Abstract
Objective
Ablation of the globus pallidus internus (pallidotomy) is an effective surgical intervention for dystonia. However, the current literature on the efficacy and safety of pallidotomy for dystonia is derived only from single‐case reports and small cohort studies.
Methods
We retrospectively analyzed patients with primary dystonia who underwent pallidotomy at our institution between 2014 and 2019. Neurological conditions were evaluated using the Burke‐Fahn‐Marsden Dystonia Rating Scale (BFMDRS, range: 0–120). We evaluated the total BFMDRS score and each subitem score (nine body regions) in the patients who underwent unilateral and bilateral pallidotomy before surgery and at last available follow‐up. Moreover, postoperative complications were analyzed.
Results
We found that 69 and 20 patients underwent unilateral and bilateral pallidotomy respectively. The mean age at dystonia onset was 40.4 ± 15.2 years. The mean clinical follow‐up period was 17.2 ± 11.6 months. Unilateral pallidotomy significantly improved the total BFMDRS score from 11.2 ± 14.7 preoperatively to 5.4 ± 7.6 at last available follow‐up (51.8% improvement, p < 0.001). Furthermore, there was a significant and independent improvement in all midline BFMDRS subitems, including eyes, mouth, speech/swallow, and neck, after unilateral pallidotomy. Bilateral pallidotomy significantly improved the total BFMDRS score from 14.6 ± 10.2 preoperatively to 3.8 ± 8.2 at last available follow‐up (74.0% improvement, p < 0.001). However, bilateral pallidotomy induced medically refractory parkinsonism (postural instability and gait disturbance) in five patients, dysarthria in three patients, and dysphagia in one patient.
Interpretation
Unilateral radiofrequency pallidotomy remains a viable treatment option for patients with some forms of dystonia. Bilateral pallidotomy cannot be recommended due to unacceptably high complication rates.
Introduction
Dystonia is a common movement disorder that is characterized by sustained and repetitive movements or abnormal postures. 1 It develops in every body part and its distribution ranges from focal to generalized dystonia. Conservative dystonia treatments include botulinum toxin injections and oral medications. 2 However, widely spread dystonia involving cranio‐cervico‐axial regions is often refractory to these conservative treatments.
Deep brain stimulation (DBS) of the globus pallidus internus (GPi) is the most applied surgical treatment for dystonia and has been validated by numerous clinical studies. 3 , 4 , 5 However, several studies have reported a high prevalence (11%–25.3%) of hardware‐related complications associated with DBS surgery. 6 , 7 , 8 , 9
GPi ablation (pallidotomy) is an alternative surgical option for dystonia that is rarely used in most developed countries where DBS is widely available. The small cohort studies report the efficacies of pallidotomy and GPi‐DBS for dystonia are comparable. 10 , 11 , 12 , 13 , 14 Pallidotomy plays an especially important role in dystonia treatment among individuals opposed to device implantation or those unable to access DBS for economic or geographical reasons. 15 However, the current literature on the efficacy of pallidotomy for dystonia is solely derived from single case reports and small cohort studies. Therefore, the efficacy and safety of pallidotomy for dystonia remains unclear. 16
In this study, we aimed to perform a detailed assessment of the efficacy and safety of pallidotomy, including bilateral procedures. To the best of our knowledge, this is the largest study to assess the efficacy and safety of pallidotomy for dystonia.
Patients and Methods
Methods
This retrospective study was approved by the ethics committee of our institution, which waived the requirement for informed patient consent given the observational nature of this study.
Patient population
Between 2014 and 2019, 95 consecutive patients with primary dystonia underwent unilateral or bilateral pallidotomy at our institution. The inclusion criteria were a minimum of 1‐year post‐symptom onset and a previous diagnosis of primary dystonia by a neurologist or neurosurgeon specialized in movement disorders. The exclusion criteria were secondary dystonia (drug‐/trauma‐/stroke‐induced dystonia, etc.) and having undergone previous neurosurgical treatments, including other intracranial surgeries such as DBS and selective peripheral denervation. We excluded six patients (5: drug‐induced dystonia, 1: stroke‐induced dystonia) and included 89 patients in the final analysis.
