Abstract
By performing a single-atom replacement within common fluorophores, we have developed a facile and general strategy to prepare a broad-spectrum class of colorimetric and fluorogenic probes for the selective detection of mercury ions in aqueous environments. Thionation of carbonyl groups from existing fluorophore cores results in a great reduction of fluorescence quantum yield and loss of fluorescence emission. The resulting thiocaged probes are efficiently desulfurized to their oxo derivatives in the presence of mercury ions, leading to pronounced changes in chromogenic and fluorogenic signals. Because these probes exhibit high selectivity, excellent sensitivity, good membrane-permeability, and rapid responses towards mercury ions, they are suitable for visualization of mercury in both aqueous and intracellular environments.
Keywords: Mercury, detection, fluorogenic, colorimetric, thiocaged, desulfurization
Graphical Abstract
1. INTRODUCTION
Mercury is a serious environmental pollutant and toxin,[1] linked to severe pathogenic effects on the nervous system, immune system, and kidneys.[2,3] Thus, the development of new and generally-applicable methods for the selective detection and visualization of Hg2+ ions and other heavy metals is critically important.[4–10] Among a range of sophisticated Hg2+ sensors, including nanoparticles,[11–15] polymers,[16–19] enzymes,[20–24] and DNA-based probes,[25–29] chemosensors are particularly attractive because of their high sensitivity, low cost, and ease of use.[30–36] Based on the strong thiophilic affinity of Hg2+, fluorescent changes associated with Hg2+-induced cyclization,[37–40] coordination,[41–44] ring opening complexation,[45–48] and hydrolysis[49–53] have been used in the design of Hg2+ sensors. In particular, the hydrolysis reaction of thiocarbonyl group mediated by S-Hg bond formation is of great interest due to ease of design, excellent selectivity, and sensitivity to Hg2+ ions. Fluorophore scaffolds with short wavelength emission, including coumarin,[53] and 8-hydroxyquinoline,[54] have been exclusively used for the design of Hg2+ sensors based on this hydrolysis mechanism.
In 2019, we employed a single sulfur-for-oxygen atom substitution as a general approach to preparing photoactivatable probes or heavy-atom free photosensitizers.[55–57] We found that a single sulfur-for-oxygen replacement in the carbonyl groups of existing fluorophore cores (e.g., Nile red, naphthalimide, acridone, and coumarin) leads to a substantial reduced fluorescence due to a photoinduced electron transfer-quenching mechanism. Compared to the corresponding carbonyl compounds, thio-based fluorophores exhibit significant red-shifts and enhanced absorption due to the reduced energy gap between the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbitals (LUMO) in these fluorophores. The significant decrease in quantum yields of all tested thio-based fluorophores suggests that replacement of a single oxygen atom with sulfur can be a general approach for preparing fluorogenic Hg2+ sensors with diverse structures.
In this study, we have used a single sulfur-for-oxygen atom substitution as a general strategy to prepare a class of colorimetric and fluorogenic chemosensors for the selective detection of Hg2+ in aqueous environments (Figure 1). Upon addition of Hg2+, thio-caged sensors are efficiently desulfurized to their oxo derivatives, thus restoring strong emission of the fluorophores across a broad spectral range (410 nm - 625 nm). Furthermore, our newly developed thio-imide probes exhibit a much faster response to Hg2+ than the previously reported thiocoumarins, probably due to stronger electron-donating ability of the nitrogen atom compared to oxygen atom.
Fig. 1. General reaction mechanism of the desulfurization reaction.
Changes in the fluorescence intensity of thiocaged fluorophores (5.0 × 10−6 M) were monitored in H2O/CH3CN (50:50) in the presence of different metal ions (5.0 × 10−4 M). The following excitation and emission wavelengths are used: SACD (390 nm, 408 nm), SCou (375 nm, 467 nm), SDMAP-NH (370 nm, 504 nm), SDMN-NH (430 nm, 540 nm), SDMNP-NH (370 nm, 513 nm), SNile Red (550 nm, 700 nm).
2. EXPERIMENTAL DETAILS
2.1. Materials and apparatus
1H and 13C NMR spectra were measured on a Bruker AVANCE III HD 600 MHz spectrometer in CDCl3 and D2O/CD3CN 1:1. UV-vis absorption spectra were measured on a Thermo Scientific Evolution 220 UV-Vis Spectrophotometer. Fluorescence measurements were conducted on a Horiba FluoroMax-4 spectrofluorometer. ESI mass spectroscopy was performed on a Bruker MicroToF ESI LC-MS System. The cation solutions were prepared from NaCl, MgCl2, KCl, CaCl2, MnCl2, FeCl3, CoCl2, NiCl2, CuCl2, ZnCl2, Pd(OAc)2, AgNO3, CdCl2, SnCl2, CsCO3, PtCl2, PbCl2; in 10 mM HEPES buffer (pH = 7.4), respectively at a concentration of 1 mM.
2.2. Synthesis
SCou, SDMAP, and SNile Red were synthesized according to the previously reported protocol.[55] DMN-O and DMNP-O were synthesized using the reported synthetic route.[56] DMN-NH and DMNP-NH were synthesized using the procedure reported previously.[58]
DMN-NH
DMN-O (80 mg, 0.37 mmol) and urea (45 mg, 0.74 mmol, 2 eq) were heated at 170 °C for 2 h in a Schlenk tube. The mixture was cooled to room temperature, triturated with water (10 mL) and extracted three times with DCM. The organic phase was dried over Na2SO4, and the solvent was removed under reduced pressure. The residue was purified by silica gel column chromatography (30% ethyl acetate in hexanes) to afford DMN-NH (73 mg, 82% yield) as a yellow solid. 1H NMR (600 MHz, Chloroform-d) δ 8.56 (dd, J = 7.3, 1.1 Hz, 1H), 8.47 (dd, J = 7.3, 1.1 Hz, 1H), 8.47 (d, J = 8.2 Hz, 1H), 8.34 (s, 1H), 7.67 (dd, J = 8.4, 7.3 Hz, 1H), 7.13 (d, J = 8.2 Hz, 1H), 3.13 (s, 6H). 13C NMR (151 MHz, CDCl3) δ 164.47, 163.84, 157.73, 132.63, 132.07, 131.82, 131.05, 125.78, 124.96, 123.07, 114.66, 113.36, 44.95. ESIMS [M+H]+ calcd. for C14H12N2O2 241.1, found 241.0.
