986; Bieri et al. 2004; Halterman and Wise 2004). To determine which Mla alleles may be affected by the rar3 mutation, m11526 was crossed to barley lines harboring a range of Mla alleles (Moseman 1972), and the resultant F1 were self-pollinated to make large F2 populations. F2 segregants were analyzed 7 days after infection with the appropriate Bgh isolate (5874: AVRa1, AVRa6 and AVRa12, or CC148: AVRa1, AVRa7, AVRa9, AVRa10, and AVRa13) and Chi-squared tests were used to ascertain whether the observed ratios differed significantly from the expected genetic interactions.
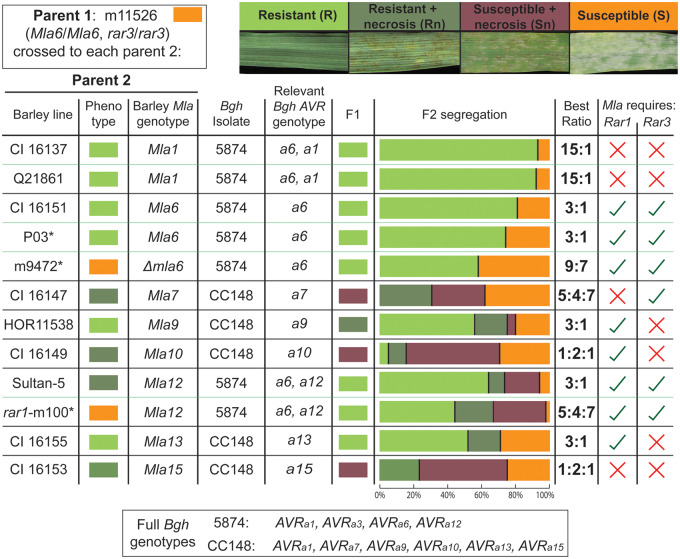
First, to verify that the m11526 phenotype is caused by a single locus, we examined the F2 progeny from crosses m11526 × CI 16151 (Mla6), m11526 × P03 (Pallas with introgressed Mla6), and m11526 × Sultan-5 (Mla12). F2 progeny from these three crosses all segregated 3R: 1S (P = 0.16, 0.68, and 0.77, respectively) as expected for a single gene (Figure 3). Second, to test whether Rar3 segregates independently of Mla6 and Rar1, we examined F2 progeny from crosses among the susceptible lines, m11526 × m9472 (mla6, Rar1, Rar3) and m11526 × rar1-m100 (Mla12, rar1, Rar3). The 9R: 7S phenotypic ratios resulting from the cross to m9472 (P = 0.68) did not differ significantly from what was expected for independent segregation. Though the rar1-m100 cross did not produce the expected 9R: 7S ratio for independently segregating genes (P = 0.015), it also did not fit the 1R: 1S ratio expected for linked genes (P = 0.000), suggesting that Rar1 and Rar3 are unlinked. In fact, the ratio of 11R: 5S which generated more resistant F2 than predicted, suggests some genetic combination that confers greater resistance overall. This may involve some contribution from the Sultan-5 background interacting with the rar1 or rar3 loci.
Figure 3.
F2 segregation results from crosses between m11526 (Mla6, rar3) and multiple near-isogenic or diverse Mla-carrying lines. As Bgh isolates with different AVR genes were used, the Bgh AVR genes relevant to a cross are indicated under “Relevant Bgh AVR genotype” and the AVRa genotypes of each strain are displayed in the box below. Representative images for each plant infection phenotype are shown above the table. Raw percentages of F2 populations are displayed, and a chi-squared test was used to determine which of the potential segregation ratios was most suitable according to the lowest P-value. Ticks and X’s are used to show whether an Mla variant appears to, or not to, require the indicated gene, respectively. * P03 [Pallas background, Mla6 (Kolster et al. 1986)], m9472 (CI 16151-derived fast-neutron mutant, mla6), m100 [Sultan-5 background, Mla12, rar1 (Torp and Jørgensen 1986)]. Raw data are documented in Supplementary Table S6.
Segregation ratios produced from crosses of m11526 to lines carrying Mla1 (CI 16137 and Q21861, 15R: 1S), Mla9 (Hor11358, 3R: 1S), and Mla13 (CI 16155, 3R: 1S) suggest that these alleles do not require Rar3 to function as they segregate as dominant genes (Figure 3; Supplementary Table S6). Though the CI 16149 (Mla10) F2 ratio did not significantly fit any of the models, the closest applicable ratios were a 3R: 1S at P = 0.004 and 1R: 2Sn: 1S at P = 0.015, which would suggest a Rar3 independent, semi-dominant model. Additionally, while Mla9, Mla10, and Mla13 do not appear to require Rar3 to function (Figure 3), they do require Rar1 (Halterman and Wise 2004).
Curiously, the m11526 × CI 16147 (Mla7), HOR11358 (Mla9), and CI 16153 (Mla15) crosses generated F1 hybrids with a different phenotype to either of the parents. The m11526 × HOR11358 (Mla9) F1 displays a resistant with necrosis (Rn) phenotype, and there is a small number of F2 that display susceptible with necrosis (Sn) phenotypes. Together these observations suggest that the rar3 mutation may lessen the strength of the Mla9 resistance, but not to an extent to overtly change the phenotype, that is, rar3 converts resistant (R) phenotype to Rn, instead of susceptible (S). Crosses to CI 16149 (Mla10) also generated F1 with the intermediate susceptible with necrosis phenotype but this is due to Mla10 being semi-dominant, which does not seem to be dependent on Rar3. The F2 phenotypic ratios resulting from m11526 × CI 16147 (Mla7) suggest that Mla7 does require Rar3 to fully function (5R: 11S, P = 0.83, 5Rn: 4Sn: 7S, P = 0.09). Together with the observation of intermediate function in the F1 (Supplementary Figure S3), these F2 phenotypic ratios suggest a dosage dependence model whereby Mla7 partially requires Rar3 to function. This model would indicate that when both loci have only a single functioning allele, the resistance does not reach a threshold necessary to prevent colonization (see Supplementary Table S7 for table showing hypothetical models).
