Abstract
Background
To investigate the uniportal video‐assisted thoracoscopic surgery (VATS) technique and safety of non‐small cell lung cancer (NSCLC) patients treated with uniportal and three‐port VATS.
Methods
We retrospectively evaluated 146 consecutive patients with NSCLC who underwent VATS lobectomy between January 2018 and May 2018. The general clinical date, perioperative data and life quality were individually compared and analyzed between the two groups.
Results
Intraoperative blood loss was significantly lower in the uniportal than in the three‐port group (p = 0.035), and significantly shorter chest tube drainage and postoperative hospital stay durations were found in the uniportal than in the three‐port group (p = 0.022 and p = 0.008). The postoperative 24 and 72 h numerical rating scale (NRS) scores were significantly lower in the uniportal group than in the three‐port group (p < 0.001 and p < 0.001). There were no significant differences between the two groups in the number or stations of total lymph node dissected (p = 0.222 and p = 0.159). There were no significant differences between the two groups in the postoperative total or respiratory complications (p = 0.917 and p = 0.930).
Conclusions
Uniportal VATS is a safe and effective alternative for patients with NSCLC. It is a preferable option for appropriate cases as it is conducive to patients’ postoperative recovery and quality of life.
Keywords: carcinoma, non‐small cell lung, pulmonary (lungs), surgical outcomes, video‐assisted thoracoscopic surgery
To investigate the uniportal video‐assisted thoracoscopic surgery (VATS) technique and safety of non‐small cell lung cancer (NSCLC) patients treated with uniportal and three‐port VATS.
INTRODUCTION
The incidence and mortality of lung cancer has been reported to be the highest among all malignant tumors worldwide. 1 Comprehensive treatment based on surgery is the main treatment method for resectable non‐small cell lung cancer (NSCLC). It has been widely recognized that video‐assisted thoracoscopic surgery (VATS) is minimally invasive and enables rapid recovery compared with thoracotomy. 2 , 3 The 2006 National Cancer Comprehensive Network (NCCN) guidelines recommended VATS as a standard surgical procedure for lung cancer. 4 Currently, most medical centers use multiport (2–4 port) VATS as a minimally invasive surgical approach for treating lung cancer. 5 , 6 , 7 Rocco et al. reported the first uniportal VATS wedge pulmonary resection in 2004. 8 Gonzalez et al. published the first application of uniportal VATS lobectomy for treating early‐stage lung cancer in 2011. 9 Uniportal VATS has quickly gained popularity as a surgical approach and has been rapidly adopted worldwide due to its further reduced invasiveness and increased recovery. 7 , 10 , 11 In our department, multiportal VATS lobectomy has been performed since 2005, and uniportal VATS was performed in 2014, starting with simple wedge resection and gradually progressing to lobectomy, segmentectomy, pneumonectomy and sleeve lobectomy. Numerous studies have reported the advantages of multiport VATS in the treatment of lung cancer compared with thoracotomy, 2 , 3 , 4 , 5 but there have been few reports comparing uniportal and three‐port VATS. The purpose of this study was to investigate the uniportal VATS technique and safety of NSCLC patients treated with uniportal and three‐port VATS.
METHODS
This study was approved by the ethics committee of the Anhui Provincial Hospital, affiliated with Anhui Medical University. Written informed consent was obtained from all patients prior to the operation. We retrospectively evaluated 216 consecutive patients with NSCLC who underwent VATS lobectomy between January 2018 and May 2018. Patients were selected based on the following eligibility criteria: (i) histopathologically proven NSCLC, (ii) uniportal or three‐port VATS lobectomy and systemic mediastinal lymph node dissection, (iii) no neoadjuvant therapy, (iv) clinical T1‐3N0‐1M0 disease prior to surgery, and (v) no known distant metastasis. Patients were excluded based on the following criteria: (i) palliative resection, or (ii) incomplete medical records. Based on these criteria, 146 patients were enrolled for analysis in this retrospective study. According to the surgical method, the patients were divided into the uniportal (n = 80) and three‐port (n = 66) groups.