DMNP-NH
DMNP-O (40 mg, 0.18 mmol) and urea (23 mg, 0.37 mmol, 2 eq) were stirred at 170 °C for 2 h under nitrogen. The mixture was cooled to room temperature, triturated in water, and extracted five times with DCM. The organic phase was dried over Na2SO4 and the solvent was removed under reduced pressure and purified by silica gel column chromatography (30 to 50% ethyl acetate in hexanes) to give DMNP-NH (35 mg, 78% yield) as a yellow solid. 1H NMR (600 MHz, DMSO-d6) δ 11.23 (s, 1H), 8.20 (s, 1H), 8.09 (s, 1H), 8.02 (d, J = 9.2 Hz, 1H), 7.39 (dd, J = 9.2, 2.5 Hz, 1H), 7.24 (d, J = 2.5 Hz, 1H), 3.09 (s, 6H). 13C NMR (151 MHz, DMSO) δ 168.63, 168.53, 149.65, 136.65, 130.45, 128.68, 126.26, 123.48, 122.90, 121.14, 117.32, 106.88, 39.26. ESIMS [M+H]+ calcd. for C14H12N2O2 241.1, found 241.0.
SDMN-NH
DMN-NH (12 mg, 0.05 mmol), and Lawesson’s reagent (40 mg, 0.1 mmol) were stirred in dry toluene (5 mL) under nitrogen at 110 °C, in a Schlenk tube for 36 h. The reaction was cooled to room temperature, the solvent was removed under reduced pressure and purified by silica gel chromatography (10% ethyl acetate in hexanes) to give SDMP-NH as a dark purple solid (8.6 mg, 63% yield).1H NMR (600 MHz, DMSO-d6) δ 13.58 (s, 1H), 8.81 (d, J = 7.6 Hz, 1H), 8.63 (d, J = 8.9 Hz, 1H), 8.57 (d, J = 8.3 Hz, 1H), 7.66 (t, J = 8.0 Hz, 1H), 7.16 (d, J = 9.0 Hz, 1H), 3.27 (s, 6H). 13C NMR (151 MHz, DMSO) δ 189.16, 186.10, 158.43, 138.10, 136.17, 134.28, 129.00, 127.72, 125.44, 122.63, 120.03, 113.83, 44.95. ESIMS [M+H]+ calcd. for C14H12N2S2 273.1, found 273.0.
SDMNP-NH
DMN-NH (12 mg, 0.05 mmol), and Lawesson’s reagent (40 mg, 0.1 mmol) were stirred in dry toluene under nitrogen at 110 °C, in a Schlenk tube for 12 h. The reaction was cooled to room temperature, the solvent was removed under reduced pressure and purified by silica gel chromatography (15% ethyl acetate in hexanes) to give SDMP-NH as a dark purple solid (9.8 mg, 72% yield). 1H NMR (600 MHz, DMSO-d6) δ 13.73 (s, 1H), 8.22 (s, 1H), 8.10 (s, 1H) 8.09 (d, J = 9.2 Hz, 2H), 7.38 (dd, J = 9.2, 2.5 Hz, 1H), 7.35 (d, J = 2.5 Hz, 1H), 3.11 (s, 6H). 13C NMR (151 MHz, DMSO) δ 198.09, 197.27, 150.05, 136.63, 133.14, 131.38, 128.09, 125.87, 124.22, 121.46, 117.41, 107.74, 39.24. ESIMS [M+H]+ calcd. for C14H12N2S2 273.1, found 273.0.
2.3. Fluorescence assays
Thionated fluorophores (10 μM) in acetonitrile, were mixed with 1mM solutions of each corresponding metal dissolved in 10mM HEPES buffer. For detection limit experiments, 10 μM solutions of thionated fluorophores in acetonitrile were mixed with the corresponding concentration of mercury (2.5, 5, 7.5, and 10 μM). Blank measurements were performed with 5 μM solutions of the corresponding fluorophore in the solution containing 50% acetonitrile and 50% 10 mM HEPES.
2.4. UV-Vis experiments
Blank reference was measured using an acetonitrile/water (1:1) mixture. 10 μM solutions of thionated fluorophores in acetonitrile, were mixed with 1mM solutions of each corresponding metal dissolved in 10mM HEPES buffer.
2.5. NMR experiments
Fluorophore (1 mM) were prepared in 1:1 CD3CN/D2O (DMSO-d6 for SDMNP-NH), followed by the addition of HgCl2 (10 eq). 1H NMR spectra were monitored at different time points until no change was observed.
2.6. Quantum yield determination
Quantum yields measurements were determined using fluorescein (quantum yield of 0.92 in 0.1 M NaOH), Quinine sulfate (quantum yield of 0.546 in H2SO4 0.5 M), and Rhodamine B (quantum yield of 0.31 in water) as standards.
The fluorescence quantum yield, Φf (sample), was calculated according to the equation below:
Φf: quantum yield of fluorescence;
I: integrated emission intensity;
OD: optical density at the excitation wavelength;
d: refractive index of solvents, dDMSO=1.48; dwater=1.33.
