Abstract
Here we investigated the inhibitory effects in rats of mature Citrus unshiu peel (Chenpi) and its component hesperidin on aspirin-induced oxidative damage. The content of hesperidin in Chenpi extract was approximately 11.4%. Wistar rats were orally administered Chenpi extract or hesperidin (20 mg/kg body weight) and then were orally administered aspirin (200 mg/kg body weight) to induce oxidative damage to the stomach, liver, and kidneys. Such damage was evaluated using the formamidopyrimidine DNA glycosylase-modified comet assay. We also measured the amount of the oxidative marker 8-oxo-7,8-dihydroguanine (8-oxodG) in the stomach. Aspirin-induced damage to the gastric mucosa was evaluated using a bleeding score. Chenpi extract and hesperidin significantly inhibited aspirin-induced oxidative DNA damage. The bleeding score of the aspirin-induced gastric mucosa was significantly reduced by treatment with Chenpi extract and hesperidin. To investigate the effects of Chenpi extract and hesperidin on the analgesic effect of aspirin on ddY mice, we employed the acetic acid-induced writhing response test. Chenpi extract and hesperidin did not significantly affect the analgesic effect of aspirin. These results suggest that Chenpi extract and hesperidin significantly inhibit aspirin-induced gastric mucosal damage.
Keywords: Chenpi; hesperidin; aspirin-induced oxidative mucosal damage; gastric ulcer; 8-oxo-7,8-dihydroguanine
Introduction
Peptic ulcer is a serious gastrointestinal disease. The main causes of clinically observed gastric and duodenal ulcers are Helicobacter pylori (H. pylori) infection and nonsteroidal anti-inflammatory drugs (NSAIDs), which are referred to as H. pylori gastric ulcer and NSAIDs gastric ulcer, respectively. NSAIDs are typically taken to relieve pain, fever, and various inflammatory diseases. Acetylsalicylic acid (aspirin), which is one of the most widely used NSAIDs worldwide, has anti-inflammatory, antipyretic, antithrombotic, and analgesic effects.(1) NSAIDs are typically used as first-line analgesics, despite the risk of associated adverse events such as severe gastrointestinal bleeding.(2)
Aspirin irreversibly inhibits the enzymatic activities of COX-1 and COX-2 to block the arachidonic acid cascade. Blocking COX-2 activity using NSAIDS suppresses inflammatory responses and pain, although NSAIDs suppresses COX-1 activity and induces injury to the gastric mucosa.(3) Under inflammatory conditions such as NSAIDs-induced gastric ulcer, inflammatory cells release reactive oxygen species and reactive nitrogen species, which damage DNA through the formation of 8-oxo-7,8-dihydroguanine (8-oxodG).(4–6)
Inhibiting COX-1 activity using NSAIDs induces the leukotriene synthesis pathway and causes oxidative damage to various organs. The major adverse effects of NSAIDs include significant gastrointestinal upset, gastritis, ulceration, hemorrhage, and death.(7) In Japan, the prevalence of upper gastrointestinal lesions in patients prescribed NSAIDs is 62%.(8)
A substantial amount of aspirin is hydrolyzed to salicylic acid by esterase,(9) and approximately 60% of salicylic acid is nonenzymatically converted to the primary metabolites 2,3-dihydroxybenzoic acid (2,3-DHBA) and 2,5-dihydroxybenzoic acid.(10) Further, 2,3-DHBA increases the production of 8-oxodG in the PANC-1 human pancreatic cancer cell line, and 2,3-DHBA causes oxidative DNA damage in the presence of Cu(II) and NADH.(11)
Many patients must take other drugs during treatment with NSAIDs to avoid causing gastrointestinal-associated adverse effects. Various methods are employed to minimize such adverse effects induced by NSAIDs. For example, the traditional Chinese medicines pogostone and patchouli alcohol of Pogostemon cablin (Blanco) Benth (Labiatae) have potential anti-inflammatory and antiulcer effects.(12,13)
Dried Citrus peels are widely used as a traditional medicine in Korea, China, and Japan. The mature peel of Citrus unshiu (Chenpi) is used for improving bronchial and asthmatic conditions as well as for improving the circulation of cardiac and peripheral blood.(14,15) Citrus peels contain more flavonoids, phenolic acids, and limonoids than citrus juice.(16) The immature and mature peels of Citrus unshiu contain flavonoids such as hesperidin (Fig. 1A), rutin, nobiletin, and naringenin.(17) Hesperidin exerts significant pharmacological effects and is abundant in Citrus unshiu peel.(18) Although citrus is an economically important fruit in Japan, Citrus unshiu peel is considered as a waste. Citrus unshiu are commonly either consumed fresh or used to produce juice. This means that a large amount of Citrus unshiu peel waste are produced each year. Therefore, to enhance the added value of Chenpi may lead to increasing its market value.
Fig. 1.
