Abbreviations
- AUC
area under the curve
- CAT
catalase
- CYBA
cytochrome b‐245 alpha polypeptide
- GEO
Gene Expression Omnibus
- GPX3
glutathione peroxidase 3
- HMOX1
heme oxygenase 1
- IHC
immunohistochemical
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- KIRC
kidney renal clear cell carcinoma
- NOS1
nitric oxide synthase 1
- NQO
NADPH dehydrogenase quinone
- PI3K
phosphoinositide 3‐Kinase
- ROC
receiver operating characteristic
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TCGA
The Cancer Genome Atlas
- VHL/HIF
von Hippel‐Lindau tumor suppressor/hypoxia‐inducible factor
Dear editor,
Oxidative stress induced by reactive oxygen species (ROS) production plays a key role in tumor initiation and progression [1, 2]. Emerging evidence has shown that oxidative stress‐related genes could be used as biomarkers for predicting the progression and prognosis of malignant tumors [3]. Our previous study showed that the expression and genotypes of oxidative stress‐related genes could be used for predicting the chemosensitivity and clinical outcomes of biliary tract cancer patients [4]. However, the associations between oxidative stress‐related genes and kidney renal clear cell carcinoma (KIRC) remain undefined. In this study, we characterized oxidative stress‐related genes’ expression in KIRC by systematically analyzing data from 8 independent KIRC databases, namely 4 from the Gene Expression Omnibus (GEO; n = 130, https://www.ncbi.nlm.nih.gov/geo), 1 from The Cancer Genome Atlas (TCGA; n = 535, http://gdac.broadinstitute.org), and 3 from our KIRC verification cohorts (cohort 1 from Ruijin hospital, n = 48; cohort 2 from OutdoBiotech, n = 150; cohort 3 from Shanghai Ninth People's hospital, n = 306; Supplementary Materials and Methods; Supplementary Table S1). Then, we developed an individualized oxidative stress‐related scoring system for predicting the prognosis of KIRC patients.
The expression of 21 oxidative stress‐related genes, all of which are key enzymes or regulators in controlling ROS production and oxidative stress response (Supplementary Figure S1), was firstly examined in the 4 GEO databases (GSE16449, GSE36895, GSE16441, and GSE71963). As compared with normal kidney tissues, catalase (CAT), glutathione peroxidase 3 (GPX3), nitric oxide synthase 1 (NOS1), NADPH dehydrogenase quinone (NQO) 1 (NQO1), NQO2, superoxide dismutase (SOD) 1 (SOD1), and SOD3 were significantly decreased in KIRC tissues, cytochrome b‐245 alpha polypeptide (CYBA) and heme oxygenase 1 (HMOX1) were instead obviously increased (Supplementary Figure S2A, B). These differentially expressed genes were then validated in the TCGA‐KIRC cohort and our KIRC verification cohort 1, which contained 48 paired KIRC and normal kidney tissues. Interestingly, besides NQO1 and NQO2, the same changes were found with the other 7 oxidative stress‐related genes (CAT, CYBA, GPX3, HMOX1, NOS1, SOD1, and SOD3) (Supplementary Figure S2C, D). Moreover, the transcripts of the 7 oxidative stress‐related genes were closely correlated in KIRC samples (Supplementary Figure S3A). Protein‐protein interactions were identified by using the online STRING and HIGHCHARTS databases, and we also found that the 7 oxidative stress‐related genes could interact with each other at the protein level (Supplementary Figure S3B). Finally, the receiver operating characteristic curve (ROC) analysis was performed in our verification cohort 1 to evaluate the possibilities of these 7 genes as biomarkers for KIRC. As shown in Supplementary Figure S4, all these 7 genes achieved an area under the curve (AUC) value > 0.80, demonstrating their high sensitivity for screening KIRC. The above results suggested that oxidative stress‐related genes could be useful biomarkers for the prognostication of KIRC.
To determine the possible implications of the 7 differentially expressed oxidative stress‐related genes for predicting KIRC patients’ prognosis, Kaplan‐Meier survival analyses were performed using the TCGA database. The results showed that the transcripts of CAT, NOS1, and CYBA were significantly associated with the clinical outcomes of KIRC patients (Supplementary Figure S5A‐G). Then, two other KIRC verification cohorts comprising of 456 KIRC samples (cohort 2, n = 150; cohort 3, n = 306) were collected. They were classified into low and high expression groups according to CAT, NOS1, or CYBA protein expression levels, as measured by tissue microarray immunohistochemical (IHC) staining (Supplementary Figure S6A). Consistently, these two other KIRC verification cohorts confirmed the correlations between the expression of the 3 oxidative stress‐related genes and the patients’ survival rate (Supplementary Figure S6B‐D).
