Abstract
Carbon nanotube (CNT) fiber microelectrodes have been developed as electrode materials for the detection of neurotransmitters using fast scan cyclic voltammetry (FSCV). We have used acid-wet spinning to create “neat” carbon nanotube fibers and utilized them as electrode materials. Thirty-forty micron diameter acid spun CNT fiber microelectrodes were more sensitive than PEI-CNT fiber microelectrodes, with a 3 nM limit of detection. They also had faster electron transfer kinetics and a greater reversibility for the oxidation of dopamine using FSCV than CFMEs and other carbon nanomaterials. The acid spun CNT fiber microelectrodes also displayed a frequency independent response for the peak oxidation current of dopamine. This property was also seen in other CNT materials such as PEI-CNT fiber microelectrodes and CNT-Yarn microelectrodes. Upon varying the frequency from 10 Hz to 100 Hz, there was no decrease in sensitivity. When scanning at 2,000 V/s, there was no decrease in sensitivity upon changing the frequency from 10 Hz to 500 Hz. This could potentially allow for a 2 ms sampling rate for FSCV, comparable to those used with amperometry as opposed to 100 ms temporal resolution of traditional FSCV, an almost two orders of magnitude difference. Since the frequency independent response is seen with many CNT fibers/yarns, it suggests it is a fundamental property of the CNTs shared by many types of CNT fibers and yarns.
Introduction
Carbon nanotube (CNT) based electrodes have found much use in the electrochemical detection of biomolecules (1,2). The high conductivity of carbon nanotubes allows for fast electron-transfer kinetics for the detection of rapid fluctuations of neurotransmitters in vivo(3,4). The cylindrical CNT structure provides a relatively large aspect ratio (surface area: volume) for the adsorption of biomolecules(5,6). Also, carbon nanotubes have a higher density of edge-plane carbon on the ends, which is thought to be the catalytic site for neurotransmitter oxidation(7). Because of all of these properties, carbon nanotubes have been frequently incorporated into sensor technology(3). Wang’s group has explored carbon nanotube based electrodes for the detection of peptides(8), neurotransmitters(9), DNA(10,11), cholesterol(12), proteins(13), insulin(13), and other biomolecules(14–17). Many of these sensors have been carbon nanotube fiber microelectrodes whose electrochemical properties have not been fully examined.
More recently, carbon nanotube based electrode technology has been used with fast scan cyclic voltammetry (FSCV)(18). Before CNT fiber microelectrodes were developed, the Wightman group pioneered the usage of the T-650 carbon-fiber microelectrode as the standard for the field(19–24). Carbon nanotubes have been incorporated onto CFMEs via dip coating the carbon fiber into a suspension of carbon nanotubes in an organic solvent such as dimethylformamide (DMF). The method increases sensitivity towards dopamine and other neurotransmitters, but also produces much noise and is not reproducible(6,25–28). The development of carbon nanotube fiber and yarn microelectrodes is an alternative method of electrode construction to incorporate purely carbon nanotube-based materials into electrode technology for sensing applications(29,30).
Carbon nanotube fibers are produced by wetspinning(31). In contrast, CNT yarns are produced through a method developed by textile industry where they are produced via dry-spinning from the furnace and twisted into yarns(32). Carbon nanotube fibers and yarns were made into microelectrodes that have been examined preliminarily via voltammetry. PEI-CNT(33) and CNT yarn(32) microelectrodes both have lower limits of detection than CFMEs due to their larger electroactive surface areas. As shown previously, PEI-CNT fiber microelectrodes have shown a resistance to surface fouling by 5-HT and 5-HIAA and faster electron transfer kinetics than PVA-CNT fiber microelectrodes(33). Here, we examine an additional type of CNT fiber, the acid-spun CNT fiber, as way to avoid polymer and surfactant impurities produced through polymer wet spinning. CNTs are dissolved in chlorosulfonic acid and syringed into an acetone bath that displaces the acid to spontaneously form a fiber. The acid wet spinning technique precludes the use of sonication used in polymer wet spinning, which cuts CNTs to shorter lengths and reduces conductivity. Also, dissolving the CNTs in acid could possibly oxide functionalize the CNTs, which would make the negatively charged electrode more sensitive to the cationic dopamine(34,35). Acid spun CNT fibers have high sensitivities towards dopamine, although they are larger in diameter than the PEI-CNT fibers and CNT yarns.
The sensitivity of CNT yarn based electrodes towards dopamine was found to be independent of the waveform application frequency, which could greatly improve the temporal resolution of current electrode technology(32). This phenomenon occurs in CNT yarn microelectrodes because the rate of desorption of dopamine and the oxidation product, dopamine-orthoquinone (DOQ), are equal at CNT based electrodes, while the desorption of DOQ is much faster at CFMEs. Here, we find that PEI and acid-spun CNT fiber microelectrodes also have this frequency independent response. We can perform measurements at higher frequencies (500 Hz) and scan rates (2,000 V/s) where the time for the triangle waveform is now 2 ms instead of the traditional 100 ms (at 400 V/s), an almost two orders of magnitude difference. This new property could enable measurements of dopamine release at the millisecond timescale using FSCV. We show that the high temporal resolution of the PEI and acid-spun CNT fiber and CNT yarn microelectrodes is an inherent property of the carbon nanotubes and not dependent on the manner of the fiber/yarn construction.
