Abstract
With its unique system of intramural and extramural research programs, funding for academic and corporate product development, along with its supporting foundations, the National Institutes of Health (NIH) has created a vibrant public “innovation ecosystem” that has changed not only the face of healthcare, but has also led to the creation of the biotech industry in the U.S. Whether your interest in the overall healthcare environment is scientific, medical, educational or commercial, there is something here for you.
INTRODUCTION
The start of National Institutes of Health (NIH) and thus the origins of its “innovation ecosystem” begins in 1887, when a one-room laboratory was created within the Marine Hospital Service (MHS), predecessor agency to the U.S. Public Health Service (PHS). The MHS itself had been charged by Congress in the 1880s for examining passengers on arriving ships for clinical signs of infectious diseases, especially for the dreaded diseases cholera and yellow fever, in order to prevent epidemics. Joseph J. Kinyoun, a young MHS physician trained in the new bacteriological methods being reported in Europe, was chosen to set up a one-room laboratory in the Marine Hospital at Stapleton, Staten Island, New York (Photo 1). Dr. Kinyoun (in essence the first NIH Director), called this facility a “laboratory of hygiene” to indicate that the laboratory’s purpose was to serve the public’s health. Within only a few months, Kinyoun had identified the cholera bacillus in suspicious medical cases and used his Zeiss microscope to demonstrate it to his colleagues as confirmation of their clinical diagnoses. In stimulating and assisting other parties for the improvement of healthcare we see the very beginnings of this unique innovation ecosystem around NIH.
Photo 1:
Dr Joseph J.Kinyoun, NIH Founder
Besides being the founding NIH Director, Dr. Kinyoun also focused on what we could call today bioentrepreneurship and technology transfer. In working first as a federal employee and later in the private sector Kinyoun invented and patented multiple industrial disinfecting machines used in quarantine operations such as the “Kinyoun Portable Bed Disinfectors”. He also developed the first smallpox immune serum and his “Kinyoun Method” of smallpox vaccination used until the 1960s. The “Kinyoun Stain” that he discovered for TB is still in use today. Late in his career he even worked in pharma for a firm that became a predecessor to Merck.1 Clearly Kinyoun led by example in founding NIH not only as an institution but also as an innovation ecosystem.
NIH TODAY
Despite his own remarkable vision and activities, Dr. Kinyoun could hardly have imagined the size and scope of the NIH’s present programs and the supportive environment for biomedical research and product development that is fostered today. From its humble beginnings as a single laboratory, the NIH has evolved into a comprehensive program of 27 institutes and centers (ICs) that is both national and international in scope.
As a result of the numerous scientific opportunities and funding programs that make up today’s NIH, the environment that NIH fosters continues to foster even more significant contributions to human health, new medical products and economic development. The 1986 Federal Technology Transfer Act codified and fostered partnerships between NIH intramural research and private-sector development of new medical products.
Around 90 percent of NIH’s $41.7 billion FY 2020 final budget allocation went to more than 300,000 research personnel at over 2,500 universities, medical schools, companies and other research institutions in every state and throughout the world. The remaining 10% of this funding was spent on internal NIH R&D projects (intramural research) carried out by the approximately 6,000 scientists employed by the NIH. Dozens of NIH-supported scientists from around the world have received Nobel Prizes for their groundbreaking achievements in Physiology or Medicine; Chemistry; Physics; and Economic Sciences. To date, 163 NIH supported researchers have been sole or shared recipients of 96 Nobel Prizes. Included here are also individuals who have served as NIH staff scientists in the NIH Intramural Research Program. The 1980 Bayh-Dole Technology Act codified and fostered partnerships between NIH-funded extramural research and private-sector development of new medical products.2
As a continuous process, biomedical research and product development requires a supportive environment and an innovative ecosystem. For new research to truly yield new drugs, devices, and reagents, both public and private sector institutions need to use the ecosystem to refine and build upon basic knowledge to enable the development of even better products. Uniquely for NIH, it does not matter whether an idea originates in a supported university laboratory, its own intramural research program, or even in the private sector. Each new medical idea can be evaluated and supported based upon its own scientific and product merits, regardless of its origin. Collaborations, publications and research tool sharing also help ensure that important findings percolate through and invigorate the entire scientific community. For NIH’s innovation ecosystem, new findings serve as a building blocks for establishing a deeper understanding of human health and disease and can be supported through a wide variety funding, educational, training and developmental programs.
STRUCTURE OF THE NIH INNOVATION ECOSYSTEM
To truly function as the foundation of an ecosystem, an institution or organization must realistically be able to help stimulate and sustain two primary functions — for biomedicine this would be both new research as well as product development. Most biomedical products have some history of their research and development that can be traced back to basic research institutions with the original research often funded by NIH or other governmental programs. Licensing and technology transfer programs at these federal labs, or other non-profit basic research organizations, then provide a means for getting new inventions to the market for public use and benefit. From a research institution’s perspective, this portion of the innovation ecosystem is quite desirable since the public and commercial use of inventions typically come with new recognition of the value of basic research programs at the university or organization that originated it. These inventions also serve as helpful means to attract new R&D resources and partnerships within the ecosystem to these laboratories. Through licensing or other technology-transfer mechanisms, these institutions also receive a “return on investment” whether that is measured in terms of financial, educational or societal parameters, or some combination thereof.