2.7. Cell culture
HeLa cells were cultured in DMEM (Invitrogen, Carlsbad, CA), supplemented with 10% fetal bovine serum in a humidified atmosphere of 5% CO2 at 37 °C. For imaging experiments, exponentially growing cells were seeded in 8-chamber (Lab-Tek 155411). The cells were cultured at 37 °C in a 5% CO2 atmosphere for 24 h before adding reagents.
2.8. Cell imaging
For labeling, the medium was removed and the cells were rinsed three times with PBS (pH 7.4). Then, the HeLa cells were incubated with probes SCou, SDMAP, and SDMNP-NH with a final concentration of 4 μM in PBS at 37 °C for 30 min as the control. For Hg2+ imaging, another set of HeLa cells was preloaded with the corresponding probes with a final concentration of 4 μM in PBS at 37 °C for 30 min, rinsed three times with PBS, and further treated with increasing amounts of Hg2+ (2, 5, and 10 μM) in PBS at 37 °C for additional 30 minutes. The cells were rinsed three times with PBS, then the imaging was carried out on a Carl Zeiss Confocal LSM800 microscope with 40× lenses. All probes were scanned by 405 laser light at 70% laser intensity.
3. RESULTS AND DISCUSSION
To determine if a single sulfur-for-oxygen atom replacement within common fluorophore scaffolds represents a general approach to preparing Hg2+ sensors, we prepared thio-acridone (SACD), thio-coumarin (SCou), thio-4-dimethylaminophthalimide (SDMAP-NH), thio-4-dimethylamino-1,8-naphthalimide (SDMN-NH), thio-4-dimethylaminonaphthalimide (SDMNP-NH), and thio-based Nile Red (SNile Red) from their oxo forms using Lawesson’s reagent in toluene according to our previously described methodology.[55,56] All compounds were fully characterized by 1H NMR, 13C NMR, and mass spectrometry as described in the supporting information. Compared to carbonyl analogs, thiocarbonyl fluorophores exhibit a red-shifted absorption spectrum and larger extinction coefficients (Table S1).
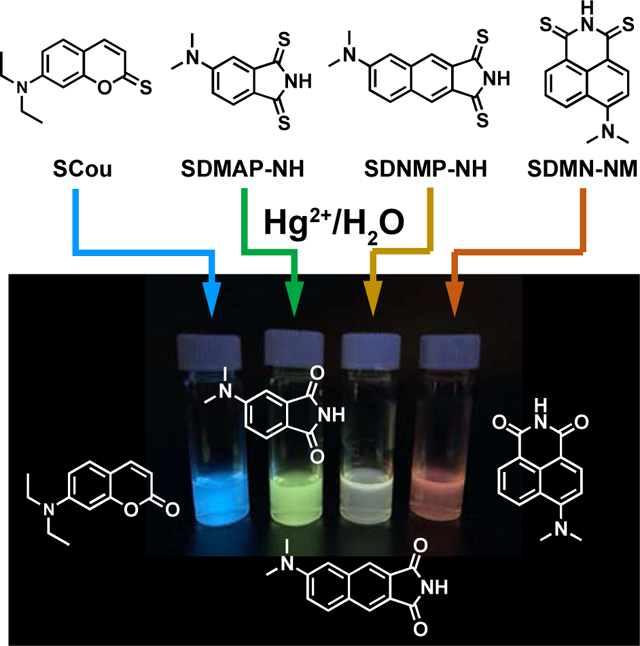
Importantly, the fluorescence quantum yield of thio-caged fluorophores in DMSO is significantly lower than that of the corresponding oxo forms, indicating the likelihood that the Hg2+-induced hydrolysis reaction might be used to re-activate the fluorescence of thio-caged probes. To test the selectivity of thio-caged fluorophores as Hg2+ sensors, the fluorescence intensity changes in different thio-caged probes were evaluated 30 minutes after the addition of different metal ions (Figure 1). In the presence of Hg2+ ions, SCou, SDMAP-NH, SDMN-NH, SDMNP-NH, but not SACD and SNile Red, exhibited remarkable increases in fluorescence intensity, probably due to differences in the electron-donating ability of the atom adjacent to the carbonyl group. In contrast, the addition of other metal ions, including Pt2+, Pb2+, Na+, Mg2+, K+, Ca2+, Mn2+, Fe2+, and others, did not result in a significant change in fluorescence intensity (Figure 1). Additionally, competing experiments showed the probe selectivity reacts mercury. Using the mixture of Hg2+ and other ions listed in Figure 1, the fluorescent response was similar to the single addition of HgCl2 (Figure S1). The only exceptions were the addition of Ag+ to SCou and SDMN-NH. Silver ions are known to form complexes with thionated dyes. Therefore, with the addition of Ag+, the fluorescence intensity was relatively lower. SDMAP and SDMNP-NH react quickly with Hg2+, and no significant difference was observed when adding silver ions (Figure S1).
