Abstract
Background
The extent to which co-occurrence of cardiovascular disease (CVD) and cancer is due to shared risk factors or other mechanisms is unknown.
Objectives
This study investigated the association of standard CVD risk factors, CVD biomarkers, pre-existing CVD, and ideal cardiovascular (CV) health metrics with the development of future cancer.
Methods
This study prospectively followed Framingham Heart Study and PREVEND (Prevention of Renal and Vascular End-Stage Disease) study participants free of cancer at baseline and ascertained histology-proven cancer. This study assessed the association of baseline CV risk factors, 10-year atherosclerotic (ASCVD) risk score, established CVD biomarkers, prevalent CVD, and the American Heart Association (AHA) Life’s Simple 7 CV health score with incident cancer using multivariable Cox models. Analyses of interim CVD events with incident cancer used time-dependent covariates.
Results
Among 20,305 participants (mean age 50 ± 14 years; 54% women), 2,548 incident cancer cases occurred over a median follow-up of 15.0 years (quartile 1 to 3: 13.3 to 15.0 years). Traditional CVD risk factors, including age, sex, and smoking status, were independently associated with cancer (p < 0.001 for all). Estimated 10-year ASCVD risk was also associated with future cancer (hazard ratio [HR]: 1.16 per 5% increase in risk; 95% confidence interval [CI] 1.14 to 1.17; p < 0.001). The study found that natriuretic peptides (tertile 3 vs. tertile 1; HR: 1.40; 95% CI: 1.03 to 1.91; p = 0.035) were associated with incident cancer but not high-sensitivity troponin (p = 0.47). Prevalent CVD and the development of interim CV events were not associated with higher risk of subsequent cancer. However, ideal CV health was associated with lower future cancer risk (HR: 0.95 per 1-point increase in the AHA health score; 95% CI: 0.92 to 0.99; p = 0.009).
Conclusions
CVD risk, as captured by traditional CVD risk factors, 10-year ASCVD risk score, and natriuretic peptide concentrations are associated with increased risk of future cancer. Conversely, a heart healthy lifestyle is associated with a lower risk of future cancer. These data suggest that the association between CVD and future cancer is attributable to shared risk factors.
Key Words: lifestyle risk factors, prevention
Abbreviations and Acronyms: AHA, American Heart Association; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CI, confidence interval; CV, cardiovascular; CVD, cardiovascular disease; DM, diabetes mellitus; HF, heart failure; HR, hazard ratio; HTN, hypertension; hs-cTn, high-sensitivity cardiac troponin; MI, myocardial infarction; NP, natriuretic peptide; NT-pro-BNP, N-terminal pro-B-type natriuretic peptide; SBP, systolic blood pressure
Central Illustration
Cardiovascular disease (CVD) and cancer are among the leading causes of morbidity and mortality worldwide (1). A growing body of evidence supports a potential link between the 2 disease entities (2), with studies showing an increased risk of CVD among cancer patients (3). In particular, cancer treatment-related cardiotoxicities have emerged as an unintended consequence of evolving therapies and improved cancer-related survival, and the presence of CVD risk factors has been shown to potentiate cardiotoxicity (4). The need to better understand and treat CVD occurring after cancer has led to the development of the emerging field of cardio-oncology (5).
Conversely, recent investigations have suggested that CVD itself may lead to increased risk of cancer development, although data are conflicting. In particular, a number of studies support an increased risk of cancer among patients with heart failure (HF) (6, 7, 8), although this has not been true across all studies (9). Furthermore, CVD risk factors and ideal cardiovascular (CV) health as outlined by the American Heart Association (AHA) 2020 Impact Goal, have previously been associated with cancer risk (10). Despite a growing body of literature examining the relationship between cancer and CVD, whether the association between CVD and cancer is due to shared risk factors or other mechanisms remains unclear.
In this context, we hypothesized that both CV risk factors and the development of CVD itself would be associated with increased risk of future cancer in otherwise healthy individuals. We sought to investigate the association of standard CVD risk factors with incident cancer among participants in the FHS (Framingham Heart Study) and the PREVEND (Prevention of Renal and Vascular End-Stage Disease) study. In this sample, incident cancer outcomes were rigorously ascertained and adjudicated based on histological and pathological evidence. Leveraging this longitudinal study design, we examined the effect of traditional CVD risk factors, 10-year atherosclerotic CVD (ASCVD) risk scores, CVD biomarkers, prevalent CVD, the development of interim CVD events, and ideal CV health metrics (AHA Life’s Simple 7 CV health score) on subsequent cancer incidence.
Methods
Study population
The FHS and PREVEND are prospective, longitudinal community-based observational cohort studies. The characteristics and enrollment of FHS and PREVEND participants have been previously described (11, 12, 13). For FHS, we included participants in the original cohort who attended examination cycles 16 (1979 to 1982; n = 2,351) and 24 (1995 to 1998; n = 831); the offspring cohort who attended examination cycles 2 (1979 to 1983; n = 3,863) and 6 (1995 to 1998; n = 3,532); and the third generation cohort who attended examination cycle 1 (2002 to 2005; n = 4,095). For PREVEND, we included participants who attended examination 1 (1997 to 1998; n = 8,592). The study sample was a pooled sample of FHS and PREVEND participants.
For analyses of prevalent CVD, we excluded subjects with end-stage renal disease (n = 90), prevalent cancer (n = 922), missing covariates (n = 720), and missing follow-up data (n = 1), which yielded a final sample of 21,531 participants. For all other analyses, we further excluded prevalent major CVD (n = 1,020) and prevalent atrial fibrillation (n = 206), which yielded a final sample of 20,305 participants.
All participants provided written informed consent, and institutional review board approval was obtained at all participating institutions.
Clinical assessment
All participants underwent a comprehensive medical history, physical examination, anthropometry, and phlebotomy. The primary exposures of interest included traditional CV risk factors, including age, sex, hypertension (HTN) treatment, systolic blood pressure (SBP) diabetes mellitus (DM), body mass index (BMI), total cholesterol/high-density lipoprotein ratio, smoking status, and statin use. Smoking status was stratified into 3 groups: nonsmoker, former smoker, and present smoker. Secondary exposures of interest included the estimated glomerular filtration rate and ASCVD risk score (14).
