Abstract
Rationale:
Chronic health conditions (CHCs) are costly and difficult to manage. Patients often struggle with behavioral adherence to complex treatment regimens and experience psychiatric distress. Acceptance and Commitment Therapy (ACT) is a transdiagnostic behavioral approach that aims to improve functioning and quality of life (QoL), which are important treatment outcomes for this population. Preliminary efficacy of multi-session ACT in patients with CHCs has been demonstrated, and single-session ACT interventions have since been developed to increase feasibility, acceptability, and accessibility. The purpose of this systematic review and meta-analysis was to describe the literature on single-session ACT intervention studies in CHC populations with regards to (1) study design and methodology, (2) patient characteristics and conditions targeted, and (3) efficacy for outcomes across various domains, using narrative and quantitative methods.
Methods:
PsycINFO, PubMed, and Web of Science were systematically searched in August 2020. Studies of single-session ACT interventions in adult patients with CHCs that reported quantitative outcomes in any of the following domains were included: (a) functioning and related domains (e.g., disability, QoL, well-being); (b) mental health; (c) physical health; (d) ACT processes. Both controlled and uncontrolled studies were included. Study quality was assessed using the Psychotherapy Outcome Study Methodology Rating Scale (POMRF). Between-group random effects meta-analysis was conducted on general functioning outcomes.
Results:
Fourteen manuscripts reporting outcomes from 13 studies (N = 793) met inclusion criteria. Ten studies were identified by their authors as pilot or feasibility trials. Eight studies used comparison or control groups. Twelve studies delivered the ACT content in workshop format. Studies recruited for a variety of conditions. Narrative review found that between- and within-group effect sizes showed generally positive results favoring single-session ACT overall (69%), especially for measures of functioning and related domains (88%), mental health (67%), and ACT processes (73%). Meta-analysis found that ACT did not significantly outperform comparison groups on measures of general functioning (Hedges’ g: −0.51, 95% confidence interval: [−1.19, 0.16]; I2 = 86%; K = 5) despite a medium-sized pooled effect.
Discussion:
Use of single-session ACT interventions in CHC populations is an emergent field. There is preliminary evidence for the acceptability, feasibility, and efficacy of these interventions, which provides support for further testing in fully-powered RCTs. Additional RCTs will enable larger meta-analyses and stronger conclusions about efficacy. Recommendations for future trials are provided.
Keywords: ACT, acceptance and commitment therapy, brief, systematic review, chronic health conditions, workshop, meta-analysis
Physical illness is part of the human experience. While some illnesses are transient and curable, many become chronic and present ongoing challenges to the individual, their families and communities, and the healthcare system. An estimated 60% of Americans have at least one chronic health condition (CHC; Hartzler, Castle, Lewis, & Zakaria, 2020). These conditions account for approximately 75% of healthcare expenditures and can lead to hospitalization, long-term disability, reduced quality of life (QoL), and death (Raghupathi & Raghupathi, 2018). Treatment for CHCs often requires a shift from trying to get rid of the condition to managing it and preventing complications. CHC management often requires health behavior change (e.g., diet, exercise, medication adherence) and ongoing engagement with the healthcare system (e.g., in-office treatments, routine checkups, specialist visits).
Many individuals living with CHCs also experience psychiatric symptoms. Psychiatric distress might be pre-existing and become exacerbated by the condition, appear following diagnosis or CHC-related impairment, or occur as a response to difficulties managing the condition (e.g., diabetes distress; Skinner, Joensen, & Parkin, 2019). For example, the prevalence of comorbid depression ranges from 30–60% in chronic pain (Bair, Robinson, Katon, & Kroenke, 2003), 20–40% in cardiovascular disease (Davidson et al., 2006), 11–30% in diabetes (Anderson, Freedland, Clouse, & Lustman, 2001), and 16–30% in irritable bowel syndrome (Mykletun et al., 2010; Van Oudenhove et al., 2016). Co-occurring depression is associated with more severe medical pathology, poorer prognosis, and higher healthcare utilization compared to having a CHC only (Arnow et al., 2006; Celano & Huffman, 2011; Jeong et al., 2017; Löwe et al., 2008; Merikangas et al., 2007). Similarly, the prevalence of anxiety disorders in CHC is higher than in the general population, including reports of 25–29% in chronic pain (Asmundson & Katz, 2009), 11–14% in cardiovascular disease (Celano, Daunis, Lokko, Campbell, & Huffman, 2016), 14% in diabetes (Grigsby, Anderson, Freedland, Clouse, & Lustman, 2002), and 37% in irritable bowel syndrome (Grover et al., 2021). Anxiety is associated with greater pain severity (Csupak, Sommer, Jacobsohn, & El-Gabalawy, 2018), an elevated risk of cardiovascular mortality (Emdin et al., 2016), suboptimal diabetes control (Anderson et al., 2002), and greater impairment in irritable bowel syndrome (Lee et al., 2009).
There is a bidirectional relationship between physical and psychiatric symptoms (Evans et al., 2005). Comorbid psychiatric symptoms and disruptions to daily living contribute to poor QoL in people with CHCs (Megari, 2013). Interventions that maximize self-management behaviors and behavioral activation in the face of complex treatment regimens and accompanying mental health challenges hold promise for maximizing patients’ QoL while decreasing healthcare burden.
Acceptance and Commitment Therapy (ACT), a third-wave cognitive-behavioral therapy, is a promising adjunctive treatment for people with CHCs (Dindo, Van Liew, & Arch, 2017). ACT’s primary treatment target is not amelioration of symptoms, but rather improved functioning-related outcomes specifically aligned with an individual’s values. Though symptom reduction often does occur following ACT treatment (A-Tjak et al., 2015), ACT is particularly well-suited for patients with CHCs, for whom symptom remission may not be a reasonable treatment outcome. ACT is hypothesized to improve functioning and QoL via increases in psychological flexibility. Psychological flexibility is the synthesis of six interrelated processes, which include: (1) acceptance (rather than avoidance) of any and all valanced internal and external experiences; (2) cognitive defusion (that is, de-identification with thoughts and feelings, rather than fusion with or attachment to one’s thoughts and feelings as absolute truth); (3) present-moment awareness (rather than maladaptive past- or future-focus); (4) self-as-context (rather than personal identification with one’s thoughts or attachment to one’s imagined self); (5) defining personally-relevant valued life directions (rather than lack of values clarity); and (6) committed action consistent with those values (rather than inaction or values-incongruent action) (Luoma, Hayes, & Walser, 2007).
ACT is considered a transdiagnostic intervention, as processes can be applied flexibly to address multiple overlapping issues. For example, patients with CHCs may attempt to avoid the disappointment and discomfort associated with having their condition by not taking medication, avoiding activities where modifications must be made or that are related to symptom increase (e.g., physical activity in chronic pain), and engaging in unhealthy coping behaviors (e.g., substance use, overeating). While these behaviors may serve short-term goals (e.g., decreased stress, enjoyment of unhealthy foods, etc.), in the long-term these behaviors paradoxically increase distress and illness severity, and often lower QoL. Personal identification with and attachment to thoughts and feelings about one’s condition can be targeted with ACT (e.g., using cognitive defusion and self-as-context exercises) to disrupt these behavioral repertoires. Ruminating thoughts about life before the condition, worries about future complications, or concerns about symptom flare-ups can be addressed using acceptance and present moment awareness exercises. Values identification and committed action exercises can then be used to help patients identify and commit to behaviors that would make their lives personally meaningful given the unique context of their diagnosis and prognosis.
A prior systematic review of full-length ACT interventions for CHCs found preliminary evidence for efficacy (Graham, Gouick, Krahe, & Gillanders, 2016). However, across the 18 studies reviewed, mean number of treatment sessions was 6.5, with some interventions delivering as many as 11 or 12 sessions. For patients with CHCs, briefer interventions may be more feasible, acceptable, and appropriate, as they can be delivered in traditional healthcare settings and require less resource investment from patients and providers. Many patients live in remote areas, making it logistically and financially challenging to attend multiple sessions. Additionally, the modal number of treatment sessions attended in psychotherapy interventions is one (Gibbons et al., 2011). Thus, using single-session protocols specifically may increase adherence and potentially effectiveness because they ensure every participant receives all intervention components. Although a small narrative review (k = 6) of single-session ACT interventions for CHCs found preliminary support (Dindo, 2015), that review was neither systematic nor did it include a meta-analytic component.
The overarching purpose of the present review is to bolster the literature by providing a current, systematic review of single-session ACT for CHC interventions, including preliminary meta-analysis of efficacy. The scope of the present review is broader than prior reviews in its inclusion of both peer-reviewed and grey literature publications. While five included studies were previously reported in Graham et al. (2016) and Dindo (2015), several relevant studies have been published since, including nine studies newly reported here. Primary aims of the present manuscript are to describe the state of the literature of single-session ACT interventions for CHCs with regards to (1) study design and methodology, (2) patient characteristics and conditions targeted, and (3) efficacy for outcomes across various domains, using narrative and quantitative summary methods.
Methods
This systematic review with meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher, Liberati, Tetzlaff, Altman, & Prisma Group, 2009).
Eligibility Criteria
We used the five PICOS components (participants, interventions, comparators, outcomes, and study design) to design our research question and eligibility criteria (Moher et al., 2009). Studies were required to meet the following inclusion criteria: (P) sample was adults, 18 years and older, with physical illness(es); (I) delivered a single-session intervention based on the ACT model; (C) due to the nascent stage of the literature, both controlled and uncontrolled studies were included; (O) assessed any of the following outcomes quantitatively: functioning and related domains (e.g., disability, well-being, QoL, health behaviors), mental health (e.g., symptom severity, diagnoses changes), physical health (e.g., symptom severity, biomarkers), or ACT processes (e.g., experiential avoidance, psychological flexibility); and (S) used a prospective design. Studies were excluded if: (P) the condition, population, or sole outcome of interest was a health behavior outside the context of a CHC (e.g., physical activity or eating behavior in adults without a discrete physical illness) or the intervention was designed to treat caregivers of individuals with a CHC exclusively; (I) a self-help or web-based design was used with no substantive therapist interaction; (O) only qualitative outcomes were reported; or (C/S) study design was cross-sectional, case study, or case series. Only manuscripts published in English were included.
Information Sources and Search Strategy
The online databases of PsycINFO, PubMed, and Web of Science were systematically searched in August 2020. No search limitations or filters were imposed. We reviewed both peer-reviewed manuscripts and grey literature (i.e., theses and dissertations) for inclusion. No lower limit to year of publication was imposed. Manuscripts were identified using a pre-defined search strategy developed with the assistance of a research librarian. The following search terms were entered, joined by the operator “AND”:
“acceptance and commitment therapy” OR “acceptance”
“brief” OR “single-session” OR “One-day” OR “focused” OR “workshop” OR “FACT” OR “adjunct*” OR “single-session” OR “Pilot” OR “short”
“chronic” OR “disease*” OR “health” OR “physical” OR “medical” OR “illness*” OR “condition*” OR “diabetes” OR “cancer” OR “pain” OR “migraine*” OR “HIV”
An example search is included in Supplemental material. Reference lists of included manuscripts, as well as Dindo (2015) and Dindo et al. (2017), were hand-searched. We also searched the Association for Contextual Behavioral Science (http://contextualscience.org) website and contacted the email listserv of that professional organization for peer review of the final inclusion list.