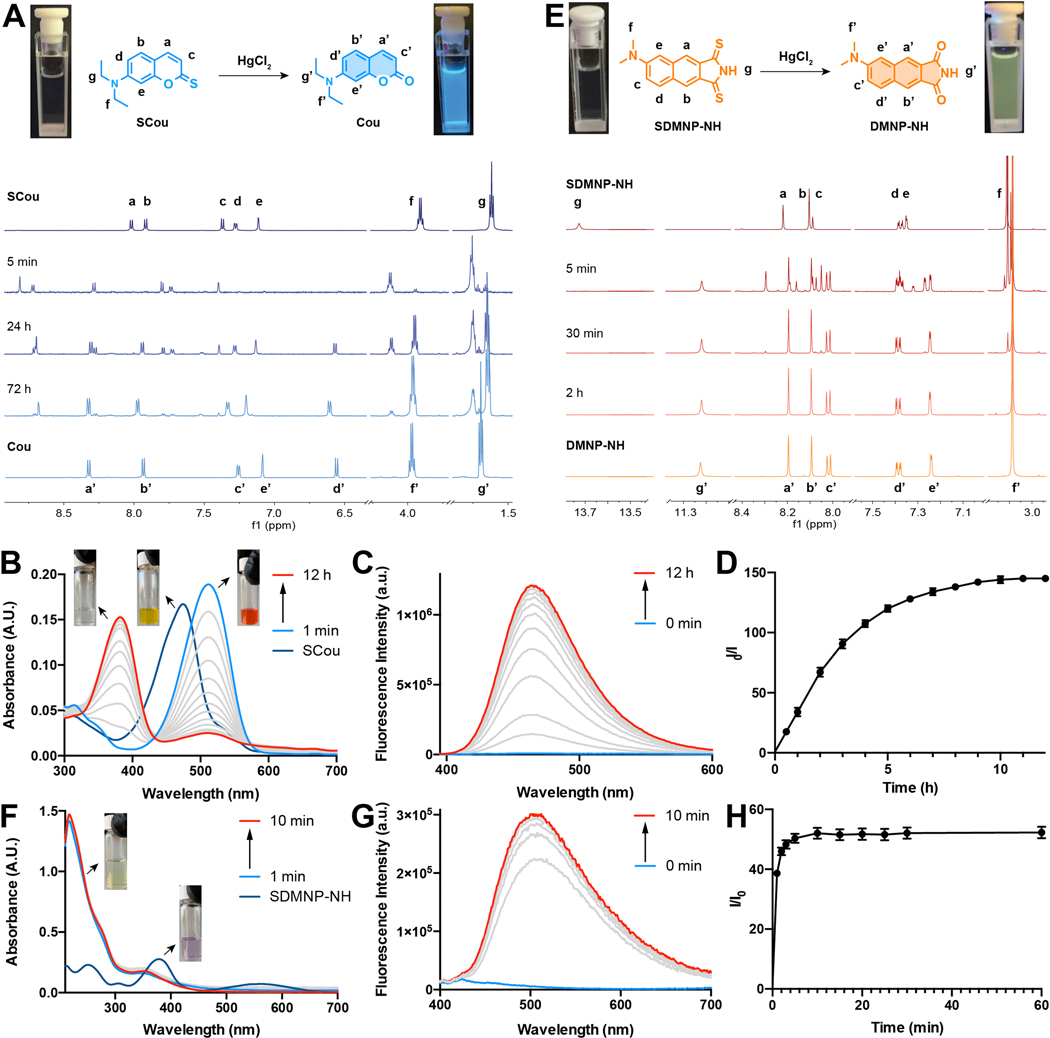
To further characterize the Hg2+-triggered fluorescent activation process in SCou, SDMAP, and SDMNP, we recorded 1H NMR, absorption, and emission spectra. Upon addition of Hg2+ ions, aromatic protons of SCou shift from 8.02, 7.92, 7.37, 7.27, and 7.12 ppm to 8.72, 8.29, 7.80, 7.73, 7.41 ppm, corresponding to the formation of the SCou-Hg complex. Within 72 h, signals corresponding to the SCou-Hg complex gradually disappeared and were replaced by new peaks appearing at 8.32, 7.97, 7.33, 7.20, and 6.61 ppm, in good agreement with the spectrum of commercially available Cou (Figure 2A). The absorption peak of SCou at 472 nm was initially red-shifted to 511 nm, followed by a blue-shift to 381 nm with the addition of Hg2+ ions (Figure 2B). After 12h of exposure to Hg2+, a 144-fold increase in fluorescence intensity was observed at 467 nm (Figure 2C and 2D).
Fig. 2.
Spectral changes of SCou and SDMNP-NH after Hg2+ addition. A) 1H NMR monitoring of SCou 1 mM with 10 eq. of HgCl2 in CD3CN/D20 1:1. Image of samples before (left) and after (right) addition of HgCl2 under UV light (365 nm) irradiation. B) UV-vis monitoring of 5 μM SCou after the addition of HgCl2 (500 μM) in CH3CN/HEPES buffer 1:1. C) Fluorescence spectra monitoring of 5 μM SCou after the addition of HgCl2 (500 μM) in CH3CN/HEPES buffer 1:1. D) Turn-on fold increase of 5 μM SCou after the addition of Hg 2+ (500 μM) at 467 nm in CH3CN/HEPES buffer 1:1. E) 1H NMR monitoring of SDMNP-NH 1 mM with 10 eq. of HgCl2 in DMSO-d6. Images of samples before (left) and after (right) addition of HgCl2 under UV light (365 nm) irradiation. F) UV-vis monitoring of 5 pM SDMNP-NH after the addition of HgCl2 (500 μM) in CH3CN/HEPES buffer 1:1. G) Fluorescence spectra monitoring of 5 pM SDMNP-NH after the addition of HgCl2 (500 μM) in CH3CN/HEPES buffer 1:1. H) Turn-on fold increase of 5 μM SDMNP-NH after the addition of HgCl2 (500 μM) at 513 nm in CH3CN/HEPES buffer 1:1.
SDMAP-NH and SDMNP-NH exhibited more rapid responses to Hg2+ ions. Upon treatment of SDMAP-NH and SDMNP-NH with 10 equiv. of HgCl2 in aqueous solution, changes in 1H NMR, absorption, and emission spectra were recorded. As shown in Figure 2E, signals corresponding to SDMNP-NH phenyl moiety protons shifted from 8.22, 8.10, 8.09, 7.38, 7.35 ppm to 8.20, 8.09, 8.02, 7.39, 7.24 ppm, respectively. After the addition of Hg2+, SDMAP-NH and SDMNP-NH exhibit a purple color, with absorption bands at 557 nm (Figure S2) and 558 nm (Figure 2F), that rapidly disappears within 10 min, yielding colorless solutions. Furthermore, SDMAP-NH and SDMNP-NH exhibited fluorescence turn-on ratios of 38 at 504 nm (Figure S2) and 52 at 513 nm (Figure 2G and H), respectively. Therefore, our data suggest that SDMAP-NH and SDMNP-NH can be fully desulfurized within 10 mins, compared to 12h for SCou. SDMN-NH exhibits a similar 34-fold fluorescence turn-on ratio at 540 nm after 10 h (Figure S3). The solution colors change immediately from yellow to red for SCou, purple to light-yellow for SDMAP and SDMNP-NH, and purple to pink for SDMN-NH. These changes allow for the naked-eye detection of mercury ions at varying concentrations (Figure S4).
