Abstract
Genetic approaches in the fruit fly, Drosophila melanogaster, have led to a major triumph in the field of sensory biology—the discovery of multiple large families of sensory receptors and channels. Some of these families, such as transient receptor potential channels, are conserved from animals ranging from worms to humans, while others, such as “gustatory receptors,” “olfactory receptors,” and “ionotropic receptors,” are restricted to invertebrates. Prior to the identification of sensory receptors in flies, it was widely assumed that these proteins function in just one modality such as vision, smell, taste, hearing, and somatosensation, which includes thermosensation, light, and noxious mechanical touch. By employing a vast combination of genetic, behavioral, electrophysiological, and other approaches in flies, a major concept to emerge is that many sensory receptors are multitaskers. The earliest example of this idea was the discovery that individual transient receptor potential channels function in multiple senses. It is now clear that multitasking is exhibited by other large receptor families including gustatory receptors, ionotropic receptors, epithelial Na+ channels (also referred to as Pickpockets), and even opsins, which were formerly thought to function exclusively as light sensors. Genetic characterizations of these Drosophila receptors and the neurons that express them also reveal the mechanisms through which flies can accurately differentiate between different stimuli even when they activate the same receptor, as well as mechanisms of adaptation, amplification, and sensory integration. The insights gleaned from studies in flies have been highly influential in directing investigations in many other animal models.
Keywords: FlyBook, nervous system and behavior, Drosophila, phototransduction, TRP channels, rhodopsin, taste, gustation, gustatory receptor, ENaC, TMC, smell, pheromones olfaction, olfactory receptor, somatosensation, touch, temperature sensation, hearing
Introduction
A few years ago, on a tour of the “East Baltimore Community School,” I spotted a bulletin board outside of a kindergarten classroom reviewing the five classical senses defined by Aristotle (2015) around 350 bce (Figure 1). For these kindergarten students and many of us, our education about sight, taste, smell, hearing, and touch was our earliest introduction to neuroscience.
Figure 1.

A poster describing the five senses to kindergarten students. The poster was displayed outside a kindergarten classroom in the East Baltimore Community School in 2011.
The mechanisms that we and other animals use to sense the world are among the most fundamental and fascinating questions in neuroscience. On the most basic level, even kindergarten students are curious about the senses. How is it possible that the visual system has the sensitivity to detect a dim star in the night sky and not be blinded by brilliant images under a bright summer sky? The dim star and the summer sky represent differences in light intensities of more than a billion-fold. Our auditory system is so exquisitely sensitive that we can detect miniscule sounds that cause vibrations in our eardrum in the range of picometers (Dalhoff et al. 2007). How do the olfactory and gustatory systems detect enormous diversities of volatile and non-volatile chemicals (Bushdid et al. 2014) and allow animals to discriminate safe and dangerous stimuli? Touch is so sensitive that we can detect vibrations with displacements in the nanometer range (Johnson 2001).
In addition to the five classical senses defined by Aristotle (2015), there are other senses. However, it is tricky to provide an exact number, since this depends on how the various senses are grouped. Touch is only one of several senses and is used to receive information from the body surface. There is also nociception, which includes the detection of aversive chemical, mechanical, and thermal stimuli (Julius 2013). These various body surface sensations may be collectively referred to as somatosensation. The sense of balance and spatial orientation can be referred to as the vestibular sense. Proprioception—the sense of the position of one’s own body parts—is yet another sense. Moreover, not all animals have precisely the same repertoire of senses. Unlike humans, several species of fish and insects detect electric fields, and many birds and insects monitor the earth’s magnetic field, which aids them during navigation (Clarke et al. 2013, 2015; Alerstam and Backman 2018; Mouritsen 2018; Reppert and de Roode 2018).
Major goals in sensory biology have been to define the sensory receptor cells and their intrinsic cell surfaces proteins that detect external stimuli. Do the receptor proteins initiate multicomponent pathways that promote signal amplification, or are they functioning as receptors and cation channels [ionotropic receptors (IRs)] so that they serve both to detect external stimuli and to activate the sensory receptor cells?
Multiple model organisms have been exploited to answer these fundamental questions, including the vinegar fly, Drosophila melanogaster, which is more commonly referred to as the fruit fly. There are several cogent reasons for focusing on Drosophila. This model organism is small, has a short 10-day generation time at 25°C, and can be maintained on simple food in small vials enabling large numbers of individuals to be maintained in the laboratory. Plus, flies offer an unparalleled combination of molecular genetic tools, behavioral assays, and electrophysiological approaches to identify and dissect the roles of sensory receptor cells and proteins essential for their senses.
Flies have provided insights into sensory reception in humans, since they respond to a similar set of external cues as we do. Indeed, the transient receptor potential (TRP) channel protein, which was originally identified through work on fly phototransduction (Cosens and Manning 1969; Montell and Rubin 1989), is the founding member of a large family of proteins in humans and other mammals, which function in taste, temperature, and light sensation (Venkatachalam and Montell 2007).
Drosophila also serves as a model for other Dipteran insects including mosquitoes that spread diseases such as malaria, dengue, and yellow fever, which affect hundreds of millions of people annually, and kill nearly a million people each year (Benelli and Mehlhorn 2016; Ferguson 2018; Fernandes et al. 2018). Female mosquitoes employ multiple senses to locate the image, smell, taste, and skin temperature of human hosts so that they can take a blood meal (Montell and Zwiebel 2016). Thus, unraveling the identities of sensory receptor proteins that are exclusive to insects is also of great value. It can pinpoint new protein targets to conduct screens to identify chemicals that control mosquitoes but do not harm humans.
In this review, I focus on the state of our current knowledge of the sensory receptor cells and proteins that are critical for each of the senses in Drosophila. Prior to the discoveries described here, the prevailing view was that detection of each type of external stimuli, such as light, olfactory, and gustatory cues, depended on distinct receptors. However, there are a number of startling surprises, such as the discovery that the classical light receptors, rhodopsins, function in temperature sensation and hearing and the findings that “gustatory receptors” (GRs) also contribute to olfaction, temperature, and light sensation. Another unexpected realization is that taste and smell each depend on not just one or two but multiple types of chemosensory receptors. The discovery that the primary sensory neurons are not restricted to the peripheral nervous system was also unanticipated. An example is that neurons in the brain also express some of the same receptor proteins important in vision, temperature sensation, and taste and allow the brain to directly sense light, temperature changes, and sugars. These revelations, and others, are highlighted in the current review.
Light receptors
Animals, such as flies, sense light for multiple purposes. Photon detection provides flies the ability to see images, set circadian rhythms, and either move toward or away from light depending on the wavelength and developmental stage. Due to the many roles of light sensation, there are multiple types of receptors, and their cellular distributions are not limited to photoreceptor cells in the eyes.
A genetic triumph in sensory biology: clarifying the entire phototransduction cascade in the compound eye
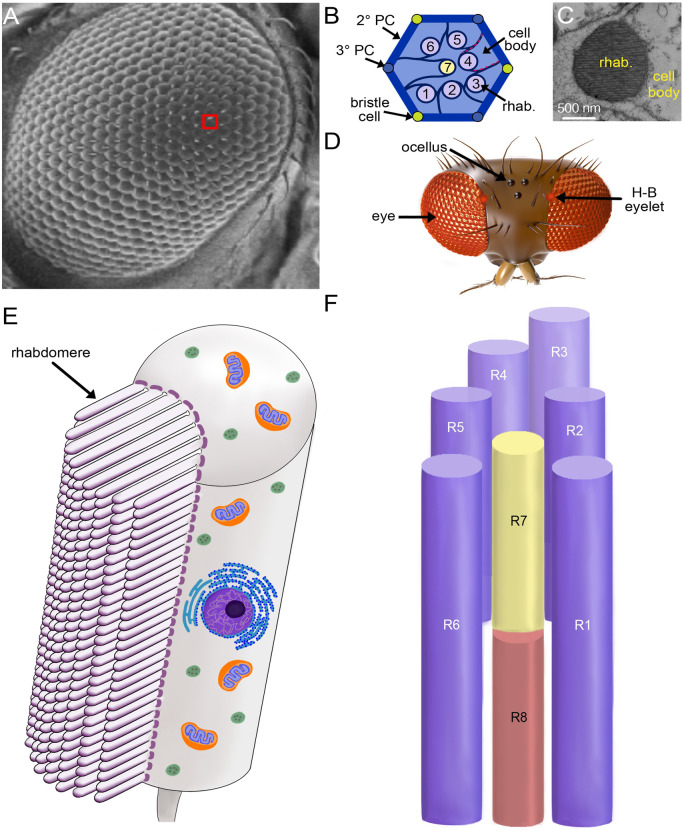
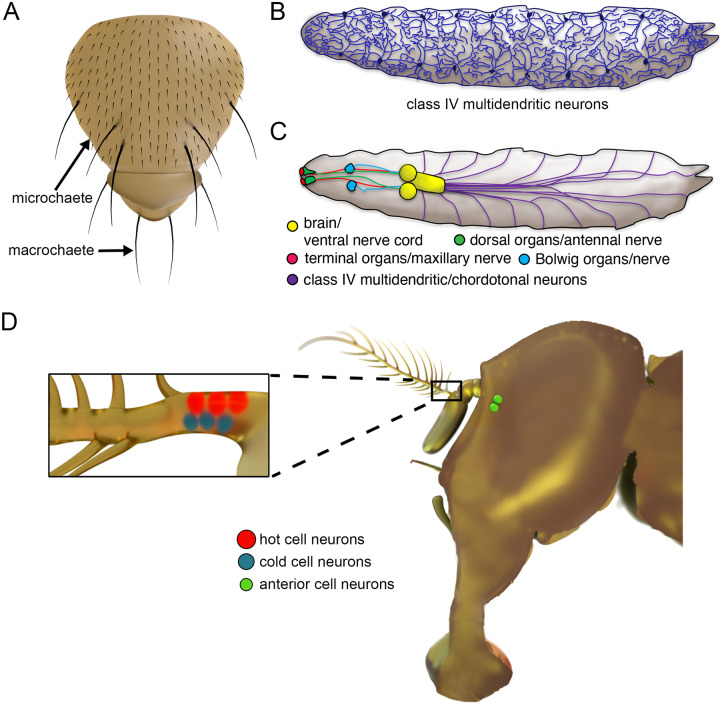
Drosophila is endowed with multiple types of eyes. In adults, image formation depends on the compound eyes, which are comprised of ∼800 repetitive units, ommatidia (Figure 2A). Each ommatidium contains 20 cells including 8 photoreceptor cells and several other cell types (Figure 2B). The light-sensing portions of the photoreceptor cells are large domains with 30,000–50,000 microvilli called rhabdomeres, which are the fly functional equivalent of human rod and cone outer segments (Figure 2, C and E). Six of the eight photoreceptor cells (R1-R6) occupy the periphery of the ommatidia and extend the full depth of the retina (Figure 2F). The R7 and R8 cells are restricted to the distal (top) and proximal (bottom) portions of each ommatidia (Figure 2F). They contain centrally positioned rhabdomeres, which are slightly smaller than those in the R1–6 cells and are stacked one on top of one another. Adult flies are also endowed with three much smaller light sensitive organs (ocelli) at the top of their head (Figure 2D). Ocelli appear to provide information about light levels (Krapp 2009). The main light-sensing organ in larvae is the Bolwig organ, which is the progenitor of a small group of photoreceptor cells in adults, the Hofbauer–Buchner (H-B) eyelet, which is situated between the retina and optic lobes (Figure 2D). The H-B eyelet appears to function in modulating circadian rhythms (Helfrich-Förster et al. 2002).
Figure 2.
Light-sensitive organs and photoreceptor cells. (A) Scanning electron micrograph of an adult compound eye. The red square outlines one of the ∼800 ommatidia. (B) Cartoon of a cross-sectional view through the distal region of an ommatidium. Shown are seven photoreceptor cells including the six outer photoreceptor cells, R1–6 (1–6), and the R7 photoreceptor (7). The circles represent the rhabdomeres. The red dashes outline the cell body of one photoreceptor cell (R4). 2° PC, secondary pigment cell; 3° PC, tertiary pigment cell; rhab., rhabdomere. (C) Transmission electron micrograph of a photoreceptor cell. (D) Cartoon of a top view of the head showing light-sensing organs. H-B, Hofbauer–Buchner. (E) Longitudinal representation of a single photoreceptor cell. The microvilli comprising the rhabdomere are indicated. Shown are far fewer microvilli than the 30,000–50,000 that normally comprise a rhabdomere. (F) Longitudinal view of the 8 rhabdomeres in a single ommatidium. The R1–6 cells extend the full depth of the retinal while the R7 and R8 cells occupy the distal and proximal regions of each ommatidium, respectively.
The Drosophila visual cascade has been studied for decades and is the best characterized of all of the sensory signaling cascades in flies (Pak 2010; Montell 2012; Hardie and Juusola 2015). As in rods and cones, light reception occurs through stimulation of the classical G-protein coupled receptor (GPCR) called rhodopsin. Rhodopsin consists of two components: an opsin protein with seven transmembrane domains (TMDs), and a vitamin A derivative (3-hydroxy 11-cis-retinal), which is covalently bound to a lysine in the seventh transmembrane domain (Figure 3A). Light induces a cis to trans isomerization of the retinal, which releases an inhibitory constraint, leading to activation of rhodopsin (Figure 3B). Unlike the visual pigments in rods and cones, retinal does not usually dissociate from fly opsins following light excitation. Rather, the conversion from the all-trans back to the cis conformation is also light dependent (Pak et al. 2012). Rhodopsin is highly concentrated in the rhabdomeres, which provides a massive plasma membrane surface to pack in high levels of the light receptor, thereby enabling efficient photon capture.
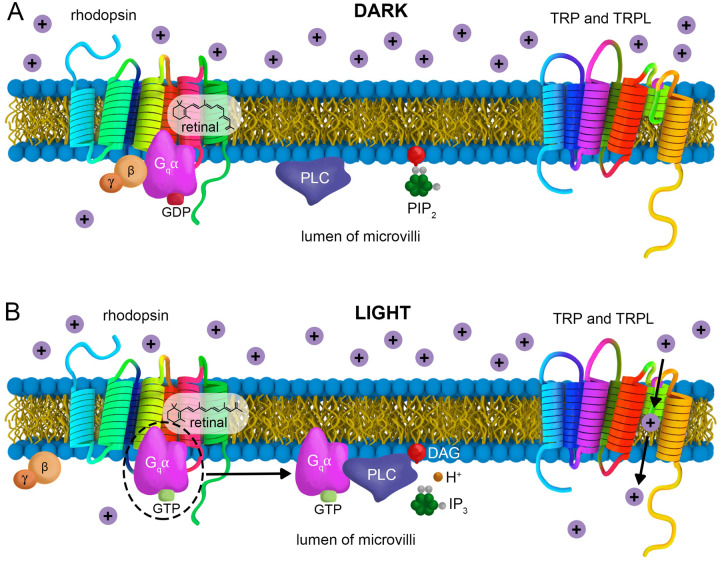
Figure 3.
Phototransduction cascade. (A) Inactive (dark) state of the phototransduction cascade. Rhodopsin associates with 3-hydroxy 11-cis-retinal. The Gqα is bound to GDP and associates with Gβγ. The TRP and TRPL channels are in the closed state. (B) Light-activated phototransduction cascade. Light induces the isomerization of 3-hydroxy 11-cis-retinal to 3-hydroxy all trans-retinal. This activates rhodopsin, causing an exchange of GTP for the GDP that was bound to the Gqα, and dissociation of the Gβγ. The Gqα-GTP is then released from rhodopsin, PLCβ is then activated , leading to hydrolysis of PIP2 to create DAG, IP3, and H+. The cascade culminates with activation of TRP and TRPL and cation influx. The purple circles with a “+” represent cations (Na+ or Ca2+).
Flies encode seven rhodopsins, five of which are expressed in the compound eyes in non-overlapping subsets of photoreceptor cells. Rh1 absorbs light maximally in the blue range and is the most abundant rhodopsin in the eye since it is expressed in the R1-6 photoreceptor cells (O’Tousa et al. 1985; Zuker et al. 1985, 1988). Two ultraviolet (UV)-sensitive rhodopsins, Rh3 and Rh4, are restricted to ∼30% and 70% of R7 cells, respectively (Montell et al. 1987; Zuker et al. 1987; Feiler et al. 1992). Similarly, ∼30% and ∼70% of R8 cells express Rh5 and Rh6, which maximally absorb violet/blue and green light, respectively (Chou et al. 1996; Huber et al. 1997; Papatsenko et al. 1997; Salcedo et al. 1999). With some minor exceptions at the dorsal rim of the eyes, ommatidia that express Rh3 in R7 cells are paired with Rh5 in R8 cells, while Rh4 and Rh6 are coordinately expressed in other ommatidia (Fortini and Rubin 1990; Papatsenko et al. 1997; Chou et al. 1999). A violet rhodopsin (Rh2) is present in ocelli but not the compound eyes (Mismer et al. 1988; Pollock and Benzer 1988; Zuker et al. 1988). The Bolwig organ and H-B eyelet express Rh5 and Rh6 (Yasuyama and Meinertzhagen 1999; Malpel et al. 2002; Sprecher et al. 2007). An additional rhodopsin (Rh7) is a violet/blue light receptor in the brain (Ni et al. 2017) and in multidendritic neurons (Lazopulo et al. 2019). Rh7 is also reported to be expressed at low levels in R8 photoreceptor cells, but it does not appear to promote phototransduction but rather to decrease sensitivity to bright light in dark-adapted flies through a mechanism that remains to be clarified (Senthilan and Helfrich-Förster 2016; Senthilan et al. 2019).
The signaling cascade that is critical for fly vision has been revealed through genetic approaches beginning with pioneering work in the Benzer and Pak laboratories (Hotta and Benzer 1969, 1970; Pak et al. 1969, 1970; Deland and Pak 1973). Mutations in the gene (ninaE) encoding Rh1 not only cause a large reduction in the photoresponse, but this discovery provided the first genetic link in any animal between alterations in rhodopsin expression and retinal degeneration (Ostroy et al. 1974; Scavarda et al. 1983; O’Tousa et al. 1985, 1989; Zuker et al. 1985).
Photo-stimulation of rhodopsins triggers a multistep amplification cascade, which is critical for vision. Light activation of rhodopsin causes the exchange of GDP for GTP on the Gqα subunit (Gα49B) of the trimeric G-protein (Figure 3) (Scott et al. 1995; Gu et al. 2020). Activated Gqα, in turn, stimulates an enzyme phospholipase Cβ (PLCβ), which hydrolyzes the membrane lipid, phosphatidylinositol 4,5-bisphosphate (PIP2) (Inoue et al. 1985). There are two genes encoding PLCβs: norpA and plc21C. Mutations disrupting norpA dramatically impair vision and provided the earliest genetic evidence in any animal that a rhodopsin is coupled to a phosphoinositide signaling system (Inoue et al. 1985; Bloomquist et al. 1988).
A very small remaining light response in null norpA mutants is due to coupling of at least three rhodopsins (Rh1, Rh5, and Rh6) to Gq, which in turn activate PLC21C (Szular et al. 2012; Ogueta et al. 2018). The PLC21C-dependent pathway appears to operate on a much a slower time scale than the canonical NORPA pathway and participates in the synchronization of circadian cycles in response to medium light intensities (Szular et al. 2012; Ogueta et al. 2018, 2020). The NORPA pathway functions in synchronizing the clock to low levels of light (Saint-Charles et al. 2016). The cation influx channel(s) that are activated following PLC21C stimulation in the compound eyes have not been described, but they might be the same as those functioning downstream of NORPA.
The NORPA-dependent phototransduction cascade culminates with the activation of the TRP cation channel (Montell and Rubin 1989; Hardie and Minke 1992). TRP proteins, which are conserved from worms to humans, share the common features of six TMDs and conduct cations (Wes et al. 1995; Zhu et al. 1995; Venkatachalam and Montell 2007). We now know that TRP channels are critical for many sensory cascades throughout animal phylogeny (Venkatachalam and Montell 2007). In addition to Drosophila TRP, a related cation channel, TRP-Like (TRPL) also contributes to phototransduction (Phillips et al. 1992; Niemeyer et al. 1996). TRP and TRPL are defining members of the TRPC subfamily. Members of four other subfamilies of TRPs (TRPV, TRPA, TRPM, and TRPN) share clear primary amino acid homology to the TRPCs and are collectively referred to as Group 1 TRPs (Montell 2005) (Table 1). Two other subfamilies (TRPML and TRPP) comprise the Group 2 TRPs and include members that share only weak sequence similarity to the Group 1 TRPs (Montell 2005).
Table 1.
Two groups and seven subfamilies of TRP channels
| Group | Subfamily | Protein |
|---|---|---|
| 1 | TRPC | TRP |
| TRPL | ||
| TRPγ | ||
| 1 | TRPV | Nan |
| Iav | ||
| 1 | TRPA | TRPA1 |
| Pain | ||
| Pyx | ||
| WTRW | ||
| 1 | TRPM | TRPM |
| 1 | TRPN | NOMPC |
| 2 | TRPML | TRPML |
| 2 | TRPP | AMO (PKD2) |
| Brv1 | ||
| Brv2 | ||
| Brv3 |
The multi-component phototransduction cascade serves two critical functions—signal amplification and adaptation. Amplification arises due to sequential engagement of multiple (∼5) G-proteins with a single light-activated rhodopsin (Hardie et al. 2002). Each Gqα protein then activates PLCβ, resulting in opening of virtually all of the TRP and TRPL channels (∼25) in a single microvillus (Henderson et al. 2000). Thus, each of the ∼50,000 microvilli in the larger R1–6 cell, which are only ∼50 nm wide and ∼1 µm in length, defines the limit of the response to a photon. Adaptation is primarily a Ca2+-regulated process (Gu et al. 2005; Wang et al. 2005). Consequently, adaptation depends largely on TRP, which is modestly Ca2+ selective (∼40:1), while TRPL is a non-selective cation channel (Reuss et al. 1997; Xu et al. 1997; Wang et al. 2005; Liu, Wang, et al. 2007).
The mechanism of activation of TRP and TRPL is not resolved. However, one model is quite intriguing as it posits that the channels are mechanically gated. Hydrolysis of PIP2 leads to production of diacylglycerol (DAG), inositol 1,4,5-trisphosphate (IP3), and H+ (Figure 3B). Therefore, in principle, gating of the channels could be through a ligand-binding mechanism involving any of these products, metabolites of DAG and IP3, reduction in PIP2, or any combination of these possibilities. Depletion of PIP2, in combination with local acidification, appears to activate the channels (Huang et al. 2010). What is remarkable is the mechanism through which a reduction of PIP2 is proposed to gate TRP and TRPL. Light-induced hydrolysis of PIP2 and release of IP3 cause a conformational change in the rhabdomeral membrane, since the DAG that remains in the membrane is smaller than PIP2 (Figure 3B). This might then lead to opening of the TRP and TRPL channels through a mechanical gating mechanism (Hardie and Franze 2012). This possible mode of activation is remarkable since the stimulus that initiates the cascade is light! Another open question is whether the biophysical features of TRP channels are regulated by single transmembrane domain auxiliary subunits as is the case for the structurally related voltage-gated K+ channels (Abbott 2016). Excellent candidates are INAF-B and INAF-C, which are single transmembrane proteins that bind to TRP, co-localize with the channels in the rhabdomeres and are mutually required on each other for their stability in the rhabdomeres (Chen and Montell 2020).
Phototransduction in adult ocelli and in the Bolwig organ in larvae appears to be virtually identical to the cascade in the compound eye. The same signaling components are expressed in the photoreceptor cells in these various types of light-sensing organs (Mishra et al. 2016). The main distinction is that Rh2 is the opsin expressed in the ∼90 photoreceptor cells in each of the three ocelli (Feiler et al. 1988; Mismer et al. 1988; Pollock and Benzer 1988), while Rh5 and Rh6 are detected in 4 and 8 distinct photoreceptor cells in the Bolwig organ, respectively (Sprecher et al. 2007; Mishra et al. 2013).
Relationship between fly and mammalian phototransduction
The phototransduction cascades in the compound eye, ocelli and Bolwig organ are distinct from the cascades in mammalian rods and cones. In these latter photoreceptor cells, the second messenger is cGMP, and phototransduction culminates with closing of cGMP-gated cation channels (Yau and Nakatani 1985; Haynes et al. 1986; Kaupp et al. 1989). Thus, for many years, it was thought that fly and mammalian phototransductions were vastly different. However, it turns out that a small subset of retinal ganglion cells in the mammalian retina are photosensitive and employ a phototransduction cascade that is nearly identical to the phototransduction cascade in the fly compound eye (Provencio et al. 1998; Berson et al. 2002; Panda et al. 2005; Qiu et al. 2005; Xue et al. 2011). Light sensation through these intrinsically photosensitive retinal ganglion cells (ipRGCs) contributes to photo-entrainment of circadian rhythms, pupillary constriction to light, and indirectly impact certain aspects of vision such as adaptation (Berson et al. 2002; Panda et al. 2002; Ruby et al. 2002; Hattar et al. 2003; Lucas et al. 2003; Prigge et al. 2016).
The discovery of the ipRGCs highlights the critical importance of using Drosophila as a powerful genetic animal model for basic research in sensory signaling. Once the ipRGCs were uncovered, this entire mammalian cascade was defined quickly in part through the insights provided by the genetic approaches focusing on fly phototransduction. The elucidation of the phototransduction cascade in Drosophila represents one of the many triumphs of fly genetics and is the first sensory cascade revealed through genetic approaches.
UV-light detection independent of rhodopsins
Exposure to UV light inhibits feeding in many insects including Drosophila (Mazza et al. 1999, 2002; Du et al. 2016) and is also aversive for egg laying (oviposition) (Zhu et al. 2014; Guntur et al. 2017). Avoidance of bright UV light is not dependent on phototransduction in the eyes. Rather, the UV aversion relies on the TRPA1 channel expressed in bitter-responsive gustatory receptor neurons (GRNs) in the proboscis (Du et al. 2016; Guntur et al. 2017). TRPA1 is not directly sensing light but is proposed to be activated by H2O2 that is produced by exposure to bright UV, which in turn activates TRPA1 (Guntur et al. 2015; Du et al. 2016). Thus, in contrast to rhodopsin, which senses light through a retinal subunit, TRPA1 detects light indirectly following a photochemical reaction.
UV, violet, and blue light also cause avoidance in larvae, and this behavior is also independent of rhodopsins. The light aversion depends on expression of a member of the “GR” family (GR28b) and TRPA1 in class IV dendritic arborization (da) neurons, which send out extensive arbors throughout the body, including the body wall (Xiang et al. 2010). The relationship between GR28b and TRPA1 is not clear, and it is possible that they are both sensing high-intensity light indirectly through production of reactive oxygen species, such as H2O2. Alternatively, based on work on LITE-1—a related protein in Caenorhabditis elegans—it is conceivable that GR28b is a direct light sensor. Reminiscent of GR28b, the LITE-1 protein is required in worms for UV avoidance (Edwards et al. 2008; Liu et al. 2010), and purified LITE-1 is a direct light sensor (Gong et al. 2016). However, two tryptophan residues required for photon absorption in LITE-1 (Gong et al. 2016) are not conserved in GR28b, lessening the possibility that it is a direct light sensor.