Routine preoperative examination included routine blood tests, electrocardiograms, pulmonary function tests, chest computed tomography (CT), fiberoptic bronchoscopy, brain magnetic resonance imaging (MRI), bone scintigraphy, abdominal and adrenal ultrasonography and echocardiography. Patients whose CT scan indicated a possible N2 or N3 node greater than 1.0 cm along its shortest axis or any suspected M1 disease underwent fluorodeoxyglucose positron emission tomography (FDG‐PET). TNM staging was based on the eighth edition of the International Association for Lung Cancer Research (IASLC) guidelines. Postoperative complications were evaluated by the Clavien‐Dindo classification criteria. 12 , 13 Clavien‐Dindo 1–2 complications were classified as minor, and Clavien‐Dindo 3–5 complications were classified as major. Patients were interrogated for pain by a numerical rating scale (NRS) scoring system at rest and during cough by an intensivist blinded to the study groups at 24 and 72 h. Pain was classified as painless, mild, moderate, severe and sharp for the analysis (painless, NRS 0; mild, NRS 1–3; moderate, NRS 4–6; severe, NRS 7–9; and sharp, NRS 10).
Surgical technique
For uniportal VATS, double‐lumen endotracheal intubation and single‐lung ventilation were performed with the patient in a lateral position on the healthy side. The operator stood on the patient's abdominal side, and the assistant and the camera‐holder stood on the opposite side. A surgical incision (3.0–5.0 cm in length) was made in the fourth or fifth intercostal space between the anterior and posterior axillary lines, and a wound protector (Beijing HangTian KaDi Technology) was placed to stretch the incision. A high‐definition 30° 10 mm thoracoscope was applied for a panoramic view and placed in the posterior part of the incision throughout the surgery. Thoracoscopy was performed to detect the presence of adhesions, effusion and disseminated nodules in the thoracic cavity and to determine the specific location of the lesion and the anatomy of the lung. In patients with a confirmed preoperative diagnosis, lobectomy and systemic mediastinal lymph node dissection were directly performed. In patients without a pathological diagnosis, a wedge resection was performed, and the cryosection was checked first; lobectomy and systemic mediastinal lymph node dissection were performed only if the cryosection showed malignancy. If the pulmonary fissure developed well, the arteries were treated preferentially; if not, a “single‐direction” method 14 was used to remove lobes. Vessels below 5 mm were treated with an ultrasonic harmonic scalpel (Ethicon Endo‐Surgery) after double ligation, and vessels above 5 mm, the trachea and dysplastic lung fissures were removed by endoscopic staplers (Ethicon Endo‐Surgery). In all cases, systematic mediastinal and hilar lymph node dissections were performed. The lymph node stations addressed typically included 2A, 3A, 3P, 4R, 7, 8, 9, 10, 11 and 12 for right‐sided resections and 4L, 5, 6, 7, 8, 9, 10, 11 and 12 for left‐sided resections. A 28‐F chest tube was inserted through the uniport after surgery.
For three‐port VATS, three incisions were used in standard fashion for visualization and mobilization of the pulmonary hilum. A 3 to 5 cm anterior utility incision was made at approximately the fourth interspace directly over the hilum, a 10 mm camera port incision was made at the seventh interspace on the midaxillary line, and a 1.5 cm incision was made at the ninth interspace on the posterior axillary line. The hilum was typically dissected anterior‐to‐posterior or upper‐to‐lower, and major vascular structures, as well as the interlobar fissure, were sectioned with endoscopic staples (Ethicon Endo‐Surgery). The anesthesia, surgeon position and lymph node dissection were the same as in the uniportal group. A 28‐F chest tube was inserted through the camera port after surgery.
In both groups, the chest tube was removed according to the following criteria: (i) the amount of daily chest drainage was less than 200 ml without air leakage, and (ii) no pneumothorax or localized pleural effusion was observed on chest X‐rays.
Statistical analysis
All analyses were performed using SPSS (IBM SPSS Statistics for Windows, Version 24.0). For quantitative variables, a t‐test was used for evaluating normally distributed data. Non‐normally distributed data were analyzed with the Mann‐Whitney test. Qualitative variables were examined with Pearson's χ2 test when appropriate. Data are expressed as the median and interquartile range. Survival curves were depicted by the Kaplan‐Meier method and compared among groups with the log‐rank test. p‐values <0.05 were considered statistically significant.