Methods and Materials
Chemicals and Materials
Dopamine was purchased from Sigma (St. Louis, MO, U.S.). A 10 mM stock solution was prepared in 0.1 M perchloric acid and diluted to 1.0 μM daily with phosphate-buffered saline (PBS) (131.5 mM NaCl, 3.25 mM KCl, 1.2 mM CaCl2, 1.25 mM NaH2PO4, 1.2 mM MgCl2, and 2.0 mM Na2SO4 with the pH adjusted to 7.4) (all from Fisher Scientific, Fair Lawn, New Jersey, U.S.). All aqueous solutions were made with deionized water (EMD Millipore, Billerica, MA, U.S.). Armstrong C7 Resin and Armstrong A2 Activator were obtained from Ellsworth Adhesives (Germantown, WI, U.S.). Diethylenetriamine hardener (DETA) was used as received from Fisher Scientific.
Instrumentation
Fast scan cyclic voltammetry (FSCV) was performed using a ChemClamp potentiostat (Dagan, Minneapolis, MN, U.S.). Data were collected and analyzed with Tarheel CV software (gift of Mark Wightman, UNC, Chapel Hill, NC, U.S.) using custom data acquisition hardware previously described.(36) A triangle waveform was applied to the electrode from a holding potential of −0.4 V to 1.3 V and back at a scan rate of 400 V/s and a frequency of 10 Hz unless otherwise noted. A silver-silver chloride wire was used as the reference electrode. Samples were tested in a flow injection analysis system consisting of a six-port, stainless steel HPLC loop injector mounted on a two-position air actuator (VICI Valco Instruments, Co., Houston, TX, U.S.). Buffer and samples were pumped through the flow cell at 2 mL/min using a syringe pump (Harvard Apparatus, Holliston, MA, U.S.).
Scanning electron microscopy
Scanning electron microscope (SEM) images were collected on a FEI Quanta 650 microscope with a secondary electron detector using an accelerating voltage of 5 kV and a working distance of 5.6 mm.
Carbon Nanotube Fiber and Yarn Microelectrode Preparation
All carbon nanotube fiber microelectrodes were made with epoxy insulation(18). Nanotubes are either separated by the use of a surfactant or acid and are then syringed into a solution of polymer (polyethyleneimine, PEI) or acetone, respectively. We call these fibers PEI-CNT fibers(33) and acid-spun CNT fibers, respectively(34). A mold was made in Teflon with 60-70 μm wide and deep channels(18). Under a stereoscope, Armstrong Resin C7 and 0.8% Armstrong Activator A2 were syringed into each channel using a 30 gauge needle. A single carbon nanotube fiber or carbon fiber was manually inserted into each channel, and the epoxy was allowed to cure for three hours at 165°C before being removed from the mold. Silver epoxy (H20E, equal portions of Parts A and B, Epoxy Technology, Billerica, MA, U.S.) was applied with a syringe to the other end of the epoxied carbon fiber and connected to a gold pin (0.035” x 0.249”, Digikey, Thief River Falls, MN, U.S.) to connect to the potentiostat. The silver epoxy was cured for one hour at 150°C. CNT fibers were cut at the surface to form “disk-like” electrodes. Carbon fibers were cut at 100 μm to give them more surface area.
Poly (vinyl alcohol) (PVA) carbon nanotube (CNT) fibers were prepared as previously described(31). Gloves and glasses are recommended to be worn when handling raw powders of nanotubes. A suspension of 0.35% HiPCO CNTs (high pressure carbon monoxide, Unidym, Sunnyvale, CA) in 1.2% sodium dodecylbenzenesulfonic acid (SDBS, Sigma) in water was pumped through a 30 G syringe needle (flow rate 0.5 mL/minute) into a 4% aqueous solution of poly(vinyl alcohol) (PVA) (Aqua Solutions, Deer Park, TX, MW = 124,000-186,000). The PVA solution was revolved using a custom built rotating stage. CNT ribbons were subsequently purified and rinsed in water and then methanol, which washed away the excess polymer. Ribbons collapsed into fibers upon being allowed to dry in air and then in the oven for 1 hour at 180°C.
Polyethyleneimine (PEI) CNT fibers were formed as previously described(37). HiPCO CNTs (0.4%) were suspended in water with SDBS (1.2%) and were syringed into a streaming solution of 40% PEI (branched, MW = 50,000 – 100,000, MP Biomedicals, LLC, Santa Ana, CA) in methanol. The CNT ribbons were subsequently purified in methanol. CNT fibers were dried in the oven for approximately one hour at 180°C to remove any excess impurities. All CNT fiber microelectrodes were equilibrated in the flow cell by scanning with the applied waveform for 1 hour before testing(33). The limit of detection (LOD) was calculated using a S/N ratio of 3 from 100 nM measurements for dopamine.
Acid spun CNT fibers were made as previously described.(34) 1-8% HiPCO CNTs were dissolved into chlorosulfonic acid (Fluka Analytical/Sigma, St. Louis, MO, U.S.). They were then syringed into a bath of acetone on a rotating stage with a Harvard Apparatus pump. Fibers were spontaneously formed in the acetone bath and were removed with forceps. They were washed with water and then dried in an oven for one hour at 150°C.