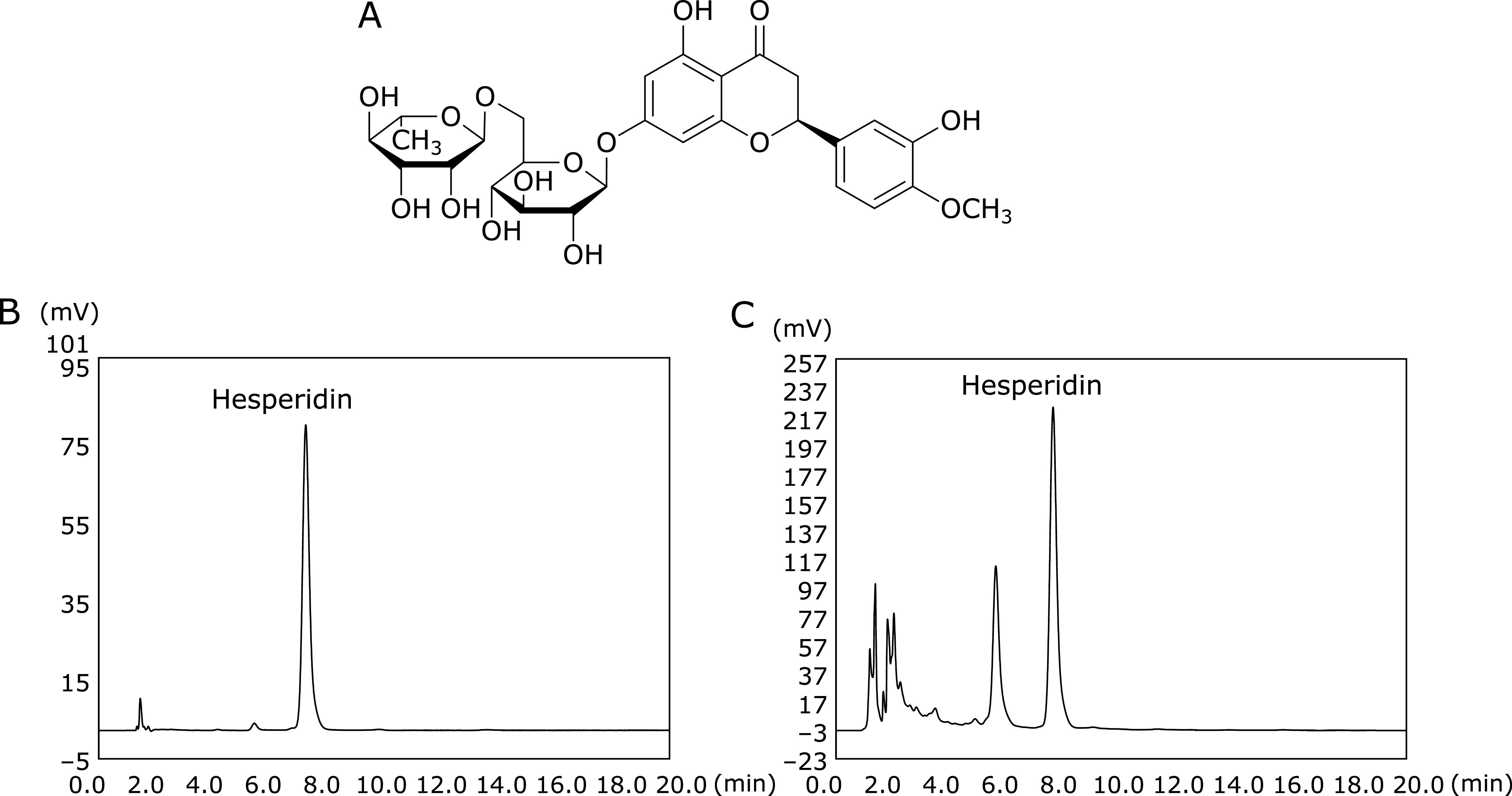
Analysis of hesperidin in Chenpi extract. (A) Structure of hesperidin, (B) HPLC chromatograms of a hesperidin standard (100 ppm), and (C) a Chenpi extract (10,000 ppm).
Although Chenpi is used as a traditional medicine to treat indigestion and inflammatory syndromes of the respiratory tract, its protective effects against aspirin-induced oxidative mucosal damage and gastric ulcer are unknown. Furthermore, although the inhibitory effect of hesperidin on alcohol/NSAID indomethacin-induced gastric damage has been reported, the inhibitory effect of Chenpi and its ingredient, hesperidin on the oxidative DNA damage (oxidative genotoxicity) is also unclear. It is thought that the carcinogenic mechanism of chemicals would be related to its genotoxicity. To fill this gap in our knowledge, here we investigated the inhibitory effects of Chenpi extract and hesperidin on the oxidative DNA damage and injury to the gastric mucosa in aspirin-treated rats and mice, respectively. In some study aimed at evaluating the gastric mucosal damage by non-steroidal anti-inflammatory drugs, mice were used to evaluate the analgesic effect and rats were used to evaluate gastric mucosal damage.(19,20) With these reference data, the analgesic effects of Chenpi extract and hesperidin were evaluated using mice, and the inhibitory effect of Chenpi extract and hesperidin on aspirin-induced oxidative damage was evaluated using rats, respectively.
Materials and Methods
Drugs and chemicals
Aspirin (Wako Pure Chemical Industries, Ltd., Osaka, Japan) was ground in a mortar and dissolved in 1% carboxymethyl cellulose sodium salt solution to 20 mg/ml carboxyl methylcellulose (CMC) and hesperidin were analytical-grade chemicals purchased from Wako. Chenpi powder was provided by AFC-HD AMS Life Science Co., Ltd., (Shizuoka, Japan).
Chenpi extract
MilliQ water (700 ml) was added to the 50 g of the Chenpi powder, and the mixture was refluxed at 60°C for 90 h. After extraction, the suspension was vacuum-filtered using an aspirator. This filtrate was lyophilized, and the yield of the residue (dry weight) was 16.6 g (33.2%).
HPLC analysis of hesperidin derived from Chenpi extract
Chenpi extract was dissolved in methanol to 10 mg/ml and then subjected to HPLC analysis using a Capcell Pak UG120 column (4.6 mm i.d. × 150 mm; Shiseido Co., Ltd., Tokyo, Japan). The mobile phase was 1.25% acetic acid:acetonitrile (8:2), and the flow rate was 1.0 ml/min for 20 min. The column temperature was maintained at 30°C, the injection volume was 10 µl, and the detection wavelength was 280 nm. The calibration curves were generated using 5, 10, 50, 100, 200, and 1,000 ppm of hesperidin.
Rodents
We used nine-week-old male Wistar rats (180–220 g) for evaluating oxidative damage and five-week-old male ddY mice (24–31 g) for evaluating analgesic activity. The rodents were purchased from Japan SLC Inc. (Hamamatsu, Japan) and were first housed in plastic laboratory cages for 1 week under normal laboratory conditions (room temperature, approximately 24.5 ± 0.5°C and photoperiodicity, 12 h light/12 h dark). Rodents were fed a commercial diet (CE-2, Nihon CLEA Inc., Tokyo) and water ad libitum. All animal experimental procedures were conducted according to the guidelines of the University of Shizuoka, Japan, which are based on those of the American Association for Laboratory Animal Science (Permit Number: 145056 and 145075).