CVD events, including major CVD, were documented using established protocols by FHS and PREVEND study investigators after review of all available hospital records. Major CVD events included myocardial infarction (MI), coronary heart disease, HF, stroke, and peripheral vascular disease (11). Cohort-specific details are listed in the Supplemental Appendix. Prevalent CVD, MI, and HF were present at or before baseline examination, and interim CVD, MI, and HF events occurred after the baseline examination.
Biomarker measurements
CVD biomarkers (natriuretic peptides [NPs] and high-sensitivity troponin [hs-cTn]) were ascertained among 9,575 subjects, including participants who attended the FHS Offspring examination 6 and the PREVEND baseline examination. Methods for biomarker measurements were previously reported and details of assay specific assays are presented in Supplemental Table 1 (15, 16, 17). For NPs, B-type natriuretic peptide (BNP) was measured in FHS and its amino terminal pro-peptide equivalent (N-terminal pro-B-type natriuretic peptide [NT-proBNP]) was measured in the PREVEND study. For hs-cTn, hs-cTnI was measured in FHS and hs-cTnT was measured in PREVEND.
Life’s simple 7 cardiovascular health metrics
CV health metrics, defined by the AHA Life’s Simple 7 metrics, were available for FHS participants only (10). Ideal CV health metrics included: 1) never or former smoker; 2) body BMI <23 kg/m2; 3) ≥75 min/week of vigorous intensity physical activity or ≥150 min/week of moderate intensity physical activity; 4) intake of 4 to 5 of the following: ≥450 g/day of fruits and/or vegetables, ≥198 g/week of fish, ≥85 g/day of fiber-rich whole grains, sodium <1,500 mg/day, ≤1 l/week of sugar-sweetened beverages; 5) total cholesterol of <200 mg/dl; 6) blood pressure of <120 mm Hg; and 7) fasting glucose of <100 mg/dl. Each ideal CV health metric was assigned 2 points, and all points were added up to produce a CV health score with a range of 0 to 14 points. CV health scores were classified as poor (0 to 6), average (7 to 9), and optimal (10 to 14).
Ascertainment of cancer outcomes
Participants were prospectively followed for the occurrence of incident cancer. Cancer cases were identified through surveillance of routine examinations, health updates, hospital admissions, or from death records through December 31, 2016. All available medical records and pathology reports were reviewed and coded based on topology and morphology and were graded by 2 independent reviewers. The primary outcome of interest was incident cancer, and secondary outcomes included specific cancer subtypes. Incident cancer included all malignancies, except non-melanoma skin cancers. Specific cancer subtypes included gastrointestinal, lung, prostate, and breast cancers. Specifically, gastrointestinal cancers were defined using International Classification Disease-O topology codes 150 to 157, behavior codes 2 or 3, and grade codes 1 to 4. Cancer outcomes included histologically confirmed cancer and cancer deaths (in the absence of previous histological confirmation). Cancer mortality was defined as subjects with a diagnosis of cancer in whom cancer was identified as the primary cause of death. Cause of death was ascertained by review of death certificates, hospital admission records, and medical records. Cancer deaths (without previous histologically confirmed nonfatal diagnosis) constituted 1% of the total adjudicated cases. Cancer death events were included in the primary analyses but were excluded from all cancer subtype specific analyses due to the lack of confirmed histology.
Statistical analysis
Mean ± SD or median (25th and 75th percentiles [quartile 1 to quartile 3]) for continuous variables and percentages for dichotomous variables were reported for baseline characteristics.
In our primary analysis, we used multivariable Cox proportional hazards regression models to evaluate the association of traditional CVD risk factors with incident cancer outcomes. Models were adjusted for baseline age, sex, HTN treatment, SBP, DM, BMI, total cholesterol/high-density lipoprotein ratio, smoking status, and statin use. Regression splines were used to evaluate the linearity assumption for the relationship between the continuous variables examined in the primary analysis and incident cancer (Supplemental Figure 1). Although the relationship of age with incident cancer statistically violated the linearity assumption, visual inspection of the regression spline demonstrated that the association was approximately linear.
Secondary models also included estimated glomerular filtration rate, interim CVD, interim MI, and interim HF separately. Interim events were treated as time-dependent covariates. Finally, in a sensitivity analysis, we restricted ascertainment of follow-up to events that occurred 1 year after baseline examination to minimize the effect of potentially undiagnosed cancers present at baseline.
We next examined the association of the 10-year ASCVD risk score with incident cancer and cancer outcomes using Cox models (14,18). We examined the association of 10-year ASCVD risk categories (low <5%, borderline 5% to 7.5%, intermediate 7.5% to 20%, and high >20%) with cancer outcomes. We further explored estimated 10-year ASCVD risks as continuous variables. To evaluate the relationship between CV risk and cancer development beyond age and sex, we removed age and sex from the ASCVD risk equation and re-derived the beta coefficients of the remaining risk score components based on CV events available in our dataset. We subsequently examined the re-derived risk score (without age and sex) in relation to incident cancer in the secondary analyses in univariable analyses, as well as in models, adjusting for age and sex as separate covariates.
We assessed the association of 2 established CVD biomarkers (NPs and hs-cTn), with cancer-related outcomes using multivariable Cox hazards models, adjusting for age, sex, HTN treatment, SBP, DM, BMI, total cholesterol/high-density lipoprotein ratio, smoking status, and statin use. Because of skewed distributions, biomarker concentrations were natural log-transformed. Because of interassay and cohort-specific differences, biomarker concentrations were standardized within each cohort to center mean values to zero and set each unit change to 1 SD before conducting pooled analyses (17). Associations were examined for continuous biomarkers and across sex-specific tertiles. Cox models for NPs (BNP/NT-proBNP) and hs-cTn violated the proportional hazards assumption. To account for non-proportionality of biomarker-related cancer risk, we added a biomarker × time interaction variable to the model.
Finally, we examined the association of the AHA Life’s Simple 7 CV health score with cancer-related outcomes using Cox proportional hazards models, adjusting for age and sex. Analyses were restricted to FHS participants because CV health metrics were not available for the PREVEND participants. Associations were examined for continuous scores and across CV health score groups: poor, average, and ideal.