A 1 – 2 cm length of commercially available CNT Yarn of 10-25 μm diameter (General Nano, LLC, Cincinnati, OH) was either (1) inserted into a polyimide coated fused-silica capillary while submerged in 2-propanol, to reduce friction and ease insertion (45 μm I.D. × 90 μm O.D., Polymicro Technologies, Phoenix, AZ), or (2) inserted into a 0.68 mm I.D. × 1.2 mm O.D. glass capillary that had been pulled into a glass pipette and cut to have an opening of about 50 μm diameter(32). The solvent was allowed to fully evaporate from inside of the capillary before the CNT yarn was sealed into the capillary with Loctite brand 5-minute epoxy and was allowed to fully cure for 24 hours. The resulting microelectrode was polished at about a 90° angle on a Sutter Instruments polishing wheel with the coarse and fine polishing disks to make a disk CNTYME. For comparison, carbon fiber microelectrodes (CFMEs) were also fabricated, insulated, and polished in a similar manner using 7 μm diameter T-650 carbon fibers (Cytec Technologies, Woodland Park, NJ)(32).
Results
Synthesis of Carbon Nanotube Fibers and Yarns
In this study, we compare three different types of carbon nanotube microelectrodes (PEI-CNT fiber, acid-spun CNT fiber, and CNT yarn) to determine the extent to which their electrochemical properties are a function of the fiber/yarn construction or are an inherent property of the carbon nanotubes. Polyethyleneimine (PEI) CNT fibers are formed by wet spinning. HiPCO CNTs are suspended in an aqueous solution of water via tissue sonication and the addition of a surfactant, SDBS (sodium dodecyl benzenesulfonic acid) to prevent van der Waals aggregation. When pushed into a streaming solution of polymer, the CNTs collapse into ribbons as the polymer displaces the surfactant. The CNT ribbons are then washed in water to remove the excess polymer to form thin CNT fibers (~20 microns in diameter). Despite washing, rinsing, and heating to remove impurities, some remain. The presence of polymer on the surface of the CNT fiber could be blocking sites for further adsorption of biomolecules to the CNT surface. PEI was chosen to replace the traditional, less-conductive PVA because the amine group of the PEI physisorbs to the walls of the CNTs to induce an intermolecular charge transfer, which increases conductivity 100-fold more than PVA-wetspun CNT fibers(37). This increase in conductivity makes PEI-CNT fibers very attractive as electrode-based materials for neurotransmitter detection with fast scan cyclic voltammetry (FSCV).
More recently, wet-spinning with acids instead of polymers has been investigated. Using sulfuric acid, neat carbon nanotube fibers have been constructed(35). The mechanism of fiber formation involves the oxides of the sulfuric acid separating each CNT bundle through an electrostatic repulsion. Once pushed into water or acetone, which displaces the acid, the CNTs are then oriented into vertically aligned carbon nanotube fibers. Chlorosulfonic acid has recently been shown to be a solvent for carbon nanotubes, which can dissolve the CNTs(34). The oxide group of the chlorosulfonic acid can separate CNT bundles and align them into fibers(34). Using chlorosulfonic acid is advantageous since it does not require the use of polymer or surfactant impurities for fiber formation, which can hinder biomolecule adsorption and electron transfer. It also does not require sonication, which cuts CNTs to shorter lengths, thus reducing conductivity(34).
CNT yarns are synthesized by growing CNTs using CVD on a substrate, and slowly pulling a bundle of CNTs off the substrate and twisting them into a yarn(32). The dry spinning technique directly from the furnace was developed from the textile industry and can produce meter length fibers for industrial purposes(32). Again, these CNT yarns are relatively impurity free, which could account for their enhanced properties when used as electrode materials. The twisting of the fibers into yarns creates a larger surface area for neurotransmitter detection.
Surface Characterization
Scanning electron microscope images show PEI-CNT fibers that have diameters of 15 to 25 μm (Fig. 1A)(33). The diameter is dependent on the flow rate of the syringe pump and the rotation speed of the stage and can be controlled by varying these two parameters. Fig. 1A shows the side of a fiber. The surface of the fiber is primarily comprised of SWCNTs with distinct regions of PEI that were not fully removed during the rinse. Fewer regions of polymer impurities are observed on the outside of the CNT fiber walls for PEI-CNT fibers than for PVA-CNT fibers(31). Fig. 1B shows an end of a CNT fiber. The CNTs appear to be in thick bundles and thin CNT bundles are seen protruding from the surface. Fig. 1 C is an SEM image of an acid-spun CNT fiber made with chlorosulfonic acid. The fiber has a larger diameter than PEI-CNT fibers. Vertically aligned CNT bundles form the CNT fiber. The fiber also has more pits and is not as round as the PEI-CNT fiber.
Figure 1: SEM Images of Carbon Nanotube Fibers and Yarn.