Damage response
Nine-week-old male Wistar rats were divided into the groups (n = 5 per group) as follows: normal rat group (control group), aspirin administration group (aspirin group), aspirin + Chenpi extract administration group (aspirin + Chenpi group), and aspirin + hesperidin administration group (aspirin + hesperidin group). After fasting for 24 h, water, Chenpi extract (20 mg/kg body weight), and hesperidin (20 mg/kg body weight) were orally administered. After 1 h, 1% CMC solution (control group) or 200 mg/kg body weight aspirin (aspirin, aspirin + Chenpi and aspirin + hesperidin group) was orally administered. The rats were dissected 4 h later. Stomachs, livers, and kidneys were collected to determine the gastric bleeding score, to perform the formamidopyrimidine DNA glycosylase (Fpg)-modified comet assay, and to detect 8-oxodG. Furthermore, as the bleeding scores, the number of gastric bleeding per rat was counted.
Histopathology
Gastric tissue was removed and fixed in 10% formalin and made to the paraffin section. Thin sections were prepared and stained using the Hematoxylin-Eosin (HE) method. The histopathology examination was carried out using common optical microscope.
Fpg-modified comet assay
Stomachs, livers, and kidneys (200 mg each) were suspended in 2 ml of mincing solution (30 mM EDTA-0.9% KCl) and minced. The glass slide were coated with 0.7% normal melting-point (NMP) agarose the day before the preparation of specimens for the alkaline comet assay (single-cell gel electrophoresis). Samples (75 µl) were mixed with an equal volume of 1.4% low melting-point agarose. The mixture (75 µl) was layered onto 0.7% NMP agarose and covered with a cover glass. The slide was placed on ice for >20 min. The cover glass was removed, and the slide was immersed in 150 ml of chilled lysing solution (2 M NaOH, 2.5 M NaCl, 100 mM EDTA-2Na, 10 mM Tris, 1.1% sodium N-lauroyl sarcosinate, 1% Triton-X100, and 10% DMSO, pH 10) at 4°C for 1 h. A sample of the cell lysate was immersed in Fpg buffer (100 mM NaCl, 0.01% BSA, 12.5 mM Tris-HCI, 1 mM EDTA), which was replaced three times every 5 min. Fpg solution (80 µl, 1 µg/ml) and the Fpg buffer control were each added dropwise, covered with a cover glass, and placed in an incubator at 37°C for 15 min.
The cells embedded in agarose, overlaid with Fpg (1 mg/ml) or Fpg buffer (100 mM NaCl, 0.01% BSA, 12.5 mM Tris-HCI, 1 mM EDTA) at 37°C for 15 min. The slides were placed in alkaline electrophoresis buffer (0.3 M NaOH and 1 mM EDTA) for 20 min. Electrophoresis was performed at 25 V, 0.3 A for 20 min at 4°C. After electrophoresis, the slide was immersed in neutralization buffer for 20 min. The slide was dehydrated by immersion into absolute ethanol (99.6%) for 10 min and incubated with 40 µl of SYBR Gold Nucleic Acid Gel Stain [Invitrogen Paisley, UK; diluted 1:10,000 in TE buffer (10 mM Tris-HCl, 1.0 mM EDTA, pH 8.0)]. Slides were analyzed using a fluorescence microscope (Nikon, Tokyo, Japan) equipped with an image analyzer (Comet Analyzer, You Works, Tokyo, Japan). The percentage tail moment (TM), a measure of DNA damage in comet assays, was calculated according to the degree of DNA damage degree using Comet Assay IV software (Perceptive Instruments, UK) (100 cells per slide, 200× magnification).
DNase digestion
DNA from stomach tissue (100 mg) was extracted using a Puregene Core Kit A (Qiagen, Tokyo, Japan), dried, and the residue was dissolved in 100 µl of distilled water. DNA concentrations were determined using a NanoDrop ND-1000 Spectrophotometer (Nano Drop Technologies Inc., Rockland, DE). For LC/MS/MS experiments, each DNA sample (2 µg/20 µl) was mixed with 20 µl of digestion buffer (17 mM sodium succinate, 8 mM CaCl2, pH 6.0) containing 12 µl of Nuclease Mix (GE Healthcare). A DNA adduct [15N5]-8-oxodG (0.5 ng/µl) was added to the solution to serve as an internal standard. After incubation at 37°C overnight, 0.8 µl (150 units) of alkaline phosphatase (Sigma-Aldrich, St. Louis, MO), 1.6 µl of 1 M ZnCl2, and 1.6 µl of 1 M Tris-HCl (pH 8.5) were added. The solution was then incubated for 4 h at 37°C. The digested DNA solution was concentrated to approximately 20 µl using a SpeedVac Vacuum Concentrator, and 100 µl of methanol was added to precipitate enzymes and excess salt. The supernatant was collected, and the precipitate was washed with 100 µl of methanol. The supernatant and the methanol fractions were combined, evaporated to dryness, and 40 µl of 50% methanol was added to dissolve the residue.