The cross between m11526 and CI 16153 (Mla15) may shed light on the interaction between Mla7 and Rar3. Mla7 and Mla15 share the same sequence but appear to act slightly differently, as evidenced by Bgh infection kinetics (Wise and Ellingboe 1983), and also by interpretation of the different segregation ratios. Whereas the Mla7 cross generated a ratio of 5R: 11S, or if we take necrosis into account 5Rn: 4Sn: 7S, the Mla15 cross generated the ratio 1R: 3S, or 1Rn: 2Sn: 1S, indicating a single semi-dominant gene.
Bulk segregant analysis with exome capture positions rar3 on chromosome 3H
At 5 Gb, the large and repetitive barley genome can be a challenge for isolating unknown genes (Mascher et al. 2017). To overcome these challenges and position rar3 in m11526, we used a capture approach with ∼2 million exome probes to reduce the sequenced portion of the genome to about 61.6 Mb (Mascher et al. 2013).
Then, in order to generate a large set of diverse SNPs for exome capture, we generated F2 populations from the m11526 × P03 (Mla6) and m11526 × Sultan-5 (Mla12) F1 hybrids. These populations were selected because (1) both Mla6 and Mla12 require Rar3 for resistance function and (2) P03 and Sultan-5 are two-row European lines that have genetic backgrounds distinct from the 6-row Manchuria background in CI 16151 and its m11526 derivative. Two-hundred segregating F2 progeny from each population were challenged with Bgh 5874 (AVRa6, AVRa12) and scored for resistance or susceptibility, creating four distinct groups based on genetic cross and infection phenotype. Both populations segregated 3R: 1S as shown in Figure 3. DNA extractions were performed on tissue pooled from leaves within each group, amplified using the standard exome capture method and sent for Illumina sequencing.
Nearly 450,000 SNPs were generated from the exome capture and aligned to the barley reference genome (IBSC_V2, Mascher et al. 2017), as well as our reference genome for CI 16151 (PRJNA630064). For each SNP, the number of reads with SNP’s matching the reference genome (reference SNPs) was measured for each group as a proportion of the total number of reads for each SNP location. SNPs that did not match the reference genome (alternative SNPs) must either be from the alternative parent (either P03 or Sultan-5), novel mutations in m11526, or sequencing errors which were filtered from the data set. The SNP frequencies in each pool were averaged over 5 Mb intervals to reduce noise, and then plotted across the seven chromosomes. The results for most chromosomes did not display any major differences between the frequencies of resistant and susceptible pools in either cross. However, chromosome 3H revealed multiple regions where the resistant and susceptible frequencies diverged considerably, suggesting regions that may cosegregate with the observed phenotypes (Figure 4A).
Figure 4.
Mapping of the Rar3 cosegregating region to chromosome 3H. (A) Results of the exome capture allele frequencies. Allele frequencies were determined relative to proportion of reads that had SNPs which mapped to the reference genome, out of the total number of reads. Allele frequencies were averaged over 5 Mb for the resistant and susceptible pools prior to plotting. Red and blue lines indicate allele frequencies for susceptible and resistant pools, respectively. (B) Physical map of chromosome 3H, Mb locations of genetic markers is indicated by black vertical lines with numbers. The orientation of the centromere is indicated by an arrow. The number of X’s represents the number of observed crossover events between the flanking markers. The position of Sgt1 is shown with a green dashed line at position 417.32 Mb. (C) Zoomed in location of closest flanking markers to Sgt1. (D) Alignment of wild-type and m11526 Sgt1 cDNA sequences covering the region containing the rar3-m11526 mutation. Protein alignment and structure included for reference, red dashed line indicates location of mutation.
High-resolution recombination mapping delineates Rar3 to 12 Mb on chromosome 3H flanking Sgt1
To fine-map the Rar3 locus, Cleaved Amplified Polymorphic Sequence (CAPS) markers were designed spanning chromosome 3H and tested on a small m11526 × P03 F2 population of 94 individuals. Markers with the closest linkage to Rar3 were between ∼325 and ∼450 Mb, which coincided with the largest cosegregating region from the exome capture. Fine mapping, using CAPS markers on 3470 m11526 × P03 F2 individuals, identified 15 recombination events that reduced the co-segregating region between 408.3 Mb (HORVU3Hr1G054770) and 420.2 Mb (HORVU3Hr1G056080) on chromosome 3H (Figure 4C).
This region contains 67 predicted genes, according to Ensembl, Assembly IBSC_V2 (annotations by the International Barley Sequencing Consortium, IBSC). Reads generated by the capture protocol aligned to each of the genes within the entire 12 Mb cosegregating region, and the only difference between CI 16151 and m11526 was a small deletion in HORVU3Hr1G055920, corresponding to Sgt1 (GenBank: AF439974.1). Subsequent re-isolation of full-length Sgt1 from both m11526 and CI 16151 cDNA confirmed the 6 bp in-frame deletion to be at position 922–927 bp (Figure 4D). This comprises exactly two amino acids, lysine (K) 308 and leucine (L) 309 in the SGS domain, the domain that directly interacts with NLRs (Zhang et al. 2008). These data indicate that the Sgt1ΔKL308–309 mutation in m11526, as delineated by recombination, is the only candidate for causing susceptibility.