NIH INNOVATION ECOSYSTEM KEYSTONE: BAYH-DOLE AND THE BIRTH OF TECHNOLOGY TRANSFER
Picking up from the momentum of the policies of Presidents John F. Kennedy and Richard Nixon, in 1980 Senators Birch Bayh and Robert Dole enacted legislation that gave universities, nonprofits, and small-businesses the right to own inventions made by their employees for federal government-funded research. The Bayh-Dole Act of 1980 (P.L. 96-517) reversed the presumption of title ownership by NIH in NIH grants and permitted a university, small business, or nonprofit institution to elect and pursue ownership of an invention in preference to the government. The underlying spirit of this important piece of legislation was to maximally utilize the outstanding research at these universities and other recipients for the good of the public who funded the research through their tax dollars and thus setting the stage for explosive growth of a new innovation ecosystem built around government biomedical funding agencies such as NIH.
The ownership right that universities and other funding recipients have to these inventions comes with obligations, but these obligations also stimulated activity in the ecosystem. The primary obligation for these institutions is to actively market and attempt to commercialize the invention, preferably through U.S.-based business enterprises (including start-ups) to benefit the public. Thus, was born the field of “technology transfer” and the establishment and growth of technology-transfer offices (TTOs) now found on every research campus. Prior to Bayh-Dole, 28,000 patents were owned by the U.S. government, less than 5 percent of which were commercialized. Since the enactment of Bayh-Dole, more than 6,500 new companies that were created are still operational, resulting in billions of dollars of direct economic impact within the United States and more than 800 new products put in the market during those years—all based upon NIH or other agency funded research.3
Similarly, in the 1980s, federal intramural laboratories, including NIH, were also given a statutory mandate under the Stevenson-Wydler Technology Innovation Act (P.L. 96-480), the Federal Technology Transfer Act (P.L. 99-502), and Executive Order 12591, to ensure that new technologies developed in federal laboratories were similarly transferred to the private sector and commercialized.
Within the innovation ecosystem, NIH and NIH-funded universities have developed a more strategic focus for their technology-transfer activities that is focused on working with entrepreneurs. Maximization of licensing revenue is not the goal of the NIH supported ecosystem. Instead, research organizations find themselves also looking for increasing product launches, company formation and new jobs creation based upon NIH-funded inventiveness, supporting faculty recruitment and retention, enhancing access to follow-on research funding, and in general creating an entrepreneurial culture that will help attract venture investment. The economic development aspects of research are being recognized as a fourth mission for such institutions—going along with education, research, and public service. Entrepreneurs play a key role in this “fourth mission” by establishing companies driven by new research discoveries and thus helping to build out the innovation ecosystem.
ACCESSING TECHNOLOGIES AND COLLABORATIONS IN THE NIH INNOVATIVE ECOSYSTEM
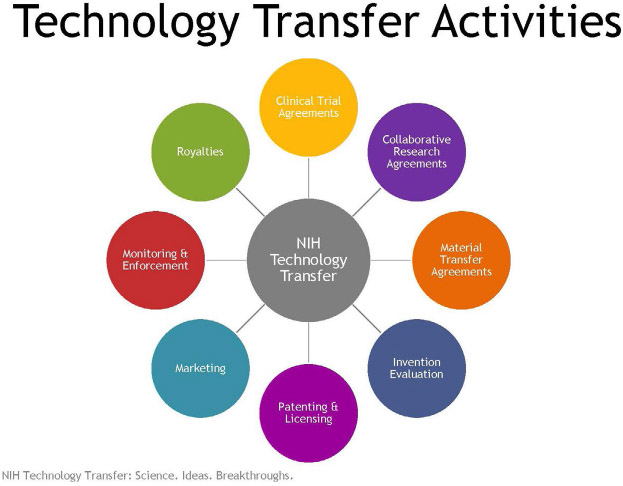
Generally, bioentrepreneurs can directly access NIH-supported research and inventions for product development from three main sources as shown in Table 1. For research funded by grants and contracts from NIH (extramural research), the individual university or small business would control commercial rights. Biomedical research conducted by NIH itself (intramural research program) is licensed directly through the individual IC technology transfer offices or their service centers at NIH.4 The full spectrum of NIH intramural technology transfer activities is shown in Table 2.