Detection of 8-oxodG
The 50% methanol fraction (20 µl) was injected and separated using an Alliance HT 2795 HPLC (Waters Corporation, Milford, MA) and eluted at 400 µl/min. A Shim-pack FC-ODS (150 mm × 4.6 mm, Shimadzu, Kyoto, Japan) column was used. The separation was performed using a water (solvent A) and methanol (solvent B) gradient as follows: 5% B for 5 min to 30% B in 30 min, isocratic 85% B for 10 min to 5% B in 12 min. The method was optimized using 8-oxodG and [15N5]-8-oxodG standards. The LC system was coupled online with a triple quadrupole mass spectrophotometer (Quattro Ultima Platinum Micromass, Waters Corporation) with an ESI source. The ion source temperature was 120°C, the desolvation gas temperature was 340°C, and the voltage was held constant at 35 V. Nitrogen was used as the desolvation gas (660 L/h). Positive ions were acquired in multiple reaction monitoring mode (MRM). The MRM transitions were as follows: [15N5]-8-oxodG (m/z 288.8 → 172.8) and 8-oxodG (m/z 283.8 → 167.8), respectively. The amount of 8-oxodG in the DNA sample was determined by compared with that of [15N5]-8-oxodG. The number of 8-oxodG lesions was estimated as follows:
Analgesic activity
Mice were used to investigate the effect on analgesic activity since mice were more susceptible to writhing response pain than rats.(21) Five-week-old male ddY mice were randomly divided into 4 groups (n = 6 per group) as follows: normal mouse group (control group), aspirin administration group (aspirin group), aspirin + Chenpi extract administration group (aspirin + Chenpi group), and aspirin + hesperidin administration group (aspirin + hesperidin group). After fasting for 16 h, water, Chenpi extract, or hesperidin suspension (20 mg/kg body weight) was orally administered, and after 1 h, 1% CMC solution (control group) or 200 mg/kg body weight aspirin (aspirin, aspirin + Chenpi, and aspirin + hesperidin group) was orally administered. After 45 min, mice were intraperitoneally injected with 10 ml/kg of 0.6% acetic acid. The number of abdominal writhes was counted during a 10 min period, starting 10 min after the administration of acetic acid. A writhe was defined as a contraction of the abdominal muscles followed by an elongation of the body and extension of the hind limbs. A significant reduction of writhes in test animals compared with controls was designated an antinociceptive response. The percentage inhibition of writhing was calculated as follows:(22)
Nc: average number of writhes of the control group, Nt: average number of writhes of the test group.
Statistical analysis
The results were analyzed using Microsoft Excel 2016 (Microsoft, Redmond, WA). Excluding the bleeding score, the Tukey-Kramer test was used to compare differences between each other. The bleeding score was compared using the original scale and indicated the need for non-parametric testing. The Steel-Dwass test, a non-parametric multiple comparisons procedure, was used to test for between-group differences. All statistical tests were conducted at the two-sided 5% significance level.
Results and Discussion
Concentrations of hesperidin in Chenpi extract
Hesperidin (Fig. 1A), which may contribute to the antioxidant effects of Chenpi, was detected in Chenpi extract. Identification of hesperidin was performed according to its retention time compared with that of the hesperidin standard (Fig. 1B and C). The amount of hesperidin in the Chenpi extract was calculated according to the calibration curve for the hesperidin standard. The Chenpi extract contained 93.7 µg/g of hesperidin (11.4%). The concentration of hesperidin in Chenpi peels determined here was higher than that reported for three citrus cultivars (5.89–6.25%) cultivated in the United States, China, Japan, and Spain.(23)
Inhibitory effect of Chenpi extract and hesperidin on aspirin-induced oxidative damage
To evaluate the inhibitory effects of Chenpi extract and hesperidin on aspirin-induced oxidative DNA damage to the stomach, liver and kidney, we used an Fpg-modified comet assay. The comet assay serves as test for genotoxicity through its ability to detect DNA damage (e.g., double- and single-strand breaks, alkali-labile sites, DNA base damage, DNA strand crosslinking, DNA adducts) caused by chemicals such as pesticides, pharmaceuticals, and toxins.(24) Fpg is a DNA repair enzyme that cleaves oxidized bases. Therefore, Fpg is used in the comet assay to detect oxidative DNA damage with high sensitivity.(25) The Fpg-modified comet assay is used as well to evaluate the inhibitory effects of plant-derived substances on the oxidative toxicities of oxidants.(26)
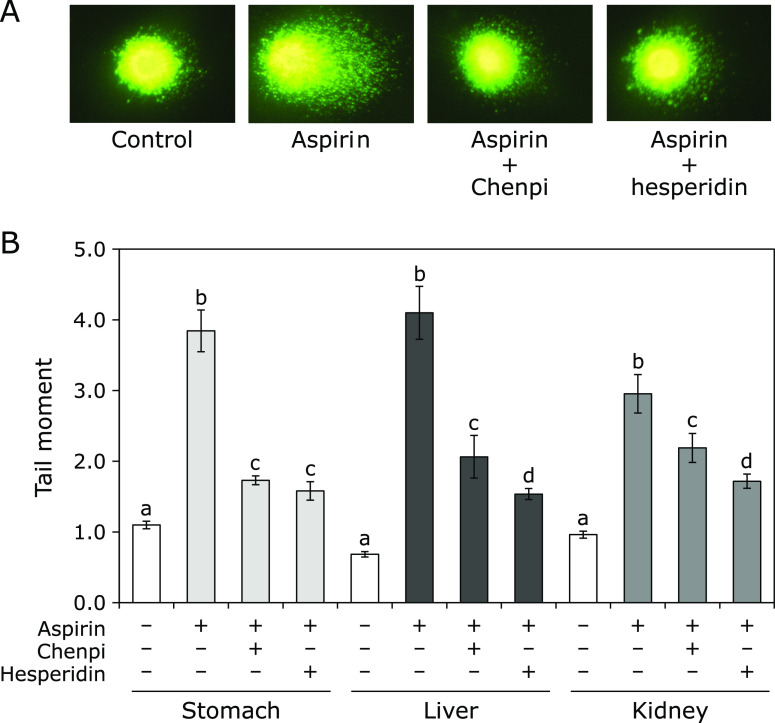
When we examined the inhibitory effect of hesperidin on the oxidative DNA damage with the dose 10 or 20 mg/kg body weight for oral administration, the inhibitory effect was high at 20 mg/kg body weight (data not shown). Therefore, Chenpi extract administration was determined to be 20 mg/kg body weight. The extent of DNA damage to stomach, liver, and kidney tissues of each experimental group was assessed using fluorescence microscopy (Fig. 2A) and TM (Fig. 2B). Rats treated with aspirin alone showed significantly higher levels of TM in extracts of stomach, liver, and kidney compared with those of the control group (p<0.05). However, rats treated with aspirin plus Chenpi extract or hesperidin had significantly lower levels of TM than those treated with aspirin alone (p<0.05).