For all survival analyses, patients were followed until time of event, death, or 15 years of follow-up, whichever occurred first. To account for competing risk of noncancer death, we compared cumulative incidence functions across strata of ASCVD risk and Life’s Simple 7 CV health score using Gray’s test in unadjusted analyses and performed cause-specific Cox regression models in adjusted analyses. For Cox regressions, we performed Schoenfeld residuals to confirm that the proportionality hazards assumption was met. In analyses that examined the association of CVD risk factors with incident cancer and incident cancer-specific subtypes, a Bonferroni-corrected p value threshold of p = 0.006 (0.05/9 risk factors) was deemed statistically significant. For other secondary analyses, a 2-sided p value <0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, North Carolina).
Results
A total of 20,305 participants were included for analysis, with mean age of 50 ± 14 years, 54% women, and an average BMI of 26.5 ± 4.8 kg/m2 (Table 1). More than one-half of participants were either former (33%) or present smokers (29%). Medical comorbidities included DM (4%), use of antihypertensive medications (15%), and hyperlipidemia that required statin therapy (4%), with similar distributions among FHS and PREVEND participants (Supplemental Table 2). The mean estimated 10-year ASCVD risk was 8.2%.
Table 1.
Baseline Demographic and Clinical Characteristics
| Total Cohort (N = 20,305) | Incident Cancer (n = 2,548) | No Cancer∗ (n = 17,757) | |
|---|---|---|---|
| Age, yrs | 50 ± 14 | 59 ± 12 | 49 ± 14 |
| Men | 9,426 (46) | 1,328 (52) | 8,098 (46) |
| SBP, mm Hg | 126 ± 19 | 132 ± 20 | 125 ± 19 |
| DBP, mm Hg | 75 ± 10 | 77 ± 10 | 75 ± 10 |
| HTN treatment | 3,097 (15) | 624 (25) | 2,473 (14) |
| BMI, kg/m2 | 26.5 ± 4.8 | 27.0 ± 4.7 | 26.4 ± 4.8 |
| DM | 839 (4) | 171 (7) | 668 (4) |
| Former smoker | 6,750 (33) | 1,035 (41) | 5,715 (32) |
| Current smoker | 5,822 (29) | 786 (31) | 5,036 (28) |
| Total cholesterol, mg/dl | 210 ± 42 | 215 ± 42 | 209 ± 42 |
| HDL, mg/dl | 52 ± 16 | 50 ± 16 | 52 ± 16 |
| Statin therapy | 820 (4) | 140 (6) | 680 (4) |
| eGFR, ml/min1/1.73 m2 | 85 ± 25 | 74 ± 21 | 87 ± 26 |
| 10-yr ASCVD risk, % | 8.2 ± 11.9 | 13.9 ± 13.5 | 7.4 ± 11.4 |
Values are mean ± SD or n (%).
ASCVD = atherosclerotic cardiovascular disease; BMI = body mass index; DBP = diastolic blood pressure; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; HDL = high-density lipoprotein; HTN = hypertension; SBP = systolic blood pressure.
No cancer group included participants who died, were lost to follow-up, or had not developed cancer by the end of follow-up.
A total of 2,548 incident cancer cases (fatal and nonfatal) occurred over a median follow-up of 15.0 years (quartile 1 to 3: 13.3 to 15.0 years) (Supplemental Table 3). The most common cancer subtypes included gastrointestinal (20%), lung (11%), prostate (16%), and breast (18%) cancers. Compared with participants who did not develop cancer, participants who developed cancer had higher rates of current and/or former smoking (cancer vs. no cancer: 72% vs. 61%), DM (7% vs. 4%), use of antihypertensive medications (25% vs. 14%), and hyperlipidemia that required statin therapy (6% vs. 4%). Estimated 10-year ASCVD risk was 13.9% in participants who developed cancer compared with 7.4% for participants who did not develop cancer. Over the observation period, a total of 1,454 incident CVD events occurred (687 MI events and 681 HF events) with similar proportions among those who developed cancer versus those who remained cancer free at last contact (Supplemental Table 4).
Association of standard CV risk factors with cancer outcomes
We evaluated the association of standard CVD risk factors with future cancer and cancer-related outcomes. In multivariable-adjusted Cox models, we observed that age, male sex, and smoking status were independently associated with future risk of cancer (Bonferroni-corrected p value threshold <0.006 for all) (Table 2; cohort specific analyses in Supplemental Table 5). Specifically, a 1-SD increase in age was associated with >2-fold increased hazard of incident cancer (hazard ratio [HR]: 2.12; 95% confidence interval [CI]: 2.00 to 2.26). By contrast, SBP, HTN treatment, DM, BMI, and statin use were not associated with future overall cancer risk (Bonferroni-corrected p value threshold >0.006 for all). Next, in secondary analyses, we examined the association of CVD risk factors with site-specific cancer and found that age and male sex were consistently associated with cancer subtypes (Table 3). In addition, DM and smoking status were associated with incident gastrointestinal cancers (Bonferroni-corrected p value threshold <0.006 for all), whereas smoking status and BMI were positively and negatively associated with incident lung cancer, respectively. Results were similar after also adjusting for the estimated glomerular filtration rate (Supplemental Table 6) and pack-years (Supplemental Table 7) in the secondary analyses.
Table 2.