Table 1:
Sources for Accessing NIH-Funded Research In The Ecosystem
 |
Table 2:
Intramural NIH Technology Transfer Ecosystem Activities
 |
Both NIH and NIH-supported research institutions have a robust research program “pipeline” that provides novel, fundamental research discoveries available for commercial applications. NIH, for instance, as both a large-scale provider and consumer, represents a sort of “supermarket” of research products or tools for its commercial partners and suppliers. Additionally, overall product sales of all types by NIH licensees generally are around $6 billion annually. Most NIH intramural technology transfer activities date from the Federal Technology Transfer Act of 1986 which authorized formal research partnerships with industry and provided incentives for these NIH programs to license technology by allowing the federal laboratory to, for the first time, keep its license royalties and share them between the individual inventors and their laboratories or institutes.
Research collaborations or research assistance from NIH or NIH funded institutions can take several forms as these researchers and clinicians can work with industry under different collaborative modalities. For example, research institutions may seek to access technologies developed by industry—an imaging tool, a sequencing platform, or a drug discovered and in development by a company. The technology transfer office then works with companies and clinical partners to memorialize the understanding between the scientists and/or clinicians to allow the collaborations to happen. The key components of these collaboration agreement are terms related to inventions, rights to inventions, confidentiality versus publication, managing conflicts of interest, and finally, indemnification, especially for work involving patient care.
INDUSTRY COLLABORATIONS IN THE NIH INNOVATION ECOSYSTEM
There are several types of research or collaboration-related agreements that biotech companies will commonly encounter in working with NIH and NIH-funded institutions:
Confidential Disclosure/Nondisclosure Agreements (CDA/NDA):
Prior to engaging in any collaboration, each party may need to disclose to the other party some proprietary information that if passed on to third parties might be detrimental to the interest of the disclosing party. Such a discussion is a necessary first step to determine the interest in, and the breadth and scope of any potential collaboration. The parties will negotiate a CDA/NDA that ensures the information disclosed is held confidential, is only used for establishing the collaboration, stipulates a term of how long the information needs to be held confidential, and describes the consequences of nonadherence to the terms of the agreement.
Material Transfer Agreement (MTA), Sponsored Research Agreement (SRA), Research Collaboration Agreement (RCA), Clinical Trial Agreement (CTA) and Cooperative Research and Development Agreement (CRADA):
Companies, both small and large, typically need to invest a significant research and development funds toward developing drugs or other biomedical products. NIH and NIH-funded research institutions have several programs that are key towards understanding the fundamental biology underlying a wide variety of commercial products. When companies and research institutions seek to collaborate, they often will have very different focuses. A company often is hoping to learn more about their product concept, get mechanistic insights that can be used to position their product better in the marketplace, and have discoveries come out of this collaboration which may improve the usefulness and utility of their eventual product. In the case of collaborations with NIH supported clinical programs, it may also be possible to access to patient samples in addition to the valuable clinical insights the company hopes will guide them through clinical validation of their product whether it be a potential drug, medical device, or diagnostic. The NIH or university investigator are often interested to test various compounds from various companies to build a scientific insight or medical knowledge that will be publishable. It will also be possible under CRADAs or SRAs for the investigator to receive funding support from the company for basic or clinical research programs that may need it.
MTAs and SRAs are agreements that dictate the terms of the transfer of material and/or money from the company to the academic institution. Similarly, at NIH, joint projects with companies for basic research or clinical studies can be formalized as CRADAs or if there no IP options or funding provided then RCAs. Because of their clinical hospitals and centers as well as other networks and facilities, the NIH and at least some of its supported universities can also take some medical discoveries (or those of their partners) into clinical trials through CTAs. A case study about how the Foundation for the National Institutes of Health (FNIH)as an NIH-supporting foundation helps to “fill the collaboration gaps” in the NIH innovation ecosystem is given in Appendix A.
LICENSING TECHNOLOGIES FROM THE NIH INNOVATION ECOSYSTEM
Basic Licensing Principles of University and Federal Laboratories:
Compared to technology licensing with corporations, NIH and NIH-supported institutions bring a different focus and perspective to the table when negotiating technology transfer agreements. Because these agreements are used to further overall institutional missions, representatives from such nonprofit institutions consider the public consequences of such licenses as their priority, not the financial terms that may be involved. For example, NIH-funded nonprofit institutions, compared with their peers in industry, have the mandate to make new technology as broadly available as possible. This means that there is a strong preference to limit the scope of a license to only what is needed to develop specific products. Exclusive licenses are quite typical for biomedical products such as vaccines, therapeutics, and others where the underlying technologies require substantial private risk and investment (and a prior public notice and comment period in the Federal Register in the case of NIH laboratories). In their agreements, NIH laboratories and universities would also typically expect to retain the right to permit further research use of the technology whether to be conducted either in the NIH intramural program, universities, or companies. Because the commercial rights granted represent institutional (and public) assets, these agreements have enforceable performance benchmarks to ensure that the public will eventually receive the benefit (through commercialized products) of the research it funded. Regulations governing the license negotiation of federally-owned technologies and their mandated requirements are described in more detail at 37 Code of Federal Regulations (CFR), Part 404, while those for federally-funded technologies can be found at 37 CFR Part 401.