Surgical procedures
Under local anesthesia, a Leksell stereotactic frame (Elekta, Stockholm, Sweden) was fixed onto the patient’s skull. T1‐weighted axial and T2‐weighted coronal magnetic resonance imaging (MRI) and computed tomography scans were used to determine the stereotactic GPi target. The tentative target was a location 2–4 mm below, 18.5–22 mm lateral, and 2 mm anterior to the midpoint of the anterior and posterior commissures. A radiofrequency probe (1.0 mm diameter with a 4.0 mm uninsulated tip) and Leksell neurogenerator were used for stimulation and coagulation. Surgery was performed under local anesthesia without microelectrode recording. After ensuring the absence of capsular response and phosphene through macrostimulation via the electrode (130 Hz, 100 µsec pulse width, and 5–10 mA), coagulation was performed at 70°C for 30–40 sec. Immediate post‐surgery MRI was used to examine for coagulated lesions and hemorrhagic complications. Detailed information regarding the pallidotomy procedure has been described in previous articles. 17
Treatment strategy
All patients except for two patients who underwent simultaneous bilateral pallidotomy, underwent initial unilateral pallidotomy. The surgery site for unilateral pallidotomy was decided based on the dystonia distribution. Generally, the contralateral hemisphere to the most affected side by dystonia was chosen as the surgical site. For torticollis, the contralateral hemisphere to the direction of head deviation was chosen as the surgical side, for example, right‐side rotation–left pallidotomy or left‐side rotation–right pallidotomy. For laterocollis, the contralateral side to the direction of neck tilting was chosen as the surgical side, for example, right laterocollis–left pallidotomy. Based on the patients’ discretion, they underwent contralateral side surgery or continued follow‐up at 6 months after unilateral pallidotomy. Patients seeking further dystonia improvement were scheduled to undergo contralateral side pallidotomy. There was an interval of ≥6 months between the first and second surgery.
Evaluation procedures
We used the Burke‐Fahn‐Marsden Dystonia Rating Scale (BFMDRS) to evaluate the patients’ dystonic conditions preoperatively and at last available follow‐up. The BFMDRS, which has a score that ranges between 0 (minimum/best) and 120 (maximum/worst), is used to evaluate dystonia severity in 9 body regions, including the eyes (0–8), mouth (0–8), speech/swallow (0–16), neck (0–8), trunk (0–16), right arm (0–16), left arm (0–16), right leg (0–16), and left leg (0–16). We evaluated the total BFMDRS score and each subitem score in patients who underwent unilateral and bilateral pallidotomy. Regarding unilateral pallidotomy evaluation, the subitems for the arms and legs were divided into the contralateral or ipsilateral surgery side. Moreover, we analyzed postoperative complications.
Statistical analysis
We performed a Shapiro–Wilk normality test to confirm data normality. Since the data were non‐normally distributed, the Wilcoxon signed‐rank test was used to compare the preoperative total and subitem BFMDRS scores with those at last available follow‐up. All statistical analyses were performed using the SPSS 25.0 (SPSS Inc., Chicago, IL, USA). All statistical tests were two‐tailed and statistical significance was set at p‐value <0.05.
Results
Table 1 shows detailed patients’ and demographic characteristics. There were 69 (29 right side: 40; left side) and 20 patients who underwent unilateral and bilateral pallidotomy respectively. The mean age at onset of dystonia was 40.4 ± 15.2 years. The mean clinical follow‐up period was 17.2 ± 11.6 months. The dystonia distribution was as follows: focal dystonia (54 patients), segmental dystonia (24 patients), and generalized dystonia (11 patients). The mean pre‐operative BFMDRS score was 13.8 ± 1.5.
Table 1.
Patient characteristics.
| Number of patients | 89 |
| Male | 59 |
| Female | 30 |
| Age at onset (year) | 40.4 ± 15.2 |
| Age at surgery (year) | 48.1 ± 14.1 |
| Mean follow‐up period (month) | 17.2 ± 11.6 |
| BFMDRS | 13.8 ± 1.5 |
| Distribution of dystonia | |
| Generalized dystonia | 11 |
| Segmental dystonia | |
| Meige syndrome | 17 |
| Other segmental dystonia | 7 |
| Focal dystonia | |
| Cervical dystonia | 33 |
| Focal arm/hand dystonia | 8 |
| Mouth dystonia | 7 |
| Tongue dystonia | 3 |
| Blepharospasm | 2 |
| Spasmodic dysphonia | 1 |
| Unilateral pallidotomy | 69 |
| Rt | 29 |
| Lt | 40 |
| Bilateral pallidotomy | 20 |
| Simultaneous | 2 |
| Staged | 18 |
Data are presented as mean ± standard deviation.
BFMDRS, Burke‐Fahn‐Marsden Dyatonia Rating Scale.
Unilateral pallidotomy
There were 29 and 40 patients who underwent right‐side and left‐side pallidotomy, respectively (Fig. 1A and B). The mean follow‐up period was 15.3 ± 10.9 months. The total BFMDRS score significantly improved from 11.2 ± 14.7 preoperatively to 5.4 ± 7.6 at last available follow‐up (51.8% improvement, p < 0.001). Arm and leg dystonia in the contralateral side was significantly improved compared with those in the ipsilateral side. There was a significant improvement in all midline BFMDRS subitems, including eyes, mouth, speech/swallow, and neck, after unilateral pallidotomy at the last available follow‐up. Table 2 presents the detailed clinical outcomes.
Figure 1.