The Lys-Leu deletion is associated with disruption of H2O2 accumulation and hypersensitive cell death
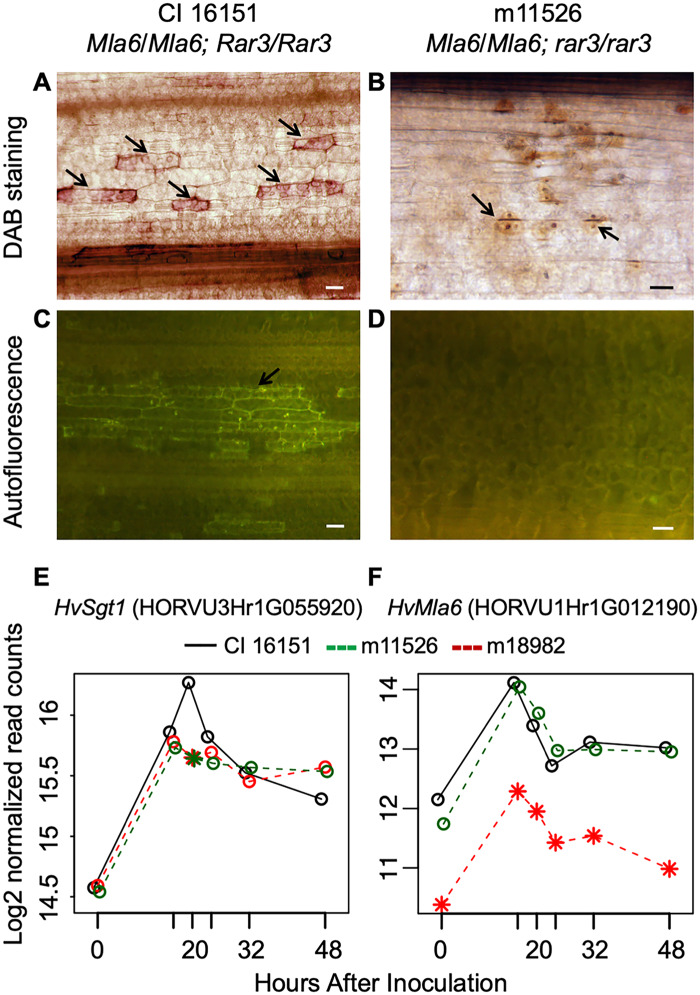
Rapid localized cell death, termed the hypersensitive reaction or HR, is often elicited by host recognition of invading pathogens. One of the major components of HR is the generation of ROS, such as hydrogen peroxide (H2O2), which act as late stage signaling molecules (Lehmann et al. 2015). Moreover, flavonoids, which function as antimicrobials, are often released as part of plant defense responses and will autofluoresce when examined under green fluorescence protein microscope filters (Cushnie and Lamb 2011). We investigated whether the rar3 mutation disrupted accumulation of H2O2 by DAB (3,3′-diaminobenzidine) staining. We observed obvious accumulation of H2O2 in Bgh challenged epidermal cells for CI 16151 (Figure 5A), but not in the m11526 loss-of-function mutant (Figure 5B). Moreover, while we see rampant autofluorescence in and around cells challenged by Bgh in CI 16151 (Figure 5C), we did not observe any autofluorescence in m11526 (Figure 5D).
Figure 5.
Rar3 is required for Mla-mediated H2O2 accumulation and the hypersensitive reaction (HR). (A) H2O2 accumulation evidenced by intense brown coloration produced by 3,3′-diaminobenzidine (DAB) staining in epidermal cells from CI 16151 (Mla6) at 24 HAI with Bgh isolate 5874 (AVRa6). Bar, 25 μm. (B) Decreased accumulation of H2O2, as indicated by fewer, and less intense, DAB-stained (brown) cells, was observed in the m11526 loss-of-function mutant. (C) Consistent with ROS accumulation, whole-cell autofluorescence is observed in epidermal cells from wild-type CI 16151 at 24 HAI with Bgh isolate 5874 (AVRa6). Bar, 25 μm. (D) Autofluorescence was absent in the m11526 loss-of-function mutant. (E) Transcript accumulation of Sgt1 (HORVU3Hr1G055920) in wild-type CI 16151 (black solid lines) vs. m11526 (dashed green lines) and m18982 (dashed red lines). * Indicates significant difference from CI 16151 at P < 0.001. (F) Transcript accumulation of Mla6 (HORVU1Hr1G012190) in wild-type CI 16151 vs. m11526.
Expression of Sgt1 in CI 16151 and the rar3 mutant m11526
Coincident with the absence of H2O2 accumulation and HR, we considered that accumulation of Sgt1 transcripts may differ in the m11526 mutant as compared to its CI 16151 progenitor. As shown in Figure 5E, RNA transcript profiling revealed that Sgt1 was significantly differentially expressed at 20 HAI just after Bgh penetration, but prior to development of haustoria, with marked up-regulation in CI 16151 plants as compared to the m11526 loss-of-function mutant (P-value cutoff = 0.001). However, when we compared the expression of Sgt1 in m11526 at 20 HAI to the susceptible mutant m18982 (mla6), there was no significant difference. This suggests that the change in expression of Sgt1 at 20 HAI may not be directly attributed to the rar3 mutation, but is indirectly associated with susceptibility, per se. Subsequently, transcript levels decreased post penetration at 24–48 HAI in wild-type plants but stabilize for the m11526 mutant. As expected, transcript accumulation was equivalent for the Mla6 NLR in CI 16151 and m11526 (Figure 5F). Though there appeared to be some residual expression attributed to Mla6 in the m18982 deletion mutant, this may be due to confounding by reads from Mla6 paralogs present in the progenitor CI 16151 mapping to this gene.