Study Selection
Study selection proceeded in three stages. In stage 1 (screening), all manuscripts returned from database searches were imported into reference management software. These manuscripts received title/abstract review. Studies that clearly failed to meet inclusion criteria or met exclusion criteria were removed. In stage 2 (selection), remaining studies received a full-text review. Ineligible studies were removed and categorized according to exclusion reason (see Figure 1). In stage 3 (hand-searching), the reference section of included studies as well as Dindo (2015) and Dindo et al. (2017) were reviewed to identify relevant manuscripts not previously identified through database searches. Two independent reviewers (C.D. and M.S.H.) conducted stages 1–3. Disagreements about final study inclusion (k = 2; 2%) were discussed in a consensus meeting with other co-authors.
Figure 1.

Study screening and selection flowchart (adapted from Moher et al., 2009).
Data Extraction and Management
EndNote X8 was used to store results from database and hand searches, and to categorize manuscripts according to inclusion and exclusion decisions. Duplicates were removed using the EndNote X8 “remove duplicates” feature and by hand. Study coding and data extraction occurred in Excel. Data extraction was performed by the primary author (C.D.) and verified by co-authors (J.S.W. and M.W.L.).
Data regarding sociodemographic characteristics (employment, gender, race/ethnicity, education), study population/condition, study design (e.g., pilot, randomized controlled trial), setting, intervention duration, assessment schedule, and intervention and comparator conditions were extracted. Data from functioning and related outcomes, mental health outcomes, physical health outcomes, and ACT process outcomes were extracted. Results from intention-to-treat (ITT) analyses and analyses controlling for relevant covariates were extracted when reported. Where two measures were used to examine the same construct, results from the measure identified by the study authors as primary were extracted. Where outcomes were assessed at multiple follow-up intervals, results from three-month (12-week) follow-up were reported, as this was the most common interval across studies (k = 10; 77%). Otherwise, the follow-up interval closest to three months was selected. Authors of included studies were contacted for additional information as needed. Effect size information were calculated by the primary author (C.D.) and confirmed by a co-author (J.S.W.) when not reported. A Cohen’s d or Hedges’ g value of .2 is considered small, .5 is considered medium, and .8 is considered large. A phi value of .1 is considered small, .3 is considered medium, and .5 is considered large.
Meta-analysis
Quantitative analysis of treatment efficacy in controlled studies for functioning-related outcomes was conducted using random effects meta-analysis. Functioning-related outcomes were chosen as they are central transdiagnostic treatment targets of ACT. To avoid dependence among multiple effect sizes from the same study, one outcome per controlled study was selected. Where studies reported more than one relevant outcome, the general (rather than condition-specific) measure was selected to reduce heterogeneity.
Analysis was conducted in R version 3.6.1 (R Core Team, 2013) using the metafor and dmetar packages (Harrer, Cuijpers, Furukawa, & Ebert, 2019), using the inverse variance method and Hedges’ g as the standardized mean difference. Random effects modeling was used due to considerable statistical heterogeneity among the analyzed effects (i.e., I2 > 75%, per Cochrane guidelines). All measures included in the meta-analysis were scored such that higher scores indicated poorer results, except for one (the World Health Organization Quality of Life-BREF psychological subscale reported in Dindo et al., 2015), which was reverse scored for consistency of interpretation. Given concerns about inflation in pre-post effect size estimates (Cuijpers, Weitz, Cristea, & Twisk, 2017), within-group effect sizes are depicted graphically but not meta-analyzed.
Statistical heterogeneity was assessed using I2, Cochran’s Q-statistic, and τ2 (using DerSimonian-Laird estimator) (Higgins, Thompson, Deeks, & Altman, 2003). I2 is the percentage of variability in effect sizes due to heterogeneity rather than sampling error. The Q-statistic is the weighted sum of squared differences between individual study effects and the pooled effect, from which I2 is derived. The Q-statistic chi-squared significance test is known to be low-powered for analyses with few studies and should be interpreted with caution (Higgins et al., 2003). τ2 is another metric of between-study variance in effect sizes (Deeks, Higgins, Altman, & Cochrane Statistical Methods Group, 2019). A prediction interval, which accounts for between-study variance and is less sensitive to number of studies than standard heterogeneity estimates, was also calculated (Harrer et al., 2019). Prediction intervals provide a range in which future study effects are predicted to fall based on present evidence in the meta-analysis.
Individual Study Quality Assessment
Study quality was assessed using the Psychotherapy Outcome Study Methodology Rating Form (POMRF; Öst, 2008). The POMRF is a 22-item measure that assesses methodology and reporting for psychotherapy trials. Each item is rated on a 3-point scale where 0 = poor, 1 = fair, 2 = good. Given that this review includes studies focused on CHC populations rather than strictly psychiatric populations, POMRF items #2 (severity/chronicity of the disorder), #4 (reliability of the diagnosis in question), and #21 (clinical significance) were deemed not directly applicable and were not assessed. For pilot/feasibility studies, item #11 (power analysis) was not assessed. For uncontrolled studies, items #10 (design) and #22 (equality of therapy) were not assessed. Because some items were not assessed and because it is unclear if the total score is a reliable and valid measure of study quality (Liberati et al., 2009), total POMRF scores are not reported. Two reviewers (C.D. and J.S.W.) independently assessed quality of each study and met to reach consensus. Risk of bias was not formally assessed but was approximated at the study level based on whether or not the trial was pre-registered.
Results
Figure 1 shows the number of manuscripts identified throughout the screening, hand-searching, and selection phases. Half of the manuscripts excluded during the selection phase were ACT interventions for CHC that delivered more than one session (k = 37). Fourteen manuscripts reporting results from 13 unique studies met inclusion criteria. Two manuscripts (Dindo, Recober, Marchman, O’Hara, & Turvey, 2014; Dindo, Recober, Marchman, Turvey, & O’Hara, 2012) report separate outcomes from a single study. Five of the 14 included manuscripts (36%; Gregg, Callaghan, Hayes, & Glenn-Lawson, 2007; Sheppard, Forsyth, Hickling, & Bianchi, 2010; Dindo et al., 2012; Dindo et al., 2014; Dindo, Marchman, Gindes, & Fiedorowicz, 2015) were included in prior reviews by Dindo (2015) and Graham et al. (2016). Of the nine newly reported studies, two were RCTs, one was a dissertation, and six were pilot/feasibility trials.
Quality Assessment
POMRF item scores for each manuscript are presented in Table 1. Overall, studies ensured inclusion of representative samples, used valid and reliable outcome measures specific to the condition and treatment targets, delivered the intervention by clinical psychologists with advanced training, and used appropriate analytic methods with thorough reporting. Most manuscripts presented ITT analysis. Three studies reported both ITT and dropout analysis (Dindo et al., 2018; Gregg, Callaghan, Hayes, & Glenn-Lawson, 2007; Pedersen et al., 2019). All studies reported attrition rates. Rates of attrition for intervention completion (i.e., % of participants that were consented but did not complete the 1-day ACT intervention) ranged from 0% to 35%. The highest attrition rates were in patients with diabetes (35%), irritable bowel syndrome (28%), Veterans with chronic pain and traumatic brain injury (26%), and patients with comorbid headache and depression (21%).
Table 1.
Study quality assessment using the Psychotherapy Outcome Study Methodology Rating Scale (POMRF)
| Study | Gregg (2007) | Sheppard (2010) | Dindo (2012) | Dindo (2014)† | Welch (2014) | Dindo (2015) | Hou (2017) | Dindo (2018) | Ferreira (2018) | Huddleston (2018) | Hadland smyth (2019) | Pedersen (2019) | Dindo (2020a) | Dindo (2020b) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pilot/Feasibility | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N |
| Controlled | Y | N | Y | Y | N | Y | N | Y | N | N | Y | Y | Y | Y |
| 1: Clarity of sample description | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 |
| 2: Severity/chronicity of disorder | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 3: Representative-ness of sample | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 4: Reliability of diagnosis | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 5: Specificity of outcome measures | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 6: Reliability and validity of outcome measures | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 |
| 7: Use of blind evaluators | 1 | n/a | 0 | 0 | n/a | 0 | n/a | 1 | n/a | n/a | n/a | 0 | 0 | 2 |
| 8: Assessor training | 1 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | n/a | 0 | 0 | 0 |
| 9: Assignment to treatment | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 2 |
| 10: Design | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 2 |
| 11: Power analysis | 2 | 0 | n/a | n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | 2 | n/a | 0 |
| 12: Assessment points | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 2 | 0 | 1 |
| 13: Manualized, replicable, specific treatment programs | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 2 | 2 |
| 14: Number of therapists | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| 15: Therapist training/experience | 1 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 0 | 2 | 2 | 2 | 2 |
| 16: Checks for treatment adherence | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| 17: Checks for therapist competence | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 0 |
| 18: Control of concomitant treatments | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 19: Handling of attrition | 2 | 1 | 1 | 1 | 0 | 1 | 0 | 2 | 1 | 0 | 0 | 2 | 1 | 1 |
| 20: Statistical analyses and presentation of results | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 2 |
| 21: Clinical significance | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 22: Equality of therapy hours | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 0 | n/a | 2 |
Note. Pilot/feasibility determination made based on source author’s use of the terms to describe study.
Same parent study as Dindo (2012)
The majority of studies with comparison conditions implemented random assignment to treatment and one also randomly assigned to therapist within condition. Two studies reported power analyses (Gregg et al., 2007; Pedersen et al., 2019). Equality of therapy hours in studies with treatment as usual (TAU) control conditions was difficult to assess. Many studies did not provide descriptions of assessor training and blinding, checks for treatment adherence or therapist supervision, or attempts to control for concomitant treatment (particularly other psychotherapy interventions). Many studies used two or more therapists, but none controlled for therapist effects in statistical analyses. Only one study was pre-registered (Dindo et al., 2020b).
Study Design and Methodology
The design and methodology of each study is described in detail in Table 2 and the specific content of intervention and control conditions is shown in Supplement Table 1. Sample sizes ranged from 15–136 participants (M = 61, SD = 36). The range of female participant inclusion was 0–100% of the sample (M = 61%, SD = 32%); the range of participants identifying as white was 24–92% of the sample (M = 61%, SD = 24%). Mean sample ages ranged from 33–63 years (median: 46.5; M = 46, SD = 8.5). Employment rates ranged from 19–89% and unemployment or disability rates ranged from 11–62%.
Table 2.