Chemosensory receptors
The senses of taste and smell enable animals to detect both non-volatile and volatile chemicals, respectively. These are ancient senses that are critical for allowing flies to sense chemical cues from the environment, including foods, oviposition substrates, and conspecific individuals. While some chemicals are primarily detected by either taste or smell, many others, such as acids, carbonation, and even water, are sensed robustly through both taste and smell. The receptors used for detection of these latter chemicals will be described in a section following the introduction of taste and smell.
Taste receptors
Flies rely on contact chemosensation for multiple behaviors, including feeding, mating, and oviposition. Due to space limitations, I have limited the synopsis of taste receptors primarily to the adult stage.
Gustatory organs, the neurons, and the coding mechanism
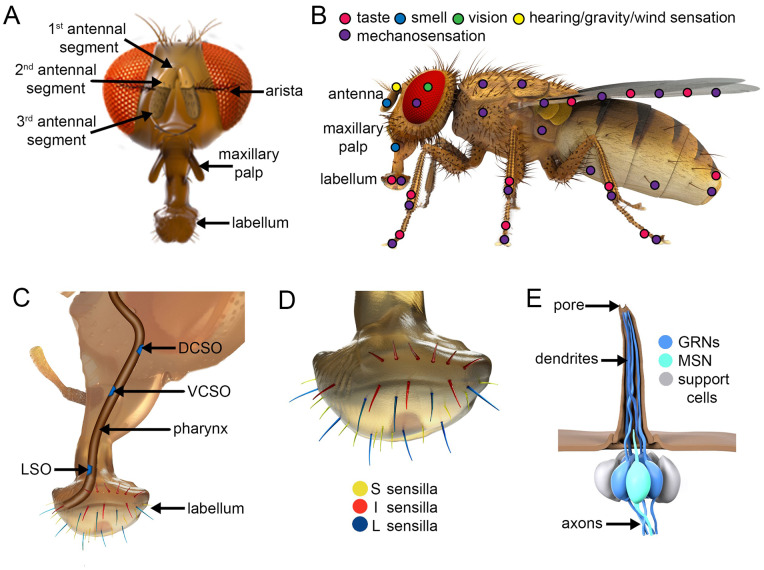
Flies and humans respond to a similar repertoire of tastes, including sweet, bitter, sour, amino acids, high and low Na+, and Ca2+ (Liman et al. 2014; Chen and Dahanukar 2020). These similarities are notable in view of the striking differences in the types and evolutionary origins of receptors and the morphology of the taste organs including the taste receptor cells. Humans taste foods using taste buds, which are restricted to the tongue and other parts of the mouth (Liman et al. 2014). However, flies evaluate chemicals in food and pheromones through multiple external taste organs and employ gustatory neurons, rather than neuroepithelial cells as is the case in humans (Liman et al. 2014; Chen and Dahanukar 2020). The closest fly equivalent to the mammalian tongue are two bilaterally symmetrical labella situated at the end of the proboscis (Figure 4, A and B). The proboscis also includes the pharynx, which houses several internal taste organs lining the esophagus (Chen and Dahanukar 2017, 2020) (Figure 4C). Other external gustatory organs are distributed on the leg tarsi, wing margins, and the female ovipositor, located at the tip of the abdomen (Figure 4B).
Figure 4.
Sensory organs in adults. (A) Front view of a head. (B) Distribution of body parts that function in different senses. (C) Cutout from the side view of head showing the pharynx. Internal taste organs are indicated in blue: labral sense organs (LSOs), the dorsal cibarial sense organ (DCSO), and the ventral cibarial sense organ (VCSO). (D) S-, I- and L-type sensilla decorate the labellum. (E) An I-type taste sensillum showing two GRNs, the MSN, and support cells. The hair has a single pore at the distal end.
The largest taste organs, the labella, are bilaterally symmetric tissues at the end of the proboscis. Each of the two labella is decorated with 31 hair-like sensilla, which fall into three size classes: small (S), intermediate (I), and large (L) (Figure 4D). These bristles contain the dendrites extending from the GRNs (Figure 4E). The 9 L-type and 11 S-type sensilla are associated with four GRNs, while the 11 I-type contain two GRNs (Stocker 1994; Shanbhag et al. 2001) (Table 2). When the labella or legs touch a substrate the chemicals enter a pore at the tip of each sensillum (Figure 4E) and either dissolve directly in the endolymph bathing the dendrites or bind to members of the large family of “odorant binding proteins” (OBPs) (McKenna et al. 1994; Pikielny et al. 1994; Galindo and Smith 2001). Once the fly opens its labella, a set of 30–40 conically shaped taste pegs on the surface of the labella gain access to the food and participate in the evaluation of food quality (Falk et al. 1976; Steck et al. 2018; Zhou et al. 2019). Each taste peg is associated with only a single GRN (Shanbhag et al. 2001). The anterior wing margins contain about 40 curved sensilla that house GRNs, which also detect tastants and pheromones (Raad et al. 2016; He et al. 2019; Yanagawa et al. 2019).
Table 2.
Types of GRNs in taste hairs
| Sensilla | # GRNs | GRN | Markers | Former GRN names | Activators | Suppressors |
|---|---|---|---|---|---|---|
| S-type | 4 | |||||
| A | Gr64f | “Sweet” | Sugars, low Na+, glycerol, fatty acids, acetic acida | Bitter, Ca2+, acids | ||
| Bb | Gr66a | “Bitter” | Bitter, high Na+, acids, polyamines, tryptophan, L-canavanine, cool temperatures | |||
| C | Ppk28 | “Water” | H2O (hypo-osmolarity) | Osmolytes, salts (e.g. Na+) | ||
| Dsc | Ppk23, VGlut | “Cation” | High cations (e.g. Ca2+, Na+, K+) | |||
| I-type | 2 | |||||
| A | Gr64f | “Sweet” | Sugars, low Na+, glycerol, fatty acids, acetic acida | Bitter, Ca2+, acids | ||
| B | Gr66a | “Bitter” | Bitter, acids, cool temperatures | |||
| L-type | 4 | |||||
| A | Gr64f | “Sweet” | Sugars, low Na+, glycerol, acetic acida | Bitter, Ca2+, acids | ||
| C | Ppk28 | “Water” | H2O (hypo-osmolarity) | Osmolytes, salts (e.g. Na+) | ||
| DL | Ppk23, VGlut | “Cation” | High cations (e.g. Na+, K+) | |||
| E | Ir94e | “Low salt” | Low Na+ |
A GRNs are more responsive to acetic acid in starved flies.
A subset of these B neurons are also positive for Ppk23 and ChAT.
These GRNs respond non-selectively to cations with particularly strong responses to Ca2+.
The activities of the GRNs rather than the receptors expressed in the neurons define the logic dictating whether or not a chemical is attractive or aversive. An illustration of this “labeled line” mechanism makes use of ectopic expression of a mammalian TRPV, which is the receptor for capsaicin—the pungent ingredient in hot chili peppers (Caterina et al. 1997). Flies are normally not very responsive to capsaicin. However, ectopic expression of TRPV1 causes opposite attractive and aversive reactions to capsaicin, depending on whether the TRPV1 transgene is expressed in A GRNs, which respond to sugars, or B GRNs, which are activated by bitter chemicals (Marella et al. 2006; Lee et al. 2018).
The response profiles of different taste sensilla on the labellum vary (Weiss et al. 2011; Jaeger et al. 2018; Dweck and Carlson 2020) (Table 2). Moreover, most, if not all, GRNs respond to multiple types of tastes, typically with the same valence (either a positive or negative effect on feeding). Examples include a subset of GRNs that are activated by sugars, glycerol, and fatty acids, which stimulate feeding (Thorne et al. 2004; Wang et al. 2004; Wisotsky et al. 2011; Ahn et al. 2017; Tauber et al. 2017; Kim et al. 2018) (A GRNs; Table 2). Other GRNs are stimulated by bitter compounds and very low pH carboxylic acids (B GRNs; Table 2), which deter feeding (Charlu et al. 2013; Rimal et al. 2019). Thus, the coding mechanism for chemical taste conforms to a “valence labeled line” mechanism (Liman et al. 2014). What defines the urge to accept or reject a food is whether positive or negative GRNs are activated, and the same GRNs can be activated by different ingredients that elicit the same valence.
A chorus of taste receptors required for sweet and bitter taste
In mice, the gustatory detection of sugars, bitter compounds, and L-glutamate (umami) depends primarily on a small set of 38 GPCRs, which couple to a Gq, PLCβ, and the TRPM4 and TRPM5 channels (Zhang et al. 2003; Chandrashekar et al. 2006; Damak et al. 2006; Liman et al. 2014; Dutta Banik et al. 2018). However, flies employ multiple families of chemosensory receptors for sweet, bitter, and amino acid taste, which are not homologous to mammalian taste receptors. One of the large families of fly taste receptors is the 68-member “GR” family (Clyne et al. 2000; Dunipace et al. 2001; Scott et al. 2001; Robertson et al. 2003) (Figure 5A). Nevertheless, the name “gustatory receptors” belies the unexpectedly broad roles of these proteins in light sensation as described above and in olfaction and thermosensation as described below. GRs may be the most ancient family of chemosensory receptors in insects. In addition to their prevalence in Arthropoda, they are also found in Cnidaria and Placozoa (Robertson 2015; Eyun et al. 2017), but have no mammalian homologs. This lack of relatedness of GRs and several other types of insect chemosensory receptors to mammalian receptors provides new opportunities for developing chemical approaches to control insects without interfering with the activities of related proteins in humans.
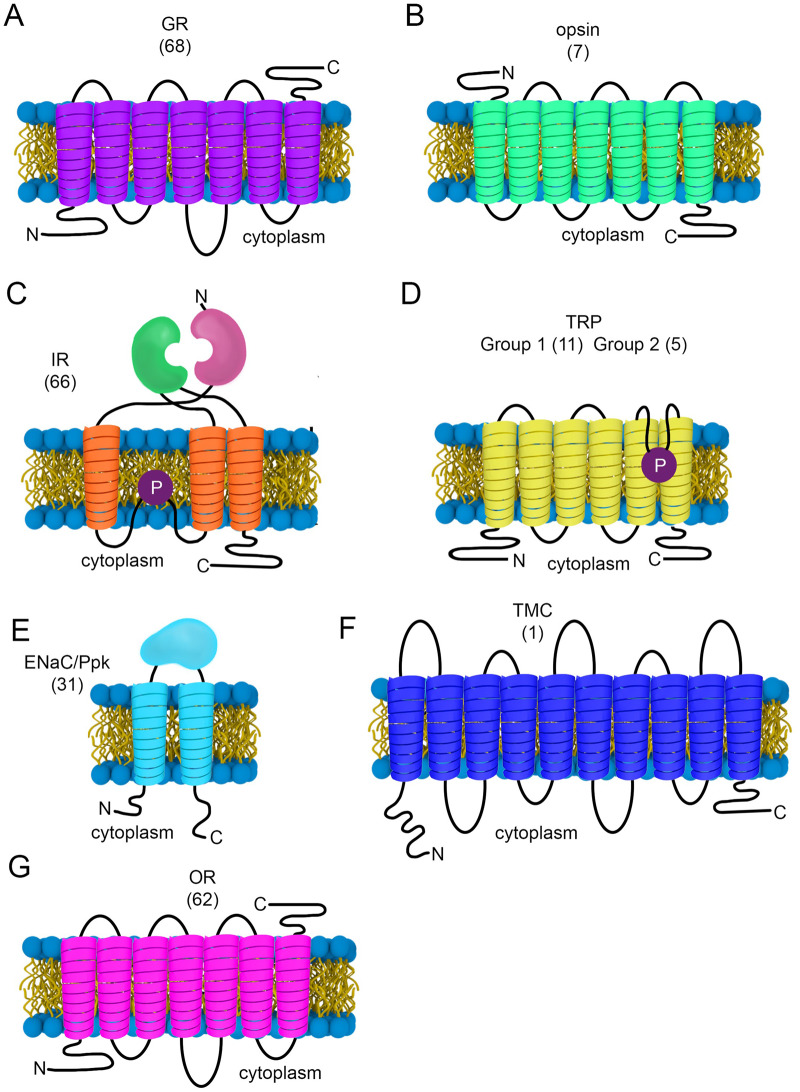
Figure 5.
Transmembrane topologies of various sensory receptors and channels. The number of family members are indicated in brackets. (A) GR. (B) Opsin. (C) IR. (D) TRP. (E) ENaC/Ppk. (F) TMC. (G) OR. P, pore-loop.
GRs are comprised of seven TMDs (Figure 5A); however, they are not GPCRs like rhodopsins (Figure 5B). GRs have a cytoplasmic N-terminus (Figure 5A)—a topology opposite to GPCRs (Zhang et al. 2011) (Figure 5B). Based on in vitro expression studies, and ectopic expression of GRs in vivo, at least some GRs are cation-permeable ionotropic receptors (Sato et al. 2011; Shim et al. 2015; Kim et al. 2018).
In addition to GRs, there are at least four other classes of fly taste receptors one of which is the 66-member “IR” family (Benton et al. 2009). As their names indicate, IRs are both receptors and cation channels. Family members are expressed in neurons that function in taste, smell, temperature sensation, and hygrosensation and are therefore also polymodal receptors (Benton et al. 2009; Croset et al. 2010; Koh et al. 2014; Enjin et al. 2016; Knecht et al. 2016; Ni et al. 2016). IRs have a predicted structure similar to mammalian ionotropic glutamate receptors (iGluRs; Figure 5C), although there is only minor sequence homology to iGluRs. IRs are comprised of a two-part extracellular ligand-binding domain, three TMDs, and a pore loop (Figure 5C). Two other taste receptor families include several TRP channels (Figure 5D and Table 1) (Al-Anzi et al. 2006; Kang, Pulver, et al. 2010; Kim et al. 2010; Zhang, Raghuwanshi, et al. 2013; Soldano et al. 2016; Leung et al. 2020) and Ppk (Pickpocket) channels (Cameron et al. 2010; Chen et al. 2010; Liu et al. 2012, 2018; Lu et al. 2012; Starostina et al. 2012; Thistle et al. 2012; Toda et al. 2012; Vijayan et al. 2014) (Figure 5E). A surprise is that multiple opsins are also taste receptors, and this represents a light-independent function (Leung et al. 2020). Thus, all five known families of Drosophila taste receptors are polymodal sensors.
Sweet taste
Not all animals sense sugars—cats being a notable example (Li et al. 2005). However, like us, flies have a “sweet tooth.” They sense sugars through GRNs in all three size classes of taste hairs (Table 2) (Marella et al. 2006). We refer to the “sweet” GRN as the A GRN rather than as an S GRN as suggested formerly (Meunier et al. 2003) since these GRNs are not activated exclusively by sugars (Table 2). The repertoire of labelar GRs that sense sugars includes GR5a (Dahanukar et al. 2001; Ueno et al. 2001) and most, if not all, GRs encoded by a polycistronic cluster of six related genes (Gr64a–Gr64f) all of which are expressed in Gr5a GRNs (Dahanukar et al. 2007; Jiao et al. 2007, 2008; Slone et al. 2007; Freeman et al. 2014; Fujii et al. 2015; Uchizono et al. 2017). Expression of different combinations of GR64 proteins in distinct subset of sugar neurons in the labellum and in the tarsi may function in the responses to different sugars (Fujii et al. 2015). However, there is a lack of consensus as to whether GR64a functions in sweet sensation in the labellum (Dahanukar et al. 2007; Jiao et al. 2007) or tarsi (Fujii et al. 2015).
In most cases, the full subunit composition of sugar receptors is not known and is likely to require multiple GRs (Jiao et al. 2008). An exception is GR43a, which is sufficient to generate a fructose-activated cation channel in vitro (Sato et al. 2011). GR43a functions in fructose detection in leg tarsi as well as in the brain where it serves to monitor fructose levels, which change in the hemolymph (fly blood) depending on whether or not they have fed on a sugar-containing meal (Miyamoto et al. 2012). In hungry flies, activity of the Gr43a neurons in the brain enhances the feeding urge. In satiated flies, activity of the same neurons inhibits feeding.
In addition to the role of GR43a in controlling feeding based on internal fructose levels, other mechanisms suppress feeding on sucrose-containing foods. For example, IR60b is expressed and functions in just two neurons in the pharynx (Joseph et al. 2017). What is remarkable is that IR60b serves exclusively to inhibit rather than to promote feeding in response to sucrose (Joseph et al. 2017). If such a mechanism existed in humans, it would limit obesity. Nevertheless, it is not known if IR60b is sufficient to serve as a sucrose-activated channel or requires other subunits.
Bitter taste
Roles of GRs in bitter taste
Flies and many other animals reject foods with bitter taste since many of these compounds are toxic, especially at high doses. Activation by bitter compounds is limited to the “B GRNs,” which are associated with S- and I-type sensilla (Table 2) (Meunier et al. 2003; Marella et al. 2006; Weiss et al. 2011). The “B GRNs” have different response profiles in two classes of S-type (S-a and S-b) and two classes of I-type sensilla (I-a and I-b) (Weiss et al. 2011).
Bitter compounds are usually present in foods with nutrients, such as sugars that activate A GRNs and promote feeding. Therefore, as a safeguard to prevent consumption of potentially dangerous foods, bitter compounds also inhibit A GRNs (French et al. 2015) and do so through at least two mechanisms. One mechanism involves association of bitter compounds with an OBP (OBP49a) in the endolymph surrounding the dendrites of GRNs. OBP49a then binds to sugar-activated GRs in A GRNs, thereby inhibiting their activity (Jeong et al. 2013). A second mechanism involves activated B GRNs stimulating a GABAergic interneuron in the primary taste processing center in the brain, the subesophageal zone (SEZ), which in turn inhibits A GRNs (Chu et al. 2014).
Unlike sugars, which have relatively similar structures, bitter-tasting compounds exhibit considerable structural diversity. Therefore, it should come as no surprise that B GRNs employ a larger repertoire of GRs to detect bitter compounds, than the GRs used by A GRNs to detect sugars. The detection of bitter compounds is mediated largely through complex combinations of GRs. Six GRs are widely expressed in all bitter GRNs in the labellum (Gr32a, Gr33a, Gr39a.a, Gr66a, Gr89a, and Gr93a) and are referred to as “commonly expressed receptors” (CERs) (Lee et al. 2009; Weiss et al. 2011; Dweck and Carlson 2020). Mutations affecting any of three CERs (GR32a, GR33a, and GR66a) disrupt repulsion to a very broad set of bitter chemicals (Lee et al. 2009; Moon et al. 2009; Lee, Kim, et al. 2010; Dweck and Carlson 2020). However, no CER is required for detection of all bitter compounds (Dweck and Carlson 2020). Other GRs are more narrowly tuned than the CERs and appear to contribute to bitter taste specificity. These include GR22e, and GR47a, which are required for sensing only a small number of bitter compounds (Lee et al. 2009, 2015; Poudel et al. 2017). Mutations disrupting some CERs increase the responses to some chemicals or confer responses to chemicals to which particular GRNs are normally unresponsive suggesting that CERs can also inhibit the binding of other GRs to some ligands (Dweck and Carlson 2020). This inhibitory function is not limited to CERs, as other GRs also act to suppress the activities of GRs.
To identify sets of GRs needed for the responses to different repertoires of bitter compounds, one approach is to ectopically express combination of GRs in sugar GRNs. The next step is to perform extracellular electrophysiological recordings (tip recordings) to assess whether the sugar GRNs produce action potentials in response to bitter chemicals. Bitter GRs appear to include multiple subunits. Four CERs are required to confer sugar GRNs with responsiveness to a set of bitter chemicals (Dweck and Carlson 2020). In addition, either GR22e or GR59c is needed in combination with two CERs (GR32a and GR66a) to endow sugar GRNs with sensitivity to various subsets of bitter compounds (Sung et al. 2017).
Opsins, bitter taste, and insights into ancestral role for opsins
Surprisingly, opsins comprise a newly discovered class of bitter taste receptor. Mutations eliminating any of three opsin genes (rh1, rh4, or rh7) greatly impair the responses to certain bitter chemicals, such as aristolochic acid (Leung et al. 2020). This gustatory function is independent of the retinal chromophore and is not affected by light (Leung et al. 2020). In the visual system, rhodopsins initiate a multistep amplification cascade thereby allowing for exquisite light sensitivity. In contrast, GRs act as both receptors and cation channels. Therefore, there is no intervening amplification between reception of a chemical by GRs and cation conductance. Consistent with the model that the opsins promote signal amplification in GRNs as they do in photoreceptor cells, the opsins are only required for sensing and rejecting relatively low levels of aristolochic acid (Leung et al. 2020). Moreover, aristolochic acid detection appears to employ an amplification cascade similar to phototransduction as mutations affecting the Gqα, the NORPA PLCβ and a TRP channel (TRPA1) also impair the ability to sense low levels of aristolochic acid (Kim et al. 2010; Leung et al. 2020). The opsins are dispensable for detection of high levels of aristolochic acid that are sufficient to directly activate TRPA1.
Based on ectopic expression in sugar GRNs and in vitro expression in tissue culture cells, the opsins are directly activated by aristolochic acid (Leung et al. 2020). Moreover, molecular modeling indicates that aristolochic acid associates with the opsins via a similar binding pocket as the chromophore. The requirement for three opsins for sensing aristolochic acid suggests that they are subunits of a single receptor. Consistent with this idea, there is growing evidence that GPCRs can function as tetramers (Petrin and Hebert 2012; Redka et al. 2014; Cordomi et al. 2015; Navarro et al. 2016; Sleno and Hebert 2019).
The discovery that opsins are chemosensors raises intriguing questions as to whether the primordial opsins were light sensors or chemosensors. Chemosensation is an ancient sense required for survival, as it enables organisms to differentiate between nutrients and toxic chemicals. Given that aristolochic acid and retinal associate with the opsin via a similar binding pocket, we posit that an ancestor of the light-sensitive rhodopsins was a chemosensor and later co-opted as a light sensor due to association with a light-activated chemical—retinal (Leung et al. 2020).
The observation that TRPA1 is directly activated by high levels of aristolochic acid raises questions as to whether other TRP channels also function as ionotropic receptors in GRNs. TRPL is directly activated by camphor (Zhang, Raghuwanshi, et al. 2013), which unlike chemicals such as strychnine and quinine is not toxic. Flies that have never been exposed to camphor avoid this ingredient. However, if the camphor is the only nutritive food source, then the flies slowly increase their acceptance of the camphor-laced food. This behavioral change occurs because of a camphor-induced decline in expression of TRPL and a subsequent reduction in synaptic boutons in the B GRNs, which collaborate to cause a decline in camphor repulsion (Zhang, Raghuwanshi, et al. 2013). Painless (Pain) is another TRP channel that impacts on taste, since mutation of pain or trpA1 impairs the aversion to allyl-isothiocyanate (AITC) (Al-Anzi et al. 2006; Mandel et al. 2018). However, unlike TRPA1, which is activated directly by AITC in vitro (Kang, Pulver, et al. 2010), Pain is not (Sokabe et al. 2008) and so its role in AITC sensation is unclear.
Amino acid taste
Flies are incapable of synthesizing half of the 20 standard amino acids (Sang and King 1961). Therefore, they must consume proteins for egg production and development, larval growth, and to achieve maximum adult lifespan (Lee and Micchelli 2013). Adult flies show differential behavioral responses to individual amino acids (Ganguly et al. 2017; Park and Carlson 2018). The attraction of adult flies to amino acids is subtle relative to sugars (Park and Carlson 2018), and there is no clear relationship between the degree of attraction and whether or not the amino acid is essential. Nevertheless, consumption of amino acids increases in flies raised on amino acid deficient food and in females following mating (Ribeiro and Dickson 2010; Vargas et al. 2010; Toshima and Tanimura 2012; Ganguly et al. 2017; Steck et al. 2018; Yang et al. 2018). The L- and I-type sensilla are virtually unresponsive to amino acids, while different S-type sensilla show distinct patterns of activities to different amino acids (Dahanukar et al. 2007; Park and Carlson 2018). Tryptophan elicits particularly strong responses relative to other amino acids, and it appears to be sensed by B neurons in S-type sensilla (Park and Carlson 2018).
The receptors required for amino acid attraction in the labellum are not known. However, OBP19b functions in the labellum for sensing a subset of amino acids such as phenylalanine and glutamine (Rihani et al. 2019). The amino acid responses of GRNs in leg tarsi (Ling et al. 2014) depends on IR76b and IR20a, and ectopic expression of both IRs in sweet GRNs endows them with amino acid sensitivity (Ganguly et al. 2017). In larvae, low and high concentrations of some of the same amino acids result in opposing attractive and aversive feeding responses, and IR76b contributes to these behaviors (Croset et al. 2016).
In addition to synthesizing bitter compounds to ward off insects and other predators, plants also produce a bevy of toxic amino acid derivatives such as L-canavanine (Rodgers 2014). According to one report, a GPCR (DmXR) encoded by the mangetout (mtt) gene functions in sensing L-canavanine (Mitri et al. 2009). However, in another study, mtt mutant sensilla display normal L-canavanine-induced action potentials and behavioral avoidance to L-cananavine (Lee et al. 2012). Thus, while DmXR is activated in vitro by L-cananavine (Mitri et al. 2009), it does not function in vivo for sensation of this toxic amino acid derivative (Lee et al. 2012). Rather, three GRs (GR8a, GR66a, and GR98a) are required in a subset of B neurons in S-type sensilla for sensing L-canavanine (Lee et al. 2012; Shim et al. 2015). These GRs form an L-canavanine-activated channel since they are sufficient to generate an L-canavanine activated channel in vitro, and to convert L-canavanine to an attractive compound after expression of these GRs in sugar-sensing GRNs (Shim et al. 2015).
The taste of Na+
Animals differ in their sensitivities and abilities to sense sugars, bitter compounds, sour, and amino acids. However, among all of the different basic tastes, the taste of Na+, which is popularly referred to as salt taste, is the most universal. Even animals, such as dolphins and whales, which lack sugar, bitter, and umami sensation, are endowed with the gustatory detection of Na+ (Feng et al. 2014). In flies and humans, sugar and bitter compounds have a negative and positive valence, respectively (Liman et al. 2014). However, salt taste is bivalent. This makes sense since animals require a certain level of Na+ beyond which it can be deleterious.
