Abstract
Stem cell therapy has the potential to improve tissue remodeling and repair. For cardiac stem cell therapy, methods to improve the injection and tracking of stem cells may help to increase patient outcomes. Here we describe a multimodal approach that combines ultrasound imaging, photoacoustic imaging, and magnetic particle imaging (MPI). Ultrasound imaging offers real-time guidance, photoacoustic imaging offers enhanced contrast, and MPI offers high-contrast, deep-tissue imaging. This work was facilitated by a poly(lactic-co-glycolic acid) (PLGA)-based iron oxide nanobubble labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide (DiR) as a trimodal contrast agent. The PLGA coating facilitated the ultrasound signal, the DiR increased the photoacoustic signal, and the iron oxide facilitated the MPI signal. We confirmed that cell metabolism, proliferation, differentiation, and migration were not adversely affected by cell treatment with nanobubbles. The nanobubble-labeled cells were injected intramyocardially into live mice for real-time imaging. Ultrasound imaging showed a 3.8-fold increase in the imaging intensity of labeled cells postinjection compared to the baseline; photoacoustic imaging showed a 10.2-fold increase in the cardiac tissue signal postinjection. The MPI intensity of the nanobubble-treated human mesenchymal stem cells injected into the hearts of mice was approximately 20-fold greater than the negative control.
Keywords: stem cell therapy, magnetic particle imaging, photoacoustic imaging, ultrasound, iron oxide, DiR, nanobubbles
Graphical Abstract
INTRODUCTION
Stem cells have regenerative potential to remodel bone, cartilage, and cardiac tissue. Recent studies using nanolayered hybrids of ligand and ligand activators1 as well as bioadhesive supramolecular gelatin hydrogels2 have been used to facilitate the stem cell regeneration of bone tissue. Self-assembled N-cadherin mimetic peptide hydrogels have also been shown to regenerate cartilage through canonical Wnt/β-catenin signaling.3 Studies of ligand oscillation integrin used an oscillating magnetic field to characterize the adhesion as well as differentiation of the stem cells.4 After myocardial infarction, stem cell therapy can increase the regeneration of cardiac tissue5-9 and has been used in multiple clinical trials10,11 for thousands of patients. A metaanalysis of these trials has shown a modest improvement in cardiac function, but the mechanism of this improvement remains somewhat unclear, with both differentiation and paracrine effects implicated.12,13 One challenge to the efficacy of stem cell therapy is the poor viability of transplanted stem cells,14-17 which adversely affects tissue regeneration.18 Additional challenges include cell contamination, cell misdelivery,5 and the interaction of cells with their host environment.19
Imaging can help to improve stem cell therapy and ensure proper cell delivery while also monitoring the location, number, and fate of delivered cells.20 For cardiac stem cell trafficking, imaging can help to determine the best route for cell delivery as well as the biodistribution of delivered stem cells.21 Stem cell imaging typically involves labeling a cell with a reporter gene14,18 or an exogenous contrast agent.22 Various types of imaging have been used for tracking stem cells—with pros and cons for each. Magnetic resonance imaging (MRI) is the gold standard because of the established use of iron oxide nanoparticles, ferric nanoparticles, and gadolinium chelates23,24 and has shown the detection of single cells.25 Recently, a 2D graphene oxide-based nanomaterial was used as a T1 contrast agent in MRI to label stem cells.26 However, MRI suffers from low contrast, low specificity, long scan times, and T2 saturation.27 Positron emission tomography (PET) has also been used with reporter genes to track stem cells in vivo28,29 or with exogenous radiolabeled nanoparticles.30 While PET offers high resolution, sensitivity, and quantification,31 it suffers from high cost32 and poor spatial resolution.
More recently, we and others33-35 have shown that ultrasound (US)-based imaging has utility in imaging stem cells. The advantages of US include up to 300 Hz temporal resolution and good penetration through tissue. In addition, the established use of echocardiography in clinics makes US a natural fit for cardiac-based stem cell therapy. Mesoporous silica has been used to image stem cells with US.35 However, US is limited by the lack of contrast of stem cells above background tissue. Photoacoustic (PA) imaging can further increase contrast36-43 and has recently been used in imaging of protein sulfenic acids,44 multimodal imaging of tumors,45 and biological imaging in the second near-IR window,46 but PA imaging alone has limitations in deep-tissue imaging. Contrast agents used to image stem cells for PA imaging include Prussian Blue–poly(l-lysine) nanocomplexes47 as well as DiR.48 While PA imaging offers enhanced contrast over US imaging, the penetration depth is limited. One recent solution to low contrast and poor penetration depth in molecular imaging is magnetic particle imaging (MPI).
MPI applies a magnetic gradient field with a central field-free point to the imaging FOV; this field saturates the nonlinear magnetization of magnetic particles.49 Electromagnets scan the field-free point to form an image, and the MPI signal is generated when the field-free point passes an area with magnetic particles.50 The magnetization of the magnetic particles changes in orientation and magnitude, which is detected via a coil, and the resulting voltage is used to reconstruct the MPI image. MPI most often utilizes Resovist superparamagnetic iron oxide,51 which is cleared via the hepatobiliary system and approved for clinical use.52 MPI has been used to track stem cells in vivo50 and image the cardiovascular system.53 Additionally, MPI shows a linear response to the iron oxide dose, unlike T2 MRI, which saturates at high concentrations. Recently, MPI has been used in combination with magnetic fluid hyperthermia, showing the potential therapeutic coupling capability of MPI with other superparametric iron oxide-based modalities,54 especially for deep-tissue imaging. Additionally, MPI has been used to image labeled mesenchymal stem cells and neural stem cells in the mouse brain to detect approximately 50000 implanted cells.55 However, MPI suffers from low temporal resolution and offers no details on the surrounding anatomy.
To overcome the limitations of each of these modalities alone, a multimodal approach combines the advantages of each modality by combining the multifunctionality of nanoparticles56 and advances in biomedical imaging. For example, the combination of MRI with a PA/surface-enhanced Raman scattering contrast agent was used to define brain tumor margins and guide resection.57 Additionally, trimodal imaging probes using PET, optical microscopy, and radiometal chelates have been created by combining 64Cu-DOTA and 111In-DOTA, with dual MRI/optical probes.58 Sulfonate-based saline inorganic–organic hybrid nanoparticles have been developed as multimodal contrast agents for optical imaging, PA imaging, and MRI and showed improved T1 values over gadoliniumbased particles.56
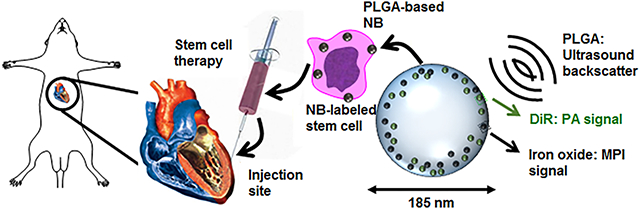
Here, we report a combination approach that uses a high frame rate of acoustic imaging with the good contrast and penetration depth of MPI. We report a hybrid nanobubble with US, PA, and MPI signals. Because the success of stem cell injections has previously been hindered by inaccurate injections,59 we utilized acoustic modalities for real-time-guided injections and MPI for long-term, high-resolution imaging. This PLGA-based nanobubble formulation contains iron oxide nanoparticles with a core size of 4.2 nm and a mean hydrodynamic diameter of 62 nm to generate the MPI signal and a 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide (DiR)60 fluorophore to enhance the PA signal.61 The bubble conformation increases the US signal.62 We studied the effect of the nanobubbles on the viability, differentiation, cytokine expression, and migration ability of human mesenchymal stem cells (hMSCs). We then used them to label hMSCs and injected these labeled cells intracardially into living mice. This multimodal contrast agent tracks cells and can also be used in other imaging applications that require good contrast, temporal resolution, and high penetration depth.
MATERIALS AND METHODS
Reagents.
The following materials were acquired and used as received: Vivotrax iron oxide nanoparticle solution (5.5 mg/mL, Magnetic Insight, Inc.); poly(lactic-co-glycolic acid) (PLGA; Sigma-Aldrich); poly(vinyl alcohol) (PVA; MD Biochemicals); chloroform (Fisher Chemical); DiR (Invitrogen); phosphate-buffered saline (PBS; Thermo Fisher); matrigel (Corning); 5% silver nitrate (American Mastertech Scientific, Inc.); 5% sodium thiosulfate (American Mastertech Scientific, Inc.); Oil Red O (Sigma-Aldrich).
Instrumentation.
Dynamic light scattering (DLS) measured the size and ζ potential (Zetasizer-90, Malvern Instruments) of the nanobubbles in water. Transmission electron microscopy (TEM) was performed with a JEOL JEM-1200 EXII micrscope operating at 120 kV. We performed photoacoustic (PA) and ultrasound (US) imaging with the Vevo 2100 instrument (Visualsonics) with a 21-MHzcentered transducer as described previously.63 The distance between the imaging transducer and phantom was maintained throughout the scans. We collected at least eight fields of views (FOVs) for each sample. MPI was performed using the Magnetic Insight MOMENTUM imager.64 MPI utilized 6T/m gradients with a 45 kHz receiver bandwidth, 20 mT peak amplitude, and X-space reconstruction. All absorbance measurements utilized a SpectraMax M5 spectrophotometer from Molecular Devices. Inductively coupled plasma optical emission spectrometer (ICP-OES; Optima 3000DV, PerkinElmer) was used to determine the number of nanobubbles endocytosed by the cells.