Manuscripts reporting studies of single-session ACT interventions for medical populations (K =14)
| First author (year) | Population/Condition | Participant characteristics | Study design, setting, intervention duration, assessment schedule | Intervention and comparator conditions | Outcomes and measures | Key Findings | |
|---|---|---|---|---|---|---|---|
| 1 | Gregg (2007) | Adults with diabetes mellitus type 2 | N = 81; Mage = 50.9 years; female = 46.9%; white = 23.5%; employment = 10% working full-time, 9% working part-time, 25% unemployed looking for work, 6% unemployed not looking for work, 8% retired, 28% disabled/unable to work; education = 57% with some education greater than High School; income not reported; MBMI = 32.6 | Randomized controlled trial; 7-hour workshop; Setting: community health center; Baseline, 3-month FU | (1) ACT + Diabetes management education (n = 43) (2) Diabetes management education (n = 38) |
(a) HbA1c (b) Diabetic Control (HbA1c < 7.0%) (c) Diabetes self-management (3 self-report items based on exercise, diet, glucose monitoring) (d) Diabetes acceptance (Acceptance and Action Diabetes Questionnaire/AADQ) |
(a) Nonsignificant trend for greater improvement in HbA1c in Group (1) > Group (2) at FU; Cohen’s d = 0.35 (b) Significant increase in rate of diabetic control in Group (1) at FU, p < .01, but not Group (2); Cohen’s d = 0.61 (c) Group (1) increase > Group (2) increase at FU, both p < .05; Cohen’s d = 0.68 (d) Group (1) increase significant (p < .01) and > Group (2) increase at FU; Cohen’s d = 0.78 |
| 2 | Sheppard (2010) | Adults with multiple sclerosis (MS) | N = 15; Mage (SD) = 53.13 years (7.68); female = 80%; white = 66.7 %; employment = 46% unemployed or receiving disability payments; education = 60% with Bachelors degree or higher; income not reported | Uncontrolled feasibility trial; 5-hour workshop; Setting: local hotel conference venue; Baseline, 3-month FU | ACT + MS education | (a) Depression (Beck Depression Inventory-II/BDI-II) (b) Impact of Fatigue (Modified Fatigue Impact Scale/MFIS) (c) Pain Effects on mood/behavior (Pain Effects Scale/PES) (d) Physical Health (SF-36) (e) Mental Health (SF-36) (f) Quality of Life (Quality of Life Inventory/QOLI) (g) Thought Suppression (White Bear Suppression Inventory/WBSI) (h) Mindful Attention Awareness Scale (MAAS) |
(a) Baseline to FU mean difference = −8.31; Hedge’s g = −0.73 (b) Baseline to FU mean difference = −11.21; Hedge’s g = −0.50 (c) Baseline to FU mean difference = 4.98; Hedge’s g = −0.98 (d) Baseline to FU mean difference for physical health = −0.86; Hedge’s g = −0.10 (e) Baseline to FU mean difference for mental health = 4.23; Hedge’s g = 0.36 (f) Baseline to FU mean difference = 1.11; Hedge’s g = 0.45 (g) Baseline to FU mean difference = 5.35; Hedge’s g = −0.33 (h) Baseline to FU mean difference = 0.22; Hedge’s g = −0.22 |
| 3 | Dindo (2012) | Adults with migraine and depression (current depressive episode) | N = 45; Mage (SD) = 32.8 years (13.2); female = 94%; white = 92%; employment = 89% working or in school; education = 89% > 12 years; income not reported | Non-randomized controlled pilot trial; 5-hour workshop; Setting: university hospital and clinics; Baseline, 2-, 6-, 12-week FU | (1) ACT + migraine psychoeducation (n = 31) (2) Waitlist/TAU (n = 14) |
(a) Depression Sx (Hamilton Rating Scale for Depression/HRSD) (b) General functioning (World Health Organization Disability Assessment Schedule II/WHODAS) M (c) Migraine-related disability (Headache Disability Inventory/HDI) |
(a) Group (1) decrease > Group (2) decrease at 12-week FU; Cohen’s d = 1.18 (b) Group (1) increase > Group (2) increase at 12-week FU; Cohen’s d = 0.98 (c) Group (1) decrease > Group (2) decrease at 12-week FU; Cohen’s d = 1.03 |
| 4 | Dindo (2014)† | Adults with migraine and depression (current depressive episode) | N = 45; Mage (SD) = 31.4 years (12.3); female = 94%; white = 87%; employment = 90% working or in school; education = 92% > 12 years; income not reported | Non-randomized controlled pilot trial; 5-hour workshop; Setting: university hospital and clinics; Baseline, 2-, 6-, 12-week FU | (1) ACT + migraine psychoeducation (n = 38) (2) Waitlist/TAU (n = 22) | (a) Headache frequency/severity (b) Acute headache medication use (c) Leisure and work disability (d) Visit to healthcare professional Note: all outcomes measured using daily headache diary |
(a) Group (1) decreased in frequency (OR=0.57) and severity (OR=0.41) at 12-week FU, no change for Group (2) (OR=0.84, 1.0) (b) Group (1) decreased in acute medication use (OR=0.64) at 12-week FU, no change for Group (2) (OR=0.97) (c) Group (1) decreased in leisure disability (OR=0.56) and work disability (OR=1.0) at 12-week FU, no change for Group (2) (OR=0.78, 1.8) (d) At baseline, 20% of Group (1) and 22% of Group (2) attended medical visit in last month; at 12-week FU, 3% of Group (1) and 33% of Group (2) |
| 5 | Welch (2014) | Adults with diabetes mellitus type 2 with significant distress (either diabetes management regimen distress or emotional burden) | N = 31; Mage (SD)= 43 years (9.1); female = 70%; white = 45%; employment status not reported; Myears education (SD) = 13.9 (1.2); income not reported | Uncontrolled pilot trial; 8-hour workshop; Setting: psychology graduate school; Baseline, post-treatment, 2-week FU | ACT | (a) Self-care behavior (Summary of Diabetes Self-Care Activities scale revised version/SDSCA) (b) Diabetes-related distress (Diabetes Distress Scale 17/DDS17) total (c) Diabetes acceptance (AADQ) (d) Thought suppression (WBSI) (e) Depression (DASS-21) (f) Anxiety (DASS-21) (g) Stress (DASS-21) |
(a) Baseline to FU mean difference on subscales: (1) General diet = 0.82; Cohen’s d = 0.52 (2) Diabetes-specific diet = 0.62; Cohen’s d = 0.61 (3) Exercise = −2.12; Cohen’s d = 1.62 (4) Blood-glucose testing = 0.58; Cohen’s d = 0.29 (5) Foot care = −1.29; Cohen’s d = 0.76 (6) Smoking status = −0.9; Cohen’s d = 0.22 (b) Baseline to FU mean difference on total score = −1.51; Cohen’s d = 1.74 (c) Baseline to FU mean difference = 18.85; Cohen’s d = 1.95 (d) Baseline to FU mean difference = 19.15; Cohen’s d = 1.58 (e) Baseline to FU mean difference = −3.1; Cohen’s d = 0.57 (f) Baseline to FU mean difference = −4.4; Cohen’s d = 0.42 (g) Baseline to FU mean difference = −5.7; Cohen’s d = 0.72 |
| 6 | Dindo (2015) | Adults at risk for vascular disease* with clinically significant anxiety or depression Sx | N = 44; Mage = 45 years; female = 67%; white = 74%; employment not reported; education = 70% completed college; income not reported | Randomized controlled pilot trial; 6-hour workshop; Setting: university hospital and clinics; Baseline, 12- and 24-week FU | (1) ACT + psychoeducation (n = 30) (2) TAU (n = 14) |
(a) General wellbeing (World Health Organization Quality of Life-BREF/WHOQOL-BREF) M (b) Depression (HRSD) (c) Anxiety (Hamilton Rating Scale for Anxiety/HSRA) (d) Psychological Flexibility/decentering (Experiencing Questionnaire/EQ) |
(a) In Group (1), all four domains (physical, social, psychological, environment) improved at 24-week FU; in Group (2), only psychological domain was improved (b) Group (1) decrease > Group (2) decrease at 12- and 24-week FU; unspecified effect size = 1.4 at 24-week FU (c) Group (1) decrease > Group (2) decrease at 12- and 24-week FU; unspecified effect size = 1.5 at 24-week FU (d) Increase in Group (1) at unspecified FU; Group (2) results not reported |
| 7 | Hou (2017) | Adults with inflammatory bowel disease (IBD) and clinically significant anxiety or depression symptoms | N = 20; Mage = 51 years; female = 30%; % white not reported; employment not reported; education not reported; income not reported; n=9 with Crohn’s disease, n=10 with ulcerative colitis, n=1 IBD unclassified | Uncontrolled feasibility study; 5-hour workshop; Setting: Veterans Affairs medical center and university medical school; Baseline, 3-month FU | ACT + IBD education | (a) Health-related Quality of Life (the Short IBD Questionnaire/SIBDQ) (b) Depression (DASS-21) (c) Anxiety (DASS-21) (d) Stress (DASS-21) (e) IBD activity (Harvey Bradshaw Index for pts. with Crohn’s/HBI) (f) IBD activity (partial Mayo score for pts with Ulcerative Colitis/pMayo) |
(a) Baseline to FU mean difference = 0.5; Hedge’s g = 0.41 (b) Baseline to FU mean difference = −2.1; Hedge’s g = −0.39 (c) Baseline to FU mean difference = −2.6; Hedge’s g = −0.65 (d) Baseline to FU mean difference = −1.9; Hedge’s g = −0.39 (e) Baseline to FU mean difference = −0.5; Hedge’s g = −0.15 (f) Baseline to FU mean difference = −1.2; Hedge’s g = −0.47 |
| 8 | Dindo (2018) | Veterans receiving orthopedic surgery at risk for chronic pain or prolonged opioid use** | N = 88; Mage (SD) = 63 years (10); female = 7%; white = 82.5%; employment not reported; education = 68% with some education greater than High School; income not reported | Randomized controlled pilot trial; 5-hour workshop; Setting: Veterans Affairs medical center; Baseline and 3-month FU for CPAQ and CPVI; DLPM submitted weekly for 14 weeks post-treatment | (1) ACT workshop + 1 individualized “booster” session by phone 2–4 wks following workshop (n = 44) (2) TAU (n = 44) |
(a) Pain cessation (Daily Log of Pain and Pain Medication/ DLPM) (b) Opioid use/ cessation (DLPM) (c) Pain acceptance (Chronic Pain Acceptance Questionnaire total/CPAQ) (d) Values-based behavior (Chronic Pain Values Inventory/ CPVI) |
(a) Median days to pain cessation was 66 for Group (1) and 74 for Group (2); HR = 1.42 [95% CI: 0.68, 2.95] (b) 29% of Group (1) taking opioids at 7 weeks v. 52% of Group (2); HR = 1.44 [95% CI: 0.74, 2.78] (c) Mean difference between Group (1) and (2) at FU = 2.07 [95% CI: −7.46, 11.61]; HR = 1.42 [95% CI: −7.11, 9.57] (d) Mean difference between Group (1) and (2) on ‘mean success’ at FU = −0.50 [95% CI: −1.01, 0.01]; HR = 0.13 [95% CI: −0.33, 0.59]; mean difference between Group (1) and (2) on ‘discrepancy score’ at FU = 0.21 [95% CI: −0.23, 0.65]; HR = −0.42 [95% CI = −0.85, 0.01] |
