Abstract
We conducted a retrospective cohort study in Kaiser Permanente Southern California from 1 January 2020 to 30 September 2020. We found that rituximab‐treated persons with multiple sclerosis (pwMS, n = 1895) were more likely be hospitalized (n = 8, 33.3%), but not die (n = 0) from COVID‐19, compared to the 4.81 million non‐MS population (5.8% and 1.4%, respectively). Time in months (adjusted OR = 0.32, 95% CI = 0.15–0.69, p = 0.0033) and receiving 1000 mg compared to lower doses at last infusion (adjusted OR = 6.28, 95% CI = 1.38–28.5, p = 0.0173) were independent predictors of COVID‐19 severity. Rituximab‐treated pwMS should be counseled to take extra precautions in the 5 months following each infusion. Using extended dosing intervals and lower doses could be considered.
Introduction
The B‐cell depleting multiple sclerosis (MS) treatments, rituximab and ocrelizumab, are associated with an increased risk of serious infections. 1 , 2 Whether rituximab or ocrelizumab is associated with an increased risk of moderate‐to‐severe COVID‐19 is unclear. Reports to date have suggested either no effect 3 , 4 , 5 , 6 , 7 or a slightly increased risk of hospitalization but not death from COVID‐19. 8 , 9 , 10 However, significant methodological limitations that constrain interpretation include unknown number of rituximab/ocrelizumab‐treated persons with MS (pwMS) at risk, 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 lack of comparison to the general population, 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 incomplete ascertainment of comorbidities, 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 inability to account for geographic variation in COVID‐19 outbreaks, 3 , 7 , 8 , 10 reliance on registries, 3 , 8 , 10 and/or inclusion of unconfirmed COVID‐19 cases with non‐specific symptoms. 3 , 4 , 7 , 9
The aim of this study was to determine whether rituximab‐treated pwMS were at higher risk of more severe COVID‐19 infection compared to the general population, and if so, whether this is best explained by known risk factors for moderate‐to‐severe COVID‐19, MS‐related disability, or rituximab treatment characteristics.
Methods
We conducted a retrospective cohort study utilizing Kaiser Permanente Southern California’s (KPSC) complete electronic health record (EHR). KPSC provides care to over 4.8 million members in Southern California. Approximately 20% of the general population in the geographic areas served belong to KPSC. The sociodemographic characteristics of KPSC members are generally representative of the underlying population. 11
The EHR was electronically searched from 1 January 2020 to 31 August 2020 to identify the following: all SARS‐CoV‐2‐infected patients; rituximab‐treated pwMS; rituximab treatment, clinical and demographic characteristics; and COVID‐19 course. The EHR of rituximab‐treated persons with at least one MS or MS‐like ICD10 diagnostic code were reviewed to confirm MS diagnosis. 12 SARS‐CoV‐2 infection required confirmation by polymerase chain reaction (PCR) or positive serum antibody testing. We included patients with positive antibody tests (available starting in June) without prior PCR testing (n = 0 rituximab‐treated pwMS, n = 1620 non‐MS population) to capture mild COVID‐19 cases because early in the pandemic, PCR testing was restricted to patients who required hospitalization. The study protocol was approved by the KPSC institutional review board (#11302).
The primary outcome was the maximum severity of COVID‐19 defined as mild (not requiring hospitalization), moderate (requiring hospitalization), or severe (death). To allow for a minimum of 30‐day follow‐up, outcomes were assessed through 30 September 2020.