Discussion
Sgt1ΔKL308–309 joins a list of invaluable mutants for elucidating molecular functions
Phenotypes observed by mutagenesis often result from deletions of sections of DNA that result in loss of whole genes, frame shifts, truncations, and/or loss of functionality. However, substitutions of two, or even a single amino acid, can have a profound effect on protein function. For example, targeted in-frame deletions have been critical to unraveling complex dystrophin phenotypes of Duchenne/Becker muscular dystrophy (Aartsma-Rus et al. 2006; Bello et al. 2016). A single amino acid change in the envelope protein gene, E1-A226V, is responsible for Chikungunya virus adaptation to Aedes albopictus mosquitoes, resulting in increased host range and associated with a 2006 epidemic on Reunion island (Tsetsarkin et al. 2007). Likewise, a two-amino acid difference in the coat protein of satellite panicum mosaic virus is responsible for differential synergistic interactions with panicum mosaic virus (Chowda-Reddy et al. 2019). Moreover, a single G to D substitution in sedoheptulose 1,7-bisphosphatase reduces growth and yield in rice (Li et al. 2020), and a double L92A/I94A mutation in the gravitropism gene LAZY1 changed direction of auxin accumulation relative to gravity in Arabidopsis (Yoshihara and Spalding 2020). In barley, a single G to D substitution in MLA6 or MLA13 at position 721 alters the requirement for the co-chaperone RAR1 for resistance to powdery mildew (Halterman and Wise 2004).
To further characterize NLR-based disease defense, a fast-neutron screen was used to identify rar3-m11526, an Sgt1 allele that compromises Mla6-, Mla7-, and Mla12-mediated immunity, but does not disrupt resistance specified by Mla1, Mla9, Mla10, or Mla13. This is reminiscent of the differential requirement of Mla alleles for Rar1, but the allele set that requires Rar3 is divergent from that of Rar1 (Shirasu et al. 1999; Shen et al. 2003; Bieri et al. 2004; Halterman and Wise 2004), and Rar3 segregates independently of both Mla6 and Rar1. Furthermore, though some of the alleles, such as Mla9, may not fully require Rar3 to function, the rar3 mutation does appear to lessen the strength of their resistance, evidenced by the appearance of the new resistant with necrosis phenotype of the F1 heterozygote. We delineated the causal mutation to a 6 bp (Lys-Leu) in-frame deletion within the SGS domain of Sgt1 (Sgt1ΔKL308–309), the structural region that interacts with NLR proteins (Zhang et al. 2008). SGT1 is a highly conserved protein that is essential for life processes including growth, development, kinetochore formation, auxin, and jasmonate recognition, in addition to disease defense (Kitagawa et al. 1999; Gray et al. 2003; Hoser et al. 2013; Zhang et al. 2015). Most diploids like barley have a single copy of Sgt1, though Arabidopsis and soybean have two copies that have diverged enough to not be entirely redundant (Austin et al. 2002; Fu et al. 2009).
Potential molecular effects of the ΔKL308–309 mutation on Sgt1
Stable silencing of Sgt1 in N. benthamiana and hexaploid wheat results in severely stunted growth (Peart et al. 2002; Wang et al. 2015) and is lethal in rice and tomato (Bhattarai et al. 2007; Thao et al. 2007). Furthermore, lethality is common for Sgt1 mutants, including Arabidopsis double mutants, (Azevedo et al. 2006) and transposon insertions in Drosophila (Martins et al. 2009). Even a single change in the phosphomimic SGT1-S361D is enough for lethality in yeast and human cells (Bansal et al. 2009). Transient virus-induced-gene silencing of Sgt1 in barley by barley stripe mosaic virus (BSMV) does not lead to any obvious growth defects, however, it is suggested that this could be because the virus’ used in other systems are able to infect meristematic tissue and thus cause silencing at an earlier stage of shoot development (Hein et al. 2005).
Protein structure prediction with iTasser (Yang et al. 2015) found little difference in the structure of the SGS domain between wild-type SGT1 and SGT1ΔKL308–309. Furthermore, X-ray crystallography showed that the SGS region of SGT1 is highly disordered as it lacks a fixed tertiary structure (Dunker et al. 2008; Taube et al. 2014). This decreases the likelihood that a two-amino-acid mutation could disorder the tertiary structure any further. These suggest that the difference in phenotype is not due to misfolding, but rather the loss of function that is tied directly to one or both of the deleted amino acids.
SGT1 consists of three highly conserved functional domains, the tetratricopeptide repeat (TPR), CHORD, and Sgt1 (CS) and Sgt-specific (SGS) domains, separated by two unconserved variable (Vr) domains (Zhang et al. 2008). The SGS domain is responsible for interacting with the LRR domain of MLA proteins and is necessary for SGT1-mediated accumulation of resistance proteins (Boter et al. 2007; Shirasu 2009). As lysines are known to be involved in protein–protein interactions by creating salt bridges, the K308 could be an important residue specifically for the interaction between MLA and SGT1. Moreover, the SGS region is not involved in kinetochore function or implicated in growth, which would explain why the plant appears to grow normally.
Genetic analysis of m11526 crossed to lines with various Mla alleles uncovered that, even though the susceptibility phenotype was similar to that of plants that contained rar1, those Mla alleles which required Rar3 were different (Figure 3). Having now discovered that the rar3-m11526 mutation is in the SGS domain of SGT1, we can look at the results of these crosses in a different light. If we consider that the requirement for RAR1 may be a need for a molecular “crutch” to assist the interaction with SGT1, we can rate MLA variants for how strongly they interact with SGT1 by their need for RAR1 and/or RAR3. MLA1 would be the strongest interactor by not requiring either, whereas MLA7, MLA10, and MLA13 would be intermediate for only requiring one or the other and MLA6 and MLA12 would be the weakest by requiring both. MLA7 and MLA10 could be considered lesser interactors due to their necrotic phenotype, which is indicative of a late defense response, also known as trailing necrosis (Morel and Dangl 1999). However, MLA7 and MLA10 have amino acid differences in the highly conserved CC domain (108LE insertion and E41D, respectively), the region that associates with transcription factors to transmit the defense response, which are not present in other variants tested here. Therefore, the necrotic phenotypes may be caused by the signal not being transduced effectively after being initiated due to reduced binding with transcription factors.