Mass spectrometry analysis of samples incubated with Hg2+ indicates losses of 16 amu for SCou and 32 amu for SDMAP-NH, SDMN-NH, and SDMNP-NH, corresponding to the regeneration of fluorophores in the oxo form (Figures S5-S8). The results obtained with UV-vis absorbance and 1H NMR agree with the two-step desulfurization mechanism described previously.[53] After the addition of mercury, a complex is formed immediately, assisted by a neighboring electron-donating atom (oxygen or nitrogen). The second step involves the hydrolysis of mercury sulfide, restoring the corresponding fluorescent dye. (Figure 1).
Detection limits for SCou, SDMAP, SDMN, and SDNMP were measured according to the methodology used by Talukdar.[59] Low mercury concentrations were used to obtain linear relationships between fluorescence intensity and Hg2+ concentration (Figure S9). SCou proved to be the most sensitive of the probes tested, with a detection limit of 2.70 ± 0.06 nM after an incubation time of 8 h. With a similar incubation time, SDMN has an Hg2+ detection limit of 160 nm. By comparison, SDMAP and SDMNP exhibit detection limits of 288 ± 7 nM and 770 ± 8 nM, respectively, measured 15 minutes after Hg2+ ion addition. A comparison with other sensing platforms is also summarized in table S2.
Next, we studied the utility of thio-caged probes for the detection of Hg2+ ions in living cells. Hela cells were incubated for 30 min with 4 μM SCou, SDMAP-NH, and SDMNP-NH, followed by the addition of different concentrations of HgCl2 for an additional 20 min. As shown in Figure 3, cells without HgCl2 treatment exhibited no fluorescence signal. By comparison, SCou-, SDMAP-NH-, and SDMNP-NH-treated cells exhibited distinct intracellular fluorescence in the prescence of more than 5 μM Hg2+ ions. This illustrates both the membrane permeability of thio-caged probes and their ability to detect intracellular Hg2+. Importantly, no obvious cytotoxicity was observed in cells treated with these probes.
Fig. 3.
Confocal microscope images of HeLa cells incubated with SCou, SDMAP-NH, and SDMNP-NH (4 μM) with varying concentrations of HgCl2 (0, 2, 5, and 10 μM, respectively). Images were obtained 30 min after mercury addition. Scalebar = 20 μm.
4. CONCLUSION
In conclusion, we have used a single sulfur-for-oxygen atom substitution within available fluorophores to develop a general method for the preparation of colorimetric and fluorogenic Hg2+ chemosensors. Using this approach, we developed a series of thio-caged probes across a broad spectral range. Fluorescence spectroscopy can be used alongside these probes to provide rapid recognition of Hg2+ ions, with high selectivity and sensitivity in aqueous samples. The colorimetric assay developed in this work also allows for the immediate, qualitative detection of Hg2+. SDMAP and SDMNP-NH offer rapid detection, while SCou requires a longer incubation time but affords higher sensitivity. Furthermore, the detection of intracellular Hg2+ using these thio-caged chemosensors suggests their potential applications in biological and environmental research.
Supplementary Material
HIGHLIGHTS.
Single-atom replacement can quench fluorescent dyes via one-step modification.
Mercury ions can activate thiocaged dyes in aqueous and cellular environments.
Thiocaged dyes are applied to detect mercury based on colorimetric and fluorescent signals.
ACKNOWLEDGMENTS
This work was supported by the Cancer Prevention Research Institute of Texas (CPRIT RR170014), NIH (R35-GM133706), the Robert A. Welch Foundation (C-1970), the John S. Dunn Foundation Collaborative Research Award, and the Hamill Innovation Award. H. X. is a Cancer Prevention & Research Institute of Texas (CPRIT) Scholar in Cancer Research.
Footnotes
Declaration of competing interest
The authors declare no competing financial interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at:
Axel Loredo: Conceptualization, Methodology, Formal analysis, Writing- Original draft preparation, Writing- Reviewing and Editing. Lushun Wang: Conceptualization, Methodology, Formal analysis, Writing- Reviewing and Editing. Shichao Wang: Conceptualization, Methodology, Formal analysis, Writing- Reviewing and Editing. Han Xiao: Conceptualization, Methodology, Formal analysis, Writing- Reviewing and Editing, Supervision.
