Abstract
Later onset of puberty has been associated with lower body mass index (BMI) in adulthood independent of childhood BMI. However, how the relationship between time of onset of puberty and BMI in adulthood is associated with neurocognitive outcomes is largely unstudied. Here, women were sampled from the Human Connectome Project 1200 PTN release. Inclusion criteria were: 4 (15 minute) resting state fMRI scans, current measured BMI, self-reported age at onset of menstruation (a proxy of age at onset of puberty), and no endocrine complications (e.g., polycystic ovarian syndrome). The effect of age at onset of menstruation, measured BMI at scan date, and the interaction of age at onset of menstruation by BMI on brain functional correlation was modeled using FSLnets controlling for race and age at scan. Corrected significance was set at pFWE<0.05. A final sample of n=510 (age 29.5y±3.6; BMI at scan 25.9±5.6; age at onset of menstruation 12.7±1.6) were included. Age at onset of menstruation was negatively associated with BMI at scan (r=−0.19, p<0.001). The interaction between age at onset of menstruation and BMI at scan was associated with stronger correlation between a somatosensory and visual network (t= 3.45, pFWE= 0.026), and a visual network and cingulo-opercular task control network (t= 4.74, pFWE= 0.0002). Post hoc analyses of behavioral/cognitive measures showed no effect of the interaction between BMI and age at onset of menstruation on behavioral/cognitive measures. However, post hoc analyses of heritability showed adult BMI and the correlation between the visual and somatosensory networks have high heritability. In sum, we show increased correlation between visual, taste-associated, and self-control brain regions in women at high BMI with later age at onset of menstruation
Keywords: Puberty, fMRI, cingulo-opercular, somatosensory, BMI
Introduction
Obesity is a global epidemic, with the burden of obesity and related comorbidities greater among women (1). The majority of obesity research in women has focused on reproductive capacity, noting that women with elevated body mass index (BMI) have decreased reproductive capacity and higher infant mortality (2). The health risks associated with high BMI and reproduction are well known, yet the onset of reproductive maturation - puberty - represents an understudied and potentially modifiable childhood risk factor for adult obesity through pharmacology (3,4).
Puberty, the rapid development of reproductive capacity, represents a period of high physical and cognitive development (5). This sudden increase in development presents challenges for all children, however developing earlier than one’s peers conveys additional challenges especially for girls (6). Earlier puberty is related to a host of health problems including gynecological, psychiatric, musculoskeletal, and respiratory conditions (7). Previous research shows that early puberty independently increases the risk of diseases that are seemingly unrelated to reproduction or development, such as: cardiovascular disease (8), metabolic syndrome (9–11), type 2 diabetes (T2D) (10–13), asthma (14), certain cancers (15), and overall reduced life expectancy (16). A common element associated with all of these disease states is elevated BMI (3,17,18). It is possible that BMI drives the increased risk of these conditions associated with early puberty. In support, Gill and colleagues demonstrated a potential causal positive effect of earlier puberty on elevated BMI in adulthood in a large European sample, with the stipulation that hormonal and psychological effects may drive this relationship (19).
Both psychological and hormonal responses are coordinated in the brain, making the brain a strong candidate site for changes contributing to the health problems associated with earlier puberty. Despite the known relationship between earlier onset of puberty, elevated BMI, and psychosocial problems, no study to date has examined the relationship between the onset of puberty, adult BMI, and the brain. This gap in knowledge is surprising considering that the onset of puberty is rooted in neuroendocrine function driven by the brain and body weight. The brain integrates somatic condition, energy balance, genetic predisposition, and seasonality signals, which together trigger human gonadotropin releasing hormone (GnRH) secretion from the hypothalamus (5). GnRH drives the production and release of luteinizing and follicular stimulating hormones from the pituitary, and stimulates steroid hormone production (5). In turn, the brain, which initiated this increased production of steroid hormones, is sensitive to hormonal reorganization of the hypothalamic, limbic, and prefrontal executive control systems (20–22). Hypothalamic, limbic, and prefrontal executive control regions of the brain have been implicated in both the development of psychiatric disorders and in reward based eating (23,24). The premature flood of steroid hormones in early puberty has been hypothesized to abbreviate adolescent brain reorganization and may potentially underlie the increased onset of psychiatric disorders (20,22). Considering that psychological disturbances in childhood can last through adulthood (22), premature exposure to pubertal hormones may underlie the higher rate of psychological and physical diseases such as anxiety, depression, disordered eating, and obesity in women (25).
While there is a paucity of longitudinal resting state functional magnetic resonance imaging (rfMRI) studies evaluating neural risk factors for obesity from childhood to adulthood, some cross sectional studies (26–28) and two task-based longitudinal imaging studies suggest neural differences exist across the BMI spectrum and may predict weight gain (29,30). The few resting state studies that have examined functional correlations associated with BMI have found the sensorimotor cortex, putamen, and insula correlations associated with BMI (31–33). A cross sectional investigation using Human Connectome Project data found functional correlations in obesity have shown differences in the default mode network (DMN), reward-related, executive, and somatosensory brain regions (33), whereas task-based longitudinal studies have shown response in the operculum and caudate (regions related to taste and reward respectively) predicts weight gain in adolescents at risk for high BMI as adults (29,30). Therefore, the present study aimed to assess the relationship between onset of puberty (operationalized as age at onset of menstruation), adult BMI, and resting state functional neural correlations in a large sample of women from the Human Connectome Project (34). We hypothesized that earlier puberty would be related to increased BMI in adulthood. Within resting state functional correlations, the interaction between puberty and BMI will be related to increased connectivity between reward-related (striatum, somatosensory cortex, and limbic) regions of the brain previously associated with weight gain from adolescents to adulthood (29,30) shown to be sensitive to BMI (31–33). Specifically, in women with earlier puberty, BMI would be more strongly positively associated with reward network connectivity than in women with later puberty. If there is no interaction effect, we still expect main effects of age at onset of menarche and BMI, i.e. women with earlier puberty will show stronger correlations within reward-related regions at all BMIs.
Methods
Sample
Female subjects were selected from the Human Connectome Project (HCP) 1200 parcellation, timeseries, and netmats1 release (PTN) (35). Participants in the HCP gave written informed consent approved by both the Washington University in St. Louis and the University of Minnesota Institutional Review Boards. Women were excluded if they did not provide BMI or age at onset of menstruation data, or if they reported any endocrine conditions. Of the 656 females in the full sample, 648 reported their first menstrual cycle was before 24 years of age, 628 did not report any endocrine conditions, and 510 had full scan data. Sample characteristics are described in Table 1.
Table 1:
Sample characteristics by reported race
| n | BMI(kg/m2) | Age at Scan (y) | Age of Menses Onset (y) | Hemoglobin A1c (mmol/mol) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| mean | SD | mean | SD | mean | SD | n | mean | SD | ||
| Native American | 1 | 29.23 | NA | 35.00 | NA | 16.00 | NA | 1 | 5.90 | NA |
| Asian, Native Hawaiian, or Pacific Islander | 33 | 21.62 | 3.62 | 26.97 | 4.29 | 12.33 | 1.49 | 21 | 5.23 | 0.31 |
| Black or African American | 78 | 29.12 | 6.42 | 29.54 | 3.43 | 12.35 | 1.84 | 44 | 5.40 | 0.35 |
| More than one race | 12 | 25.15 | 4.13 | 26.17 | 3.49 | 12.92 | 1.51 | 8 | 5.39 | 0.26 |
| Unknown or chose not to report | 10 | 28.53 | 4.73 | 28.10 | 4.23 | 12.30 | 1.83 | 8 | 5.29 | 0.40 |
| White | 376 | 25.60 | 5.31 | 29.87 | 3.43 | 12.82 | 1.51 | 245 | 5.21 | 0.37 |
| Total | 510 | 25.94 | 5.62 | 29.52 | 3.61 | 12.72 | 1.58 | 327 | 5.25 | 0.37 |
Data description and preprocessing
Data collection, preprocessing, and generation of the PTN release has been detailed extensively previously (34,36,37). Briefly, participants completed 4 rfMRI over two days on a 3T Siemens Skyra magnet, totaling 58 min and 12 s of rfMRI data per participant. The HCP data was gathered with a standard protocol using an eight-factor multiband, gradient echo EPI sequence (TR: 720 ms, TE: 33.1 ms, flip angle: 52 degrees, slice thickness: 2.0 mm) (38,39). Participants viewed a light crosshair on a dark background projected into their field of view. The PTN release was extensively preprocessed, and no additional preprocessing was performed locally (36).
Group ICA
Dense connectomes were generated using the incremental group-principal components analysis (PCA) from the Multivariate Exploratory Linear Optimized Decomposition into Independent Components (MELODIC) software (40,41). These dense connectomes were subsequently parcellated using group-independent components analysis (ICA) to create 15 spatial-ICA network maps (42). Because multiple components in a network may include overlap of anatomical regions with other components, independent components (IC) will be referred to by their number and anatomical regions(s) (ex. IC 3 primary visual network).
Individual component timeseries and creation of netmats1
Participant timeseries from the 4 scans were concatenated and spatially mapped to the corresponding network map. Dual regression was used to regress each group network map against the individual timeseries to create individual participant network matrices (netmats1). Therefore, the netmats1 represent neural activity in the IC over time.
Statistical analyses
Imaging analyses were performed in FSLNets (Version 0.6, FMRIB, Oxford, UK). Each IC was correlated with every other IC (15×15) to create individual correlation matrices with normalized covariances. A general linear model was then fitted assessing the interaction between BMI at scan, age at onset of menstruation (43,44) (as a proxy of pubertal status) on functional correlations between ICs controlling for race and age at scan. The race variable was categorical with the white group as the referent category.
The following contrasts were assessed: 1) positive interaction between BMI and age at onset of menses on brain connectivity; 2) negative interaction between BMI and age at onset of menses; 3) positive effect of BMI; 4) negative effect of BMI; 5) positive effect of age at onset of menses; 6) negative effect of age at onset of menses. To correct for potential false positives, non-parametric permutation testing was used through FSL’s Randomize tool with 10,000 permutations (45). Results were considered significant at pFWE < 0.05. Results were visualized using workbench view (https://github.com/Washington-University/workbench). To better characterize what functional networks were present, each IC was parcellated into the 333 area Gordon 2016 functional parcellation for finer detail (46), and Yeo 2011 17-resting state parcellation to assess larger network participation (47). Non-imaging data analyses were performed in R (v. 3.6.1) (48). To visualize the interactions, the top and bottom age at onset of menstruation tertiles were selected and graphed against BMI and the pearson-z corrected correlations between ICs.