Association of CV Risk Factors, Biomarkers, and CVD With Cancer
| Cancer (n/N = 2,548/20,305) |
||
|---|---|---|
| HR (95% CI) | p Value | |
| CV risk factors∗ | ||
| Age | 2.12 (2.00−2.26) | <0.001 |
| Male | 1.39 (1.28−1.51) | <0.001 |
| SBP | 0.99 (0.94−1.03) | 0.49 |
| HTN treatment | 1.10 (1.00−1.22) | 0.06 |
| BMI | 1.03 (0.99−1.08) | 0.20 |
| DM | 1.10 (0.94−1.30) | 0.24 |
| Former smoker | 1.30 (1.18−1.43) | <0.001 |
| Current smoker | 1.74 (1.57−1.93) | <0.001 |
| TC/HDL | 0.96 (0.91−1.00) | 0.048 |
| Statin use | 0.92 (0.77−1.10) | 0.36 |
| Risk scores† | ||
| 10-yr ASCVD risk | 1.16 (1.14−1.17) | <0.001 |
| Low | Ref. | |
| Borderline | 1.88 (1.63−2.18) | <0.001 |
| Intermediate | 2.70 (2.44−3.00) | <0.001 |
| High | 3.71 (3.29−4.19) | <0.001 |
| Biomarkers∗ | ||
| NP tertile 1 | Ref. | ptrend = 0.02 |
| Tertile 2 | 1.10 (0.90−1.34) | 0.35 |
| Tertile 3 | 1.40 (1.02−1.91) | 0.035 |
| NP × time interaction‡ | 0.87 (0.81−0.95) | 0.001 |
| Continuous | 1.26 (1.12−1.41) | <0.001 |
| hs-cTn tertile 1 | Ref. | ptrend = 0.47 |
| Tertile 2 | 1.24 (1.01−1.53) | 0.043 |
| Tertile 3 | 1.16 (0.84−1.61) | 0.37 |
| hs-cTn × time interaction‡ | 0.95 (0.88−1.03) | 0.18 |
| Continuous | 1.10 (0.99−1.21) | 0.07 |
| Previous events∗ | ||
| CVD, n = 1,020 | 0.96 (0.82−1.12) | 0.61 |
| MI, n = 793 | 1.03 (0.87−1.22) | 0.71 |
| HF, n = 116 | 0.66 (0.37−1.17) | 0.15 |
| Interim events∗ | ||
| CVD, n = 1454 | 0.99 (0.85−1.16) | 0.91 |
| MI, n = 687 | 0.99 (0.79−1.25) | 0.95 |
| HF, n = 681 | 1.07 (0.84−1.36) | 0.59 |
CI = confidence interval; CVD = cardiovascular; HF = heart failure; hs-cTn = high-sensitivity cardiac troponin; MI = myocardial infarction; NP = natriuretic peptide; other abbreviation as in Table 1.
Multivariable model adjusts for age, sex, SBP, HTN treatment, BMI, DM, total cholesterol/high-density lipoprotein (TC/HDL), statin use, smoking status. HR (hazards ratio) per dichotomous variable or 1 SD increase in a continuous variable.
Continuous 10-yr ASCVD risk: HR per 5% increase in estimated 10-yr risk. ASCVD categories: HR of ASCVD risk category compared with ASCVD low-risk category.
Time interaction variable is displayed to account for violation of the proportionality hazards assumption,
Table 3.
Association of Traditional CV Risk Factors With Incident Site Specific Cancer Subtypes
| GI Cancer (n/N = 514/20,305) |
Lung Cancer (n/N = 287/20,305) |
Prostate Cancer (n/N = 397/9,426) |
Breast Cancer (n/N = 449/10,879) |
|||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age | 2.48 (2.17−2.85) | <0.001 | 2.92 (2.41−3.52) | <0.001 | 2.77 (2.37−3.24) | <0.001 | 1.32 (1.14−1.53) | <0.001 |
| Male | 1.91 (1.58−2.30) | <0.001 | 1.70 (1.33−2.18) | <0.001 | — | — | — | — |
| SBP | 0.96 (0.87−1.05) | 0.36 | 1.02 (0.90−1.15) | 0.75 | 1.00 (0.90−1.12) | 0.94 | 1.06 (0.95−1.17) | 0.30 |
| HTN treatment | 1.11 (0.90−1.38) | 0.33 | 1.24 (0.93−1.65) | 0.14 | 1.01 (0.79−1.30) | 0.92 | 1.17 (0.91−1.51) | 0.21 |
| BMI | 1.14 (1.03−1.26) | 0.008 | 0.77 (0.67−0.90) | 0.001 | 1.02 (0.89−1.16) | 0.82 | 1.01 (0.99−1.03) | 0.43 |
| DM | 1.63 (1.20−2.21) | 0.002 | 0.97 (0.58−1.60) | 0.89 | 0.73 (0.48−1.12) | 0.15 | 0.88 (0.54−1.44) | 0.61 |
| Former smoker | 1.36 (1.09−1.69) | 0.006 | 3.90 (2.45−6.20) | <0.001 | 0.93 (0.73−1.17) | 0.52 | 1.08 (0.86−1.35) | 0.51 |
| Current smoker | 1.75 (1.37−2.22) | <0.001 | 13.0 (8.24−20.6) | <0.001 | 0.79 (0.59−1.06) | 0.12 | 1.32 (1.04−1.66) | 0.021 |
| TC/HDL | 0.86 (0.77−0.95) | 0.004 | 1.07 (0.99−1.16) | 0.08 | 0.97 (0.87−1.08) | 0.53 | 0.97 (0.86−1.10) | 0.62 |
| Statin use | 1.13 (0.78−1.63) | 0.51 | 0.99 (0.59−1.66) | 0.97 | 0.66 (0.41−1.08) | 0.10 | 0.79 (0.48−1.31) | 0.36 |
Sensitivity analyses restricting follow-up to events occurring >1 year after the baseline visit demonstrated consistent findings (Supplemental Table 8). Age, male sex, and baseline smoking status were significant predictors of future cancer, with only a slight attenuation of effect size (p < 0.005 for all).