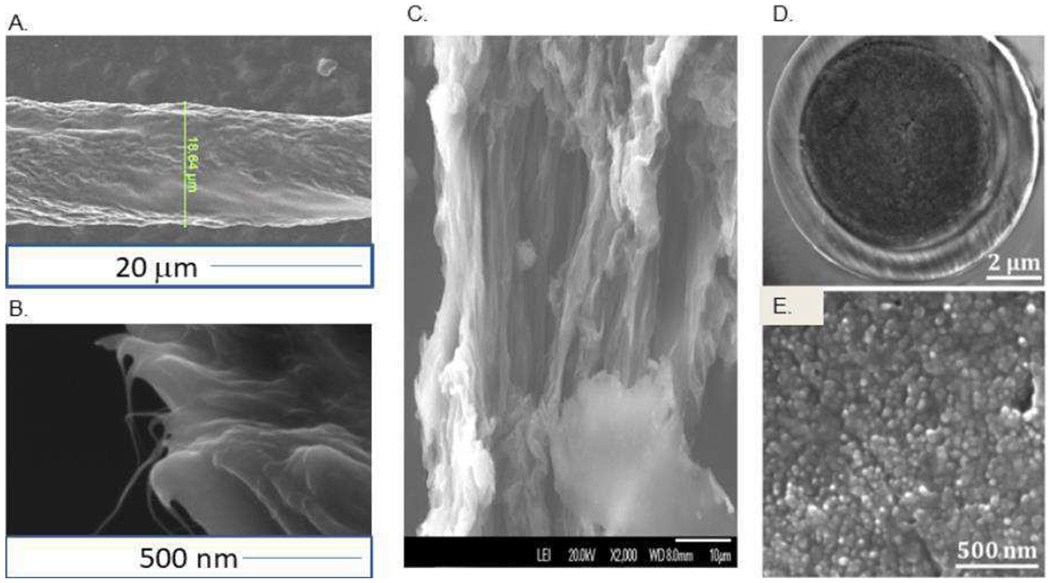
(A). SEM Image of a PEI CNT fiber with darker regions containing more conductive CNTs. (B) SEM image of a PEI CNT fiber end. Thin whiskers of individual CNTs protrude from the bundles in the cross-section. (C). SEM of Acid Spun CNT fiber. (D) A beveled end a CNT yarn microelectrode. (E) High magnification CNT yarn with 30-50 nm diameter CNTs bundled tightly together to form a nanostructured surface. Panels D and E are taken from reference (32).
Carbon nanotube yarns (CNTYs) consist of two or more CNT threads twisted together, each typically around 5 μm in diameter(32). Because multiple threads are twisted together, CNTYs do not have perfectly circular cross-sections, and often have localized areas that vary in CNT density. The cross-section of the CNTY typically ranges between 5 μm and 30 μm, but microelectrode fabrication in pulled glass capillaries can yield carbon nanotube yarn microelectrodes (CNTYMEs) with electrode tips smaller than the original measured cross-section of the CNTY because the yarn can compress(32). The SEM in Figure 1D shows an example of a pulled glass capillary disk microelectrode, with a tip diameter of 10 μm. High magnification SEM images of the CNTYME surface, Figure 1E, shows a multitude of small circles, each about 10-30 nm in diameter, which suggests the surface consists primarily of CNT ends(32).
Electrochemical Characterization
Acid-spun CNT fiber microelectrodes are a novel electrode material for neurotransmitter sensing using fast scan cyclic voltammetry (FSCV). Figure 2A shows an example cyclic voltammogram for 1 μM dopamine using an acid-spun CNT fiber microelectrode, while Figure 2B shows the example background charging current, which is dependent on the surface area of the electrode material. The acid-spun fibers produced through wetspinning are approximately 30-50 microns in diameter. The acid-spun fibers were tested with a waveform going to 1.0 V because the surface area was larger and they often overloaded, with background currents over 10 μ A, with the 1.3 V switching potential. The oxidation and reduction peaks for dopamine in Fig. 2A are large and nearly identical in size, indicating the reaction is more reversible. The background current is about 6000 nA in Fig. 2B, which indicates a large surface area. The acid-spun fiber electrodes are very sensitive, with about 100 nA of current for 1 μM dopamine, as they have larger surface areas made up of vertically aligned CNTs. Future work could concentrate on reducing the diameter of the acid spun fibers. For example, at the Smalley Institute, they have a custom built compression system that continually compresses and stretches the CNT fibers to smaller diameters and more vertically aligned orientations(35).
Figure 2:
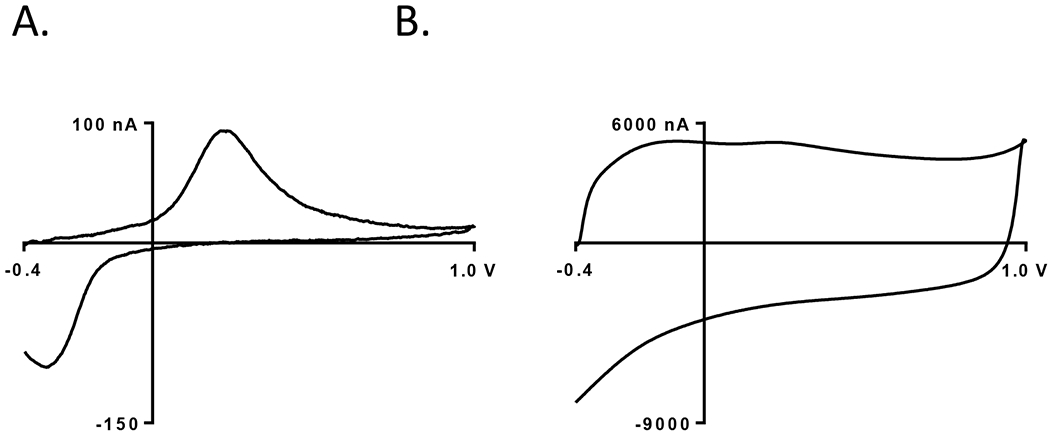
Acid Spun-CNT Fiber Microelectrode Data A. Cyclic Voltammogram for 1 μM dopamine (background subtracted). B. Background charging current. Dopamine is detected with a repetitive scan and background subtraction. The acid spun CNT fiber is 40 μm in diameter and are cut at the tip to form a disk-like electrode (performed at pH = 7.4).