PLGA Nanobubble Synthesis.
PLGA/iron oxide/DiR nanobubbles were fabricated via an approach modified from the literature.65 An iron oxide nanoparticle solution (5.5 mg/mL) was added to 1 mL of chloroform. Then, 100 μL of DiR [10 mg/mL in dimethyl sulfoxide (DMSO)] was added to the solution and vortexed for 10 s (Fisher Scientific, Fixed Speed Vortexer). Next, 30 mg of PLGA was dissolved in this solution, and 200 μL of Millipore water was added to the solution and sonicated (Branson Sonifier 450) for 1 min in an ice bath, forming a water-in-oil emulsion. A total of 6 mL of Millipore water with 2% PVA was added to initiate a secondary emulsion. The solution was sonicated for 5 min and stirred for 24 h to evaporate chloroform unless otherwise noted. The iron oxide and DiR-containing nanobubbles were centrifuged (Fisher Scientific Legend XR1) at 22000g for 25 min. The nanobubbles were then washed three times with Millipore water to remove excess PVA, iron oxide, and DiR and diluted in Millipore water.
Cell Culture.
Poietic hMSCs (Lonza PT-2501) were grown in supplemented media (Lonza, PT-3001) and seeded in a T75 flask at a concentration of 5000 cells/cm2. These cells were labeled with nanobubbles and incubated under standard conditions for 6 h unless otherwise noted. The hMSCs were washed three times with PBS to remove free nanobubbles and detached using TrypLE Express (Life Technologies). The cell viability was determined using the Cell Viability Kit 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Biotium). A total of 10 μL of the MTT solution was added to 100 μL of the medium in each well, mixed briefly, and incubated at 37 °C for 4 h; 200 μL of DMSO was added to each well and pipetted to dissolve the resulting formazan salt. The absorbance was read at 570 nm on a spectrophotometer. MTT assays were conducted by plating 8000 cells/well in replicate (n = 8) in 96-well plates and treated at varying time points (0–24 h) at a constant concentration (240 μg/mL) as well as at varying concentrations (0–480 μg/mL) at a constant time (8 h unless otherwise noted). Wells were analyzed in replicate (n = 8). For differentiation studies, adipogenic cells were stained with Oil Red O, and osteogenic-differentiated cells were stained with a Von Kossa stain as described previously.66
In Vitro Phantom Preparation and US, PA, and MPI Imaging.
For US and PA imaging of cells, PLGA nanobubbles or cells (100000 unless otherwise noted) labeled with nanobubbles were immobilized in a 1% agarose phantom gel. Nanobubble or cell solutions were sonicated and mixed with the 1% agarose solution at a 1:1 ratio. A total of 100 μL of the mixture was pipetted into the phantom gel. A 1% agarose solution was used to cover the samples after solidification.
For PA imaging of the nanobubbles, PLGA nanobubbles were loaded into polyethylene tubes, sealed, and placed in a 3D-printed phantom holder to maintain a constant height of 1 cm between the transducer and phantom.67 The gain was set to 30–40 dB and read at a wavelength of 730 nm. Eight FOVs were collected for each sample. For MPI imaging, PLGA nanobubbles or cells (100000) labeled with nanobubbles were loaded into polyethylene tubes, sealed, and fixed in a sample holder.
Data Analysis.
ImageJ 1.48v 58 was used to quantitate the B-mode, PA, and MPI signals for nanobubbles via the region-of-interest (ROI) analysis in arbitrary units for 8-bit images.68 The US, PA, and MPI means and standard error were calculated from eight FOVs in each sample. Error bars represent the standard error of measurements unless otherwise noted. ImageJ was also used to quantitate osteogenic and adipogenic differentiation via ROI for induced and noninduced cells.
Cytokine Expression.
We plated cells with and without nanobubbles at 200000 cells/well in a 6-well plate and cultured for 24 h. We removed the media from the positive and control cells and compared it to the control cells. Protein analysis was performed by MyriadRBM Inc. (Austin, TX).
Flow Cytometry.
For flow cytometry studies, labeled and unlabeled hMSCs (400 × 103 cells, respectively) were resuspended in PBS; 1 μL of monoclonal antibody specific for mesenchymal stem cells (CD73-PE/CD90-FITC/CD105-APC) was added to the cells. Flow cytometry analysis was run by FACSCalibur (BD Biosciences, San Jose, CA) and FlowJo software was used to analyze the results (Becton Dickinson and Company).
In Vivo Animal Studies.
Adult nu/nu mice (12 weeks old) were used for this study. Animal studies were conducted in accordance with our institution’s IACUC animal use committee. Mice were anesthetized with 2% isoflurane in oxygen at 2 L/min, which was confirmed with a tail pinch. The labeled cell pellets were resuspended in 50 μL of PBS and mixed in a 1:1 ratio with matrigel. They were loaded into a 24-gauge syringe and then injected using B-mode US for injection guidance. For MPI imaging, mice were anesthetized, sacrificed, and injected intramyocardially with nanobubble-labeled hMSCs. Mice were then frozen and sent to Magnetic Insight, Inc. (Alameda, CA) to conduct MPI. For animal studies, Matlab was used to colormap the signal from MPI. Images were overlaid on stock computed tomography images of adult mice.
RESULTS AND DISCUSSION
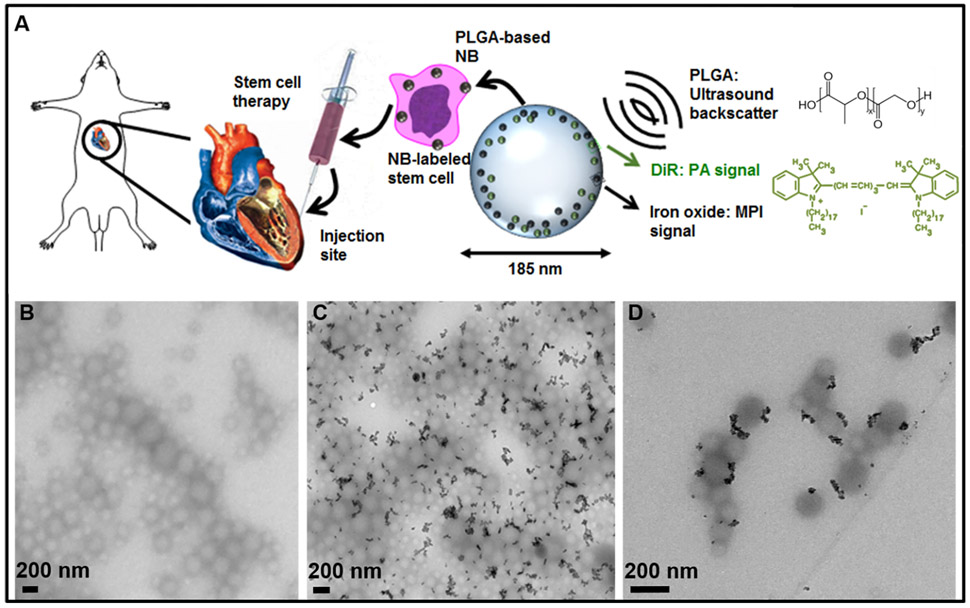
Figure 1A presents a schematic of this work. A PLGA-based nanobubble with DiR and iron oxide was used as a MPI/PA/US contrast agent to image stem cells in mice. Nanobubbles were synthesized with DiR and iron oxide, used to label hMSCs, injected intramyocardially into mice, and imaged. DiR was used to enhance the PA signal of the particle, and iron oxide provided the MPI contrast. Our results showed that the PLGA–nanobubble increased the US signal 3.8-fold in vivo, building off of previous studies.69
Figure 1.
Nanobubble schematic and synthesis. (A) Schematic of cells labeled with the trimodal nanobubble being injected intracardially into a murine model. The PLGA and DiR molecular structures are shown. (B) TEM image of PLGA nanobubbles without iron oxide. (C) TEM image of iron oxide-loaded PLGA particles. (D) Higher-magnification TEM image of iron oxide-loaded PLGA particles.
Synthesis and Physical Characterization.
TEM imaging showed that the nanobubbles were spherical in shape and uniform in size of approximately 185 nm. Figure 1B shows TEM imaging of the PLGA nanobubbles without iron oxide. Parts C and D of Figure 1 show TEM imaging of the nanobubbles with iron oxide; the black particles are the iron oxide nanoparticles. TEM imaging showed that the iron oxide particles were present in approximately 70% of the PLGA nanobubbles (Figure 1C and Table S.1).
Optimization, Viability, and Cellular Uptake.