By mining an RNA transcript profiling dataset, we observed a difference in Sgt1 expression that does not appear to be caused directly by the rar3 deletion. Indeed, the expression profile of Sgt1 was not significantly different when m11526 was compared to the susceptible mla6 mutant m18982 (Figure 5F), suggesting the difference in expression could be associated with susceptibility. Thus, our current hypothesis is that the SGT1ΔKL308–309 mutation appears to affect the function of SGT1 protein, and other proteins that interact with its SGS domain, such as COI1, HSC70 (Noël et al. 2007), or perhaps an unknown interactor, rather than transcript accumulation of Sgt1 (or Mla6). However, there may be a feedback or feed forward mechanism that indirectly affects the accumulation of transcripts.
Summary
To date, research on Sgt1 has been hindered by the fact that all organisms are unable to survive when it has been deleted. Notable exceptions to this requirement are yeast and organisms that have evolved duplicates, such as Arabidopsis, though these organisms would not be able to survive without all duplicates so it’s questionable whether they are in fact exceptions. The data presented here indicate that the rar3-m11526 allele, Sgt1ΔKL308–309, affects only interactions involved in disease resistance, while leaving other Sgt1-mediated processes intact.
The observation that the necessity of SGT1 by NLRs can be localized to a single protein domain provides a framework for other genes that interact with the SGS domain. For example, targeting Sgt1ΔKL308–309 by directed CRISPR-Cas editing could uncover how SGT1 interacts with proteins for Sr33-mediated Ug99 stem rust- (Periyannan et al. 2013) or Lr21-specified leaf rust resistance in wheat (Scofield et al. 2005; Huang et al. 2009). Recently, pathogen effectors have been identified that specifically target the HRS machinery, such as RipAC in Ralstonia solanacearum that interacts with the SGS domain to block interactions with MAP kinases (Yu et al. 2020). By understanding how plant and fungal proteins specifically interact with SGT1, it could be possible to engineer crops with SGT1 variants without fungal effector interaction sites, whilst maintaining interactions with resistance proteins.
Acknowledgments
The authors thank Liz Miller, Charles Warwick, Eunice Kong, Morgan Bixby, and Jessica Faust for expert assistance during the mutant screen and laboratory protocols. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture or the National Science Foundation. USDA is an equal opportunity provider and employer.
Funding
Research supported in part by the National Science Foundation—Plant Genome Research Program grant 13-39348 and USDA—Agricultural Research Service project 3625-21000-067-00D to R.P.W. V.V.Z. was supported by Fulbright—Minciencias 2015 & Schlumberger Faculty for the Future fellowships. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
None declared.
Literature cited
- Aartsma-Rus A, Van Deutekom JCT, Fokkema IF, Van Ommen G-JB, Den Dunnen JT. 2006. Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve. 34:135–144. [DOI] [PubMed] [Google Scholar]
- Austin MJ, Muskett P, Kahn K, Feys BJ, Jones JDG, et al. 2002. Regulatory role of SGT1 in early R gene-mediated plant defenses. Science. 295:2077–2080. [DOI] [PubMed] [Google Scholar]
- Azevedo C, Betsuyaku S, Peart J, Takahashi A, Noël L, et al. 2006. Role of SGT1 in resistance protein accumulation in plant immunity. EMBO J. 25:2007–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai S, Liu J, Chang C, Zhang L, Maekawa T, et al. 2012. Structure-function analysis of barley NLR immune receptor MLA10 reveals its cell compartment specific activity in cell death and disease resistance. PLoS Pathog. 8:e1002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal PK, Mishra A, High AA, Abdulle R, Kitagawa K. 2009. Sgt1 dimerization is negatively regulated by protein kinase CK2-mediated phosphorylation at Ser³61. J Biol Chem. 284:18692–18698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello L, Campadello P, Barp A, Fanin M, Semplicini C, et al. 2016. Functional changes in Becker muscular dystrophy: implications for clinical trials in dystrophinopathies. Sci Rep. 6:32439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 57:289–300. [Google Scholar]
- Bhattarai KK, Li Q, Liu Y, Dinesh-Kumar SP, Kaloshian I. 2007. Mi-1-mediated pest resistance requires Hsp90 and Sgt1. Plant Physiol. 144:312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieri S, Mauch S, Shen Q-H, Peart J, Devoto A, et al. 2004. RAR1 positively controls steady state levels of barley MLA resistance proteins and enables sufficient MLA6 accumulation for effective resistance. Plant Cell. 16:3480–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boter M, Amigues B, Peart J, Breuer C, Kadota Y, et al. 2007. Structural and functional analysis of SGT1 reveals that its interaction with HSP90 is required for the accumulation of Rx, an R protein involved in plant immunity. Plant Cell. 19:3791–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldo RA, Nettleton D, Wise RP. 2004. Interaction-dependent gene expression in Mla-specified response to barley powdery mildew. Plant Cell. 16:2514–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari S, Moore J, Chen C, Webb D, Periyannan S, et al. 2016. Cytosolic activation of cell death and stem rust resistance by cereal MLA-family CC–NLR proteins. Proc Natl Acad Sci USA. 113:10204–10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Yu D, Jiao J, Jing S, Schulze-Lefert P, et al. 2013. Barley MLA immune receptors directly interfere with antagonistically acting transcription factors to initiate disease resistance signaling. Plant Cell. 25:1158–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowda-Reddy RV, Palmer N, Edme S, Sarath G, Kovacs F, et al. 2019. A two-amino acid difference in the coat protein of satellite panicum mosaic virus isolates is responsible for differential synergistic interactions with panicum mosaic virus. Mol Plant Microbe Interact. 32:479–490. [DOI] [PubMed] [Google Scholar]
- Cushnie TPT, Lamb AJ. 2011. Recent advances in understanding the antibacterial properties of flavonoids. Int J Antimicrob Agents. 38:99–107. [DOI] [PubMed] [Google Scholar]
- Draz IS, Esmail SM, Abou-Zeid MAE-H, Essa TAE-M. 2019. Powdery mildew susceptibility of spring wheat cultivars as a major constraint on grain yield. Ann Agric Sci. 64:39–45. [Google Scholar]
- Dunker AK, Silman I, Uversky VN, Sussman JL. 2008. Function and structure of inherently disordered proteins. Curr Opin Struct Biol. 18:756–764. [DOI] [PubMed] [Google Scholar]
- Ellingboe AH. 1972. Genetics and physiology of primary infection by Erysiphe graminis. Phytopathology. 62:401–406. [Google Scholar]
- Fu D-Q, Ghabrial S, Kachroo A. 2009. GmRAR1 and GmSGT1 are required for basal, R gene-mediated and systemic acquired resistance in soybean. Mol Plant Microbe Interact. 22:86–95. [DOI] [PubMed] [Google Scholar]
- Graves S, Piepho H-P, Selzer L, Dorai-Raj S. 2019. multcompView: visualizations of paired comparisons. R package version 0.1-8.