Post hoc cognitive performance, twin, and network analyses
The relationship between the interaction of BMI and the age at onset of menstruation with cognitive performance measures, glycemia, stress, taste sensitivity, physical activity, socio-economic status (SES), and genetic contributions was also assessed. Cognitive performance measures included: delayed discounting for $200 and $4000, NIH Toolbox Picture Sequence Memory, NIH Toolbox Dimensional Change Card Sort Test, NIH Toolbox Flanker Inhibitory Control and Attention Test, NIH Toolbox Cognition Fluid Composite, NIH Toolbox Cognition Total Composite Score, NIH Toolbox Cognition Crystallized Composite, depressive symptoms and major episodes, and the NEO-Five Factor Inventory composite scores for neuroticism, openness, agreeableness, and conscientiousness.
A subsample of twins (n = 227) was used to test for genetic effects on the interaction between BMI, onset of menses, and functional connectivity. Genetic effects were assessed through the “Mets” package (v. 1.2.6) in R using the “twinlm” function to fit a classical twin model for quantitative traits (49,50). This assessed the following variation: Additive genetic (A), common shared family (C), genetic dominance (D), and nonshared environmental (E) effects. Bonferroni corrections were used to prevent false positives. Significance was set at p < 0.003.
MZ Latent Variable Model
IC correlation.1 ~ a1+c1+d1+e1+BMI.1+ Age at onset of menses.1+Age_in_Yrs.1+Race.1+motion.1+BMI.1: Age at onset of menses.1
IC correlation.1.2 ~ a1+c1+d1+e2+BMI.2+ Age at onset of menses.2+Age_in_Yrs.2+Race.2+motion.2+BMI.2: Age at onset of menses.2
DZ Latent Variable Model
IC correlation.1.1 ~ a1+c1+d1+e1+BMI.1+ Age at onset of menses.1+Age_in_Yrs.1+Race.1+motion.1+BMI.1: Age at onset of menses.1
IC correlation.1.2 ~ a2+c1+d2+e2+BMI.2+ Age at onset of menses.2+Age_in_Yrs.2+Race.1+motion.2+BMI.2: Age at onset of menses.2
To assess the network structure within each IC associated with the interaction between BMI and age at onset of menstruation (ICs 2, 3, 6, 12, 13), we examined differences in network topology measures between an earlier onset of menses group (<12 y, n=87) and later onset of menses group (>13 y, n=125). The data was split into menses groups for ease of interpretation and to be consistent with figure 2 interpretation. Each netmats2 per subject containing z-scored timeseries per IC were parcellated into 379 regions from the Glasser parcellation (51), derived from HCP data, and subcortical regions from the Gordon parcellation (supplemental figure 1) (46). Z-scores less than 3.5 and parcels with greater than 75% missing data were set to zero. Per participant and per IC, parcels along with covariates of interest (including BMI, age at onset of menstruation, the interaction of BMI and age at onset of menstruation, race, age, and head motion) were correlated to form connectivity matrices (379 brain parcels and 6 covariates for a 385×385 matrix). Matrices were then averaged per group (early and later menses).Communities of densely connected nodes were detected using the Louvain method (52) and were grouped into modules. Participation coefficient (a measure of inter-modular connectivity) and intra-modular degree (a measure hubness) were used to assess how the node is interacting with the graph at large. Hubness and nodal roles were assigned according to Meunier et al. (53). Briefly, nodes were considered hubs if they had an intra-modular degree greater than 2.5. Nodal roles depended on hubness: for hubs, a participant coefficient between 0 and .3 was considered a provincial hub, between .3 and .75 was considered a connector hub, and between .75 and 1 was a kinless hub. Non-hub nodes were considered an ultra-peripheral node if the participation coefficient was between 0 and .05, a peripheral node if the participation coefficient was between .05 and .62, and a connector node if the participation coefficient was between .63 and .8, and kinless nodes if the participation coefficient was between .8 and 1 (53). This post hoc analysis used connectome workbench (v. 1.2.3) to parcellate the netmats2 and to generate initial text files. Networks were built and topologic measures calculated with networkx (v. 2.2) in python (v. 3.6.0). All statistical analysis was performed in R (v. 3.6.1) (54).
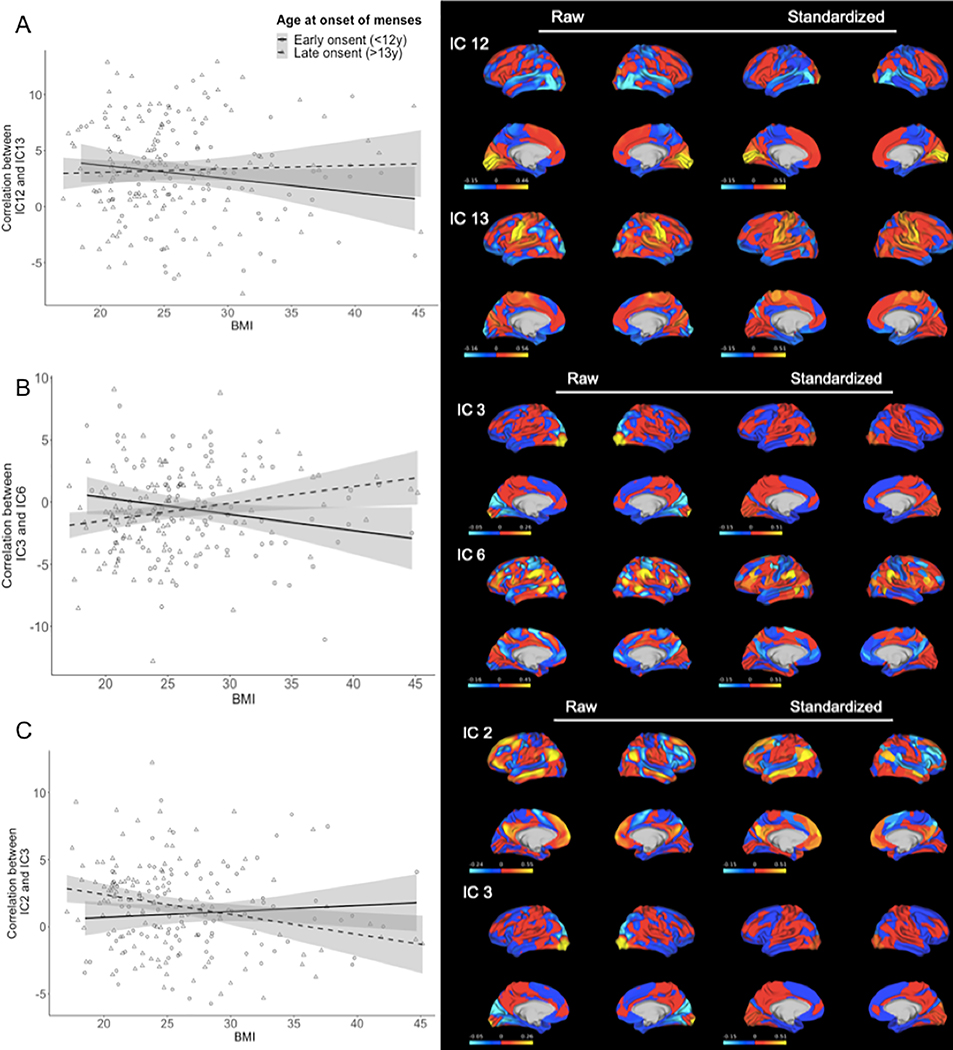
Figure 2. Interaction of Body Mass Index (BMI) and Age at Onset of Menses on Functional Correlation of Brain Networks (n=510).
A) shows the overall negative relationship between functional correlation of visual cortex (IC12) and mouth somatosensory area (IC13) with body mass index (BMI) in bottom (early) and top (late) age at onset of menstruation tertiles. The interaction with age at onset of menses is such that for women with early onset, the relationship is negative, and for those with late onset the relationship is positive. B) shows the relationship between functional correlation of primary visual cortex (IC3) and cingulo-opercular network (IC6) with BMI and the interaction with age at onset of menses, which shows that for women with early onset, the relationship is negative, and for those with late onset the relationship is positive. C) shows the overall positive functional correlation of default mode network (IC2) and primary visual cortex (IC3) with BMI. The interaction with age at onset of menses is such that for women with early onset, the relationship is positive, and for those with late onset the relationship is negative. Raw maps represent the fisher z-correlation values per independent component. Standardized maps represent the fisher z-correlation per the Gordon parcellation (46). This allows for better generalization of the IC within the framework of an established map.
Results
Demographics
Sample characteristics can be found in Table 1 and relationship to BMI and age at onset of menstruation in Figure 1. Overall, the sample was predominantly white (73%), with an average age just under 30 years (mean= 29.5 y, SD= 3.6 y). On average women in this sample reported their first menses just before 13 years (mean= 12.7 y, SD= 1.6 y), which is in line with United States national average (55). Compared to white women, Black or African American women reported their first menses earlier (β= −0.11, p= 0.02). Additionally, compared to white women, Asian American, Native Hawaiian, and or Pacific Islander women had lower BMI (β= −0.17, p= 0.00005) whereas Black or African American women had higher BMI on average in this sample (β= 0.22, p= 0.0000002). Asian American, Native Hawaiian, and or Pacific Islander women (β= −0.20, p= 0.000006) and women reporting more than one race (β= −0.15, p= 0.0003) were also on average younger at time of scan than the white women.
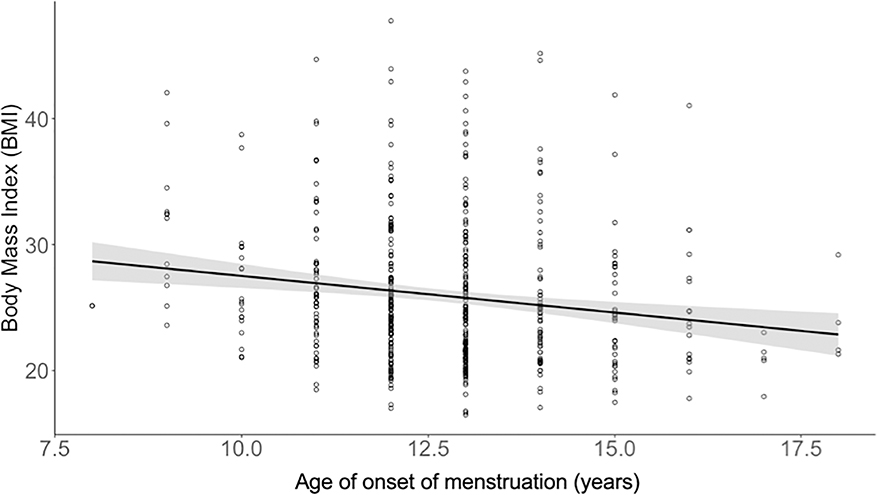
Figure 1. Relationship between BMI and Age of Onset of Menses (n=510).