The acid-spun CNT fiber microelectrodes display many interesting characteristics (Table I). The high sensitivity and low limit of detection, 3 nM, are a function of the large electroactive surface area of the acid spun CNT fiber. The background charging current is 5-fold greater than PEI-CNT fiber microelectrodes that are also disk-like. Even though the CFMEs are cylindrical in shape, their background charging currents are still markedly smaller than acid-spun CNT fiber microelectrodes. This is most likely due to the higher conductivity and larger diameter of the CNT fiber. Also, the electron transfer kinetics are markedly faster than CFMEs and PEI-CNT fiber microelectrodes by about 100 mV (Table I). This is thought to occur due to the higher conductivity of the CNT materials over the traditional CFMEs with no impurity present to hinder electron transfer.
TABLE I:
Electrochemical Data. Some data is reproduced with permission from reference 32. A chart of the approximate area, limit of detection for dopamine, overpotentials, and ratios of peak reductive to peak oxidative currents comparing CFMEs to ACID CNTFMEs, PEI CNTFMEs, PVA-CNTFMEs, and CNTYMEs. The geometric approximations for areas were calculated using the formulas: πr2 + 2πrh for cylinder electrodes and πr2 for disc electrodes with r being the radius of the fiber and h being the length protruding.
| 1 μM DA | n | Approximate Area (μm2) | LOD (nM) | ΔEp (mV) | ip,c/ip,a |
|---|---|---|---|---|---|
| CFME | 4 | 362 π | 24 ± 2 nM | 680 ± 5 | 0.63 ± 0.01 |
| ACID-CNTFME | 6 | 400 π | 3 ± 0.5 nM | 570 ± 7 | 0.95 ± 0.01 |
| PEI-CNTFME | 6 | 156 π | 5 ± 1 nM | 670 ± 6 | 0.78 ± 0.01 |
| PVA-CNTFME | 6 | 156 π | 53 ± 5 nM | 970 ± 3 | 0.72 ± 0.01 |
| CNTYME | 5 | 156 π | 10 ± 0.8 nM | 580 ± 3 | 0.77 ± 0.01 |
CNT fibers and CNT yarns have been made into electrode materials for testing with fast-scan cyclic voltammetry for a comparison study. CNT fibers were epoxy insulated and attached to a gold pin, while CNT yarns were placed in a polyimide coated fused silica capillary that was slid into a glass capillary and then polished(32). Zestos et. al have already shown that the sensitivity, temporal resolution, and electron transfer kinetics of a carbon-fiber microelectrode is independent of the electrode insulation(18). The electrochemical properties of different carbon nanotube fibers/yarns were compared (Table I). The limits of detection of each carbon nanotube/yarn are a function of the surface area. Geometric areas were approximated using the diameter of the fiber/yarn and simple geometrical formulas for the areas of a circle or cylinder. This assumes that the CFMEs were cylindrical electrodes of 100 μm length while the CNT fibers and yarns were disk electrodes. Because PEI-CNT and acid-spun fibers were cut instead of polished, the surface roughness may be greater than approximated by the calculations. The large size of the backgrounds for the acid-spun fibers suggests that they may be substantially larger than the geometric area indicates. The CNT yarn and fiber microelectrodes, with the exception of the less conductive PVA-CNT fiber that has non-conductive polymer blocking sites for adsorption, have lower limits of detection than CFMEs, but similar or smaller geometric areas. This could be explained by an electrocatalytic effect of the edge-plane carbon that the CNTs have for dopamine oxidation. The surface areas of acid-spun CNT fiber microelectrodes are approximately 4-times greater than PEI-CNT fibers, which is similar to the increase in background charging currents and peak oxidative currents that are 4-5 greater than PEI-CNT fiber microelectrodes (see Figure 1).
PEI CNT fiber microelectrodes have ΔEp values that are comparable to CFMEs. PVA-CNT fiber microelectrodes have ΔEp values that are approximately 300 mV greater. Non-conductive PVA likely coats the surface of the CNT fiber, which slows electron transfer. The PEI fiber is more conductive because the amine group of the PEI undergoes an intermolecular charge transfer with the sidewall of the CNTs, which increases conductivity over 100-fold more than the PVA fiber.(37) The acid-spun fibers and CNT yarns have ΔEp values that are approximately 100 mV less than CFMEs and polymer-CNT fiber electrodes because they have no polymer or surfactant impurities on the surface (see Table I). The conductivity of the acid spun CNT fiber is solely a function of the CNTs without any other surface impurities present to diminish electron transfer.