In a license agreement, the academic entity essentially grants rights to a company to make, use, and sell products that were it not for the license, would infringe on the patent rights that the academic center owns and/or controls. In some instances, the academic center also grants the company rights to use technological information/know-how or materials that goes together with the information in the patent application and that is valuable to the company as it hopes to commercialize the technology into products. Licensing is at the heart of operations of a technology transfer office since neither NIH, or NIH-funded universities, function as nonprofits, and do not, and cannot, have a product commercialization arm. NIH or NIH-funded universities may also not themselves convert inventions into commercial products and processes. They must partner with industry to do that as is also often the case with NIH-funded small businesses under the Small Business Innovation Research (SBIR) programs. Thus, these out-licensing activities are the key for research programs to fulfil the core of the Bayh-Dole Act and other federal mandates of commercializing inventions that arise from NIH funding.
Licensing from NIH & NIH-Funded Laboratories:
Commercializing technologies, such as vaccines or drugs, and then marketing them successfully in worldwide markets, cannot be the responsibility or mission of research institutions or government agencies. As is the case with its funded universities, the NIH is not able to commercialize its discoveries even with its considerable size and resources—it relies instead upon partners. Companies with access to the needed expertise financial resources are needed to undertake continued development of these inventions from the NIH or other research institutions into final products. Typically, a royalty-bearing license agreement with the right to sublicense is given to a company from NIH (if NIH-owned) or the university (if university-owned) to use patents, materials, or other assets to bring a therapeutic, vaccine, or other product concept to market. Exclusivity is almost always the norm for the U.S. Food and Drug Administration (FDA)-regulated products due to the risk involved in time, money, and regulatory pathways involved for companies and their investors. Financial terms of the license agreement are negotiable but do typically reflect the nascent, high-risk nature of the discovery. Because the technologies coming from NIH or NIH-funded research are most typically preclinical inventions, most licensees are early-stage companies or start-ups, rather than larger firms who typically want more proven ideas for new products. In addition to the license agreement, there will often be research collaborations between the licensee and the NIH or university to assist with additional work needed on the product technology. When the NIH licensee can sufficiently “de-risk” the technology through its various efforts, these companies then sublicense, partner, or get acquired by larger biotech or pharmaceutical firms for the final, most expensive stages of development with the large company expected to sell the product once it reaches the market.
Start-Ups as Licensing Vehicles in The NIH Innovation Ecosystem:
Since the 1980s, federally-funded health research institutions have developed an active but increasingly strategic focus on improving public health through technology-transfer activities. As such, they are particularly interested in working with start-ups and other early-stage companies in the healthcare area that are looking to develop and deliver innovative products. Rather than just seeking a financial return through revenue generation, these institutions are looking to utilize licensing of nascent inventions to increase new company formation, support faculty recruitment and retention, enhance research funding, and create in general a more entrepreneurial culture within the organization, attracting venture investment and development to their specific geographic region (universities) or to the health sector in general (NIH).
The licensing practices for most NIH-funded nonprofit research institutions have changed significantly over time with respect to biomedical inventions.5 With its ever-increasing consolidation, large pharmaceutical firms are typically no longer looking to directly license early-stage technologies for commercialization, whereas the number of licenses signed with start-ups as well as small – to medium-sized biotechnology companies is on the rise. Indeed, typically around 70 percent of the total licenses are executed with start-ups and small biotech firms. Unlike 20 or so years ago, when all or most of the important medical products based on licenses from university or federal laboratory research came from direct agreements with large pharmaceutical firms, most of the latest success stories tend to be from those originally partnered with biotech or other smaller companies at the time of the original license agreement. Some examples from the NIH licensing program are Kepivance® (a human growth factor used to treat oral sores arising from chemotherapy licensed to Amgen), Velcade® (a small molecule proteasome inhibitor used to treat multiple myeloma from Millennium), Synagis® (a recombinant monoclonal antibody for preventing serious lung disease caused by respiratory syncytial virus in premature infants from MedImmune), Prezista® (an HIV protease inhibitor used to treat drug-resistant AIDS patients from Tibotec) and Taxus Express® (a paclitaxel drug-eluting coronary stent used to prevent restenosis from Angiotech). Although these firms or their successors are all substantive, well-known companies now, at the time the underlying technology was licensed to them, they were not large corporations.
FUNDING IN THE NIH INNOVATION ECOSYSTEM
NIH is well known as the largest public funder of biomedical research in the world and invests more than $37 billion a year with outside institutions to enhance life and reduce illness and disability. This level of funding supports a strong research ecosystem that has led to breakthroughs and new treatments, helping people live longer, healthier lives, and building the research foundation that drives discovery. NIH offers funding for many types of grants, contracts, and even programs that help repay loans for researchers
While perhaps best known for grants to academic scientists, NIH also provides private sector entities with nondilutive funding through the SBIR (Small Business Innovation Research) and STTR (Small Business Technology Transfer Research) programs.6 The NIH SBIR program is perhaps the most valuable and stable funding source for new companies and unlike small business loans or convertible notes, SBIR grant funds do not need to be repaid.