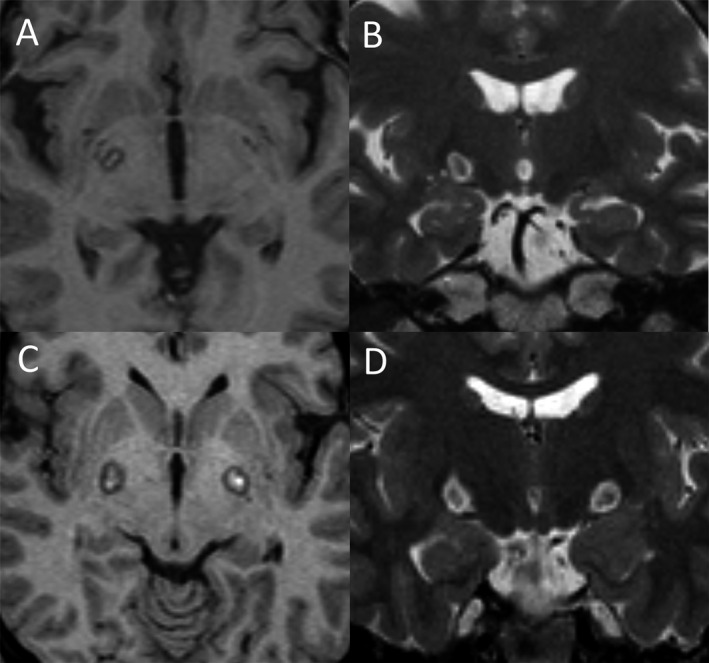
MRI images of the patients after undergoing pallidotomy. (A–B) show postoperative T1‐ and T2‐weighted MRI after undergoing unilateral pallidotomy. (C–D) show postoperative T1‐ and T2‐weighted MRI after undergoing simultaneous bilateral pallidotomy.
Table 2.
Clinical outcomes of unilateral pallidotomy.
| Number of affected patients | Before surgery | Last follow‐up | % improvement | p * | ||
|---|---|---|---|---|---|---|
| BFMDRS | Total | 69 | 11.2 ± 14.7 | 5.4 ± 7.6 | 51.80% | <0.001 |
| Contralateral side | ||||||
| Arm | 18 | 7 ± 4.3 | 2.1 ± 3.4 | 70.00% | <0.001 | |
| Leg | 2 | 14 ± 2.8 | 1.5 ± 0.7 | 89.30% | 0.180 | |
| Ipsilateral side | ||||||
| Arm | 6 | 9.7 ± 6.0 | 7.9 ± 5.7 | 18.60% | 0.317 | |
| Leg | 2 | 12 ± 5.7 | 7 ± 2.6 | 41.70% | 0.317 | |
| Midline | ||||||
| Eyes | 15 | 4.5 ± 2.2 | 2.5 ± 2.5 | 44.40% | 0.0205 | |
| Mouth | 18 | 3.6 ± 2.3 | 1.7 ± 2.2 | 52.80% | 0.005 | |
| Speech/swallow | 17 | 5.8 ± 3.3 | 2.3 ± 2.8 | 60.30% | 0.002 | |
| Neck | 40 | 5.4 ± 2.6 | 2.7 ± 2.5 | 50.00% | <0.001 | |
| Trunk | 10 | 9.5 ± 4.5 | 5.4 ± 5.8 | 43.20% | 0.016 | |
| Follow‐up period (month) | 15.3 ± 10.9 | |||||
Data are presented as mean ± standard deviation.
BFMDRS, Burke‐Fahn‐Marsden Dyatonia Rating Scale.
Wilcoxon signed‐rank test.
Bilateral pallidotomy
There were 2 and 18 patients who underwent simultaneous and staged bilateral pallidotomy, respectively (Fig. 1C and D). The mean interval between staged surgeries was 8.2 ± 3.8 months. The total BFMDRS score significantly improved from 14.6 ± 10.2 preoperatively to 3.8 ± 8.2 at last available follow‐up (74.0% improvement, p < 0.001). Regarding midline dystonia distribution, all subitems other than “trunk,” were significantly improved after bilateral pallidotomy. Table 3 presents the detailed clinical outcomes.
Table 3.
Clinical outcomes of bilateral pallidotomy.
| Number of affected patients | Before surgery | Last follow‐up | % Improvement | p * | ||
|---|---|---|---|---|---|---|
| BFMDRS | Total | 20 | 14.6 ± 10.2 | 3.8 ± 8.2 | 74.00% | <0.0001 |
| Right side | ||||||
| Arm | 4 | 11.3 ± 3.6 | 0.7 ± 1.2 | 93.80% | 0.068 | |
| Leg | 0 | – | – | |||
| Left side | ||||||
| Arm | 4 | 8 ± 1.4 | 2.3 ± 4.5 | 71.30% | 0.109 | |
| Leg | 1 | 12 | 0 | 100% | 0.317 | |
| Midline | ||||||
| Eyes | 8 | 6.3 ± 1.3 | 3.3 ± 3.4 | 47.60% | 0.026 | |
| Mouth | 6 | 4.3 ± 2.0 | 2.4 ± 3.3 | 44.20% | 0.039 | |
| Speech/swallow | 7 | 4.0 ± 1.6 | 1.2 ± 1.6 | 70.00% | 0.026 | |
| Neck | 16 | 5.2 ± 2.0 | 0.9 ± 1.7 | 73.10% | <0.001 | |
| Trunk | 4 | 5.0 ± 2.6 | 1.5 ± 3 | 70.00% | 0.109 | |
| Follow‐up period (month) | 23.6 ± 12.2 | |||||
| Interval between staged surgeries (month) | 8.2 ± 3.8 | |||||
Data are presented as mean ± standard deviation.