Flies prefer foods with ≤100 mM Na+ and tend to exhibit less interest in foods with higher levels of Na+. Using tip recordings to assay Na+-induced action potentials, nearly all sensilla respond to both low and high Na+, although some sensilla exhibit very large differences in responsiveness to different levels of Na+ (Zhang, Ni, et al. 2013). For example, two L-type sensilla (L4 and L6) exhibit the highest frequencies of action potentials in response to low Na+, but still respond to high Na+. Three S-type sensilla exhibit the most neuronal firing in response to high Na+, but also respond to a lesser degree to low Na+ (Zhang, Ni, et al. 2013). This led to the model that the behavioral output to low and high Na+ (positive and negative) is defined by the relative activities of the opposing GRNs to low and high Na+. At low levels, there is more neuronal firing by the GRNs that respond to low salt and feeding is promoted. However, at high Na+ concentrations, the firing by GRNs that are activated by high salt dominates and feeding is therefore inhibited (Zhang, Ni, et al. 2013). Thus, the attraction or repulsion to low and high Na+ is a consequence of competition between the activities of these different types of GRNs.
Using a genetically encoded Ca2+ sensor as a proxy for monitoring excitation and inhibition (GCaMP6f), four out of five classes of GRNs respond positively to Na+ (Table 2) (Jaeger et al. 2018). Low Na+ increases GCaMP6f fluorescence in A GRNs, which also respond to sugars (Jaeger et al. 2018). In addition, low Na+ induces GCaMP6f responses in E GRNs (Table 2) (Jaeger et al. 2018), which most likely corresponds to the L-type GRNs that produce the highest frequencies of action potentials in response to low Na+ (Zhang, Ni, et al. 2013). High salt increases the GCaMP6f responses of B (bitter) GRNs in S- and I-type sensilla and of the D GRNs in S- and L-type sensilla (Table 2) (Jaeger et al. 2018). Interestingly, if the animals are salt deprived, the responses of the D GRNs to high Na+ diminish, presumably to decrease aversion to Na+ under these conditions. High Na+ suppresses the responses of another class of GRNs (C GRNs), which are activated by water (Jaeger et al. 2018). The low salt responses in both A and E GRNs depend on IR76b (Zhang, Ni, et al. 2013; Lee et al. 2017; Jaeger et al. 2018), as well as IR25a (Jaeger et al. 2018). These IRs also appear to be required for high salt (Lee et al. 2017; Jaeger et al. 2018); however, only one of the two GRNs that are activated by high salt depends on IR76b (Jaeger et al. 2018), perhaps explaining why IR76b initially appeared to be dispensable for the responses of high Na+-activated GRNs (Zhang, Ni, et al. 2013).
Ca2+ taste avoidance
All animals depend on Ca2+ for survival, and humans and other vertebrates are capable of tasting Ca2+ (Tordoff 2001). However, high concentrations of Ca2+ can be toxic. Therefore, it is a rational expectation that flies might display a bivalent response to Ca2+ as is the case for Na+. However, flies are indifferent to low levels of Ca2+, but can taste Ca2+ since they and are repulsed by high levels (Lee et al. 2018).
The aversion to high Ca2+ occurs through effects on two types of GRNs: suppression of A GRNs and activation of a class of avoidance GRNs (Lee et al. 2018). The GRNs that are activated by Ca2+ are not bitter-responsive B GRNs but are marked by an epithelial Na+ channel (ENaC) called Ppk23 (Lee et al. 2018). However, loss of Ppk23 does not impact Ca2+ avoidance. Rather, the activation of Ppk23 GRNs by high (≥1 mM) Ca2+ depends on IR25a, IR62a, and IR76b. These three IRs do not appear to be sufficient for conferring Ca2+ avoidance, since they do not confer Ca2+ sensitivity to GRNs that do not normally respond to Ca2+. Therefore, the set of IRs that comprise the high Ca2+ sensor is likely to include ≥4 IR subunits.
There are two classes of Ppk23 neurons: D neurons that express the glutamate transporter (VGlut) and a subset of B GRNs that express choline acetyltransferase (ChAT) (Jaeger et al. 2018). The D GRNs are the Ca2+-sensing neurons since the Ir mutant phenotypes are rescued by driving expression of the Irs in all ppk23 neurons (D neurons plus a subset of B GRNs) but not by driving expression in B neurons only (Lee et al. 2018) (Table 2).
Fatty acid taste
In addition to amino acids, consumption of fatty acids contributes to egg production and larval growth. Consequently, fatty acids are appetitive but only at low and modest concentrations, while high concentrations are aversive (Masek and Keene 2013; Ahn et al. 2017). The attraction to fatty acids depends on GRNs in taste pegs (Sánchez-Alcañiz et al. 2018), leg tarsi, and taste hairs (Masek and Keene 2013; Ahn et al. 2017; Kim et al. 2018). However, only a subset of A GRNs in taste hairs are activated by fatty acids (Tauber et al. 2017; Kim et al. 2018). Most S-type sensilla respond robustly to the medium chain fatty acid, hexanoic acid, while I-type A GRNs display a modest response (Kim et al. 2018). However, A GRNs in L-type sensilla are unresponsive to hexanoic acid (Kim et al. 2018). Flies can behaviorally discriminate between sugars and fatty acids (Tauber et al. 2017; Kim et al. 2018), and this ability may be possible because only a subset of A GRNs are activated by fatty acids.
The PLCβ encoded by norpA (Masek and Keene 2013) and Gr64e (Kim et al. 2018) are required for fatty acid taste. Ectopic expression of TRPA1, which can function downstream of PLCβ pathways, can restore fatty acid taste in the Gr64e mutant GRNs (Kim et al. 2018). Since TRPA1 is not normally required for fatty acid sensation, one interpretation of the rescue result is that GR64e is acting as a cation channel downstream of PLCβ. However, it is not clear if GR64e is a subunit of a larger channel, or acting in parallel with another channel downstream of PLCβ. The fatty acid receptor that initiates the PLCβ cascade is also unknown. Given the contribution of a PLCβ, it seems likely to be GPCR, which couples to a Gq.
In addition to acting downstream of a PLCβ signaling cascade, Gr64e also appears to be directly activated by glycerol (Wisotsky et al. 2011). The finding that GR64e can act either directly as a taste receptor or downstream of PLCβ is reminiscent of multiple TRP channels, such as fly TRPA1, which can be directly activated by aristolochic acid as well as indirectly by aristolochic acid through a PLCβ signaling cascade (Kim et al. 2010; Leung et al. 2020).
Fatty acid detection also depends on three IRs (IR25a, IR76b, and IR56d) in taste peg GRNs and in a subset of A GRNs for the attraction to low levels of medium-chain fatty acids (Ahn et al. 2017; Tauber et al. 2017; Sánchez-Alcañiz et al. 2018). Among these three IRs, only IR56d appears to be restricted to sugar GRNs (Ahn et al. 2017; Tauber et al. 2017). It remains to be determined whether IR25a, IR76b, and IR56d are subunits of a fatty-acid activated channel. Whether these IRs act in the PLCβ pathway or another pathway also needs to be reconciled.
Chemicals dually detected through taste and smell
While sweet, salt, and amino acids, are detected exclusively through the sense of taste, other chemicals, such as acids, water, carbonation, and polyamines, are recognized through receptors in both gustatory and olfactory neurons. The mechanisms underlying the detection of these chemicals are reviewed following the final section on taste and after presenting an overview of the olfactory system.
Sensation of food texture and temperature on sugar feeding behavior
The selection of food depends on more than chemicals . It is also influenced by the texture, temperature, and color of food. Texture, including hardness, and viscosity provide information about whether a botanical food is ripe or spoiled. Evaluating the hardness of a food is also necessary for an animal to apply the appropriate force to process the food prior to ingestion.
Regardless of the texture of a substrate, flies show no propensity to feed unless there is nutrient value. Therefore, several studies have evaluated food texture in the context of sucrose and found that flies prefer soft food over harder options (Jeong et al. 2016; Zhang et al. 2016; Sánchez-Alcañiz et al. 2017) and liquid foods with low vs high viscosity (Zhang et al. 2016). These analyses demonstrate that two types of mechanosensory neurons (MSNs) function in food texture discrimination. One is a single mechanically activated multidendritic neuron (md-L neuron) in each of the two bilaterally symmetrical labella (Zhang et al. 2016). The md-L neurons extend dendrites to the bases of ∼70% of the sensilla most of which are L-type sensilla. In contrast to the labeled line coding mechanisms used by GRNs, the md-L neurons employ an intensity coding mechanism. Low levels of activity of md-L neurons appear to simulate soft food and provoke feeding on sugar, while high levels of activity simulate hard food and suppress feeding (Zhang et al. 2016).
The ability of md-L neurons to sense texture depends at least in part on the Transmembrane Channel-like (TMC) protein (Figure 5F), which is homologous to proteins required for audition in mammals (Kurima et al. 2002; Vreugde et al. 2002). In vitro and in vivo studies indicate that vertebrate TMCs are mechanosensitive channels (Pan et al. 2018; Jia et al. 2020) suggesting that Drosophila TMC is also mechanically activated. Mutation of Drosophila tmc impairs the ability of the animals to reject foods that are very hard or liquid food with high viscosity. The tmc mutation also reduces the firing of md-L neurons upon mechanical stimulation of taste sensilla (Zhang et al. 2016). The md-L and A GRNs send their axons to overlapping regions of the primary taste center in the brain, the SEZ. Therefore, it is possible that the md-L neurons and A GRNs coordinate their activities by converging onto the same command interneurons (Fdg neurons), which control feeding (Flood et al. 2013).
Each gustatory hair and taste peg includes a single MSN (Falk et al. 1976), and some or all of the MSNs are also required for sensing food hardness (Jeong et al. 2016; Sánchez-Alcañiz et al. 2017). According to one report, the MSNs make direct synaptic connections with A GRNs, and MSNs inhibit A GRNs through release of the neurotransmitter, GABA (Jeong et al. 2016). Moreover, the requirement for the MSNs for sensing food hardness depends on the TRPV channel, Nanchung (Nan) (Jeong et al. 2016). Another study concludes that mechanical stimulation of MSNs and the contribution of these neurons to the selection of soft foods depend on the TRPN channel, NOMPC (Sánchez-Alcañiz et al. 2017). The basis for the different conclusions concerning requirements for NAN and NOMPC in these two studies is unclear. Additional questions are whether flies are able to discriminate foods particles based on size or shape and if so, what are the underlying cellular and molecular mechanisms?
In addition to food texture, the temperature of sugary foods also has a significant impact on its appeal (Li et al. 2020). The propensity of flies to feed on sugar is so sensitive to temperature that even a drop in temperature of the food from 23 to 19°C causes a significant suppression in the palatability of sugar (Li et al. 2020). The reduced consumption of cooler sugar may reflect the slower activity and rate of development of Drosophila at slightly lower temperatures, such as 19°C. If there is no balance between metabolic need and food intake, there can be a fitness cost.
The diminished urge to feed on cooler sugar-containing food is not due to a reduction in sugar-induced action potentials in A GRNs. Rather, B GRNs in S- and I-type sensilla and MSNs in L-type sensilla are activated by cool temperatures in the 17–19°C range (Li et al. 2020). While the thermosensor in the MSNs has not yet been defined, an opsin (Rh6) is expressed and required in B neurons in S-type sensilla (Li et al. 2020). This finding, along with the prior demonstration that Rh6 and two other opsins function in thermotaxis in larvae (Shen et al. 2011; Sokabe et al. 2016), raises the question as to whether opsins function directly as thermosensors.
The neurons that are required for sensing cool temperatures in the labellum are also activated by either bitter chemicals or mechanical stimuli. Thus, the question arises as to how flies differentiate between sensing the coolness of food from bitterness or food texture. In considering the coding mechanism, it is notable that disrupting the activities of any of the subsets of neurons that are activated by cooling is sufficient to disrupt a fly’s ability to sense coolness in sugar-laced foods. Inactivation of just MSNs, B neurons, or mutation of rh6, which functions only in B neurons in S-type sensilla, all eliminate the ability to suppress feeding if the food is cool (Li et al. 2020). If a fly is in a cool environment, then all cool-activated neurons in the labellum will be activated. However, bitter chemicals activate B neurons only, and food texture is sensed by MSNs. Therefore, in order to interpret the sensation of coolness in food, all classes of cool-activated neurons must be activated simultaneously.
Receptors required for smell
The olfactory systems in flies and many other animals are faced with the daunting problem as to how to recognize an immense and chemically diverse array of volatile chemicals.
Olfactory organs, the neurons, and the coding mechanism
The fly olfactory organs and olfactory receptors (ORs) are as divergent from their mammalian counterparts as are the fly and mammalian taste organs and receptors. Remarkably, despite striking molecular and anatomical differences, flies solve the complex problem of interpreting vast arrays of olfactory cues using similar logic at the circuit level, which is described below.
The Drosophila equivalent of the mammalian nose is comprised of two organs, the third antennal segment, and the maxillary palp (Figure 4A). These organs are decorated with olfactory sensilla, which have multiple pores, and house 1–4 olfactory receptor neurons (ORNs) and support cells (Figure 6A). Among the functions of the accessory, cells is the production of OBPs, which are secreted into the endolymph surrounding the dendrites and bind to and present low-solubility odorants to the ORs (Sun et al. 2018). Most olfactory sensilla are subdivided into three morphologically distinct types (Figure 6B). The basiconic are largely responsible for sensing food odors and are distributed on both the antenna and maxillary palp (Vosshall and Stocker 2007; Su et al. 2009). The trichoid sensilla are restricted to the antenna and detect volatile pheromones, while the coeloconic sensilla, which are situated on the antenna, sense acids, and amines (Vosshall and Stocker 2007; Su et al. 2009). Most ORNs fire action potentials in the absence of ligands. Consequently, some ligands increase while others decrease this baseline activity (de Bruyne et al. 2001).
Figure 6.
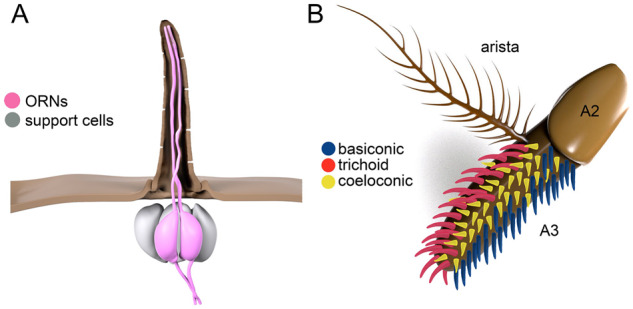
Olfactory sensilla. (A) Olfactory sensilla housing two neurons. The support cells and the distribution of pores are also indicated. (B) Antenna. The distributions of three types of olfactory sensilla are indicated. A2, second antennal segment; A3, third antennal segment.
The ORNs send their axons into the brain’s antennal lobes, each of which is organized into 52 discrete glomeruli (Vosshall and Stocker 2007; Su et al. 2009). As in humans, the glomeruli form an odotopic map such that each is targeted by ORNs that express the same receptors (Gao et al. 2000). Consequently, each glomerulus responds to different sets of odorants.
Classes of olfactory receptors
ORs
Several classes of receptors function in Drosophila olfaction. A large and well-characterized class is a set of 62 “ORs,” which are present only in insects and are unrelated to any mammalian proteins (Clyne et al. 1999; Gao and Chess 1999; Vosshall et al. 1999; Robertson et al. 2003; Eyun et al. 2017). Their predicted topology of seven membrane spanning domains with a cytoplasmic N-terminus is opposite to GPCRs but is reminiscent of Drosophila GRs (Figure 5G) (Benton et al. 2006). One OR (ORCO; formerly OR83b) serves as a co-receptor, which is required for trafficking of the ligand binding ORs to the dendrites (Larsson et al. 2004). The ORs are widely expressed in ORNs in basiconic and trichoid sensilla but with one exception (OR35a) are excluded from coeloconic sensilla.
An early challenge following the identification of ORs was to identify their cognate ligands. To “deorphanize” the ORs (find the ligands), John Carlson’s group pioneered a strategy that involves first genetically removing the native ligand-binding ORs in an ORN and then ectopically expressing a single orphan OR in the “empty neuron.” This allowed them to test a battery of 110 odorants to determine the effects on the activity of the empty neuron (Hallem and Carlson 2006). Using this approach, a number of concepts emerged. First, some ORs are narrowly tuned and respond to only one odorant, while others respond to a large repertoire of odorants. Second, the spontaneous firing rate in the absence of any odorant is dependent on the particular OR that is expressed in the ORN. Third, the same OR can be either excited or inhibited by different odorants. Fourth, some odorants are capable of activating many ORs. Fifth, the kinetics of response termination is a feature of the OR.
In vitro expression studies indicate that ORs are ionotropic receptors (Sato et al. 2008; Wicher et al. 2008). In addition, one study also concludes that OR22a activates a stimulatory G protein (Gs), suggesting that this OR is a GPCR in addition to functioning as an ionotropic receptor (Wicher et al. 2008). However, given that ORs have a topology opposite to GPCRs, the concept that ORs directly engage G-proteins remains speculative.
IRs
Proteins related to the 66 member Drosophila “IR” family (Benton et al. 2009) are present in Mollusca and Nematoda (Croset et al. 2010; Eyun et al. 2017). Thus, IRs appear to be more ancient in origin that the ORs but not as ancient as the GRs (Croset et al. 2010; Eyun et al. 2017). A minimum of 17 IRs are expressed in coeloconic sensilla (Benton et al. 2009; Croset et al. 2010), which largely exclude ORs. The spontaneous activity of coeloconic ORNs depends in part on the expression of IRs, and this activity can be increased or decreased depending on whether an IR interacts with an excitatory or inhibitory olfactory cue (Grosjean et al. 2011; Rytz et al. 2013). The olfactory ligands for most of the IR-expressing ORNs are distinct from OR-expressing ORNs. While OR-expressing ORNs respond robustly to esters, alcohols, and ketones, IR-expressing ORNs in coeloconic sensilla are primarily responsive to carboxylic acids, amines, and aldehydes (Yao et al. 2005; Silbering et al. 2011). Moreover, OR-expressing ORNs respond faster and with greater sensitivity and in many cases adapt more rapidly than IR-expressing ORNs (Yao et al. 2005; Silbering et al. 2011; Getahun et al. 2012). Thus, IR-sensitive ORNs respond best to sustained odorant stimulation, while OR-expressing ORNs are better suited to sensing transient and low levels of stimuli.
The IRs that function in olfaction fall into several classes. Some, such as IR8a and IR25a, may serve as co-receptors to promote trafficking of other ligand binding IRs in different subset of ORNs (Benton et al. 2009; Abuin et al. 2011). Other IRs may be ligand-binding subunits. Loss-of-function mutations of various Irs, such as Ir64a, Ir84a, and Ir92a, demonstrate that they are required for the olfactory responses to specific subsets of amines, ammonia, and acids (Ai et al. 2010, 2013; Abuin et al. 2011; Grosjean et al. 2011; Min et al. 2013). In some cases, expression of IRs in vitro, such as in Xenopus oocytes, is sufficient to confer olfactory responses to a given volatile ligand but only if a co-receptor such as IR8a is co-expressed to facilitate receptor trafficking (Abuin et al. 2011).
Signal amplification through IRs
A classical mechanism for signal amplification involves coupling of receptors to G-protein signaling cascades that culminate with activation or inhibition of ion channels. In the olfactory system, an example of this type of mechanism occurs in the detection of citronellal by TRPA1 (Kwon, Kim, et al. 2010). While some TRPA1 isoforms, such as one expressed in Anopheles gambiae can be directly activated by citronellal, in Drosophila, this insect repellent is also sensed through a Gq- PLCβ signaling cascade, which culminates with activation of TRPA1 (Kwon, Kim, et al. 2010). Ca2+ influx through TRPA1 then subsequently turns on a Ca2+-activated K+ channel called Slowpoke. However, this pathway only appears to function in the modulation of citronellal-induced action potentials, and an ORCO-dependent pathway may be the main mode for sensing citronellal. The specific ORs that couple to ORCO for sensing citronellal have not been defined.
Due to the dual roles of ORs and IRs as receptors and channels, it has been unclear whether there is any signal amplification following activation of these proteins. However, two groups of ORNs in males that sense courtship pheromones employ signal amplification following activation of OR47a and IR84a (Ng et al. 2019). The Ca2+ influx through these cation channels, in turn, activate the Ppk25 channel through a calmodulin binding site. Moreover, the extent of amplification is dictated by the level of Ppk25 expression. This mode of amplification provides an explanation for the age-dependent increase in sensitivity of OR47a neurons to pheromones (Lin et al. 2016) since Ppk25 levels increase with age (Ng et al. 2019).
Chemicals sensed through taste and smell
A vast array of chemicals, including acids, carbonation, water, polyamines, pheromones, and insect repellents, such as DEET (N,N-diethyl-m-toluamide), some bitter compounds, and fatty acids, are dually sensed through taste and smell.
Acid sensing
Taste of acids
The gustatory response of flies to acids varies enormously depending on the chemical composition and concentration. Some carboxylic acids, such as lactic acid, are highly attractive at a concentration of 5%. Even at a level of 10% and a pH of 1.9, lactic acid is attractive (Rimal et al. 2019). Lactic acid is not aversive until the concentration reaches 20%. Conversely, flies normally exhibit only minimal attraction to low levels of acetic acid, and are repulsed by 5% acetic acid even though the pH (2.5) is not as low as 10% lactic acid (pH 1.9), which is attractive (Rimal et al. 2019). If the flies are starved, they dramatically increase their acceptance of acetic acid and use it as a source of nutrients (Devineni et al. 2019). However, flies do not increase their acceptance of HCl under starvation conditions, demonstrating that the internal-state-dependent acetic acid acceptance is not driven simply by pH (Devineni et al. 2019).
In the gustatory system, the rejection of sugar mixed with highly concentrated acids occurs through activation of aversive GRNs and suppression of A GRNs (Charlu et al. 2013). This dual system for avoiding strong acids is reminiscent of bitter taste, which also causes gustatory avoidance through activation of B GRNs, which suppress feeding, and inhibition of sugar-activated A GRNs.
Sensing of aversive levels of acids occurs in least in part through activation of B GRNs in S-type (Charlu et al. 2013; Rimal et al. 2019) and possibly I-type sensilla (Charlu et al. 2013). IR7a is required in a subset of B GRNs for avoiding high levels of acetic acid. Surprisingly, IR7a is narrowly tuned, as it is dispensable for the rejection of other carboxylic acids tested as well as HCl (Rimal et al. 2019). Two IRs (IR25a and IR76b) are required in leg tarsi for choosing egg-laying sites (Chen and Amrein 2017). However, neither IR25a nor IR76b seems to contribute significantly to the gustatory decision to reject high levels of acetic acid (Rimal et al. 2019). Because IR7a is the only receptor identified thus far that impacts on sour taste, this raises the possibility that there may be multiple receptors expressed in the labellum that are used to detect subsets of organic acids . The capacity to discriminate between different types of acids such as acetic acid and lactic acid might endow flies with the ability to access whether a prospective food is laden with microorganisms, such as Acetobacter or Lactobacillus, which grow on plants and produce acetic acid and lactic acid, respectively.
Smell of acids
Many acids are volatile and inhibit feeding in part through detection by the olfaction system. IR8a and IR64b are expressed in ORNs in coeloconic sensilla and form a complex required for sensing carboxylic acids, such as acetic acid, and HCl (Ai et al. 2010, 2013; Abuin et al. 2011). In the absence of IR8a, trafficking and stability of IR64a is compromised (Abuin et al. 2011; Ai et al. 2013). Of significance, these two proteins form an acetic acid-activated cation channel in Xenopus oocytes (Ai et al. 2013). Because the IR8a/IR64a olfactory channel is more broadly tuned than IR7a, it appears that the discrimination between acetic acid and other acids is mediated by acid detection through taste rather than smell.
Detection of carbonation
Taste of carbonation
While the smell of carbonation (CO2) is aversive to Drosophila, the taste of carbonated water is appealing. The gustatory attraction to carbonation is mediated by GRNs in taste pegs (Fischler et al. 2007). The levels of carbonation sensed by the taste peg neurons (0.2–0.4%) is within the range produced by growing yeast, leading to the suggestion that these GRNs enable flies to recognize and consume foods with yeast and other microorganisms (Fischler et al. 2007). The gustatory attraction to CO2 requires IR56d, which acts in taste peg neurons in combination with the more broadly expressed co-receptors, IR25a and IR76b (Sánchez-Alcañiz et al. 2018). These three IRs are the same repertoire of receptor that function in sensing fatty acids. However, these three IRs have not been shown to be sufficient for detecting either COs or fatty acids. Thus, the full fatty acid or carbonation receptors may require additional subunits that enable the flies to distinguish between these tastes.
Smell of carbonation
Stressed flies emit CO2 as a part of a stress response (Suh et al. 2004). As a result, the smell of even slightly elevated levels of CO2 is repulsive, as it is a signal to these animals to escape from a potentially dangerous environment. The olfactory repulsion to CO2 is mediated by a subset of ORNs in large basiconic sensilla in the third antennal segment (ab1C class) (de Bruyne et al. 2001). These ORNs are distinct from other ORNs, in that they express two “GRs,” GR21a and GR63a (Faucher et al. 2006; Jones et al. 2007; Kwon et al. 2007), which together form a CO2 receptor (Jones et al. 2007; Kwon et al. 2007; Kumar et al. 2020). Among the multiple lines of evidence in support of this conclusion is the observation that ectopic expression of Gr21a and Gr63a confers CO2 sensitivity to ORNs that normally are unresponsive to CO2 (Jones et al. 2007; Kwon et al. 2007). GR21a and Gr63a also function in the detection of other odorants (see below) and inhibition of these GRs leads to behavioral attraction while receptor activation causes behavioral aversion (MacWilliam et al. 2018). Mosquito vectors, such as Anopheles gambiae, express homologs of GR21a and GR63a in their CO2-responsive organ, the maxillary palps (Jones et al. 2007). However, in contrast to Drosophila, the smell of high levels of CO2 is attractive to these mosquitoes, as CO2 plumes emanating from humans are important for host-seeking in combination with other cues.
In addition to GR21a and GR63a, a signal transduction pathway that includes a Gqα (Yao and Carlson 2010), PLCβ (PLC21C), and all three TRPC channels: TRP, TRPL, and TRPγ (Badsha et al. 2012) also impacts on CO2 avoidance. This signal transduction cascade operates in the same ab1C neurons as GR21a and GR63a, and mutations disrupting this pathway decrease the CO2 response mediated by the GR21a and GR63a. However, the receptor that initiates this cascade is not known, and a mechanistic explanation for the impact of this cascade on the CO2 response is unclear. One possibility is that the TRP-dependent Ca2+-influx sensitizes the GRs to activation by CO2. Conversely, activation of the GRs may somehow stimulate the Gq/PLCβ/TRP cascade, which contributes to depolarization of the ab1C neurons.