Other noteworthy advantages of NIH SBIR programs for small companies include retention by the company of any intellectual property rights from the research funding, receipt of early-stage funding that doesn’t impact stock or shares in any way (e.g., no dilution of capital), national recognition for the firm, verification and visibility for the underlying technology and the generation of a leveraging tool that can attract other funding from venture capital or angel investors.
The SBIR program itself was established in 1982 by the Small Business Innovation Development Act to increase the participation of small, high technology firms in federal R&D activities. Under this program, departments and agencies with R&D budgets of $100 million or more are required to set aside 3.2 percent of their R&D budgets to sponsor research at small companies. The STTR program was established by the Small Business Technology Transfer Act of 1992 and requires federal agencies with extramural R&D budgets over $1 billion to administer STTR programs using an annual set-aside of 0.45 percent. In FY 2018 NIH’s combined SBIR and STTR grants totaled over $1.059 billion.7
The STTR and SBIR programs are similar in that both seek to increase small business participation and private-sector commercialization of technology developed through federal R&D. The SBIR program funds early-stage research and development at small businesses. The unique feature of the STTR program is the requirement for the small business applicant to formally collaborate with a research institution in Phase I and Phase II.
Thus, the SBIR and STTR programs at NIH differ in two major ways. First, under the SBIR program, the principal investigator must have their primary employment with the small business concern at the time of the award and for the duration of the project period. However, under the STTR program, primary employment is not so stipulated. Second, the STTR program requires research partners at universities and other nonprofit research institutions to have a formal collaborative relationship with the small business concern. At least 40 percent of the STTR research project is to be conducted by the small business concern and at least 30 percent of the effort is to be conducted by the single “partnering” research institution.
As a major mechanism at the NIH for achieving the goals of enhancing public health through the commercialization of new technology, the SBIR and STTR grants present an excellent funding source for start-up and other small biotechnology companies. The NIH SBIR and STTR programs themselves are structured in three primary phases: Phase I (feasibility), Phase II (development) and Phase III (commercialization).
In addition to receiving funding through the NIH SBIR and STTR programs, small companies may also be eligible for technical and management assistance programs designed to increase their chances for successful commercialization of the funded technology. These are a key part of the NIH innovation ecosystem and would include:
Niche Assessment Program –
For SBIR/STTR Phase I Awardees, this program is designed to help small businesses “jump start” their commercialization efforts by providing market insight and data that can be used to help such companies strategically position their technology in the marketplace. The results of this program can help small businesses develop their commercialization plans for their Phase II application and be exposed to potential commercial partners.
Innovation Corps (I-Corps) at NIH —
The I-Corps program provides funding, mentoring, and networking opportunities to help SBIR Phase I awardees commercialize promising biomedical technology. During this 8-week, hands-on program, companies learn how to focus their business plans and get the tools to bring their treatment to market. Program benefits include funding up to $55,000 to cover direct program costs; training from biotech sector experts; expanding professional networks; creating a comprehensive business model; and gaining entrepreneurial skills.
Commercialization Accelerator Program (CAP) –
NIH CAP is a nine-month program open to SBIR/STTR Phase II awardees that is well-regarded for its combination of deep domain expertise and access to industry connections, which have resulted in measurable gains and accomplishments by participating companies. Offered since 2004 to address the commercialization objectives of companies across the spectrum of experience and stage, 1000+ companies have participated in the CAP. The program enables participants to establish market and customer relevance, build commercial relationships, and focus on revenue opportunities available to them.
USING NIH BASIC AND CLINICAL RESEARCH ASSISTANCE TO DEVELOP THE INNOVATION ECOSYSTEM
Basic and clinical research assistance from the NIH institutes may also be available to companies or other partners through specialized services such as drug candidate compound screening and preclinical and clinical drug development and testing services, which are offered by several programs. These initiatives are particularly targeted towards developing and enhancing new clinical candidates in the disease or health area of focus at various NIH institutes. The largest and perhaps best-known programs of these types at the NIH are those currently run in the National Cancer Institute (NCI)8. The NCI has played an active role in the development of drugs for cancer treatment for over 50 years. This is reflected in the fact that approximately one half of the chemotherapeutic drugs currently used by oncologists for cancer treatments were in some form discovered and/or developed with NCI. The Developmental Therapeutics Program (DTP) promotes all aspects of drug discovery and development before testing in humans (preclinical development) and is a part of the Division of Cancer Treatment and Diagnosis (DCTD). NCI also funds an extensive clinical (human) trials network to ensure that promising agents are tested in humans. NCI’s Cancer Therapy Evaluation Program (CTEP), also a part of the DCTD, administers clinical drug development. Compounds can enter at any stage of the development process with either very little or extensive prior testing. Drugs developed through these programs include well-known products such as cisplatin, paclitaxel, and fludarabine.