Fig. 2.
Inhibitory effects in rats of Chenpi extract and hesperidin on aspirin-induced oxidative DNA damage (tail moment) in stomach, liver, and kidney. (A) The levels of DNA damage, which were measured using a comet assay, and the tissue extracts were visualized using fluorescence microscopy. (B) The mean of the parameters was calculated from 100 independent cells per sample. Tail moment = tail intensity × (tail DNA mean–head DNA mean). The amount of DNA in the tail moment is represented as the mean ± SE (n = 5, per group). Means with the same alphabet are not significantly different from each organ (Tukey-Kramer test, p<0.05).
We next measured the amounts of 8-oxodG to determine the effects of Chenpi extract and hesperidin on aspirin-induced oxidative gastric mucosal injury. Oxidative stress increases the amount of 8-oxodG, which is an oxidized derivative of deoxyguanosine and the major product of DNA oxidation.(27) In mammalian cells, 8-oxodG is a premutagenic lesion in DNA.(28) Moreover, numerous studies show that the formation of 8-oxodG causes misreplication of DNA, which may lead to cancer.(29)
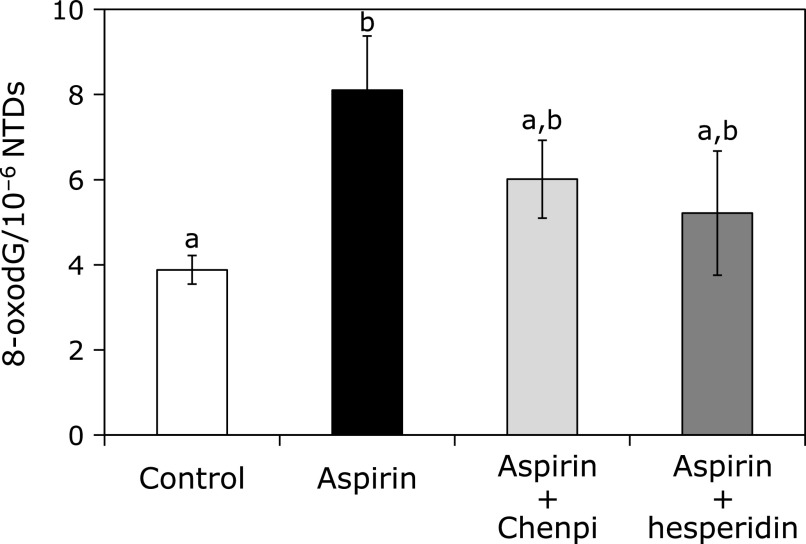
The potency of aspirin to induce oxidative damage in stomach was determined by measuring the content of 8-oxodG (Fig. 3). A significant (p<0.05) increase in 8-oxodG content was observed after the administration of aspirin. However, pretreatment with Chenpi extract and hesperidin reduced the content of 8-oxodG.
Fig. 3.
Inhibitory effects of Chenpi extract and hesperidin on the formation of 8-oxodG in the gastric mucosa of rats treated with aspirin. The data represent the mean ± SD (n = 5, per group). Means with the same alphabet are not significantly different from each other (Tukey–Kramer test, p<0.05).
The state of the stomachs of each experimental group after treatment is shown in Fig. 4A. In the aspirin group, heavy bleeding was observed in several places, and minimal bleeding was observed in the Chenpi extract and hesperidin-treated groups. HE staining revealed severe degenerative changes in epithelial folds and connective septa in mucosal tissue of rats treated with aspirin. In contrast, gastric mucosal and submucosal tissues of the Chenpi extract and hesperidin-treated groups appeared normal compared with those of the control group (Fig. 4B). The number of bleeding in the aspirin group was 19 ± 3.7, while that in the Chenpi extract and hesperidin-treated groups were 5.4 ± 1.2 and 6.4 ± 1.0, respectively, which was significantly reduced (p<0.05) (Fig. 4C). These findings indicate that Chenpi extract and hesperidin inhibited aspirin-induced oxidative damage.
Fig. 4.

The effect of Chenpi extract or hesperidin on the macroscopic appearance of the gastric mucosa in aspirin-induced gastric mucosal lesions in rats. (A) Photomicrographs showing the macroscopic appearance of the stomach. Macroscopic appearance of the gastric mucosa of the control group did not reveal lesions in the gastric mucosa. Macroscopic appearance of the gastric mucosa in the aspirin group revealed multiple hemorrhagic lesions in the gastric mucosa (arrows). Macroscopic appearance of the gastric mucosa in aspirin + Chenpi and aspirin + hesperidin groups revealed minimal lesions in the gastric mucosa (arrows). (B) Photomicrograph of HE-stained stomachs of rats. The black arrow points to the base of an ulcer. (C) The number of gastric bleeding in rats. The data represent the mean ± SD (n = 5, per group). Statistical analysis was performed with Steel–Dwass test. *p<0.05.
Polyphenols efficiently scavenge free radicals because of the high reactivities of their hydroxyl substituents.(30) Chenpi contains the phenolic acids caffeic acid, p-coumaric acid, ferulic acid, sinapic acid, protocatechuic acid, p-hydroxybenzoic acid, and vanillic acid as well as flavonoids such as hesperidin, rutin, nobiletin, and naringenin.(17,31) It was reported that the phenolic content in dried Citrus unshiu peel could be highly correlated with antioxidive activity.(32,33) Therefore, the antioxidants in Chenpi reduce oxidative damage induced by aspirin.