To further evaluate the combined effect of CAT, NOS1, and CYBA in predicting KIRC prognosis, a previously described oxidative stress scoring method [4] was used. Specifically, risk factors for KIRC prognosis (low CAT, low NOS1, or high CYBA expression) were assigned “0”, and protective factors for KIRC prognosis (high CAT, high NOS1, or low CYBA expression) were assigned “1” in this study. Thus, each KIRC sample was given an oxidative stress score ranging from 0 to 3, and the patients were divided into 2 groups according to their scores (low: 0‐1, high: 2‐3; Supplementary Figure S6A). In both the TCGA‐KIRC and our verification KIRC cohorts, the overall survival rates were significantly improved in the high oxidative stress score group as compared with the low oxidative stress score group (Supplementary Figure S5H and S6E). These results implied that the clustering of oxidative stress‐related genes could be a suitable prognosis‐stratification method for KIRC patients. Multivariate Cox proportion hazard model revealed that age, pathologic stage, distant metastasis, and pTNM stage were independent risk factors, whereas a high oxidative stress score was an independent protective factor for KIRC patients (Supplementary Table S2). These findings suggested that the oxidative stress score signature was associated with KIRC prognosis.
We further evaluated the correlations between oxidative stress score and pathological indicators utilizing the clinical data from the combined KIRC verification cohort 2 and cohort 3. Intriguingly, univariate analysis showed that patients with low oxidative stress score were consistently prone to have advanced pathologic stage, pT classification, pN stage, and pTNM stage, while multivariate logistic regression analysis showed that oxidative stress score was an independent predictor of lymph node metastasis (Figure 1A, B, and Supplementary Table S3). This result was validated in the KIRC verification cohort 1 (Figure 1C). To find out the underlying mechanisms between oxidative stress‐related genes’ expression and lymph node metastasis, the oxidative stress score‐related genes were assessed from the 4 GEO‐KIRC and TCGA‐KIRC databases. Hallmark Gene Sets and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis using Metascape software [5] converged the commonly altered genes into 3 gene sets including regulation of cell focal adhesion, Rap1, and phosphoinositide 3‐Kinase (PI3K)‐Akt signaling pathways (Figure 1D‐F). Protein‐protein interaction analysis of the oxidative stress‐related genes also showed enrichment in the cell focal adhesion, Rap1, and PI3K‐Akt signaling pathways (Figure 1G). PI3K‐Akt signaling is the conductor of Rap1 and directly suppresses cell adhesion and epithelial‐mesenchymal transition, which could ultimately promote the metastasis of tumor cells [6, 7]. In KIRC, the PI3K‐Akt pathway was found related to the von Hippel‐Lindau tumor suppressor/hypoxia‐inducible factor (VHL/HIF) pathway in contributing to cell proliferation, migration, and invasion [8]. Thus, oxidative stress‐related genes might regulate the lymph node metastasis of KIRC through Rap1‐PI3K‐Akt signaling and cancer cell focal adhesion. More studies are needed to verify the mechanism involved.
FIGURE 1.
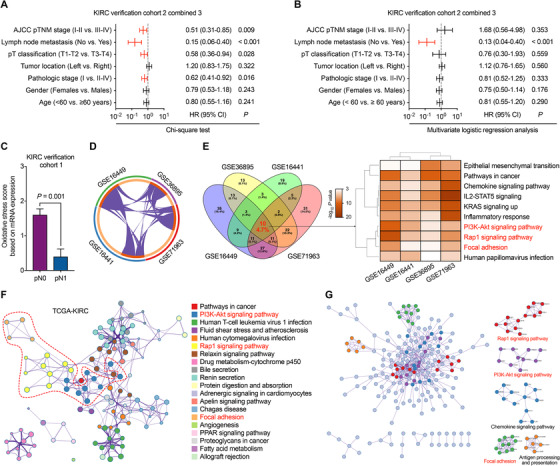
Correlation between oxidative stress score and KIRC patients’ clinicopathological features. A‐B, Univariate and multivariate analyses between clinicopathological characteristics and oxidative stress score in verification KIRC cohort 2 combined 3. C, oxidative stress score calculated in KIRC verification cohort 1 grouped by pN stage. Bar, SEM; Student's t‐test. D, Analysis of genes associated with oxidative stress score in 4 GEO‐KIRC databases. E, Pathway and process enrichment analyses of oxidative stress score‐related genes in 4 GEO‐KIRC databases. Terms with a P < 0.05, a minimum count of 3, and an enrichment factor > 1.5 are collected and grouped into clusters based on their membership similarities. F, Top 20 network of enriched terms associated with oxidative stress score in the TCGA‐KIRC database. Terms with a similarity > 0.3 are connected by edges. G, Protein‐protein interaction enrichment analysis of oxidative stress score‐related genes in TCGA‐KIRC database.