Beginning in 2012 the NIH established a new center called the National Center for Advancing Translational Sciences (NCATS) that is designed to assist companies with the many costly, time-consuming bottlenecks that exist in translational product development.9 Working in partnership with both the public and private organizations, NCATS seeks to develop innovative ways to reduce, remove, or bypass such bottlenecks to speed the delivery of new drugs, diagnostics, and medical devices to patients. NCATS is not a drug development company but focuses more on using science to create powerful new tools and technologies that can be adopted widely by translational researchers in all sectors. NCATS-supported programs and projects have also produced numerous tools to help basic and clinical researchers advance translational science.
Programs of note for the NIH innovation ecosystem from NCATS include Bridging Interventional Development Gaps (BrIDGs) which enables research collaborations to advance candidate therapeutics for both common and rare diseases into clinical testing; Clinical and Translational Science Awards (CTSA) support a national network of medical research institutions that work together to improve the translational research process to get more treatments to more patients more quickly; and Therapeutics for Rare and Neglected Diseases (TRND) offers collaborative opportunities to access rare and neglected disease drug-development capabilities, expertise, and clinical/regulatory resources.
There is additional assistance available from other NIH institutes in a variety of disease areas including infectious diseases, drug abuse, and others—many more than can be highlighted here. All in all, such efforts can provide a wide variety of technical assistance (often at modest or no cost) for preclinical and even clinical development of novel therapies or other biomedical products by a variety of partners within the NIH innovation ecosystem.
CONTRACTING OPPORTUNITIES WITH NIH AND NIH-FUNDED INSTITUTIONS
One of the most overlooked opportunities by biomedical-focused companies is the ability to sell products and services to the NIH and NIH-funded centers. Indeed, for start-up companies looking to develop new products used in conducting basic or clinical research, the NIH may be their first customer. With an intramural staff of about 18,000 employees, laboratories in several regions of the country (with the Bethesda campus in Maryland home to the majority), and an annual intramural budget of about $4 billion, the NIH is perhaps the largest individual institutional consumer of bioscience research reagents and instruments in the world. A variety of mechanisms for selling products and services to the NIH are possible, including stocking in government storerooms and general contracting opportunities. Companies that provide products and services to NIH laboratories and programs can not only generate cash flow and revenues to fuel their own R&D, but also begin to demonstrate their commercial acumen to would-be partners and investors. Being a large research organization, the NIH has numerous R&D contracting opportunities. Specific information on such opportunities can be found by visiting the NIH Office of Acquisition Management and Policy website.10
The annual NIH Research Festival is also an excellent starting point for companies hoping to sell products to the NIH11. This event is held at the Bethesda, Maryland campus and the Frederick, Maryland campus. Part scientific, part social, part informational, and part inspirational, this event draws a variety of small – to medium-sized bioscience firms to exhibit their product and services available to NIH.
TRAINING AND EDUCATION IN THE NIH INNOVATION ECOSYSTEM
In addition to traditional scientific training supported at all educational levels, NIH and NIH-funded universities have set up or have access to educational programs that train scientists and engineers to have a greater appreciation as to the importance of commercialization. These programs are often funded and supported at NIH institute training offices. In addition, the NIH Office of Intramural Training and Education (OITE) provides resources and information to enhance the educational experience of NIH trainees and can assist with finding appropriate workshops, arranging individual career counseling and identifying other NIH resources to meet trainee needs. OITE resources are also available for trainees in the extramural NIH community. Other options for education and training include entrepreneurship centers and small business assistance programs at many universities and such things as the “Advanced Studies in Technology Transfer” program given at the Foundation for Advanced Education in the Sciences (FAES) Graduate School at NIH.12 A case study on how FAES as an NIH-supporting foundation helps to “fill the educational gaps” in the NIH innovation ecosystem is given in Appendix B.
NIH INNOVATION ECOSYSTEM HAS SPURRED BIOTECHNOLOGY INDUSTRY GROWTH
As previously noted, the economic development potential of biomedical research is being recognized as a fourth mission for research institutions such as the NIH —going along with education, research, and public service. Thus, it is in this “fourth mission” that bioentrepreneurs and NIH find themselves again sharing the common goal of having new companies established based upon developing innovative research discoveries.
The economic importance of licensing and technology transfer has become better recognized in recent years and some of the figures can be quite striking. For example, the overall product sales of all types by licensees of NIH intramural research reported by the NIH Office of Technology Transfer as being around $6 billion annually, the equivalent of mid-tier Fortune 500 companies. Economic development also was the focus of the October 28, 2011 U.S. Presidential Memorandum entitled “Accelerating Technology Transfer and Commercialization of Federal Research in Support of High-Growth Businesses” .13 This directive from the White House recognized the economic aspects of innovation and technology transfer for federal research in the way it fuels economic growth as well as creating new industries, companies, jobs, products and services, and improving the global competitiveness of U.S. industries. The directive requires federal laboratories such as the NIH to support high-growth entrepreneurship by increasing the rate of technology transfer and the economic and societal impact from federal R&D investments. During this period, federal laboratories such as the NIH will be establishing goals and measuring progress towards commercialization, streamlining the technology transfer and commercialization processes, especially for licensing, collaborations, and grants to small companies, and also facilitating the commercialization of new technology and the formation of new start-up firms through local and regional economic development partnerships.