While stimulation of ab1C neurons elicits avoidance, such as when there are stressed flies in the environment (Suh et al. 2004), it is not always in the animals’ best interest to evade CO2, as some excellent food sources are laden with microorganisms that emit CO2. It turns out that some odorants in foods such as 1-hexanol and 2,3-butanedione inhibit GR21a/GR63a, thereby suppressing CO2 avoidance (Turner and Ray 2009). Moreover, flies can also be attracted to CO2 (Wasserman et al. 2013; van Breugel et al. 2018). The appeal of CO2 only occurs when the flies are foraging, and this behavior is dependent on IR25a (van Breugel et al. 2018). It is likely that there are other co-receptors that function in combination with IR25a for CO2 attraction, and the specific IR25a-expressing ORNs that mediate CO2 attraction are not defined.
Taste and smell of polyamines
Polyamine detection by GRNs
Polyamines are essential for an array of physiological processes, and high-polyamine content in food results in a dramatic increase in the number of progeny per mating (Hussain et al. 2016). Polyamines are synthesized in flies through endogenous metabolic pathways and by their microbial flora. However, flies need to consume additional polyamines to meet their nutritional needs. The roles of polyamines in influencing food consumption have not been reported. Nevertheless, GRNs detect polyamines and this information influences oviposition sites. Females prefer laying eggs on polyamine-containing sites but only if the polyamines are present in combination with other nutrients, such as sugars (Hussain et al. 2016). Oviposition sites with pure polyamines are aversive. The sensation of polyamines depends on GRNs in taste pegs and in B neurons in S-type sensilla. IR76b contributes to the gustatory detection of polyamines, possibly through functioning in the pegs (Hussain et al. 2016). The receptors that are critical for sensing polyamines in B GRNs remain to be identified (Hussain et al. 2016).
Smell of amines and polyamines
The odors of pure polyamines, such as cadaverine, putrescine, spermidine, and many others, are attractive to flies. This positive response to volatile polyamines depends in part on two IRs that act in the same ORNs: IR41a and IR76b (Hussain et al. 2016). Whether IR41a and IR76b are sufficient to confer polyamine sensitivity is not known. An olfactory CO2 receptor, GR63a, also functions in the attraction to spermidine and other polyamines and does so through an intriguing mechanism—reducing the baseline activity of CO2-responsive ab1C neurons, thereby attenuating aversive behavior (MacWilliam et al. 2018).
Ammonia and monoamines are also attractive to flies (Min et al. 2013), as they alert the animals to rotting organic matter, which is potentially nutritious. The appeal of these chemicals requires IR92a, which is expressed in a subpopulation of ORNs in coeloconic sensilla (Min et al. 2013). In contrast, the attraction to volatile polyamines is not affected significantly by loss of IR92a consistent with the finding that this sensation requires IR41a and IR76b. Nevertheless, monoamines also inhibit the baseline activity of ab1C neurons through a GR63a-dependent mechanism (MacWilliam et al. 2018). Whether loss of the other CO2-receptor subunit (GR21a) also causes this same phenotype is not known.
Water and dry sensation
Taste of water
Water is so essential for survival that one of the four GRNs in all S- and L-type sensilla (C GRNs) is dedicated primarily to water sensation (Inoshita and Tanimura 2006) (Table 2). The detection of water in C GRNs depends on Ppk28, which is sufficient for water detection because ectopic expression of this channel in B GRNs endows them with water sensitivity (Cameron et al. 2010; Chen et al. 2010). Moreover, introduction of Ppk28 in mammalian HEK293 cells caused them to respond to hypo-osmotic solutions (Cameron et al. 2010).
Water vapor detection
Hygrosensation allows flies to sense the level of humidity and to select either moist or dry environments depending on its state of desiccation. This is of great importance to these small animals to prevent them from drying out. Hygrosensation also enables females to choose oviposition sites with the ideal level of wetness for their eggs. Humidity is detected primarily by sensilla in an invaginated portion near the proximal region of the third antennal segment called the sacculus. The sacculus is comprised of three recessed chambers, including chamber II, which contains humidity-sensitive sensilla. These sensilla are distinct from olfactory sensilla, in that they are poreless. Neurons that are activated by dry and moist air are housed in the same hygrosensory sensilla along with a third neuron that is cold activated (Enjin et al. 2016; Knecht et al. 2016).
Despite the importance of preventing desiccation, excessive humidity is aversive. Flies that are given a choice between environments with 0% humidity and those with 100% humidity strongly prefer the dry option (Liu, Li, et al. 2007). RNAi mediated knockdown of water witch (wtrw; a TRPA channel) or mutation of nanchung (nan; a TRPV channel) have been reported to be required for this behavior (Liu, Li, et al. 2007). Electrophysiological analyses suggest that WTRW functions in the detection of moist air and Nan is required for detecting dry air (Liu, Li, et al. 2007). While subsequent studies support the conclusion that mutations that disrupt nan or wtrw reduce discrimination between different humidity levels, this sensation is not eliminated in these mutants (Enjin et al. 2016; Knecht et al. 2016). Moreover, wtrw and nan do not function in sacculus neurons that respond to moist or dry air (Knecht et al. 2016), suggesting that other receptors act in hygrosensation. The neurons that are activated by dry air require IR25a, IR93a, and IR40a (Enjin et al. 2016; Knecht et al. 2016), and the moist-activated neurons depend on IR25a, IR93a, and IR68a (Frank et al. 2017; Knecht et al. 2017). Thus, the IR subunits that enable discrimination between dry and moist air are IR40a and IR68a, respectively. The inclusion of a cold-sensitive neuron in the same sensillum might facilitate hygrosensation by activating these neurons through evaporation-induced cooling (Tichy et al. 2017). The cold neurons could in turn affect the activities of the hygrosensory neurons.
Detection of DEET
DEET remains the most effective insect repellent available (Travis et al. 1949). However, this synthetic compound has limitations including low olfactory potency (∼30% is used for this purpose), it can damage clothing containing plastic or nylon, and some individuals find the smell and feel of DEET unappealing. During the many decades since it was released for public use, there have been numerous efforts to find more potent and long-lasting alternatives, but none have emerged. Because DEET is the most commonly used repellent worldwide, there is tremendous interest in clarifying its mechanism of action. While there has been progress, there remain multiple competing hypotheses to explain olfactory repellency (see below: Smell of DEET).
Taste of DEET
In contrast to the controversy concerning the mode of olfactory detection, we have a clearer concept as to how DEET causes repulsion through the taste system. The gustatory aversion to DEET is incredibly sensitive, as 0.05% suppresses feeding (Lee, Kim, et al. 2010). DEET directly activates GRNs, and 0.02% DEET is sufficient to produce action potentials in B GRNs (Lee, Kim, et al. 2010). GRNs in both the proboscis and legs respond to DEET, and the gustatory response to DEET depends on GR32a, GR33a, and GR66a (Lee, Kim, et al. 2010; Guo et al. 2020). However, these three GRs are not sufficient to confer a DEET response to GRNs that are insensitive to DEET. Thus, the DEET receptor most likely includes a minimum of four GRs, further highlighting the large repertoire of subunits comprising many GR complexes functioning in aversive taste. DEET is also detected through the taste system in multiple mosquito species (Sanford et al. 2013; Sparks and Dickens 2016). In Aedes aegypti DEET is sensed through the legs (Dennis et al. 2019). The Aedes proboscis is not sufficient for the gustatory repulsion of DEET (Dennis et al. 2019); however, it has not been excluded that GRNs in the proboscis are activated by DEET.
Smell of DEET
Despite the widespread use of DEET, it does not have high volatility relative to many other odorants such as the human attractive odorant, 1-octen-3-ol (0.00167 and 0.53 mmHg at 25°C, respectively) (DeGennaro 2015). There are multiple proposals to account for the olfactory repellency of DEET. According to one of three primary models, DEET inhibits ORNs that are activated by attractive odorants, thereby rending humans invisible or difficult to detect (Davis and Sokolove 1976; Dogan et al. 1999; Ditzen et al. 2008). In support of this “masking mechanism,” the electrophysiological responses to attractive odorants are suppressed by DEET in Drosophila, Aedes, and Anopheles, and these effects are mediated through ORs (Ditzen et al. 2008; Bohbot and Dickens 2010; Afify et al. 2019).
A second model is that DEET modulates the activities of many types of ORNs, and therefore serves as a “confusant,” distorting the patterns of activities that would normally lead to attractive and avoidance behavior (Pellegrino et al. 2011). According to this model, DEET does not result in the host becoming invisible as in the masking model but causes the host olfactory signals to be confusing. This confusant model is supported by studies in mosquitoes indicating that DEET has a complexity of stimulatory and inhibitory effects on different ORs (Bohbot and Dickens 2010, 2012; Bohbot et al. 2011; Pellegrino et al. 2011).
The third model is that DEET directly activates receptors in ORNs that induce avoidance behavior. Supporting this “direct activation” model are two-electrode voltage clamp studies in Xenopus oocytes indicating that DEET alone activates some ORs from Anopheles gambiae (Xia et al. 2008; Bohbot and Dickens 2010). Moreover, DEET is sufficient to activate a subset of ORNs in other mosquitoes including Aedes aegypti and Culex quinquefasciatus (Boeckh et al. 1996; Syed and Leal 2008). Co-expression of two Culex ORs (OR136 and ORCO) in Xenopus oocytes is sufficient to generate a DEET-induced current and RNAi knockdown of Or136 eliminates DEET repellency (Xu et al. 2014). Thus, in addition to inhibiting contact chemosensation (taste), DEET might exert its effects through multiple types of olfactory mechanisms: masking, serving as a confusant, and direct activation of ORs. However, the relative contributions of each of these mechanisms and whether olfactory receptors other than ORs function in DEET repellency remain to be worked out.
Pheromone detection
Pheromones are chemicals that promote communication between members of the same species (Karlson and Luscher 1959). They are produced by one animal for the purpose of impacting the behavior of a conspecific. In insects, such as Drosophila, pheromones stimulate or suppress courtship and mating, aggression toward members of their own sex, and post-mating behaviors in females. Many pheromones are long-chain hydrocarbons, most of which are produced in oenocytes and secreted onto the cuticle. A few are synthesized in the male ejaculatory bulb and transferred to females during mating. Some cuticular pheromones are sufficiently volatile to be detected through the sense of smell, while most are detected through direct contact through taste receptors. The initial cues that flies use to evaluate a mate are vision and smell, followed by hearing, taste, and touch. These senses, combined with past experience and the metabolic state help an animal to decide whether to initiate courtship and mate or fight or ignore the other animal.
Contact pheromone sensation
The cuticular hydrocarbons that serve as pheromones tend to have low volatility. Consequently, many female pheromones are detected by males through leg tapping or licking as part of a multi-sensory courtship ritual. There are also male pheromones that are sensed through direct contact. The GRNs in the front legs that are employed in pheromone detection are male-specific and depend on expression of a male-specific isoform of the transcription factor Fruitless (FruM) for their development. Two of the four GRNs on male legs express FruM and are referred to as M or F neurons depending on whether they respond to male and female pheromones, respectively (Thistle et al. 2012; Toda et al. 2012). Pheromone sensation appears to follow a labeled-line mechanism. As with GRNs that sense either positive or negative chemicals in food and stimulate or inhibit feeding, distinct neurons are activated by male or female pheromones and induce opposite effects on courtship.
Only a handful of Drosophila pheromones have been studied in detail, and several have been associated with specific taste receptors. Well-characterized fly pheromones include (Z,Z)-7,11-heptacosadiene (7,11-HD) and (Z,Z)-7,11-nonacosadiene (7,11-ND), which are distributed on the female cuticle and are aphrodisiacs for males (Antony et al. 1985). Other pheromones, such as (z)-7-tricosene (7-T), and (3R,11Z,19Z)-3-acetoxy-11,19-octacosadien-1-ol (CH503), decorate the male cuticle and are anti-aphrodisiacs (Scott 1986; Lacaille et al. 2007; Billeter et al. 2009; Yew et al. 2009). 7-T and CH503 inhibit courtship to mated females after males transfer these pheromones to females during copulation (Yew et al. 2009). GRs, Ppks, and IRs are three families of receptors that detect pheromones through contact chemosensation and are described below.
GRs and contact pheromones
Multiple GRs are required for responding to pheromones that inhibit courtship behavior including the commonly expressed receptor (CER), GR32a, which is not expressed in FruM-positive neurons but in neurons that contact FruM neurons (Fan et al. 2013). GR32a senses 7-T and is required to suppress courtship in males toward (1) mated females, (2) other males, and (3) females of other Drosophila species (Miyamoto and Amrein 2008; Wang et al. 2011; Fan et al. 2013). Moreover, sensation of 7-T by GR32a facilitates the aggression-promoting effect of another pheromone, cis-vaccenyl acetate (cVA) (Wang et al. 2011), which is detected primarily through smell (see below). While GR32a is a subunit of a receptor for 7-T, the set of GRs that are sufficient for responding to this pheromone is not known. Nevertheless, another CER, GR33a, is an additional candidate 7-T subunit since it is required for suppressing male-male courtship (Moon et al. 2009). Both GR32a and GR33a are CERs suggesting that the complete 7-T receptor includes at least one additional ligand-specific GR subunit.
The role of GR68a in courtship appears to be complex. GR68a is expressed in the male forelegs (Bray and Amrein 2003; Shankar et al. 2015) and contributes to courtship suppression, where it is required for sensing the anti-aphrodisiac, CH503 (Shankar et al. 2015). An earlier study suggested that GR68a is required for detecting an attractive pheromone produced by females (Bray and Amrein 2003), which is counter to the finding that GR68a is the receptor for an anti-aphrodisiac (Shankar et al. 2015). The potential explanation for the earlier proposal that GR68a responds to an attractive female pheromone emerged after the realization that GR68a has a second, non-chemosensory role. In addition to expression in GRNs, Gr68a is also present in auditory neurons and other types of MSNs and is required to detect moving females, presumably through acoustic cues (Ejima and Griffith 2008). However, given its broad distribution in other types of mechanosensory organs, it cannot be excluded that GR68a is employed to detect surface vibrations produced by moving females (Ejima and Griffith 2008). Nevertheless, the mechanosensory requirement for GR68a for detecting moving females may explain the earlier conclusion that this receptor responds to an attractive female pheromone (Bray and Amrein 2003).
GR39a may also sense pheromones. All four Gr39a isoforms appear to be expressed in the legs and labellum, and a P-element insertion affecting all Gr39a isoforms reduces courtship of males to females (Watanabe et al. 2011; Ling et al. 2014). The pheromone(s) that signal(s) through GR39a is (are) not known, but likely candidates include the aphrodisiacs 7,11-HD and 7,11-ND.
Ppks and contact pheromones
Three Na+ channel subunits that belong to the ENaC family, Ppk23, Ppk25 and Ppk29, function in the detection of female and male pheromones through contact chemosensation (Lin et al. 2005; Liu et al. 2012; Lu et al. 2012; Starostina et al. 2012; Thistle et al. 2012; Toda et al. 2012). Ppk23 is expressed in both M and F neurons in the forelegs (Lu et al. 2012; Thistle et al. 2012). In contrast, Ppk25 is expressed in the F neurons only, while Ppk29 is localized to either one or both of these neurons depending on the sensilla (Liu et al. 2012; Starostina et al. 2012; Thistle et al. 2012). Consistent with these expression studies, half of the fru-positive neurons in the forelegs respond to the stimulatory female pheromone 7, 11-HD, and all three Ppks contribute to male–female courtship (Lu et al. 2012; Starostina et al. 2012; Thistle et al. 2012; Toda et al. 2012; Liu et al. 2018). The M neurons, which respond to male pheromones, appear to depend on Ppk23 but not Ppk25 since mutation of ppk23 but not ppk25 increases male courtship (Starostina et al. 2012; Thistle et al. 2012). Ppk channels also appear to function in pheromone detection in females, since mutations disrupting ppk23, ppk25 or ppk29 reduce their receptivity to males. However, this phenotype is only revealed if the antenna or arista are surgically removed (Vijayan et al. 2014). Since these organs are required for audition, this suggests that hearing and contact pheromones act redundantly to stimulate receptivity in females.
IRs, contact chemosensation, and sexual behaviors
IR52c and IR52d may be activated by contact pheromones as they are expressed in sexually dimorphic neurons in the male forelegs that do not express GRs or Ppks (Koh et al. 2014). However, Ir52c-positive neurons do not express fru, although they make contacts with these neurons (Koh et al. 2014) similar to the situation with Gr32a (Fan et al. 2013). Ir52c and Ir52d have evolved rapidly (Koh et al. 2014) consistent with the proposal that this is a signature of genes involved in sexual behaviors (Civetta and Singh 1998). Males missing one or the other of these genes exhibit a delay in the time to copulation supporting the concept that Ir52c and Ir52d respond to a female pheromone (Koh et al. 2014). In contrast to Ir52c and Ir52d, the Ir52a reporter is expressed in GRNs in all legs (Koh et al. 2014). Ir52a is also expressed in GRNs in wing margins, which sense pheromones from other flies, and functions in regulating sexual behaviors in both males and females (He et al. 2019). The contact pheromones that stimulate these IRs remain to be defined.
Detection of cVA by the olfactory system
All 147 trichoid sensilla respond to volatile pheromones (van der Goes van Naters and Carlson 2007). However, different ORNs in these sensilla have different sensitivities to odors from males and from virgin females. Trichoid sensilla harbor either one, two, or three ORNs, and are referred to as T1, T2, and T3, respectively. Although all three classes of trichoid sensilla respond to male pheromones, the ORNs in T1 sensilla are most sensitive to male pheromones (van der Goes van Naters and Carlson 2007). In contrast, ORNs in T2 and T3 sensilla are the most sensitive to pheromones from virgin females, thereby enabling flies to distinguish odors from males and virgin females (van der Goes van Naters and Carlson 2007).
To achieve high reproductive success, it is not enough for a male to mate with a female. The male must also minimize the chance that the female will re-mate with another male (polyandry) since most of the progeny are the product of the last mating. To discourage polyandry, the male transfers multiple inhibitory pheromones during mating, which reduces female attractiveness to other males. Two of these pheromones (7-T and CH503) are transferred directly to the cuticle and sensed through contact chemosensation as described above. A third is cVA, which is male-specific (Butterworth 1969). cVA is introduced to the female in the ejaculate and then sensed primarily through olfaction. While a male is motivated to block a female from re-mating with another male, the female attempts to eject cVA from her reproductive tract to restore attractiveness to other males (Laturney and Billeter 2016). The other anti-aphrodisiacs that remain on the female, such as 7-T, are insufficient to effectively suppress polyandry.
The molecular mechanisms underlying the olfactory detection of cVA in males and females has been studied in greater detail than for any other pheromone. cVA enters trichoid sensilla and binds to an OBP, LUSH, which increases the solubility of cVA in the endolymph bathing the ORN dendrites (Laughlin et al. 2008). cVA then directly activates an OR complex comprised of OR67d and the co-receptor, ORCO (Ha and Smith 2006; Ejima et al. 2007; Kurtovic et al. 2007; van der Goes van Naters and Carlson 2007; Jin et al. 2008; Gomez-Diaz et al. 2013). The rapid activation and inactivation of OR67d by cVA depends on SNMP1 (Li et al. 2014)—a CD36 family member with two transmembrane segments separated by a large extracellular domain. It has been proposed that this ectodomain serves to promote the release of cVA from the OBP (LUSH) and provides a tunnel to direct cVA to OR67d/OCRO while insulating the hydrophobic pheromone from the aqueous endolymph (Gomez-Diaz et al. 2016).
cVA does more than suppress the attractiveness of mated females to other males. cVA on the male stimulates receptiveness to mating in virgin females (Kurtovic et al. 2007). These opposite reactions by males and females to cVA (courtship repulsion and receptivity) are defined by distinct neuronal circuits controlled by fru (Datta et al. 2008). cVA also promotes aggregation in both sexes (Bartelt et al. 1985; Xu et al. 2005). In addition, females deposit cVA on eggs to mark favorable egg-laying sites. This encourages additional females to deposit their eggs on the same site possibly so that the larval density is sufficient to keep growth of microorganisms under control and process the food (Duménil et al. 2016).
cVA impacts on male–male aggression (Wang and Anderson 2010; Liu et al. 2011). However, whether cVA stimulates or inhibits aggression depends on whether the males are exposed acutely or long term to this pheromone. The positive or negative effects of cVA occurs through distinct receptors and ORNs. Acute cVA exposure stimulates aggression through activating OR67d (Wang and Anderson 2010; Liu et al. 2011). A high density of males also increases aggression, and this presumably occurs through elevated levels of volatile cVA in the presence of many males (Wang and Anderson 2010). Opposite to the effects of acute cVA in promoting aggression, chronic cVA exposure or long-term exposure to males suppresses male aggression (Liu et al. 2011). This inhibition depends on OR65a, which is expressed in ORNs distinct from OR67d.
In contrast to cVA, which is a volatile anti-aphrodisiac, methyl laurate (ML), methyl myristate (MM), and methyl palmitate (MP) are attractive olfactory pheromones for both males and females (Dweck et al. 2015). The ORNs that sense these pheromones are housed in trichoid sensilla in males that are fruM positive. Ectopic expression in empty neurons indicates that OR47a is tuned to ML but not MM and MP, while OR88a responds to ML and MM. Mutation of Or47a impairs male–female courtship. However, loss of Or88a has no effect on mating behavior, suggesting that there is a redundant receptor for OR88a.
While cVA is produced by males and is sensed at a relatively close range, (Z)-4-undecenal (Z4-11Al) is synthesized by females and attracts flying males and females at a long distance (Lebreton et al. 2017). Z4-11Al is reported to activate a basiconic sensillum (ab9) rather than trichoid sensilla (Lebreton et al. 2017), which respond to cVA (van der Goes van Naters and Carlson 2007). Two OR69a isoforms (OR69aA and OR69aB) appear to be the Z4-11Al receptor since they are expressed in ab9, and introduction of OR69aA and OR69aB in neurons that do not normally respond to Z4-11Al confers sensitivity to this pheromone (Lebreton et al. 2017). These receptors are also tuned to food odors raising the possibility that the combination of pheromone and food signaling brings flies together at food sources. However, the behavioral effects caused by knocking out Or69a have not been tested.
IR84a is a receptor that impacts on courtship through an unusual mechanism. The ORNs that express IR84a are FruM positive; however, they are not activated by pheromones (Grosjean et al. 2011). Rather, the IR84a-ORNs sense the volatile fruit-derived chemicals phenylacetic acid and phenylacetaldehyde (Yao et al. 2005; Grosjean et al. 2011). IR84a appears to be a direct receptor for both phenylacetic acid and phenylacetaldehyde since introduction of IR84a into neurons that are normally insensitive to phenylacetic acid and phenylacetaldehyde confers responsiveness to these botanical chemicals (Grosjean et al. 2011). The key finding is that IR84a is required in males for achieving the normally high levels of male–female courtship even though IR84a is activated by fruit-derived chemicals rather than pheromones. This fascinating discovery is supported by the observations that Drosophila melanogaster has a bias for mating on food sources, including fruit (Spieth 1974; Markow and O’Grady 2008). Such a mechanism would have selective advantages as females need to lay their eggs on sites that are sufficiently nutrient rich to support larval growth.
Somatosensation
Somatosensation includes senses that perceive information received at the body surface such as the detection of gentle and noxious touch, thermosensation as well as the static positions and dynamic movements of joints, which are referred to as proprioception. Due to space limitations, this section on somatosensation focuses on exteroreception and exteroreceptors that enable Drosophila to sense external, somatosensory stimuli.
Touch with tough skin
How do adult flies sense soft touch when they are covered with an external exoskeleton, which by design serves as a shield to protect against mechanical assaults? A major part of the solution is provided by bristles that protrude through the cuticular exoskeleton and respond to physical movements. These external sensory organs, which are the primary organs used for detecting external mechanical stimuli, are distributed on many body parts including the thorax. The bristles point toward the rear of the fly and come in two sizes—the larger macrochaetes and smaller microchaetes (Figure 7A). Several cell types are associated with each bristle, including a mechanically activated neuron, which extends a dendrite into the base of the endolymph-filled hair shaft. Because the dendrites are fixed to the base of the bristle, movements of the hairs open mechanically-gated channels. Deflections that bend the bristles toward the body are the most effective in activating the MSNs (Walker et al. 2000). The level of K+ in the endolymph is high. Consequently, upon bending of the bristles, K+ is the primary cation that enters and depolarizes the neurons. Other types of mechanoreceptive organs include dome-shaped campaniform sensilla, which function in sensing stresses in the surrounding cuticle, hair plates, which sense joint movements, and stretch-sensitive chordotonal organs situated internally beneath the cuticle (Tuthill and Wilson 2016). Some chordotonal organs contribute to hearing as described in the section on audition and others for proprioception, which also depends on external sensory organs (Kernan 2007). All of these sensory organs contain ciliated, microtubule-containing dendrites (type 1 sensory organs).
Figure 7.
Mechanosensory sensilla on an adult thorax, thermosensory neurons in the arista, and sensory organs and neurons in larvae. (A) Distribution of microchaetae and macrochaetae on the adult thorax. (B) Arborization of class IV multidendritic neurons, which tile the body wall. (C) Sensory organs in larvae. (D) Side view of a head showing the positions of the anterior cell neurons in the brain (green) as well as the hot cell neurons (red cells) and cold cell neurons (blue) in the arista, a portion of which is magnified to the left.
arvae also use non-ciliated sensory neurons called type II multidendritic (md) neurons to detect mechanical and thermal stimuli. The type II md neurons, which are not contained within a specialized structure such as a bristle or chordotonal organ, are categorized in larvae into three subtypes: (1) tracheal dendrite (md-td) neurons, (2) neurons with bipolar dendrites (md-bd), and (3) dendritic arborization (md-da) neurons (Bodmer and Jan 1987). The larval md-da neurons are further subdivided into classes I, II, III, and IV based on the increasing complexity of the dendrites, which tile the body wall (Grueber et al. 2002). Some of these da neurons persist in the adult (Shimono et al. 2009).
Models for activation of mechanosensory channels
Deflection of mechanosensory bristles in Drosophila elicits electrical responses in microseconds (Walker et al. 2000) comparable to the rapid mechanosensory kinetics recorded in mammals. The speed of mechanotransduction is considerably greater than the 10-ms timescale for phototransduction, which is the fastest known chemical transduction cascade. The rapid kinetics of mechanotransduction is made possible by direct activation of channels by mechanical force.
There are at least two models as to how mechanosensory channels might be activated. According to the “membrane conformation model,” changes in curvature of the lipid bilayer lead to gating of the channels. A modification in membrane conformation could be induced by direct physical force on the lipid bilayer. As appears to occur in fly photoreceptor cells, alteration of the conformation of the membrane could also be mediated by an enzymatically induced mechanism, such as PLC-dependent hydrolysis of PIP2 (Hardie and Franze 2012; Liu and Montell 2015). However, this latter membrane conformation mechanism is inconsistent with sub-millisecond kinetics of mechanosensation.