- Gray WM, Muskett PR, Chuang H-W, Parker JE. 2003. Arabidopsis SGT1b is required for SCF-TIR-1-mediated auxin response. Plant Cell. 15:1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halterman D, Zhou F, Wei F, Wise RP, Schulze-Lefert P. 2001. The MLA6 coiled-coil, NBS-LRR protein confers AvrMla6-dependent resistance specificity to Blumeria graminis f. sp. hordei in barley and wheat. Plant J. 25:335–348. [DOI] [PubMed] [Google Scholar]
- Halterman DA, Wise RP. 2004. A single-amino acid substitution in the sixth leucine-rich repeat of barley MLA6 and MLA13 alleviates dependence on RAR1 for disease resistance signaling. Plant J. 38:215–226. [DOI] [PubMed] [Google Scholar]
- Hein I, Barciszewska-Pacak M, Hrubikova K, Williamson S, Dinesen M, et al. 2005. Virus-induced gene silencing-based functional characterization of genes associated with powdery mildew resistance in barley. Plant Physiol. 138:2155–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoser R, Żurczak M, Lichocka M, Zuzga S, Dadlez M, et al. 2013. Nucleocytoplasmic partitioning of tobacco N receptor is modulated by SGT1. New Phytol. 200:158–171. [DOI] [PubMed] [Google Scholar]
- Huang L, Brooks S, Li W, Fellers J, Nelson JC, et al. 2009. Evolution of new disease specificity at a simple resistance locus in a crop-weed complex: reconstitution of the Lr21 gene in wheat. Genetics. 182:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt M, Banerjee S, Surana P, Liu M, Fuerst G, et al. 2019. Small RNA discovery in the interaction between barley and the powdery mildew pathogen. BMC Genomics. 20:610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaswal R, Kiran K, Rajarammohan S, Dubey H, Singh PK, et al. 2020. Effector biology of biotrophic plant fungal pathogens: Current advances and future prospects. Microb Res. 241:126567. [DOI] [PubMed] [Google Scholar]
- Jordan T, Seeholzer S, Schwizer S, Töller A, Somssich IE, et al. 2011. The wheat Mla homologue TmMla1 exhibits an evolutionarily conserved function against powdery mildew in both wheat and barley. Plant J. 65:610–621. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Skowyra D, Elledge SJ, Harper JW, Hieter P. 1999. SGT1 encodes an essential component of the yeast kinetochore assembly pathway and a novel subunit of the SCF ubiquitin ligase complex. Mol Cell. 4:21–33. [DOI] [PubMed] [Google Scholar]
- Klingenberg H, Meinicke P. 2017. How to normalize metatranscriptomic count data for differential expression analysis. PeerJ. 5:e3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolster P, Munk L, Stolen O, Lohde J. 1986. Near-isogenic barley lines with genes for resistance to powdery mildew. Crop Sci. 26:903–907. [Google Scholar]
- Kourelis J, van der Hoorn RAL. 2018. Defended to the nines: 25 years of resistance gene cloning identifies nine mechanisms for R protein function. Plant Cell. 30:285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krattinger SG, Keller B. 2016. Molecular genetics and evolution of disease resistance in cereals. New Phytol. 212:320–332. [DOI] [PubMed] [Google Scholar]
- Kwaaitaal M, Nielsen ME, Böhlenius H, Thordal-Christensen H. 2017. The plant membrane surrounding powdery mildew haustoria shares properties with the endoplasmic reticulum membrane. J Exp Bot. 68:5731–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langin G, Gouguet P, Üstün S. 2020. Microbial effector proteins—a journey through the proteolytic landscape. Trends Microbiol. 28:523–535. [DOI] [PubMed] [Google Scholar]
- Lehmann S, Serrano M, L’Haridon F, Tjamos SE, Metraux J-P. 2015. Reactive oxygen species and plant resistance to fungal pathogens. Phytochemistry. 112:54–62. [DOI] [PubMed] [Google Scholar]
- Lenth RV. 2016. Least-squares means: the R package lsmeans. J Stat Soft. 69:33. [Google Scholar]
- Li C, Li N, Huang R, Chen C, Guo J, et al. 2020. A single nucleotide substitution at the 3′-end of SBPase gene involved in Calvin cycle severely affects plant growth and grain yield in rice. BMC Plant Biol. 20. Article number 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics. 25:1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Presti L, Lanver D, Schweizer G, Tanaka S, Liang L, et al. 2015. Fungal effectors and plant susceptibility. Annu Rev Plant Biol. 66:513–545. [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Kracher B, Saur IML, Bauer S, Ellwood SR, et al. 2016. Allelic barley MLA immune receptors recognize sequence-unrelated avirulence effectors of the powdery mildew pathogen. Proc Natl Acad Sci USA. 113:E6486–E6495, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa T, Kracher B, Vernaldi S, Ver Loren van Themaat E, Schulze-Lefert P. 2012. Conservation of NLR-triggered immunity across plant lineages. Proc Natl Acad Sci USA. 109:20119–20123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mago R, Zhang P, Vautrin S, Šimková H, Bansal U, et al. 2015. The wheat Sr50 gene reveals rich diversity at a cereal disease resistance locus. Nat Plants. 1:15186. [DOI] [PubMed] [Google Scholar]