RESULTS
Patient characteristics
Among the 146 patients with NSCLC, 79 were men and 67 were women; there were 113 cases of adenocarcinoma, 30 of squamous cell carcinoma, and three of other pathological types. There were 93 stage IA cases, 11 stage IB cases, 12 stage IIA cases, 20 stage IIB cases and 10 stage IIIA cases. The two groups were similar in terms of sex, age, smoking history, tumor location, histological classification, pathological stage and ASA grade, with no significant differences (p > 0.05) (Table 1).
TABLE 1.
Comparison of clinical characteristics between the uniportal and three‐port groups
| Uniportal group (n = 80) | Three‐port group (n = 66) | t/χ2 | p‐value | |
|---|---|---|---|---|
| Sex | 0.583 | 0.445 | ||
| Male | 41 (51.3%) | 38 (57.6%) | ||
| Female | 39 (48.7%) | 28 (42.4%) | ||
| Age | 61.28 ± 8.085 | 62.00 ± 11.326 | −0.450 | 0.653 |
| Smoking history | 0.035 | 0.851 | ||
| Yes | 18 (22.5%) | 14 (21.2%) | ||
| No | 62 (77.5%) | 52 (78.8%) | ||
| Pathological types | 3.317 | 0.190 | ||
| Adenocarcinoma | 63 (78.8%) | 50 (75.8%) | ||
| Squamous cell carcinoma | 14 (17.5%) | 16 (24.2%) | ||
| Others | 3 (3.8%) | 0 (0%) | ||
| Tumor location | 1.036 | 0.904 | ||
| RUL | 22 (27.5%) | 16 (24.2%) | ||
| RML | 11 (13.8%) | 12 (18.2%) | ||
| RLL | 13 (16.3%) | 13 (19.7%) | ||
| LUL | 16 (20.0%) | 12 (18.2%) | ||
| LLL | 18 (22.5%) | 13 (19.7%) | ||
| Differentiation | 2.582 | 0.275 | ||
| WD | 47 (58.8%) | 35 (53.0%) | ||
| MD | 27 (33.8%) | 29 (43.9%) | ||
| PD | 6 (7.5%) | 2 (3.0%) | ||
| TNM stage | 3.440 | 0.487 | ||
| IA | 53(66.3%) | 40 (60.6%) | ||
| IB | 8 (10.0%) | 3 (4.5%) | ||
| IIA | 6 (7.5%) | 6 (9.1%) | ||
| IIB | 9 (11.3%) | 11 (16.7%) | ||
| IIIA | 4 (5.0%) | 6 (9.1%) | ||
| ASA grade | 0.500 | 0.779 | ||
| I | 23 (28.8%) | 16 (24.2%) | ||
| II | 44 (55.0%) | 40 (60.6%) | ||
| III | 13 (16.3%) | 10 (15.2%) | ||
| IV | 0 (0%) | 0 (0%) | ||
| V | 0 (0%) | 0 (0%) |
Abbreviations: ASA, American Society of Anesthesiologists; LLL, left lower lobe; LUL, left upper lobe; MD, moderately differentiated; PD, poorly differentiated; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe; WD, well differentiated.
Operative and postoperative data
Intraoperative blood loss was significantly lower in the uniportal than in the three‐port group (p = 0.035), and significantly shorter chest tube drainage and postoperative hospital stay durations were found in the uniportal than in the three‐port group (p = 0.022 and p = 0.008). The postoperative 24 and 72 h NRS scores were significantly lower in the uniportal than in the three‐port group (p < 0.001 and p < 0.001). There was no significant difference in the operative time between the two groups (p = 0.347) (Table 2).
TABLE 2.
Comparison of perioperative parameters between the uniportal and three‐port groups
| Variables | Uniportal group (n = 80) | Three‐port group (n = 66) | χ2 | p‐value |
|---|---|---|---|---|
| Intraoperative blood loss (ml) | 58.44 ± 45.775 | 98.03 ± 143.540 | −2.329 | 0.035 |
| Postoperative hospital stay (day) | 5.58 ± 2.540 | 7.21 ± 4.663 | −2.694 | 0.008 |
| Operation time (main) | 143.54 ± 70.378 | 185.47 ± 75.979 | −0.944 | 0.347 |
| Postoperative thoracic drainage (ml) | 250.89 ± 100.524 | 321.14 ± 197.805 | −2.773 | 0.006 |
| Chest tube duration (day) | 4.14 ± 2.453 | 5.09 ± 2.491 | −2.321 | 0.022 |
| Total number of lymph nodes dissected | 16.10 ± 8.377 | 14.47 ± 7.506 | 1.226 | 0.222 |
| Total number of lymph node stations dissected | 5.35 ± 5.774 | 4.30 ± 1.823 | 1.415 | 0.159 |
| 24 h postoperative pain NRS score | 3.83 ± 0.839 | 4.52 ± 0.769 | −5.136 | <0.001 |
| 72 h postoperative pain NRS score | 2.70 ± 0.624 | 3.20 ± 0.775 | −4.304 | <0.001 |
Note: Continuous variables are shown as mean ± standard deviation and categorical variables as number (%).