All of the of CNT yarns/fiber microelectrodes have a greater reversibility of dopamine oxidation with respect to CFMEs as seen in the ratio of peak cathodic current to peak anodic current in Table I. A ratio of 1 would indicate all the dopamine that was oxidized was reduced to dopamine on the return scan. The oxidation of dopamine appears to be almost completely reversible at acid-spun fibers. This property has also been observed with CNT yarn microelectrodes, but not CFMEs. (19,30) For CFMEs, the oxidation of dopamine is quasi-reversible, meaning that not all of the DOQ that is oxidized is reduced back to dopamine. For CNT-based electrode, more of the DOQ is reduced back to dopamine. A hypothesis for this phenomenon is that less DOQ desorbs from the electrode at CNT fibers than at CFMEs.
Frequency Independent Response
We recently discovered an interesting property of CNT yarn microelectrodes: their sensitivity for dopamine is independent of the wave application frequency (32). Traditionally with CFMEs, the peak oxidative current of dopamine decreases as the frequency is increased because there is less time for dopamine to adsorb at higher frequencies. Thus, there is less time at the negative holding potential which electrostatically attracts the positively charged dopamine. The rate of desorption of DOQ is 10 times faster than the rate of desorption of dopamine from the surface of the CFME (32). The rate of desorption of dopamine and DOQ are approximately equal at the surface of CNT yarn microelectrodes based on previous modeling studies of dopamine adsorption kinetics on CNT yams.
Here, we tested the extent to which PEI-CNT fibers and acid spun fibers would exhibit the same frequency response. As shown in Figure 3 A, upon increasing the frequency from 10 Hz to 90 Hz, peak oxidative current for dopamine decreases 75% for CFMEs. There is no decrease in peak oxidative current for dopamine for CNTYMEs upon increasing the frequency from 10 to 90 Hz (Fig. 3B). Similar results are observed for PEI-CNT and acid-spun CNT fiber microelectrodes (Figures 3C–D). There is no drop in peak oxidative current for dopamine upon increasing the wave application frequency from 10 Hz to 90 Hz. Again, this is hypothesized to occur because dopamine and DOQ desorb at equal rates from the nano structured CNT fiber/yarn.
Figure 3:
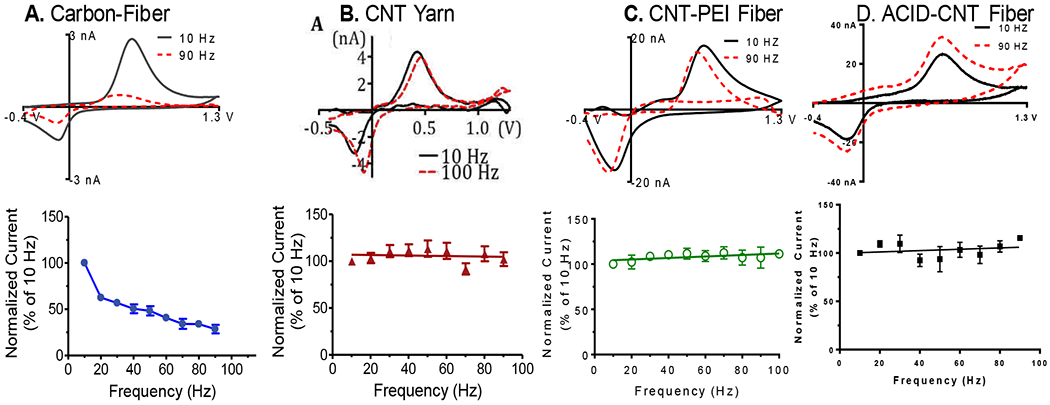
Frequency Independent Response. Current for 1μM dopamine for different microelectrodes as the frequency of the applied FSCV waveform is increased. Some of the data are reproduced with permission from reference 32. A. The current at a diskcarbon-fiber microelectrode drops dramatically by 90 Hz. B. For a disk CNT yarn microelectrode, the current is steady over all the frequencies. C. For a CNT-PEI fiber microelectrode, the current does not decrease with increasing frequency. D. There is also no decrease in sensitivity for acid spun CNT fiber microelectrodes upon increasing the frequency from 10 Hz to 90 Hz. The pH is 7.4 and scan rate is 400 V/s for all. n = 4-6.
CNTYMEs, PEI-CNTFMEs, and Acid CNTFMEs all display a sensitivity that is independent of the frequency. Since all three materials are composed almost exclusively of CNTs, then it is assumed that the catalytic properties of the CNTs cause this phenomenon. However, CFMEs that were chemically modified with vertically aligned CNT forests do not exhibit a sensitivity that is independent of the frequency. (27) We hypothesize that the less conductive carbon fiber core convolutes the signal and slows down electron transfer, which is why we do not see this phenomenon for the CNT modified CFME. The self-assembled CNTs also do not appear to be as well aligned as in the CNT yarns and fibers, so the CNT alignment and structure may also play a role. The temporal response is likely due to aligned, purely CNT-based electrode materials such as the CNT yarns and CNT fibers.
A useful application of having a frequency independent response for the peak oxidative current of dopamine is the ability to use faster scan rates. With the traditional waveform (400 V/s), the triangle takes about 10 ms, therefore, the maximum repetition rate is approximately 100 Hz. At faster scan rates, the time for the triangle can be decreased. At 2000 V/s, the time for the triangle is about 2 ms; therefore, a 500 Hz wave application frequency can be utilized. Figure 4 A shows a CFME at 2,000 V/s at 10 and 500 Hz, respectively. The peak oxidative current for dopamine drops dramatically at 500 Hz. On the other hand, there is no decrease in peak oxidative current for CNTYMEs (Fig 4 B) and PEI-CNT fiber microelectrodes (Fig. 4 C), respectively upon increasing the frequency from 10 to 500 Hz.