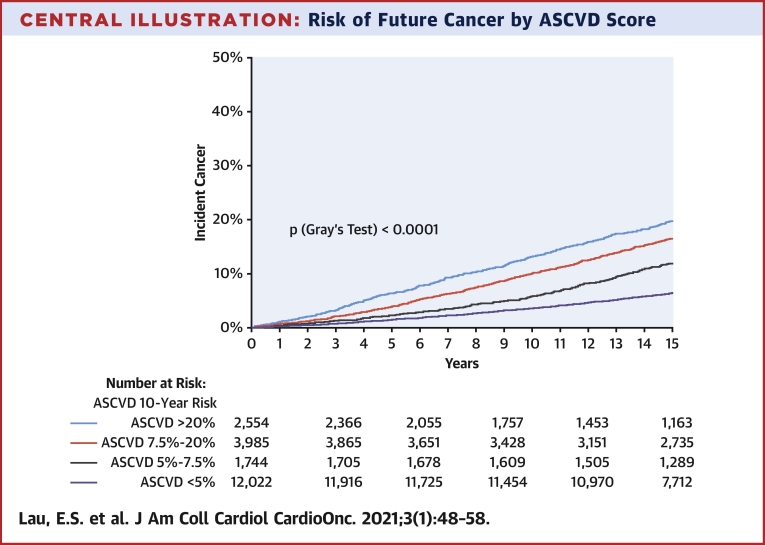
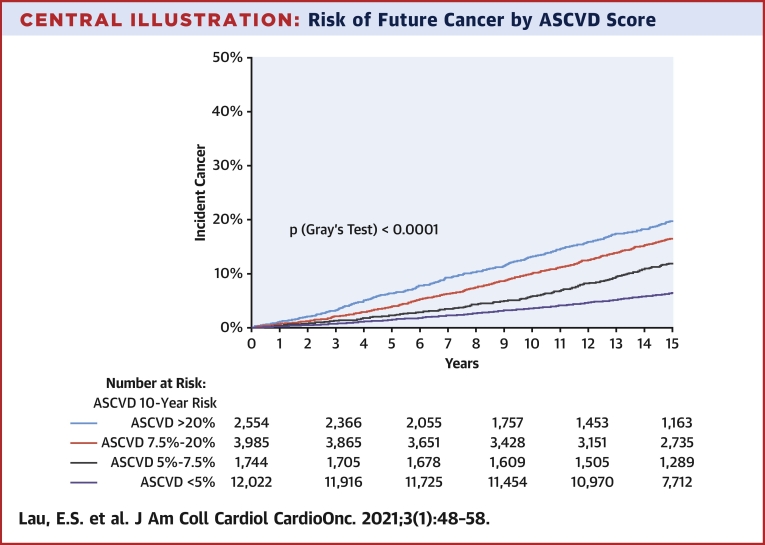
ASCVD risk score predicts cancer outcomes
To further capture the association of CV risk and future cancer, we examined the association of 10-year estimated ASCVD risk with cancer outcomes. Cumulative incidence plots showed greater risk of incident cancer among those with a greater estimated 10-year ASCVD risk (Central Illustration) (p log rank <0.0001). In Cox models, the 10-year ASCVD risk score was associated with incident cancer (ASCVD: HR per 5% increase in risk estimate: 1.16; 95% CI: 1.14 to 1.17; p < 0.001) (Table 2). Specifically, subjects with high (>20%) 10-year ASCVD risk had a 3.7-fold increased risk of future cancer compared with low-risk (<5%) subjects (HR: 3.71; 95% CI: 3.29 to 4.19; p < 0.001). To explore the relationship between CV risk and cancer development beyond age and sex, we removed age and sex from the ASCVD equation, re-derived the beta coefficients of the remaining risk score components, and evaluated the association of these remaining components with future cancer. Residual estimated 10-year ASCVD predicted future cancer in univariable analyses (HR: 1.31 per 1-SD increase in score; 95% CI: 1.27 to 1.35; p < 0.001) but not after adjustment for age and sex (HR: 1.03; 95% CI: 1.00 to 1.07; p = 0.10) (Supplemental Table 9). Finally, estimated 10-year ASCVD risk was also associated with greater risk of incident cancer by subtypes, including gastrointestinal, lung, prostate, breast, colorectal, hematologic, and bladder cancers (Supplemental Table 9 for full results).
Central Illustration.
Risk of Future Cancer by ASCVD Score
Incident cancer among subjects classified as atherosclerotic cardiovascular disease (ASCVD) low risk (<5%) (purple), borderline risk (5% to 7.5%) (gray), intermediate risk (7.5% to 20%) (red), and high risk (>20%) (blue) for developing cancer.
NPs, but not troponin, were associated with incident cancer
We evaluated the association of NPs and hs-cTn with future cancer risk. In multivariable models, NP was significantly associated with incident cancer, with a 26% increased hazard of future cancer per 1-SD increase in NP (HR: 1.26; 95% CI: 1.12 to 1.41; p < 0.001). Similarly, subjects in the highest NP tertile had a 1.4-fold increased risk of developing cancer compared with subjects in the lowest tertile (NP tertile 3 vs. tertile 1: HR: 1.40; 95% CI: 1.03 to 1.91; p = 0.034) (Table 2). However, the effect was attenuated over time (BNP × time interaction: HR: 0.87; 95% CI: 0.81 to 0.95; p = 0.001). When we examined BNP and NT-proBNP separately among FHS and PREVEND samples, respectively, the association with incident cancer was more robust for NT-proBNP compared with BNP, although results were qualitatively similar (Supplemental Table 5). In contrast to NP, there was no association of hs-cTn with future cancer in either continuous or tertile analyses (p > 0.05 for both). There were no significant associations between biomarkers and specific cancer subtypes (Supplemental Table 10).
Prevalent and interim CVD events do not predict subsequent cancer
We further examined the association of prevalent CVD and the development of interim CV events with subsequent cancer. We found that prevalent CV events, including MI, HF, and CVD, were not associated with future risk of cancer (p > 0.05 for all) (Table 2 and Supplemental Table 11). Similarly, the development of interim CV events did not increase risk of subsequent cancer (p > 0.05 for all) (Table 2). The median time between interim CV event and subsequent cancer diagnosis was 3.4 years (quartile 1 to 3: 1.3 to 6.1 years).
A heart healthy lifestyle is associated with lower risk of cancer
To investigate the impact of ideal CV health on cancer risk, we examined the association of ideal CV health as ascertained by the AHA Life’s Simple 7 CV health score with subsequent cancer. Optimal CV health was associated with a significantly decreased risk of subsequent cancer, with a 5% reduced hazard of future cancer per 1-U increase in CV health score (age- and sex-adjusted HR: 0.94; 95% CI: 0.90 to 0.99; p = 0.01). Compared with subjects with poor CV health, those with average and optimal CV health were less likely to develop incident cancer (p < 0.001) (Figure 1 and Supplemental Table 12).
Figure 1.