BFMDRS, Burke‐Fahn‐Marsden Dyatonia Rating Scale.
Wilcoxon signed‐rank test.
Adverse events
Table 4 presents the detailed adverse events. Intracerebral hemorrhage occurred in 6 of 109 procedures (5.5%); moreover, all hemorrhage cases were asymptomatic and confirmed only in the site of pallidotomy lesion. Of 109 procedures, five (4.6%) presented with cerebral infarction on the posterior limb of internal capsule, which resulted in temporary hemifacial palsy or hemiparesis (Fig. 2). However, none of the cerebral infarction cases resulted in prolonged neurological deficits. All infarction cases developed between a few weeks and one month after surgery.
Table 4.
Adverse events.
| Unilateral pallidotomy | Bilateral pallidotomy | |
|---|---|---|
| Cerebral infarction | 3 | 1 |
| Hemorrhage | 5 | 1 |
| Asymptomatic | 0 | 0 |
| Symptomatic | ||
| Parkinsonism 1 | 3 | 5 |
| Hemiparesis 1 | 2 | 0 |
| Visual disturbance 1 | 1 | 0 |
| Dysarthria 1 | 0 | 4 |
| Dysphagia 1 | 0 | 1 |
Adverse events not associated with cerebral infarction and hemorrhage.
Figure 2.
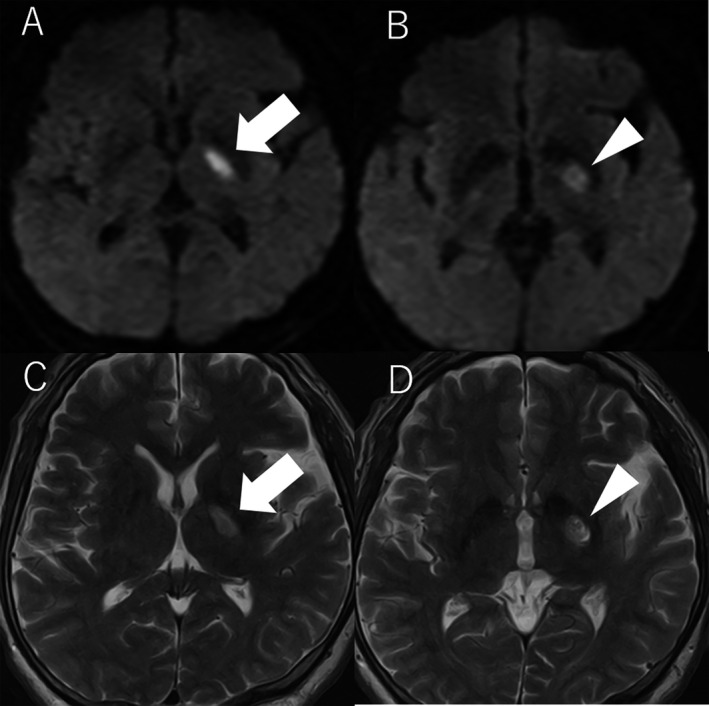
Diffusion‐weighted (A and B) and T2‐weighted (C and D) MRI images of cerebral infarction 1 month after pallidotomy. A and C show acute cerebral infarction on the left posterior limb of internal capsule (arrow). B and D show left pallidotomy lesion (arrowhead).
Parkinsonism was observed in five (25%) and 2 (2.9%) patients who underwent bilateral and unilateral pallidotomy. Those five patients who underwent bilateral pallidotomy presented with postural instability and gait disturbance; among them, two cases were severe with assistance from another person being required. During the follow‐up period, postural instability and gait disturbance did not improve in all patients. Micrographia was observed in two and three patients who underwent unilateral and bilateral pallidotomy respectively. Oral levodopa intake was attempted but was not effective for all patients. There was a postoperative spontaneous improvement of micrographia for 3–6 months. However, postural instability and gait disturbance persisted in all patients at the last available follow‐up evaluation. Persistent dysarthria and dysphagia were observed in four and one patient respectively. Among four patients presenting permanent dysarthria, one patient had concomitant parkinsonism (postural instability and gait disorder).
Discussion
This study has three major findings. First, unilateral pallidotomy achieved 51.8% improvement of the total BFMDRS score, which was consistent with the findings of open‐label studies on bilateral GPi‐DBS for dystonia (45%–51% BFMDRS score improvement). 4 , 5 Second, unilateral pallidotomy significantly improved all midline BFMDRS subitems (eyes, mouth, speech/swallow, neck, and trunk). Third, bilateral pallidotomy was associated with parkinsonism, including postural instability and gait disturbance, which was medically refractory and could not improve spontaneously.