Abbreviations: KIRC, kidney renal clear cell carcinoma; AJCC, American Joint Committee on Cancer; HR, hazard ratio; CI, confidence interval; IL2‐STAT5, interleukin 2‐signal transducer and activator of transcription 5; KRAS, Kirsten rat sarcoma 2 viral oncogene homolog; PI3K, phosphoinositide 3‐Kinase; PPAR, peroxisome proliferator‐activated receptor; GEO, Gene Expression Omnibus; TCGA, The Cancer Genome Atlas.
In summary, by first analyzing data from 5 independent KIRC databases and further validation in our 3 independent KIRC cohorts, we constructed an oxidative stress‐related model composed of three genes that could be used to predict the prognosis of KIRC patients. The calculated oxidative stress score was correlated with lymph node metastasis, and its related genes were mainly enriched in the cell focal adhesion, Rap1, and PI3K‐Akt signaling pathways. These findings indicate that the expression of oxidative stress‐related genes could also serve as a promising biomarker for screening high metastasis risk patients and guide the treatment of KIRC patients. However, further validation of the oxidative stress score for clinical usage in KIRC patients needs to be performed in large prospective studies to confirm our findings.
DECLARATIONS
AUTHORS’ CONTRIBUTIONS
MZ and ZW designed the research. XJW, HX, and MMG conducted the experiments. MZ, XJW, HX, YTS, and PZL acquired and analyzed the data. MZ, XJW, and ZW wrote the manuscript. MZ and ZW revised the manuscript.
COMPETING INTERESTS
The authors declare that no competing interests exist.
AVAILABILITY OF DATA AND MATERIALS
Online KIRC expression profile data sets GSE16449, GSE36895, GSE16441, GSE71963, and TCGA‐KIRC and corresponding clinical data in this study were directly downloaded from the GEO database and the open‐access tiers of the TCGA data portal.
FUNDING
This study was supported by grants from the National Natural Science Foundation of China (81970656).
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Ethical Committee of Shanghai Ninth People's Hospital and Ruijin Hospital, School of Medicine, Shanghai Jiao Tong University, and all of the subjects were provided with written informed consent.
CONSENT FOR PUBLICATION
Not applicable
Supporting information
Supporting Information
ACKNOWLEDGMENTS
Not applicable.
Contributor Information
Zhong Wang, Email: zhanming@shsmu.edu.cn.
Ming Zhan, Email: zhongwang2000@sina.com.
REFERENCES
- 1. Costa A, Scholer‐Dahirel A, Mechta‐Grigoriou F. The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Semin Cancer Biol. 2014;25:23‐32. [DOI] [PubMed] [Google Scholar]
- 2. Huang X, He C, Hua X, Kan A, Mao Y, Sun S, et al. Oxidative stress induces monocyte‐to‐myofibroblast transdifferentiation through p38 in pancreatic ductal adenocarcinoma. Clin Transl Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klaunig JE. Oxidative Stress and Cancer. Curr Pharm Des. 2018;24(40):4771‐8. [DOI] [PubMed] [Google Scholar]
- 4. Zhan M, Wang H, Xu SW, Yang LH, Chen W, Zhao SX, et al. Variants in oxidative stress‐related genes affect the chemosensitivity through Nrf2‐mediated signaling pathway in biliary tract cancer. EBioMedicine. 2019;48:143‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist‐oriented resource for the analysis of systems‐level datasets. Nat Commun. 2019;10(1):1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Okada T, Sinha S, Esposito I, Schiavon G, Lopez‐Lago MA, Su W, et al. The Rho GTPase Rnd1 suppresses mammary tumorigenesis and EMT by restraining Ras‐MAPK signalling. Nat Cell Biol. 2015;17(1):81‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rossetto A, De Re V, Steffan A, Ravaioli M, Miolo G, Leone P, et al. Carcinogenesis and Metastasis in Liver: Cell Physiological Basis. Cancers (Basel). 2019;11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo H, German P, Bai S, Barnes S, Guo W, Qi X, et al. The PI3K/AKT Pathway and Renal Cell Carcinoma. J Genet Genomics. 2015;42(7):343‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
Online KIRC expression profile data sets GSE16449, GSE36895, GSE16441, GSE71963, and TCGA‐KIRC and corresponding clinical data in this study were directly downloaded from the GEO database and the open‐access tiers of the TCGA data portal.