Hesperidin chelates metals and inhibits the superoxide-mediated Fenton reaction, which is an important source of highly reactive hydroxyl radicals.(34) Thus, hesperidin may reduce aspirin-induced DNA damage by scavenging free radicals. For example, orange (Citrus sinensis) peel aqueous extracts and hesperidin protect against alcohol-induced oxidative stress and peptic ulcer in rats.(35) Further, hesperidin inhibits gastric damage and ulcer formation caused by the NSAID indomethacin.(36,37)
Here we show that Chenpi extract and hesperidin suppressed aspirin-induced oxidative damage. These findings suggest that the inhibitory effect of Chenpi extract and hesperidin on aspirin-induced gastric mucosal damage was caused by an activity related to antioxidation. Furthermore, the inhibitory effect on aspirin-induced gastric mucosal damage may be caused by the activities unrelated to antioxidative effects. For example, Rikkunshinto, which comprises eight drugs such as Chenpi, enhances the effect of the cisplatin-induced delay in gastric emptying.(38) Thus, the pharmacological efficacy of Chenpi-derived hesperidin may enhance gastric motility via antagonism of the 5-HT3 receptor-mediated pathway.(39) Therefore, the inhibitory effects of Chenpi or hesperidin on aspirin-induced damage to the stomach may be explained by each of their antioxidative effects as well as by their contributions to promoting excretion.
Analgesic activity
Acetic acid induces the writhing reaction, a model of inflammatory pain, which has long been used to evaluate the analgesic or anti-inflammatory properties of new drugs. Acetate acts indirectly by inducing the release of endogenous mediators that stimulate nociceptive neurons that are sensitive to NSAIDs and opioids.(16,31) Here we used the acetic acid-induced writhing test to investigate the analgesic effects of Chenpi extract and hesperidin (Fig. 5A). The numbers of writhes were as follows: control group, 30.7 ± 8.1; aspirin group, 15.7 ± 3.2 (49% inhibition) (Fig. 5B). These findings indicate that pain caused by intraperitoneal administration of acetic acid was suppressed by aspirin.
Fig. 5.
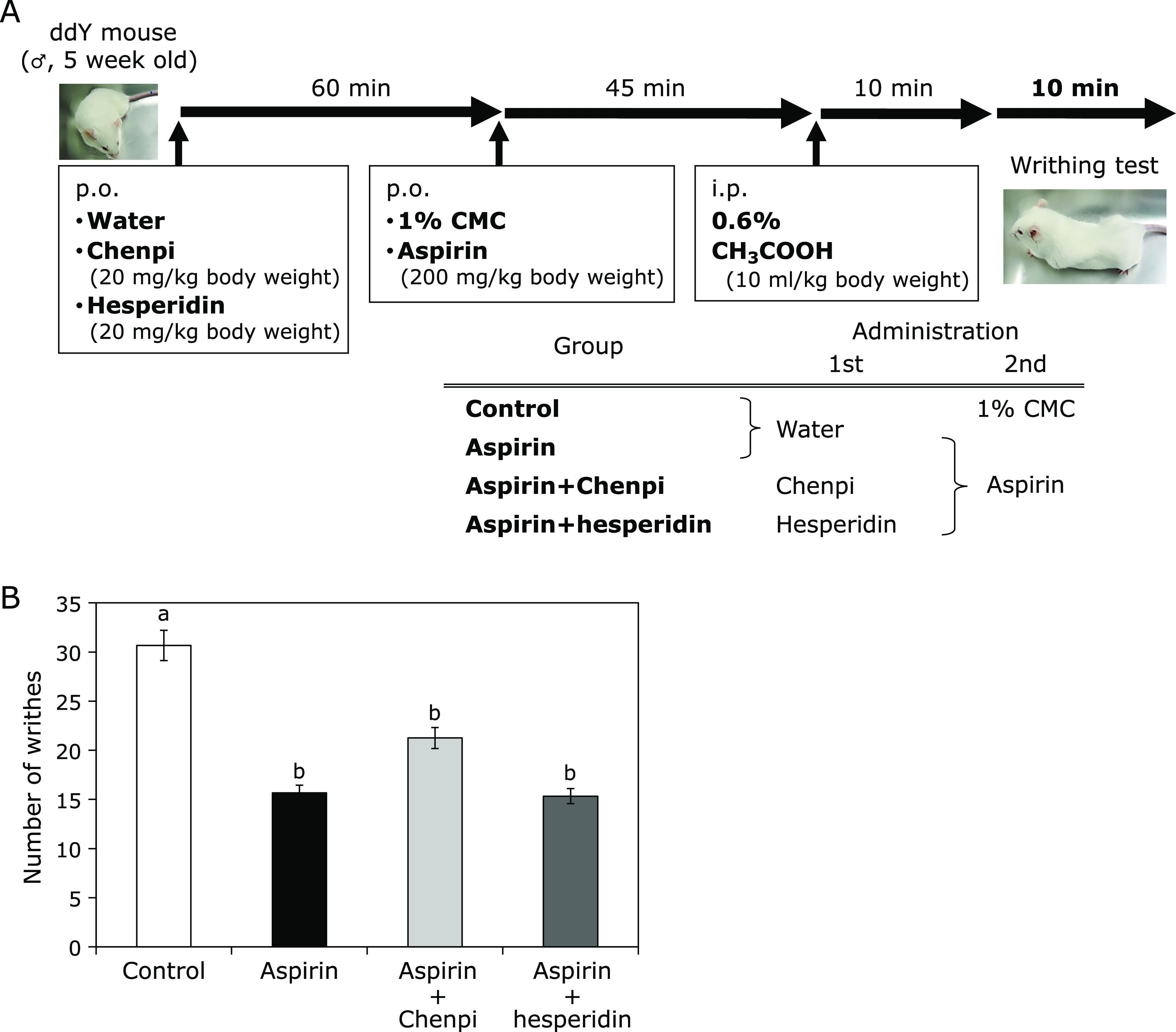
Effects of Chenpi extract or hesperidin on acetic acid-induced writhing in mice treated with aspirin. (A) Time schedule of the experiment. Vehicle (control), aspirin (200 mg/kg body weight), aspirin + Chenpi (20 mg/kg body weight), or hesperidin (20 mg/kg body weight) were administered i.p. before acetic acid injection. Abdominal writhes were counted during a 10 min period, starting 10 min after the administration of acetic acid solution. (B) Chenpi extract and hesperidin did not affect the number of writhes. Each column represents the mean ± SD (n = 6, per group). Means with the same alphabet are not significantly different from each other (Tukey-Kramer test, p<0.05).