Pallidotomy for dystonia was commonly used until the 1990s. 18 In the mid‐1980s, studies reported significant effects of pallidotomy on dyskinesia and dystonia in patients with Parkinson’s disease (PD), 19 which promoted the use of pallidotomy for patients with primary dystonia. 14 , 20 , 21 However, the advent of GPi‐DBS in the 1990s led to a gradual abandonment of pallidotomy as a dystonia treatment. Within the last decade, only seven patients with primary and secondary dystonia have been reported to undergo pallidotomy for dystonia treatment with our patients excluded. 22 , 23 , 24 , 25 , 26 , 27 This could be attributed to the lack of evidence for pallidotomy. 18 There has been no randomized controlled study for confirming the safety and efficacy of pallidotomy for dystonia. The current literature on the efficacy of pallidotomy is derived from small case studies and reports using objective evaluating scales such as the BFMDRS or Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS).
Previous studies on bilateral pallidotomy for dystonia have reported excellent results with a BFMDRS score improvement of 50%–90%, which is consistent with our findings (74.0% improvement of BFMDRS). 10 , 12 , 14 , 23 , 28 , 29 A recent study by Gross et al reported that bilateral pallidotomy using laser interstitial thermal therapy in two dystonia patients led to a 40.4% and 45.7% BFMDRS score improvement at 1‐year follow‐up. 22 Contrastingly, Lin et al. reported BFMDRS score improvement of only 13% after bilateral pallidotomy at 1‐year follow‐up in 18 patients with generalized dystonia. 30 Moreover, significant improvements have been limited to the craniocervical region. 30 In our study, all BFMDRS subitems of craniocervical region improved with statistical significance. The “trunk” improved with 70.0% improvement without statistical significance. This could be attributed to inadequate statistical power resulting from small sample sizes.
There have been few reports of unilateral pallidotomy for dystonia. 17 , 26 , 27 , 31 , 32 Ondo et al. reported that unilateral pallidotomy led to a 50%–73.9% improvement in the BFMDRS score of two and one patients with trauma‐induced and hereditary dystonia respectively. 32 Alkhani et al. reported an 84% improvement of the Unified Dystonia Rating Scale (UDRS) at the 2‐year postoperative follow‐up in a patient with hemidystonia of unknown origin. 31 Moreover, Tripathi et al. presented a patient with hemidystonia who underwent unilateral pallidotomy using gamma knife, which achieved a 61% improvement of the UDRS score at the 6‐year follow‐up. 27 Moreover, we previously reported a patient with blepharospasm who completely improved after unilateral pallidotomy 33 with the good condition being maintained at the 50‐month follow‐up. We recently reported that unilateral pallidotomy achieved a 47.9% improvement of the TWSTRS score at the 6‐month follow‐up. GPi‐DBS is widely used as a surgical intervention for patients with bilateral, but not unilateral, craniocervical, or truncal dystonia. 3 , 4 , 5 However, few studies have reported the effects of unilateral pallidotomy or GPi‐DBS for patients with midline dystonia distribution using objective evaluation scales. 17 , 26 , 33 Escamilla‐Sevilla et al. reported a patient with cervical dystonia who underwent unilateral GPi‐DBS, which achieved a 94.1% improvement of the BFMDRS score. 34 Torres et al. reported that unilateral GPi‐DBS in a patient with cervical dystonia achieved a 60% improvement in the TWSTRS score at the 10‐month follow‐up. 35 These clinical findings are consistent with our findings where all midline BFMDRS subitems significantly improved from 43.2% to 60.3%.
This is the first study to report complications of parkinsonism induced by pallidotomy for dystonia. Recent studies have reported GPi‐DBS‐induced parkinsonism, including bradykinesia, postural instability, and gait disturbance. 36 , 37 , 38 , 39 However, there has been no report of pallidotomy‐induced parkinsonism in patients with dystonia, which could be due to the limited studies on pallidotomy safety for patients with dystonia. Many studies have reported the use of pallidotomy for patients with PD. There have been several reports of pallidotomy worsening pre‐existing parkinsonism, including bradykinesia, postural instability, and gait disturbance. 40 , 41 , 42 We found that bilateral pallidotomy induced postural instability and gait disturbance with an unacceptable rate of onset (40%). All these cases did not improve spontaneously and were refractory to levodopa. Two of these cases involved severe postural instability and gait disturbance, which resulted in frequent falling and severely worsened quality of life. Consistent with our findings, Parkin et al. reported deterioration of freezing of gait in patients with PD who underwent bilateral pallidotomy after analyzing 51 and 52 unilateral and bilateral pallidotomies, respectively. 41
Previous studies have indicated that bilateral pallidotomy has a much higher risk than unilateral pallidotomy. In our study, unilateral pallidotomy did not induce dysarthria and dysphagia. One patient who underwent bilateral pallidotomy presented with severe dysarthria and dysphagia without parkinsonism. Except for parkinsonism, the remaining adverse events were very mild, which did not interfere with daily activities. The adverse events associated with unilateral pallidotomy were considered acceptable. However, there was an unacceptably high incidence of postural instability and gait disturbance, which was refractory to levodopa and unpredictable, after bilateral pallidotomy. Regarding avoiding parkinsonism associated with bilateral pallidal interventions, we recently reported that the pallidothalamic tract could be a possible treatment target for dystonia. 43 Combined unilateral pallidotomy with contralateral pallidothalamic tractotomy achieved 74.3% improvement of the BFMDRS score in 11 patients with dystonia but did not induce parkinsonism. 43 There is a need for further studies to confirm the safety and efficacy of this combined ablative treatment. Recent reports have indicated that bilateral GPi‐DBS induced parkinsonism including postural instability and gait disturbance in patients with dystonia. 36 , 39 , 44 However, adjustments of stimulation parameters can relieve stimulation‐induced parkinsonism. When performing bilateral pallidal intervention, we consider DBS should be selected to avoid irreversible severe parkinsonism.