Age of onset of menstruation (years) is negatively associated with adult body mass index.
Relationship Between Puberty and BMI
Age at onset of menses was negatively related to BMI as an adult, controlling for age at scan and race (Table 2, partial η2= 0.03, β= −0.16, CI= −0.86, −0.27, p= 0.0002). BMI was also significantly related to head motion (partial η2= 0.41, β=0.66, CI=103.4,113.38 p< 0.0001). However, when motion parameters were added to the model, age at onset of menses remained related to BMI though the effect size was attenuated (partial η2=0.012, β=−0.11, CI= −0.51, −0.29, p< 0.0001).
Table 2:
Relationship between BMI and age of onset of menses
| Without head motion parameters1 | With head motion parameters2 | |||||||
|---|---|---|---|---|---|---|---|---|
| b | SE | t | p | b | SE | t | p | |
| Intercept | 29.06 | 2.76 | 10.52 | 0.00 | 21.42 | 21.43 | 10.06 | 0.00 |
| Motion | 101.36 | 5.24 | 19.33 | 0.00 | ||||
| Menstrual age began (y) | −0.56 | 0.15 | −3.74 | 0.00 | −0.47 | 0.11 | −4.09 | 0.00 |
| Age (y) | 0.13 | 0.07 | 1.86 | 0.06 | 0.05 | 0.05 | 1.06 | 0.29 |
| Native American or Alaskan† | 4.77 | 5.33 | 0.89 | 0.37 | 0.10 | 4.04 | 0.03 | 0.98 |
| Asian, Native Hawaiian, or Pacific Islander† | −3.89 | 0.98 | −3.95 | 0.00 | −2.92 | 0.74 | −3.91 | 0.00 |
| Black or African American† | 3.29 | 0.66 | 4.96 | 0.00 | 1.04 | 0.51 | 2.01 | 0.04 |
| More than one race† | 0.07 | 1.57 | 0.04 | 0.96 | −0.50 | 1.19 | −0.42 | 0.67 |
| Unknown or chose not to report† | 2.85 | 1.70 | 1.67 | 0.73 | 1.10 | 1.29 | 0.91 | 0.36 |
Race was coded as a categorical variable with White as the referent category
Brain Correlation Results
Interaction of menstruation and BMI
The interaction between age at onset of menstruation and BMI was positively associated with functional correlations between IC 12 comprised of the visual cortex and IC 13 comprised of the mouth somatosensory area (Figure 2A, t= 3.45, pFWE= 0.026). Additionally, the interaction of BMI and age at onset of menstruation was positively associated with the functional correlation between IC 3 of the primary visual cortex and IC 6 comprised of the cingulo-opercular network and dorsolateral prefrontal cortex (Figure 2B, t= 4.74, p= 0.0002). Overall, those women who reported earlier menses showed decreased connectivity between the visual cortex and the somatosensory area and cingulo-opercular network with increasing BMI.
The interaction between age at onset of menstruation and BMI was negatively associated with functional correlation between IC 2 the default mode network and IC 3 the primary visual area (Figure 2C, t= 4.03, pFWE= 0.004). As such, those women who reported later onset of menses showed decreased functional correlations between the DMN and primary visual area.
When head motion was included in the model, both the positive and negative relationships remained (Table 3).
Table 3.
BMI, age at onset of menstruation, and the interaction of BMI and age at onset of menstruation models predicting independent component correlations
| Without head motion | With head motion | |||||
|---|---|---|---|---|---|---|
| Contrast | ICs | T value | pFWE | ICs | T value | pFWE |
| Positive interaction between age at onset of menstruation and BMI | 3–6 | 4.74 | 0.0002 | 3–6 | 4.74 | 0.0002 |
| 12–13 | 3.45 | 0.026 | 12–13 | 3.51 | 0.022 | |
| Negative interaction between age at onset of menstruation and BMI | 2–3 | 4.03 | 0.004 | 2–3 | 4.03 | 0.004 |
| Positive BMI | 5–10 | 3.69 | 0.012 | 1–8 | 3.49 | 0.023 |
| 5–7 | 4.09 | 0.003 | 6–11 | 3.34 | 0.036 | |
| 9–11 | 4.16 | 0.003 | 6–12 | 4.12 | 0.002 | |
| 10–13 | 4.26 | 0.002 | 8–9 | 3.33 | 0.037 | |
| 12–14 | 3.35 | 0.037 | 8–12 | 5.25 | 0.0002 | |
| 12–14 | 3.78 | 0.007 | ||||
| Negative BMI | 1–6 | 3.32 | 0.044 | 1–12 | 6.26 | 0.0002 |
| 3–4 | 3.30 | 0.046 | 3–9 | 3.62 | 0.014 | |
| 4–8 | 3.63 | 0.016 | 3–5 | 4.16 | 0.002 | |
| 4–15 | 4.27 | 0.002 | 4–12 | 3.95 | 0.004 | |
| 10–15 | 3.67 | 0.014 | 6–7 | 4.01 | 0.003 | |
| 10–12 | 4.01 | 0.006 | 7–8 | 3.72 | 0.01 | |
| 9–12 | 5.12 | 0.0002 | ||||
| 9–13 | 3.61 | 0.015 | ||||
| 12–13 | 5.47 | 0.0002 | ||||
| Positive age at onset of menstruation | 12–15 | 3.26 | 0.048 | 12–15 | 3.28 | 0.045 |
Correlation with BMI
BMI was positively associated with multiple correlations between ICs (Table 3). In particular, IC 5, defined as the fronto-parietal network, was correlated with IC 10 (the ventral attention network; t=3.69, pFWE=0.023) and IC 7 (the right fronto-parietal network including the orbital frontal cortex; t=4.09, pFWE=0.003) and these correlations were positively associated with BMI. Additionally, IC 10 (ventral attention) was correlated with IC 13 (mouth somatosensory cortex) and this was positively associated with BMI (t=4.26, pFWE=0.002).
When head motion was included in the model, correlations including IC 5 were lost (Table 3). However, IC 6 (cingulo-opercular) showed correlations between ICs 11 (dorsal attention t= 3.34, pFWE=0.036) and 12 (the third and sixth visual areas, t= 4.12, pFWE= 0.002) and was positively associated with BMI. IC 8 is made up of subcortical areas including the caudate, nucleus accumbens, thalamus, and ventral diencephalon, and was correlated with both IC 1 (dorsal stream visual area, t= 3.49, pFWE= 0.023), 9 (parietal opercular network, t= 3.33, pFWE= 0.037), and 12 (dorsal stream visual area, t= 5.25, pFWE= 0.0002).
BMI was negatively associated with correlations involving IC 4, which incorporates the visual and dorsal attention network. IC 4 was correlated with ICs 3 (primary visual area, t= 3.30, pFWE= 0.046), 8 (subcortical areas, t= 3.63, pFWE= 0.016), and 15 (medial parietal network, t= 4.27, pFWE= 0.002). When head motion was included as a covariate in the model, IC 4 was only correlated with IC 12 (dorsal stream visual area, t= 3.95, pFWE= 0.004). Full list of correlations can be found in Table 3.
Correlation with age at onset of menstruation
Age at onset of menstruation was only positively associated with the correlation between ICs 12, the dorsal stream visual area, and 15, the medial parietal network (t= 3.26, pFWE= 0.048). When head motion was included in the model, the relationship between age at onset of menstruation and ICs 12 and 15 remained (t= 3.28, pFWE= 0.045).
Post Hoc Cognitive Performance and Twin Analysis Results
Cognitive performance
SES, cognitive function, glycemia, stress, physical activity, and taste were not related to the interaction of BMI and age at onset of menstruation (p’s= 0.24–1).
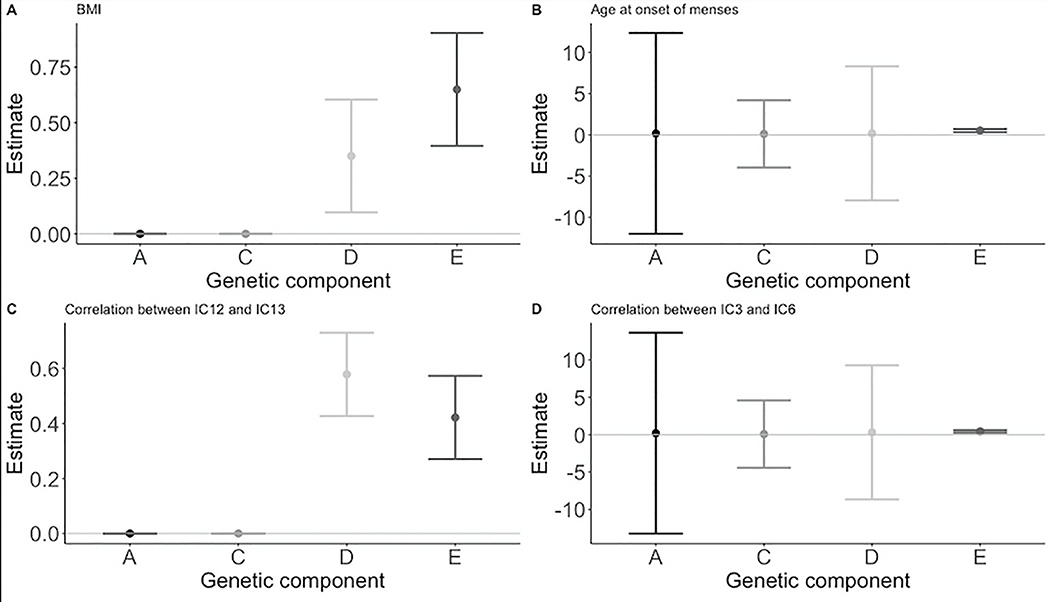
Twin Analysis
The HCP dataset is enriched with twin pairs. Table 4 outlines the distribution of twins in the sample. Additive genetic (A), common shared family (C), genetic dominance (D), and nonshared environmental (E) effects are shown in Table 5 and Figure 3. Overall, nonshared environment (E) accounted for the most significant variance for adult BMI, age at onset of menstruation, and correlations between IC 12 and IC 13, and IC 3 and IC 6. BMI and the correlation between ICs 12 (dorsal stream visual areas) and 13 (functional description) showed significant heritability (h2= 0.35, h2= 0.58 respectively). Additionally, dominant genetic factors accounted for a significant proportion of variance in both BMI (d2=0.35) and the correlation between ICs 12 and 13 (d2=0.58). The correlation between ICs 2 and 3 did not show any effect of heritability, rather common and non-shared environment accounted for a significant portion of the variability (c2=0.43, e2=0.57).