The association between demographic characteristics, comorbidities, MS‐related, and/or rituximab‐related characteristics and moderate‐to‐severe COVID‐19 was examined using unconditional logistic regression. We first examined the factors associated with moderate‐to‐severe COVID‐19 in the total non‐MS population. The final model included age (continuous in years), male sex, Hispanic ethnicity (yes/no), and the Elixhauser comorbidity index (continuous). 13
We conducted crude analyses to determine whether rituximab treatment characteristics and factors identified in the non‐MS population were associated with a moderate‐to‐severe COVID‐19 course among all rituximab‐treated pwMS. Factors with p < 0.20 were included in the final adjusted model (time and dose since last infusion, cumulative dose, and Hispanic ethnicity). Sensitivity analyses using the Charlson comorbidity index, 13 restricting to rituximab‐treated pwMS who received an infusion in 2020 (n = 953) or comparison to glatiramer acetate (Appendix S1), were conducted.
The means and standard deviations (SD) of normally distributed variables were compared using 2‐sample t tests; for variables with non‐normal distributions, the Wilcoxon rank‐sum test was used; and for binary or categorical variables, Chi‐square with the Fisher’s exact test was used. Statistical significance was set at p = 0.05. No adjustment for multiple comparisons was made. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
Rituximab‐treated pwMS were slightly older, predominantly female, less likely to be Hispanic and had a higher Elixhauser but similar Charlson comorbidity index, compared to the general non‐MS population (Table 1).
Table 1.
Clinical and demographic characteristics among all and COVID‐19 rituximab‐treated pwMS compared to KPSC’s non‐MS population.
| Total population | COVID‐19 population | |||||
|---|---|---|---|---|---|---|
| RTX‐MS | non‐MS | p‐value | RTX‐MS | non‐MS | p‐value | |
| n = 1895 | n = 4,813,365 | n = 24 | n = 65,520 | |||
| Age, y, mean (SD) | 44.5 (12.3) | 38.9 (22.2) | <0.0001 | 42.4 (10.1) | 40.9 (17.4) | 0.4799 |
| Sex, females, n (%) | 1364 (72.0) | 2,470,408 (51.3) | <0.0001 | 15 (62.5) | 34,611 (52.8) | 0.6368 |
| Race/ethnicity, n (%) | <0.0001 | 0.0210 | ||||
| White | 867 (45.8) | 1,442,350 (30.0) | 7 (29.2) | 10,132 (15.5) | ||
| Black | 293 (15.5) | 362,922 (7.5) | 4 (16.7) | 3736 (5.7) | ||
| Hispanic | 598 (31.6) | 1,927,709 (40.0) | 13 (54.2) | 43,274 (66.0) | ||
| Asian/Pacific | 51 (2.7) | 524,788 (10.9) | 0 (0) | 3976 (6.1) | ||
| Other | 86 (4.5) | 555,596 (11.5) | 0 (0) | 4402 (6.7) | ||
| Charlson comorbidity index, mean (SD) | 0.6 (1.2) | 0.5 (1.2) | <0.0001 | 1.2 (2.0) | 0.5 (1.3) | 0.0138 |
| Elixhauser comorbidity index, mean (SD) | 2.3 (1.7) | 0.9 (1.6) | <0.0001 | 3.0 (2.7) | 1.0 (1.6) | <0.0001 |
| Maximum COVID‐19 severity, n (%) | ||||||
| Mild (not hospitalized) | — | — | 16 (66.7) | 60,799 (92.8) | 0.0002 | |
| Moderate (hospitalized) | — | — | 8 (33.3) | 3799 (5.8) | <0.0001 | |
| Death | — | — | 0 (0) | 922 (1.4) | 1.0 | |
| Hospital stay duration, mean (SD) | 6.9 (3.5) | 11.0 (11.2) | 0.4115 | |||
Abbreviations: MS, multiple sclerosis; pwMS, persons with multiple sclerosis; RTX, rituximab; SD, standard deviation; y, years.
Independent predictors of a moderate or severe COVID‐19 course in the non‐MS population were Hispanic ethnicity (OR = 3.66, 95% CI = 3.44–3.88); older age (OR = 1.037, 95% CI = 1.035–1.038); male sex (OR = 1.65, 95% CI = 1.55–1.74); and higher Elixhauser comorbidity index (OR = 1.18, 95% CI = 1.17–1.20) in a mutually adjusted model.