Statistically significant (p < 0.05).
Abbreviation: NRS, numerical rating score.
There were no significant differences between the two groups in the number or stations of total lymph node dissected (16.10 ± 8.377 per patient in the uniportal group vs. 14.47 ± 7.506 per patient in the three‐port group, p = 0.222, 5.35 ± 5.774 per patient in the uniportal group vs. 4.30 ± 1.823 per patient in the three‐port group, p = 0.159).
Survival and postoperative complications
The median follow‐up time was 31 months. The 30‐day mortality was 0, and the overall survival at 12 months was 91.1% vs. 90.8% in the uniportal and multiportal groups, respectively, and 30 months was 67.0% vs. 71.3% in the uniportal and multiportal groups, respectively (Figure 1).
FIGURE 1.
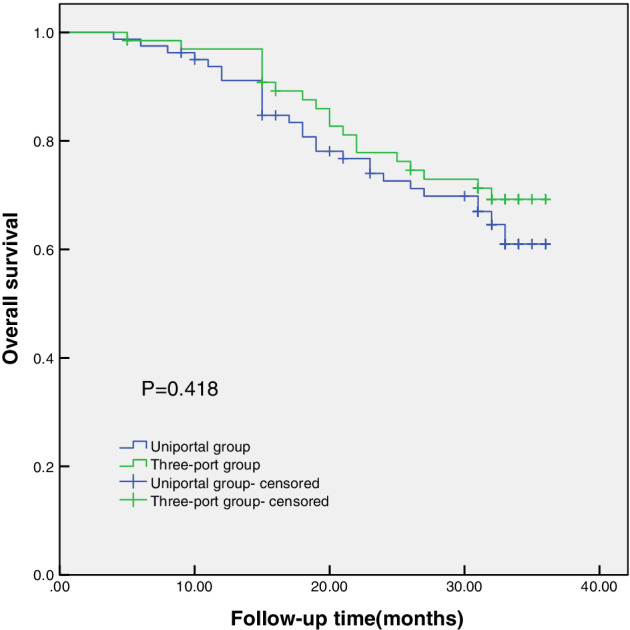
Survival after uniportal or three‐port video‐assisted thoracoscopic surgery (VATS) lobectomy for lung cancer
No deaths occurred during surgery in either group. Complications according to the Clavien‐Dindo classification were reported in 37 patients (25.3%). Major complications (Clavien‐Dindo grades 3–5) occurred in eight (5.5%) of 146 patients, and minor complications (Clavien‐Dindo grades 1–2) occurred in 35 (30.0%) patients. There were no significant differences between the two groups in the postoperative total or respiratory complications (25.0% in the uniportal group vs. 25.8% in the three‐port group, p = 0.917, 18.8% in the uniportal group vs. 18.2% in the three‐port group, p = 0.930). Further stratification showed that there were no significant differences between the two groups in pulmonary leaks (p = 0.723), pulmonary infection (p = 0.591) or atelectasis (p = 0.409) (Table 3).
TABLE 3.
Comparison of postoperative complications between the uniportal and three‐port groups
| Uniportal group (n = 80) | Three‐port group (n = 66) | χ2 | p‐value | |
|---|---|---|---|---|
| Minor complications (Clavien‐Dindo grades 1–2) | ||||
| Pulmonary leakage | 10 (12.3%) | 7 (10.6%) | 0.126 | 0.723 |
| Pulmonary infection | 3 (3.8%) | 2 (3.0%) | 1.000 | 0.591 |
| Atelectasis | 2 (2.5%) | 3 (4.5%) | 0.658 | 0.409 |
| Incisional infection | 1 (1.3%) | 2 (3.0%) | 0.590 | 0.428 |
| Arrhythmia | 3 (3.8%) | 2 (3.0%) | 1.000 | 0.591 |
| Major complications (Clavien‐Dindo grade 3–5) | ||||
| Pulmonary infection | 2 (2.5%) | 2 (3.9%) | 1.000 | 0.641 |
| Chylothorax | 1 (1.3%) | 1 (1.5%) | 1.000 | 0.701 |
| Reoperation | 1 (1.3%) | 1 (1.5%) | 1.000 | 0.701 |
| Postoperative complication rate | 20 (25.0%) | 17 (25.8%) | 0.538 | 0.917 |
| Pulmonary complication rate | 15 (18.8%) | 12 (18.2%) | 0.103 | 0.930 |
Note: Categorical variables are shown as number (%). Statistically significant (p < 0.05).