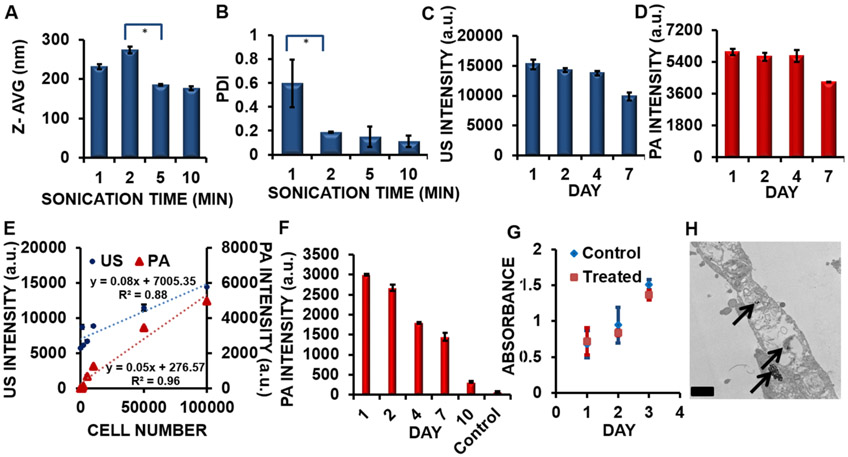
Figure 2 shows optimization parameters of the nanobubble synthesis, cell number detection, viability assay of hMSCs, and uptake of the nanobubbles. The effects of the sonication time on the size (z-avg, nm) and polydispersity index (PDI) of the nanobubbles are shown in parts A and B of Figure 2, respectively. The size of the nanobubbles was tuned by changing the sonication time from 1 to 10 min for the double emulsion, and the impact on the size was negligible after 5 min of sonication. This produced nanobubbles with a size of 185 ± 2 nm (Figure 2A). The PDI decreased from 0.60 to 0.19 with increasing sonication time from 1 to 2 min; the PDI decreased to 0.15 after 5 min of sonication, indicating a monodisperse solution (Figure 2B). Additionally, we varied the amount of iron oxide loading from 0 to 1000 μL and showed no significant effect on the size (Figure S1A) or PDI (Figure S1B) as measured by DLS. This indicates that the amount of iron oxide present in the nanobubbles did not significantly change the monodispersity of the solution. The final conditions chosen for the nanobubble synthesis were 5 min of sonication and 1000 μL of iron oxide loading. These conditions resulted in iron oxide in approximately 70% of the nanobubbles (Table S1) and a PDI of 0.15.
Figure 2.
Optimization of synthesis parameters and cell number detection. (A) Increased sonication time after 2 min resulted in a smaller nanobubble size, with the effects negligible after 5 min of sonication. (*) p value < 0.05. (B) Increasing the sonication time from 1 to 2 min resulted in a decrease in the PDI from 0.60 to 0.19. (*) p value < 0.05. (C) The US signal of the nanobubble remained stable for 4 days and decreased by approximately 35% after 7 days (p value < 0.05). (D) The PA signal of the nanobubble remained stable for 4 days and decreased by approximately 28% after 7 days (p value < 0.05). (E) The limit of detection of hMSCs labeled with nanobubbles (incubation time = 6 h; concentration = 240 μg/mL) was 10461 cells for US imaging and 996 cells for PA imaging. The primary y axis indicates the US intensity (blue circles) of hMSCs treated with nanobubbles from 0 to 100000 cells. The secondary y axis indicates the PA intensity (red triangles) of hMSCs treated with nanobubbles from 0 to 100000 cells. Error bars represent the standard error (n = 8). (F) PA intensity data show that cells (100000 cells; incubation time = 6 h; concentration = 240 μg/mL) were detectable for 10 days in a phantom (p value < 0.05). (G) A cell viability assay showing the growth kinetics of hMSCs. Treated cells (red squares) and control cells (blue diamonds) doubled in approximately 3 days. Error bars represent the standard error (n = 8). (H) TEM imaging of a hMSC treated with nanobubbles. The black arrows indicate the location of the nanobubbles within the cytoplasm of the hMSC. Scale bar = 0.5 μm.
We measured the US (Figure 2C) and PA (Figure 2D) intensities of the nanobubbles in solution for 7 days and found consistent US and PA signals over days 1–4. After 7 days, the US signal of the nanobubbles decreased by 35%; the PA signal of the nanobubbles decreased by 28%. We monitored the size (Figure S1C) and PDI (Figure S1D) of the nanobubbles in solution at room temperature for 4 days and found no significant difference in these parameters as measured by DLS, indicating good stability of the nanobubble conformation for 4 days. Finally, we compared the US signal of control nanobubbles without DiR and iron oxide to the US signal of the nanobubbles with these contrast agents (Figure S1E). We found that the US signal of the nanobubbles with contrast agents was 13% higher than the US signal of the nanobubbles without the contrast agents. This increase in the US signal may be due to the higher impedance mismatch of the particle with both iron oxide and PLGA than with PLGA alone.
We imaged nanobubble-labeled cells in a phantom and found the detection level of hMSCs. Figure 2E shows the US and PA detection levels of hMSCs labeled with nanobubbles (incubation time = 6 h; concentration = 240 μg/mL) in a phantom. The limit of detection of hMSCs labeled with nanobubbles was 10461 cells for US imaging and 996 cells for PA imaging. Previous research has indicated that the limit of detection for MPI is approximately 200 cells and the theoretical detection limit of a single cell.70 We measured the PA signal of 100000 labeled cells (incubation time = 6 h; concentration = 240 μg/mL) in a phantom and found that the PA signal was detectable over the control nonlabeled cells for 10 days (Figure 2F). We also conducted a viability analysis to show that the growth kinetics of the hMSCs were not affected by cell treatment with nanobubbles (Figure 2G). There were no significant differences in the viability (p > 0.05; two-tailed t test) for hMSCs that were treated (incubation time = 6 h; concentration = 240 μg/mL) compared to control cells; both cell populations doubled in approximately 3 days. TEM imaging of the treated hMSCs confirmed the cellular uptake of the nanobubbles in the cytoplasm of the cell, as indicated by the black arrows (Figure 2H).
The ζ potential of the PLGA-only nanobubble was 1.25 mV compared to −0.169 mV for the version with iron oxide and DiR. The ζ potential of the iron oxide nanobubbles without DiR in water was −0.222 mV, consistent with the slightly negative ζ potential of the final nanobubble. This indicates that the addition of the iron oxide caused the ζ potential to change from slightly positive to slightly negative in the final nanobubble.
Concentration Dependence and Cell Labeling Optimization.
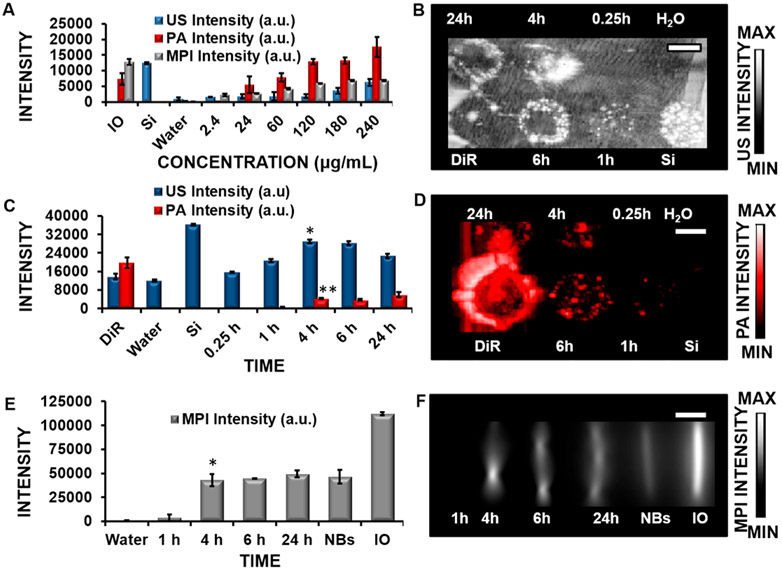
We imaged the nanobubbles in a phantom and found a linear relationship between the concentration of the nanobubble and the intensities for US, PA, and MPI (Figure 3A). This is an important relationship to quantify the amount of hMSCs present in cell-tracking applications. Figure S1F details the linear relationship between the imaging modalities and shows R2 values of above 0.80 for all three modalities. We also identified the optimum incubation time and concentration for cell labeling. Dosage conditions were optimized so that the MPI, US, and PA signals could be detected in hMSCs with no adverse effects on cell differentiation, protein expression, or toxicity.
Figure 3.
Signal intensity of the multimodal contrast agent and the effect of the incubation time on labeled cells. (A) US, PA, and MPI signals of the nanobubbles are shown as a function of the concentration in a phantom. Iron oxide nanoparticles (IO; 5.5 mg/mL) and Stoba silica (SiO2) nanoparticles (Si; 5 mg/mL) are positive controls for MPI and US, respectively; water is a negative control. There is a linear relationship in detection in these modalities, which is an important aspect in quantifying the number of labeled cells present. (B) US imaging of hMSCs treated with nanobubbles from 0.25 to 24 h (concentration = 240 μg/mL). (C) US and PA imaging quantification at various times up to 24 h. The signal was detected at 4 h of incubation for both modalities, compared to the negative control (water). * and ** indicate p values < 0.05 compared to the negative control. (D) PA imaging of hMSCs treated with nanobubbles from 0.25 to 25 h (concentration = 240 μg/mL). (E) MPI quantification of hMSCs treated with nanobubbles from 1 to 24 h (concentration = 240 μg/mL). * indicates p values < 0.05 compared to the negative control (water); nanobubbles (NBs, 24 μg/mL) and IO (5.5 mg/mL) are positive controls. (F) MPI imaging data of hMSCs treated with nanobubbles at varying times from 1 to 24 h (concentration = 240 μg/mL). The MPI signal was detected at 4 h of incubation. Error bars represent the standard error (n = 8). Scale bars are 4 mm.