- Martins T, Maia AF, Steffensen S, Sunkel CE. 2009. Sgt1, a co-chaperone of Hsp90 stabilizes Polo and is required for centrosome organization. EMBO J. 28:234–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascher M, Gundlach H, Himmelbach A, Beier S, Twardziok SO, et al. 2017. A chromosome conformation capture ordered sequence of the barley genome. Nature. 544:427–433. [DOI] [PubMed] [Google Scholar]
- Mascher M, Richmond TA, Gerhardt DJ, Himmelbach A, Clissold L, et al. 2013. Barley whole exome capture: a tool for genomic research in the genus Hordeum and beyond. Plant J. 76:494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer KF, Waugh R, Brown JW, Schulman A, Langridge P, et al. 2012. A physical, genetic and functional sequence assembly of the barley genome. Nature. 491:711–716. [DOI] [PubMed] [Google Scholar]
- McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, et al. 2010. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20:1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Moscou M, Wise R. 2009. Blufensin1 negatively impacts basal defense in response to barley powdery mildew. Plant Physiol. 149:271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel JB, Dangl JL. 1999. Suppressors of the Arabidopsis lsd5 cell death mutation identify genes involved in regulating disease resistance responses. Genetics. 151:305–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscou MJ, Lauter N, Caldo RA, Nettleton D, Wise RP. 2011. Quantitative and temporal definition of the Mla transcriptional regulon during barley–powdery mildew interactions. Mol Plant Microbe Interact. 24:694–705. [DOI] [PubMed] [Google Scholar]
- Moscou MJ, Lorang J, Immaculada H-P, Tinker N, Leonard JM, et al. 2018. Map-based cloning of Pc2/Vb: a doubled-edge sword of immunity. Plant and Animal Genome XXVI, San Diego, CA. https://pag.confex.com/pag/xxvi/meetingapp.cgi/Paper/28867.
- Moseman JG. 1972. Isogenic barley lines for reaction to Erysiphe graminis F. sp. hordei. Crop Sci. 12:681–682. [Google Scholar]
- Murray G, Brennan J. 2010. Estimating disease losses to the Australian barley industry. Aust Plant Pathol. 39:85–96. [Google Scholar]
- Noël LD, Cagna G, Stuttmann J, Wirthmüller L, Betsuyaku S, et al. 2007. Interaction between SGT1 and cytosolic/nuclear HSC70 chaperones regulates Arabidopsis immune responses. Plant Cell. 19:4061–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peart JR, Lu R, Sadanandom A, Malcuit I, Moffett P, et al. 2002. Ubiquitin ligase-associated protein SGT1 is required for host and nonhost disease resistance in plants. Proc Natl Acad Sci USA. 99:10865–10869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periyannan S, Moore J, Ayliffe M, Bansal U, Wang X, et al. 2013. The gene Sr33, an ortholog of barley Mla genes, encodes resistance to wheat stem rust race Ug99. Science. 341:786–788. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2019. A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Redkar A, Hoser R, Schilling L, Zechmann B, Krzymowska M, et al. 2015. A secreted effector protein of Ustilago maydis guides maize leaf cells to form tumors. Plant Cell. 27:1332–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridout CJ, Skamnioti P, Porritt O, Sacristan S, Jones JDG, et al. 2006. Multiple avirulence paralogues in cereal powdery mildew fungi may contribute to parasite fitness and defeat of plant resistance. Plant Cell. 18:2402–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur IML, Bauer S, Kracher B, Lu X, Franzeskakis L, et al. 2019. Multiple pairs of allelic MLA immune receptor-powdery mildew AVRA effectors argue for a direct recognition mechanism. eLife. 8:e44471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield SR, Huang L, Brandt AS, Gill BS. 2005. Development of a virus-induced gene-silencing system for hexaploid wheat and its use in functional analysis of the Lr21-mediated leaf rust resistance pathway. Plant Physiol. 138:2165–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeholzer S, Tsuchimatsu T, Jordan T, Bieri S, Pajonk S, et al. 2010. Diversity at the Mla powdery mildew resistance locus from cultivated barley reveals sites of positive selection. Mol Plant Microbe Interact. 23:497–509. [DOI] [PubMed] [Google Scholar]
- Shen Q-H, Saijo Y, Mauch S, Biskup C, Bieri S, et al. 2007. Nuclear activity of MLA immune receptors links isolate-specific and basal disease-resistance responses. Science. 315:1098–1103. [DOI] [PubMed] [Google Scholar]
- Shen Q-H, Zhou F, Bieri S, Haizel T, Shirasu K, et al. 2003. Recognition specificity and RAR1/SGT1 dependence in barley Mla disease resistance genes to the powdery mildew fungus. Plant Cell. 15:732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasu K. 2009. The HSP90-SGT1 chaperone complex for NLR immune sensors. Annu Rev Plant Biol. 60:139–164. [DOI] [PubMed] [Google Scholar]
- Shirasu K, Lahaye T, Tan M-W, Zhou F, Azevedo C, et al. 1999. A novel class of eukaryotic zinc-binding proteins is required for disease resistance signaling in barley and development in C. elegans. Cell. 99:355–366. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zhu Y-X, Balint-Kurti PJ, Wang G-F. 2020. Fine-tuning immunity: players and regulators for plant NLRs. Trends Plant Sci. 25:695–713. [DOI] [PubMed] [Google Scholar]