DISCUSSION
Uniportal VATS lobectomy is safe, feasible and minimizes surgical trauma without affecting the surgical resection range. 10 , 15 Uniportal VATS has become a trend in the development of VATS in recent years, 16 with some theoretical advantages, as follows. First, the use of fewer incisions can reduce the nerve damage around the incision and reduce the pain of the incision. Second, intraoperative bleeding caused by main operating incisions and auxiliary surgical incisions can be avoided because of the use of a soft incision protector. Third, the camera and the operating instrument can be inserted through the same incision, rendering the surgical field more similar to that of traditional open surgery, which is beneficial for the learning curve and more convenient for the dissection of pleural adhesions. Finally, patients are more likely to accept the procedure because the incision is more aesthetic and more likely to improve the quality of life after surgery. This study found that uniportal VATS can achieve the same range of tumor resection while not increasing the operation time and or postoperative complication rate compared to three‐port VATS. At the same time, the uniportal group showed significantly better results than the three‐port group in terms of the intraoperative blood loss, postoperative NRS score and postoperative hospital stay duration. The status and advantages of uniportal VATS for lung cancer were further demonstrated by the minimal invasiveness of the surgery.
To date, a number of studies have demonstrated acceptable short‐term outcomes of multiportal VATS lobectomy in terms of intraoperative blood loss and chest tube drainage and postoperative hospital stay durations. 17 , 18 In the present study, uniportal VATS was superior to three‐port VATS in terms of intraoperative blood loss and postoperative thoracic drainage and postoperative hospital stay durations, similar to previous reports. 11 , 19 These data suggest that fewer surgical ports lead to reduced surgical trauma and accelerate patient recovery. We believe that the main reasons for these findings are as follows. First, the use of fewer surgical ports results in reduced intraoperative blood loss and postoperative local edema. Second, the view provided by uniportal VATS is more similar to that provided by thoracotomy, which is more precise under the microscope and reduces accidental injury during the operation, thereby further reducing intraoperative blood loss and postoperative thoracic drainage.
Postoperative pain is significantly associated with short‐term quality of life of lung cancer patients after surgery. 20 Postoperative acute pain with poor control can induce chronic pain, and chronic pain seriously affects the postoperative long‐term quality of life of patients. Postoperative pain relief can help patients cough and expectorate after surgery and increases the compliance of patients with early postoperative rehabilitation activities. 20 , 21 Our results showed that, compared with three‐port VATS, uniportal VATS has significant advantages in terms of the 24 and 72 h postoperative NRS scores. Tamura et al. 22 published a retrospective study showing that the 24 h postoperative VAS score was significantly lower in the uniportal than in the three‐port group. Yang et al. 23 performed a meta‐analysis and found that the 24 and 72 h postoperative VAS scores in the uniportal group were significantly lower than those in the three‐port group. The main reason for this is that a uniportal approach minimizes intercostal nerve damage. In addition, the use of the soft port protector avoids the postoperative pain caused by repeated extrusion of the incision and the friction caused by use of the instrument.