Cerebral infarction in the posterior limb of internal capsule (PLIC) adjacent to the pallidotomy lesion developed after a few postoperative weeks in four (4.5%) patients, which caused temporary hemifacial palsy or hemiparesis. There have been few reports of delayed infarction associated with pallidotomy, including 3 (6%) cases reported by Lim et al after radiofrequency pallidotomy. 45 All infarctions were on the PLIC adjacent to the pallidotomy lesion, which occurred on postoperative days 10, 51, and 117, respectively. 45 Moreover, they reported that a history of vascular disease could increase the risk for post‐pallidotomy delayed capsular infarction. In our study, two patients with delayed infarction had a previous history of cerebral infarction and coronary artery disease; moreover, all infarctions developed after about one postoperative month. The detailed mechanism of delayed injury to adjacent tissue remains unclear. Lesion involvement of perforating arteries arising from the middle cerebral artery to supply blood to the PLIC may contribute to the post‐pallidotomy onset of cerebral infarction in the PLIC.
This study has several limitations. First, open‐label and retrospective studies have observer and patient bias. Subtle neurological deficits may be missed given the lack of preoperative unified evaluation procedures. Second, given the limited number of patients who underwent bilateral pallidotomy, we could not determine the effect of bilateral pallidotomy on focal affected regions other than the neck. Third, recommended dystonia rating scales for each focal dystonia (e.g. eyes: Blepharospasm Disability Index, neck: Toronto Western Spasmodic Torticollis Rating Scale) was not performed in this study. 46 Alternatively, subscale of BFMDRS‐MS was used to evaluate each focal dystonia, which is not strictly accurate compared to recommended dystonia rating scales. Fourth, the overall follow‐up period was short. The effects of pallidotomy for dystonia have the possibility of waning benefits in the long run.
In conclusion, we found that unilateral and bilateral pallidotomy provided 51.8% and 74.0% improvement, respectively, in the total BFMDRS score. Bilateral pallidotomy often induced medically refractory parkinsonism, which are newly reported adverse effects of bilateral pallidotomy. The advent of MRI‐guided focused ultrasound procedures, which do not require skin incision, has prompted the reappraisal of ablative treatments. Unilateral radiofrequency pallidotomy remains a viable treatment option for patients with some forms of dystonia. Bilateral pallidotomy cannot be recommended due to unacceptably high complication rates.
Authors’ Contributions
SH and TT conceived the study. SH, AF, NT and TT collected data. SH and TT designed the study. AF performed the statistical analysis. SH analyzed the data. SH and TT interpreted the data. SH performed literature search and made figures.
Conflict of Interest
The authors declare no conflicts of interest.
Data Sharing Statement
The deidentified participant data will be available for a qualified investigator. Please email the corresponding author for more information.
Funding Information
Takeda Science Foundation.
Funding Statement
This work was funded by Takeda Science Foundation grant .