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Onyido I, Norris AR, Buncel E. Biomolecule−Mercury InteracVons: ModaliVes of DNA Base−Mercury Binding Mechanisms. RemediaVon Strategies. Chem Rev 2004;104:5911–30. 10.1021/cr030443w. [DOI] [PubMed] [Google Scholar]
- [2].Harris HH, Pickering IJ, George GN. The Chemical Form of Mercury in Fish. Science 2003;301:1203–1203. 10.1126/science.1085941. [DOI] [PubMed] [Google Scholar]
- [3].Liu J, Lu Y. Rational Design of “Turn-On” Allosteric DNAzyme Catalytic Beacons for Aqueous Mercury Ions with Ultrahigh Sensitivity and Selectivity. Angew Chem Int Ed 2007;46:7587–90. 10.1002/anie.200702006. [DOI] [PubMed] [Google Scholar]
- [4].Ali I, Jain CK. Advances in arsenic speciation techniques. Int J Environ Anal Chem 2004;84:947–64. 10.1080/03067310410001729637. [DOI] [Google Scholar]
- [5].Youn NJ, Chang S-K. Dimethylcyclam based fluoroionophore having Hg2+- and Cd2+-selective signaling behaviors. Tetrahedron Lett 2005;46:125–9. 10.1016/j.tetlet.2004.11.003. [DOI] [Google Scholar]
- [6].Kim HN, Ren WX, Kim JS, Yoon J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem Soc Rev 2012;41:3210–44. 10.1039/C1CS15245A. [DOI] [PubMed] [Google Scholar]
- [7].Gupta VK, Kumar S, Singh R, Singh LP, Shoora SK, Sethi B. Cadmium (II) ion sensing through p-tert-butyl calix[6]arene based potentiometric sensor. J Mol Liq 2014;195:65–8. 10.1016/j.molliq.2014.02.001. [DOI] [Google Scholar]
- [8].Dehghani MH, Sanaei D, Ali I, Bhatnagar A. Removal of chromium(VI) from aqueous solution using treated waste newspaper as a low-cost adsorbent: Kinetic modeling and isotherm studies. J Mol Liq 2016;215:671–9. 10.1016/j.molliq.2015.12.057. [DOI] [Google Scholar]
- [9].Wu G, Li M, Zhu J, Lai KWC, Tong Q, Lu F. A highly sensitive and selective turn-on fluorescent probe for Pb(II) ions based on a coumarin–quinoline platform. RSC Adv 2016;6:100696–9. 10.1039/C6RA19734E. [DOI] [Google Scholar]
- [10].Liu B, Zhuang J, Wei G. Recent advances in the design of colorimetric sensors for environmental monitoring. Environ Sci Nano 2020;7:2195–213. 10.1039/D0EN00449A. [DOI] [Google Scholar]
- [11].Gao W, Xu Y, Wei W, Wang D, Shi X. Ultrasensitive determination of mercury ions (Ⅱ) by analysis of the degree of quantum dots aggregation. Talanta 2018;188:644–50. 10.1016/j.talanta.2018.06.039. [DOI] [PubMed] [Google Scholar]
- [12].Wang N, Liu G, Dai H, Ma H, Lin M. Spectroscopic evidence for electrochemical effect of mercury ions on gold nanoparticles. Anal Chim Acta 2019;1062:140–6. 10.1016/j.aca.2019.02.037. [DOI] [PubMed] [Google Scholar]
- [13].Liu C, Chen X, Zong B, Mao S. Recent advances in sensitive and rapid mercury determination with graphene-based sensors. J Mater Chem A 2019;7:6616–30. 10.1039/C9TA01009B. [DOI] [Google Scholar]
- [14].Tsao Y-H, Husain RA, Lin Y-J, Khan I, Chen S-W, Lin Z-H. A self-powered mercury ion nanosensor based on the thermoelectric effect and chemical transformation mechanism. Nano Energy 2019;62:268–74. 10.1016/j.nanoen.2019.05.032. [DOI] [Google Scholar]
- [15].Gan Y, Sun J, Liang T, Tu J, Hu N, Wan H, et al. An Ultrasensitive Gold Nanoband Aptasensor for Mercury(II) Detection in Aquatic Environment. J Electrochem Soc 2019;166:B793. 10.1149/2.1061910jes. [DOI] [Google Scholar]
- [16].Liu X, Tang Y, Wang L, Zhang J, Song S, Fan C, et al. Optical Detection of Mercury(II) in Aqueous Solutions by Using Conjugated Polymers and Label-Free Oligonucleotides. Adv Mater 2007;19:1471–4. 10.1002/adma.200602578. [DOI] [Google Scholar]
- [17].Balamurugan A, Lee H. Water-Soluble Polymeric Probes for the Selective Sensing of Mercury Ion: pH-Driven Controllable Detection Sensitivity and Time. Macromolecules 2015;48:1048–54. 10.1021/ma502350p. [DOI] [Google Scholar]
- [18].Shan Y, Yao W, Liang Z, Zhu L, Yang S, Ruan Z. Reaction-based AIEE-active conjugated polymer as fluorescent turn on probe for mercury ions with good sensing performance. Dyes Pigments 2018;156:1–7. 10.1016/j.dyepig.2018.03.060. [DOI] [Google Scholar]
- [19].Zhang D, Yao Y, Wu J, Protsak I, Lu W, He X, et al. Super Hydrophilic Semi-IPN Fluorescent Poly(N-(2-hydroxyethyl)acrylamide) Hydrogel for Ultrafast, Selective, and Long-Term Effective Mercury(II) Detection in a Bacteria-Laden System. ACS Appl Bio Mater 2019;2:906–15. 10.1021/acsabm.8b00761. [DOI] [PubMed] [Google Scholar]
- [20].Han S, Zhu M, Yuan Z, Li X. A methylene blue-mediated enzyme electrode for the determination of trace mercury(II), mercury(I), methylmercury, and mercury–glutathione complex. Biosens Bioelectron 2001;16:9–16. 10.1016/S0956-5663(00)00114-7. [DOI] [PubMed] [Google Scholar]
- [21].Pirvutoiu S, Surugiu I, Dey E S, Ciucu A, Magearu V, Danielsson B. Flow injection analysis of mercury(ii) based on enzyme inhibition and thermometric detection. Analyst 2001;126:1612–6. 10.1039/B102723A. [DOI] [Google Scholar]