Systemic mediastinal lymph node dissection is an important part of lung cancer surgery. Standardized lymph node dissection is crucial for postoperative staging and guiding postoperative treatment. It has been widely accepted that multiportal VATS can achieve the same extent of lymph node dissection compared with thoracotomy. 16 , 17 In this study, there were no significant differences between the two groups in the number or stations of total lymph node dissected. Liu et al. 24 published a retrospective study showing that the number and lymph node stations dissected in the uniportal group were 12.8 and 7.5, respectively, while the number and lymph node stations dissected in the three‐port group were 13.6 and 7.1, respectively. There were no significant differences between the two groups in the number or stations of total lymph node dissected. In an opinion analysis reported by Shen et al., 25 between 115 patients who underwent uniportal VATS and 296 patients who underwent three‐port VATS, there was no significant difference in the number of total lymph nodes dissected (21.4 and 20.9, respectively). All of the above studies indicate that uniportal surgery can achieve the same extent of lymph node dissection compared to other methods. We are of the opinion that the proper placement and local exposure of intraoperative instruments are key to uniportal VATS lymph node dissection. Our experience is that the thoracoscopic lens is always located on the upper edge of the incision; for pulling, the exposed loop clamp gauze is located at the lower edge of the incision, and using energy and suction devices, all locations requiring lymph node dissection can be reached through the center of the incision. In addition, in the process of lymphadenectomy, it is necessary to remove the outer membrane of the lymph nodes as far as possible, and the groups of regional lymph nodes should be completely excised to reduce intraoperative bleeding and avoid affecting the surgical field.
Compared with thoracotomy, multiportal VATS significantly reduces surgical trauma and postoperative complication rates and improves the quality of life after surgery. 26 Multiportal VATS is also significantly superior to thoracotomy in terms of the rates of surgical incision infection and postoperative cardiac and pulmonary complications. 27 , 28 , 29 This study shows that the rates of postoperative and pulmonary complications were similar between the groups. French et al. 30 presented their results from a prospective comparative study showing that there was no significant difference in the postoperative complication rate between the uniportal (18%) and three‐port (26%) groups. McElnay et al. 31 also published a retrospective study, and they found that the postoperative complication rate was not significantly different between these two groups. These results indicate that uniportal VATS can guarantee the required surgical resection range without increasing the incidence of complications. The advantages of uniportal VATS in postoperative complications may possibly be revealed with increased sample sizes.
This study has several limitations. First, analysis bias might exist because the sample size of this study was small and it was a single institution retrospective analysis. Second, the rates of postoperative complications may be underestimated due to reporting bias. Third, long‐term follow‐up data were missing. Therefore, it is necessary to include more patients and longer follow‐up periods in future studies.
In conclusion, our results suggest that uniportal VATS is a safe and effective alternative for treating patients with NSCLC. Compared with three‐port VATS, there were no significant differences in lymph node dissection or the rate of postoperative complications. Uniportal VATS leads to better outcomes than three‐port VATS with regard to intraoperative blood loss, postoperative pain, chest tube drainage duration, postoperative thoracic drainage and postoperative hospital stay duration. Therefore, uniportal VATS is a preferable option for appropriate cases as it is conducive to patients’ postoperative recovery and quality of life.
CONFLICT OF INTEREST
No authors report any conflict of interest.
ACKNOWLEDGMENTS
This work was supported by the grants from the National Natural Science Foundation of China, The Fundamental Research Funds for the Central Universities and Key research and development projects in Anhui Province (No. 81973643, No.WK9110000021 and 202004j07020017).
Li T, Xia L, Wang J, et al. Uniportal versus three‐port video‐assisted thoracoscopic surgery for non‐small cell lung cancer: A retrospective study. Thorac Cancer. 2021;12:1147–1153. 10.1111/1759-7714.13882
Funding information Key research and development projects in Anhui Province, Grant/Award Number: 202004j07020017; The Fundamental Research Funds for the Central Universities, Grant/Award Number: WK9110000021; the National Natural Science Foundation of China, Grant/Award Number: 81973643
Contributor Information
Tian Li, Email: 75078511@qq.com.