A second model postulates that there exists a flexible tether linking the mechanically gated channel to a fixed internal or external structure, such as the cytoskeleton, or an extracellular structure (Gillespie and Walker 2001; Howard and Bechstedt 2004). According to this “tether model,” force-induced movements of the rigid structure cause movements in the tether resulting in activation of the channel. The flexible tether (gating spring) could be part of the channel itself or a separate structure interfacing the rigid structure and the channel.
Gentle touch receptors
Gentle touch is critical for multiple behaviors. It allows flies and larvae to sense their environment, which is necessary for navigation. Soft touch enables adults to detect light debris, which initiates a repetitive grooming sequence (Seeds et al. 2014; Mueller et al. 2019) and the presence of small pests and kicks them off (Li et al. 2016). Gentle touch contributes to courtship since males tap females as one of the initial events in this multistep behavior (Bontonou and Wicker-Thomas 2014). Mild physical encounters between flies also impact on social interactions (Ramdya et al. 2015).
In larvae, light touch is mediated through class II and class III md-da neurons (Walker et al. 2000; Tsubouchi et al. 2012; Yan et al. 2013), which are distinct from the neurons that mediate noxious touch as described below. In adults, light touch is enabled in part through MSNs associated with macrochaetes and microchaetes (Walker et al. 2000). MSNs in recurved bristles on the wing margin harbor MSNs also function in light touch (Li et al. 2016).
At least two TRP channels are involved in light touch in adults. These include the TRPV channel, Nan, which function in MSNs in the wing margin (Li et al. 2016). The TRPN channel, NOMPC, is required for light touch in adults and in larvae (Walker et al. 2000; Tsubouchi et al. 2012; Yan et al. 2013).
The first demonstration that NOMPC channels are mechanically gated emerged from work on the NOMPC homolog in C. elegans (TRP-4), which is expressed in cephalic (CEP) MSNs (Li et al. 2006; Kang, Gao, et al. 2010; Kang et al. 2011). A conductance in CEP neurons is mechanically activated in microseconds and is dependent on TRP-4 (Kang, Gao, et al. 2010). Moreover, the current is altered by mutations in the pore loop (Kang, Gao, et al. 2010) providing strong evidence that TRP-4 is a pore-forming subunit of a mechanically-activated channel in vivo. Drosophila NOMPC is also mechanically gated, as ectopic expression of this channel in vivo is sufficient to endow neurons with mechanosensitivity (Gong et al. 2013; Yan et al. 2013; Zhang et al. 2015; Jin et al. 2017).
A feature of mechanically-gated channels is the employment of a gating spring, which provides a compliant element so that physical force opens the channel. NOMPC includes a record setting 29 ankyrin repeats, which are situated N-terminal to the first transmembrane segment. These ankyrin repeats appear to be a critical part of the “gating spring” (Howard and Bechstedt 2004; Liang et al. 2013; Knecht et al. 2015; Zhang et al. 2015; Jin et al. 2017).
Another protein that functions in gentle touch in larvae is referred to as Brivido-1 (Brv1). Brv1 is related to the TRPP family of proteins, which along with TRPML defines the group 2 TRPs (Table 1). As with the group 1 TRPs (TRPC, TRPV, TRPA, TRPM and TRPN), the two group 2 TRPs (TRPML and AMO) include six TMDs. However, Brv proteins, which are related to TRPP1 (The International Polycystic Kidney Disease Consortium 1995), consist of a greater number of TMDs (up to 11). The C-terminal six TMDs of TRPP1 proteins bear sequence similarity to TRPP2 channels, suggesting that they might be cation channels. Indeed, functional expression of Brv1 in tissue culture cells indicates that it is a pore-forming subunit of a stretch-activated channel (Zhang et al. 2018).
Similar to the requirement for NOMPC, Brv1 is required in class III md-da neurons for gentle touch (Zhang et al. 2018). Thus, the question arises as to the function of two mechanically-gated channels in the same cells and whether they form distinct or heteromultimeric channels. Based on the results of in vitro patch clamp recordings, it is proposed that Brv1 functions autonomously as a stretch-activated channel and also as a modulator of NOMPC (Zhang et al. 2018).
Noxious touch
The ability to sense noxious mechanical stimuli is critical for survival, as it allows larvae and flies to detect assaults that cause injury, including attacks from parasitic wasps and mites (Hwang et al. 2007; Li et al. 2016). Strong mechanical stimulation is sensed in adults by the same MSNs associated with macrochaetes and microchaetes that are involved in light touch. Thus, the interpretation of whether the mechanical stimulus is soft or noxious may be defined by action potential frequencies. The MSNs in the recurved bristles on the wing margins may also be involved in both light and harsh touch (Li et al. 2016).
In larvae, mechanical nociception is detected through class IV neurons, which are endowed with the most extensive and highly branched dendritic arbors among all the da neurons (Figure 7B) (Grueber et al. 2002; Hwang et al. 2007). These neurons respond to several types of noxious cues including harsh mechanical stimuli, short wavelength light as described above, and noxious heat, as described below. In adults, mechanical nociception is detected by movement of external mechanosensory bristles (Walker et al. 2000; Li et al. 2016).
Multiple classes of channels function in class IV neurons in larvae for detection of noxious mechanical stimuli. These include the TRPA channels (Pain) (Tracey et al. 2003), and TRPA1 (Zhong et al. 2012) as well as three ENaCs: Ppk (Ppk1), Ppk26 (Balboa), and Ppk30 (Zhong et al. 2010; Guo et al. 2014; Mauthner et al. 2014; Jang et al. 2019). Ppk1 and Ppk26 may form subunits of a heteromultimer (Mauthner et al. 2014). Given that ENaCs include three subunits (Hanukoglu 2017), it is plausible that the functional channel may consist of a Ppk1/Ppk26/Ppk30 heterotrimer. The Drosophila homolog of mammalian Piezo channels, which are mechanically gated (Coste et al. 2010), is also required in larvae for mechanical nociception (Kim et al. 2012). Mammalian Piezo channels are comprised of 38 TMDs and form a trimer with extended arms resembling a propeller (Guo and MacKinnon 2017; Saotome et al. 2018; Zhao et al. 2018).
While multiple channels have been uncovered that function in mechanical nociception in larvae, the channels required for the detection of harsh touch in adult flies have not been explicitly identified. However, they are presumably NOMPC and Nan. The MSNs in external bristles that express these proteins detect both low- and high-intensity mechanical stimuli, and most likely do so through these TRP channels.
Temperature sensation
The ability to sense environmental temperatures is critical for survival, as it alerts animals to suboptimal or dangerously hot and cold conditions. The capacity to sense and respond to ambient temperature is especially important for very small, poikilothermic organisms, such as Drosophila whose body temperature equilibrates with the environment. Moreover, the rate of development and body size is very sensitive to the surrounding temperature (Ray 1960). While 18–25°C is the ideal range for Drosophila, an increase from just 18°C to 25°C accelerates the rate of development twofold. If the flies are forced to remain at 10°C, they go into diapause and cease reproduction. Long-term exposure to temperatures ≥35°C greatly decreases survival. Due to the major consequences of even relatively small differences in temperature on physiology, both larvae and adults are endowed with a variety of thermosensory neurons and molecular mechanisms to sense hot, cold, and slightly suboptimal temperatures in the comfortable range.
Behavioral responses by larvae to noxious and suboptimal temperatures
Larvae avoid uncomfortably high and low temperatures by orchestrating a set of discrete behaviors that allow them to sense a temperature gradient and then adjust their direction of movement (Kwon, Shen, et al. 2010; Luo et al. 2010; Lahiri et al. 2011; Klein et al. 2015). To assess the environmental temperature at a given time, a larva stops its forward movement, and sweeps its head from side to side one or more times. If the animal is moving in the direction of a less desirable temperature, the forward run lengths decrease, and the total number of turns and the average turning angle increases (Kwon, Shen, et al. 2010; Luo et al. 2010; Lahiri et al. 2011; Klein et al. 2015). However, if a larva comes into contact with a hot probe that contacts a discrete point on its body (e.g. 44°C), it attempts to quickly escape by initiating a very rapid rolling response perpendicular to its body axis (Tracey et al. 2003). Larvae will also initiate a rolling escape response at much lower warm temperatures if the entire body is subjected to a very fast rise in temperature (Luo et al. 2017). In contrast to the rapid rolling escape behavior induced by dangerous heat, larvae react to noxious cold (≤14°C) by contracting their bodies (Turner et al. 2016).
Channels required in larvae for detecting noxious heat and cold
Multiple channels are activated directly by hot temperatures, and contribute to the detection of uncomfortable or noxious heat in larvae, including the TRPA channels, Pain, and TRPA1. Mutations affecting either of these channels impair the rolling response to a hot probe of ∼44°C (Tracey et al. 2003; Neely et al. 2011; Zhong et al. 2012; Gu et al. 2019). In addition, Pain and TRPA1 promote thermal allodynia and hyperalgesia, respectively (Babcock et al. 2011). The contributions of Pain and TRPA1 to heat nociception are mediated through class IV multidendritic neurons (Figure 7B), which also sense harsh touch (Tracey et al. 2003; Neely et al. 2011; Hwang et al. 2012).
The requirements for Pain to detect noxious mechanical and thermal stimuli appear to be mediated by distinct isoforms. Pain is alternatively spliced resulting in production of versions with different numbers of N-terminal ankyrin repeats (two, six, and eight) (Hwang et al. 2012). Based on genetic rescue of a strong pain allele, the isoform with eight ankyrin repeats (Painp103) functions in thermal but not mechanical nociception, while the isoform with only two ankyrin repeats (Painp60) is needed for mechanical but not thermal nociception (Hwang et al. 2012). These results argue against the concept that a large string of ankyrin repeats serve as gating spring, at least for Pain (Hwang et al. 2012).
TRPA1 is expressed as five isoforms (TRPA1-A–TRPA1-E) (Kwon, Kim, et al. 2010; Kang et al. 2012; Zhong et al. 2012; Gu et al. 2019) all of which share the same 13 N-terminal ankyrin repeats, TMDs and C-termini, but include distinct combinations of two alternative N-termini and two short sequences between the ankyrin repeats and TMDs. Multiple studies demonstrate that TRPA1-A and TRPA1-D are heat activated in vitro and function in temperature sensation in vivo (Viswanath et al. 2003; Kang et al. 2012; Zhong et al. 2012; Luo et al. 2017; Gu et al. 2019). TRPA1-B and TRPA1-C may also be heat-activated at least in vitro (Gu et al. 2019). Although there are variations in the thermal thresholds in different heterologous expression systems, TRPA1-A appears to be turned on at approximately ≥27°C, while TRPA1-D is activated at approximately ≥34°C. The TRPA1-E isoform may not be functional.
Both TRPA1-C and TRPA1-D are expressed in class IV neurons, where they have different nociceptive functions. Class IV neurons mediate the writhing response due to exposure to UV-C, which promotes production of H2O2 (Kim and Johnson 2014). TRPA1 is activated by H2O2 (Guntur et al. 2015), and the TRPA1-C isoform in class IV neurons is required for the nociceptive response to UV-C (Guntur et al. 2017). TRPA1-D in these same class IV neurons functions in thermal nociception (Gu et al. 2019).
Many animals including Drosophila are sensitive not only to the absolute temperature but also to the rate of temperature change. Larvae exhibit a robust nociceptive rolling response at lower temperatures when the rate of temperature increase is very rapid vs very slow (29 vs 34°C) (Luo et al. 2017). These rolling behaviors, which are stimulated by heating the entire body, are evoked by temperatures considerably lower than the ≥39°C needed to induce rolling with a heat probe applied to a small spot on the larvae. The ability to sense the rate of temperature change depends on expression of TRPA1-A in a small subset of neurons in the brain (Luo et al. 2017). This is distinct from the roles of TRPA1-C and TRPA1-D in class IV neurons in sensing a much hotter noxious heat probe applied to specific portion of the body.
In addition to TRP channels, an anoctamin family member called Subdued functions in sensing noxious heat in larvae. Anoctamin proteins have 8 TMDs, and some family members are Ca2+-activated Cl− channels. Similar to mammalian Ano1 (Cho et al. 2012), Subdued is activated by Ca2+ and ≥40°C and contributes to depolarization. The channel is required in class IV neurons for the rolling escape response that is stimulated by a hot probe. The Subdued channel may function in concert with TRPA1 and Pain. Heat activation of these TRP channels may provide the Ca2+ that augments the thermal activation of Subdued, thereby contributing to heat-induced depolarization (Jang et al. 2015).
In contrast to the class IV neurons that respond to noxious heat, larvae detect noxious cold (≤10°C) through class III multidendritic neurons (Turner et al. 2016). The contractions of larvae in response to cold are mediated by three TRP channels in class III neurons: NOMPC, TRPM, and PKD2 (Turner et al. 2016) (PKD2 is also known as AMO) (Watnick et al. 2003). It remains to be determined if any of these channels are directly cold activated.
Larval detection of slightly warm and cool temperatures
Distinct from the rolling escape and body contractions that larvae employ in response to noxious heat and cold, larvae use navigation to avoid slightly uncomfortable temperatures (Kwon, Shen, et al. 2010; Luo et al. 2010; Lahiri et al. 2011; Klein et al. 2015). Thermotaxis down a temperature gradient from excessively warm temperatures (31–35°C) toward 24°C depends on TRPA1 (Rosenzweig et al. 2005).
Larvae prefer temperatures in the 18–24°C range (Kwon et al. 2008), although their ideal temperature within this range varies with the developmental stage. When placed in an 18–25°C gradient, mid- or late-third instar larvae accumulate in the 18°C zone due to avoidance of the warmer temperatures (Sokabe et al. 2016). TRPA1 is also required for temperature discrimination within the 18–24°C (Kwon et al. 2008), which is below the approximate ≥27°C required for direct activation of the lowest threshold TRPA1 isoform (Viswanath et al. 2003; Kang et al. 2012; Zhong et al. 2012; Gu et al. 2019).
In the comfortable temperature range (18–25°C) TRPA1 is indirectly activated through a Gq, PLCβ signaling cascade (Kwon et al. 2008; Shen et al. 2011; Sokabe et al. 2016). The GPCRs that initiate this cascade are three rhodopsins: Rh1, Rh5, and Rh6 (Shen et al. 2011; Sokabe et al. 2016). Thus, TRPA1 is thermally activated through two mechanisms. Noxious or uncomfortable heat (≥27°C) directly activates TRPA1. However, temperatures in the comfortable range that are below the threshold for activating any TRPA1 isoform indirectly activate TRPA1 through an amplification cascade that this is initiated by multiple rhodopsins. Since GPCRs may be tetrameric (Petrin and Hebert 2012; Redka et al. 2014; Cordomi et al. 2015; Navarro et al. 2016; Sleno and Hebert 2019), the three rhodopsins required for initiating the amplification cascade may form a heteromultimeric receptor. It remains to be determined whether the rhodopsins are direct thermosensors. If so, the environment of the thermosensory neurons must enable rhodopsins to overcome the high thermal stability of these proteins in photoreceptor cells (Luo et al. 2011).
Larvae also use a navigation strategy to locate slightly warmer temperatures over temperatures that are cool but not noxious. This behavior depends on three bilaterally symmetrical neurons in each dorsal organ near the fly head (Figure 7C) (Klein et al. 2015), which require IR21a, IR25a, and IR93a (Knecht et al. 2016; Ni et al. 2016). Cool sensing does not only appear to be limited to the head region but also depends on chordotonal neurons and the TRPV channel, Iav, since multiple alleles eliminate the ability of third instar larvae to discriminate between 17.5 and 14°C in a two-way choice assay (Kwon, Shen, et al. 2010). Another study concluded that Iav does not play a role because expression of the tetanus toxin light chain in chordotonal neurons has no impact on temperature discrimination (Klein et al. 2015). However, temperature preferences change significantly during larval development, and this latter study was conducted using an earlier larval stage (second instar) and under different temperature conditions (15–22°C gradient).
Sensing innocuous warm and cool temperatures in adults
Adult flies sense slight warming and cooling through different sets of neurons and multiple channels. Mild heating is detected in part through “anterior cell” neurons in the brain (Hamada et al. 2008) and by activation of three of the six neurons in the arista—the “hot cell” neurons (also called heating cells) (Foelix et al. 1989; Gallio et al. 2011; Ni et al. 2013) (Figure 7D). Gentle cooling is through stimulation of neurons in the sacculus and the other three neurons in the arista referred to as cold cells (also called cooling cells; Figure 7D), which have dendrites consisting of complex layers of lamellae, decorated on the outside with small particles (BOSS-structures) (Foelix et al. 1989; Gallio et al. 2011; Budelli et al. 2019).
The sensation of cooling by the cold cells depends on the three Brv proteins (Brv1, Brv2, and Brv3) (Gallio et al. 2011). Loss of any of these Brv proteins impairs the behavioral discrimination of 25°C from cool temperatures, such as 15°C (Gallio et al. 2011). A subsequent study concludes that the Brv proteins are dispensable for activating cold cells (Budelli et al. 2019). Rather, cool activation of the cold cells is reported to depend on the same three IRs (IR21a, IR25a, and IR93a), which function in the larval dorsal organ (Budelli et al. 2019). However, the paradigms tested in the two studies were quite different. First, the study focusing on the IRs did not examine a contribution of the Brv proteins in the cool range. Rather, they tested roles for the IRs and Brvs in cooling from 30 to 25°C (Budelli et al. 2019). Second, the analysis by Budelli et al. (2019) did not address the behavioral effects of loss of the Brv proteins for detecting cool stimuli. Thus, the Brvs and IRs may all contribute to sensing either cool temperatures or cooling but in different ranges.
In addition to functioning in cool sensation, mutations eliminating IR21a, IR25a, or IR93a also disrupt the morphology of the cold cells (Budelli et al. 2019). If morphogenesis depends on the IRs, this finding would be reminiscent of the dual requirements of rhodopsin 1 (Rh1) for light sensation and for morphogenesis of fly photoreceptor cells (Montell 2012). However, an alternative explanation is that the Ir mutations cause rapid degeneration of structures in the cold cells.
The behavioral responses to slow and rapid warming in the innocuous range appear to be mediated through distinct mechanisms. The slow response depends on TRPA1 in anterior cells while the rapid response to increasing temperature is impaired by loss of Gr28b(D), which is expressed in hot cells (Figure 7D) (Ni et al. 2013). Both TRPA1 and GR28b(D) are required in adults for sensing the rate of temperature change, as well as the absolute temperature (Soto-Padilla et al. 2018). However, the behavioral deficits elicited by the Gr28b mutants are only partial (Soto-Padilla et al. 2018; Budelli et al. 2019). The response to rapid warming also depends on the cold cells and expression of the IRs (Budelli et al. 2019) through a mechanism that remains to be described.
Sensors for noxious temperatures in adults
Currently, none of the cold channels/receptors have been defined. However, three warm or hot activated TRP channels are required for sensing noxious heat in adults, all of which belong to the TRPA subfamily: TRPA1, Pain, and Pyrexia (Pyx) (Viswanath et al. 2003; Lee et al. 2005; Xu et al. 2006; Sokabe et al. 2008; Neely et al. 2010, 2011; Zhong et al. 2012; Gu et al. 2019). Mutations in trpA1 and pain greatly decrease the fly’s capacity to sense a very hot environment such as ∼45°C (Xu et al. 2006; Neely et al. 2010, 2011). As a consequence, the mutant animals remain in zones with noxious heat and become incapacitated (Neely et al. 2010, 2011) or are impaired in a heat-induced jump response (Xu et al. 2006). While the specific neurons required for detection of high temperatures are unknown, behavioral experiments using flies with different appendages surgically removed suggest that the neurons may be situated in the antenna and proboscis (Neely et al. 2011). The third heat-activated TRPA channel, Pyx contributes to heat tolerance since pyx mutant animals are quickly paralyzed (<3 min) in a 40°C environment (Lee et al. 2005).
Hearing, vestibular, wind, and gravity sensation in adults
Hearing contributes to multiple social and defensive behaviors in adults including courtship, aggression, and helping larvae evade predators (Shorey 1962; Zhang, Yan, et al. 2013; Versteven et al. 2017). To enhance courtship success, males extend one wing and initiate wing vibrations to produce sounds, which help the female determine that the male is a conspecific (Shorey 1962; Hall 1994; Riabinina et al. 2011; Albert and Göpfert 2015; Ishikawa and Kamikouchi 2016). There are three types of “courtship song”—two pulse and one sine type, and the probability of displaying one or the other is influenced by visual feedback (Clemens et al. 2018; Deutsch et al. 2019). If the courtship song stimulates female receptivity, she then slows down to accept the male (Hall 1994; Crossley et al. 1995). In the absence of a courtship song, the flies still mate, but the rate is reduced (Bennet-Clark and Ewing 1969).
Males become aggressive when competing with other males for mating partners, food, and territory. Aggression is displayed and communicated through a complexity of behaviors and senses including acoustic stimuli created by vibration of both wings extended out from their bodies (Jonsson et al. 2011; Versteven et al. 2017). The auditory signals produced by aggressive males are not confused with the courtship song, because they lack a sine-like pattern and are characterized by longer interpulse intervals (Jonsson et al. 2011).
Larvae are also endowed with the ability to sense auditory stimuli. They detect sounds of predators, such as wasps and yellow jackets, and respond with a startle response and by attempting to escape by burrowing into their food (Zhang, Yan, et al. 2013). Larvae detect only low- but not high-frequency sounds.
In adult flies, the ear is located in the antenna and includes the Johnston’s organ (JO), which harbors the largest set of MSNs in the fly (∼475) (Kamikouchi et al. 2006). Sound is captured by a feathery appendage called the arista, which extends out from the largest of three antennal segments (the third antennal segment; A3; also known as the funiculus; Figure 8A). Sound-induced vibrations of the arista in the range of ∼10–1000 Hz as well as larger, lower frequency vibrations and static deflections produced by wind and gravity (Budick et al. 2007; Kamikouchi et al. 2009; Yorozu et al. 2009; Patella and Wilson 2018) cause the main part of A3 to turn along its longitudinal axis. The rotation of A3 activates neurons in the JO in the second antennal segment (A2) also known as the pedicel (Figure 8A). Each JO contains ∼200 repeat chordotonal units (scolopidia) most of which (∼90%) contain two ciliated, bipolar JO neurons in addition to support cells (Figure 8B) (Todi et al. 2004; Ishikawa et al. 2020). One end of each chordotonal unit is attached to the A2/A3 joint, while the other is attached to the A2 cuticle. These physical attachments contribute to the mechanical activation of JO neurons. The ciliated dendrite is separated into two parts by a ciliary dilation—a distal region (tip) that comprises about a fourth of the dendrite, and a proximal region that makes up most of the dendrite.
Figure 8.
Auditory organ. (A) Antenna showing location of Johnston’s organ (JO) in the 2nd antennal segment (A2). The arista and 3rd antennal segment are indicated. Shown are chordotonal neurons in the JO that function in detecting sound (purple) and wind/gravity (green). (B) A single scolopidium, which is the repeat unit in chordotonal organs such as the Johnston’s organ. Each scolopidium in the JO is comprised of multiple cells including two neurons, a ligament cell, a scolopale cell, and a cap cell. The various parts of the neuron are indicated to the right.
The JO neurons that sense sound (JO-A and JO-B) are distinct from those that detect wind and gravity (JO-C and JO-E) (Kamikouchi et al. 2009; Yorozu et al. 2009; Matsuo and Kamikouchi 2013; Matsuo et al. 2014; Ishikawa et al. 2020). The precise role of JO-D neurons is not clear, although they are activated by both vibrations and static deflections (Matsuo et al. 2014). Many JO neurons are also activated during flight and contribute to proprioception (Mamiya and Dickinson 2015).
The different classes of JO neurons express three TRP channels—NOMPC, NAN, and IAV. In JO-A/B neurons and other chordotonal neurons, NOMPC is spatially restricted to the distal dendrite, while the two TRPV channels, Nan and Iav, which are proposed to form a heteromultimeric channel, are localized to proximal region (Figure 8B) (Kim et al. 2003; Gong et al. 2004; Cheng et al. 2010; Lee, Moon, et al. 2010; Liang et al. 2011). The segregation of these TRP proteins to different segments of JO neuron dendrites depends on a protein called TULP, which is necessary for protein trafficking (Park et al. 2013). Interestingly, mammalian homologs of this protein are required for the localization of some sensory receptors such as rhodopsin (Hagstrom et al. 2001).
Currently, the mechanism of auditory transduction is controversial. According to one model, NOMPC is the mechanically-activated channel in auditory JO-A/B neurons and contributes to signal amplification largely through promoting subsequent activation of the Nan/Iav channel (Göpfert and Robert 2003; Göpfert et al. 2006; Kamikouchi et al. 2009; Lee, Moon, et al. 2010; Effertz et al. 2011, 2012; Albert and Göpfert 2015). The less sensitive wind/gravity-sensing JO-C/E neurons do not express NOMPC and therefore depend on another mechanically-gated channel in the distal dendrite that remains to be identified (Kamikouchi et al. 2009; Albert and Göpfert 2015). Using sensitive electrophysiological recordings from giant fiber neurons, which are electrically coupled to JO neurons, another study concludes that Nan/Iav is the transduction channel, and NOMPC contributes to sensitivity of the JO-A/B neurons (Lehnert et al. 2013). Expression of NOMPC in vitro leads to a rapidly activated cation conductance upon mechanical stimulation (Gong et al. 2013; Yan et al. 2013; Zhang et al. 2015; Jin et al. 2017) supporting the proposal that NOMPC might be an auditory transduction channel. On the other hand, the Nan/Iav heteromultimer has not been shown to be mechanically gated, although each of the two individual subunits are activated in vitro by bathing the cells in a hypotonic solution, which causes membrane stretch (Kim et al. 2003; Gong et al. 2004). Nevertheless, the preponderance of evidence indicates that NOMPC and NAN/IAV contribute to hearing (Eberl et al. 2000; Kim et al. 2003; Gong et al. 2004; Göpfert et al. 2006; Effertz et al. 2011, 2012; Lehnert et al. 2013). Intriguingly, two opsins (Rh5 and Rh6) and some of the same proteins that function in phototransduction are expressed in JO neurons and function in amplification of the response to sound (Senthilan et al. 2012). However, the mechanism through which these proteins promote amplification is unclear.
In addition to Nan/Iav, gravity sensation also depends on two TRPA channels, Pain and Pyx, which are expressed in the JO (Lee et al. 2005; Al-Anzi et al. 2006; Sun et al. 2009). pain is detected in JO neurons, while pyx is localized to cap cells (Figure 8B) (Sun et al. 2009). The contributions of pain and pyx to gravity sensation raise a number of unresolved questions. Are either Pain or Pyx directly mechanically gated? Does activation of Pyx in cap cells contribute to activation of Nan/Iav in JO-C/E neurons? Another question is whether Pyx is localized to the distal tip of the JO-C/E as is the case for NOMPC in JO-A/B neurons.