Figure 4:
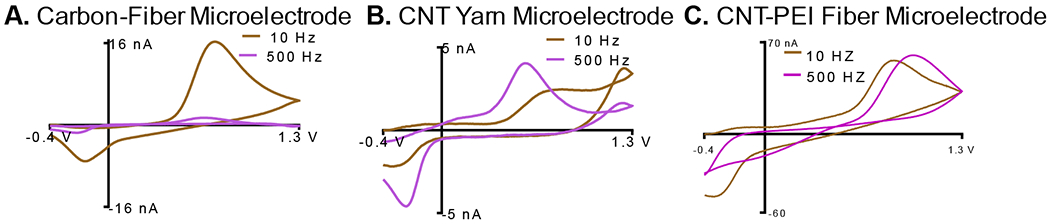
High Temporal Resolution Measurements. FSCV at 2000 V/s, pH 7.4. Example CVs of 1 μM dopamine at 10 Hz and 500 Hz repetition rates at A. CFMEs, B. CNT yarn microelectrodes and C. CNT-PEI fiber microelectrodes. (4B Reproduced with permission from Reference 32)
This markedly increases the sampling rate at our electrodes from 100 ms to 2 ms, about a two-order of magnitude difference. This makes the sampling rate comparable to that used with amperometry (38), however, holding at single potential provides no chemical information about the molecules being detected. The work could possibly provide a breakthrough in neurotransmitter detection by providing the first FSCV measurement of dopamine release on the millisecond timescale. Therefore, it may even allow for measurements of dopamine during individual pulses of burst firing. Obviously, future in vivo measurements are necessary with CNT fiber/yarn microelectrodes to realize this claim.
Conclusion and Discussion
We have shown that CNT fiber and CNT yarn microelectrodes have enhanced properties for neurotransmitter detection. The PEI-CNT and acid-CNT fibers were produced by wet spinning with polymers/surfactants and acids/acetone, respectively, while CNT yarns were dry-spun directly from the furnace and twisted into yarns using techniques developed by the textile industry. SEM images show high vertical alignment of CNTs on all three electrode materials. The enhanced electrochemical properties, apart from PVA-CNTFMEs, are evident as faster electron transfer kinetics, lower limits of detection, and increased cathodic/anodic peak ratios. The kinetics of dopamine oxidation are adsorption controlled for all CNT fibers and yarns, similar to CFMEs. The sensitivity towards dopamine is independent of the wave application frequency, which is a function of DA and DOQ desorbing from the surface of the CNT yarns and fibers at equal rates, while the DOQ desorbs from the electrode about 10-times faster than dopamine at the surface of CFMEs. CFMEs chemically modified with a CNT-forest did not exhibit a frequency independent response. Therefore, it is thought that the carbon-fiber core of the heterogeneous electrode convoluted the enhanced electron transfer and the electrochemical properties of the CNTs.
In our initial studies, the frequency independent response only occurs for electrode materials whose sole carbon source comes from vertically aligned CNTs. Therefore, we argue that the enhanced electrochemical properties of the CNT fiber and yarn microelectrodes are a function of the intrinsic properties of the CNTs and not the manner of the construction of fibers or yarns, respectively. The frequency independent response allows for testing at 500 Hz and 2,000 V/s, which increases the temporal resolution to 2 ms from the previous 100 ms. Using CNT fiber or yarn microelectrodes could potentially enable the first measurement of dopamine release on the millisecond timescale using fast scan cyclic voltammetry. More rapid measurements would allow a better understanding of the dopamine signaling in the brain on a millisecond time scale.