| 9 | Ferreira (2018) | Adults with refractory Irritable Bowel Syndrome (IBS) | N = 79; Mage (SD) = 48 years (13); female = 93%; race/ethnicity not reported; employment not reported; education = 68% with post-secondary education; income not reported. | Uncontrolled pilot trial; 6-hour workshop followed by 2 months of bibliotherapy and 2 FU support calls; Setting: gastroenterology outpatient clinic; Enrollment, pre-treatment, post-treatment (2 months following pre-treatment), 6-month FU | ACT + IBS education workshop, bibliotherapy, 2 individualized support calls | (a) IBS Acceptance (IBS Acceptance and Action Questionnaire; IBSAAQ) (b) Symptom severity (IBS Symptom Severity Scale; IBSSSS) (c) Quality of life (IBS Impact on Quality of Life Scale; IBS36) (d) IBS avoidant behaviors (Behavioural Responses Questionnaire; IBS-BRQ) (e) Gastrointestinal specific anxiety (Visceral Sensitivity Index; VSI) |
(a) Pre- to post-treatment mean difference = 7.24; Cohen’s d = 0.32; Pre-treatment to FU mean difference = 9.82; Cohen’s d = 0.50 (b) Pre- to post-treatment mean difference = −41.48; Cohen’s d = 0.41; Pre-treatment to FU mean difference = −49.78; Cohen’s d = 0.47 (c) Pre- to post-treatment mean difference = −17.41; Cohen’s d = 0.41; Pre-treatment to FU mean difference = −23; Cohen’s d = 0.55 (d) Pre- to post-treatment mean difference = −8.57; Cohen’s d = 0.32; Pre-treatment to FU mean difference = −10.18; Cohen’s d = 0.39 (e) Pre- to post-treatment mean difference = −6.3; Cohen’s d = 0.76; Pre-treatment to FU mean difference = −8.73; Cohen’s d = 1.10 |
| 10 | Huddleston (2018) | Veterans with migraines and co-occurring depression (current depressive episode) | N = 32; Age = 36% under 45, 36% 45–55 years, 24% 56–65 years, 1% over 65 years; female = 36%; white = 24%; employment = 25% employed full- or part-time, 6% retired, 34% unemployed, 22% disabled, 13% student; education = 56% with some education greater than High School; income not reported | Uncontrolled pilot trial; 5-hour workshop; Setting: Veterans Affairs medical center; Baseline, 3-month FU | ACT + migraine education | (a) Depression Sx (HRSD) (b) Anxiety Sx (HRSA) (c) General functioning (WHO Disability Assessment Schedule II/WHODAS) (d) Headache-related disability (HDI) (e) Pain acceptance (CPAQ) (f) Values-based behavior (CPVI) (g) Psychological flexibility (Acceptance and Action Questionnaire/AAQ-II) |
(a) Baseline to FU mean difference = −7.95; Cohen’s d = −1.93 (b) Baseline to FU mean difference = −6.57; Cohen’s d = −1.84 (c) Baseline to FU mean difference = −5.38; Cohen’s d = −0.38 (d) Baseline to FU mean difference = −7.44; Cohen’s d = −0.39 (e) Baseline to FU mean difference = 8.48; Cohen’s d = 0.48 (f) Baseline to FU mean difference = 0.5; Cohen’s d = 0.48 (g) Baseline to FU mean difference = −5.53; Cohen’s d = −0.71 |
| 11 | Hadlandsmyth (2019) | Adult women undergoing surgery for breast cancer or ductal carcinoma in situ at risk for persistent postsurgical pain*** | N = 62; Mage (SD) = 53 years (12); female = 100%; white = 87%; employment not reported; education not reported; income = 17% <$40,000, 24% $40,000–79,999, 59% $80,000+ | Randomized controlled pilot trial; 2-hour individual session 2 weeks post-surgery; Setting: comprehensive cancer center; Baseline, 3-month FU | (1) ACT therapy + TAU (n = 24) (2) TAU (medical care) (n = 30) |
(a) Pain intensity (0–10 scale) (b) Pain catastrophizing (Pain Catastrophizing Scale/PCS) (c) Depression (Patient Health Questionnaire-8) (d) Anxiety (Generalized Anxiety Disorder-7) (e) Pain acceptance (CPAQ) |
(a) At FU, 8.3% in Group (1) reported moderate-to-severe pain v. 13.3% in Group (2); phi = 0.08 (b) At FU, 4.2% in Group (1) reported elevated pain catastrophizing v. 3.3% in Group (2); phi = 0.02 (c) At FU, 12.5% in Group (1) reported elevated depression v. 13.3% in Group (2); phi = 0.01 (d) At FU, 4.2% in Group (1) reported elevated anxiety v. 13.3% in Group (2); phi = 0.16 (e) Mean difference at FU = 1.66, favoring Group (1); Cohen’s d = 0.10 |
| 12 | Pedersen (2019) | Adults with multiple functional somatic syndromes (FSS) | N = 121 (for conditions of interest); Mage (SD) = 39 years (9); female = 83%; race/ ethnicity not reported; employment = 26% employed or student, 31% unemployed, 31% disability pension or flexible work; education = 31% greater than basic school (Denmark); income not reported; Functional Somatic Syndromes: 29% Irritable Bowel Syndrome, 79% Chronic Fatigue Syndrome, 74% Fibromyalgia, 74% tension headaches, 55% non-cardiac chest pain; average number of FSS = 3.9 | Randomized, controlled, 3-arm trial; Setting: university general hospital; Baseline, 6-, 14, and 20-month FU | (1) ACT (6-hour workshop) + Enhanced Care (1–1.5 hour psychoeducation consultation with a physician 1–2 weeks after randomization) (n = 61) (2) Enhanced Care only (n = 60) (3) Extended ACT (nine 3-hr group sessions) + Enhanced Care (n = 59) Note: this review reports results comparing Groups (1) and (2) only |
(a) Patient-rated overall health (5-pt clinical global improvement scale/CGI) (b) Physical Health (SF-36) (c) Mental Health (SF-36) Note: total of 18 secondary outcomes assessed, including: depression Sx, anxiety and somatic Sx (Hopkins Symptom Checklist/SCL-92; BDS checklist); illness worry (Whiteley-7); disability (WHODAS 2.0) M |
(a) No difference between Groups (1) and (2) at 14-month FU (b) No difference between Groups (1) and (2), p = .98 at 14-month FU (c) No difference between Groups (1) and (2), p = .59 at 14-month FU Note: no significant differences between Groups (1) and (2) in change over time on the 18 secondary outcomes |
| 13 | Dindo (2020a) | Veterans with chronic pain, mild Traumatic Brain Injury (mTBI), and current diagnosis of MDD, GAD, or PTSD | N = 39; Mage (SD) = 36.6 years (6.2); female = 0%; white = 42%; employment = 51% employed full- or part-time; Myears education (SD) = 14.2 (1.7); Past month diagnoses: 68% PTSD, 54% MDD, 16% GAD; most severe TBI = 26% Stage 1 mTBI, 55% Stage 2 mTBI, 19% Stage 3 mTBI | Randomized controlled pilot trial; 5-hour workshop; Setting: Veteran Affairs medical center; Baseline, 3-month FU | (1) ACT + psychoeducation (n = 20) (2) TAU (n = 12) |
(a) PTSD (Posttraumatic Stress Disorder Checklist; PCL-C) (b) Depression, Anxiety, and Stress (DASS-21 total) (c) Reintegration (Military to Civilian Questionnaire/M2C-Q) (d) Disability (WHODAS 2.0) M (e) Pain Severity (Brief Pain Inventory/BPI) (f) Pain Interference (BPI) (g) Psychological flexibility (AAQ-II) |
(a) Group (1) decrease > Group (2) decrease at FU; Cohen’s d = 0.33 (b) Group (1) decrease > Group (2) increase at FU; Cohen’s d = 0.68 (c) Group (1) improved, Group (2) worsened at FU; Cohen’s d = 0.47 (d) Group (1) decrease > Group (2) decrease at FU; Cohen’s d = 0.44 (e) No difference in Group (1) and Group (2) decrease at FU; Cohen’s d = 0.10 (f) Group (2) decrease > Group (1) decrease at FU; Cohen’s d = 0.78 (g) Group (1) increase > Group (2) increase at FU; Cohen’s d = 0.56 |
| 14 | Dindo (2020b) | Adults with migraines and co-occurring depression (current depressive episode) | N = 136; Mage (SD) = 35.8 years (13.9); female = 83%; white = 76%; employment = 81% employed or in school; education = 57% with more than 12 years of education; age of onset of migraines M (SD) = 19 (10.6); 31% taking antidepressants; number of migraine/headache days during month prior to baseline M (SD) = 7.4 (3.4); 87% taking abortive anti-migraine medication; 35% taking preventative anti-migraine medication | Randomized controlled trial; 5 to 6-hour workshop; Setting: hospital; Baseline, 3-, and 6-month FU | (1) ACT + migraine education (n = 56) (2) Support + migraine education (n = 47) |
(a) Depression Sx (Hamilton Rating Scale of Depression/HRSD) (b) Current depressive episode (depression module of SCID-IV) (c) Anxiety (Structured Interview Guide for the Hamilton Anxiety Rating Scale/SIGH-A) (d) Headache-related disability (HDI) (e) General Functioning (WHODAS 2.0) M (f) Social relationship functioning (World Health Organization Quality of Life/WHO-QOL) (g) Environment (WHO-QOL) (h) Psychological well-being (WHO-QOL) (i) Physical health (WHO-QOL) |
(a) Group (1) proportion of treatment responders > Group (2) at 3-month FU, p < .05, OR = 3.10 (b) Nonsignificant result for group (1) proportion of participants meeting criteria < Group (2) at 3-month FU, p = .33, OR = 0.54 (c) Nonsignificant result for group (1) proportion of treatment responders > Group (2) at 3-month FU, p = .11, OR = 2.45 (d) Group (1) proportion of treatment responders (i.e., ≥ 29 decline in total score) mean decrease > Group (2) at 3-month FU, p < .05, OR = 4.47 (e) Nonsignificant difference in mean improvement at 3-month FU, p = .23, Cohen’s d = 0.33 (f) Group (1) increase > Group (2) increase at 3-month FU, p = .01, Cohen’s d = 0.62 (g) Group (1) increase > Group (2) increase at 3-month FU, p = .05, Cohen’s d = 0.47 (h) Nonsignificant trend for Group (1) increase > Group (2) increase at 3-month FU, p = .06, Cohen’s d = 0.46 (i) Nonsignificant difference in mean increase at 3-month FU, p = .40, Cohen’s d = 0.27 |
Note. N reflects the number of participants randomized (for studies with > 1 condition) or assigned to treatment (for single-arm studies); FU = follow-up; Sx = symptoms; M = mean; SD = standard deviation; TAU = treatment as usual; OR = odds ratio; HR = hazard ratio; SCID-IV = Structured Clinical Interview for DSM-IV; PTSD = posttraumatic stress disorder; MDD = major depressive disorder; GAD = generalized anxiety disorder. Unable to calculate effect sizes for Pedersen (2019). Effect sizes reported for studies with comparison conditions are between-group effects; effect sizes reported those studies without comparison conditions are within-group effects.