Concluding remarks: sensory receptors as molecular Swiss Army Knives
The work on Drosophila sensory reception has contributed greatly to overturning the long-standing view that each sense functions through specialized receptors dedicated to detecting only one specific type of stimulus. According to this dogma, vision depends on rhodopsin—a protein that functions exclusively in light reception. Somatosensation and hearing employ receptors that have been honed to perfection for these senses only, while smell and taste are made possible by proteins uniquely capable of binding volatile and nonvolatile ligands, respectively.
Due to the many discoveries using Drosophila molecular genetics, the former view that receptors are sculpted to serve only one type of sense is now replaced with the concept that sensory receptors have multimodal functions. TRP channels represent the first and best-documented example of evolutionarily conserved multimodal sensory receptors (Venkatachalam and Montell 2007). They are instrumental for senses ranging from vision to thermosensation, touch, hearing, smell, taste, and hygrosensation. More recent is the realization that opsins are not just light sensors, but also function in thermosensation, hearing, and taste. In addition, another large family of receptors that are also multitaskers are the so-called “gustatory receptors” (GRs). In spite of their name, they are much more than taste receptors. They also function in smell, thermosensation, and most surprisingly, in light-reception. Similarly, the “Ionotropic Receptors” (IRs) were originally characterized as a class of olfactory receptor but have additional roles in taste, thermosensation, and hygrosensation.
A central question is the coding mechanism that allows flies to accurately differentiate between different stimuli that activate the same receptor. For example, since light and gustatory input both activate the same opsins, how do flies tell the difference between light and chemicals? What allows the animals to accurately identify the type of stimulus is dictated by the neurons that are activated or inhibited, rather than the particular type of receptor. Activation of opsins in photoreceptor cells is interpreted as light sensation, while activation of opsins in bitter GRNs is interpreted as an aversive chemical. An even further complication is that the same neurons can be activated by different distinct stimuli. Class B GRNs are activated by bitter compounds and by cool temperatures. In addition, MSNs in the labellum are activated by food texture and by cool temperatures. Thus, if only the class B GRNs are activated, then the animal interprets the stimuli as bitter. If MSNs are activated, the fly is sensing food texture. However, only if class B GRNs in S- and I-type sensilla, as well as MSNs, are activated does the animal interpret the external signal as a cool temperature.
In conclusion, molecular genetic approaches in flies have revealed many families of sensory receptors that can be thought of as collections of molecular Swiss Army Knives. An unopened Swiss Army Knife could be confused at first with a simple pocket knife. However, upon investigating the inner workings of different Swiss Army Knives, what emerges is a diversity of types from the classic version with two functions (a knife and scissors) to very complex variations with an impressive array of tools that serve many functions. Similarly, Drosophila molecular genetics has been enormously successful in revealing the many families of multitasking sensory receptors some of which are evolutionarily conserved throughout animal phylogeny (TRPs and rhodopsins). Others are restricted to certain insects and other invertebrates (GRs and IRs). While it is already established that mammalian TRP channels are polymodal sensory receptors, exciting questions for the future include investigations into unconventional roles for mammalian sensory receptors including opsins, gustatory receptors, olfactory receptors, and mechanically-gated channels that function in hearing and somatosensation.
Acknowledgments
The author thanks Brian Long and Sophie Nebeker for help with the figures.
Funding
Work in the author’s laboratory was supported by grants from the National Institute on Deafness and Other Communication Disorders (DC007864 and DC016278), National Eye Institute (EY008117 and EY010852), and the National Institute of Allergy and Infectious Diseases (AI153334).
Conflicts of interest
None declared.
Literature cited
- Abbott GW. 2016. KCNE1 and KCNE3: the yin and yang of voltage-gated K+ channel regulation. Gene. 576:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuin L, Bargeton B, Ulbrich MH, Isacoff EY, Kellenberger S, et al. 2011. Functional architecture of olfactory ionotropic glutamate receptors. Neuron. 69:44–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afify A, Betz JF, Riabinina O, Lahondère C, Potter CJ. 2019. Commonly used insect repellents hide human odors from Anopheles mosquitoes. Curr Biol. 29:3669–3680.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn JE, Chen Y, Amrein H. 2017. Molecular basis of fatty acid taste in Drosophila. Elife. 6:e30115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai M, Blais S, Park JY, Min S, Neubert TA, et al. 2013. Ionotropic glutamate receptors IR64a and IR8a form a functional odorant receptor complex in vivo in Drosophila. J Neurosci. 33:10741–10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai M, Min S, Grosjean Y, Leblanc C, Bell R, et al. 2010. Acid sensing by the Drosophila olfactory system. Nature. 468:691–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Anzi B, Tracey WD Jr, Benzer S. 2006. Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr Biol. 16:1034–1040. [DOI] [PubMed] [Google Scholar]
- Albert JT, Göpfert MC. 2015. Hearing in Drosophila. Curr Opin Neurobiol. 34:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alerstam T, Backman J. 2018. Ecology of animal migration. Curr Biol. 28:R968–R972. [DOI] [PubMed] [Google Scholar]
- Antony C, Davis TL, Carlson DA, Pechine JM, Jallon JM. 1985. Compared behavioral responses of male Drosophila melanogaster (Canton S) to natural and synthetic aphrodisiacs. J Chem Ecol. 11:1617–1629. [DOI] [PubMed] [Google Scholar]
- Aristotle. 2015. On Sense and the Sensible. English ed. Whitefish, MT: Kessinger Publishing LLC, p. 38. [Google Scholar]
- Babcock DT, Shi S, Jo J, Shaw M, Gutstein HB, et al. 2011. Hedgehog signaling regulates nociceptive sensitization. Curr Biol. 21:1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badsha F, Kain P, Prabhakar S, Sundaram S, Padinjat R, et al. 2012. Mutants in Drosophila TRPC channels reduce olfactory sensitivity to carbon dioxide. PLoS One. 7:e49848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelt RJ, Schaner AM, Jackson LL. 1985. cis-vaccenyl acetate as an aggregation pheromone in Drosophila melanogaster. J Chem Ecol. 11:1747–1756. [DOI] [PubMed] [Google Scholar]
- Benelli G, Mehlhorn H. 2016. Declining malaria, rising of dengue and Zika virus: insights for mosquito vector control. Parasitol Res. 115:1747–1754. [DOI] [PubMed] [Google Scholar]
- Bennet-Clark HC, Ewing AW. 1969. Pulse Interval as a critical parameter in courtship song of Drosophila melanogaster. Anim Behav. 17:755–759. [Google Scholar]
- Benton R, Sachse S, Michnick SW, Vosshall LB. 2006. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 4:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. 2009. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 136:149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. 2002. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 295:1070–1073. [DOI] [PubMed] [Google Scholar]
- Billeter JC, Atallah J, Krupp JJ, Millar JG, Levine JD. 2009. Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature. 461:987–991. [DOI] [PubMed] [Google Scholar]
- Bloomquist BT, Shortridge RD, Schneuwly S, Perdew M, Montell C, et al. 1988. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 54:723–733. [DOI] [PubMed] [Google Scholar]
- Bodmer R, Jan YN. 1987. Morphological differentiation of the embryonic peripheral neurons in Drosophila. Rouxs Arch Dev Biol. 196:69–77. [DOI] [PubMed] [Google Scholar]
- Boeckh J, Breer H, Geier M, Hoever FP, Kruger BW, et al. 1996. Acylated 1,3-aminopropanols as repellents against bloodsucking arthropods. Pesticide Sci. 48:359–373. [Google Scholar]
- Bohbot JD, Dickens JC. 2010. Insect repellents: modulators of mosquito odorant receptor activity. PLoS One. 5:e12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohbot JD, Dickens JC. 2012. Odorant receptor modulation: ternary paradigm for mode of action of insect repellents. Neuropharmacology. 62:2086–2095. [DOI] [PubMed] [Google Scholar]
- Bohbot JD, Fu L, Le TC, Chauhan KR, Cantrell CL, et al. 2011. Multiple activities of insect repellents on odorant receptors in mosquitoes. Med Vet Entomol. 25:436–444. [DOI] [PubMed] [Google Scholar]
- Bontonou G, Wicker-Thomas C. 2014. Sexual Communication in the Drosophila genus. Insects. 5:439–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray S, Amrein H. 2003. A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron. 39:1019–1029. [DOI] [PubMed] [Google Scholar]
- Budelli G, Ni L, Berciu C, van Giesen L, Knecht ZA, et al. 2019. Ionotropic receptors specify the morphogenesis of phasic sensors controlling rapid thermal preference in Drosophila. Neuron. 101:738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budick SA, Reiser MB, Dickinson MH. 2007. The role of visual and mechanosensory cues in structuring forward flight in Drosophila melanogaster. J Exp Biol. 210:4092–4103. [DOI] [PubMed] [Google Scholar]
- Bushdid C, Magnasco MO, Vosshall LB, Keller A. 2014. Humans can discriminate more than 1 trillion olfactory stimuli. Science. 343:1370–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth FM. 1969. Lipids of Drosophila: a newly detected lipid in the male. Science. 163:1356–1357. [DOI] [PubMed] [Google Scholar]
- Cameron P, Hiroi M, Ngai J, Scott K. 2010. The molecular basis for water taste in Drosophila. Nature. 465:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, et al. 1997. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 389:816–824. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. 2006. The receptors and cells for mammalian taste. Nature. 444:288–294. [DOI] [PubMed] [Google Scholar]
- Charlu S, Wisotsky Z, Medina A, Dahanukar A. 2013. Acid sensing by sweet and bitter taste neurons in Drosophila melanogaster. Nat Commun. 4:2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Amrein H. 2017. Ionotropic receptors mediate Drosophila oviposition preference through sour gustatory receptor neurons. Curr Biol. 27:2741–2750.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YD, Dahanukar A. 2017. Molecular and cellular organization of taste neurons in adult Drosophila pharynx. Cell Rep. 21:2978–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YD, Dahanukar A. 2020. Recent advances in the genetic basis of taste detection in Drosophila. Cell Mol Life Sci. 77:1087–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Montell C. 2020. A family of auxiliary subunits of the TRP cation channel encoded by the complex inaF locus. Genetics. 215:713–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Wang Q, Wang Z. 2010. The amiloride-sensitive epithelial Na+ channel PPK28 is essential for Drosophila gustatory water reception. J Neurosci. 30:6247–6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng LE, Song W, Looger LL, Jan LY, Jan YN. 2010. The role of the TRP channel NompC in Drosophila larval and adult locomotion. Neuron. 67:373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Yang YD, Lee J, Lee B, Kim T, et al. 2012. The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat Neurosci. 15:1015–1021. [DOI] [PubMed] [Google Scholar]
- Chou WH, Hall KJ, Wilson DB, Wideman CL, Townson SM, et al. 1996. Identification of a novel Drosophila opsin reveals specific patterning of the R7 and R8 photoreceptor cells. Neuron. 17:1101–1115. [DOI] [PubMed] [Google Scholar]
- Chou WH, Huber A, Bentrop J, Schulz S, Schwab K, et al. 1999. Patterning of the R7 and R8 photoreceptor cells of Drosophila: evidence for induced and default cell-fate specification. Development. 126:607–616. [DOI] [PubMed] [Google Scholar]
- Chu B, Chui V, Mann K, Gordon MD. 2014. Presynaptic gain control drives sweet and bitter taste integration in Drosophila. Curr Biol. 24:1978–1984. [DOI] [PubMed] [Google Scholar]
- Civetta A, Singh RS. 1998. Sex-related genes, directional sexual selection, and speciation. Mol Biol Evol. 15:901–909. [DOI] [PubMed] [Google Scholar]
- Clarke D, Whitney H, Sutton G, Robert D. 2013. Detection and learning of floral electric fields by bumblebees. Science. 340:66–69. [DOI] [PubMed] [Google Scholar]
- Clarke SE, Longtin A, Maler L. 2015. Contrast coding in the electrosensory system: parallels with visual computation. Nat Rev Neurosci. 16:733–744. [DOI] [PubMed] [Google Scholar]
- Clemens J, Coen P, Roemschied FA, Pereira TD, Mazumder D, et al. 2018. Discovery of a new song mode in Drosophila reveals hidden structure in the sensory and neural drivers of behavior. Curr Biol. 28:2400–2412.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne PJ, Certel SJ, de Bruyne M, Zaslavsky L, Johnson WA, et al. 1999. The odor specificities of a subset of olfactory receptor neurons are governed by Acj6, a POU-domain transcription factor. Neuron. 22:339–347. [DOI] [PubMed] [Google Scholar]
- Clyne PJ, Warr CG, Carlson JR. 2000. Candidate taste receptors in Drosophila. Science. 287:1830–1834. [DOI] [PubMed] [Google Scholar]
- Cordomi A, Navarro G, Aymerich MS, Franco R. 2015. Structures for G-protein-coupled receptor tetramers in complex with G proteins. Trends Biochem Sci. 40:548–551. [DOI] [PubMed] [Google Scholar]
- Cosens DJ, Manning A. 1969. Abnormal electroretinogram from a Drosophila mutant. Nature. 224:285–287. [DOI] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, et al. 2010. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 330:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croset V, Rytz R, Cummins SF, Budd A, Brawand D, et al. 2010. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 6:e1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croset V, Schleyer M, Arguello JR, Gerber B, Benton R. 2016. A molecular and neuronal basis for amino acid sensing in the Drosophila larva. Sci Rep. 6:34871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley SA, Bennet-Clark HC, Evert HT. 1995. Courtship song components affect male and female Drosophila differently. Anim Behav. 50:827–839. [Google Scholar]
- Dahanukar A, Foster K, van der Goes van Naters WM, Carlson JR. 2001. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat Neurosci. 4:1182–1186. [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Lei YT, Kwon JY, Carlson JR. 2007. Two Gr genes underlie sugar reception in Drosophila. Neuron. 56:503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalhoff E, Turcanu D, Zenner HP, Gummer AW. 2007. Distortion product otoacoustic emissions measured as vibration on the eardrum of human subjects. Proc Natl Acad Sci USA. 104:1546–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Pérez CA, et al. 2006. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 31:253–264. [DOI] [PubMed] [Google Scholar]
- Datta SR, Vasconcelos ML, Ruta V, Luo S, Wong A, et al. 2008. The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature. 452:473–477. [DOI] [PubMed] [Google Scholar]
- Davis EE, Sokolove PG. 1976. Lactic acid-sensitive receptors on antennae of mosquito, Aedes aegypti. J Comp Physiol. 105:43–54. [Google Scholar]
- de Bruyne M, Foster K, Carlson JR. 2001. Odor coding in the Drosophila antenna. Neuron. 30:537–552. [DOI] [PubMed] [Google Scholar]
- DeGennaro M. 2015. The mysterious multi-modal repellency of DEET. Fly (Austin). 9:45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deland MC, Pak WL. 1973. Reversibly temperature sensitive phototransduction mutant of Drosophila melanogaster. Nat New Biol. 244:184–186. [DOI] [PubMed] [Google Scholar]
- Dennis EJ, Goldman OV, Vosshall LB. 2019. Aedes aegypti mosquitoes use their legs to sense DEET on contact. Curr Biol. 29:1551–1556.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch D, Clemens J, Thiberge SY, Guan G, Murthy M. 2019. Shared song detector neurons in Drosophila male and female brains drive sex-specific behaviors. Curr Biol. 29:3200–3215.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devineni AV, Sun B, Zhukovskaya A, Axel R. 2019. Acetic acid activates distinct taste pathways in Drosophila to elicit opposing, state-dependent feeding responses. Elife. 8:e47677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzen M, Pellegrino M, Vosshall LB. 2008. Insect odorant receptors are molecular targets of the insect repellent DEET. Science. 319:1838–1842. [DOI] [PubMed] [Google Scholar]
- Dogan EB, Ayres JW, Rossignol PA. 1999. Behavioural mode of action of deet: inhibition of lactic acid attraction. Med Vet Entomol. 13:97–100. [DOI] [PubMed] [Google Scholar]
- Du EJ, Ahn TJ, Wen X, Seo DW, Na DL, et al. 2016. Nucleophile sensitivity of Drosophila TRPA1 underlies light-induced feeding deterrence. Elife. 5:e18425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duménil C, Woud D, Pinto F, Alkema JT, Jansen I, et al. 2016. Pheromonal cues deposited by mated females convey social information about egg-laying sites in Drosophila Melanogaster. J Chem Ecol. 42:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunipace L, Meister S, McNealy C, Amrein H. 2001. Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr Biol. 11:822–835. [DOI] [PubMed] [Google Scholar]
- Dutta Banik D, Martin LE, Freichel M, Torregrossa AM, Medler KF. 2018. TRPM4 and TRPM5 are both required for normal signaling in taste receptor cells. Proc Natl Acad Sci USA. 115:E772–E781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dweck HK, Ebrahim SA, Thoma M, Mohamed AA, Keesey IW, et al. 2015. Pheromones mediating copulation and attraction in Drosophila. Proc Natl Acad Sci USA. 112:E2829–E2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dweck HKM, Carlson JR. 2020. Molecular logic and evolution of bitter taste in Drosophila. Curr Biol. 30:17–30.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl DF, Hardy RW, Kernan MJ. 2000. Genetically similar transduction mechanisms for touch and hearing in Drosophila. J Neurosci. 20:5981–5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SL, Charlie NK, Milfort MC, Brown BS, Gravlin CN, et al. 2008. A novel molecular solution for ultraviolet light detection in Caenorhabditis elegans. PLoS Biol. 6:e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effertz T, Nadrowski B, Piepenbrock D, Albert JT, Göpfert MC. 2012. Direct gating and mechanical integrity of Drosophila auditory transducers require TRPN1. Nat Neurosci. 15:1198–1200. [DOI] [PubMed] [Google Scholar]
- Effertz T, Wiek R, Göpfert MC. 2011. NompC TRP channel is essential for Drosophila sound receptor function. Curr Biol. 21:592–597. [DOI] [PubMed] [Google Scholar]
- Ejima A, Griffith LC. 2008. Courtship initiation is stimulated by acoustic signals in Drosophila melanogaster. PLoS One. 3:e3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejima A, Smith BP, Lucas C, van der Goes van Naters W, Miller CJ, et al. 2007. Generalization of courtship learning in Drosophila is mediated by cis-vaccenyl acetate. Curr Biol. 17:599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjin A, Zaharieva EE, Frank DD, Mansourian S, Suh GS, et al. 2016. Humidity sensing in Drosophila. Curr Biol. 26:1352–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyun SI, Soh HY, Posavi M, Munro JB, Hughes DST, et al. 2017. Evolutionary history of chemosensory-related gene families across the Arthropoda. Mol Biol Evol. 34:1838–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk R, Bleiser-Avivi N, Atidia J. 1976. Labellar taste organs of Drosophila melanogaster. J Morphol. 150:327–341. [DOI] [PubMed] [Google Scholar]
- Fan P, Manoli DS, Ahmed OM, Chen Y, Agarwal N, et al. 2013. Genetic and neural mechanisms that inhibit Drosophila from mating with other species. Cell. 154:89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucher C, Forstreuter M, Hilker M, de Bruyne M. 2006. Behavioral responses of Drosophila to biogenic levels of carbon dioxide depend on life-stage, sex and olfactory context. J Exp Biol. 209:2739–2748. [DOI] [PubMed] [Google Scholar]
- Feiler R, Bjornson R, Kirschfeld K, Mismer D, Rubin GM, et al. 1992. Ectopic expression of ultraviolet-rhodopsins in the blue photoreceptor cells of Drosophila: visual physiology and photochemistry of transgenic animals. J Neurosci. 12:3862–3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiler R, Harris WA, Kirschfeld K, Wehrhahn C, Zuker CS. 1988. Targeted misexpression of a Drosophila opsin gene leads to altered visual function. Nature. 333:737–741. [DOI] [PubMed] [Google Scholar]
- Feng P, Zheng J, Rossiter SJ, Wang D, Zhao H. 2014. Massive losses of taste receptor genes in toothed and baleen whales. Genome Biol Evol. 6:1254–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson NM. 2018. Challenges and opportunities in controlling mosquito-borne infections. Nature. 559:490–497. [DOI] [PubMed] [Google Scholar]
- Fernandes JN, Moise IK, Maranto GL, Beier JC. 2018. Revamping mosquito-borne disease control to tackle future threats. Trends Parasitol. 34:359–368. [DOI] [PubMed] [Google Scholar]
- Fischler W, Kong P, Marella S, Scott K. 2007. The detection of carbonation by the Drosophila gustatory system. Nature. 448:1054–1057. [DOI] [PubMed] [Google Scholar]
- Flood TF, Iguchi S, Gorczyca M, White B, Ito K, et al. 2013. A single pair of interneurons commands the Drosophila feeding motor program. Nature. 499:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foelix RF, Stocker RF, Steinbrecht RA. 1989. Fine structure of a sensory organ in the arista of Drosophila melanogaster and some other dipterans. Cell Tissue Res. 258:277–287. [DOI] [PubMed] [Google Scholar]
- Fortini ME, Rubin GM. 1990. Analysis of cis-acting requirements of the Rh3 and Rh4 genes reveals a bipartite organization to rhodopsin promoters in Drosophila melanogaster. Genes Dev. 4:444–463. [DOI] [PubMed] [Google Scholar]
- Frank DD, Enjin A, Jouandet GC, Zaharieva EE, Para A, et al. 2017. Early integration of temperature and humidity stimuli in the Drosophila brain. Curr Biol. 27:2381–2388.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman EG, Wisotsky Z, Dahanukar A. 2014. Detection of sweet tastants by a conserved group of insect gustatory receptors. Proc Natl Acad Sci USA. 111:1598–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French AS, Sellier MJ, Agha MA, Guigue A, Chabaud MA, et al. 2015. Dual mechanism for bitter avoidance in Drosophila. J Neurosci. 35:3990–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Yavuz A, Slone J, Jagge C, Song X, et al. 2015. Drosophila sugar receptors in sweet taste perception, olfaction, and internal nutrient sensing. Curr Biol. 25:621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo K, Smith DP. 2001. A large family of divergent Drosophila odorant-binding proteins expressed in gustatory and olfactory sensilla. Genetics. 159:1059–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallio M, Ofstad TA, Macpherson LJ, Wang JW, Zuker CS. 2011. The coding of temperature in the Drosophila brain. Cell. 144:614–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A, Pang L, Duong VK, Lee A, Schoniger H, et al. 2017. A molecular and cellular context-dependent role for Ir76b in detection of amino acid taste. Cell Rep. 18:737–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Chess A. 1999. Identification of candidate Drosophila olfactory receptors from genomic DNA sequence. Genomics. 60:31–39. [DOI] [PubMed] [Google Scholar]
- Gao Q, Yuan B, Chess A. 2000. Convergent projections of Drosophila olfactory neurons to specific glomeruli in the antennal lobe. Nat Neurosci. 3:780–785. [DOI] [PubMed] [Google Scholar]
- Getahun MN, Wicher D, Hansson BS, Olsson SB. 2012. Temporal response dynamics of Drosophila olfactory sensory neurons depends on receptor type and response polarity. Front Cell Neurosci. 6: Article 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie PG, Walker RG. 2001. Molecular basis of mechanosensory transduction. Nature. 413:194–202. [DOI] [PubMed] [Google Scholar]
- Gomez-Diaz C, Bargeton B, Abuin L, Bukar N, Reina JH, et al. 2016. A CD36 ectodomain mediates insect pheromone detection via a putative tunnelling mechanism. Nat Commun. 7:11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Diaz C, Reina JH, Cambillau C, Benton R. 2013. Ligands for pheromone-sensing neurons are not conformationally activated odorant binding proteins. PLoS Biol. 11:e1001546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Wang Q, Wang Z. 2013. NOMPC is likely a key component of Drosophila mechanotransduction channels. Eur J Neurosci. 38:2057–2064. [DOI] [PubMed] [Google Scholar]
- Gong J, Yuan Y, Ward A, Kang L, Zhang B, et al. 2016. The C. elegans taste receptor homolog LITE-1 Is a photoreceptor. Cell. 167:1252–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Z, Son W, Chung YD, Kim J, Shin DW, et al. 2004. Two interdependent TRPV channel subunits, Inactive and Nanchung, mediate hearing in Drosophila. J Neurosci. 24:9059–9066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göpfert MC, Albert JT, Nadrowski B, Kamikouchi A. 2006. Specification of auditory sensitivity by Drosophila TRP channels. Nat Neurosci. 9:999-1000. [DOI] [PubMed] [Google Scholar]
- Göpfert MC, Robert D. 2003. Motion generation by Drosophila mechanosensory neurons. Proc Natl Acad Sci USA. 100:5514–5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean Y, Rytz R, Farine JP, Abuin L, Cortot J, et al. 2011. An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature. 478:236–240. [DOI] [PubMed] [Google Scholar]
- Grueber WB, Jan LY, Jan YN. 2002. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 129:2867–2878. [DOI] [PubMed] [Google Scholar]
- Gu P, Gong J, Shang Y, Wang F, Ruppell KT, et al. 2019. Polymodal nociception in Drosophila requires alternative splicing of TrpA1. Curr Biol. 29:3961–3973.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Wu J, Tian Y, Cheng S, Zhang ZC, et al. 2020. Gαq splice variants mediate phototransduction, rhodopsin synthesis, and retinal integrity in Drosophila. J Biol Chem. 295:5554–5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Oberwinkler J, Postma M, Hardie RC. 2005. Mechanisms of light adaptation in Drosophila photoreceptors. Curr Biol. 15:1228–1234. [DOI] [PubMed] [Google Scholar]
- Guntur AR, Gou B, Gu P, He R, Stern U, et al. 2017. H2O2-sensitive isoforms of Drosophila melanogaster TRPA1 act in bitter-sensing gustatory neurons to promote avoidance of UV during egg-laying. Genetics. 205:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntur AR, Gu P, Takle K, Chen J, Xiang Y, et al. 2015. Drosophila TRPA1 isoforms detect UV light via photochemical production of H2O2. Proc Natl Acad Sci USA. 112:E5753–E5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Kunwar K, Smith D. 2020. Multiple channels of DEET repellency in Drosophila. Pest Manag Sci. 76:880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Wang Y, Wang Q, Wang Z. 2014. The role of PPK26 in Drosophila larval mechanical nociception. Cell Rep. 9:1183–1190. [DOI] [PubMed] [Google Scholar]
- Guo YR, MacKinnon R. 2017. Structure-based membrane dome mechanism for Piezo mechanosensitivity. Elife. 6:e33660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha TS, Smith DP. 2006. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J Neurosci. 26:8727–8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstrom SA, Adamian M, Scimeca M, Pawlyk BS, Yue G, et al. 2001. A role for the Tubby-like protein 1 in rhodopsin transport. Invest Ophthalmol Vis Sci. 42:1955–1962. [PubMed] [Google Scholar]