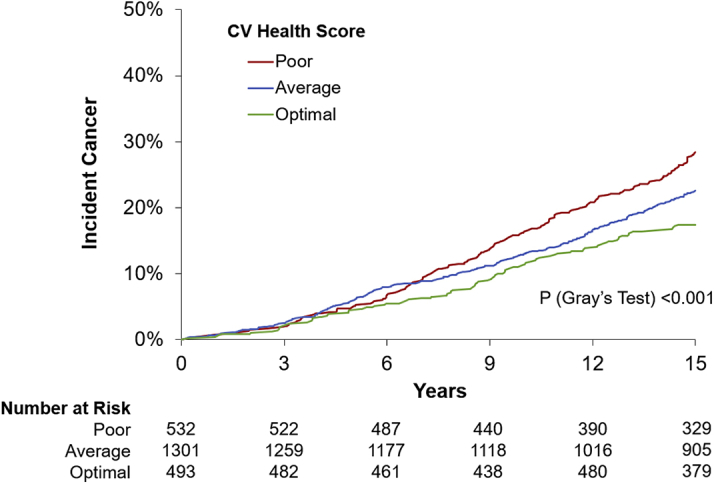
Time to Incident Cancer by Categories of the AHA’s Life Simple 7 CVH Score
The American Heart Association (AHA) Life Simple 7 cardiovascular health (CVH) score ranged from 0 to 14 and was classified into poor (0 to 6), average (7 to 9), and optimal (10 to 14) scores.
Discussion
Our study leveraged 2 longitudinal cohorts of subjects who were followed prospectively for incident histologically proven cancer outcomes. Within these inception cohorts, we showed that CV risk, as captured by traditional CV risk factors, CV risk scores, and biomarkers, was significantly associated with an increased risk of incident cancer. Specifically, traditional CV risk factors, including age, sex, and smoking status, were found to be independent predictors of future cancer risk, and estimated 10-year estimated ASCVD risk was associated with future cancer. Other risk factors, including DM and BMI, were variably associated with cancer subtypes but not overall incident cancer. Higher levels of NPs were associated with increased risk of subsequent cancer. Finally, although a history of CVD and the development of interim CV events were not associated with subsequent cancer development, ideal CV health, represented by the AHA Life’s Simple CV health score, was associated with decreased cancer risk. Together, these findings support the assertion that the association of CVD with future cancer was attributable to shared risk factors. By contrast, we did not find that either prevalent or incident CVD itself was associated with subsequent increased risk of future cancer, which was notable in light of previous studies that suggested otherwise. These data highlight the need for further studies to better understand underlying mechanisms linking CVD and cancer development.
Standard CV risk factors and incident cancer
We found that estimated 10-year ASCVD risk was associated with future cancer risk, with those at high CV risk having a >3-fold increased risk of cancer compared with low CV risk subjects. After removing age and sex, residual ASCVD risk remained associated with future cancer events but modestly. Our studies were in concert with existing studies that support shared risk factors between CVD and cancer (19,20).
When we examined the components of 10-year ASCVD risk most strongly associated with future cancer risk, we found age, sex, and smoking status to be the most strongly associated. These data underscore the importance of smoking cessation as a modifiable risk factor in the prevention of both CVD and cancer. Moreover, although neither BMI nor DM were associated with greater risk of future overall cancer in our cohort, we found associations with BMI and DM with increased risk of gastrointestinal cancers. Previous studies showed an excess of incident cancer among hospitalized subjects with obesity compared with their nonobese counterparts (21,22). Most studies focused on the association of obesity with cancer subtypes, which might explain the lack of association between BMI and overall cancer risk. Obesity and DM are modifiable cardiometabolic risk factors; their association with specific cancer subtypes offers further evidence supporting the importance of aggressive obesity and diabetes prevention and management. Finally, this observation suggests that risk factors for different cancer subtypes may be distinct and that future investigations of CVD and cancer examining specific cancer subtypes may identify clinically actionable risk factors for the prevention of CVD and cancer.
CV biomarkers and incident cancer
Our findings indicate that NPs, markers of cardiac stress, but not troponins, are associated with increased risk of cancer. NPs were previously associated with prevalent cancers. For example, in a study of patients with newly diagnosed cancer, CV biomarkers were sometimes elevated and had prognostic significance (23). Recently, in a study of 555 patients with a diagnosis of cancer not yet treated with chemotherapy, NT-proBNP and hsTnT levels were elevated in patients with cancer, particularly in those in an advanced tumor stage (24). Although troponins are often elevated in those with prevalent cancer, our data suggest that HF-related pathways rather than atherosclerosis might be the common link between CVD and incident cancer. Support for this hypothesis comes from a mechanistic study by Meijers et al. (25), which showed that HF in a murine model of pre-cancerous polyps led to marked progression of tumor growth in response to cardiac-derived secreted factors.
Prevalent and interim CV events do not predict future cancer
Although our findings support a link between traditional CV risk factors and BNP with future cancer, we did not find an association between prevalent or interim CVD with future cancer. Epidemiological data on cancer incidence among patients with CVD is conflicting (6, 7, 8, 9). A number of studies in HF samples showed greater incident cancer and cancer deaths (6,7). Investigators recently explored the role of CVD itself in promoting cancer and found that cardiac-derived secreted factors stimulated tumor growth in an animal model of pre-cancerous polyps, although investigations in humans demonstrated associations of NT-proBNP as a clinical surrogate of HF, but not HF itself, with incident cancer (25). As such, a recent analysis from the Physicians’ Health Study found no association between HF and incident cancer among male physicians (9). The association of atherosclerosis with incident cancer was also examined although the data were more limited. In a previous study of FHS participants, statin eligibility predicted incident cancer risk, although the coronary artery calcium score did not modify the association, which led the investigators to hypothesize that shared risk factors and not ASCVD underlay the association (26).
Adopting a heart healthy lifestyle may be associated with decreased cancer risk
Finally, we found that ideal CV health, as measured by the AHA Life’s Simple 7 CV health score, was associated with decreased risk of cancer. These findings add to the growing body of evidence that strongly supports the inverse relationship between ideal CV health and cancer risk, emphasizing the role of risk factor modification in the prevention of both CVD and cancer. Previous analyses examined the association of Life’s Simple 7 with future cancer, including an analysis of 13,253 participants in the ARIC (Atherosclerosis Risk In Communities) study, in which participants who met 6 to 7 of Life’s Simple 7 ideal CV health metrics had a 51% lower risk of incidence cancer compared with participants who met zero ideal CV health metrics (27). Similarly, in 6,506 men and women in the MESA (Multi-Ethnic Study of Atherosclerosis) cohort found that ideal CV health significantly reduced the burden of cancer and other chronic non-CV diseases (28). Both studies ascertained non-CVD outcomes, including cancer, using International Classification of Disease codes; we expand upon these previous investigations and demonstrate a significant association with AHA Life’s Simple 7 CV health score and a decreased risk of histology-proven cancer outcomes.