The US, PA, and MPI signals of the labeled cells at various time points were studied to determine the signal intensity and proper conditions for cell labeling and in vivo studies. For US and PA studies, phantoms were prepared in a 1% agar gel for imaging. For MPI studies, cells were placed in tubes, sealed, and imaged. The concentration and time were held constant across the three different modalities, and these images were processed as maximum-intensity projections to help normalize the data across the modalities. Uptake of the nanobubbles (concentration = 240 μg/mL) was observed in US, PA, and MP imaging at 4 h (Figures 3B,D,F) with p values < 0.05, indicating a statistically significant signal over the negative control (water). Flow cytometry data indicated that the fluorescent signal from the nanobubbles increases from 0.25 to 1 h of incubation (Figure S2A). Figure 3C quantifies the US and PA image intensities. The MPI signal was observed at 4 h of incubation with nanobubbles with a p value < 0.05 (Figures 3E and 4F).
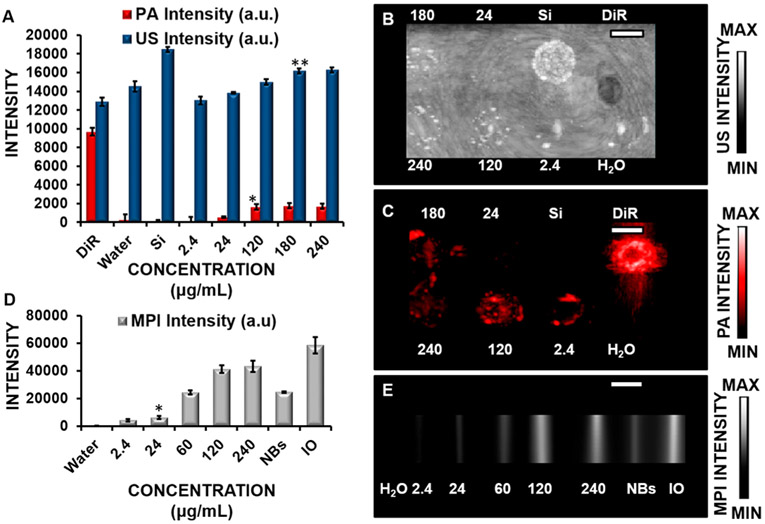
Figure 4.
Effect of the concentration on the US, PA, and MPI signals in labeled cells. (A) Quantification of US and PA imaging data of cells treated from 2.4–240 μg/mL of nanobubbles for 6 h. Stoba silica (SiO2) nanoparticles (Si; 5 mg/mL) and DiR (5 mg/mL) were positive controls for US and PA imaging, respectively. Water was a negative control. * and ** indicate p values < 0.05 compared to the negative control (water). (B) US imaging of cells (100000) treated with 2.4–240 μg/mL of nanobubbles for 6 h. (C) PA imaging of cells (100000) treated with 2.4–240 μg/mL of nanobubbles for 6 h. (D) Quantification of MPI data from cells treated with 2.4–240 μg/mL of nanobubbles for 6 h where NBs = nanobubbles and IO = iron oxide (1 mg/mL) as a positive control. * indicates p values < 0.05 compared to the negative control (water). (E) MPI imaging data of cells (100000) treated with 2.4–240 μg/mL of nanobubbles for 6 h. Error bars represent the standard error (n = 8). Scale bars are 4 mm.
MTT assays were performed to evaluate the toxicity of the nanobubbles. The results suggested that there was a slight decrease of approximately 14% in the viability of hMSCs treated for 24 h (Figure S3A). Therefore, the optimal incubation time of the hMSCs with nanobubbles was determined to be 6 h to produce sufficient US, PA, and MPI signals while maintaining maximum cell viability. Other studies have found similar incubation times of nanoparticle-treated hMSCs ranging from 10 min to 24 h. Uptake of mesoporous silica labeled with fluorescein isothiocyanate was found to be internalized as early as 10 min and maximized at 2–4 h in hMSCs;71 uptake of magnetic silica nanoparticles in hMSCs was observed at 1 h.72 Transfection agents combined with superparamagnetic iron oxides have also been used to study iron oxide uptake in various cell lines including hMSCs and showed uptake from 2 to 48 h of incubation time.73
Next, we studied the impact that the concentration (Figure 4) had on the hMSCs’ signal intensities. The lowest concentration of nanobubbles that produce a signal in hMSCs and was significantly elevated relative to the untreated hMSCs was 180 μg/mL for US (Figure 4A,C), 120 μg/mL for PA (Figure 4B,D), and 24 μg/mL for MPI (Figure 4E,F). Flow cytometry data further confirmed the effects of the concentration on the intensity and showed increasing intensity as the concentration increased from 2.4 to 60 μg/mL (Figure S2B). MTT assay was also performed and showed no effect on the toxicity of the cells at a concentration of up to 480 μg/mL for cells treated for 8 h (Figure S3B).
ICP-OES analysis was also conducted. ICP-OES measurements showed that there were 1.04 × 103, 1.40 × 103, 4.54 × 103, 9.07 × 103, 11.1 × 103, and 11.2 × 103 iron oxide particles in cells treated with concentrations of 2.4, 24, 60, 120, 180, and 240 μg/mL nanobubbles, respectively, for 6 h. This corresponds to 24, 32, 103, 205, 251, and 255 nanobubbles/cell, respectively, as determined from the diameter of the iron oxide nanoparticles and their initial concentrations. It is possible that this in vivo concentration resides in vacuoles within the cytoplasm,74 which prevents toxic effects.
Pluripotency and Cytokine Analysis.
Next, we used differentiation reagents to determine if the nanobubbles affected the pluripotency of the hMSCs. The hMSCs labeled with nanobubbles differentiated into lineages of adipocytes and osteocytes, similar to the untreated hMSCs with similar morphology and staining. We labeled cells with nanobubbles (240 μg/mL, 6 h) and washed the cells 3 times with PBS to remove nonendocytosed nanobubbles. We then monitored the differentiation of the cells for 3 weeks in induction and control media.
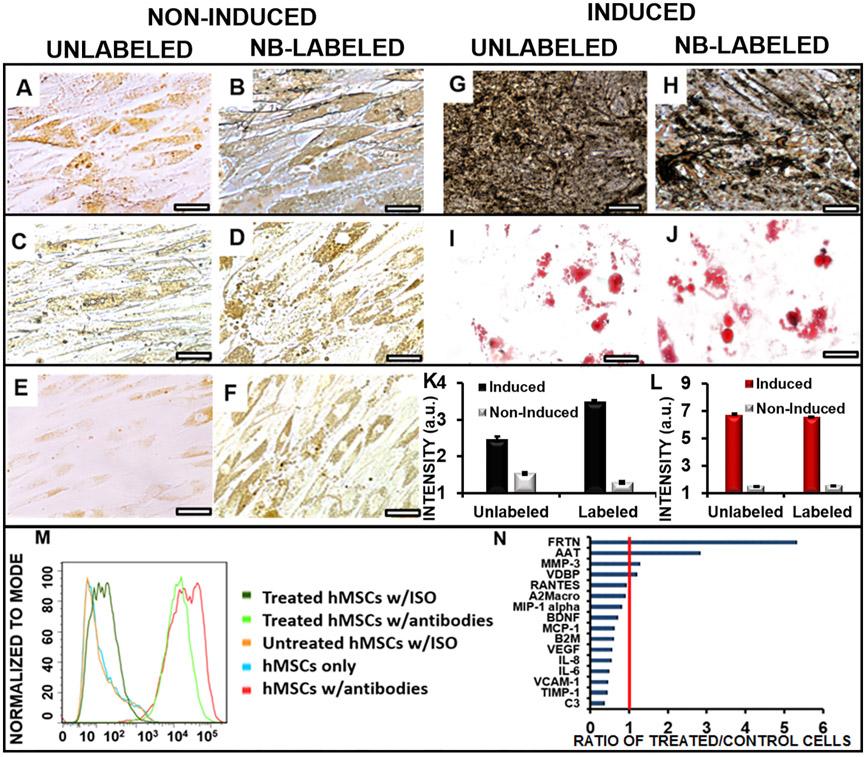
Parts A and B of Figure 5 show Von Kossa staining for osteocytes of noninduced, unlabeled, and labeled cells. There was no significant black calcium staining for these noninduced cells. Parts C and D of Figure 5 show Oil Red O staining for adipocytes; no red staining was observed for these cells. Parts E and F of Figure 5 show unlabeled and labeled cells in typical media. There were no significant morphological changes, suggesting that the nanobubble does not induce spontaneous differentiation by itself. Unlabeled and labeled hMSCs differentiated into osteogenic (Figure 5G,H) and adipogenic (Figure 5I,J) cell lines with the use of induction media. Osteogenic induction produced black calcium staining from the Von Kossa stain in the treated and untreated cells. Adipogenic induction produced characteristic lipid-containing vacuoles75 and red staining with Oil Red O in the treated and untreated cells. Image analysis of the osteogenic cell differentiation images (Figure 5K) and adipogenic cell differentiation images (Figure 5L) showed that labeling the cells with nanobubbles did not reduce the number of differentiated cells. These findings indicated that the hMSCs still retained the ability to differentiate into adipogenic and osteogenic cells. Flow cytometry data indicated that the labeled hMSCs retained surface markers CD90, CD73, and CD105 (Figures 5M and S4A and S4B) compared to unlabeled hMSCs, indicating that the cells were still expressing characteristic stem cell phenotypes. Additionally, a cell migration assay was conducted to ensure that the hMSCs retained the ability to migrate (Figure S5A-F). We found that cells treated with nanobubbles (6 h, 240 μg/mL) migrated at a rate similar to that of untreated cells over 7 days. Overall, these results suggest that the nanobubble treatment did not affect the differentiation, surface marker expression, or migration ability of the hMSCs compared to untreated hMSCs.