- Taube M, Pieńkowska JR, Jarmołowski A, Kozak M, Silman I. 2014. Low-resolution structure of the full-length barley (Hordeum vulgare) SGT1 protein in solution, obtained using small-angle X-ray scattering. PLoS One. 9:e93313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thao NP, Chen L, Nakashima A, Hara S-I, Umemura K, et al. 2007. RAR1 and HSP90 form a complex with Rac/Rop GTPase and function in innate-immune responses in rice. Plant Cell. 19:4035–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torp J, Jørgensen JH. 1986. Modification of barley powdery mildew resistance gene Ml-a12 by induced mutation. Can J Genet Cytol. 28:725–731. [Google Scholar]
- Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. 2007. A single mutation in Chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 3:e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wersch S, Tian L, Hoy R, Li X. 2020. Plant NLRs: the whistleblowers of plant immunity. Plant Commun. 1:100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G-F, Fan R, Wang X, Wang D, Zhang X. 2015. TaRAR1 and TaSGT1 associate with TaHSP90 to function in bread wheat (Triticum aestivum L.) seedling growth and stripe rust resistance. Plant Mol Biol. 87:577–589. [DOI] [PubMed] [Google Scholar]
- Wei F, Wing RA, Wise RP. 2002. Genome dynamics and evolution of the Mla (powdery mildew) resistance locus in barley. Plant Cell. 14:1903–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R, Ellingboe A. 1985. Fine structure and instability of the MI-a locus in barley. Genetics. 111:113–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RP, Ellingboe AH. 1983. Infection kinetics of Erysiphe graminis f. sp. hordei on barley with different alleles at the Ml-a locus. Phytopathology. 73:1220–1222. [Google Scholar]
- Xi L, Moscou M, Meng Y, Xu W, Caldo R, et al. 2009. Transcript-based cloning of RRP46, a regulator of rRNA processing and R gene-independent cell death in barley-powdery mildew interactions. Plant Cell. 21:3280–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Meng Y, Wise RP. 2014. Mla- and Rom1-mediated control of microRNA398 and chloroplast copper/zinc superoxide dismutase regulates cell death in response to the barley powdery mildew fungus. New Phytol. 201:1396–1412. [DOI] [PubMed] [Google Scholar]
- Yang J, Yan R, Roy A, Xu D, Poisson J, et al. 2015. The I-TASSER Suite: protein structure and function prediction. Nat Methods. 12:7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara T, Spalding EP. 2020. Switching the direction of stem gravitropism by altering two amino acids in AtLAZY1. Plant Physiol. 182:1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Xian L, Xue H, Yu W, Rufian JS, et al. 2020. A bacterial effector protein prevents MAPK-mediated phosphorylation of SGT1 to suppress plant immunity. bioRxiv: 641241. [DOI] [PMC free article] [PubMed]
- Yu G-X, Braun E, Wise RP. 2001. Rds and Rih mediate hypersensitive cell death independent of gene-for-gene resistance to the oat crown rust pathogen Puccinia coronata f. sp. avenae. Mol Plant Microbe Interact. 14:1376–1383. [DOI] [PubMed] [Google Scholar]
- Zhang M, Botër M, Li K, Kadota Y, Panaretou B, et al. 2008. Structural and functional coupling of Hsp90- and Sgt1-centred multi-protein complexes. EMBO J. 27:2789–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XC, Millet YA, Cheng Z, Bush J, Ausubel FM. 2015. Jasmonate signalling in Arabidopsis involves SGT1b-HSP70-HSP90 chaperone complexes. Nat Plants. 1:15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains and plasmids are available upon request. Supplementary files available at FigShare: https://doi.org/10.25386/genetics.12935036. Supplementary Figure S1 contains categorization of Bgh spore growth stages. Supplementary Figure S2 contains phenotypes of m9450 and m9455 at 3, 5, and 7 DPI. Supplementary Figure S3 phenotypes of crosses between m11526 and CI 16147 or CI 16155. Supplementary Table S1 contains molecular markers used for fine mapping of Rar3. Supplementary Table S2 contains a summary of fast-neutron mutants, phenotypes, and genes identified by them. Supplementary Table S3 contains morphology data of mutant and wild-type barley under normal growing conditions. Supplementary Table S4 contains measurements and analysis of mla6, bln1, and rar3 mutants from seed-to-seed during development. Supplementary Table S5 contains morphology data of mutant and wild-type barley under stressed growing conditions. Supplementary Table S6 contains F2 infection-type results from crosses among m11526 and other lines. Supplementary Table S7 contains expected ratios of F2 from cross m11526 (Mla6, rar3) and CI 16147 (Mla7, Rar3). Supplementary Table S8 contains acronyms used.
Illumina sequence data to construct the CI 16151 reference genome are available in NCBI’s Gene Expression Omnibus (GEO) under the accession number PRJNA630064. Illumina sequence data supporting the Exome capture is available under GEO accession number PRJNA645935. HvSgt1 (HORVU3Hr1G055920) from CI 16151 and rar3-m11526 are available under GenBank Accessions AF439974 and MT787218, respectively. RNA-Seq datasets are available in NCBI-GEO under the accession number GSE101304 (https://www.ncbi.nlm.nih.gov/gds/? term=GSE101304).