- Hall JC. 1994. The mating of a fly. Science. 264:1702–1714. [DOI] [PubMed] [Google Scholar]
- Hallem EA, Carlson JR. 2006. Coding of odors by a receptor repertoire. Cell. 125:143–160. [DOI] [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, et al. 2008. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 454:217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanukoglu I. 2017. ASIC and ENaC type sodium channels: conformational states and the structures of the ion selectivity filters. FEBS J. 284:525–545. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Franze K. 2012. Photomechanical responses in Drosophila photoreceptors. Science. 338:260–263. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Juusola M. 2015. Phototransduction in Drosophila. Curr Opin Neurobiol. 34:37–45. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Martin F, Cochrane GW, Juusola M, Georgiev P, et al. 2002. Molecular basis of amplification in Drosophila phototransduction: roles for G protein, phospholipase C, and diacylglycerol kinase. Neuron. 36:689–701. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Minke B. 1992. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 8:643–651. [DOI] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, et al. 2003. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 424:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes LW, Kay AR, Yau KW. 1986. Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature. 321:66–70. [DOI] [PubMed] [Google Scholar]
- He Z, Luo Y, Shang X, Sun JS, Carlson JR. 2019. Chemosensory sensilla of the Drosophila wing express a candidate ionotropic pheromone receptor. PLoS Biol. 17:e2006619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Förster C, Edwards T, Yasuyama K, Wisotzki B, Schneuwly S, et al. 2002. The extraretinal eyelet of Drosophila: development, ultrastructure, and putative circadian function. J Neurosci. 22:9255–9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson SR, Reuss H, Hardie RC. 2000. Single photon responses in Drosophila photoreceptors and their regulation by Ca2+. J Physiol (Lond). 524:179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta Y, Benzer S. 1969. Abnormal electroretinograms in visual mutants of Drosophila. Nature. 222:354–356. [DOI] [PubMed] [Google Scholar]
- Hotta Y, Benzer S. 1970. Genetic dissection of the Drosophila nervous system by means of mosaics. Proc Natl Acad Sci USA. 67:1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J, Bechstedt S. 2004. Hypothesis: a helix of ankyrin repeats of the NOMPC-TRP ion channel is the gating spring of mechanoreceptors. Curr Biol. 14:R224–R226. [DOI] [PubMed] [Google Scholar]
- Huang J, Liu CH, Hughes SA, Postma M, Schwiening CJ, et al. 2010. Activation of TRP channels by protons and phosphoinositide depletion in Drosophila photoreceptors. Curr Biol. 20:189–197. [DOI] [PubMed] [Google Scholar]
- Huber A, Schulz S, Bentrop J, Groell C, Wolfrum U, et al. 1997. Molecular cloning of Drosophila Rh6 rhodopsin: the visual pigment of a subset of R8 photoreceptor cells. FEBS Lett. 406:6–10. [DOI] [PubMed] [Google Scholar]
- Hussain A, Zhang M, Ucpunar HK, Svensson T, Quillery E, et al. 2016. Ionotropic chemosensory receptors mediate the taste and smell of polyamines. PLoS Biol. 14:e1002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang RY, Stearns NA, Tracey WD. 2012. The ankyrin repeat domain of the TRPA protein Painless is important for thermal nociception but not mechanical nociception. PLoS One. 7:e30090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang RY, Zhong L, Xu Y, Johnson T, Zhang F, et al. 2007. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr Biol. 17:2105–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoshita T, Tanimura T. 2006. Cellular identification of water gustatory receptor neurons and their central projection pattern in Drosophila. Proc Natl Acad Sci USA. 103:1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Yoshioka T, Hotta Y. 1985. A genetic study of inositol trisphosphate involvement in phototransduction using Drosophila mutants. Biochem Biophys Res Commun. 132:513–519. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Fujiwara M, Wong J, Ura A, Kamikouchi A. 2020. Stereotyped combination of hearing and wind/gravity-sensing neurons in the Johnston's organ of Drosophila. Front Physiol. 10:1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y, Kamikouchi A. 2016. Auditory system of fruit flies. Heart Res. 338:1–8. [DOI] [PubMed] [Google Scholar]
- Jaeger AH, Stanley M, Weiss ZF, Musso PY, Chan RC, et al. 2018. A complex peripheral code for salt taste in Drosophila. Elife. 7:e37167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang W, Kim JY, Cui S, Jo J, Lee BC, et al. 2015. The anoctamin family channel subdued mediates thermal nociception in Drosophila. J Biol Chem. 290:2521–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang W, Lee S, Choi SI, Chae HS, Han J, et al. 2019. Impairment of proprioceptive movement and mechanical nociception in Drosophila melanogaster larvae lacking Ppk30, a Drosophila member of the Degenerin/Epithelial Sodium Channel family. Genes Brain Behav. 18:e12545. [DOI] [PubMed] [Google Scholar]
- Jeong YT, Oh SM, Shim J, Seo JT, Kwon JY, et al. 2016. Mechanosensory neurons control sweet sensing in Drosophila. Nat Commun. 7:12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong YT, Shim J, Oh SR, Yoon H, Kim ICH, et al. 2013. An odorant-binding protein required for suppression of sweet taste by bitter chemicals. Neuron. 79:725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Zhao Y, Kusakizako T, Wang Y, Pan C, et al. 2020. TMC1 and TMC2 proteins are pore-forming subunits of mechanosensitive ion channels. Neuron. 105:310–321.e3. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Moon SJ, Montell C. 2007. A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proc Natl Acad Sci USA. 104:14110–14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Moon SJ, Wang X, Ren Q, Montell C. 2008. Gr64f is required in combination with other gustatory receptors for sugar detection in Drosophila. Curr Biol. 18:1797–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, Bulkley D, Guo Y, Zhang W, Guo Z, et al. 2017. Electron cryo-microscopy structure of the mechanotransduction channel NOMPC. Nature. 547:118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Ha TS, Smith DP. 2008. SNMP is a signaling component required for pheromone sensitivity in Drosophila. Proc Natl Acad Sci USA. 105:10996–11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KO. 2001. The roles and functions of cutaneous mechanoreceptors. Curr Opin Neurobiol. 11:455–461. [DOI] [PubMed] [Google Scholar]
- Jones WD, Cayirlioglu P, Grunwald Kadow I, Vosshall LB. 2007. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 445:86–90. [DOI] [PubMed] [Google Scholar]
- Jonsson T, Kravitz EA, Heinrich R. 2011. Sound production during agonistic behavior of male Drosophila melanogaster. Fly (Austin). 5:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph RM, Sun JS, Tam E, Carlson JR. 2017. A receptor and neuron that activate a circuit limiting sucrose consumption. Elife. 6:e24992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D. 2013. TRP channels and pain. Annu Rev Cell Dev Biol. 29:355–384. [DOI] [PubMed] [Google Scholar]
- Kamikouchi A, Inagaki HK, Effertz T, Hendrich O, Fiala A, et al. 2009. The neural basis of Drosophila gravity-sensing and hearing. Nature. 458:165–171. [DOI] [PubMed] [Google Scholar]
- Kamikouchi A, Shimada T, Ito K. 2006. Comprehensive classification of the auditory sensory projections in the brain of the fruit fly Drosophila melanogaster. J Comp Neurol. 499:317–356. [DOI] [PubMed] [Google Scholar]
- Kang K, Panzano VC, Chang EC, Ni L, Dainis AM, et al. 2012. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature. 481:76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, et al. 2010. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 464:597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Gao J, Schafer WR, Xie Z, Xu XZ. 2010. C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron. 67:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Wescott S, Li W, Xu XZ. 2011. In touch—the molecular basis of mechanosensory transduction. Biochem (Lond). 33:18–20. [PMC free article] [PubMed] [Google Scholar]
- Karlson P, Luscher M. 1959. Pheromones': a new term for a class of biologically active substances. Nature. 183:55–56. [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Niidome T, Tanabe T, Terada S, Bonigk W, et al. 1989. Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature. 342:762–766. [DOI] [PubMed] [Google Scholar]
- Kernan MJ. 2007. Mechanotransduction and auditory transduction in Drosophila. Pflugers Arch Eur J Physiol. 454:703–720. [DOI] [PubMed] [Google Scholar]
- Kim H, Kim H, Kwon JY, Seo JT, Shin DM, et al. 2018. Drosophila Gr64e mediates fatty acid sensing via the phospholipase C pathway. PLoS Genet. 14:e1007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Chung YD, Park DY, Choi S, Shin DW, et al. 2003. A TRPV family ion channel required for hearing in Drosophila. Nature. 424:81–84. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Johnson WA. 2014. ROS-mediated activation of Drosophila larval nociceptor neurons by UVC irradiation. BMC Neurosci. 15:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. 2012. The role of Drosophila Piezo in mechanical nociception. Nature. 483:209–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Lee Y, Akitake B, Woodward OM, Guggino WB, et al. 2010. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc Natl Acad Sci USA. 107:8440–8445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, Afonso B, Vonner AJ, Hernandez-Nunez L, Berck M, et al. 2015. Sensory determinants of behavioral dynamics in Drosophila thermotaxis. Proc Natl Acad Sci USA. 112:E220–E229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht ZA, Gaudet R, Garrity PA. 2015. The touching tail of a mechanotransduction channel. Cell. 162:1214–1216. [DOI] [PubMed] [Google Scholar]
- Knecht ZA, Silbering AF, Cruz J, Yang L, Croset V, et al. 2017. Ionotropic receptor-dependent moist and dry cells control hygrosensation in Drosophila. Elife. 6:e26654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht ZA, Silbering AF, Ni L, Klein M, Budelli G, et al. 2016. Distinct combinations of variant ionotropic glutamate receptors mediate thermosensation and hygrosensation in Drosophila. Elife. 5:e17879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh TW, He Z, Gorur-Shandilya S, Menuz K, Larter NK, et al. 2014. The Drosophila IR20a clade of ionotropic receptors are candidate taste and pheromone receptors. Neuron. 83:850–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp HG. 2009. Ocelli. Curr Biol. 19:R435–R437. [DOI] [PubMed] [Google Scholar]
- Kumar A, Tauxe GM, Perry S, Scott CA, Dahanukar A, et al. 2020. Contributions of the conserved insect carbon dioxide receptor subunits to odor detection. Cell Rep. 31:107510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurima K, Peters LM, Yang Y, Riazuddin S, Ahmed ZM, et al. 2002. Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nat Genet. 30:277–284. [DOI] [PubMed] [Google Scholar]
- Kurtovic A, Widmer A, Dickson BJ. 2007. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 446:542–546. [DOI] [PubMed] [Google Scholar]
- Kwon JY, Dahanukar A, Weiss LA, Carlson JR. 2007. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci USA. 104:3574–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Kim SH, Ronderos DS, Lee Y, Akitake B, et al. 2010. Drosophila TRPA1 channel is required to avoid the naturally occurring insect repellent citronellal. Curr Biol. 20:1672–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Shen WL, Shim HS, Montell C. 2010. Fine thermotactic discrimination between the optimal and slightly cooler temperatures via a TRPV channel in chordotonal neurons. J Neurosci. 30:10465–10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Shim HS, Wang X, Montell C. 2008. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat Neurosci. 11:871–873. [DOI] [PubMed] [Google Scholar]
- Lacaille F, Hiroi M, Twele R, Inoshita T, Umemoto D, et al. 2007. An inhibitory sex pheromone tastes bitter for Drosophila males. PLoS One. 2:e661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri S, Shen K, Klein M, Tang A, Kane E, et al. 2011. Two alternating motor programs drive navigation in Drosophila larva. PLoS One. 6:e23180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, et al. 2004. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 43:703–714. [DOI] [PubMed] [Google Scholar]
- Laturney M, Billeter JC. 2016. Drosophila melanogaster females restore their attractiveness after mating by removing male anti-aphrodisiac pheromones. Nat Commun. 7:12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin JD, Ha TS, Jones DN, Smith DP. 2008. Activation of pheromone-sensitive neurons is mediated by conformational activation of pheromone-binding protein. Cell. 133:1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazopulo S, Lazopulo A, Baker JD, Syed S. 2019. Daytime colour preference in Drosophila depends on the circadian clock and TRP channels. Nature. 574:108–111. [DOI] [PubMed] [Google Scholar]
- Lebreton S, Borrero-Echeverry F, Gonzalez F, Solum M, Wallin EA, et al. 2017. A Drosophila female pheromone elicits species-specific long-range attraction via an olfactory channel with dual specificity for sex and food. BMC Biol. 15:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Moon S, Cha Y, Chung YD. 2010. Drosophila TRPN(=NOMPC) channel localizes to the distal end of mechanosensory cilia. PLoS One. 5:e11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Sung HY, Jo H, Kim HW, Choi MS, et al. 2017. Ionotropic receptor 76b is required for gustatory aversion to excessive Na+ in Drosophila. Mol Cells. 40:787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WC, Micchelli CA. 2013. Development and characterization of a chemically defined food for Drosophila. PLoS One. 8:e67308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kang MJ, Shim J, Cheong CU, Moon SJ, et al. 2012. Gustatory receptors required for avoiding the insecticide L-canavanine. J Neurosci. 32:1429–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kim SH, Montell C. 2010. Avoiding DEET through insect gustatory receptors. Neuron. 67:555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Lee Y, Lee J, Bang S, Hyun S, et al. 2005. Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat Genet. 37:305–310. [DOI] [PubMed] [Google Scholar]
- Lee Y, Moon S, Wang Y, Montell C. 2015. A Drosophila gustatory receptor required for strychnine sensation. CHEMSE. 40:525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Moon SJ, Montell C. 2009. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc Natl Acad Sci USA. 106:4495–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Poudel S, Kim Y, Thakur D, Montell C. 2018. Calcium taste avoidance in Drosophila. Neuron. 97:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnert BP, Baker AE, Gaudry Q, Chiang AS, Wilson RI. 2013. Distinct roles of TRP channels in auditory transduction and amplification in Drosophila. Neuron. 77:115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung NY, Thakur DP, Gurav AS, Kim SH, Pizio AD, et al. 2020. Function of opsins in Drosophila taste. Curr Biol. 30:1367–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhang W, Guo Z, Wu S, Jan LY, et al. 2016. Defensive kicking behavior in response to mechanical stimuli mediated by Drosophila wing margin bristles. J Neurosci. 36:11275–11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, DeBeaubien NA, Sokabe T, Montell C. 2020. Temperature and sweet taste integration in Drosophila. Curr Biol. 30:2051–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Feng Z, Sternberg PW, Xu XZ. 2006. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature. 440:684–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Li W, Wang H, Cao J, Maehashi K, et al. 2005. Pseudogenization of a sweet-receptor gene accounts for cats' indifference toward sugar. PLoS Genet. 1:e3–e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Ni JD, Huang J, Montell C. 2014. Requirement for Drosophila SNMP1 for rapid activation and termination of pheromone-induced activity. PLoS Genet. 10:e1004600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Madrid J, Gartner R, Verbavatz JM, Schiklenk C, et al. 2013. A NOMPC-dependent membrane-microtubule connector is a candidate for the gating spring in fly mechanoreceptors. Curr Biol. 23:755–763. [DOI] [PubMed] [Google Scholar]
- Liang X, Madrid J, Saleh HS, Howard J. 2011. NOMPC, a member of the TRP channel family, localizes to the tubular body and distal cilium of Drosophila campaniform and chordotonal receptor cells. Cytoskeleton (Hoboken). 68:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER, Zhang YV, Montell C. 2014. Peripheral coding of taste. Neuron. 81:984–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Mann KJ, Starostina E, Kinser RD, Pikielny CW. 2005. A Drosophila DEG/ENaC channel subunit is required for male response to female pheromones. Proc Natl Acad Sci USA. 102:12831–12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HH, Cao DS, Sethi S, Zeng Z, Chin JS, et al. 2016. Hormonal modulation of pheromone detection enhances male courtship success. Neuron. 90:1272–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling F, Dahanukar A, Weiss LA, Kwon JY, Carlson JR. 2014. The molecular and cellular basis of taste coding in the legs of Drosophila. J Neurosci. 34:7148–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Montell C. 2015. Forcing open TRP channels: mechanical gating as a unifying activation mechanism. Biochem Biophys Res Commun. 460:22–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Wang T, Postma M, Obukhov AG, Montell C, et al. 2007. In vivo Identification and manipulation of the Ca2+ selectivity filter in the Drosophila Transient Receptor Potential channel. J Neurosci. 27:604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ward A, Gao J, Dong Y, Nishio N, et al. 2010. C. elegans phototransduction requires a G protein-dependent cGMP pathway and a taste receptor homolog. Nat Neurosci. 13:715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Li Y, Wang R, Yin C, Dong Q, et al. 2007. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature. 450:294–298. [DOI] [PubMed] [Google Scholar]
- Liu T, Starostina E, Vijayan V, Pikielny CW. 2012. Two Drosophila DEG/ENaC channel subunits have distinct functions in gustatory neurons that activate male courtship. J Neurosci. 32:11879–11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Wang Y, Tian Y, Zhang J, Zhao J, et al. 2018. The receptor channel formed by ppk25, ppk29 and ppk23 can sense the Drosophila female pheromone 7,11-heptacosadiene. Genes Brain Behav. 2018:e12529. [DOI] [PubMed] [Google Scholar]
- Liu W, Liang X, Gong J, Yang Z, Zhang YH, et al. 2011. Social regulation of aggression by pheromonal activation of Or65a olfactory neurons in Drosophila. Nat Neurosci. 14:896–902. [DOI] [PubMed] [Google Scholar]
- Lu B, LaMora A, Sun Y, Welsh MJ, Ben-Shahar Y. 2012. ppk23-Dependent chemosensory functions contribute to courtship behavior in Drosophila melanogaster. PLoS Genet. 8:e1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, et al. 2003. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 299:245–247. [DOI] [PubMed] [Google Scholar]
- Luo DG, Yue WW, Ala-Laurila P, Yau KW. 2011. Activation of visual pigments by light and heat. Science. 332:1307–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Shen WL, Montell C. 2017. TRPA1 mediates sensation of the rate of temperature change in Drosophila larvae. Nat Neurosci. 20:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Gershow M, Rosenzweig M, Kang K, Fang-Yen C, et al. 2010. Navigational decision making in Drosophila thermotaxis. J Neurosci. 30:4261–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacWilliam D, Kowalewski J, Kumar A, Pontrello C, Ray A. 2018. Signaling mode of the broad-spectrum conserved CO2 receptor is one of the important determinants of odor valence in Drosophila. Neuron. 97:1153–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpel S, Klarsfeld A, Rouyer F. 2002. Larval optic nerve and adult extra-retinal photoreceptors sequentially associate with clock neurons during Drosophila brain development. Development. 129:1443–1453. [DOI] [PubMed] [Google Scholar]
- Mamiya A, Dickinson MH. 2015. Antennal mechanosensory neurons mediate wing motor reflexes in flying Drosophila. J Neurosci. 35:7977–7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel SJ, Shoaf ML, Braco JT, Silver WL, Johnson EC. 2018. Behavioral aversion to AITC requires both Painless and dTRPA1 in Drosophila. Front Neural Circuits. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, et al. 2006. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 49:285–295. [DOI] [PubMed] [Google Scholar]
- Markow TA, O’Grady P. 2008. Reproductive ecology of Drosophila. Funct Ecol. 22:747–759. [Google Scholar]
- Masek P, Keene AC. 2013. Drosophila fatty acid taste signals through the PLC pathway in sugar-sensing nurons. PLoS Genet. 9:e1003710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo E, Kamikouchi A. 2013. Neuronal encoding of sound, gravity, and wind in the fruit fly. J Comp Physiol A. 199:253–262. [DOI] [PubMed] [Google Scholar]
- Matsuo E, Yamada D, Ishikawa Y, Asai T, Ishimoto H, et al. 2014. Identification of novel vibration- and deflection-sensitive neuronal subgroups in Johnston's organ of the fruit fly. Front Physiol. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauthner SE, Hwang RY, Lewis AH, Xiao Q, Tsubouchi A, et al. 2014. Balboa binds to pickpocket in vivo and is required for mechanical nociception in Drosophila larvae. Curr Biol. 24:2920–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza CA, Izaguirre MM, Zavala J, Scopel AL, Ballare CL. 2002. Insect perception of ambient ultraviolet-B radiation. Ecol Lett. 5:722–726. [Google Scholar]
- Mazza CA, Zavala J, Scopel AL, Ballare CL. 1999. Perception of solar UVB radiation by phytophagous insects: behavioral responses and ecosystem implications. Proc Natl Acad Sci USA. 96:980–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MP, Hekmat-Scafe DS, Gaines P, Carlson JR. 1994. Putative Drosophila pheromone-binding proteins expressed in a subregion of the olfactory system. J Biol Chem. 269:16340–16347. [PubMed] [Google Scholar]
- Meunier N, Marion-Poll F, Rospars JP, Tanimura T. 2003. Peripheral coding of bitter taste in Drosophila. J Neurobiol. 56:139–152. [DOI] [PubMed] [Google Scholar]
- Min S, Ai M, Shin SA, Suh GS. 2013. Dedicated olfactory neurons mediating attraction behavior to ammonia and amines in Drosophila. Proc Natl Acad Sci USA. 110:E1321–E1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra AK, Bargmann BOR, Tsachaki M, Fritsch C, Sprecher SG. 2016. Functional genomics identifies regulators of the phototransduction machinery in the Drosophila larval eye and adult ocelli. Dev Biol. 410:164–177. [DOI] [PubMed] [Google Scholar]
- Mishra AK, Tsachaki M, Rister J, Ng J, Celik A, et al. 2013. Binary cell fate decisions and fate transformation in the Drosophila larval eye. PLoS Genet. 9:e1004027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mismer D, Michael WM, Laverty TR, Rubin GM. 1988. Analysis of the promoter of the Rh2 opsin gene in Drosophila melanogaster. Genetics. 120:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitri C, Soustelle L, Framery B, Bockaert J, Parmentier ML, et al. 2009. Plant insecticide L-canavanine repels Drosophila via the insect orphan GPCR DmX. PLoS Biol. 7:e1000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Amrein H. 2008. Suppression of male courtship by a Drosophila pheromone receptor. Nat Neurosci. 11:874–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Slone J, Song X, Amrein H. 2012. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell. 151:1113–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. 2005. The TRP superfamily of cation channels. Sci STKE. 2005:re3. [DOI] [PubMed] [Google Scholar]
- Montell C. 2012. Drosophila visual transduction. Trends Neurosci. 35:356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C, Jones K, Zuker C, Rubin G. 1987. A second opsin gene expressed in the ultraviolet-sensitive R7 photoreceptor cells of Drosophila melanogaster. J Neurosci. 7:1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C, Rubin GM. 1989. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 2:1313–1323. [DOI] [PubMed] [Google Scholar]
- Montell C, Zwiebel LJ. 2016. Mosquito sensory systems. In: Raikhel AS, editor. Advances in Insect Physiology. Oxford: Academic Press. p. 294–328. [Google Scholar]
- Moon SJ, Lee Y, Jiao Y, Montell C. 2009. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr Biol. 19:1623–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen H. 2018. Long-distance navigation and magnetoreception in migratory animals. Nature. 558:50–59. [DOI] [PubMed] [Google Scholar]
- Mueller JM, Ravbar P, Simpson JH, Carlson JM. 2019. Drosophila melanogaster grooming possesses syntax with distinct rules at different temporal scales. PLoS Comput Biol. 15:e1007105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro G, Cordomi A, Zelman-Femiak M, Brugarolas M, Moreno E, et al. 2016. Quaternary structure of a G-protein-coupled receptor heterotetramer in complex with Gi and Gs. BMC Biol. 14:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely GG, Hess A, Costigan M, Keene AC, Goulas S, et al. 2010. A genome-wide Drosophila screen for heat nociception identifies α2d3 as an evolutionarily conserved pain gene. Cell. 143:628–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely GG, Keene AC, Duchek P, Chang EC, Wang QP, et al. 2011. TrpA1 regulates thermal nociception in Drosophila. PLoS One. 6:e24343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng R, Salem SS, Wu ST, Wu M, Lin HH, et al. 2019. Amplification of Drosophila olfactory responses by a DEG/ENaC channel. Neuron. 104:947–959.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni JD, Baik LS, Holmes TC, Montell C. 2017. A rhodopsin in the brain functions in circadian photoentrainment in Drosophila. Nature. 545:340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L, Bronk P, Chang EC, Lowell AM, Flam JO, et al. 2013. A gustatory receptor paralogue controls rapid warmth avoidance in Drosophila. Nature. 500:580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L, Klein M, Svec KV, Budelli G, Chang EC, et al. 2016. The ionotropic receptors IR21a and IR25a mediate cool sensing in Drosophila. Elife. 5:e13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. 1996. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 85:651–659. [DOI] [PubMed] [Google Scholar]
- O’Tousa JE, Baehr W, Martin RL, Hirsh J, Pak WL, et al. 1985. The Drosophila ninaE gene encodes an opsin. Cell. 40:839–850. [DOI] [PubMed] [Google Scholar]
- O’Tousa JE, Leonard DS, Pak WL. 1989. Morphological defects in oraJK84 photoreceptors caused by mutation in R1-R6 opsin gene in Drosophila. J Neurogenet. 6:41–52. [DOI] [PubMed] [Google Scholar]
- Ogueta M, Hardie RC, Stanewsky R. 2018. Non-canonical phototransduction mediates synchronization of the Drosophila melanogaster circadian clock and retinal light responses. Curr Biol. 28:1725–1735.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogueta M, Hardie RC, Stanewsky R. 2020. Light sampling via throttled visual phototransduction robustly synchronizes the Drosophila circadian clock. Curr Biol. 30:2551–2563. [DOI] [PubMed] [Google Scholar]
- Ostroy SE, Wilson M, Pak WL. 1974. Drosophila rhodopsin: photochemistry, extraction and differences in the norpAP12 phototransduction mutant. Biochem Biophys Res Commun. 59:960–966. [DOI] [PubMed] [Google Scholar]