Figure 5.
Differentiation ability of hMSCs unaffected by the nanobubble labeling (240 μg/mL nanobubbles; 6 h of incubation). Panels A–F indicate control cells without induction media. Panels G–J indicate cells with induction media. (A) Osteogenic staining of noninduced, unlabeled cells did not produce black calcium staining. (B) Osteogenic staining of noninduced, labeled cells did not produce significant black calcium staining. (C) Adipogenic staining of noninduced, unlabeled cells did not produce characteristic red staining. (D) Adipogenic staining of noninduced, labeled cells did not produce characteristic red staining. (E) Unlabeled cells in typical media. (F) Labeled cells in typical media. (G) Induced, unlabeled cells showed characteristic black calcium staining from the Von Kossa stain. (H) Induced, labeled cells showed characteristic black calcium staining from the Von Kossa stain. (I) Unlabeled cells in adipogenic media showed characteristic red staining and globular morphology from the Oil Red O stain. (J) Labeled cells in adipogenic media showed characteristic red staining and globular morphology from the Oil Red O stain. Scale bar for all images = 100 μm. (K) Image analysis of the osteogenic cell differentiation images showed that labeled cells did not reduce the number of differentiated cells for cells treated with induction media. (L) Image analysis of the adipogenic cell differentiation images showed that labeled cells did not reduce the number of differentiated cells for cells treated with induction media. (M) CD73 flow cytometry data showing that treated hMSCs retain the ability to express characteristic phenotypes. (N) Cytokine analysis of labeled hMSCs showing the ratio of expression to unlabeled cells. Abbreviations: ferritin, FRTN; alpha-1 antitrypsin, AAT; matrix metalloproteinase-3, MMP-3; vitamin D binding protein, VDBP; T-cell-specific protein RANTES, RANTES; alpha-2 macroglobulin, A2Macro; macrophage inflammatory protein-1 alpha, MIP-1 alpha; brain-derived neurotrophic factor, BDNF), ;monocyte chemotactic protein 1, MCP-1; beta-2 microglobulin, B2M; vascular endothelial growth factor, VEGF; interleukin-8, IL-8; interleukin-6, IL-6; vascular cell adhesion molecule-1, VCAM-1; tissue inhibitor of metalloproteinases 1, TIMP-1; complement C3, C3.
There are three main types of endocytosis processes involving nanomaterials including caveolae-mediated endocytosis, clathrin-mediated endocytosis, and micropinocytosis. We used Dynasore, a clathrin-mediated endocytosis inhibitor, to treat the cells to determine if cell labeling was hindered. Cells treated with Dynasore and labeled with nanobubbles did not show fluorescence (Figure S4C) compared to cells labeled with nanobubbles (Figure S4D), indicating clathrin-mediated endocytosis as the mechanism of cellular uptake.
We also studied the secretome of the nanobubble-treated and control cells by measuring 17 cytokines in cell culture media to determine if expression patterns changed with the nanobubble treatment. The treated hMSCs showed increased expression over untreated cells for ferritin and alpha-1 antitrypsin of 5.3 and 2.8 times, respectively (Figure 5N). Other cytokines tested did not show significantly increased expression over untreated cells. Ferritin, an iron binding protein, has been implicated in the biodegradation of magnetic iron oxide nanoparticles, suggesting that hMSCs were metabolizing the iron oxide.76 Alpha-1 antitrypsin has been shown to increase fibroblast proliferation by inducing the activation of p42MAPK and p44MAP pathways,77 which may suggest that the treated cells had increased fibroblast production compared to the untreated cells. However, the other 15 cytokines measured were detected at the same or lower levels in treated cells compared to untreated cells, indicating that the expression levels of these proteins were not dysregulated by treating the hMSCs with nanobubbles.
In Vivo Imaging.
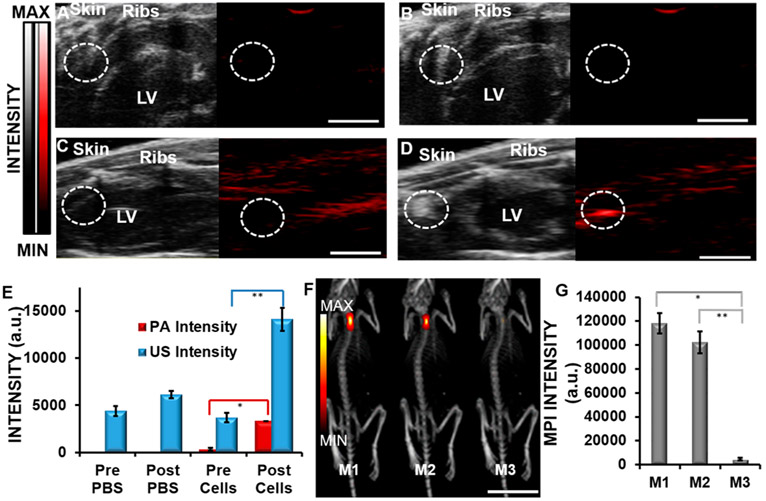
Figure 6 shows real-time PA and US imaging for clear anatomic differentiation and high contrast of injections of labeled hMSCs into nude mice. Here, 800000 hMSCs labeled with 240 μg/mL nanobubbles for 6 h were injected intramyocardially into nude mice (n = 3). US was used in real time to guide the initial injection of the stem cells, PA imaging was used to confirm the location of the stem cells in the myocardial tissue with higher contrast than US, and MPI was used to confirm the location of the stem cells long term because of its very high contrast above background and depth of penetration. Parts A–D of Figure 6 show the longitudinal axis view of the mouse hearts. Figure 6A shows preinjection of PBS, a negative control, and Figure 6B shows postinjection of PBS. Figure 6C shows preinjection of labeled cells, and Figure 6D shows postinjection of labeled cells. US imaging showed a 3.8-fold increase of labeled cells postinjection (Figure 6D) compared to the baseline, and PA imaging showed a 10.2-fold increase in the signal postinjection (Figure 6D); injection of PBS did not show any increase in the signal. Figure 6F shows MPI imaging data of mice (M1 and M2) injected with 800000 nanobubble-labeled hMSCs; M3 was a negative control. Figure 6G shows MPI quantification of the injections. The MPI intensity of the nanobubble-treated hMSCs injected into the hearts of mice (M1 and M2) was compared to that of the negative control (M3). The signal intensities from mice M1 and M2 were 25.8 and 21.2 times, respectively, higher than that of the untreated mouse M3. Previous studies have indicated a spatial resolution in MPI of approximately 1.5 mm × 3.0 mm × 3.0 mm in the z, x, and y direction, consistent with our results.56 The signal intensity in MPI is proportional to the amount of contrast agent present,56 thereby making MPI a viable option for tracking the number of stem cells in vivo. Parts A–E of Figure S6 show imaging data of 100000–800000 hMSCs injected subcutaneously into a murine model, showing the ability to discriminate between different cell numbers in vivo. It is important to track the number of injected stem cells in vivo. Additionally, the hMSCs were tracked in vivo and showed signals after 3 days of injection (Figure S7).
Figure 6.
In vivo imaging. (A) US (black and white) and PA (red) images preinjection of PBS show low signal intensity in the circled areas of the longitudinal axis of cardiac tissue. LV indicates the left ventricle. (B) US and PA images postinjection of PBS show low signal intensity. (C) US and PA images preinjection of labeled hMSCs. (D) Postinjection of 800000 labeled hMSCs (240 μg/mL nanobubbles; 6 h of incubation). Scale bar for images A–D = 0.5 cm. (E) Quantification of the US and PA signal increases postinjection of labeled cells. (F) MPI imaging of mice injected with labeled hMSCs (yellow/red images; 240 μg/mL nanobubbles; 6 h of incubation). Mice M1 and M2 were injected with 800000 labeled hMSCs. Mouse M3 was a negative control. Scale bar = 2.5 cm. (G) Quantification of MPI data. * and ** indicate p values < 0.05.