The proportion of rituximab‐treated pwMS who contracted COVID‐19 during the study period (1.27%) was similar to the non‐MS population (1.36%, p = 0.72). Among those who contracted COVID‐19, rituximab‐treated pwMS were less likely to be Hispanic and had a higher Elixhauser and Charlson comorbidity index but were similar in age and sex compared to the non‐MS population (Table 1). Rituximab‐treated pwMS with COVID‐19 were more likely to have a moderate (n = 8, 33.3%) but not severe (n = 0) course and a shorter hospital stay compared to the non‐MS population (Table 1). None of the rituximab‐treated pwMS required invasive or non‐invasive ventilation, four required supplemental oxygen up to 38–62 days after the onset of COVID‐19 symptoms. Four pwMS were hospitalized but did not require supplemental oxygen.
The increased risk of a moderate COVID‐19 course occurred shortly after the most recent rituximab treatment (median 2.5 months, range 2 days to 4.5 months, (Fig. 1 and Table 2). Rituximab‐treated pwMS with a moderate‐to‐severe COVID‐19 course were more likely to have received 1000 mg at their last infusion and a higher cumulative dose than those who did not get COVID‐19 or had a mild course (Table 2). Time since last infusion in months (adjusted OR = 0.32, 95% CI = 0.15–0.69, p = 0.0033) and receiving 1000 mg compared to a lower dose at last infusion (adjusted OR = 6.28, 95% CI = 1.38–28.54, p = 0.0173) were independent predictors of COVID‐19 severity but cumulative lifetime dose was not (adjusted OR = 1.003, 95% CI = 0.92–1.09, p = 0.9514 per 1000 mg). Hispanic ethnicity was no longer significant after adjustment for rituximab‐treatment characteristics (OR = 2.70, 95% CI = 0.61–11.96, p = 0.1903).
Figure 1.
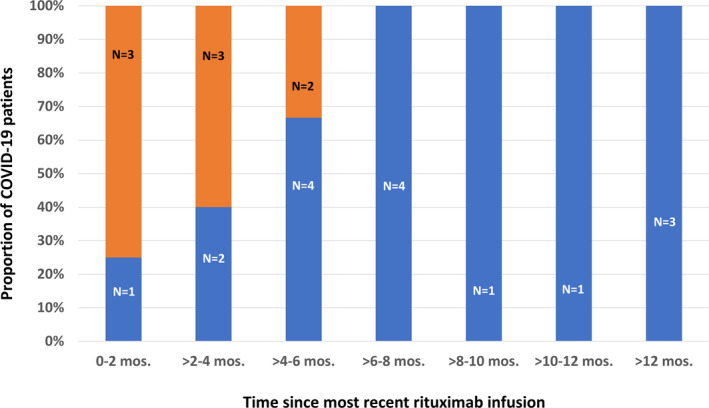
The Relationship between COVID‐19 Severity and Most Recent Rituximab Treatment for Multiple Sclerosis. Depicted is the proportion of COVID‐19 MS patients who required hospitalization (orange) and those COVID‐19 patients who did not (blue) in the months (mos.) following their most recent rituximab infusion. The sample sizes (N) are denoted above each bar. The risk of a moderate course appears highest in the first 2 months following rituximab infusion and dissipates by 6 months.
Table 2.