There was a significant (p<0.05) reduction in the mean value of abdominal constriction in aspirin + Chenpi group (21.3 ± 4.9, 31% inhibition) and aspirin + hesperidin group (15.3 ± 1.5, 50% inhibition). The value of the inhibitory effect of Chenpi extract and hesperidin in the acetic acid-induced writhing test was comparable to that of the standard drug aspirin. These results suggest that Chenpi extract and hesperidin did not inhibit the therapeutic effects of aspirin. Current pain medications in use, which are largely mu (µ) opioid receptor agonists, hesperidin does not activate the µ-opioid receptor through direct binding in vitro.(40)
In summary, we show here that Chenpi extract and its component hesperidin suppressed oxidative damage, which is a side effect of aspirin, although it did not significantly affect the pharmacological action of aspirin. We predict therefore that future research will lead to new applications of Chenpi and hesperidin in medical disciplines such as pharmacology.
Author Contributions
YS and SM conceived and designed the experiments; YS, SS, SN and SM performed the experiments; YS and SM analyzed the data; YS wrote the paper.
Acknowledgments
The authors would like to thank Enago (www.enago.jp) for the English language review.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Mehta P. Aspirin in the prophylaxis of coronary artery disease. Curr Opin Cell Cardiol 2002; 17: 552–558. [DOI] [PubMed] [Google Scholar]
- 2.McCarberg BH, Cryer B. Evolving therapeutic strategies to improve nonsteroidal anti-inflammatory drug safety. Am J Ther 2015; 22: e167–e178. [DOI] [PubMed] [Google Scholar]
- 3.Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res 2003; 110: 255–258. [DOI] [PubMed] [Google Scholar]
- 4.Murata M, Thanan R, Ma N, Kawanishi S. Role of nitrative and oxidative DNA damage in inflammation-related carcinogenesis. J Biomed Biotechnol 2012; 2012: 623019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawanishi S, Ohnishi S, Ma N, Hiraku Y, Oikawa S, Murata M. Nitrative and oxidative DNA damage in infection-related carcinogenesis in relation to cancer stem cells. Genes Environ 2017; 38: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawanishi S, Ohnishi S, Ma N, Hiraku Y, Murata M. Crosstalk between DNA damage and inflammation in the multiple steps of carcinogenesis. Int J Mol Sci 2017; 18: 1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung AM, Redlak MJ, Miller TA. Aspirin-induced mucosal cell death in human gastric cells: role of a caspase-independent mechanism. Dig Dis Sci 2009; 54: 28–35. [DOI] [PubMed] [Google Scholar]
- 8.Shiokawa Y, Nobunaga M, Saito T, Asaki S, Ogawa N. Epidemiology study on upper gastrointestinal lesions induced by non-steroidal anti-inflammatory drugs Ryumachi 1991; 31: 96–111 (in Japanese) [PubMed] [Google Scholar]
- 9.Leonards JR. Presence of acetylsalicylic acid in plasma following oral ingestion of aspirin. Proc Soc Exp Biol Med 1962; 110: 304–308. [DOI] [PubMed] [Google Scholar]
- 10.Grootveld M, Halliwell B. 2,3-Dihydroxybenzoic acid is a product of human aspirin metabolism. Biochem Pharmacol 1988; 37: 271–280. [DOI] [PubMed] [Google Scholar]
- 11.Oikawa S, Kobayashi H, Tada-Oikawa S, Isono Y, Kawanishi S. Damage to cellular and isolated DNA induced by a metabolite of aspirin. Mutat Res 2009; 661: 93–100. [DOI] [PubMed] [Google Scholar]
- 12.Chen XY, Chen HM, Liu YH, et al. The gastroprotective effect of pogostone from Pogostemonis Herba against indomethacin-induced gastric ulcer in rats. Exp Biol Med (Maywood) 2016; 241: 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu G, Peng C, Xie X, Zhang S, Cao X. Availability, pharmaceutics, security, pharmacokinetics, and pharmacological activities of patchouli alcohol. Evid Based Complement Alternat Med 2017; 2017: 4850612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyu JH, Lee HT. Effects of dried Citrus unshiu peels on gastrointestinal motility in rodents. Arch Pharm Res 2013; 36: 641–648. [DOI] [PubMed] [Google Scholar]
- 15.Choi MY, Chai C, Park JH, Lim J, Lee J, Kwon SW. Effects of storage period and heat treatment on phenolic compound composition in dried Citrus peels (Chenpi) and discrimination of Chenpi with different storage periods through targeted metabolomic study using HPLC-DAD analysis. J Pharm Biomed Anal 2011; 54: 638–645. [DOI] [PubMed] [Google Scholar]
- 16.Gorinstein S, Martin-Belloso O, Park YS, et al. Comparison of some biochemical characteristics of different citrus fruits. Food Chem 2001; 74: 309–315. [Google Scholar]
- 17.Kawaii S, Tomono Y, Katase E, Ogawa K, Yano M. Quantitation of flavonoid constituents in citrus fruits. J Agric Food Chem 1999; 47: 3565–3571. [DOI] [PubMed] [Google Scholar]
- 18.Inoue T, Yoshinaga A, Takabe K, et al. In situ detection and identification of hesperidin crystals in satsuma mandarin (Citrus unshiu) peel cells. Phytochem Anal 2014; 26: 105–110. [DOI] [PubMed] [Google Scholar]