Acknowledgments
Funding Source:
NIH R21DA037584-01
References
- 1.Deo RP, Lawrence RNS, Wang J Analyst, 129, 1076–1081, (2004). [DOI] [PubMed] [Google Scholar]
- 2.Hocevar SB; Wang J, Deo RP, Musameh M; Ogorevc B. Electroanalysis, 17, 417–422, (2005). [Google Scholar]
- 3.Britto PJ, Santhanam KSV, Ajayan PM, 41 (1), 121–125, (1996). [Google Scholar]
- 4.Britto PJ, Santhanam KSV, Rubio A, Alonso JA, Ajayan PM. Advanced Materials 11 (2), 154–157, (1999). [Google Scholar]
- 5.Jacobs CB, Vickrey TL, Venton LBJ. Wiley Encyclopedia of Chemical Biology. John Wiley & Sons, Inc. (2007). [Google Scholar]
- 6.Jacobs CB, Vickrey TL, Venton LBJ. 17 (136), 3557–3565, Analyst (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs CB, Peairs MJ, Venton LBJ. 662 (2), 105–127 Anal. Chim. Acta (2010). [DOI] [PubMed] [Google Scholar]
- 8.Zhou XJ, Moran-Mirabal JM, Craighead HG, Mceuen PL, Nature Nanotechnology, 2 (3), 185–190, (2007). [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Deo RP, Poulin P, Mangey M, J. Am. Chem. Soc, 125 (48), 14706–14707 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Kim JH, Kataoka M, Jung Y, Ko Y, Fujisawa K, Hayashi T, Kim Y, Endo M, ACS Applied Materials & Interfaces, 5 (10), 4150–4154 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Ko J, Woo JM, Ahn J, Cheon JH, Lim J, Kim SH, Chun H, Kim E, Park YJ. ACS Nano, 5 (6), 4365–4372 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Gopalan A, Lee KP, Ragupathy D. Biosensors & Bioelectronics. 24 (7), 2211–2217, (2009). [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Musameh M. Anal. Chim. Acta, 511 (1), 33–36, (2004). [Google Scholar]
- 14.Sebez B, Su L, Ogorevc B, Tong Y, Zhang XJ. Electrochemistry Communications 25, 94–97, (2012). [Google Scholar]
- 15.Zhu Z, Garcia-Gancedo L, Flewitt AJ, Xie HQ, Moussy F. Milne WI. Sensors, 12 (5), 5996–6022, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu ZG, Garcia-Gancedo L, Flewitt AJ, Moussy F, Li YL, Milne WI. Journal of Chemical Technology and Biotechnology. 87 (2), 256–262, (2012,. [Google Scholar]
- 17.Wen H, Nallathambi V, Chakraborty D, Barton SC. Microchimica Acta. 175 (3–4), 283–289, (2011),. [Google Scholar]
- 18.Zestos AG, Nguyen MD, Poe BL, Jacobs CB, Venton BJ. Sensors and Actuators B-Chemical. 182, 652–658, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bath BD, Michael DJ, Trafton BJ, Joseph JD, Runnels PL, Wightman RM. Anal. Chem. 72 (24), 5994–6002, (2000). [DOI] [PubMed] [Google Scholar]
- 20.Bunin MA, Prioleau C, Mailman RB, Wightman RM. J. Neurochem. 70 (3), 1077–1087, (1998). [DOI] [PubMed] [Google Scholar]
- 21.Forry SP, Murray JR, Heien ML, Locascio LE, Wightman RM. Anal. Chem. 76 (17), 4945–4950, (2004). [DOI] [PubMed] [Google Scholar]
- 22.Garris PA, Wightman RM. Synapse. 20 (3), 269–279, (1995). [DOI] [PubMed] [Google Scholar]
- 23.Heien ML, Johnson MA, Wightman RM. Anal. Chem, 76 (19), 5697–5704 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Hermans A, Seipel AT, Miller CE, Wightman RM. Langmuir, 22 (5), 1964–1969 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swamy BE; Venton BJ. Analyst, 132 (9), 876–884 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Peairs MJ, Ross AE, Venton BJ. Analytical Methods, 3 (10), 2379–2386, (2011). [Google Scholar]
- 27.Xiao N; Venton BJ. Analytical Chemistry, 84 (18), 7816–7822 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross AE; Venton BJ. Analyst, 137 (13), 3045–3051 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue Y, Kakihata K, Hirono Y, Horie T, Ishida A, Mimura H. Applied Physics Letters, 92 (21) (2008). [Google Scholar]
- 30.Schmidt AC, Wang X, Zhu Y, Sombers LA. 7 (9) ACS Nano (2013). [DOI] [PubMed] [Google Scholar]
- 31.Vigolo B, Penicaud A, Coulon C, Sauder C, Pailler R, Journet C, Bernier P, Poulin P. 290 (5495), 1331–1334, (2000). [DOI] [PubMed] [Google Scholar]
- 32.Jacobs CB. Carbon Nanotube-based microelectrodes for enhanced detection of neurotransmitters. University of Virginia Dissertation; . 2012. [Google Scholar]
- 33.Zestos AG, Jacobs CB, Trikantzopoulos E, Ross AE and Venton Anal BJ. Chem, 86 (17), 8568–75, (2014) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Behabtu N, Young CC, Tsentalovich DE, Kleinerman O, Wang X, Ma A, Bengio EA, Waarbeek RF, de Jong JJ, Hoogerwerf RE, Fairchild SB, Ferguson JB, Maruyama B, Kono J, Talmon Y, Cohen Y, Otto MJ, Pasquali M. Science, 339 (6116), 182–186, (2013). [DOI] [PubMed] [Google Scholar]
- 35.Ericson LM, Fan H, Peng H, Davis VA, Zhou W, Sulpizio J, Wang Y, Booker R, Vavro J, Guthy C, Parra-Vasquez AN, Kim MJ, Ramesh S, Saini RK, Kittrell C, Lavin G, Schmidt H, Adams WW, Billups WE, Pasquali M, Hwang WF, Hauge RH, Fischer JE, Smalley RE. Science 305 (5689), 1447–1450, (2004). [DOI] [PubMed] [Google Scholar]
- 36.Venton BJ, Troyer KP, Wightman RM. Anal. Chem. 74 (3), 539–546, (2002). [DOI] [PubMed] [Google Scholar]
- 37.Munoz E, Suh DS, Collins S, Selvidge M, Dalton AB, Kim BG, Razal JM, Ussery G, Rinzler AG, Martinez MT, Baughman RH.. Advanced Materials. 17 (8), 1064–+ (2005). [Google Scholar]
- 38.Chakraborty S; Raj CR. Electrochemistry Communications, 9 (6), 1323–1330, (2007). [Google Scholar]