References
- 1. Fahn S, Bressman SB, Marsden CD. Classification of dystonia. Adv Neurol 1998;78:1–10. [PubMed] [Google Scholar]
- 2. Balash Y, Giladi N. Efficacy of pharmacological treatment of dystonia: evidence‐based review including meta‐analysis of the effect of botulinum toxin and other cure options. Eur J Neurol 2004;11:361–370. [DOI] [PubMed] [Google Scholar]
- 3. Volkmann J, Mueller J, Deuschl G, et al. Pallidal neurostimulation in patients with medication‐refractory cervical dystonia: a randomised, sham‐controlled trial. Lancet Neurol 2014;13:875–884. [DOI] [PubMed] [Google Scholar]
- 4. Kupsch A, Benecke R, Müller J, et al. Pallidal deep‐brain stimulation in primary generalized or segmental dystonia. N Engl J Med 2006;355:1978–1990. [DOI] [PubMed] [Google Scholar]
- 5. Vidailhet M, Vercueil L, Houeto J‐L, et al. Bilateral deep‐brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 2005;352:459–467. [DOI] [PubMed] [Google Scholar]
- 6. Oh MY, Abosch A, Kim SH, Lang AE, Lozano AM. Long‐term hardware‐related complications of deep brain stimulation. Neurosurgery 2002;50:1268–1274; discussion 74‐6. [DOI] [PubMed] [Google Scholar]
- 7. Jitkritsadakul O, Bhidayasiri R, Kalia SK, et al. Systematic review of hardware‐related complications of Deep Brain Stimulation: do new indications pose an increased risk? Brain Stimul 2017;10:967–976. [DOI] [PubMed] [Google Scholar]
- 8. Sobstyl M, Stapińska‐Syniec A, Giziński J, et al. Deep brain stimulation hardware‐related complications and their management: a single‐center retrospective analysis of 65 patients with various dystonic conditions. J Neurol Sci 2019;407. [DOI] [PubMed] [Google Scholar]
- 9. Blomstedt P, Hariz M. Hardware‐related complications of deep brain stimulation: a ten year experience. Acta Neurochir 2005;147:1061–1064. [DOI] [PubMed] [Google Scholar]
- 10. Cersosimo MG, Raina GB, Piedimonte F, et al. Pallidal surgery for the treatment of primary generalized dystonia: long‐term follow‐up. Clin Neurol Neurosurg 2008;110:145–150. [DOI] [PubMed] [Google Scholar]
- 11. Teive HA, Sá DSD, Grande CV, et al. Bilateral pallidotomy for generalized dystonia. Arq Neuropsiquiatr 2001;59(2B):353–357. [DOI] [PubMed] [Google Scholar]
- 12. Ondo WG, Michael Desaloms J, Jankovic J, Grossman RG. Pallidotomy for generalized dystonia. Move Disord 1998;13:693–698. [DOI] [PubMed] [Google Scholar]
- 13. Vitek JL, Zhang J, Evatt M, et al. GPi pallidotomy for dystonia: clinical outcome and neuronal activity. Adv Neurol 1998;78:211–219. [PubMed] [Google Scholar]
- 14. Lozano AM, Kumar R, Gross R, et al. Globus pallidus internus pallidotomy for generalized dystonia. Mov Disord 1997;12:865–870. [DOI] [PubMed] [Google Scholar]
- 15. Moro E, Gross RE, Krauss JK. What's new in surgical treatment for dystonia? Mov Disord 2013;28:1013–1020. [DOI] [PubMed] [Google Scholar]
- 16. Gross RE. What happened to posteroventral pallidotomy for Parkinson’s disease and dystonia? Neurotherapeutics 2008;5:281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Horisawa S, Fukui A, Kohara K, et al. Unilateral pallidotomy in the treatment of cervical dystonia: a retrospective observational study. J Neurosurg 2019;20:1–7. [DOI] [PubMed] [Google Scholar]
- 18. Gross RE. What happened to posteroventral pallidotomy for Parkinson's disease and dystonia? Neurotherapeutics 2008;5:281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laitinen LV, Bergenheim AT, Hariz MI. Leksell's posteroventral pallidotomy in the treatment of Parkinson's disease. J Neurosurg 1992;76:53–61. [DOI] [PubMed] [Google Scholar]
- 20. Iacono RP, Kuniyoshi SM, Lonser RR, et al. Simultaneous bilateral pallidoansotomy for idiopathic dystonia musculorum deformans. Pediatr Neurol 1996;14:145–148. [DOI] [PubMed] [Google Scholar]
- 21. Cif L, Hariz M. Seventy years of pallidotomy for movement disorders. Mov Disord 2017;32:972–982. [DOI] [PubMed] [Google Scholar]
- 22. Gross RE, Stern MA. Magnetic resonance‐guided stereotactic laser pallidotomy for dystonia. Move Disord 2018;33:1502–1503. [DOI] [PubMed] [Google Scholar]
- 23. Minkin K, Gabrovski K, Dimova P, et al. Bilateral pallidotomy for Meige syndrome. Acta Neurochir 2017;159:1359–1363. [DOI] [PubMed] [Google Scholar]
- 24. Blomstedt P, Taira T, Hariz M. Rescue pallidotomy for dystonia through implanted deep brain stimulation electrode. Surg Neurol Int 2016;7(Suppl 35):S815–S817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hashimoto T, Naito K, Kitazawa K, et al. Pallidotomy for severe tardive jaw‐opening dystonia. Stereotact Funct Neurosurg 2010;88:105–108. [DOI] [PubMed] [Google Scholar]