Table 4:
Description of twins in sample
| Monozygotic | Dizygotic | Total | |
|---|---|---|---|
| Pairs | 64 | 31 | 95 |
| Singletons† | 16 | 21 | 37 |
| Total | 144 | 83 | 227 |
Singletons refer to individuals who are reported being twins, but their twin was not included in the sample.
Table 5.
Variance estimates and confidence intervals from heritability analysis
| Outcome | h2 | a2 | c2 | d2 | e2 |
|---|---|---|---|---|---|
| BMI† |
0.35 (0.1, 0.6) |
0.00 (0, 0) |
0.00 (0, 0) |
0.35 (0.1, 0.6) |
0.65 (0.4, 0.9) |
| Age at onset of menstruation ‡ | 0.35 (−3.7, 4.4) |
0.16 (−12.0, 12.3) |
0.12 (−3.9, 4.2) |
0.19 (−7.9, 8.3) |
0.53 (0.3, 0.7) |
| Correlation between IC 12 and IC 13 § |
0.58 (0.4, 0.7) |
0.0 (0,0) |
0.0 (0,0) |
0.58 (0.4, 0.7) |
0.4 (0.3, 0.6) |
| Correlation between IC 3 and IC 6 § | 0.49 (−4.0, 5.0) |
0.19 (−13.2, 13.6) |
0.07 (−4.4, 4.6) |
0.30 (−8.7, 9.3) |
0.43 (0.3, 0.6) |
| Correlation between IC 2 and IC 3 § | 0.0 (0, 0) |
0.0 (0,0) |
0.43 (0.2,0.6) |
0.0 (0,0) |
0.57 (0.4,0.7) |
Bold indicates p value < 0.003
model adjusted for age at scan, race, and age at onset of menstruation, head motion
model adjusted for age at scan and race, head motion
model adjusted for age at scan, age at onset of menstruation, BMI, the interaction between age at onset of menstruation and BMI, and head motion. h2 is the total heritability, a2 is the additive genetic effect, c2 is the common environment, d2 is the dominant genetic effect, and e2 is the non-shared environmental effect.
Figure 3. Effects of Additive Genetic (A), Common Environmental (C), Dominant Genetic (D), and Unique Environmental Factors (E) on BMI, Age at Onset of Menses, and Functional Neural Correlations.
A) shows the effect of additive genetic (A), common environmental (C), dominant genetic (D), and unique environmental Factors (E) on BMI. B) shows the ACDE effects on age at onset of menses. C) shows ACDE effects on the correlation between IC12 (visual cortex) and IC13 (mouth somatosensory area). D) shows the ADCE effect on the correlation between IC3 (visual cortex) and IC6 (cingulo-opercular network).
When accounting for genetic factors, motion remained significantly associated with adult BMI (z= 11.95, SE= 8.19, p<0.00001). Additionally, age at onset of menstruation remained inversely associated with adult BMI (z= −3.32, SE= 0.02, p= 0.0009) when accounting for potential genetic effects and motion. Dominant genetic effects appear to account for the most variance between the correlation between IC 12 and IC 13 as, when included in the model, the interaction between BMI and age at onset of menstruation was no longer significantly related (z= 0.0164, p= 0.14). Environmental effects accounted for significant variance between the correlation between IC 3 and IC 6 and the effect of the interaction of BMI and age at onset of menstruation diminished (z= 2.57, p= 0.01) when genetic and motion effects were controlled. However, the age at onset of menstruation remained significantly associated with the correlation between ICs 3 and 6 in this model (z= −3.00, SE= 0.34, p= 0.003). Common and individual environmental effects accounted for significant variation in the relationship between the interaction of age at onset of menstruation and BMI and the correlation of ICs 2 and 3 as they were no longer significantly associated (z= −1.73, p=0.08).
Comparison of Akaike’s Information Criterion between models with heritability estimates versus those without are summarized in Table 6. Overall, the inclusion of quantitative heritability factors resulted in more parsimonious models, even with the smaller sample sizes overall (56).
Table 6.
Akaike’s Information Criterion of heritability and standard linear models
| Model outcome | df | AIC | AICc |
|---|---|---|---|
| BMI† | |||
| Linear model | 10 | 2875.55 | 2875.99 |
| ACDE model | 12 | 1249.87 | 1252.49 |
| Age at onset of menstruation‡ | |||
| Linear model | 8 | 826.75 | 827.41 |
| ACDE model | 10 | 812.41 | 814.25 |
| Correlation between IC 12 and IC13§ | |||
| Linear model | 11 | 1012.66 | 1013.89 |
| ACDE model | 14 | 985.98 | 989.09 |
| Correlation IC 3 and IC6§ | |||
| Linear model | 11 | 888.52 | 889.75 |
| ACDE model | 14 | 867.37 | 870.48 |
| Correlation IC 2 and IC 3§ | |||
| Linear model | 11 | 849.76 | 850.99 |
| ACDE model | 14 | 840.24 | 844.47 |
model adjusted for age at scan, race, and age at onset of menstruation, head motion
model adjusted for age at scan and race, head motion
model adjusted for age at scan, age at onset of menstruation, BMI, and the interaction between age at onset of menstruation and BMI, head motion.
Network Analysis
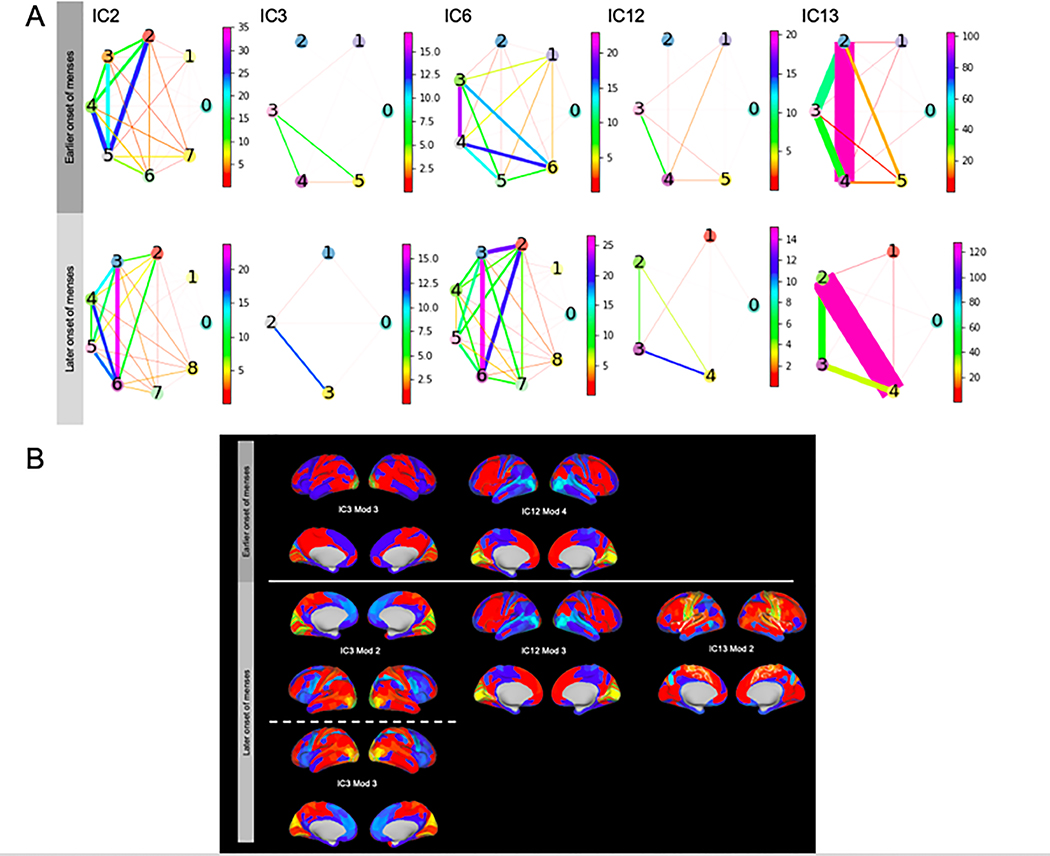
Frequency of connector nodes was significantly different between early and late onset of menses modules (chi2 = 21.67, p<0.00001). Women who were older at the onset of menstruation showed unique connector nodes in IC 13, module 2 including bilateral ROIs in the somatosensory area, premotor area, central and posterior opercular areas (Table 7, Figure 4). Module to module connectivity in IC 13 of women with later onset of menstruation showed stronger edges between module 2 and 4 compared to all other module combinations (p’s<0.05). IC 13, module 4 is made up of 16 kinless nodes bilaterally in the primary auditory complex, early auditory cortex, auditory association cortex, posterior opercular cortex, insula, and frontal opercular area. In women with later onset of menstruation within IC 3 the ventral visual stream were connector nodes in module 3, along with bilateral visual areas 1, 3, and 4 in module 2. Within IC 12 in women with later onset of menstruation, module 3 contained connector nodes comprised of bilateral visual areas 2 and 3.
Table 7.