Looking at the university and academic medical center figures reported by the Association of University Technology Managers (AUTM), we find there are similar economic indications for the impact of technology transfer and the initial funding of research from NIH and other federal programs.14 In 2018 AUTM reported 9,350 new license agreements and new research expenditures of 71.7 billion by reporting universities. In 2018, more than 6,518 start-ups were also still operational from prior years. By the end of 2018, 828 new products had been introduced into the marketplace.
NIH INNOVATION ECOSYSTEM: RESULTS TO DATE
With their leading-edge research programs and focus in the healthcare market, NIH and NIH-funded research programs have an exemplary record in providing opportunities for bioentrepreneurs to develop both high-growth companies and high-growth medical products. Indeed, a preliminary study from 2007 has shown that more than 100 drug and vaccine products approved by the U.S. FDA were based at least in part on technologies directly licensed from university and federal laboratories with federal labs (NIH) providing nearly 20 percent of the total15. Further, another study from 2009 has shown that university-licensed products commercialized by industry created at least 279,000 jobs across the United States during a 12-year period and that there was an increasing share of the United States GDP each year attributable to university-licensed products16. Additionally, a study published in the New England Journal of Medicine17 in 2011, based upon the earlier 2007 preliminary study, showed the intramural research laboratories at the NIH as by far the largest single nonprofit source of new drugs and vaccines approved by the FDA. Finally, a 2017 study from the National Cancer Institute SBIR Development Center showed that out of 690 awards, 368 (53%) had already resulted in sales. Total cumulative sales were $9.1 billion, which equates to average sales of approximately $24.8 million for each of the 368 awards.18
These sales indicate that the impact of the NIH innovation ecosystem is strong and will be increasingly effective and important into the future. Although new knowledge and product development has been a model in showing the value of the NIH innovation ecosystem from NIH and NIH-funded institutions, it is not the entire story. The final tally must include not only the full societal value and economic impact both of new companies, but also more importantly as well as the life-saving or enhancing therapeutics, vaccines, diagnostics, and other biomedical products on the market that have origins in this federally-funded research. This is believed to be the truest measure of an innovation ecosystem as well demonstrating the value and importance of having the growth of the intramural and extramural programs of the NIH since its humble origins in 1887.
CONCLUSIONS
In conclusion, there are also NIH-related programs intended to accelerate and support collaborations intended to foster entrepreneurship to support the commercialization of the inventions and discoveries that come from its laboratories, much like most innovative universities have done as well (c.f. articles included elsewhere in this Special Edition, authored by Moira Gunn at University of San Francisco, and Paul Roben and Dennis Abremski at the University of California, San Diego). We illustrate two significant NIH-related programs that are described in two concluding Sidebars: the Foundation for the National Institutes of Health, and the Foundation for Advanced Education in the Sciences.
HELPING NIH FOSTER A SYSTEM OF COLLABORATIONS: APPENDIX A
Foundation for the National Institutes of Health (FNIH)
The Foundation for the National Institutes of Health (FNIH) is a 501(c) (3) charitable organization chartered by Congress in 1996 that procures funding and manages alliances with public and private institutions in support of NIH’s mission.19 The FNIH is legally chartered to accept donations from alumni inventors and scientists, philanthropists, and high-wealth individuals to support activities designed to accelerate biomedical research and strategies to fight against diseases in the United States and across the world. FNIH organizes and administers research projects; supports education and training of new researchers; organizes educational events and symposia; and administers a series of funds supporting a wide range of health issues.
Since its founding, it has raised over $1 billion which it has used to support over 600 research programs. FNIH specializes in building public-private partnerships between government, academic, industry, nonprofit, and patient-group researchers in order to conduct research into specific disease states and research areas. Because partnerships have become increasingly important in life-sciences innovation, FNIH is an important convener and facilitator in the NIH innovation ecosystem.
HELPING NIH FOSTER A SYSTEM OF ENTREPRENEURSHIP: APPENDIX B
Foundation for Advanced Education in the Sciences (FAES) at NIH
The Foundation for Advanced Education in the Sciences at the NIH (FAES@NIH) has fostered an environment of learning in the sciences since it was established in 1959.20 The biomedical science focus has expanded to include many courses and programs intended to support the commercialization of the many biomedical innovations being created every day at the NIH. These courses and workshops include areas such as management, valuation of innovation, technology transfer and marketing of biomedical technologies.
The history of the FAES started with 11 NIH scientists seeking to create a more university-like environment for the NIH researchers. Since its beginning, FAES has offered graduate level courses and workshops to thousands of NIH researchers. This continues today, and the educational programming remains open to the general public as well as the NIH. In 2020, FAES registered almost 3,000 students in its nearly 200 courses and workshops.