- 19.Zhang B, He XL, Ding Y, Du GH. Gaultherin, a natural salicylate derivative from Gaultheria yunnanensis: towards a better non-steroidal anti-inflammatory drug. Eur J pharmacol 2006; 530: 166–171. [DOI] [PubMed] [Google Scholar]
- 20.Zheng X, Oda H, Takamatsu K, et al. Analgesic agents without gastric damage: design and synthesis of structurally simple benzenesulfonanilide-type cyclooxygenase-1-selective inhibitors. Bioorg Med Chem 2007; 15: 1014–1021. [DOI] [PubMed] [Google Scholar]
- 21.Rooks WH 2nd, Tomolonis AJ, Maloney PJ, Roszkowski A, Wallach MB. The anti-inflammatory and analgesic profile of 6, 11-dihydrodibenzo-[b.e.]-thiepin-11-one-3-acetic acid (Tiopinac). Agents and Actions 1980; 10: 266–273. [DOI] [PubMed] [Google Scholar]
- 22.Tang SY, Sivakumar M, Ng AMH, Shridharan P. Anti-inflammatory and analgesic activity of novel oral aspirin-loaded nanoemulsion and nano multiple emulsion formulations generated using ultrasound cavitation. Int J Pharm 2012; 430: 299–306. [DOI] [PubMed] [Google Scholar]
- 23.Lu Y, Zhang C, Bucheli P, Wei D. Citrus flavonoids in fruit and traditional Chinese medicinal food ingredients in China. Plant Food Huma Nutr 2006; 61: 57–65. [DOI] [PubMed] [Google Scholar]
- 24.Fairbairm DW, Olive PL, O'Neill KL. The comet assay: a comprehensive review. Mutat Res 1995; 339: 37–59. [DOI] [PubMed] [Google Scholar]
- 25.Domijan AM, Zeliezić D, Kopjar N, Peraica M. Standard and Fpg-modified comet assay in kidney cells of ochratoxin A- and fumonisin B1-treated rats. Toxicology 2006; 22: 53–59. [DOI] [PubMed] [Google Scholar]
- 26.Slameňová D, Kubošková K, Horváthová E, Robichová S. Rosemary-stimulated reduction of DNA strand breaks and FPG-sensitive sites in mammalian cells treated with H2O2 or visible light-excited Methylene Blue. Cancer Letters 2002; 177: 145–153. [DOI] [PubMed] [Google Scholar]
- 27.Haghdoost S, Czene S, Näslund I, Skog S, Harms-Ringdahl M. Extracellular 8-oxo-dG as a sensitive parameter for oxidative stress in vivo and in vitro. Free Radic Res 2005; 39: 153–162. [DOI] [PubMed] [Google Scholar]
- 28.Miller H, Prasad R, Wilson SH, Johnson F, Grollman AP. 8-OxodGTP incorporation by DNA polymerase β is modified by active-site residue Asn279. Biochemistry 2000; 39: 1029–1033. [DOI] [PubMed] [Google Scholar]
- 29.Shibutani S, Takeshita M, Grollman AP. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature 1991; 349: 431–434. [DOI] [PubMed] [Google Scholar]
- 30.Pietta PG. Flavonoids as antioxidants. J Nat Prod 2000; 63: 1035–1042. [DOI] [PubMed] [Google Scholar]
- 31.Ma YQ, Ye XQ, Fang ZX, Chen JC, Xu GH, Liu DH. Phenolic compounds and antioxidant activity of extracts from ultrasonic treatment of Satsuma mandarin (Citrus unshiu Marc.) peels. J Agric Food Chem 2008; 56: 5682–5690. [DOI] [PubMed] [Google Scholar]
- 32.Kim JS. Preliminary evaluation for comparative antioxidant activity in the water and ethanol extracts of dried citrus fruit (Citrus unshiu) peel using chemical and biochemical in vitro assays. Food Nutr Sci 2013; 4: 177–188. [Google Scholar]
- 33.Singh RP, Chidambara Murthy KN, Jayaprakasha GK. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J Agric Food Chem 2002; 50: 81–86. [DOI] [PubMed] [Google Scholar]
- 34.Kalpana KB, Devipriya N, Srinivasan M, Menon VP. Investigation of the radioprotective efficacy of hesperidin against gamma-radiation induced cellular damage in cultured human peripheral blood lymphocytes. Mutat Res Genet Toxicol Environ Mutagen 2009; 676: 54–61. [DOI] [PubMed] [Google Scholar]
- 35.Selmi S, Rtibi K, Grami D, Sebai H, Marzouki L. Protective effects of orange (Citrus sinensis L.) peel aqueous extract and hesperidin on oxidative stress and peptic ulcer induced by alcohol in rat. Lipids Health Dis 2017; 16: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bigoniya P, Singh K. Ulcer protective potential of standardized hesperidin, a citrus flavonoid isolated from Citrus sinensis. Rev Bras Farmacogn 2014; 24: 330–340. [Google Scholar]
- 37.Hamdan DI, Mahmoud MF, Wink M, El-Shazly AM. Effect of hesperidin and neohesperidin from bittersweet orange (Citrus aurantium var. bigaradia) peel on indomethacin-induced peptic ulcers in rats. Environ Toxicol Pharmacol 2014; 37: 907–915. [DOI] [PubMed] [Google Scholar]
- 38.Morimoto Y, Watanabe S, Michihara S, et al. Effects if Rikkunshito on cisplatin-induced delay in gastric emptying in rats. Kampo Med 2013; 64: 150–159. [Google Scholar]
- 39.Tominaga K, Kido T, Ochi M, et al. The traditional Japanese medicine Rikkunshito promotes gastric emptying via the antagonistic action of the 5-HT3 receptor pathway in rats. Evid Based Complement Alternat Med 2011; 2011: 248481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loscalzo LM, Yow TT, Wasowski C, Chebib M, Marder M. Hesperidin induces antinociceptive effect in mice and its aglicone, hesperetin, binds to µ-opioid receptor and inhibits GIRK1/2 currents. Pharmacol Biochem Behav 2011; 99: 333–341. [DOI] [PubMed] [Google Scholar]