Clinical, demographic, and rituximab treatment characteristics and COVID‐19 severity 1 among pwMS.
| No COVID‐19 or mild COVID‐19 | Moderate COVID‐19 | p‐value | |
|---|---|---|---|
| n = 1887 | n = 8 | ||
| Age, y, mean (SD) | 44.6 (12.3) | 40.5 (9.7) | 0.3486 |
| Sex, females, n (%) | 1359 (72.0) | 5 (62.5) | 0.5497 |
| Race/ethnicity, n (%) | 0.357 | ||
| White | 864 (45.8) | 3 (37.5) | |
| Black | 293 (15.5) | 0 (0) | |
| Hispanic | 593 (31.4) | 5 (62.5) | |
| Other | 137 (7.6) | 0 (0) | |
| Charlson comorbidity index | 0.6 (1.2) | 0.8 (1.2) | 0.6759 |
| Elixhauser comorbidity index | 2.3 (1.7) | 2.1 (1.7) | 0.5713 |
| MS‐related Disability, n (%) | 0.6481 | ||
| Walker‐dependent (EDSS = 6.5) | 178 (10.3) 2 | 0 (0) | |
| Wheelchair‐dependent or worse (EDSS ≥ 7.0) | 161 (9.3) 2 | 1 (12.5) | |
| Rituximab treatment characteristics | |||
| Time since first infusion, y, med (IQR) | 2.2 (1.2, 3.8) | 2.5 (0.7, 4.2) | 0.8366 |
| Time since last infusion, mos., med (IQR) | 7.8 (5.8, 10.8) | 2.5 (0.9, 3.5) | 0.0001 |
| Dose at last infusion, n (%) | 0.0082 | ||
| ≥1000 mg | 297 (15.7) | 4 (50.0) | |
| <1000 mg | 1590 (84.3) | 4 (50) | |
| Cumulative dose, mg, med (IQR) | 2000 (1400, 3800) | 3250 (1750, 8250) | 0.1488 |
| Cumulative dose, n (%) | 0.2994 | ||
| >8000 mg | 120 (6.4) | 2 (25.0) | |
| >3000–8000 mg | 441 (23.4) | 2 (25.0) | |
| >2000–3000 mg | 354 (18.8) | 1 (12.5) | |
| >1000–2000 mg | 542 (28.7) | 2 (25.0) | |
| ≤1000 mg | 430 (22.8) | 1 (12.5) | |
Abbreviations: EDSS, expanded disability status scale; IQR, interquartile range; med, median; mg, milligrams; mos., months; pwMS, persons with multiple sclerosis; SD, standard deviation; y, years.
Maximum COVID‐19 severity defined as requiring hospitalization (moderate) or not requiring hospitalization (mild).
Available for 1725 pwMS.
Neither age, sex, MS‐related physical disability, Elixhauser, or Charlson comorbidity indices were associated with risk of moderate‐to‐severe COVID‐19 in crude or adjusted models among rituximab‐treated pwMS (data not shown). Sensitivity analyses restricted to rituximab‐treated pwMS who received an infusion in 2020 (n = 953) showed remarkably stable estimates for the decreasing risk of moderate COVID‐19 with every passing month since last infusion (adjusted OR = 0.33, 95% CI = 0.15–0.70, p = 0.0042) and increased risk with 1000 mg or higher dose at last infusion (adjusted OR = 6.24, 95% CI = 1.38–28.31, p = 0.0177).
Discussion
Rituximab‐treated pwMS were at increased risk of hospitalization but not ventilatory support or death from COVID‐19 compared to the general population. This increased risk of moderate COVID‐19 was highest in the first few months after rituximab infusion, particularly if 1000 mg or more was given, and was not explained by risk factors for moderate‐to‐severe COVID‐19 in the general population or MS‐related disability.