CVD and cancer may have potential shared mechanisms
Our study shows that in 2 cohorts with careful longitudinal ascertainment of new cancer cases, CV risk was associated with future risk of cancer, and ideal CV health was protective against incident cancer, whereas history of CVD and development of CV events in themselves did not appear to predict cancer risk. These findings provide support for the hypothesis that shared risk factors, rather than CVD itself, contributed to the association between CVD and cancer. For example, there has been growing interest in the role of inflammation, clonal hematopoiesis, and hyperinsulinemia in both CVD and cancer development. The CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcomes) trial demonstrated a significant reduction in CVD with anti−interleukin-1ß immunotherapy in a randomized trial (29), but the unexpected result was the concomitant reduction in lung cancer and lung cancer mortality (30). Clonal hematopoiesis of indeterminate potential, an expansion of somatic blood cell clones associated with increased risk of hematological cancers, was recently implicated in CVD development, as demonstrated by a near doubling of coronary heart disease risk among clonal hematopoiesis of indeterminate potential carriers (19,31,32). Separately, angiotensin II, a hormone involved in vasoconstriction and HTN, was implicated in the link between HTN and cancer via stimulation of plasma vascular endothelial growth factor (33). Vascular endothelial growth factor is central to the pathogenesis of tumor growth by stimulating blood vessel formation and is increased in hypertensive subjects (34). The relationship between DM and cancer risk highlights the role of insulin growth factor and hyperinsulinemia. Insulin growth factor is released in response to chronic hyperinsulinemia, and high serum levels of insulin growth factor have been associated with an increased risk of colorectal cancer, prostate cancer, and pre-menopausal breast cancer (35). Furthermore, increased sex hormone levels secondary to hyperinsulinemia have been implicated in post-menopausal breast and endometrial cancers (36) and may explain the heightened risk of future cancer in women with diabetes observed in recent studies (37).
Study limitations
First, the observational nature of the analysis might be subject to residual confounding, and causal inferences cannot be drawn. However, our results were strengthened by the large study sample size, the long duration of follow-up, and prospective ascertainment of both CV and cancer outcomes. Furthermore, ascertainment of cancer cases was based on adjudicated review of histology reports, making these 2 unique cohorts of new cancer cases. We could not exclude potential ascertainment bias, which might have led to a greater likelihood of cancer diagnoses among subjects with CVD. Although reverse causality was also a potential limitation, the longitudinal nature of our study significantly reduced its probability. Moreover, although both FHS and PREVEND trials had rigorous methods in place to systematically obtain the most complete medical records for participants, rare cancer outcomes might not have been adjudicated in this analysis if medical records were not available. Finally, the study sample was predominantly white and of European ancestry, limiting our generalizability to other ethnic populations.
Conclusions
We demonstrated that CV risks, as captured by standard CV risk factors including age, sex, and smoking status, 10-year ASCVD risk score, and NP, were associated with increased risk of future cancer. Although a history of CVD and development of CV events did not predict cancer risk, maintaining healthy life habits could protect against future cancer risk. Further studies are needed to elucidate the mechanisms underlying the association of CV risk factors with cancer.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: CVD risk as captured by traditional CVD risk factors, 10-year ASCVD risk score, and NPs, were associated with increased risk of future cancer. Healthy life habits, as measured by the AHA Life’s Simple 7 CV health score, were associated with lower risk of incident cancer.
TRANSLATIONAL OUTLOOK: Future studies are needed to examine the mechanisms underlying the association of CVD with cancer.
Funding Support and Author Disclosures
Dr. Lau was supported by a grant from the National Institutes of Health (NIH) (5T32HL094301-07). Dr. Ho was supported by grants from NIH (R01-HL134893, R01-HL140224, and K24-HL153669). The Framingham Heart Study was supported by contracts from the National Heart, Lung, and Blood Institute (NO1-HC-25195, HHSN268201500001I, and 75N92019D00031). Dr. Januzzi has been supported by the Hutter Family Professorship; has been a trustee of the American College of Cardiology; has received grant support from Novartis, Applied Therapeutics, and Abbott; has received consulting income from Abbott, Janssen, Novartis, Pfizer, Merck, and Roche Diagnostics; and has participated in clinical endpoint committees/data safety monitoring boards for Abbott, AbbVie, Amgen, Bayer, CVRx, Janssen, and Takeda. Dr. Vasan has been supported in part by the Evans Medical Foundation and the Jay and Louis Coffman Endowment from the Department of Medicine, Boston University School of Medicine. Dr. Ho has received research grants from Gilead Sciences and Bayer; and has received research supplies from EcoNugenics. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
Assays for hsTnI were provided by Singulex, Inc. Singulex, Inc. did not have access to study data and had no input into the data analyses, interpretation, or preparation of the manuscript for submission.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and a figure, please see the online version of this paper.