- [22].Bontidean I, Mortari A, Leth S, Brown NL, Karlson U, Larsen MM, et al. Biosensors for detection of mercury in contaminated soils. Environ Pollut 2004;131:255–62. 10.1016/j.envpol.2004.02.019. [DOI] [PubMed] [Google Scholar]
- [23].Frasco MF, Colletier J-P, Weik M, Carvalho F, Guilhermino L, Stojan J, et al. Mechanisms of cholinesterase inhibition by inorganic mercury. FEBS J 2007;274:1849–61. 10.1111/j.1742-4658.2007.05732.x. [DOI] [PubMed] [Google Scholar]
- [24].Zhang Z, Zhang F, He P, Zhang X, Song W. Fluorometric determination of mercury(II) by using thymine-thymine mismatches as recognition elements, toehold binding, and enzyme-assisted signal amplification. Microchim Acta 2019;186:551. 10.1007/s00604-019-3683-3. [DOI] [PubMed] [Google Scholar]
- [25].Liu C-W, Huang C-C, Chang H-T. Highly Selective DNA-Based Sensor for Lead(II) and Mercury(II) Ions. Anal Chem 2009;81:2383–7. 10.1021/ac8022185. [DOI] [PubMed] [Google Scholar]
- [26].Zhuang J, Fu L, Tang D, Xu M, Chen G, Yang H. Target-induced structure-switching DNA hairpins for sensitive electrochemical monitoring of mercury (II). Biosens Bioelectron 2013;39:315–9. 10.1016/j.bios.2012.07.015. [DOI] [PubMed] [Google Scholar]
- [27].Chen J, Tang J, Zhou J, Zhang L, Chen G, Tang D. Target-induced formation of gold amalgamation on DNA-based sensing platform for electrochemical monitoring of mercury ion coupling with cycling signal amplification strategy. Anal Chim Acta 2014;810:10–6. 10.1016/j.aca.2013.12.010. [DOI] [PubMed] [Google Scholar]
- [28].Zhang J, Tang Y, Lv J, Fang S, Tang D. Glucometer-based quantitative determination of Hg(II) using gold particle encapsulated invertase and strong thymine-Hg(II)-thymine interaction for signal amplification. Microchim Acta 2015;182:1153–9. 10.1007/s00604-014-1437-9. [DOI] [Google Scholar]
- [29].Xia N, Feng F, Liu C, Li R, Xiang W, Shi H, et al. The detection of mercury ion using DNA as sensors based on fluorescence resonance energy transfer. Talanta 2019;192:500–7. 10.1016/j.talanta.2018.08.086. [DOI] [PubMed] [Google Scholar]
- [30].Yang Y-K, Yook K-J, Tae J. A Rhodamine-Based Fluorescent and Colorimetric Chemodosimeter for the Rapid Detection of Hg2+ Ions in Aqueous Media. J Am Chem Soc 2005;127:16760–1. 10.1021/ja054855t. [DOI] [PubMed] [Google Scholar]
- [31].Pal A, Bag B. Hg(II) ion specific dual mode signalling in a thiophene derivatized rhodamine based probe and their complexation cooperativity. J Photochem Photobiol Chem 2012;240:42–9. 10.1016/j.jphotochem.2012.05.010. [DOI] [Google Scholar]
- [32].Erdemir S, Kocyigit O, Malkondu S. Detection of Hg2+ ion in aqueous media by new fluorometric and colorimetric sensor based on triazole–rhodamine. J Photochem Photobiol Chem 2015;309:15–21. 10.1016/j.jphotochem.2015.04.017. [DOI] [Google Scholar]
- [33].Giri D, Bankura A, Patra SK. Poly(benzodithieno-imidazole-alt-carbazole) based π-conjugated copolymers: Highly selective and sensitive turn-off fluorescent probes for Hg2+. Polymer 2018;158:338–53. 10.1016/j.polymer.2018.10.069. [DOI] [Google Scholar]
- [34].Kraithong S, Sangsuwan R, Worawannotai N, Sirirak J, Charoenpanich A, Thamyongkit P, et al. Triple detection modes for Hg2+ sensing based on a NBD-fluorescent and colorimetric sensor and its potential in cell imaging. New J Chem 2018;42:12412–20. 10.1039/C8NJ01915K. [DOI] [Google Scholar]
- [35].Huang L, Yang Z, Zhou Z, Li Y, Tang S, Xiao W, et al. A dual colorimetric and near-infrared fluorescent turn-on probe for Hg2+ detection and its applications. Dyes Pigments 2019;163:118–25. 10.1016/j.dyepig.2018.11.047. [DOI] [Google Scholar]
- [36].Hu J, Yu X, Zhang X, Jing C, Liu T, Hu X, et al. Rapid detection of mercury (II) ions and water content by a new rhodamine B-based fluorescent chemosensor. Spectrochim Acta A Mol Biomol Spectrosc 0032020;241:118657. 10.1016/j.saa.2020.118657. [DOI] [PubMed] [Google Scholar]
- [37].Liu B, Tian H. A selective fluorescent ratiometric chemodosimeter for mercury ion. Chem Commun 2005:3156–8. 10.1039/B501913C. [DOI] [PubMed] [Google Scholar]
- [38].Lee MH, Lee SW, Kim SH, Kang C, Kim JS. Nanomolar Hg(II) Detection Using Nile Blue Chemodosimeter in Biological Media. Org Lett 2009;11:2101–4. 10.1021/ol900542y. [DOI] [PubMed] [Google Scholar]
- [39].Bera K, Das AK, Nag M, Basak S. Development of a Rhodamine–Rhodanine-Based Fluorescent Mercury Sensor and Its Use to Monitor Real-Time Uptake and Distribution of Inorganic Mercury in Live Zebrafish Larvae. Anal Chem 2014;86:2740–6. 10.1021/ac404160v. [DOI] [PubMed] [Google Scholar]