- Pak WL. 2010. Why Drosophila to study phototransduction? J Neurogenet. 24:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak WL, Grossfield J, Arnold KS. 1970. Mutants of the visual pathway of Drosophila melanogaster. Nature. 227:518–520. [DOI] [PubMed] [Google Scholar]
- Pak WL, Grossfield J, White NV. 1969. Nonphototactic mutants in a study of vision of Drosophila. Nature. 222:351–354. [DOI] [PubMed] [Google Scholar]
- Pak WL, Shino S, Leung HT. 2012. PDA (Prolonged Depolarizing Afterpotential)-defective mutants: the story of nina's and ina's-pinta and santa maria, too. J Neurogenet. 26:216–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Akyuz N, Liu XP, Asai Y, Nist-Lund C, et al. 2018. TMC1 forms the pore of mechanosensory transduction channels in vertebrate inner ear hair cells. Neuron. 99:736–753.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, et al. 2005. Illumination of the melanopsin signaling pathway. Science. 307:600–604. [DOI] [PubMed] [Google Scholar]
- Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, et al. 2002. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 298:2213–2216. [DOI] [PubMed] [Google Scholar]
- Papatsenko D, Sheng G, Desplan C. 1997. A new rhodopsin in R8 photoreceptors of Drosophila: evidence for coordinate expression with Rh3 in R7 cells. Development. 124:1665–1673. [DOI] [PubMed] [Google Scholar]
- Park J, Carlson JR. 2018. Physiological responses of the Drosophila labellum to amino acids. J Neurogenet. 32:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Lee J, Shim J, Han W, Bae YC, et al. 2013. dTULP, the Drosophila melanogaster homolog of tubby, regulates transient receptor potential channel localization in cilia. PLoS Genet. 9:e1003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patella P, Wilson RI. 2018. Functional maps of mechanosensory features in the Drosophila brain. Curr Biol. 28:1189–1203.e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino M, Steinbach N, Stensmyr MC, Hansson BS, Vosshall LB. 2011. A natural polymorphism alters odour and DEET sensitivity in an insect odorant receptor. Nature. 478:511–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrin D, Hebert TE. 2012. The functional size of GPCRs—monomers, dimers or tetramers? Subcell Biochem. 63:67–81. [DOI] [PubMed] [Google Scholar]
- Phillips AM, Bull A, Kelly LE. 1992. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron. 8:631–642. [DOI] [PubMed] [Google Scholar]
- Pikielny CW, Hasan G, Rouyer F, Rosbash M. 1994. Members of a family of Drosophila putative odorant-binding proteins are expressed in different subsets of olfactory hairs. Neuron. 12:35–49. [DOI] [PubMed] [Google Scholar]
- Pollock JA, Benzer S. 1988. Transcript localization of four opsin genes in the three visual organs of Drosophila; RH2 is ocellus specific. Nature. 333:779–782. [DOI] [PubMed] [Google Scholar]
- Poudel S, Kim Y, Gwak JS, Jeong S, Lee Y. 2017. Gustatory receptor 22e is essential for sensing chloroquine and strychnine in Drosophila melanogaster. Insect Biochem Mol Biol. 88:30–36. [DOI] [PubMed] [Google Scholar]
- Prigge CL, Yeh PT, Liou NF, Lee CC, You SF, et al. 2016. M1 ipRGCs influence visual function through retrograde signaling in the retina. J Neurosci. 36:7184–7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. 1998. Melanopsin: an opsin in melanophores, brain, and eye. Proc Natl Acad Sci USA. 95:340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, et al. 2005. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 433:745–749. [DOI] [PubMed] [Google Scholar]
- Raad H, Ferveur JF, Ledger N, Capovilla M, Robichon A. 2016. Functional gustatory role of chemoreceptors in Drosophila wings. Cell Rep. 15:1442–1454. [DOI] [PubMed] [Google Scholar]
- Ramdya P, Lichocki P, Cruchet S, Frisch L, Tse W, et al. 2015. Mechanosensory interactions drive collective behaviour in Drosophila. Nature. 519:233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray C. 1960. The application of Bergmann's and Allen's Rules to the poikilotherms. J Morphol. 106:85–108. [DOI] [PubMed] [Google Scholar]
- Redka DS, Morizumi T, Elmslie G, Paranthaman P, Shivnaraine RV, et al. 2014. Coupling of G proteins to reconstituted monomers and tetramers of the M2 muscarinic receptor. J Biol Chem. 289:24347–24365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, de Roode JC. 2018. Demystifying monarch butterfly migration. Curr Biol. 28:R1009–R1022. [DOI] [PubMed] [Google Scholar]
- Reuss H, Mojet MH, Chyb S, Hardie RC. 1997. In vivo analysis of the Drosophila light-sensitive channels, TRP and TRPL. Neuron. 19:1249–1259. [DOI] [PubMed] [Google Scholar]
- Riabinina O, Dai M, Duke T, Albert JT. 2011. Active process mediates species-specific tuning of Drosophila ears. Curr Biol. 21:658–664. [DOI] [PubMed] [Google Scholar]
- Ribeiro C, Dickson BJ. 2010. Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Curr Biol. 20:1000–1005. [DOI] [PubMed] [Google Scholar]
- Rihani K, Fraichard S, Chauvel I, Poirier N, Delompre T, et al. 2019. A conserved odorant binding protein is required for essential amino acid detection in Drosophila. Commun Biol. 2:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimal S, Sang J, Poudel S, Thakur D, Montell C, et al. 2019. Mechanism of acetic acid gustatory repulsion in Drosophila. Cell Rep. 26:1432–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson HM. 2015. The insect chemoreceptor superfamily is ancient in animals. Chem Senses. 40:609–614. [DOI] [PubMed] [Google Scholar]
- Robertson HM, Warr CG, Carlson JR. 2003. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc Natl Acad Sci USA. 100:14537–14542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers KJ. 2014. Non-protein amino acids and neurodegeneration: the enemy within. Exp Neurol. 253:192–196. [DOI] [PubMed] [Google Scholar]
- Rosenzweig M, Brenman KM, Taylor TD, Phelps P, Patapoutian A, et al. 2005. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 19:419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, et al. 2002. Role of melanopsin in circadian responses to light. Science. 298:2211–2213. [DOI] [PubMed] [Google Scholar]
- Rytz R, Croset V, Benton R. 2013. Ionotropic receptors (IRs): chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect Biochem Mol Biol. 43:888–897. [DOI] [PubMed] [Google Scholar]
- Saint-Charles A, Michard-Vanhée C, Alejevski F, Chélot E, Boivin A, et al. 2016. Four of the six Drosophila rhodopsin-expressing photoreceptors can mediate circadian entrainment in low light. J Comp Neurol. 524:2828–2844. [DOI] [PubMed] [Google Scholar]
- Salcedo E, Huber A, Henrich S, Chadwell LV, Chou WH, et al. 1999. Blue- and green-absorbing visual pigments of Drosophila: ectopic expression and physiological characterization of the R8 photoreceptor cell-specific Rh5 and Rh6 rhodopsins. J Neurosci. 19:10716–10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Alcañiz JA, Silbering AF, Croset V, Zappia G, Sivasubramaniam AK, et al. 2018. An expression atlas of variant ionotropic glutamate receptors identifies a molecular basis of carbonation sensing. Nat Commun. 9:4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Alcañiz JA, Zappia G, Marion-Poll F, Benton R. 2017. A mechanosensory receptor required for food texture detection in Drosophila. Nat Commun. 8:14192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford JL, Shields VD, Dickens JC. 2013. Gustatory receptor neuron responds to DEET and other insect repellents in the yellow-fever mosquito, Aedes aegypti. Naturwissenschaften. 100:269–273. [DOI] [PubMed] [Google Scholar]
- Sang JH, King RC. 1961. Nutritional requirements of axenically cultured Drosophila melanogaster adults. J Exp Biol. 38:793–809. [Google Scholar]
- Saotome K, Murthy SE, Kefauver JM, Whitwam T, Patapoutian A, et al. 2018. Structure of the mechanically activated ion channel Piezo1. Nature. 554:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Pellegrino M, Nakagawa T, Nakagawa T, Vosshall LB, et al. 2008. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 452:1002–1006. [DOI] [PubMed] [Google Scholar]
- Sato K, Tanaka K, Touhara K. 2011. Sugar-regulated cation channel formed by an insect gustatory receptor. Proc Natl Acad Sci USA. 108:11680–11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavarda NJ, O’Tousa J, Pak WL. 1983. Drosophila locus with gene-dosage effects on rhodopsin. Proc Natl Acad Sci USA. 80:4441–4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D. 1986. Sexual mimicry regulates the attractiveness of mated Drosophila melanogaster females. Proc Natl Acad Sci USA. 83:8429–8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K, Becker A, Sun Y, Hardy R, Zuker C. 1995. Gqα protein function in vivo: genetic dissection of its role in photoreceptor cell physiology. Neuron. 15:919–927. [DOI] [PubMed] [Google Scholar]
- Scott K, Brady R Jr., Cravchik A, Morozov P, Rzhetsky A, et al. 2001. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell. 104:661–673. [DOI] [PubMed] [Google Scholar]
- Seeds AM, Ravbar P, Chung P, Hampel S, Midgley FM Jr., et al. 2014. A suppression hierarchy among competing motor programs drives sequential grooming in Drosophila. Elife. 3:e02951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthilan PR, Grebler R, Reinhard N, Rieger D, Helfrich-Förster C. 2019. Role of rhodopsins as circadian photoreceptors in the Drosophila melanogaster. Biology (Basel). 8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthilan PR, Helfrich-Förster C. 2016. Rhodopsin 7-the unusual rhodopsin in Drosophila. PeerJ. 4:e2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthilan PR, Piepenbrock D, Ovezmyradov G, Nadrowski B, Bechstedt S, et al. 2012. Drosophila auditory organ genes and genetic hearing defects. Cell. 150:1042–1054. [DOI] [PubMed] [Google Scholar]
- Shanbhag S, Claudio P, Steinbrecht RA. 2001. Gustatory organs of Drosophila melanogaster: fine structure and expression of the putative odorant-binding protein PBPRP2. Cell Tissue Res. 304:423–437. [DOI] [PubMed] [Google Scholar]
- Shankar S, Chua JY, Tan KJ, Calvert ME, Weng R, et al. 2015. The neuropeptide tachykinin is essential for pheromone detection in a gustatory neural circuit. Elife. 4:e06914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen WL, Kwon Y, Adegbola AA, Luo J, Chess A, et al. 2011. Function of rhodopsin in temperature discrimination in Drosophila. Science. 331:1333–1336. [DOI] [PubMed] [Google Scholar]
- Shim J, Lee Y, Jeong YT, Kim Y, Lee MG, et al. 2015. The full repertoire of Drosophila gustatory receptors for detecting an aversive compound. Nat Commun. 6:8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimono K, Fujimoto A, Tsuyama T, Yamamoto-Kochi M, Sato M, et al. 2009. Multidendritic sensory neurons in the adult Drosophila abdomen: origins, dendritic morphology, and segment- and age-dependent programmed cell death. Neural Dev. 4:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorey HH. 1962. Nature of the sound produced by Drosophila melanogaster during courtship. Science. 137:677–678. [DOI] [PubMed] [Google Scholar]
- Silbering AF, Rytz R, Grosjean Y, Abuin L, Ramdya P, et al. 2011. Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J Neurosci. 31:13357–13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleno R, Hebert TE. 2019. Shaky ground—the nature of metastable GPCR signalling complexes. Neuropharmacology. 152:4–14. [DOI] [PubMed] [Google Scholar]
- Slone J, Daniels J, Amrein H. 2007. Sugar receptors in Drosophila. Curr Biol. 17:1809–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokabe T, Chen HS, Luo J, Montell C. 2016. A switch in thermal preference in Drosophila larvae depends on multiple rhodopsins. Cell Rep. 17:336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokabe T, Tsujiuchi S, Kadowaki T, Tominaga M. 2008. Drosophila Painless is a Ca2+-requiring channel activated by noxious heat. J Neurosci. 28:9929–9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldano A, Alpizar YA, Boonen B, Franco L, Lopez-Requena A, et al. 2016. Gustatory-mediated avoidance of bacterial lipopolysaccharides via TRPA1 activation in Drosophila. Elife. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Padilla A, Ruijsink R, Sibon OCM, van Rijn H, Billeter JC. 2018. Thermosensory perception regulates speed of movement in response to temperature changes in Drosophila melanogaster. J Exp Biol. 221:jeb174151. [DOI] [PubMed] [Google Scholar]
- Sparks JT, Dickens JC. 2016. Bitter-sensitive gustatory receptor neuron responds to chemically diverse insect repellents in the common malaria mosquito Anopheles quadrimaculatus. Naturwissenschaften. 103:39.doi:10.1007/s00114-00016-01367-y. [DOI] [PubMed] [Google Scholar]
- Spieth HT. 1974. Courtship behavior in Drosophila. Annu Rev Entomol. 19:385–405. [DOI] [PubMed] [Google Scholar]
- Sprecher SG, Pichaud F, Desplan C. 2007. Adult and larval photoreceptors use different mechanisms to specify the same Rhodopsin fates. Genes Dev. 21:2182–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starostina E, Liu T, Vijayan V, Zheng Z, Siwicki KK, et al. 2012. A Drosophila DEG/ENaC subunit functions specifically in gustatory neurons required for male courtship behavior. J Neurosci. 32:4665–4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck K, Walker SJ, Itskov PM, Baltazar C, Moreira JM, et al. 2018. Internal amino acid state modulates yeast taste neurons to support protein homeostasis in Drosophila. Elife. 7:e31625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker RF. 1994. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 275:3–26. [DOI] [PubMed] [Google Scholar]
- Su CY, Menuz K, Carlson JR. 2009. Olfactory perception: receptors, cells, and circuits. Cell. 139:45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, et al. 2004. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 431:854–859. [DOI] [PubMed] [Google Scholar]
- Sun JS, Xiao S, Carlson JR. 2018. The diverse small proteins called odorant-binding proteins. Open Biol. 8:180208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Liu L, Ben-Shahar Y, Jacobs JS, Eberl DF, et al. 2009. TRPA channels distinguish gravity sensing from hearing in Johnston's organ. Proc Natl Acad Sci USA. 106:13606–13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung HY, Jeong YT, Lim JY, Kim H, Oh SM, et al. 2017. Heterogeneity in the Drosophila gustatory receptor complexes that detect aversive compounds. Nat Commun. 8:1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed Z, Leal WS. 2008. Mosquitoes smell and avoid the insect repellent DEET. Proc Natl Acad Sci USA. 105:13598–13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szular J, Sehadova H, Gentile C, Szabo G, Chou WH, et al. 2012. Rhodopsin 5- and Rhodopsin 6-mediated clock synchronization in Drosophila melanogaster is independent of retinal phospholipase C-β signaling. J Biol Rhythms. 27:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber JM, Brown EB, Li Y, Yurgel ME, Masek P, et al. 2017. A subset of sweet-sensing neurons identified by IR56d are necessary and sufficient for fatty acid taste. PLoS Genet. 13:e1007059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The International Polycystic Kidney Disease Consortium. 1995. Polycystic kidney disease: the complete structure of the PKD1 gene and its protein. Cell. 81:289–298. [DOI] [PubMed] [Google Scholar]
- Thistle R, Cameron P, Ghorayshi A, Dennison L, Scott K. 2012. Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell. 149:1140–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne N, Chromey C, Bray S, Amrein H. 2004. Taste perception and coding in Drosophila. Curr Biol. 14:1065–1079. [DOI] [PubMed] [Google Scholar]
- Tichy H, Hellwig M, Kallina W. 2017. Revisiting theories of humidity transduction: a focus on electrophysiological data. Front Physiol. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda H, Zhao X, Dickson BJ. 2012. The Drosophila female aphrodisiac pheromone activates ppk23+ sensory neurons to elicit male courtship behavior. Cell Rep. 1:599–607. [DOI] [PubMed] [Google Scholar]
- Todi SV, Sharma Y, Eberl DF. 2004. Anatomical and molecular design of the Drosophila antenna as a flagellar auditory organ. Microsc Res Tech. 63:388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG. 2001. Calcium: taste, intake, and appetite. Physiol Rev. 81:1567–1597. [DOI] [PubMed] [Google Scholar]
- Toshima N, Tanimura T. 2012. Taste preference for amino acids is dependent on internal nutritional state in Drosophila melanogaster. J Exp Biol. 215:2827–2832. [DOI] [PubMed] [Google Scholar]
- Tracey WD, Wilson RI, Laurent G, Benzer S. 2003. painless, a Drosophila gene essential for nociception. Cell. 113:261–273. [DOI] [PubMed] [Google Scholar]
- Travis BV, Morton FA, Jones HA, Robinson JH. 1949. The more effective mosquito repellents tested at the Orlando, Fla.laboratory, 1942-47. J Econ Entomol. 42:686–694. [DOI] [PubMed] [Google Scholar]
- Tsubouchi A, Caldwell JC, Tracey WD. 2012. Dendritic filopodia, Ripped Pocket, NOMPC, and NMDARs contribute to the sense of touch in Drosophila larvae. Curr Biol. 22:2124–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner HN, Armengol K, Patel AA, Himmel NJ, Sullivan L, et al. 2016. The TRP channels Pkd2, NompC, and Trpm Act in cold-sensing neurons to mediate unique aversive behaviors to noxious cold in Drosophila. Curr Biol. 26:3116–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SL, Ray A. 2009. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature. 461:277–281. [DOI] [PubMed] [Google Scholar]
- Tuthill JC, Wilson RI. 2016. Mechanosensation and adaptive motor control in insects. Curr Biol. 26:R1022–R1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchizono S, Itoh TQ, Kim H, Hamada N, Kwon JY, et al. 2017. Deciphering the genes for taste receptors for fructose in Drosophila. Mol Cells. 40:731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno K, Ohta M, Morita H, Mikuni Y, Nakajima S, et al. 2001. Trehalose sensitivity in Drosophila correlates with mutations in and expression of the gustatory receptor gene Gr5a. Curr Biol. 11:1451–1455. [DOI] [PubMed] [Google Scholar]
- van Breugel F, Huda A, Dickinson MH. 2018. Distinct activity-gated pathways mediate attraction and aversion to CO2 in Drosophila. Nature. 564:420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Goes van Naters W, Carlson JR. 2007. Receptors and neurons for fly odors in Drosophila. Curr Biol. 17:606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas MA, Luo N, Yamaguchi A, Kapahi P. 2010. A role for S6 kinase and serotonin in postmating dietary switch and balance of nutrients in D. melanogaster. Curr Biol. 20:1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. 2007. TRP channels. Annu Rev Biochem. 76:387–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteven M, Vanden Broeck L, Geurten B, Zwarts L, Decraecker L, et al. 2017. Hearing regulates Drosophila aggression. Proc Natl Acad Sci USA. 114:1958–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan V, Thistle R, Liu T, Starostina E, Pikielny CW. 2014. Drosophila pheromone-sensing neurons expressing the ppk25 ion channel subunit stimulate male courtship and female receptivity. PLoS Genet. 10:e1004238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanath V, Story GM, Peier AM, Petrus MJ, Lee VM, et al. 2003. Opposite thermosensor in fruitfly and mouse. Nature. 423:822–823. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R. 1999. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell. 96:725–736. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Stocker RF. 2007. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 30:505–533. [DOI] [PubMed] [Google Scholar]
- Vreugde S, Erven A, Kros CJ, Marcotti W, Fuchs H, et al. 2002. Beethoven, a mouse model for dominant, progressive hearing loss DFNA36. Nat Genet. 30:257–258. [DOI] [PubMed] [Google Scholar]
- Walker RG, Willingham AT, Zuker CS. 2000. A Drosophila mechanosensory transduction channel. Science. 287:2229–2234. [DOI] [PubMed] [Google Scholar]
- Wang L, Anderson DJ. 2010. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature. 463:227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Han X, Mehren J, Hiroi M, Billeter JC, et al. 2011. Hierarchical chemosensory regulation of male-male social interactions in Drosophila. Nat Neurosci. 14:757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Xu H, Oberwinkler J, Gu Y, Hardie RC, et al. 2005. Light activation, adaptation, and cell survival functions of the Na+/Ca2+ exchanger CalX. Neuron. 45:367–378. [DOI] [PubMed] [Google Scholar]
- Wang Z, Singhvi A, Kong P, Scott K. 2004. Taste representations in the Drosophila brain. Cell. 117:981–991. [DOI] [PubMed] [Google Scholar]
- Wasserman S, Salomon A, Frye MA. 2013. Drosophila tracks carbon dioxide in flight. Curr Biol. 23:301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Toba G, Koganezawa M, Yamamoto D. 2011. Gr39a, a highly diversified gustatory receptor in Drosophila, has a role in sexual behavior. Behav Genet. 41:746–753. [DOI] [PubMed] [Google Scholar]
- Watnick TJ, Jin Y, Matunis E, Kernan MJ, Montell C. 2003. A flagellar polycystin-2 homolog required for male fertility in Drosophila. Curr Biol. 13:2179–2184. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Dahanukar A, Kwon JY, Banerjee D, Carlson JR. 2011. The molecular and cellular basis of bitter taste in Drosophila. Neuron. 69:258–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, et al. 1995. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci USA. 92:9652–9656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher D, Schafer R, Bauernfeind R, Stensmyr MC, Heller R, et al. 2008. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 452:1007–1011. [DOI] [PubMed] [Google Scholar]
- Wisotsky Z, Medina A, Freeman E, Dahanukar A. 2011. Evolutionary differences in food preference rely on Gr64e, a receptor for glycerol. Nat Neurosci. 14:1534–1541. [DOI] [PubMed] [Google Scholar]
- Xia Y, Wang G, Buscariollo D, Pitts RJ, Wenger H, et al. 2008. The molecular and cellular basis of olfactory-driven behavior in Anopheles gambiae larvae. Proc Natl Acad Sci USA. 105:6433–6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, et al. 2010. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 468:921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Atkinson R, Jones DN, Smith DP. 2005. Drosophila OBP LUSH is required for activity of pheromone-sensitive neurons. Neuron. 45:193–200. [DOI] [PubMed] [Google Scholar]
- Xu P, Choo YM, De La Rosa A, Leal WS. 2014. Mosquito odorant receptor for DEET and methyl jasmonate. Proc Natl Acad Sci USA. 111:16592–16597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SY, Cang CL, Liu XF, Peng YQ, Ye YZ, et al. 2006. Thermal nociception in adult Drosophila: behavioral characterization and the role of the painless gene. Genes Brain Behav. 5:602–613. [DOI] [PubMed] [Google Scholar]
- Xu XZ, Li HS, Guggino WB, Montell C. 1997. Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell. 89:1155–1164. [DOI] [PubMed] [Google Scholar]
- Xue T, Do MT, Riccio A, Jiang Z, Hsieh J, et al. 2011. Melanopsin signalling in mammalian iris and retina. Nature. 479:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Zhang W, He Y, Gorczyca D, Xiang Y, et al. 2013. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature. 493:221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa A, Couto A, Sandoz JC, Hata T, Mitra A, et al. 2019. LPS perception through taste-induced reflex in Drosophila melanogaster. J Insect Physiol. 112:39–47. [DOI] [PubMed] [Google Scholar]
- Yang Z, Huang R, Fu X, Wang G, Qi W, et al. 2018. A post-ingestive amino acid sensor promotes food consumption in Drosophila. Cell Res. 28:1013–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao CA, Carlson JR. 2010. Role of G-proteins in odor-sensing and CO2-sensing neurons in Drosophila. J Neurosci. 30:4562–4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao CA, Ignell R, Carlson JR. 2005. Chemosensory coding by neurons in the coeloconic sensilla of the Drosophila antenna. J Neurosci. 25:8359–8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuyama K, Meinertzhagen IA. 1999. Extraretinal photoreceptors at the compound eye's posterior margin in Drosophila melanogaster. J Comp Neurol. 412:193–202. [DOI] [PubMed] [Google Scholar]
- Yau KW, Nakatani K. 1985. Light-suppressible, cyclic GMP-sensitive conductance in the plasma membrane of a truncated rod outer segment. Nature. 317:252–255. [DOI] [PubMed] [Google Scholar]
- Yew JY, Dreisewerd K, Luftmann H, Muthing J, Pohlentz G, et al. 2009. A new male sex pheromone and novel cuticular cues for chemical communication in Drosophila. Curr Biol. 19:1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorozu S, Wong A, Fischer BJ, Dankert H, Kernan MJ, et al. 2009. Distinct sensory representations of wind and near-field sound in the Drosophila brain. Nature. 458:201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HJ, Anderson AR, Trowell SC, Luo AR, Xiang ZH, et al. 2011. Topological and functional characterization of an insect gustatory receptor. PLoS One. 6:e24111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Li X, Zheng H, Wen X, Chen S, et al. 2018. Brv1 Is required for Drosophila larvae to sense gentle touch. Cell Rep. 23:23–31. [DOI] [PubMed] [Google Scholar]
- Zhang W, Cheng LE, Kittelmann M, Li J, Petkovic M, et al. 2015. Ankyrin repeats convey force to gate the NOMPC mechanotransduction channel. Cell. 162:1391–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yan Z, Jan LY, Jan YN. 2013. Sound response mediated by the TRP channels NOMPC, NANCHUNG, and INACTIVE in chordotonal organs of Drosophila larvae. Proc Natl Acad Sci USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, et al. 2003. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 112:293–301. [DOI] [PubMed] [Google Scholar]
- Zhang YV, Aikin TJ, Li Z, Montell C. 2016. The basis of food texture sensation in Drosophila. Neuron. 91:863–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YV, Ni J, Montell C. 2013. The molecular basis for attractive salt-taste coding in Drosophila. Science. 340:1334–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YV, Raghuwanshi RP, Shen WL, Montell C. 2013. Food-experience induced taste desensitization modulated by the Drosophila TRPL channel. Nat Neurosci. 16:1468–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Zhou H, Chi S, Wang Y, Wang J, et al. 2018. Structure and mechanogating mechanism of the Piezo1 channel. Nature. 554:487–492. [DOI] [PubMed] [Google Scholar]
- Zhong L, Bellemer A, Yan H, Honjo K, Robertson J, et al. 2012. Thermosensory and non-thermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat sensor domains of a thermoTRP channel. Cell Rep. 1:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Hwang RY, Tracey WD. 2010. Pickpocket is a DEG/ENaC protein required for mechanical nociception in Drosophila larvae. Curr Biol. 20:429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Cao LH, Sui XW, Guo XQ, Luo DG. 2019. Mechanosensory circuits coordinate two opposing motor actions in Drosophila feeding. Sci Adv. 5:eaaw5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu EY, Guntur AR, He R, Stern U, Yang CH. 2014. Egg-laying demand induces aversion of UV light in Drosophila females. Curr Biol. 24:2797–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Chu PB, Peyton M, Birnbaumer L. 1995. Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett. 373:193–198. [DOI] [PubMed] [Google Scholar]
- Zuker CS, Cowman AF, Rubin GM. 1985. Isolation and structure of a rhodopsin gene from D. melanogaster. Cell. 40:851–858. [DOI] [PubMed] [Google Scholar]
- Zuker CS, Mismer D, Hardy R, Rubin GM. 1988. Ectopic expression of a minor Drosophila opsin in the major photoreceptor cell class: distinguishing the role of primary receptor and cellular context. Cell. 53:475–482. [DOI] [PubMed] [Google Scholar]
- Zuker CS, Montell C, Jones K, Laverty T, Rubin GM. 1987. A rhodopsin gene expressed in photoreceptor cell R7 of the Drosophila eye: homologies with other signal-transducing molecules. J Neurosci. 7:1550–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]