Subsample from Dindo (2012) study, focused on headache outcomes
At-risk defined as having hypertension, diabetes mellitus or impaired fasting glucose, dyslipidemia, or obesity
At-risk defined as having high levels of preoperative pain and clinically significant anxiety or depression
At-risk defined as under the age of 50, having a preexisting chronic pain condition, elevated anxiety, elevated depression, or elevated pain catastrophizing, assessed pre-surgery
Included in meta-analysis
Study design.
Eight of the 13 studies (62%) included a control or comparison condition, all of which used random assignment except one that assigned participants based on availability (Dindo et al., 2014; Dindo et al., 2012). Four controlled studies used medical TAU (Dindo, Marchman, Gindes, & Fiedorowicz, 2015; Dindo et al., 2018; Hadlandsmyth et al., 2019), one used waitlist/medical TAU (Dindo et al., 2014; Dindo et al., 2012), and three provided an active control condition (disease management education in Gregg et al., 2007; enhanced care in Pedersen et al., 2019; support and migraine education in Dindo et al., 2020b). The remaining five studies were single-arm trials.
Ten studies (77%) were identified by their authors as pilot or feasibility studies (Dindo et al., 2015; Dindo et al., 2014; Dindo et al., 2012; Dindo et al., 2018; Dindo et al., 2020a; Ferreira, Gillanders, Morris, & Eugenicos, 2018; Hadlandsmyth et al., 2019; Hou et al., 2017; Huddleston, Martin, Woods, & Dindo, 2018; Sheppard et al., 2010; Welch, 2014), suggesting that assessing feasibility and acceptability were primary aims for those trials. Five of 10 (50%) pilot/feasibility studies reported qualitative feedback regarding feasibility, acceptability, or treatment satisfaction, with generally positive feedback (Dindo et al., 2015; Hadlandsmyth et al., 2019; Hou et al., 2017; Huddleston et al., 2018; Welch, 2014). Among those studies, common reasons for eligible individuals declining participation included practical constraints such as time and distance (47% in Hou et al., 2017; 18% in Hadlandsmyth et al., 2019) and a general sense of overwhelm (33% in Hadlandsmyth et al., 2019).
Study methodology.
Twelve studies (92%) delivered the ACT intervention in workshop format and one delivered it in an individualized session (Hadlandsmyth et al., 2019). Two studies provided individualized follow-up calls after the workshop as part of the intervention (Dindo et al., 2018; Ferreira et al., 2018), and one included a self-guided bibliotherapy component (Ferreira et al., 2018). Across all studies, average single-session ACT intervention length was 5.5 hours, with a range of two to eight hours. Median and modal length was five hours. Likely due to the short duration of the intervention, only one study (8%; Welch, 2014) assessed outcomes at ‘post-treatment’ (i.e., directly after the single-session intervention). The modal follow-up period for assessment of post-treatment outcomes was three months (k = 10, 77%). Four studies (31%) assessed outcomes beyond three months: six months in Dindo et al. (2015), Ferreira et al. (2018), and Dindo et al. (2020b), and six, 14 and 20 months in Pedersen et al. (2019). Seven manuscripts (54%) explicitly indicated where the intervention was delivered. All interventions were delivered in-person. All interventions but two were delivered in medical settings.
Patient Characteristics and Conditions
Across 13 studies, three (23%) targeted migraine, two (15%) diabetes, two (15%) primary gastrointestinal conditions, one (8%) multiple sclerosis, one (8%) multiple functional somatic syndromes, one (8%) general chronic pain and mild traumatic brain injury, and one (8%) recruited patients characterized as “at risk” for vascular disease. Two studies (15%) targeted patients undergoing surgery who were identified a priori as being at risk for post-surgical pain and/or chronic opioid use.
Eight studies (62%) required participants to meet a minimum threshold of comorbid psychiatric distress for inclusion: three required participants to meet DSM-IV criteria for a current major depressive episode, three required participants to meet established cut-offs for clinically significant depression or anxiety symptomatology, and one required participants to meet DSM-IV criteria for either current major depressive disorder, generalized anxiety disorder, or DSM-5 criteria for posttraumatic stress disorder (PTSD). One study required significant condition-related distress (Welch, 2014). In samples from studies not requiring co-morbid psychiatric distress for inclusion, rates of clinically significant depression and anxiety ranged from 17–53% and 16–30%, respectively. One study did not report mental health comorbidities (Gregg et al., 2007).
Narrative Review: Outcomes Assessed and Intervention Efficacy
For the purpose of this review, treatment outcomes were categorized into four domains: (a) functioning and related domains; (b) mental health; (c) physical health; and (d) ACT processes. Given that pilot/feasibility trials do not have adequate power to detect statistically significant effects, results from statistical significance testing are reported for non-pilot trials only. Within-group effect sizes are reported to assess preliminary efficacy in pilot/feasibility trials.
Functioning and related domains.
Thirteen manuscripts (93%) reported 27 outcomes in this domain, including daily functioning, functioning in specific life domains, disability, QoL, healthcare utilization, pain interference, medication use and cessation, and disease self-management, all of which were measured using self-report instruments. Specific constructs and measures are listed in Table 2. Of the 27 outcomes assessed across all studies, approximately half were condition-specific and half were general to health and functioning. In the three non-pilot trial manuscripts that assessed this domain, four of seven outcomes (57%) showed significantly greater improvement in the ACT group than the control group, and one (14%) showed a trending significant (p = .06) outcome. In the 10 pilot/feasibility trial manuscripts that assessed this domain, 20 of 20 outcomes (100%) showed medium-to-large effect sizes. Effect sizes varied within and across studies (see Table 2). In total, 24 of 27 outcomes in this domain (88%) showed results favoring ACT.
Mental health.
Eleven manuscripts (79%) reported 24 mental health outcome effects total, including depression (n = 10), anxiety (n = 7), stress (n = 2), PTSD symptoms (n = 1), GI-specific anxiety (n = 1), and general or composite mental health status (n = 3). In two non-pilot trial manuscripts, one of six outcomes (17%) showed significantly greater improvement in the ACT group (depression symptoms in Dindo et al., 2020b). In nine pilot/feasibility trial manuscripts, 15 of 18 outcomes (83%) showed medium-to-large effect sizes. Those that did not were depression and anxiety in breast cancer patients (Hadlandsmyth et al., 2019) and general mental health status in adults with multiple sclerosis (Sheppard et al., 2010). In total, 16 of 24 outcomes in this domain (67%) showed results favoring ACT.
Physical health.
Ten manuscripts (71%) reported 13 physical health outcome effects total, including general physical health status (k = 3), raw HbA1c and diabetes control as assessed by HbA1c (k = 1), headache frequency and severity (k = 1), inflammatory bowel disease symptoms (k = 1), irritable bowel syndrome symptom severity (k = 1), pain cessation (k = 1), and pain severity (k = 2). In three non-pilot trial manuscripts, one of five outcomes (20%) was statistically significant in favor of the ACT group (greater increase in proportion of glycemic control in Gregg et al., 2007). In seven pilot/feasibility trial manuscripts, three of eight outcomes (38%) showed medium-to-large effect sizes: headache frequency and severity (Dindo et al., 2014), inflammatory bowel disease activity (Hou et al., 2017), and irritable bowel syndrome symptoms severity (Ferreira et al., 2018). In total, four of 13 outcomes in this domain (31%) reported results favoring ACT.
ACT processes.
Eight manuscripts (57%) reported 11 ACT process outcomes, including experiential avoidance, acceptance, psychological flexibility, and engagement in values-consistent behavior. Two studies administered the general Acceptance and Action Questionnaire-II (Bond et al., 2011), and six administered condition-specific AAQ variants. Two manuscripts examined three ACT-adjacent process constructs: thought suppression, “mindful attention awareness,” and pain catastrophizing (Hadlandsmyth et al., 2019; Sheppard et al., 2010). In one non-pilot trial, the ACT group reported significantly greater increases in diabetes acceptance at three-month follow-up than a diabetes management education condition (Gregg et al., 2007). In eight pilot/feasibility trial manuscripts, seven of 10 outcomes (70%) showed medium-to-large effect sizes. Small effects were detected for pain acceptance and values-consistent behavior among Veterans who underwent orthopedic surgery, despite greater rates of pain cessation and opioid cessation (Dindo et al., 2018), and for pain acceptance in female breast cancer patients who underwent breast surgery (Hadlandsmyth et al., 2019). Medium-to-large effect sizes were shown for thought suppression among patients with multiple sclerosis and patients with diabetes (Sheppard et al., 2010; Welch, 2014). Small effects were shown for mindful attention awareness among patients with multiple sclerosis (Sheppard et al., 2010) and pain catastrophizing among breast cancer patients (Hadlandsmyth et al., 2019). In total, eight of 11 outcomes in this domain (73%) showed results favoring ACT.
Three manuscripts conducted some form of mediational analyses using ACT process measures. Gregg et al. (2007) found that changes in diabetes acceptance and self-reported self-management behavior mediated impact of treatment on changes in HbA1c in patients with diabetes mellitus type 2. Using a proxy mediation method, Dindo et al. (2015) found that psychological flexibility (measured by the Experiencing Questionnaire) partially mediated reductions in depressive symptoms among individuals at risk for vascular disease with clinically significant anxiety or depression. Finally, Ferreria et al. (2018) used hierarchical multiple regression to examine the unique contribution of changes in irritable bowel syndrome acceptance (pre-treatment to post-treatment) in accounting for variance in change in outcome measures (pre-treatment to follow-up). They found that changes in irritable bowel syndrome acceptance significantly predicted changes in all outcomes, even when accounting for symptom severity.
Meta-analysis: Outcomes Assessed and Intervention Efficacy
Figure 2 shows effect sizes and meta-analytic results for five of eight controlled studies (63%) reporting a functioning-related outcome. Four studies assessed general functioning using the World Health Organization Disability Assessment Schedule II (WHODAS; Rehm et al., 1999). The fifth study (Dindo et al., 2015) assessed general well-being using the World Health Organization Quality of Life-BREF (WHO-QoL-BREF; Skevington, Lotfy, & O’Connell, 2004), from which scores on the psychological well-being subscale were used. Effects were estimated using data from three-month follow-up for four studies and six-month follow-up (the shortest available) for one study (Pedersen et al., 2019). Random effects meta-analysis found that ACT did not significantly outperform comparator groups on these outcomes (mean Hedges’ g = −0.51, 95% CI [−1.19, 0.16], p = .14) despite a medium pooled effect size. Study effects displayed considerable heterogeneity (I2 = 86%; Q [df=4]: 28.84, p < .001; τ2 = 0.50). The prediction interval shown in Figure 2 crosses 0, which does not suggest that future effects are expected to favor ACT. Visual examination of the funnel plot (Supplement Figure 1) and Eggers’ test of funnel plot asymmetry did not indicate the presence of publication bias (p = .20).
Figure 2.