One of the challenges of stem cell imaging is the real-time monitoring of cellular injection with differentiation of the anatomical features. Video S1 shows a video of a US-guided intramyocardial injection of nanobubble-treated hMSCs into a live mouse. The contrast increased 3.8-fold after the injection of nanobubble-treated hMSCs. Finally, histology sections of labeled hMSCs injected into the cardiac tissue of living mice showed fluorescent signals, indicating that the labeled cells were in the cardiac tissue (Figure S8A,B).
CONCLUSION
We synthesized PLGA-based iron oxide nanobubbles labeled with DiR to use as a trimodal contrast agent to label hMSCs. The nanobubbles were imaged ex vivo in agarose and 3D-printed-based phantoms; they were imaged in vivo after intramyocardial injection. We confirmed that the cell metabolism, proliferation, and differentiation ability were not adversely affected by treatment with nanobubbles. This suggests that this contrast agent can be used with hMSCs for improving stem cell therapy imaging. Clinical trials typically involve millions of cells,78,79 and ultralow cell number detection is rarely a true clinical need. However, future research directions include more careful evaluation of the detection limit and in vivo tracking capabilities of this approach. Alternative applications include immune cell trafficking such as imaging macrophages and/or Car T-cells in vivo.
Supplementary Material
ACKNOWLEDGMENTS
J.E.L. acknowledges funding from the National Institutes of Health (NIH) Institutional National Research Service Award T32 CA153915, Cancer Researchers in Nanotechnology (Dong, Kummel). J.E.L. thanks C. Isaac and P. Castillo for ICP-OES analysis and J. Gaudet and A. Clemmensen for MPI scans. J.V.J. acknowledges funding from the NIH (Grants R00 HL117048 and DP2 HL137187) and infrastructure from Grant S10 OD021821. This work was performed, in part, at the San Diego Nanotechnology Infrastructure of UCSD, a member of the National Nanotechnology Coordinated Infrastructure, which is supported by the National Science Foundation (Grant ECCS-1542148).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsanm.8b00063.
MTT assays, FACS analysis, and in vitro and in vivo PA data (PDF)
US-guided injection video showing the longitudinal axis view of a live mouse heart (AVI)
The authors declare no competing financial interest.
REFERENCES
- (1).Kang H; Kim M; Feng Q; Lin S; Wei K; Li R; Choi CJ; Kim T-H; Li G; Oh J-M; Bian L Nanolayered Hybrid Mediates Synergistic Co-Delivery of Ligand and Ligation Activator for Inducing Stem Cell Differentiation and Tissue Healing. Biomaterials 2017, 149, 12–28. [DOI] [PubMed] [Google Scholar]
- (2).Feng Q; Wei K; Lin S; Xu Z; Sun Y; Shi P; Li G; Bian L Mechanically Resilient, Injectable, and Bioadhesive Supramolecular Gelatin Hydrogels Crosslinked by Weak Host-Guest Interactions Assist Cell Infiltration and in Situ Tissue Regeneration. Biomaterials 2016, 101, 217–228. [DOI] [PubMed] [Google Scholar]
- (3).Li R; Xu J; Wong DSH; Li J; Zhao P; Bian L Self-Assembled N-Cadherin Mimetic Peptide Hydrogels Promote the Chondrogenesis of Mesenchymal Stem Cells through Inhibition of Canonical Wnt/B-Catenin Signaling. Biomaterials 2017, 145, 33–43. [DOI] [PubMed] [Google Scholar]
- (4).Kang H; Wong DSH; Yan X; Jung HJ; Kim S; Lin S; Wei K; Li G; Dravid VP; Bian L Remote Control of Multimodal Nanoscale Ligand Oscillations Regulates Stem Cell Adhesion and Differentiation. ACS Nano 2017, 11 (10), 9636–9649. [DOI] [PubMed] [Google Scholar]
- (5).Segers VF; Lee RT Stem-Cell Therapy for Cardiac Disease. Nature 2008, 451 (7181), 937–942. [DOI] [PubMed] [Google Scholar]
- (6).Masumoto H; Yamashita J Exploiting Human Ips Cell-Derived Cardiovascular Cell Populations toward Cardiac Regenerative Therapy. Stem Cell Transl. Invest. 2016, 3. [Google Scholar]
- (7).Gouadon E; Moore-Morris T; Smit NW; Chatenoud L; Coronel R; Harding SE; Jourdon P; Lambert V; Rucker-Martin C; Pucéat M Concise Review: Pluripotent Stem Cell-Derived Cardiac Cells, a Promising Cell Source for Therapy of Heart Failure: Where Do We Stand? Stem Cells 2016, 34 (1), 34–43. [DOI] [PubMed] [Google Scholar]
- (8).Terzic A; Behfar A Stem Cell Therapy for Heart Failure: Ensuring Regenerative Proficiency. Trends Cardiovasc. Med 2016, 26 (5), 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Broughton KM; Sussman MA Empowering Adult Stem Cells for Myocardial Regeneration V2. 0. Circ. Res 2016, 118 (5), 867–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Noiseux N; Mansour S; Weisel R; Stevens L-M; Der Sarkissian S; Tsang K; Crean AM; Larose E; Li S-H; Wintersperger B; Vu MQ; Prieto I; Li R-K; Roy DC; Yau TM The IMPACT-CABQ Trial: A Multicenter, Randomized Clinical Trial of CD133+ Stem Cell Therapy During Coronary Artery Bypass Grafting for Ischemic Cardiomyopathy. J. Thorac. Cardiovasc. Surg 2016, 152 (6), 1582–1588. [DOI] [PubMed] [Google Scholar]
- (11).Bhatnagar A; Bolli R; Johnstone BH; Traverse JH; Henry TD; Pepine CJ; Willerson JT; Perin EC; Ellis SG; Zhao DX; et al. Bone Marrow Cell Characteristics Associated with Patient Profile and Cardiac Performance Outcomes in the Latetime-Cardiovascular Cell Therapy Research Network (Cctrn) Trial. Am. Heart J 2016, 179, 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Gnecchi M; He H; Noiseux N; Liang OD; Zhang L; Morello F; Mu H; Melo LG; Pratt RE; Ingwall JS; Dzau VJ Evidence Supporting Paracrine Hypothesis for Akt-Modified Mesenchymal Stem Cell-Mediated Cardiac Protection and Functional Improvement. FASEB J. 2006, 20 (6), 661–669. [DOI] [PubMed] [Google Scholar]
- (13).Ryan A; Murphy M; Barry F Mesenchymal Stem/Stromal Cell Therapy. Biology and Therapeutic Application of Mesenchymal Cells; John Wiley and Sons Inc.: New York, 2017; pp 426–440. [Google Scholar]
- (14).LaBarge MA; Blau HM Biological Progression from Adult Bone Marrow to Mononucleate Muscle Stem Cell to Multinucleate Muscle Fiber in Response to Injury. Cell 2002, 111 (4), 589–601. [DOI] [PubMed] [Google Scholar]
- (15).Corbel SY; Lee A; Yi L; Duenas J; Brazelton TR; Blau HM; Rossi FM Contribution of Hematopoietic Stem Cells to Skeletal Muscle. Nat. Med 2003, 9 (12), 1528–1532. [DOI] [PubMed] [Google Scholar]
- (16).Torrente Y; Belicchi M; Sampaolesi M; Pisati F; Meregalli M; D’Antona G; Tonlorenzi R; Porretti L; Gavina M; Mamchaoui K; et al. Human Circulating Ac133+ Stem Cells Restore Dystrophin Expression and Ameliorate Function in Dystrophic Skeletal Muscle. J. Clin. Invest 2004, 114 (2), 182–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Shabbir A; Zisa D; Leiker M; Johnston C; Lin H; Lee T Muscular Dystrophy Therapy by Non-Autologous Mesenchymal Stem Cells: Muscle Regeneration without Immunosuppression and Inflammation. Transplantation 2009, 87 (9), 1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Abdelwahid E; Kalvelyte A; Stulpinas A; de Carvalho KAT; Guarita-Souza LC; Foldes G Stem Cell Death and Survival in Heart Regeneration and Repair. Apoptosis 2016, 21 (3), 252–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Srivastava AK; Kadayakkara DK; Bar-Shir A; Gilad AA; McMahon MT; Bulte JW Advances in Using Mri Probes and Sensors for in Vivo Cell Tracking as Applied to Regenerative Medicine. Dis. Models & Mech 2015, 8 (4), 323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Kircher MF; Gambhir SS; Grimm J Noninvasive Cell-Tracking Methods. Nat. Rev. Clin. Oncol 2011, 8 (11), 677–688. [DOI] [PubMed] [Google Scholar]