Evidence from previous coronavirus outbreaks have shown that T cells, but not B cells, are critical for clearing the infection and that production of antiviral antibodies plays at least some role in controlling the persistent phase of infection. 14 Thus, our findings, as well as those from previous reports that B‐cell depleting treatments are associated with an increased risk of hospitalization but not death from COVID‐19, 8 , 9 , 10 are biologically plausible, as antiviral T‐cell function is not expected to be impaired by these treatments. Our finding that this risk of moderate COVID‐19 is highest in the first few months following a rituximab infusion parallels findings of impaired vaccine‐induced antibodies in the first few months following infusions of B‐cell depleting treatments. 15
We think the absence of severe COVID‐19 cases and slightly lower infection rate among rituximab‐treated pwMS compared to the general population are probably best explained by how rituximab is used in our practice. We recommended extending rituximab dosing intervals to 12 months or more and have advised rituximab‐treated pwMS to consider themselves at high risk of severe COVID‐19 since March of 2020 due to the lack of information and the biological plausibility that impaired antiviral antibody production could contribute to a more severe COVID‐19 disease course. The recommendation to extend to annual dosing intervals is based primarily on inference with two important supporting pieces of evidence: (1) a randomized controlled trial that demonstrated efficacy sustained for at least 1 year after one cycle of rituximab 16 ; and (2) the lack of rebound disease activity ≥ 1 year after rituximab cessation. 17 , 18 The standard rituximab maintenance dose in KPSC was already 500 mg to minimize adverse events. 19 We also infrequently use RTX in pwMS with advanced disability or other comorbidities that pre‐dispose to serious infections.
This study is limited by the relatively small number of RTX‐treated pwMS with moderate‐to‐severe COVID‐19. This resulted in wide confidence limits for multiple variables including cumulative rituximab dose and dose at last infusion. Thus, we caution against overinterpreting these point estimates. Similarly, we also cannot exclude the possibility that higher cumulative rituximab dose increases the risk of severe COVID‐19.
Strengths of this study are the inclusion of a population‐based sample, large number of rituximab‐treated pwMS including those without COVID‐19, ability to compare to non‐MS population, confirmed diagnoses and outcomes, and ability to account for comorbidities. This is in contrast to previously published 3 , 7 , 8 and unpublished 10 registry studies that focused on pwMS with COVID‐19, were unable to account for geographic variation in COVID‐19 outbreaks or characteristics of B‐cell depleting therapy treatment, or thoroughly assess comorbidities—limitations that likely contribute to the conflicting findings.
Our findings suggest that rituximab‐treated pwMS should take extra precautions to avoid exposure to COVID‐19 in the 5 months following each infusion. Clinicians should consider extending dosing intervals of B‐cell depleting treatments and using the lowest effective dose to minimize the risk of moderate‐to‐severe COVID‐19. Future studies are needed to address the risks and benefits of extending dosing intervals and identifying lowest effective doses.
Group Information
The KPSC MS Specialist Group are Brandon E. Beaber, MD, Downey Medical Center, Department of Neurology, Southern California Permanente Medical Group; Sonu M Brara, MD, Panorama City Medical Center, Department of Neurology, Southern California Permanente Medical Group; Julie Debacker, MD, Los Angeles Medical Center, Department of Neurology, Southern California Permanente Medical Group; Allen Scott Nielsen, MD, Fontana Medical Center, Department of Neurology, Southern California Permanente Medical Group; Samira Amirova, NP‐C, Los Angeles Medical Center, Department of Neurology, Southern California Permanente Medical Group; and Oluwasheyi Ayeni, MD, Glenlake Medical Center, Department of Neurology, Southeast Permanente Medical Group.
Author Contributions
Conception and design of the study: ALG. Acquisition and analysis of data: ALG, JBS, and BHL. Drafting the manuscript or figures: ALG, JBS, and BHL.
Conflict of Interest
ALG has received grant support and awards from the National Institutes of Health, Patient‐Centered Outcomes Research Institute, and the National MS Society. She currently serves as a voting member on the California Technology Assessment Forum, a core program of the Institute for Clinical and Economic Review (ICER). She has received sponsored and reimbursed travel from ICER. JBS and BHL have nothing to report.
Supporting information
Appendix S1. Comparison of baseline characteristics and COVID‐19 outcomes between rituximab‐ and glatiramer acetate‐treated pwMS.
Funding Information
This study was supported by the Patient‐Centered Outcomes Research Institute (PCORI/MS‐1511‐33196). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Funding Statement
This work was funded by Patient‐Centered Outcomes Research Institute grant MS‐1511‐33196.