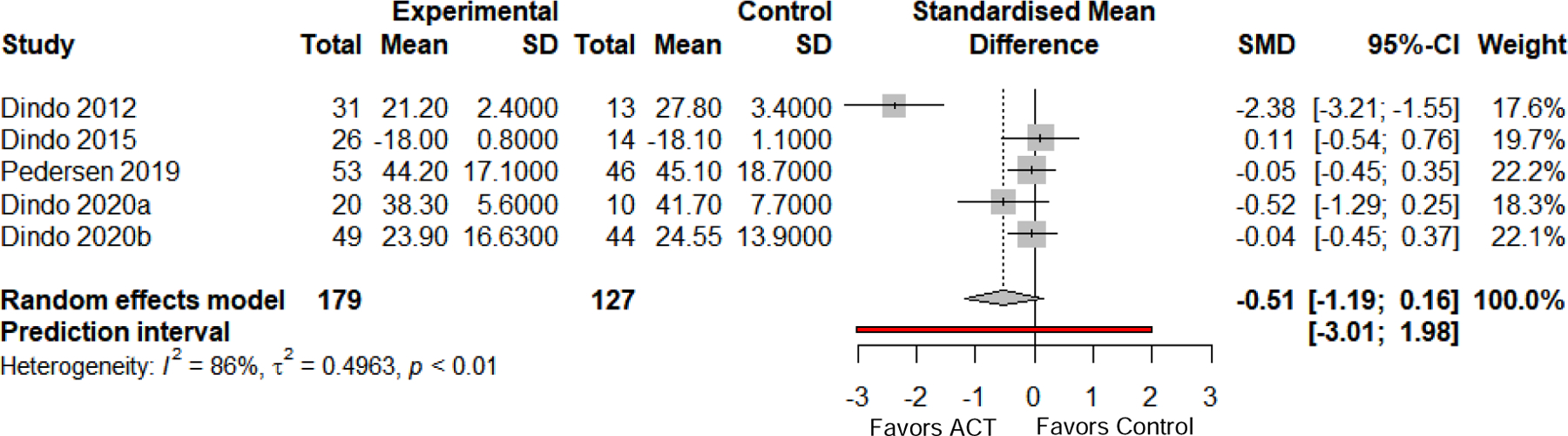
Between-group meta-analysis results and forest plot for functioning-related outcomes at follow-up. Functioning-related outcomes included measures of functioning and well-being. Where studies reported more than one relevant outcome, the general (rather than condition-specific) measure was selected to reduce heterogeneity. Error bars are 95% confidence intervals; dashed line is pooled effect size; red line is prediction interval. SD = standard deviation. SMD = standardized mean difference, calculated as Hedges’ g.
The nine within-group effects for functioning and related domains across all trial types are depicted graphically in Figure 3. Of nine effects, five were from the WHODAS (Dindo et al., 2012; Huddleston et al., 2018; Pedersen et al., 2019; Dindo et al., 2020a; Dindo et al., 2020b), one was from WHO-QoL-BREF (Dindo et al., 2015), one was from the Quality of Life Inventory (Sheppard et al., 2010), and two were from measures of condition-specific health-related QoL (the Short IBD Questionnaire in Hou et al., 2017; IBS Impact on Quality of Life Scale in Ferreira et al., 2018). Effect sizes were medium to large, with two studies clearly favoring ACT.
Figure 3.
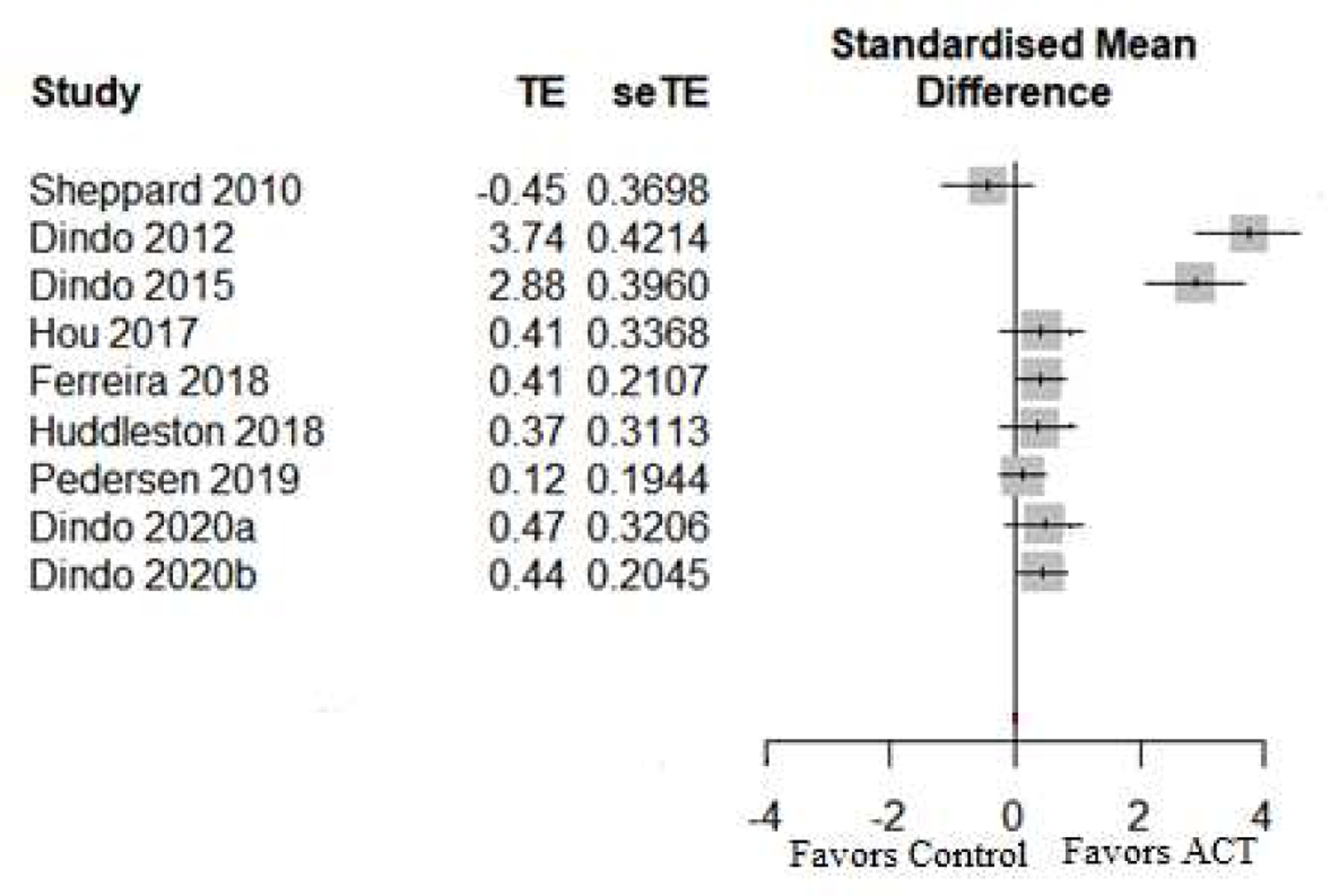
Within-group effect sizes and forest plot for functioning-related outcomes at follow-up. Functioning-related outcomes included measures of disability, symptom interference, well-being, and quality of life. Where studies reported more than one relevant outcome, the general (rather than condition-specific) measure was selected to reduce heterogeneity. Error bars are 95% confidence intervals. TE = Hedges’ g; seTE = standard error of Hedges’ g.
Discussion
To our knowledge, this is the first systematic review with meta-analysis of single-session ACT interventions in CHC populations. Our goal was to take a systematic approach to describing the state of the literature and to use both narrative and quantitative methods to estimate the efficacy of single-session ACT for CHC. Our search yielded 14 manuscripts (from 13 unique studies), nine of which were not included in prior reviews. Ten of the 13 included studies were pilot/feasibility trials and quality of all studies was variable, underscoring the nascent nature of this literature. Overall, efficacy results were promising, but varied by condition and population as well as specific outcome type. Narrative review of ACT efficacy found significant results in RCTs or medium-to-large effect sizes in pilot/feasibility trials for the majority of outcomes (69%) across the four outcome domains of interest. We found that all four studies that required participants to meet DSM diagnostic criteria for a psychiatric condition reported significant or large effect size improvements in mental health symptoms and significant or medium-to-large effect size improvements in functioning and related domains. Contrary to conclusions in Graham et al. (2016), which noted that intervention duration should be longer because CHC can be long-standing, severe, and accompanied by significant psychiatric issues, there is promise for single-session ACT in CHC, including when psychiatric distress is prominent.
For ACT process outcomes, acceptance, psychological flexibility, and values-consistent behavior were most commonly assessed, and often measured with condition-specific measures, in line with recommendations (Ong, Lee, Levin, & Twohig, 2019). Although the current review focused on direct influences of treatment on ACT process outcomes, the three studies that conducted some form of mediational analysis found evidence for partial or proxy mediation. Assessing purported mechanisms is essential for optimizing behavioral interventions for CHC populations, especially in RCTs where multiple active intervention types may be compared.
Results from meta-analysis found a medium-sized effect of single-session ACT on functioning and well-being, though the pooled estimate was not statistically significant. These results should be interpreted tentatively, since two of the five meta-analyzed effects came from pilot/feasibility trials, and the five effects displayed considerable heterogeneity. Ultimately, pilot trial results inform treatment acceptability and feasibility rather than efficacy (Bowen et al., 2009). Thus, results from pilot RCTs should be reproduced in fully powered RCTs. Data from additional RCTs will better inform the efficacy of single-session ACT for people with CHC.
Recommendations for Future Studies
While current review findings suggest promise for this treatment approach, additional high-quality trials are needed to bolster the evidence. Below we offer multiple considerations for enhancing the literature in this area, from general strategies to those specific to the single-session ACT format.
Given the variability of study quality and the preponderance of pilot/feasibility studies, additional well-designed RCTs are needed to fully examine the efficacy of single-session ACT for CHC. Such studies should include pre-specified hypotheses and outcomes, a priori power calculations, adequate sample size, blinded assessors, and therapist adherence and competency ratings. RCTs should also compare single-session ACT to an active control condition (e.g., education, relaxation) and ensure equivalence in length, intensity, therapist proficiency, and any additional treatment components (e.g., booster sessions).
Only four of the reviewed studies included follow-up periods beyond three months. Future studies would benefit from multiple follow-up assessments (perhaps up to one year) to better examine the long-term efficacy of single-session ACT. Other ACT research has emphasized the importance of longer-term follow-up periods for capturing unexpected increases in improvement over time as well as maintenance of treatment effects compared to comparator conditions (Clarke, Kingston, Wilson, Bolderston, & Remington, 2012; Gifford et al., 2011; González-Menéndez, Fernández, Rodríguez, & Villagrá, 2014). Increasing the follow-up period will inform the relative efficacy of single-session ACT and whether it can be offered as a standalone intervention or should be offered as part of a more comprehensive treatment package.