- (21).Beeres SL; Bengel FM; Bartunek J; Atsma DE; Hill JM; Vanderheyden M; Penicka M; Schalij MJ; Wijns W; Bax JJ Role of Imaging in Cardiac Stem Cell Therapy. J. Am. Coll. Cardiol 2007, 49 (11), 1137–1148. [DOI] [PubMed] [Google Scholar]
- (22).Meregalli M; Farini A; Parolini D; Maciotta S; Torrente Y Stem Cell Therapies to Treat Muscular Dystrophy. BioDrugs 2010, 24 (4), 237–247. [DOI] [PubMed] [Google Scholar]
- (23).Verwilst P; Park S; Yoon B; Kim JS Recent Advances in Gd-Chelate Based Bimodal Optical/Mri Contrast Agents. Chem. Soc. Rev 2015, 44 (7), 1791–1806. [DOI] [PubMed] [Google Scholar]
- (24).Kim T; Lee N; Park YI; Kim J; Kim J; Lee EY; Yi M; Kim B-G; Hyeon T; Yu T; Na HB Mesoporous Silica-Coated Luminescent Eu 3+ Doped Gdvo 4 Nanoparticles for Multimodal Imaging and Drug Delivery. RSC Adv. 2014, 4 (86), 45687–45695. [Google Scholar]
- (25).Ariza de Schellenberger A; Kratz H; Farr TD; Loewa N; Hauptmann R; Wagner S; Taupitz M; Schnorr J; Schellenberger EA Labeling of Mesenchymal Stem Cells for Mri with Single-Cell Sensitivity. Int. J. Nanomed 2016, 11, 1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Zhang M; Liu X; Huang J; Wang L; Shen H; Luo Y; Li Z; Zhang H; Deng Z; Zhang Z, Ultrasmall Graphene Oxide Based T1Mri Contrast Agent for in Vitro and in Vivo Labeling of Human Mesenchymal Stem Cells. Nanomedicine: Nanotechnology, Biology and Medicine 2017, 10.1016/j.nano.2017.03.019 [DOI] [PubMed] [Google Scholar]
- (27).Gerig G; Kubler O; Kikinis R; Jolesz FA Nonlinear Anisotropic Filtering of Mri Data. IEEE Transactions on medical imaging 1992, 11 (2), 221–232. [DOI] [PubMed] [Google Scholar]
- (28).Cao F; Lin S; Xie X; Ray P; Patel M; Zhang X; Drukker M; Dylla SJ; Connolly AJ; Chen X In Vivo Visualization of Embryonic Stem Cell Survival, Proliferation, and Migration after Cardiac Delivery. Circulation 2006, 113 (7), 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Love Z; Wang F; Dennis J; Awadallah A; Salem N; Lin Y; Weisenberger A; Majewski S; Gerson S; Lee Z Imaging of Mesenchymal Stem Cell Transplant by Bioluminescence and Pet. J. Nucl. Med 2007, 48 (12), 2011–2020. [DOI] [PubMed] [Google Scholar]
- (30).Sun X; Cai W; Chen X Positron Emission Tomography Imaging Using Radiolabeled Inorganic Nanomaterials. Acc. Chem. Res 2015, 48 (2), 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Tolmachev V; Stone-Elander S Radiolabelled Proteins for Positron Emission Tomography: Pros and Cons of Labelling Methods. Biochim. Biophys. Acta, Gen. Subj 2010, 1800 (5), 487–510. [DOI] [PubMed] [Google Scholar]
- (32).Wallace MB; Nietert PJ; Earle C; Krasna MJ; Hawes RH; Hoffman BJ; Reed CE An Analysis of Multiple Staging Management Strategies for Carcinoma of the Esophagus: Computed Tomography, Endoscopic Ultrasound, Positron Emission Tomography, and Thoracoscopy/Laparoscopy. Annals of Thoracic Surgery 2002, 74 (4), 1026–1032. [DOI] [PubMed] [Google Scholar]
- (33).Chen F; Ma M; Wang J; Wang F; Chern S-X; Zhao ER; Jhunjhunwala A; Darmadi S; Chen H; Jokerst JV Exosome-Like Silica Nanoparticles: A Novel Ultrasound Contrast Agent for Stem Cell Imaging. Nanoscale 2017, 9 (1), 402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Dave M; Menghini P; Sugi K; Somoza R; Lee Z; Jain M; Caplan A; Cominelli F, Ultrasound-Guided Intracardiac Injection of Human Mesenchymal Stem Cells to Increase Homing to the Intestine for Use in Murine Models of Experimental Inflammatory Bowel Diseases. J. Visualized Exp 2017, (127). 10.3791/55367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Kempen PJ; Greasley S; Parker KA; Campbell JL; Chang H-Y; Jones JR; Sinclair R; Gambhir SS; Jokerst JV Theranostic Mesoporous Silica Nanoparticles Biodegrade after Pro-Survival Drug Delivery and Ultrasound/Magnetic Resonance Imaging of Stem Cells. Theranostics 2015, 5 (6), 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Aguirre A; Ardeshirpour Y; Sanders MM; Brewer M; Zhu Q Potential Role of Coregistered Photoacoustic and Ultrasound Imaging in Ovarian Cancer Detection and Characterization. Translational Oncology 2011, 4 (1), 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Razansky D; Distel M; Vinegoni C; Ma R; Perrimon N; Koster RW; Ntziachristos V Multispectral Opto-Acoustic Tomography of Deep-Seated Fluorescent Proteins in Vivo. Nat. Photonics 2009, 3 (7), 412–417. [Google Scholar]
- (38).Chen Y-S; Frey W; Kim S; Kruizinga P; Homan K; Emelianov S Silica-Coated Gold Nanorods as Photoacoustic Signal Nanoamplifiers. Nano Lett. 2011, 11 (2), 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Bayer CL; Chen Y-S; Kim S; Mallidi S; Sokolov K; Emelianov S Multiplex Photoacoustic Molecular Imaging Using Targeted Silica-Coated Gold Nanorods. Biomed. Opt. Express 2011, 2 (7), 1828–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Yin M; Bar-Zion A; Adam D; Foster S Combined Contrast Enhanced Ultrasound and Photoacoustic Imaging Reveals Both Functional Flow Patterns and Dysfunctional Vascular Pooling in Tumor Models. Cancer Res. 2016, 76, 4197. [DOI] [PubMed] [Google Scholar]
- (41).Bar-Zion A; Yin M; Adam D; Foster FS Functional Flow Patterns and Static Blood Pooling in Tumors Revealed by Combined Contrast-Enhanced Ultrasound and Photoacoustic Imaging. Cancer Res. 2016, 76 (15), 4320–4331. [DOI] [PubMed] [Google Scholar]
- (42).Raes F; Sobilo J; Natkunarajah S; Trochet P; Fuchs D; Lerondel S; Le Pape A Novel Imaging Strategy to Assess the Antitumor Efficacy of Treatments in an Orthotopic Mouse Lung Cancer Model Using Ultrasound and Photoacoustic Imaging. Cancer Res. 2016, 76, 4200. [Google Scholar]
- (43).Hariri A; Fatima A; Mohammadian N; Mahmoodkalayeh S; Ansari MA; Bely N; Avanaki M R Development of Low-Cost Photoacoustic Imaging Systems Using Very Low-Energy Pulsed Laser Diodes. J. Biomed. Opt 2017, 22 (7), 075001. [DOI] [PubMed] [Google Scholar]
- (44).Lyu Y; Zhen X; Miao Y; Pu K Reaction-Based Semiconducting Polymer Nanoprobes for Photoacoustic Imaging of Protein Sulfenic Acids. ACS Nano 2017, 11 (1), 358–367. [DOI] [PubMed] [Google Scholar]
- (45).Jiang Y; Pu K Advanced Photoacoustic Imaging Applications of near-Infrared Absorbing Organic Nanoparticles. Small 2017, 13, 1700710. [DOI] [PubMed] [Google Scholar]
- (46).Jiang Y; Upputuri PK; Xie C; Lyu Y; Zhang L; Xiong Q; Pramanik M; Pu K Broadband Absorbing Semiconducting Polymer Nanoparticles for Photoacoustic Imaging in Second near-Infrared Window. Nano Lett. 2017, 17 (8), 4964–4969. [DOI] [PubMed] [Google Scholar]
- (47).Kim T; Lemaster JE; Chen F; Li J; Jokerst JV Photoacoustic Imaging of Human Mesenchymal Stem Cells Labeled with Prussian Blue–Poly (L-Lysine) Nanocomplexes. ACS Nano 2017, 11 (9), 9022–9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Berninger MT; Mohajerani P; Wildgruber M; Beziere N; Kimm MA; Ma X; Haller B; Fleming MJ; Vogt S; Anton M; et al. Detection of Intramyocardially Injected Dir-Labeled Mesenchymal Stem Cells by Optical and Optoacoustic Tomography. Photoacoustics 2017, 6, 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Zheng B; Lu K; Konkle JJ; Hensley DW; Keselman P; Orendorff RD; Tay ZW; Yu E; Zhou XY; Bishop M Magnetic Particle Imaging. In Design and Applications of Nanoparticles in Biomedical Imaging; Springer, 2017; pp 69–93. [Google Scholar]