- [40].Ko S-K, Yang Y-K, Tae J, Shin I. In Vivo Monitoring of Mercury Ions Using a Rhodamine-Based Molecular Probe. J Am Chem Soc 2006;128:14150–5. 10.1021/ja065114a. [DOI] [PubMed] [Google Scholar]
- [41].Rurack K, Resch-Genger U, Bricks JL, Spieles M. Cation-triggered ‘switching on’ of the red/near infra-red (NIR) fluorescence of rigid fluorophore–spacer–receptor ionophores. Chem Commun 2000:2103–4. 10.1039/B006430K. [DOI] [Google Scholar]
- [42].Long L, Tan X, Luo S, Shi C. Fluorinated near-infrared fluorescent probes for specific detection of Hg 2+ in an aqueous medium and mitochondria of living cells. New J Chem 2017;41:8899–904. 10.1039/C7NJ01327B. [DOI] [Google Scholar]
- [43].Prodi L, Bargossi C, Montalti M, Zaccheroni N, Su N, Bradshaw JS, et al. An Effective Fluorescent Chemosensor for Mercury Ions. J Am Chem Soc 2000;122:6769–70. 10.1021/ja0006292. [DOI] [Google Scholar]
- [44].Nie K, Dong B, Shi H, Liu Z, Liang B. Diketopyrrolopyrrole Amphiphile-Based Micelle-Like Fluorescent Nanoparticles for Selective and Sensitive Detection of Mercury(II) Ions in Water. Anal Chem 2017;89:2928–36. 10.1021/acs.analchem.6b04258. [DOI] [PubMed] [Google Scholar]
- [45].Tang L, Li F, Liu M, Nandhakumar R. Single sensor for two metal ions: Colorimetric recognition of Cu2+ and fluorescent recognition of Hg2+. Spectrochim Acta A Mol Biomol Spectrosc 2011;78:1168–72. 10.1016/j.saa.2010.12.072. [DOI] [PubMed] [Google Scholar]
- [46].Ma Q-J, Zhang X-B, Zhao X-H, Jin Z, Mao G-J, Shen G-L, et al. A highly selective fluorescent probe for Hg2+ based on a rhodamine–coumarin conjugate. Anal Chim Acta 2010;663:85–90. 10.1016/j.aca.2010.01.029. [DOI] [PubMed] [Google Scholar]
- [47].Rasheed T, Nabeel F, Li C, Bilal M. Rhodamine-assisted fluorescent strategy for the sensitive and selective in-field mapping of environmental pollutant Hg(II) with potential bioimaging. J Lumin 2019;208:519–26. 10.1016/j.jlumin.2019.01.032. [DOI] [Google Scholar]
- [48].Lee YH, Lee MH, Zhang JF, Kim JS. Pyrene Excimer-Based Calix[4]arene FRET Chemosensor for Mercury(II). J Org Chem 2010;75:7159–65. 10.1021/jo101113f. [DOI] [PubMed] [Google Scholar]
- [49].Choi MG, Kim YH, Namgoong JE, Chang S-K. Hg2+-selective chromogenic and fluorogenic chemodosimeter based on thiocoumarins. Chem Commun 2009:3560–2. 10.1039/B905612B. [DOI] [PubMed] [Google Scholar]
- [50].Zhang Q-Q, Ge J-F, Xu Q-F, Yang X-B, Cao X-Q, Li N-J, et al. A selective, sensitive probe for mercury(II) ions based on oxazine-thione. Tetrahedron Lett 2011;52:595–7. 10.1016/j.tetlet.2010.11.130. [DOI] [Google Scholar]
- [51].Chen J, Liu W, Wang Y, Zhang H, Wu J, Xu H, et al. Turn-on fluorescence sensor based on the aggregation of pyrazolo[3,4-b]pyridine-based coumarin chromophores induced by Hg2+. Tetrahedron Lett 2013;54:6447–9. 10.1016/j.tetlet.2013.09.052. [DOI] [Google Scholar]
- [52].Rao AS, Kim D, Wang T, Kim KH, Hwang S, Ahn KH. Reaction-Based Two-Photon Probes for Mercury Ions: Fluorescence Imaging with Dual Optical Windows. Org Lett 2012;14:2598–601. 10.1021/ol3009057. [DOI] [PubMed] [Google Scholar]
- [53].Qin S, Chen B, Huang J, Han Y. A thiocoumarin-based colorimetric and ratiometric fluorescent probe for Hg2+ in aqueous solution and its application in live-cell imaging. New J Chem 2018;42:12766–72. 10.1039/C8NJ01491D. [DOI] [Google Scholar]
- [54].Song KC, Kim JS, Park SM, Chung K- C, Ahn S, Chang S- K. Fluorogenic Hg2+-Selective Chemodosimeter Derived from 8-Hydroxyquinoline. Org Lett 2006;8:3413–6. 10.1021/ol060788b. [DOI] [PubMed] [Google Scholar]
- [55].Tang J, Robichaux MA, Wu K-L, Pei J, Nguyen NT, Zhou Y, et al. Single-Atom Fluorescence Switch: A General Approach toward Visible-Light-Activated Dyes for Biological Imaging. J Am Chem Soc 2019;141:14699–706. 10.1021/jacs.9b06237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tang J, Wang L, Loredo A, Cole C, Xiao H. Single-atom replacement as a general approach towards visible-light/near-infrared heavy-atom-free photosensitizers for photodynamic therapy. Chem Sci 2020;11:6701–8. 10.1039/D0SC02286A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tang J, Yu C, Loredo A, Chen Y, Xiao H. Site-Specific Incorporation of a Photoactivatable Fluorescent Amino Acid. ChemBioChem 2020;n/a. 10.1002/cbic.202000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Baathulaa K, Xu Y, Qian X. Unusual large Stokes shift and solvatochromic fluorophore: Synthesis, spectra, and solvent effect of 6-substituted 2,3-naphthalimide. J Photochem Photobiol Chem 2010;216:24–34. 10.1016/j.jphotochem.2010.09.002. [DOI] [Google Scholar]
- [59].Roy A, Kand D, Saha T, Talukdar P. A cascade reaction based fluorescent probe for rapid and selective fluoride ion detection. Chem Commun 2014;50:5510–3. 10.1039/C4CC01665C. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.