Several studies used general measures of functioning that assess outcomes less amenable to behavioral interventions such as ACT (e.g., mobility and self-care in the WHODAS), whereas others used condition-specific measures, which are ostensibly more sensitive to outcomes of interest to patients and providers. Selecting validated and appropriate measures of functioning and QoL as the primary outcome for single-session ACT studies of CHC is critical to establishing efficacy. Future studies should use both general and condition-specific measures when possible. Further, all included studies used self-report measures of functioning, most of which rely on retrospective recall. Investigators may consider using novel assessments of functioning that are ecologically valid and less prone to social desirability and recall biases, such as ecological momentary assessment (EMA) and accelerometry or other behavioral measures relevant to the target CHC.
There was variability in participation and dropout rates across the reviewed studies, especially high for conditions with psychiatric comorbidity or greater levels of disability (e.g., multiple sclerosis). Given that single-session interventions can facilitate access to treatment, virtual delivery may further enhance reach. Virtual delivery may increase willingness and ability to participate and may be less distressing for some individuals. Virtual delivery may also increase access among underserved populations.
Single-session interventions deliver a great amount of information in a short period. Traditional post-treatment assessments were uncommon in included studies and may not be psychometrically appropriate if they are not sensitive enough to capture change after a very short test-retest latency period. Therefore, incorporating a participant comprehension check should be considered. Instituting a comprehension check both immediately following the intervention and at follow-up may provide information that can be used to optimize single-session delivery. A recently developed measure that may be suitable for this purpose is the ACT-SQ, which captures how well ACT processes were realized during a treatment session (Probst et al., 2020).
Patient characteristics and the clinical complexity of CHCs varied widely and may have contributed to modest outcomes in some of the included studies. Examining clinical variables as moderators of treatment outcome can shed light on whether single-session ACT is adequate for those with greater disease burden. Additionally, the majority of participants in most studies were white. Given the higher prevalence of some CHCs in racial and ethnic minority groups, future studies should assess these variables as potential moderators to inform culturally sensitive approaches.
Only nine of the 14 manuscripts included ACT process measures, despite the importance of examining the impact of treatment on the purported processes of change. We strongly encourage all future single-session ACT studies to include multiple measures of ACT processes, preferably both condition-specific and general, and conduct adequately powered mediation analyses where possible. Newer measures that assess multiple ACT processes in one questionnaire may be appropriate. This is an important step to identifying underlying treatment processes that predict treatment outcomes.
Only two of the included studies delivered supplementary components (i.e., booster session in Dindo et al., 2018; bibliotherapy and support calls in Ferreira et al., 2018), and both demonstrated robust treatment findings. Future studies should specifically test whether adjunctive intervention components increase efficacy. This may include telephone- or text-based booster sessions, ACT-based smartphone applications or websites, and/or peer-support groups. Future studies also may consider implementing a multiphase optimization strategy (MOST; Collins, 2018) to better understand the briefest and most cost-effective intervention that still achieves efficacy. Relatedly, stepped-care approaches for treatment of non-responders could evaluate outcomes following a single-session intervention and provide additional intervention (e.g., full-length ACT) as indicated.
Additionally, future pilot or feasibility studies should consider the recommendations of Bowen et al. (2009) to assess outcomes in eight domains: acceptability, demand, implementation, practicality, adaptation, integration, expansion, and limited-efficacy testing. Future randomized controlled trials incorporating the above recommendations will answer important questions regarding for whom single-session ACT may be efficacious, the optimal delivery method and intervention design for specific populations, and the potential role of single-session ACT in treatment of CHC populations, for example, as “first-line” treatment in stepped-care approach.
Review Limitations
In terms of assessing study quality, there is no ideal tool for evaluating single-session psychotherapy studies, nor are we aware of a single tool that adequately assesses the quality of both pilot/feasibility studies and RCTs. The POMRF was chosen because its items assess a range of nuanced design and reporting features that are important for establishing strong empirical evidence for behavioral intervention efficacy. We modified the POMRF for our purposes, following precedent from a related review (Graham et al., 2016). However, the reliability and validity of the POMRF have not been rigorously evaluated and it has been criticized for not assessing process-based therapy variables that are especially important in ACT (Atkins et al., 2017). We encourage the development of validated quality assessment tools appropriate for brief psychotherapy interventions.
Not all controlled studies reported functioning-related outcomes, which limited the size of the meta-analysis to five effects. These five effects demonstrated considerable heterogeneity, which could not be explored using meta-regression or subgroup analyses due to the small size of the analysis. Four of the five meta-analyzed effects came from studies conducted by the same primary author, which may introduce bias. As evidence continues to accumulate, future meta-analyses should explore possible sources of heterogeneity based on both clinical and methodological factors. For example, Hadlandsmyth et al. (2019) was an outlier among included studies in terms of delivery method (individualized rather than group-based) and duration (two hours versus the modal five hours), and showed small effects across all outcomes. Meta-regression of multiple studies with varying design qualities will allow for more definitive conclusions about efficacy and optimal delivery.
It is also important to note that general (rather than condition-specific) measures of functioning and related domains were meta-analyzed to reduce statistical heterogeneity. However, condition-specific measures may be more sensitive to change. Future studies and larger meta-analyses should include both condition-specific and general measures of functioning that are sensitive to the intervention.
We calculated between- and within-group effect sizes for studies where these were not reported in the manuscript. However, these within-group effect sizes did not account for the dependence between pre- and post-test scores, which may lead to inflated estimates (Cuijpers et al., 2017; Cheung, 2019). Future studies should report appropriate effect sizes in addition to statistical significance testing. Finally, the present review did not formally assess risk of bias across studies. Of the 13 studies reviewed, only one was pre-registered, which precludes assessment of selective reporting. The funnel plot of meta-analyzed effects did not suggest publication bias, and the inclusion of grey literature should reduce publication bias in the present review. However, it will be important to systematically assess whether pre-specified analyses are reported in future reviews.
Despite these limitations, the current review has several strengths. It provides an up-to-date and broadly scoped synthesis of single-session ACT for CHC studies and is the first to meta-analyze treatment outcomes. We reported grey literature results, various outcome types, and provided detailed description of study design and intervention characteristics.
Conclusion
Brief interventions hold promise for increasing access to behavioral interventions among CHC patients, who tend to have a greater burden of healthcare needs than other psychotherapy consumers. Our systematic review identified 13 studies collectively assessing the feasibility, acceptability, and efficacy of single-session ACT as delivered to individuals with various CHCs. Studies generally reported results favoring ACT, especially in functioning and related domains, which is the primary target of ACT. However, there were few RCTs, and sample sizes were relatively small. Given the relatively limited dose of treatment delivered in a single session, and the likelihood that patients with CHCs have complex clinical presentations, it is critical to further evaluate the efficacy of brief interventions to better inform treatment design and delivery, especially in traditional and integrated healthcare settings.
Supplementary Material
Highlights:
ACT is used to improve functioning in chronic health condition populations
Single session ACT may be more feasible and acceptable than multi-session ACT
Single-session ACT appears feasible and beneficial across several chronic health conditions
Meta-analysis found a medium-sized, non-significant effect on general functioning
Larger, randomized controlled trials are warranted to test efficacy
Support:
Drs. Afari and Herbert and Ms. Dochat are partially supported by R01DK106415 from National Institute of Diabetes and Kidney Disease. Dr. Herbert is also supported by VA RR&D CDA Grant 1IK2RX002807–01A2. Dr. Wooldridge is supported by the VA Office of Academic Affiliates advanced fellowship in women’s health. The views expressed in this paper are those of the authors do not reflect the official policy or position of the funding agency, Department of Veterans Affairs, the United States Government, or any institutions with which the authors are affiliated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors have any conflicts of interest to disclose.
Registration: Review not registered
References
* References marked with an asterisk indicate studies included in the meta-analysis
- A-tjak JG, Davis ML, Morina N, Powers MB, Smits JA, & Emmelkamp PM (2015). A meta-analysis of the efficacy of acceptance and commitment therapy for clinically relevant mental and physical health problems. Psychotherapy and Psychosomatics, 84, 30–36. [DOI] [PubMed] [Google Scholar]
- Atkins PW, Ciarrochi J, Gaudiano BA, Bricker JB, Donald J, Rovner G, … & Hayes SC (2017). Departing from the essential features of a high quality systematic review of psychotherapy: A response to Öst (2014) and recommendations for improvement. Behaviour Research and Therapy, 97, 259–272. [DOI] [PubMed] [Google Scholar]
- Anderson RJ, Freedland KE, Clouse RE, & Lustman PJ (2001). The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabetes Care, 24, 1069–1078. [DOI] [PubMed] [Google Scholar]
- Anderson RJ, Grigsby AB, Freedland KE, De Groot M, McGill JB, Clouse RE, & Lustman PJ (2002). Anxiety and poor glycemic control: A meta-analytic review of the literature. The International Journal of Psychiatry in Medicine, 32, 235–247. [DOI] [PubMed] [Google Scholar]
- Arnow BA, Hunkeler EM, Blasey CM, Lee J, Constantino MJ, Fireman B, … Hayward C (2006). Comorbid depression, chronic pain, and disability in primary care. Psychosomatic Medicine, 68, 262–268. [DOI] [PubMed] [Google Scholar]
- Asmundson GJ, & Katz J (2009). Understanding the co occurrence of anxiety disorders and chronic pain: State of the art. Depression and Anxiety, 26, 888–901. [DOI] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Katon W, & Kroenke K (2003). Depression and pain comorbidity: A literature review. Archives of Internal Medicine, 163, 2433–2445. [DOI] [PubMed] [Google Scholar]
- Bond FW, Hayes SC, Baer RA, Carpenter KM, Guenole N, Orcutt HK, … & Zettle RD (2011). Preliminary psychometric properties of the Acceptance and Action Questionnaire–II: A revised measure of psychological inflexibility and experiential avoidance. Behavior Therapy, 42, 676–688. [DOI] [PubMed] [Google Scholar]
- Bowen DJ, Kreuter M, Spring B, Cofta-Woerpel L, Linnan L, Weiner D, … Fernandez M (2009). How we design feasibility studies. American Journal of Preventive Medicine, 36, 452–457. 10.1016/j.amepre.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celano CM, Daunis DJ, Lokko HN, Campbell KA, & Huffman JC (2016). Anxiety disorders and cardiovascular disease. Current Psychiatry Reports, 18(11), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celano CM, & Huffman JC (2011). Depression and cardiac disease: A review. Cardiology in Review, 19, 130–142. [DOI] [PubMed] [Google Scholar]
- Clarke S, Kingston J, Wilson KG, Bolderston H, & Remington B (2012). Acceptance and Commitment Therapy for a heterogeneous group of treatment-resistant clients: A treatment development study. Cognitive and Behavioral Practice, 19, 560–572. [Google Scholar]
- Cheung MWL (2019). A guide to conducting a meta-analysis with non-independent effect sizes. Neuropsychology Review, 29, 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM (2018). Optimization of Behavioral, Biobehavioral, and Biomedical Interventions: The Multiphase Optimization Strategy (MOST). New York: Springer. [Google Scholar]
- Csupak B, Sommer JL, Jacobsohn E, & El-Gabalawy R (2018). A population-based examination of the co-occurrence and functional correlates of chronic pain and generalized anxiety disorder. Journal of Anxiety Disorders, 56, 74–80. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Weitz E, Cristea IA, & Twisk J (2017). Pre-post effect sizes should be avoided in meta-analyses. Epidemiology and Psychiatric Sciences, 26, 364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson K, Kupfer D, Bigger J, Califf R, Carney R, Coyne J, … Freedland K (2006). National Heart, Lung, and Blood Institute Working Group. Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute working group report. Psychosomatic Medicine, 68, 645–650. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Higgins JP, Altman DG, & Cochrane Statistical Methods Group. (2019). Analysing data and undertaking meta analyses. Cochrane Handbook for Systematic Reviews of Interventions, 241–284.