Appendix
References
- 1.Members W., Mozaffarian D., Benjamin E.J. Executive summary: heart disease and stroke statistics - 2016 update: a report from the American Heart Association. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 2.Duarte C.W., Lindner V., Francis S.A., Schoormans D. Visualization of cancer and cardiovascular disease co-occurrence with network methods. JCO Clin Cancer Inform. 2017;1:1–12. doi: 10.1200/CCI.16.00071. [DOI] [PubMed] [Google Scholar]
- 3.Gernaat S.A.M., Boer J.M.A., van den Bongard D.H.J. The risk of cardiovascular disease following breast cancer by Framingham risk score. Breast Cancer Res Treat. 2018;170:119–127. doi: 10.1007/s10549-018-4723-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett-Lee P.J., Dixon J.M., Farrell C. Expert opinion on the use of anthracyclines in patients with advanced breast cancer at cardiac risk. Ann Oncol. 2009;20:816–827. doi: 10.1093/annonc/mdn728. [DOI] [PubMed] [Google Scholar]
- 5.Chang H.-M., Moudgil R., Scarabelli T., Okwuosa T.M., Yeh E.T.H. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 1. J Am Coll Cardiol. 2017;70:2536–2551. doi: 10.1016/j.jacc.2017.09.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasin T., Gerber Y., McNallan S.M. Patients with heart failure have an increased risk of incident cancer. J Am Coll Cardiol. 2013;62:881–886. doi: 10.1016/j.jacc.2013.04.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasin T., Gerber Y., Weston S.A. Heart failure after myocardial infarction is associated with increased risk of cancer. J Am Coll Cardiol. 2016;68:265–271. doi: 10.1016/j.jacc.2016.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banke A., Schou M., Videbæk L. Incidence of cancer in patients with chronic heart failure: a long-term follow-up study. Eur J Heart Fail. 2016;18:260–266. doi: 10.1002/ejhf.472. [DOI] [PubMed] [Google Scholar]
- 9.Selvaraj S., Bhatt D.L., Claggett B. Lack of association between heart failure and incident cancer. J Am Coll Cardiol. 2018;71:1501–1510. doi: 10.1016/j.jacc.2018.01.069. [DOI] [PubMed] [Google Scholar]
- 10.Rasmussen-Torvik L.J., Shay C.M., Abramson J.G. Ideal cardiovascular health is inversely associated with incident cancer. Circulation. 2013;127:1270–1275. doi: 10.1161/CIRCULATIONAHA.112.001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cupples L.A., D'Agostino R.B., Kannel W.B., Wolf P., Garrison R.J. National Heart Lung and Blood Institute; Bethesda, MD: 1988. The Framingham Study, section 35: an epidemiological investigation of cardiovascular disease: survival following initial cardiovascular events: 30 year follow-up. NIH publication No. 88-2969. [Google Scholar]
- 12.Kannel W.B., Feinleib M., McNamara P.M., Garrison R.J., Castelli W.P. An investigation of coronary heart disease in families. The Framingham Offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 13.Hillege H.L., Fidler V., Diercks G.F. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 14.Andrus B., Lacaille D. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. J Am Coll Cardiol. 2014;63:2886. doi: 10.1016/j.jacc.2014.02.606. [DOI] [PubMed] [Google Scholar]
- 15.Wang T.J., Wollert K.C., Larson M.G. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012;126:1596–1604. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang T.J., Larson M.G., Levy D. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 17.de Boer R.A., Nayor M., deFilippi C.R. Association of cardiovascular biomarkers with incident heart failure with preserved and reduced ejection fraction. JAMA Cardiol. 2018;3:215–224. doi: 10.1001/jamacardio.2017.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Agostino R.B., Sr., Vasan R.S., Pencina M.J. General cardiovascular risk profile for use in primary care. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 19.Koene R.J., Prizment A.E., Blaes A., Konety S.H. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meijers W.C., de Boer R.A. Common risk factors for heart failure and cancer. Cardiovasc Res. 2019;115:844–853. doi: 10.1093/cvr/cvz035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolk A., Gridley G., Svensson M. A prospective study of obesity and cancer risk (Sweden) Cancer Causes Control. 2001;12:13–21. doi: 10.1023/a:1008995217664. [DOI] [PubMed] [Google Scholar]
- 22.Renehan A.G., Tyson M., Egger M., Heller R.F., Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 23.Narayan V., Thompson E.W., Demissei B., Ho J.E., Januzzi J.L., Ky B. Mechanistic biomarkers informative of both cancer and cardiovascular disease: JACC state-of-the art Review. J Am Coll Cardiol. 2020;75:2726–2737. doi: 10.1016/j.jacc.2020.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pavo N., Raderer M., Hülsmann M. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart. 2015;101:1874–1880. doi: 10.1136/heartjnl-2015-307848. [DOI] [PubMed] [Google Scholar]
- 25.Meijers W.C., Maglione M., Bakker S. Heart failure stimulates tumor growth by circulating factors. Circulation. 2018;138:678–691. doi: 10.1161/CIRCULATIONAHA.117.030816. [DOI] [PubMed] [Google Scholar]
- 26.Pursnani A., Massaro J.M., D'Agostino R.B., O'Donnell C.J., Hoffmann U. Guideline-based statin eligibility, cancer events, and noncardiovascular mortality in the Framingham Heart Study. J Clin Oncol. 2017;35:2927–2933. doi: 10.1200/JCO.2016.71.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rasmussen-Torvik L.J., Shay C.M., Abramson J.G. Ideal cardiovascular health is inversely associated with incident cancer: the Atherosclerosis Risk In Communities study. Circulation. 2013;127:1270–1275. doi: 10.1161/CIRCULATIONAHA.112.001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogunmoroti O., Allen N.B., Cushman M. Association between Life's Simple 7 and noncardiovascular disease: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ridker P.M., Everett B.M., Thuren T. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 30.Ridker P.M., MacFadyen J.G., Thuren T. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1833–1842. doi: 10.1016/S0140-6736(17)32247-X. [DOI] [PubMed] [Google Scholar]
- 31.Masoudkabir F., Sarrafzadegan N., Gotay C. Cardiovascular disease and cancer: evidence for shared disease pathways and pharmacologic prevention. Atherosclerosis. 2017;263:343–351. doi: 10.1016/j.atherosclerosis.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaiswal S., Natarajan P., Silver A.J. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang Y., Park Y., Kim B. Angiotensin II stimulates the synthesis of vascular endothelial growth factor through the p38 mitogen activated protein kinase pathway in cultured mouse podocytes. J Mol Endocrinol. 2006;36:377–388. doi: 10.1677/jme.1.02033. [DOI] [PubMed] [Google Scholar]
- 34.Felmeden D.C., Spencer C., Belgore F.M., Blann A.D., Beevers D.G., Lip G. Endothelial damage and angiogenesis in hypertensive patients: relationship to cardiovascular risk factors and risk factor management. Am J Hypertens. 2003;16:11–20. doi: 10.1016/s0895-7061(02)03149-7. [DOI] [PubMed] [Google Scholar]
- 35.Renehan A.G., Zwahlen M., Minder C., O'Dwyer S.T., Shalet S.M., Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 36.Kaaks R., Lukanova A., Kurzer M.S. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11:1531–1543. [PubMed] [Google Scholar]
- 37.Ohkuma T., Peters S.A.E., Woodward M. Sex differences in the association between diabetes and cancer: a systematic review and meta-analysis of 121 cohorts including 20 million individuals and one million events. Diabetologia. 2018;61:2140–2154. doi: 10.1007/s00125-018-4664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.