The programming at FAES is kept affordable because its mission is to offer programming that is accessible by the NIH scientists at all levels. The educational programs are focused on topics that the NIH staff and researchers find relevant. In addition to hard science, one of the key areas is the Department of Technology Transfer, Business and Industry. Students can sign up for a broad selection of core courses including project management, regulatory science, intellectual property, and even courses in how to build a biotech company. FAES Academic Programs has also developed its unique “Advanced Studies Certificate in Technology Transfer” to serve the needs of scientists and engineers who want expertise in patenting, licensing, collaborative agreements, and other fundamental intellectual property transactions. This program culminates in an independent capstone project through which students demonstrate their knowledge of the theory and practice of technology transfer by completing a project of their own design at the NIH, or in their regional community.
FAES also partners with many NIH institutes to offer customized programming to help each institute meet their specific mission. One example is a partnership with the National Center for Advancing Translational Sciences. NCATS underwrites the cost of a course in bench to bedside cancer treatments so students only pay a very modest $60 total tuition. FAES@NIH also partners with several universities so that the courses often transfer and count towards a master’s degree. For instance, students interested in data science and bioinformatics may take 15 of the total thirty credits from FAES toward the University of Maryland Baltimore County Master of Professional Science in Data Science.
For decades at FAES, the philosophy has been to ‘do for the NIH what the NIH couldn’t or can’t do for itself.’ The current course offerings have all come together to create and support the ecosystem at NIH – one that fosters a culture of community and support to researchers. Beyond just education, though, the FAES has developed services that have grown to include a bookstore, coffee shops, and a social and academic center that houses classrooms and entertainment space. FAES even sponsors a music program for the NIH clinical center that features world-recognized musicians, such as the National Symphony Orchestra. Besides educational programming, FAES also offers support services such as health insurance to almost 4,000 NIH fellows, who otherwise would not have access to affordable health insurance.
When the founders of FAES@NIH created the organization, they could not have imagined the long-lasting impact it would have. Yet, 61 years later, FAES@NIH supports so many areas within the NIH community, including support for an entrepreneurial ecosystem that allows researchers to expand their research beyond the lab by supporting the transfer of their discoveries from the lab to the patients.
Contributor Information
Steven M. Ferguson, Office of Technology Transfer, National Institutes of Health, Department of Health and Human Services.
Lynn Johnson Langer, Academic Programs, Foundation for Advanced Education in the Sciences (FAES) at the National Institutes of Health.
References
- 1.Morens DM and Fauci AS, mBio. 2012. Jul-Aug; 3(4): e00139–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.https://www.nih.gov/about-nih/what-we-do (accessed October 25, 2020).
- 3.https://autm.net/AUTM/media/Surveys-Tools/Documents/AUTM_FY2018_Infographic.pdf (accessed October 25, 2020)
- 4.https://www.ott.nih.gov/tdcs (Accessed October 25, 2020).
- 5.Ben-Menachem G, Ferguson S, Balakrishnan K, Doing Business With NIH, Nature Biotechnology 2006: 24(1):17–20. [PMC free article] [PubMed] [Google Scholar]
- 6.https://sbir.nih.gov/ (Accessed October 25, 2020).
- 7.https://report.nih.gov/nihdatabook/category/8 (Accessed October 25, 2020).
- 8.https://dtp.cancer.gov/ and https://ctep.cancer.gov/ (Accessed October 25, 2020).
- 9.https://ncats.nih.gov/ (Accessed October 25, 2020).
- 10.https://oamp.od.nih.gov/ (Accessed October 25, 2020).
- 11.https://researchfestival.nih.gov/2019 and http://www.technicalsalesassociation.org/site/ (Accessed October 25, 2020).
- 12.https://faes.org/content/advanced-studies-in-technology-transfer (Accessed October 25, 2020).
- 13.https://federallabs.org/about/history (Accessed October 25, 2020).
- 14.https://autm.net/AUTM/media/SurveyReportsPDF/AUTM_FY2018_US_Licensing_Survey.pdf (Accessed October 25, 2020).
- 15.Jensen J, Wyler K, London E, Chatterjee S, Murray F, Rohrbaugh M, The Contribution of Public Sector Research to the Discovery of New Drugs. Personal communication of poster at 2007 AUTM Annual Meeting. 2007. [Google Scholar]
- 16.https://www.bio.org/sites/default/files/legacy/bioorg/docs/files/BIO_final_report_9_3_09_rev_2_0.pdf (Accessed October 25, 2020).
- 17.Stevens A, Jensen JJ, Wyller K, Kilgore P, Chatterjee S, Rohrbaugh M, “The Role of Public-Sector Research in the Discovery of Drugs and Vaccines. New England Journal of Medicine (2011) 364 535–541. [DOI] [PubMed] [Google Scholar]
- 18.https://sbir.cancer.gov/impact (Accessed October 25, 2020).
- 19.https://fnih.org/about and https://itif.org/publications/2019/03/04/bayh-dole-acts-vital-importance-us-life-sciences-innovation-system (Accessed October 25, 2020).
- 20.https://faes.org/ (Accessed October 25, 2020).