- Dindo L (2015). One-day acceptance and commitment training workshops in medical populations. Current Opinions in Psychology, 2, 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Dindo L, Johnson AL, Lang B, Rodrigues M, Martin L, & Jorge R (2020a). Development and evaluation of an 1-day Acceptance and Commitment Therapy workshop for veterans with comorbid chronic pain, TBI, and psychological distress: Outcomes from a pilot study. Contemporary Clinical Trials, 90, 105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Dindo L, Marchman J, Gindes H, & Fiedorowicz JG (2015). A brief behavioral intervention targeting mental health risk factors for vascular disease: A pilot study. Psychotherapy and Psychosomatics, 84, 183–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Dindo LN, Recober A, Calarge CA, Zimmerman BM, Weinrib A, Marchman JN, & Turvey C (2020b). One-day acceptance and commitment therapy compared to support for depressed migraine patients: A randomized clinical rial. Neurotherapeutics, 17, 743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dindo L, Recober A, Marchman J, O’Hara MW, & Turvey C (2014). One day behavioral intervention in depressed migraine patients: Effects on headache. Headache: The Journal of Head and Face Pain, 54, 528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Dindo L, Recober A, Marchman JN, Turvey C, & O’Hara MW (2012). One-day behavioral treatment for patients with comorbid depression and migraine: A pilot study. Behaviour Research and Therapy, 50, 537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dindo L, Van Liew JR, & Arch JJ (2017). Acceptance and commitment therapy: A transdiagnostic behavioral intervention for mental health and medical conditions. Neurotherapeutics, 14, 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dindo L, Zimmerman MB, Hadlandsmyth K, StMarie B, Embree J, Marchman J, … Rakel B (2018). Acceptance and commitment therapy for prevention of chronic postsurgical pain and opioid use in at-risk veterans: A pilot randomized controlled study. Journal of Pain, 19, 1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdin CA, Odutayo A, Wong CX, Tran J, Hsiao AJ, & Hunn BH (2016). Meta-analysis of anxiety as a risk factor for cardiovascular disease. The American Journal of Cardiology, 118, 511–519. [DOI] [PubMed] [Google Scholar]
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KRR, … Coyne JC (2005). Mood disorders in the medically ill: Scientific review and recommendations. Biological Psychiatry, 58, 175–189. [DOI] [PubMed] [Google Scholar]
- Ferreira NB, Gillanders D, Morris PG, & Eugenicos M (2018). Pilot study of acceptance and commitment therapy for irritable bowel syndrome: A preliminary analysis of treatment outcomes and processes of change. Clinical Psychologist, 22, 241–250. [Google Scholar]
- Gibbons MBC, Rothbard A, Farris KD, Stirman SW, Thompson SM, Scott K, … & Crits-Christoph P (2011). Changes in psychotherapy utilization among consumers of services for major depressive disorder in the community mental health system. Administration and Policy in Mental Health and Mental Health Services Research, 38, 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford EV, Kohlenberg BS, Hayes SC, Pierson HM, Piasecki MP, Antonuccio DO, & Palm KM (2011). Does acceptance and relationship focused behavior therapy contribute to bupropion outcomes? A randomized controlled trial of functional analytic psychotherapy and acceptance and commitment therapy for smoking cessation. Behavior Therapy, 42, 700–715. [DOI] [PubMed] [Google Scholar]
- González-Menéndez A, Fernández P, Rodríguez F, & Villagrá P (2014). Long-term outcomes of acceptance and commitment therapy in drug-dependent female inmates: A randomized controlled trial. International Journal of Clinical and Health Psychology, 14, 18–27. [Google Scholar]
- Graham CD, Gouick J, Krahe C, & Gillanders D (2016). A systematic review of the use of Acceptance and Commitment Therapy (ACT) in chronic disease and long-term conditions. Clinical Psychology Review, 46, 46–58. [DOI] [PubMed] [Google Scholar]
- Gregg JA, Callaghan GA, Hayes SC, & Glenn-Lawson JL (2007). Improving diabetes self-management through acceptance, mindfulness, and values: A randomized controlled trial. Journal of Consulting and Clinical Psychology, 75, 336–343. [DOI] [PubMed] [Google Scholar]
- Grigsby AB, Anderson RJ, Freedland KE, Clouse RE, & Lustman PJ (2002). Prevalence of anxiety in adults with diabetes: A systematic review. Journal of Psychosomatic Research, 53, 1053–1060. [DOI] [PubMed] [Google Scholar]
- Grover M, Kolla BP, Pamarthy R, Mansukhani MP, Breen-Lyles M, He J-P, & Merikangas KR (2021). Psychological, physical, and sleep comorbidities and functional impairment in irritable bowel syndrome: Results from a national survey of US adults. Plos One, 16, e0245323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlandsmyth K, Dindo LN, Wajid R, Sugg SL, Zimmerman MB, & Rakel BA (2019). A single session acceptance and commitment therapy intervention among women undergoing surgery for breast cancer: A randomized pilot trial to reduce persistent postsurgical pain. Psychooncology, 28, 2210–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrer M, Cuijpers P, Furukawa TA, & Ebert DD (2019). Doing Meta-Analysis in R: A Hands-on Guide. https://bookdown.org/MathiasHarrer/Doing_Meta_Analysis_in_R/.
- Hartzler ML, Castle L, Lewis C, & Zakaria L (2020). A functional approach to the chronic disease epidemic. American Journal of Health-System Pharmacy, 77, 668–672. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, & Altman DG (2003). Measuring inconsistency in meta-analyses. BMJ, 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou JK, Vanga RR, Thakur E, Gonzalez I, Willis D, & Dindo L (2017). One-day behavioral intervention for patients with inflammatory bowel disease and co-occurring psychological distress. Clinical Gastroenterology and Hepatology, 15, 1633–1634. [DOI] [PubMed] [Google Scholar]
- Huddleston C, Martin L, Woods K, & Dindo L (2018). One-day behavioral intervention for distressed veterans with migraine: Results of a multimethod pilot study. Military Medicine, 183, e184–e192. [DOI] [PubMed] [Google Scholar]
- Jeong J-H, Um YH, Ko S-H, Park J-H, Park J-Y, Han K, & Ko K-S (2017). Depression and mortality in people with type 2 diabetes mellitus, 2003 to 2013: A nationwide population-based cohort study. Diabetes & Metabolism Journal, 41, 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Wu J, Ma Y, Tsang A, Guo WJ, & Sung J (2009). Irritable bowel syndrome is strongly associated with generalized anxiety disorder: A community study. Alimentary Pharmacology & Therapeutics, 30, 643–651. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, … & Moher D (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Journal of Clinical Epidemiology, 62, e1–e34. [DOI] [PubMed] [Google Scholar]
- Löwe B, Spitzer RL, Williams JB, Mussell M, Schellberg D, & Kroenke K (2008). Depression, anxiety and somatization in primary care: Syndrome overlap and functional impairment. General Hospital Psychiatry, 30, 191–199. [DOI] [PubMed] [Google Scholar]
- Luoma JB, Hayes SC, & Walser RD (2007). Learning ACT: An Acceptance & Commitment Therapy Skills-Training Manual for Therapists. Oakland, CA: New Harbinger Publications. [Google Scholar]
- Megari K (2013). Quality of life in chronic disease patients. Health Psychology Research, 1:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Ames M, Cui L, Stang PE, Ustun TB, Von Korff M, & Kessler RC (2007). The impact of comorbidity of mental and physical conditions on role disability in the US adult household population. Archives of General Psychiatry, 64, 1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, & Prisma Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS med, 6, e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykletun A, Jacka F, Williams L, Pasco J, Henry M, Nicholson GC, … Berk M (2010). Prevalence of mood and anxiety disorder in self reported irritable bowel syndrome (IBS). An epidemiological population based study of women. BMC Gastroenterology, 10, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong CW, Lee EB, Levin ME, & Twohig MP (2019). A review of AAQ variants and other context-specific measures of psychological flexibility. Journal of Contextual Behavioral Science, 12, 329–346. [Google Scholar]
- Öst L-G (2008). Efficacy of the third wave of behavioral therapies: A systematic review and meta-analysis. Behaviour Research and Therapy, 46, 296–321. [DOI] [PubMed] [Google Scholar]
- *Pedersen HF, Agger JL, Frostholm L, Jensen JS, Ornbol E, Fink P, & Schroder A (2019). Acceptance and commitment group therapy for patients with multiple functional somatic syndromes: A three-armed trial comparing ACT in a brief and extended version with enhanced care. Psychological Medicine, 49, 1005–1014. [DOI] [PubMed] [Google Scholar]
- Probst T, Mühlberger A, Kühner J, Eifert GH, Pieh C, Hackbarth T, & Mander J (2020). Development and initial validation of a brief questionnaire on the patients’ view of the in-session realization of the six core components of acceptance and commitment therapy. Clinical Psychology in Europe, 2, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Retrieved from: http://www.R-project.org/. [Google Scholar]
- Raghupathi W, & Raghupathi V (2018). An empirical study of chronic diseases in the United States: A visual analytics approach. International Journal of Environmental Research and Public Health, 15, 431–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Üstün TB, Saxena S, Nelson CB, Chatterji S, Ivis F, & Adlaf ED (1999). On the development and psychometric testing of the WHO screening instrument to assess disablement in the general population. International Journal of Methods in Psychiatric Research, 8, 110–122. [Google Scholar]
- Sheppard SC, Forsyth JP, Hickling EJ, & Bianchi J (2010). A novel application of acceptance and commitment therapy for psychosocial problems associated with multiple sclerosis: Results from a half-day workshop intervention. International Journal of MS Care, 12, 200–206. [Google Scholar]
- Skevington SM, Lotfy M, & O’Connell KA (2004). The World Health Organization’s WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial. A report from the WHOQOL group. Quality of Life Research, 13, 299–310. [DOI] [PubMed] [Google Scholar]
- Skinner T, Joensen L, & Parkin T (2019). Twenty five years of diabetes distress research. Diabetic Medicine, 37, 393–400. [DOI] [PubMed] [Google Scholar]
- Van Oudenhove L, Levy RL, Crowell MD, Drossman DA, Halpert AD, Keefer L, … Naliboff BD (2016). Biopsychosocial aspects of functional gastrointestinal disorders: How central and environmental processes contribute to the development and expression of functional gastrointestinal disorders. Gastroenterology, 150, 1355–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch B (2014). A pilot study of the efficacy of acceptance and commitment therapy for adults with type two diabetes (Doctoral dissertation). Retrieved from ProQuest. (3582479)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