Early and late age at onset of menstruation independent component modules and nodal descriptions
| EARLY n= 87 | LATE n= 125 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC | Module | # nodes | Connector nodes | Kinless nodes | Peripheral nodes | Connector description | Module | # nodes | Connector nodes | Kinless nodes | Peripheral nodes | Connector description |
| 2 | 0 | 5 | 1 | 1 | 3 | BMI | 0 | 3 | 1 | 0 | 2 | race |
| 1 | 2 | 0 | 2 | 0 | 1 | 1 | 0 | 1 | 0 | |||
| 2 | 5 | 0 | 5 | 0 | 2 | 3 | 0 | 3 | 0 | |||
| 3 | 5 | 0 | 5 | 0 | 3 | 7 | 0 | 7 | 0 | |||
| 4 | 7 | 0 | 7 | 0 | 4 | 5 | 0 | 5 | 0 | |||
| 5 | 10 | 0 | 10 | 0 | 5 | 5 | 0 | 5 | 0 | |||
| 6 | 3 | 0 | 3 | 0 | 6 | 8 | 0 | 8 | 0 | |||
| 7 | 2 | 0 | 2 | 0 | 7 | 1 | 0 | 1 | 0 | |||
| 8 | 1 | 0 | 1 | 0 | ||||||||
| 3 | 0 | 3 | 1 | 1 | 1 | age | 0 | 3 | 0 | 1 | 2 | |
| 1 | 2 | 0 | 1 | 1 | 1 | 2 | 0 | 2 | 0 | |||
| 2 | 1 | 0 | 1 | 0 | 2 | 6 | 6 | 0 | 0 | bilateral visual area 1, 3, and 4 | ||
| 3 | 6 | 6 | 0 | 0 | bilateral visual areas 1, 3, and 4 | 3 | 4 | 2 | 2 | 0 | bilateral ventral visual stream | |
| 4 | 2 | 0 | 2 | 0 | ||||||||
| 5 | 2 | 0 | 2 | 0 | ||||||||
| 6 | 0 | 5 | 0 | 1 | 4 | 0 | 6 | 1 | 1 | 4 | age | |
| 1 | 4 | 0 | 4 | 0 | 1 | 1 | 0 | 1 | 0 | |||
| 2 | 1 | 0 | 1 | 0 | 2 | 6 | 0 | 6 | 0 | |||
| 3 | 7 | 0 | 7 | 0 | 3 | 9 | 0 | 9 | 0 | |||
| 4 | 8 | 0 | 8 | 0 | 4 | 3 | 0 | 3 | 0 | |||
| 5 | 4 | 0 | 4 | 0 | 5 | 4 | 0 | 4 | 0 | |||
| 6 | 5 | 0 | 5 | 0 | 6 | 8 | 0 | 8 | 0 | |||
| 7 | 4 | 0 | 4 | 0 | ||||||||
| 8 | 1 | 0 | 1 | 0 | ||||||||
| 12 | 0 | 3 | 2 | 0 | 1 | age, motion | 0 | 3 | 0 | 0 | 3 | |
| 1 | 2 | 0 | 2 | 0 | 1 | 2 | 0 | 2 | 0 | |||
| 2 | 1 | 0 | 1 | 0 | 2 | 3 | 0 | 3 | 0 | |||
| 3 | 4 | 0 | 4 | 0 | 3 | 6 | 4 | 2 | 0 | Bilateral visual area 2 and 3 | ||
| 4 | 7 | 7 | 0 | 0 | bilateral visual 3, right visual 2, left visual 2, left inferior opercular | 4 | 4 | 0 | 4 | 0 | ||
| 5 | 1 | 0 | 1 | 0 | ||||||||
| 13 | 0 | 4 | 1 | 1 | 2 | motion | 0 | 4 | 0 | 2 | 2 | |
| 1 | 2 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | |||
| 2 | 15 | 0 | 15 | 0 | 2 | 17 | 7 | 10 | 0 | bilateral sensory motor area (mouth), premotor area, opercular, and posterior opercular areas | ||
| 3 | 7 | 0 | 7 | 0 | 3 | 5 | 0 | 5 | 0 | |||
| 4 | 13 | 0 | 13 | 0 | 4 | 16 | 0 | 16 | 0 | |||
| 5 | 2 | 0 | 2 | 0 | ||||||||
Figure 4. Modularity of independent components by age at onset of menstruation.
A subset of 212 women’s’ netmat2 were analyzed for modularity and connectivity. Description of each module can be found in the supplemental table 1.
A. Circles represent groups of nodes grouped into modules via the Louvain algorithm. Color and width of the connections represent strength of connection.
B. Connector nodes are highlighted in lime green the IC is highlighted in white. Descriptions of the connector nodes can be found in Table 7.
Women who were younger at the onset of menstruation showed BMI as a connector node in IC 2 module 0 and showed similar connector nodes in IC 3 and IC 12 although they were assigned different modules. Motion and age represented connector nodes in ICs 3, 12, and 13 in women in the younger age at onset of menstruation group. Motion was not a connector node in any of the ICs of women who were in the older age at onset of menstruation group. Full description of nodal differences is outlined in Table 7 and a full description of nodal roles, assignment, and area represented is in supplemental materials Table 1.
Discussion
The present study assessed the relationship between self-reported onset of menstruation, adult BMI, and functional correlation of resting state BOLD response. All results presented must be interpreted with caution as the dataset and therefore the analyses presented did not account for childhood BMI (57,58). In light of limitations, we confirmed previous research (8,19) showing a negative relationship between age at onset of menstruation and adult BMI. Although we did not confirm our hypotheses that the interaction of BMI and earlier puberty would be related to increased connectivity between reward-related (striatum, somatosensory cortex, and limbic) regions of the brain, we did find the interaction between age at onset of menstruation and adult BMI was associated with increased connectivity between the visual and cingulo-opercular network. For those with late-onset puberty, visual and cingulo-opercular connectivity increased with increasing BMI, while for women with earlier onset of menses, decreased visual and cingulo-opercular connectivity was associated with increased BMI. Similarly, women with earlier puberty showed decreased connectivity between the somatosensory network and visual network with increasing BMI, whereas this relationship between connectivity and BMI was slightly positive in women with later onset of menses. Although the relationship was not as hypothesized, several hypothesized regions within the somatosensory cortex were related to the interaction between BMI and age at onset of menstruation. Additionally, we found, overall decreased connectivity between the DMN and the visual network in women with later puberty onset, compared to those with earlier onset of menses.
Post hoc tests did not show any associations between cognitive performance measures, glycemia, stress, taste sensitivity, physical activity, or socio-economic status on the interaction between age at onset of puberty and BMI as an adult. Using a subset of twins, a post hoc quantitative heritability analyses showed unshared environmental factors explained a large portion of variation in adult BMI, age at onset of puberty, and functional correlation of brain networks and that the addition of heritability and environmental factors resulted in more parsimonious models overall. Notably, the relationship between the interaction of adult BMI and age at onset of menstruation with functional correlation between the somatosensory and visual networks was nullified with the addition of heritability parameters, as were the relationships between the cingulo-opercular and visual network, and the visual network with the DMN. However, the contribution of genetics and environment varied by correlation, with dominant genetic factors contributing to the somatosensory and visual network correlation, and shared environment factors contributing to the DMN and visual correlation. Childhood obesity has been shown to have both genetic and shared common environment influence, and these results further suggests the importance childhood obesity. A subset of the data representing women with later onset of menses (after age 13) and earlier onset of menses (before 12) were used to assess intra-independent component connectivity with topological metrics. The somatosensory mouth area, premotor cortex, and areas within the operculum as well as areas in the ventral visual stream were connector nodes in women with later onset of menstruation, but not in those with earlier menstruation, whereas motion, BMI, and the 1, 3, and 4 visual areas were connector nodes in women with earlier age at menses. The connector nodes may impart better information transfer in both the somatosensory and visual networks (ICs 12 and 13) in women with later onset of menses and the lack of connector nodes may underlie the decrease in connectivity seen in those women with earlier onset of menses (Figure 4).
Overall, the interaction between BMI and age at onset of menstruation was related to functional correlation between brain networks associated with top-down control (59), interoception (60), emotional regulation (61), visualization (62), and taste perception (63). Earlier onset of menstruation was related to decreased connectivity between the visual and somatosensory networks with increasing adult BMI. The opposite was found in those with later onset of menstruation, where correlations moderately increased between the somatosensory and visual networks with increasing BMI. The somatosensory cortex is canonically made up of the pre- and postcentral gyri, the insula, and anterior opercular areas (63). Connectivity of the somatosensory cortex and visual areas are shown to be important for bodily perception and decision making (64,65). The intersection of taste, bodily perception, and decision making is important for the study of eating behaviors and disordered eating. Positron emission tomography (PET) studies have shown that individuals with high BMI have higher resting state activity in the somatosensory cortex compared to peers with BMI between 18 and 25 (66). Further, elevated BMI has been related to decreased cohesion between somatosensory and visual networks (33). Both the increased resting state activity of the somatosensory cortex and its reduced cohesion with the visual cortex are hypothesized to increase sensory driven behaviors, such as overeating (33,66). This pattern of decreased cohesion, or correlation, between the visual and somatosensory networks is similar to the pattern seen in our sample, specifically in individuals with earlier onset of menstruation, but not in those with later menstruation. The post hoc topological connectivity further suggests that a decrease in nodal connections within the somatosensory network may underlie the decrease in somatosensory and visual connectivity. Those women with later onset of menstruation showed connector nodes in the somatosensory mouth areas, premotor areas, frontal and posterior opercular areas (Figure 4, Table 7). In the visual network (IC 12), visual areas 2 and 3 were connector nodes across groups. Thus, it appears that the visual network is somewhat conserved, though reorganized, and the somatosensory network appears to drive the differential connectivity between women with earlier and later onset of menstruation. Further, reduced correlations between the somatosensory and visual network has been observed with increasing bulimia symptoms (67). These findings along with our own suggest that the relationship between earlier onset of puberty and adult BMI may be related to increased sensory driven behaviors, but decreased bodily perception (33,65,67). Similarities between functional correlations in individuals who went through puberty early and disordered eating is in line with previous research demonstrating that being more developmentally advanced compared to one’s peers is a risk factor for disordered eating in adolescence and adulthood (68–70), specifically for clinical diagnosis of bulimia nervosa (71). Similar retrospective research shows that adult women who reported going through puberty earlier than their peers were more dissatisfied with the physical changes accompanying pubertal development and this predicted eating disorder symptoms (72). Childhood BMI may underlie dissatisfaction and therefore may be related to correlation patterns seen presently. Thus, the differences in functional correlation by onset of menstruation and adult BMI may be rooted in a psychosocial etiology, however the need to test this relationship with childhood BMI remains.