- (50).Zheng B; von See MP; Yu E; Gunel B; Lu K; Vazin T; Schaffer DV; Goodwill PW; Conolly SM Quantitative Magnetic Particle Imaging Monitors the Transplantation, Biodistribution, and Clearance of Stem Cells in Vivo. Theranostics 2016, 6 (3), 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Haegele J; Duschka R; Graeser M; Luedtke-Buzug K; Schaecke C; Panagiotopoulos N; Buzug T; Barkhausen J; Vogt F 2013 International Workshop on Magnetic Particle Imaging: Kinetics of the Intravascular Signal in Vivo Magnetic Particle Imaging (IWMPI); IEEE, 2013; pp 1 and 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Saritas EU; Goodwill PW; Croft LR; Konkle JJ; Lu K; Zheng B; Conolly SM Magnetic Particle Imaging (Mpi) for Nmr and Mri Researchers. J. Magn. Reson 2013, 229, 116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Shin T-H; Choi Y; Kim S; Cheon J Recent Advances in Magnetic Nanoparticle-Based Multi-Modal Imaging. Chem. Soc. Rev 2015, 44 (14), 4501–4516. [DOI] [PubMed] [Google Scholar]
- (54).Hensley D; Tay ZW; Dhavalikar R; Zheng B; Goodwill P; Rinaldi C; Conolly S Combining Magnetic Particle Imaging and Magnetic Fluid Hyperthermia in a Theranostic Platform. Phys. Med. Biol 2017, 62 (9), 3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Bulte JW; Walczak P; Janowski M; Krishnan KM; Arami H; Halkola A; Gleich B; Rahmer J Quantitative “Hot Spot” Imaging of Transplanted Stem Cells Using Superparamagnetic Tracers and Magnetic Particle Imaging (Mpi). Tomography: J. Imaging Res 2015, 1 (2), 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Poβ M; Tower RJ; Napp J; Appold LC; Lammers T; Alves F; Glüer C-C; Boretius S; Feldmann C Multimodal [Gdo]+[Icg]− Nanoparticles for Optical, Photoacoustic, and Magnetic Resonance Imaging. Chem. Mater 2017, 29 (8), 3547–3554. [Google Scholar]
- (57).Rieffel J; Chitgupi U; Lovell JF Recent Advances in Higher-Order, Multimodal, Biomedical Imaging Agents. Small 2015, 11 (35), 4445–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Lee D-E; Koo H; Sun I-C; Ryu JH; Kim K; Kwon IC Multifunctional Nanoparticles for Multimodal Imaging and Theragnosis. Chem. Soc. Rev 2012, 41 (7), 2656–2672. [DOI] [PubMed] [Google Scholar]
- (59).Bulte JW; Walczak P; Gleich B; Weizenecker J; Markov DE; Aerts HC; Boeve H; Borgert J; Kuhn M MPI Cell Tracking: What Can We Learn from MRI?. Medical Imaging 2011: Biomedical Applications in Molecular, Structural, and Functional Imaging; International Society for Optics and Photonics, 2011; p 79650Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Mérian J; Gravier J; Navarro F; Texier I Fluorescent Nanoprobes Dedicated to in Vivo Imaging: From Preclinical Validations to Clinical Translation. Molecules 2012, 17 (5), 5564–5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Wang J; Guo F; Yu M; Liu L; Tan F; Yan R; Li N Rapamycin/Dir Loaded Lipid-Polyaniline Nanoparticles for Dual-Modal Imaging Guided Enhanced Photothermal and Antiangiogenic Combination Therapy. J. Controlled Release 2016, 237, 23–34. [DOI] [PubMed] [Google Scholar]
- (62).Huynh E; Rajora MA; Zheng G Multimodal Micro, Nano, and Size Conversion Ultrasound Agents for Imaging and Therapy. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2016, 8 (6), 796–813. [DOI] [PubMed] [Google Scholar]
- (63).Jokerst JV; Thangaraj M; Kempen PJ; Sinclair R; Gambhir SS Photoacoustic Imaging of Mesenchymal Stem Cells in Living Mice Via Silica-Coated Gold Nanorods. ACS Nano 2012, 6 (7), 5920–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Magnetic Insight, Inc. Momentum Imager.
- (65).Wassel RA; Grady B; Kopke RD; Dormer KJ Dispersion of Super Paramagnetic Iron Oxide Nanoparticles in Poly (D, L-Lactide-Co-Glycolide) Microparticles. Colloids Surf., A 2007, 292 (2), 125–130. [Google Scholar]
- (66).Kim T; Lee N; Arifin DR; Shats I; Janowski M; Walczak P; Hyeon T; Bulte JW In Vivo Micro-Ct Imaging of Human Mesenchymal Stem Cells Labeled with Gold-Poly-L-Lysine Nanocomplexes. Adv. Funct. Mater 2017, 27, 1604213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Arconada-Alvarez SJ; Lemaster JE; Wang J; Jokerst JV The Development and Characterization of a Novel yet Simple 3d Printed Tool to Facilitate Phantom Imaging of Photoacoustic Contrast Agents. Photoacoustics 2017, 5, 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Abramoff MD; Magalhães PJ; Ram SJ Image Processing with ImageJ. Biophotonics Int. 2004, 11 (7), 36–42. [Google Scholar]
- (69).Yang H; Cai W; Xu L; Lv X; Qiao Y; Li P; Wu H; Yang Y; Zhang L; Duan Y Nanobubble–Affibody: Novel Ultrasound Contrast Agents for Targeted Molecular Ultrasound Imaging of Tumor. Biomaterials 2015, 37, 279–288. [DOI] [PubMed] [Google Scholar]
- (70).Zheng B; Vazin T; Goodwill PW; Conway A; Verma A; Ulku Saritas E; Schaffer D; Conolly SM Magnetic Particle Imaging Tracks the Long-Term Fate of in Vivo Neural Cell Implants with High Image Contrast. Sci. Rep. 2015, 5, 14055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Huang D-M; Hung Y; Ko B-S; Hsu S-C; Chen W-H; Chien C-L; Tsai C-P; Kuo C-T; Kang J-C; Yang C-S; Mou C-Y; Chen Y-C Highly Efficient Cellular Labeling of Mesoporous Nanoparticles in Human Mesenchymal Stem Cells: Implication for Stem Cell Tracking. FASEB J. 2005, 19 (14), 2014–2016. [DOI] [PubMed] [Google Scholar]
- (72).Lu C-W; Hung Y; Hsiao J-K; Yao M; Chung T-H; Lin Y-S; Wu S-H; Hsu S-C; Liu H-M; Mou C-Y; Yang C-S; Huang D-M; Chen Y-C Bifunctional Magnetic Silica Nanoparticles for Highly Efficient Human Stem Cell Labeling. Nano Lett. 2007, 7 (1), 149–154. [DOI] [PubMed] [Google Scholar]
- (73).Frank JA; Miller BR; Arbab AS; Zywicke HA; Jordan EK; Lewis BK; Bryant LH Jr.; Bulte JW Clinically Applicable Labeling of Mammalian and Stem Cells by Combining Superparamagnetic Iron Oxides and Transfection Agents. Radiology 2003, 228 (2), 480–487. [DOI] [PubMed] [Google Scholar]
- (74).Yu M; Huang S; Yu KJ; Clyne AM Dextran and Polymer Polyethylene Glycol (Peg) Coating Reduce Both 5 and 30 Nm Iron Oxide Nanoparticle Cytotoxicity in 2d and 3d Cell Culture. Int. J. Mol. Sci 2012, 13 (5), 5554–5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Venugopal B; Fernandez FB; Harikrishnan V; John A Post Implantation Fate of Adipogenic Induced Mesenchymal Stem Cells on Type I Collagen Scaffold in a Rat Model. J. Mater. Sci.: Mater. Med 2017, 28 (2), 28. [DOI] [PubMed] [Google Scholar]
- (76).Volatron J; Carn F; Kolosnjaj-Tabi J; Javed Y; Vuong QL; Gossuin Y; Ménager C; Luciani N; Charron G; Hémadi M; Alloyeau D; Gazeau F Ferritin Protein Regulates the Degradation of Iron Oxide Nanoparticles. Small 2017, 13 (2), 1602030. [DOI] [PubMed] [Google Scholar]
- (77).Dabbagh K; Laurent GJ; Shock A; Leoni P; Papakrivopoulou J; Chambers RC Alpha-1-Antitrypsin Stimulates Fibroblast Proliferation and Procollagen Production and Activates Classical Map Kinase Signalling Pathways. J. Cell. Physiol 2001, 186 (1), 73–81. [DOI] [PubMed] [Google Scholar]
- (78).Bolli R; Chugh AR; D’Amario D; Loughran JH; Stoddard MF; Ikram S; Beache GM; Wagner SG; Leri A; Hosoda T; et al. Cardiac Stem Cells in Patients with Ischaemic Cardiomyopathy (Scipio): Initial Results of a Randomised Phase 1 Trial. Lancet 2011, 378 (9806), 1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- (79).Traverse JH; Henry TD; Vaughan DE; Ellis SG; Pepine CJ; Willerson JT; Zhao DX; Simpson LM; Penn MS; Byrne BJ Latetime: A Phase-Ii, Randomized, Double-Blinded, Placebo-Controlled, Pilot Trial Evaluating the Safety and Effect of Administration of Bone Marrow Mononuclear Cells 2 to 3 Weeks after Acute Myocardial Infarction. Texas Heart Inst. J 2010, 37 (4), 412. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.