- 26. Valalik I, Jobbagy A, Bognar L, Csokay A. Effectiveness of unilateral pallidotomy for meige syndrome confirmed by motion analysis. Stereotact Funct Neurosurg 2011;89:157–161. [DOI] [PubMed] [Google Scholar]
- 27. Tripathi M, Sharan S, Mehta S, et al. Gamma knife radiosurgical pallidotomy for dystonia: not a fallen angel. Neurol India 2019;67:1515–1518. [DOI] [PubMed] [Google Scholar]
- 28. Teive HA, Sa DS, Grande CV, et al. Bilateral pallidotomy for generalized dystonia. Arq Neuropsiquiatr 2001;59(2‐b):353–357. [DOI] [PubMed] [Google Scholar]
- 29. Eltahawy HA, Saint‐Cyr J, Giladi N, et al. Primary dystonia is more responsive than secondary dystonia to pallidal interventions: outcome after pallidotomy or pallidal deep brain stimulation. Neurosurgery 2004;54:613–619; discussion 9–21. [DOI] [PubMed] [Google Scholar]
- 30. Lin JJ, Lin SZ, Lin GY, et al. Treatment of intractable generalized dystonia by bilateral posteroventral pallidotomy–one‐year results. Zhonghua Yi Xue Za Zhi (Taipei) 2001;64:231–238. [PubMed] [Google Scholar]
- 31. Alkhani A, Bohlega S. Unilateral pallidotomy for hemidystonia. Move Disord 2006;21:852–855. [DOI] [PubMed] [Google Scholar]
- 32. Ondo WG, Desaloms JM, Jankovic J, Grossman RG. Pallidotomy for generalized dystonia. Move Disord 1998;13:693–698. [DOI] [PubMed] [Google Scholar]
- 33. Horisawa S, Kawamata T, Taira T. Unilateral pallidotomy for blepharospasm refractory to botulinum toxin injections. Eur J Neurol 2017;24:e39–e40. [DOI] [PubMed] [Google Scholar]
- 34. Escamilla‐Sevilla F, Mínguez‐Castellanos A, Arjona‐Morón V, et al. Unilateral pallidal stimulation for segmental cervical and truncal dystonia: which side? Move Disord 2002;17:1383–1385. [DOI] [PubMed] [Google Scholar]
- 35. Torres CV, Moro E, Dostrovsky JO, et al. Unilateral pallidal deep brain stimulation in a patient with cervical dystonia and tremor. J Neurosurg 2010;113:1230–1233. [DOI] [PubMed] [Google Scholar]
- 36. Huebl J, Brucke C, Schneider GH, et al. Bradykinesia induced by frequency‐specific pallidal stimulation in patients with cervical and segmental dystonia. Parkinsonism Relat Disord 2015;21:800–803. [DOI] [PubMed] [Google Scholar]
- 37. Blahak C, Capelle HH, Baezner H, et al. Micrographia induced by pallidal DBS for segmental dystonia: a subtle sign of hypokinesia? J Neural Transm 2011;118:549–553. [DOI] [PubMed] [Google Scholar]
- 38. Schrader C, Capelle H‐H, Kinfe T, et al. GPi‐DBS may induce a hypokinetic gait disorder with freezing of gait in patients with dystonia. Neurology 2011;77:483–488. [DOI] [PubMed] [Google Scholar]
- 39. Zauber SE, Watson N, Comella CL, et al. Stimulation‐induced parkinsonism after posteroventral deep brain stimulation of the globus pallidus internus for craniocervical dystonia. J Neurosurg 2009;110:229–233. [DOI] [PubMed] [Google Scholar]
- 40. Merello M, Starkstein S, Nouzeilles M, et al. Bilateral pallidotomy for treatment of Parkinson's disease induced corticobulbar syndrome and psychic akinesia avoidable by globus pallidus lesion combined with contralateral stimulation. J Neurol Neurosurg Psychiatry 2001;71:611–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parkin SG, Gregory RP, Scott R, et al. Unilateral and bilateral pallidotomy for idiopathic Parkinson's disease: a case series of 115 patients. Mov Disord 2002;17:682–692. [DOI] [PubMed] [Google Scholar]
- 42. Counihan TJ, Shinobu L, Eskandar EN, et al. Outcomes following staged bilateral pallidotomy in advanced Parkinson’s disease. Neurology 2001;56:799–802. [DOI] [PubMed] [Google Scholar]
- 43. Horisawa S, Fukui A, Tanaka Y, et al. Pallidothalamic tractotomy (Forel's Field H1‐tomy) for dystonia: preliminary results. World Neurosurg 2019;129:e851–e856. [DOI] [PubMed] [Google Scholar]
- 44. Berman BD, Starr PA, Marks WJ Jr, Ostrem JL. Induction of bradykinesia with pallidal deep brain stimulation in patients with cranial‐cervical dystonia. Stereotact Funct Neurosurg 2009;87:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lim JY, De Salles AA, Bronstein J, et al. Delayed internal capsule infarctions following radiofrequency pallidotomy. Report of three cases. J Neurosurg 1997;87:955–960. [DOI] [PubMed] [Google Scholar]
- 46. Albanese A, Sorbo FD, Comella C, et al. Dystonia rating scales: critique and recommendations. Move Disord 2013;28:874–883. [DOI] [PMC free article] [PubMed] [Google Scholar]