The insula has been shown to be an integral region for not only somatosensory/taste processing, but also top-down long term task control (63,73,74). Previous research from our group shows increased correlations between a functional network including the occipital pole and another comprised of the insula, central operculum, precentral gyrus, and anterior cingulate in twins with lower BMI compared to their higher BMI twin (27). On the contrary, adolescents with an elevated BMI exhibited reduced global connectivity in a region containing the insula, frontal operculum, and middle temporal gyrus (75). While the insula is important in both studies, these networks appear distinct from the somatosensory and seem to comprise the cingulo-opercular task control network (73). As the name suggests, the cingulo-opercular task control network is implicated in long term task maintenance and goals (73). In the context of the cingulo-opercular network, the insula and dorsal anterior cingulate have specific roles signaling behaviorally important events and updating attentional processes accordingly (76). It is not surprising that the cingulo-opercular network and visual network are functionally correlated, as behaviorally important information and attention often requires visual cue processing (77). Visual processing speed is highly correlated to cingulo-opercular activity (78). The present analysis showed an inverse relationship between age at onset of menstruation and adult BMI on functional correlations between the cingulo-opercular network and the primary visual network. Similar to the relationship between the somatosensory network and the visual network, individuals who went through puberty early showed decreased connectivity between the cingulo-opercular and primary visual network with increasing BMI. Alternately, women who went through puberty later exhibited increasing connectivity between the cingulo-opercular network and the primary visual network with increasing BMI. This dichotomy in cingulo-opercular network connectivity has been seen across obesity research. Increased insula and superior frontal gyrus activity has been associated with elevated BMI (77), while decreased connectivity between the cingulo-opercular and other networks has also been seen in individuals with high BMI (74). The post hoc connectivity analysis reveals that the cingulo-opercular network is largely conserved regardless of age at onset of menstruation. However, the visual network of women who achieved menses later showed the ventral visual area as a connector node. The ventral visual area has been implicated in bodily perception. Thus, women who went through puberty later and have a high BMI may increase functional correlations between the cingulo-opercular network and the visual network through the ventral visual stream reflecting increased awareness of their body. Weygandt and colleagues hypothesize that the increased correlation between the insula and visual areas may reflect better goal maintenance in the face of visual food cues (79). This improved ability to lose weight could be due to the ventral visual area updating bodily status, imparting a better perception of one’s self while trying to lose weight. Therefore, women who go through puberty later in childhood may have better success losing weight compared to women who go through puberty earlier at the same BMI because they have better bodily perception. The ventral visual cortex has been shown to develop throughout adolescence, achieving peak function in adulthood (80). Earlier onset of puberty may attenuate this development, in accordance with Schulz and colleagues’ organizational activational hypothesis, in which earlier exposure to sex hormones results in altered neural organization and adult behavior (21). The present study takes this hypothesis one step further, suggesting that earlier puberty not only results in altered neural organization and behavior, but also altered physiology and sensitivity to weight loss (79).
The interaction of adult BMI and age at puberty onset may additionally influence visual bias toward food related stimuli. Women who went through puberty earlier showed increased functional correlation between the DMN and visual network, whereas women who went through puberty later showed decreased correlation between the areas with increasing adult BMI. The DMN is a peculiar network as it is traditionally characterized by its decrease in activity during external attention tasks (81). This has led to many suggesting that the DMN is simply a rest network, or encoding subconscious neural activity (82). However, the DMN is activated in self-referential tasks, such as autobiographical memory (82). Based on the anatomical structure of the DMN, others have suggested that it represents a “sensory-visceromotor link” (81). Evidence of the sensory-visceromotor function of the DMN has been previously observed in overfed individuals with healthy BMI who exhibited increased activation in the DMN compared to those with an overweight BMI (83). This suggests the DMN is not only inactivated during a task, but also integrates internal visceral and external sensory signals (81). In light of the “sensory-visceromotor” definition, the increased connectivity between the DMN and visual network with increasing BMI and earlier puberty may suggest a shift in the DMN from visceral signals towards sensory stimuli. Similarly, increased DMN and visual network correlations with increased BMI, were previously reported in other studies using the HCP data (27,33). Additionally, regional assessment of the DMN showed an increase in the inferior parietal cortex and posterior cingulate cortex, which integrates food images with visual attention (84) and generates a bias towards food cues, respectively (85). The post hoc connectivity analysis corroborates the previous findings, as the only connector node in the DMN network was BMI in the earlier menses group. This indicates that BMI is mediating transfer of information within the DMN and potentially increasing connectivity within the DMN. The interaction between age at onset of puberty and high adult BMI may be a unique phenotype of women who are less sensitive to internal visceral signals with a visual bias to food cues.
The novel nature of the findings presented here warranted further characterization, therefore we ran a series of post hoc tests to assess the relation of socio-economic (education, household income), physiological (glycemia, taste sensitivity, physical activity, stress), cognitive (delayed discounting, working memory, inhibitory control), and heritable factors to our results. While we did not have a priori hypotheses related to these measures, all were chosen based on previous published work: socio-economic (86), physiological (25,69,77,87), cognitive (88–90), and heritability (91,92). Despite these previous relationships, we did not find any association between socio-economic, physiological, nor cognitive measures and the interaction of age at onset of puberty and adult BMI. The lack of association between HCP behavioral measures and BMI is not unique (27,33,87), and could be due to a lack of specificity of the tasks.
Previous genome wide association studies have identified multiple loci related to both earlier puberty and high BMI in adulthood independent of childhood BMI (91). The high frequency of both monozygotic and dizygotic twin pairs within the HCP dataset allowed us to run post hoc analyses exploring potential quantitative genetic factors: additive genetic (A), common environmental (C), dominant genetic (D), and unique environmental factors (E) (93). The ACDE model presumes that monozygotic twins completely share their genetic material, therefore any differences between the twins is attributed to unique environment (which also includes measurement error) (93). Dizygotic twins share an average of half of their genetic material, therefore differences between dizygotic twins could be due to non-shared genetic or non-shared environmental factors (93). Genetic effects can be further divided into additive or dominant effects (93). A factor is considered additive if the correlation of genetic effects is 50%, whereas if the correlation is near 25% it is assumed to be a dominant effect (93).
Under these model assumptions, we found that BMI remained inversely associated with age at onset of menstruation (z= −3.32, p= 0.0009) and positively associated with head motion (z= 11.95, p<0.00001) when controlling for heritability. Dominant genetic effects accounted for a significant portion of variance in BMI and the correlation between IC 12 and IC 13. When the genetic and environmental effects were included in the model, the association between the interaction between adult BMI and age at onset of menstruation and the correlation between the somatosensory and visual networks was attenuated (z= 0.0164, p= 0.14). The association between the correlation of the visual network in IC 3 and the cingulo-opercular network in IC 6 and the interaction of BMI and age at onset of menstruation was diminished when accounting for potential genetic and environmental effects as well (z= 2.57, p= 0.01). However, unshared environmental factors appear to drive this attenuation (z = 11.07, p< 0.0001). Further, the association between the interaction of BMI and age at onset of menstruation and correlations between the DMN and visual network was also curtailed with addition of potential genetic and environmental factors (z= −1.73, p=0.08). While unshared environmental factors impacted the relationship between the visual network and cingulo-opercular network, common genetic factors (those shared between siblings) accounted for the most variance between the DMN and visual networks of ICs 2 and 3. The results from this post hoc analysis suggest that the relationship between BMI, age at onset of puberty, and functional correlations are sensitive to genetic and environmental factors. However, for a given functional correlation genetic or environmental effects vary. Obesity has been shown to be multi-causal with both environmental and genetic components (94) and presently it appears that the functional correlations associated with BMI and age at onset of menstruation are also related to the environment and genes. Age at onset of menstruation showed little effect of heritability between twins, however adult BMI and the correlation between the somatosensory and visual networks both showed significant heritability. Childhood obesity and BMI have been shown to be highly heritable (a2=0.60–0.74) (95). Therefore, since the correlation between the somatosensory and visual networks and BMI showed significant dominant genetic effects, it is possible that childhood BMI is a critical source of variance for the connectivity and for adult BMI (8,19). The correlation between the DMN and visual networks in ICs 2 and 3 additionally suggest a possible role of childhood BMI, since children who live in the same home are consuming the same foods and have similar BMIs (96). These results highlight the potential importance of childhood BMI, and how it may contribute to multiple sources of both genetic and environmental variation.
Although the cross-sectional nature of this study precludes mechanistic analysis, the dopamine literature suggests a potential mechanism for future research. Dopamine synthesis has been positively correlated with cingulo-opercular network connectivity (97). Additionally, defective dopamine synthesis has been implicated in the overexpression of the orexigenic hypothalamic neuro-peptide Y (98). The cingulo-opercular network has been shown to be preferentially connected to the dopamine sensitive nucleus accumbens (NAcc) (99). Therefore, impaired dopamine synthesis may act via the NAcc to decrease cingulo-opercular connectivity, while simultaneously promoting eating behaviors through the hypothalamus. Earlier onset of menarche has been associated with higher levels of sex hormones and decreased sex hormone binding globulin, allowing for an increase in free sex hormones (13). The limbic-hypothalamic pituitary-adrenal (LHPA) axis is sensitive to sex hormones (25) and dopaminergic projections from the limbic system modulate NAcc activity (100). This additionally aligns with the organizational-activational hypothesis, and hypothesizes that early puberty in women increases free sex hormones which create a cascade effect: decreasing dopamine production and thereby protracting cingulo-opercular connectivity via the NAcc, functionally reorganizing the brain as presented here. The longitudinal adolescent, brain, cognition, and development dataset will facilitate testing this hypothesis, and shed light on the importance of childhood BMI (101).
This study has several notable limitations. First, this is a cross-sectional study with a retrospective proxy of pubertal development. While use of age at onset of menstruation has been reported to have high reproducibility (43), the validation between self-report in adolescents and again in adulthood is only moderate (102). Second, because this study relies on age at onset of menstruation it precludes subjects who do not menstruate. Therefore, these results are not generalizable to other populations. Third, this sample is predominantly white and was screened to be generally healthy. Given the known differences between racial categories on the age at onset of menstruation (103), further work is needed using more diverse samples. Fourth and finally, evidence exists of a reciprocal relationship between childhood BMI, age at onset of puberty, and adult BMI (8,10,91). These factors may not be independent, but rather synergistic. Childhood BMI may influence the onset of puberty, which in turn may predispose children to metabolic, psychological, and social adversity culminating in poor health in adulthood.
Conclusions
In a large sample of adult women, we found that pubertal onset, operationalized as age of onset of menstruation, was negatively associated with adult BMI. The interaction of pubertal onset and adult BMI was associated with resting state functional correlation of networks. The interaction was associated with decreased connectivity of visual network with somatosensory and cingulo-opercular networks. Early onset of menstruation is established as a risk factor for disease states such as obesity (19), but the present study is the first to show that the interaction of pubertal onset and adult BMI is related to neural connectivity. Our study adds to a growing body of evidence suggesting puberty is a critical period of development for immediate and distal health outcomes.