Contributor Information
Annette Langer‐Gould, Email: annette.m.langer-gould@kp.org.
the KPSC MS Specialist Group:
Brandon E Beaber, Sonu M Brara, Julie Debacker, Allen Scott Nielsen, Samira Amirova, and Oluwasheyi Ayeni
References
- 1. Luna G, Alping P, Burman J, et al. Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol 2020;77:184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wolinsky JS, Arnold DL, Brochet B, et al. Long‐term follow‐up from the ORATORIO trial of ocrelizumab for primary progressive multiple sclerosis: a post‐hoc analysis from the ongoing open‐label extension of the randomised, placebo‐controlled, phase 3 trial [published correction appears in Lancet Neurol. 2020 Nov 17;:]. Lancet Neurol. 2020;19:998–1009. 10.1016/S1474-4422(20)30342-2 [DOI] [PubMed] [Google Scholar]
- 3. Louapre C, Collongues N, Stankoff B, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol 2020;77:1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montero‐Escribano P, Matias‐Guiu J, Gomez‐Iglesias P, et al. Anti‐CD20 and COVID‐19 in multiple sclerosis and related disorders: a case series of 60 patients from Madrid, Spain. Mult Scler Relat Disord 2020;42:102185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parrotta E, Kister I, Charvet L, et al. COVID‐19 outcomes in MS: observational study of early experience from NYU Multiple Sclerosis Comprehensive Care Center. Neurol Neuroimmunol Neuroinflamm 2020;7:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaudhry F, Bulka H, Rathnam AS, et al. COVID‐19 in multiple sclerosis patients and risk factors for severe infection. J Neurol Sci 2020;418:117147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hughes R, Whitley L, Fitovski K, et al. COVID‐19 in ocrelizumab‐treated people with multiple sclerosis. Mult Scler Relat Disord 2020;49:102725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sormani MP, De Rossi N, Schiavetti I, et al. Disease‐modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. ANN NEUROL 2021:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sahraian MA, Azimi A, Navardi S, et al. Evaluation of the rate of COVID‐19 infection, hospitalization and death among Iranian patients with multiple sclerosis. Mult Scler Relat Disord 2020;46:102472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simpson‐Yap S, De Brouwer E, Kalincik T, et al. SS02.04 First results of the COVID‐19 in MS Global Data Sharing Initiative suggest anti‐CD20 DMTs are associated with worse COVID‐19 outcomes. Mult Scler 2020;26:48–49.30785358 [Google Scholar]
- 11. Koebnick C, Langer‐Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J 2012;16:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018;17:162–173. [DOI] [PubMed] [Google Scholar]
- 13. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 14. Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol 2020;92:424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baker D, Roberts CAK, Pryce G, et al. COVID‐19 vaccine‐readiness for anti‐CD20‐depleting therapy in autoimmune diseases. Clin Exp Immunol 2020;202:149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hauser SL, Waubant E, Arnold DL, et al. B‐cell depletion with rituximab in relapsing‐remitting multiple sclerosis. N Engl J Med 2008;358:676–688. [DOI] [PubMed] [Google Scholar]
- 17. Juto A, Fink K, Al Nimer F, Piehl F. Interrupting rituximab treatment in relapsing‐remitting multiple sclerosis; no evidence of rebound disease activity. Mult Scler Relat Disord 2020;37:101468. [DOI] [PubMed] [Google Scholar]
- 18. Smith JB, Hellwig K, Fink K, et al. Rituximab, MS, and pregnancy. Neurol Neuroimmunol Neuroinflamm 2020;7:e734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salzer J, Svenningsson R, Alping P, et al. Rituximab in multiple sclerosis: a retrospective observational study on safety and efficacy. Neurology 2016;87:2074–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Comparison of baseline characteristics and COVID‐19 outcomes between rituximab‐ and glatiramer acetate‐treated pwMS.