Supplementary Material
Acknowledgments
GES was responsible for the design, data analysis, and writing of the manuscript. JRS and KSB were responsible for design considerations and editing of the manuscript. AP was responsible for editing the manuscript. Data were provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases R01 DK112317 and the American Diabetes Association 1-17-JDF-031. The authors have no competing financial interests to disclose.
Data Availability
The data that support the findings of this study are openly available in connectomeDB at https://db.humanconnectome.org/app/template/Login.vm;jsessionid=FDE86FD272E119A1708AC55CDC5D3BAF, reference number HCP1200 PTN.
References
- 1.NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016. April 2;387(10026):1377–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity’s impact. Fertil Steril. 2017. March 11;107(4):840–7. [DOI] [PubMed] [Google Scholar]
- 3.Huebschmann AG, Huxley RR, Kohrt WM, Zeitler P, Regensteiner JG, Reusch JEB. Sex differences in the burden of type 2 diabetes and cardiovascular risk across the life course. Diabetologia. 2019. October;62(10):1761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Censani M, Feuer A, Orton S, Askin G, Vogiatzi M. Changes in body mass index in children on gonadotropin-releasing hormone agonist therapy with precocious puberty, early puberty or short stature. J Pediatr Endocrinol Metab. 2019. October 25;32(10):1065–70. [DOI] [PubMed] [Google Scholar]
- 5.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004. October;7(10):1040–7. [DOI] [PubMed] [Google Scholar]
- 6.Mendle J, Ryan RM, McKone KMP. Age at menarche, depression, and antisocial behavior in adulthood. Pediatrics. 2018;141(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Day FR, Elks CE, Murray A, Ong KK, Perry JRB. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: the UK Biobank study. Sci Rep. 2015. June 18;5:11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prentice P, Viner RM. Pubertal timing and adult obesity and cardiometabolic risk in women and men: a systematic review and meta-analysis. Int J Obes. 2013. August;37(8):1036–43. [DOI] [PubMed] [Google Scholar]
- 9.Widén E, Silventoinen K, Sovio U, Ripatti S, Cousminer DL, Hartikainen A-L, et al. Pubertal timing and growth influences cardiometabolic risk factors in adult males and females. Diabetes Care. 2012. April;35(4):850–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frontini MG, Srinivasan SR, Berenson GS. Longitudinal changes in risk variables underlying metabolic Syndrome X from childhood to young adulthood in female subjects with a history of early menarche: the Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2003. November;27(11):1398–404. [DOI] [PubMed] [Google Scholar]
- 11.Lim SW, Ahn JH, Lee JA, Kim DH, Seo J-H, Lim JS. Early menarche is associated with metabolic syndrome and insulin resistance in premenopausal Korean women. Eur J Pediatr. 2016. January;175(1):97–104. [DOI] [PubMed] [Google Scholar]
- 12.Farahmand M, Tehrani FR, Dovom MR, Azizi F. Menarcheal Age and Risk of Type 2 Diabetes: A Community-Based Cohort Study. J Clin Res Pediatr Endocrinol. 2017. June 1;9(2):156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elks CE, Ong KK, Scott RA, van der Schouw YT, Brand JS, Wark PA, et al. Age at menarche and type 2 diabetes risk: the EPIC-InterAct study. Diabetes Care. 2013. November;36(11):3526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minelli C, van der Plaat DA, Leynaert B, Granell R, Amaral AFS, Pereira M, et al. Age at puberty and risk of asthma: A Mendelian randomisation study. PLoS Med. 2018. August 7;15(8):e1002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Werneck AO, Coelho-E-Silva MJ, Padilha CS, Ronque ERV, Cyrino ES, Szwarcwald CL, et al. Age at menarche and cancer risk at adulthood. Ann Hum Biol. 2018. June;45(4):369–72. [DOI] [PubMed] [Google Scholar]
- 16.Avendaño MS, Vazquez MJ, Tena-Sempere M. Disentangling puberty: novel neuroendocrine pathways and mechanisms for the control of mammalian puberty. Hum Reprod Update. 2017. November 1;23(6):737–63. [DOI] [PubMed] [Google Scholar]
- 17.Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017. September;67(5):378–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez-Llorente MA, Romero R, Chueca N, Martinez-Cañavate A, Gomez-Llorente C. Obesity and asthma: A missing link. Int J Mol Sci. 2017. July 11;18(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill D, Brewer CF, Del Greco MF, Sivakumaran P, Bowden J, Sheehan NA, et al. Age at menarche and adult body mass index: a Mendelian randomization study. Int J Obes. 2018. February 26;42(9):1574–81. [DOI] [PubMed] [Google Scholar]
- 20.Piekarski DJ, Johnson CM, Boivin JR, Thomas AW, Lin WC, Delevich K, et al. Does puberty mark a transition in sensitive periods for plasticity in the associative neocortex? Brain Res. 2017. January 1;1654(Pt B):123–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009. May;55(5):597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caballero A, Granberg R, Tseng KY. Mechanisms contributing to prefrontal cortex maturation during adolescence. Neurosci Biobehav Rev. 2016. November;70:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Papies EK, Barsalou LW. A core eating network and its modulations underlie diverse eating phenomena. Brain Cogn. 2016. May 5;110:20–42. [DOI] [PubMed] [Google Scholar]
- 24.Yang X, Casement M, Yokum S, Stice E. Negative affect amplifies the relation between appetitive-food-related neural responses and weight gain over three-year follow-up among adolescents. Neuroimage Clin. 2019. November 5;24:102067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michopoulos V Stress-induced alterations in estradiol sensitivity increase risk for obesity in women. Physiol Behav. 2016. November 1;166:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chodkowski BA, Cowan RL, Niswender KD. Imbalance in Resting State Functional Connectivity is Associated with Eating Behaviors and Adiposity in Children. Heliyon. 2016. January;2(1):e00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadler JR, Shearrer GE, Burger KS. Body mass variability is represented by distinct functional connectivity patterns. Neuroimage. 2018. November 1;181:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geha P, Cecchi G, Todd Constable R, Abdallah C, Small DM. Reorganization of brain connectivity in obesity. Hum Brain Mapp. 2017;38(3):1403–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shearrer GE, Stice E, Burger KS. Adolescents at high risk of obesity show greater striatal response to increased sugar content in milkshakes. Am J Clin Nutr. 2018. June 1;107(6):859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci. 2011. March 23;31(12):4360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang Z, Spaeth AM, Ma N, Zhu S, Hu S, Goel N, et al. Altered salience network connectivity predicts macronutrient intake after sleep deprivation. Sci Rep. 2015. February 3;5:8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park B-Y, Chung C-S, Lee MJ, Park H. Accurate neuroimaging biomarkers to predict body mass index in adolescents: a longitudinal study. Brain Imaging Behav. 2019. May 7; [DOI] [PubMed] [Google Scholar]
- 33.Doucet GE, Rasgon N, McEwen BS, Micali N, Frangou S. Elevated Body Mass Index is Associated with Increased Integration and Reduced Cohesion of Sensory-Driven and Internally Guided Resting-State Functional Brain Networks. Cereb Cortex. 2018. March 1;28(3):988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Essen DC, Smith SM, Barch DM, Behrens TEJ, Yacoub E, Ugurbil K, et al. The WU-Minn Human Connectome Project: an overview. Neuroimage. 2013. October 15;80:62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, et al. Resting-state fMRI in the Human Connectome Project. Neuroimage. 2013. October 15;80:144–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013. October 15;80:105–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson EC, Jbabdi S, Glasser MF, Andersson J, Burgess GC, Harms MP, et al. MSM: a new flexible framework for Multimodal Surface Matching. Neuroimage. 2014. October 15;100:414–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012. May;67(5):1210–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Gunther M, et al. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PLoS One. 2010. December 20;5(12):e15710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004. February 1;23(2):137–52. [DOI] [PubMed] [Google Scholar]
- 41.Smith SM, Hyvärinen A, Varoquaux G, Miller KL, Beckmann CF. Group-PCA for very large fMRI datasets. Neuroimage. 2014. November 1;101:738–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hyvärinen A Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans Neural Netw. 1999;10(3):626–34. [DOI] [PubMed] [Google Scholar]
- 43.Lundblad MW, Jacobsen BK. The reproducibility of self-reported age at menarche: The Tromsø Study. BMC Womens Health. 2017. August 22;17(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pokrzywniak AS, A K. Reliability of Self-Reported Weight, Age at Menarche and Menopause, and Reason for Absence of Menses: A Cohort Study. J Womens Health, Issues Care. 2016;5(4). [Google Scholar]
- 45.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014. May 15;92:381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE. Generation and Evaluation of a Cortical Area Parcellation from Resting-State Correlations. Cereb Cortex. 2016. January;26(1):288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011. September;106(3):1125–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Team RC. R: A Language and Environment for Statistical Computing. 2019;
- 49.Scheike TH, Holst KK, Hjelmborg JB. Estimating heritability for cause specific mortality based on twin studies. Lifetime Data Anal. 2014. April;20(2):210–33. [DOI] [PubMed] [Google Scholar]
- 50.Holst KK, Scheike TH, Hjelmborg JB. The liability threshold model for censored twin data. Comput Stat Data Anal. 2016. January;93:324–35. [Google Scholar]
- 51.Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, et al. A multimodal parcellation of human cerebral cortex. Nature. 2016. August 11;536(7615):171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blondel VD, Guillaume J-L, Lambiotte R, Lefebvre E. Fast unfolding of communities in large networks. J Stat Mech. 2008. October 9;2008(10):P10008. [Google Scholar]
- 53.Meunier D, Lambiotte R, Fornito A, Ersche KD, Bullmore ET. Hierarchical modularity in human brain functional networks. Front Neuroinformatics. 2009. October 30;3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.R Foundation for Statistical Computing RCT. R: A language and environment for statistical computing.. Vienna, Austria.; 2019. [Google Scholar]
- 55.Chumlea WC, Schubert CM, Roche AF, Kulin HE, Lee PA, Himes JH, et al. Age at menarche and racial comparisons in US girls. Pediatrics. 2003. January;111(1):110–3. [DOI] [PubMed] [Google Scholar]
- 56.Burnham KP, Anderson DR. Multimodel Inference: Understanding AIC and BIC in Model Selection. Sociol Methods Res. 2004. November 1;33(2):261–304. [Google Scholar]
- 57.Holmgren A, Niklasson A, Nierop AFM, Gelander L, Aronson AS, Sjöberg A, et al. Pubertal height gain is inversely related to peak BMI in childhood. Pediatr Res. 2017;81(3):448–54. [DOI] [PubMed] [Google Scholar]
- 58.Durda-Masny M, Hanć T, Czapla Z, Szwed A. BMI at menarche and timing of growth spurt and puberty in Polish girls - longitudinal study. Anthropol Anz. 2019. March 28;76(1):37–47. [DOI] [PubMed] [Google Scholar]
- 59.Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci USA. 2007. August 14;104(33):13507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mata F, Verdejo-Roman J, Soriano-Mas C, Verdejo-Garcia A. Insula tuning towards external eating versus interoceptive input in adolescents with overweight and obesity. Appetite. 2015. October;93:24–30. [DOI] [PubMed] [Google Scholar]
- 61.Brandl F, Le Houcq Corbi Z, Mulej Bratec S, Sorg C. Cognitive reward control recruits medial and lateral frontal cortices, which are also involved in cognitive emotion regulation: A coordinate-based meta-analysis of fMRI studies. Neuroimage. 2019. October 15;200:659–73. [DOI] [PubMed] [Google Scholar]
- 62.Kullmann S, Pape A-A, Heni M, Ketterer C, Schick F, Häring H-U, et al. Functional network connectivity underlying food processing: disturbed salience and visual processing in overweight and obese adults. Cereb Cortex. 2013. May;23(5):1247–56. [DOI] [PubMed] [Google Scholar]
- 63.Small DM. Flavor is in the brain. Physiol Behav. 2012. November 5;107(4):540–52. [DOI] [PubMed] [Google Scholar]
- 64.Pleger B, Villringer A. The human somatosensory system: from perception to decision making. Prog Neurobiol. 2013. April;103:76–97. [DOI] [PubMed] [Google Scholar]
- 65.Kuehn E, Pleger B. How visual body perception influences somatosensory plasticity. Neural Plast. 2018. March 11;2018:7909684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang G-J, Volkow ND, Felder C, Fowler JS, Levy AV, Pappas NR, et al. Enhanced resting activity of the oral somatosensory cortex in obese subjects. Neuroreport. 2002. July 2;13(9):1151–5. [DOI] [PubMed] [Google Scholar]
- 67.Lavagnino L, Amianto F, D’Agata F, Huang Z, Mortara P, Abbate-Daga G, et al. Reduced resting-state functional connectivity of the somatosensory cortex predicts psychopathological symptoms in women with bulimia nervosa. Front Behav Neurosci. 2014. August 4;8:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Killen JD, Hayward C, Litt I, Hammer LD, Wilson DM, Miner B, et al. Is puberty a risk factor for eating disorders? Am J Dis Child. 1992. March;146(3):323–5. [DOI] [PubMed] [Google Scholar]
- 69.Chong LS, Chin YS, Gan WY, Nasir MTM. Associations between socio-demographic characteristics and pubertal status with disordered eating among primary school children in Selangor, Malaysia. Asia Pac J Clin Nutr. 2017. March;26(2):326–33. [DOI] [PubMed] [Google Scholar]
- 70.Zehr JL, Culbert KM, Sisk CL, Klump KL. An association of early puberty with disordered eating and anxiety in a population of undergraduate women and men. Horm Behav. 2007. November;52(4):427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaltiala-Heino R, Rimpelä M, Rissanen A, Rantanen P. Early puberty and early sexual activity are associated with bulimic-type eating pathology in middle adolescence. J Adolesc Health. 2001. April;28(4):346–52. [DOI] [PubMed] [Google Scholar]
- 72.Moore SR, McKone KMP, Mendle J. Recollections of puberty and disordered eating in young women. J Adolesc. 2016. December;53:180–8. [DOI] [PubMed] [Google Scholar]
- 73.Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci (Regul Ed). 2008. March;12(3):99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kullmann S, Heni M, Veit R, Ketterer C, Schick F, Häring H-U, et al. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp. 2012. May;33(5):1052–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moreno-Lopez L, Contreras-Rodriguez O, Soriano-Mas C, Stamatakis EA, Verdejo-Garcia A. Disrupted functional connectivity in adolescent obesity. Neuroimage Clin. 2016. July 12;12:262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Han SW, Eaton HP, Marois R. Functional Fractionation of the Cingulo-opercular Network: Alerting Insula and Updating Cingulate. Cereb Cortex. 2019. June 1;29(6):2624–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Devoto F, Zapparoli L, Bonandrini R, Berlingeri M, Ferrulli A, Luzi L, et al. Hungry brains: A meta-analytical review of brain activation imaging studies on food perception and appetite in obese individuals. Neurosci Biobehav Rev. 2018. July 30;94:271–85. [DOI] [PubMed] [Google Scholar]
- 78.Ruiz-Rizzo AL, Sorg C, Napiórkowski N, Neitzel J, Menegaux A, Müller HJ, et al. Decreased cingulo-opercular network functional connectivity mediates the impact of aging on visual processing speed. Neurobiol Aging. 2019;73:50–60. [DOI] [PubMed] [Google Scholar]
- 79.Weygandt M, Spranger J, Leupelt V, Maurer L, Bobbert T, Mai K, et al. Interactions between neural decision-making circuits predict long-term dietary treatment success in obesity. Neuroimage. 2019. January 1;184:520–34. [DOI] [PubMed] [Google Scholar]
- 80.Golarai G, Liberman A, Yoon JMD, Grill-Spector K. Differential development of the ventral visual cortex extends through adolescence. Front Hum Neurosci. 2010. February 22;3:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015. July 8;38:433–47. [DOI] [PubMed] [Google Scholar]
- 82.Buckner RL, DiNicola LM. The brain’s default network: updated anatomy, physiology and evolving insights. Nat Rev Neurosci. 2019. September 6;20(10):593–608. [DOI] [PubMed] [Google Scholar]
- 83.Tregellas JR, Wylie KP, Rojas DC, Tanabe J, Martin J, Kronberg E, et al. Altered default network activity in obesity. Obesity (Silver Spring). 2011. December;19(12):2316–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nachev P, Husain M. Disorders of visual attention and the posterior parietal cortex. Cortex. 2006. July;42(5):766–73. [DOI] [PubMed] [Google Scholar]
- 85.Mohanty A, Gitelman DR, Small DM, Mesulam MM. The spatial attention network interacts with limbic and monoaminergic systems to modulate motivation-induced attention shifts. Cereb Cortex. 2008. November;18(11):2604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parsons TJ, Power C, Logan S, Summerbell CD. Childhood predictors of adult obesity: a systematic review. Int J Obes Relat Metab Disord. 1999. November;23 Suppl 8:S1–107. [PubMed] [Google Scholar]
- 87.Sadler JR, Shearrer GE, Burger KS. Alterations in ventral attention network connectivity in individuals with prediabetes. Nutr Neurosci. 2019. April 28;1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Filbey FM, Yezhuvath US. A multimodal study of impulsivity and body weight: Integrating behavioral, cognitive, and neuroimaging approaches. Obesity (Silver Spring). 2017;25(1):147–54. [DOI] [PubMed] [Google Scholar]
- 89.Prickett C, Brennan L, Stolwyk R. Examining the relationship between obesity and cognitive function: a systematic literature review. Obes Res Clin Pract. 2015. April;9(2):93–113. [DOI] [PubMed] [Google Scholar]
- 90.Mamrot P, Hanć T. The Association of the Executive Functions with Overweight and Obesity Indicators in Children and Adolescents: A Literature Review. Neurosci Biobehav Rev. 2019. August 27; [DOI] [PubMed] [Google Scholar]
- 91.Bell JA, Carslake D, Wade KH, Richmond RC, Langdon RJ, Vincent EE, et al. Influence of puberty timing on adiposity and cardiometabolic traits: A Mendelian randomisation study. PLoS Med. 2018. August 28;15(8):e1002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vainik U, Baker TE, Dadar M, Zeighami Y, Michaud A, Zhang Y, et al. Neurobehavioral correlates of obesity are largely heritable. Proc Natl Acad Sci USA. 2018. September 11;115(37):9312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Posthuma D, Beem AL, de Geus EJC, van Baal GCM, von Hjelmborg JB, Iachine I, et al. Theory and practice in quantitative genetics. Twin res. 2003. October;6(05):361–76. [DOI] [PubMed] [Google Scholar]
- 94.Rohde K, Keller M, la Cour Poulsen L, Blüher M, Kovacs P, Böttcher Y. Genetics and epigenetics in obesity. Metab Clin Exp. 2019;92:37–50. [DOI] [PubMed] [Google Scholar]
- 95.Haworth CMA, Plomin R, Carnell S, Wardle J. Childhood obesity: genetic and environmental overlap with normal-range BMI. Obesity (Silver Spring). 2008. July;16(7):1585–90. [DOI] [PubMed] [Google Scholar]
- 96.Schrempft S, van Jaarsveld CHM, Fisher A, Herle M, Smith AD, Fildes A, et al. Variation in the heritability of child body mass index by obesogenic home environment. JAMA Pediatr. 2018. December 1;172(12):1153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McCutcheon RA, Nour MM, Dahoun T, Jauhar S, Pepper F, Expert P, et al. Mesolimbic dopamine function is related to salience network connectivity: an integrative positron emission tomography and magnetic resonance study. Biol Psychiatry. 2019. March 1;85(5):368–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Levin BE, Dunn-Meynell AA. Dysregulation of arcuate nucleus preproneuropeptide Y mRNA in diet-induced obese rats. Am J Physiol. 1997. May;272(5 Pt 2):R1365–70. [DOI] [PubMed] [Google Scholar]
- 99.Huckins JF, Adeyemo B, Miezin FM, Power JD, Gordon EM, Laumann TO, et al. Reward-related regions form a preferentially coupled system at rest. Hum Brain Mapp. 2019. February 1;40(2):361–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30(2):215–38. [DOI] [PubMed] [Google Scholar]
- 101.Uban KA, Horton MK, Jacobus J, Heyser C, Thompson WK, Tapert SF, et al. Biospecimens and the ABCD study: Rationale, methods of collection, measurement and early data. Dev Cogn Neurosci. 2018. August;32:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cooper R, Blell M, Hardy R, Black S, Pollard TM, Wadsworth MEJ, et al. Validity of age at menarche self-reported in adulthood. J Epidemiol Community Health. 2006. November;60(11):993–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bleil ME, Booth-LaForce C, Benner AD. Race disparities in pubertal timing: Implications for cardiovascular disease risk among African American women. Popul Res Policy Rev. 2017. October;36(5):717–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.