Lin Xia, Email: Xia2018051@163.com.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2. Yan TD, Black D, Bannon PG, McCaughan BC. Systematic review and meta‐analysis of randomized and nonrandomized trials on safety and efficacy of video‐assisted thoracic surgery lobectomy for early‐stage non‐small‐cell lung cancer. J Clin Oncol. 2009;27(15):2553–62. [DOI] [PubMed] [Google Scholar]
- 3. Long H, Tan Q, Luo Q, Wang Z, Jiang G, Situ D, et al. Thoracoscopic surgery versus thoracotomy for lung cancer: Short‐term outcomes of a randomized trial. Ann Thorac Surg. 2018;105(2):386–92. [DOI] [PubMed] [Google Scholar]
- 4. Yu PS, Capili F, Ng CS. Single port VATS: Recent developments in Asia. J Thorac Dis. 2016;8(Suppl 3):S302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang BY, Tu CC, Liu CY, Shih CS, Liu CC. Single‐incision thoracoscopic lobectomy and segmentectomy with radical lymph node dissection. Ann Thorac Surg. 2013;96(3):977–82. [DOI] [PubMed] [Google Scholar]
- 6. Higuchi M, Yaginuma H, Yonechi A, Kanno R, Ohishi A, Suzuki H, et al. Long‐term outcomes after video‐assisted thoracic surgery (VATS) lobectomy versus lobectomy via open thoracotomy for clinical stage IA non‐small cell lung cancer. J Cardiothorac Surg. 2014;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Drevet G, Ugalde FP. Uniportal video‐assisted thoracoscopic surgery: Safety, efficacy and learning curve during the first 250 cases in Quebec. Canada. Ann Cardiothorac Surg. 2016;5(2):100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rocco G, Martin‐Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg. 2004;77(2):726–8. [DOI] [PubMed] [Google Scholar]
- 9. Gonzalez D, Paradela M, Garcia J, dela Torre M. Single‐port video‐assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg. 2011;12(3):514–5. [DOI] [PubMed] [Google Scholar]
- 10. Fan J, Yao J, Wang Q, Chang Z. Safety and feasibility of uniportal video‐assisted thoracoscopic surgery for locally advanced non‐small cell lung cancer. J Thorac Dis. 2016;8(12):3543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu Y, Liang M, Wu W, Zheng J, Zheng W, Guo Z, et al. Preliminary results of single‐port versus triple‐port complete thoracoscopic lobectomy for non‐small cell lung cancer. Ann Transl Med. 2015;3(7):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992;111(5):518–26. [PubMed] [Google Scholar]
- 13. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6,336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu L, Che G, Pu Q, Ma L, Wu Y, Kan Q, et al. A new concept of endoscopic lung cancer resection: Single‐direction thoracoscopic lobectomy. Surg Oncol. 2010;19(2):e71–7. [DOI] [PubMed] [Google Scholar]
- 15. Lin Z, Xi J, Xu S, Jiang W, Wang L, Wang Q. Uniportal video‐assisted thoracic surgery lobectomy in semiprone position: Primary experience of 105 cases. J Thorac Dis. 2015;7(12):2389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang GS, Wang Z, Wang J, Rao ZP. Uniportal complete video‐assisted thoracoscopic lobectomy with systematic lymphadenectomy. J Thorac Dis. 2014;6(7):1011–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang J, Wu Y, Li H, Shen Q, Yu C, Chai Y. Retrospective study on video‐assisted vs. open mediastinal lymphadenectomy for non‐small cell lung cancer: A propensity‐matched analysis. J Thorac Dis. 2018;10(3):1884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reichert M, Posentrup B, Hecker A, Schneck E, Pons‐Kühnemann J, Augustin F, et al. Thoracotomy versus video‐assisted thoracoscopic surgery (VATS) in stage III empyema‐an analysis of 217 consecutive patients. Surg Endosc. 2018;32(6):2664–75. [DOI] [PubMed] [Google Scholar]
- 19. Song KS, Park CK, Kim JB. Efficacy of single‐port video‐assisted thoracoscopic surgery lobectomy compared with triple‐port VATS by propensity score matching. Korean J Thorac Cardiovasc Surg. 2017;50(5):339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bendixen M, Jorgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video‐assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: A randomised controlled trial. Lancet Oncol. 2016;17(6):836–44. [DOI] [PubMed] [Google Scholar]
- 21. Kwon ST, Zhao L, Reddy RM, Chang AC, Orringer MB, Brummett CM, et al. Evaluation of acute and chronic pain outcomes after robotic, video‐assisted thoracoscopic surgery, or open anatomic pulmonary resection. J Thorac Cardiovasc Surg. 2017;154(2):652–9. [DOI] [PubMed] [Google Scholar]
- 22. Tamura M, Shimizu Y, Hashizume Y. Pain following thoracoscopic surgery: Retrospective analysis between single‐incision and three‐port video‐assisted thoracoscopic surgery. J Cardiothorac Surg. 2013;8:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang X, Li M, Yang X, Zhao M, Huang Y, Dai X, et al. Uniport versus multiport video‐assisted thoracoscopic surgery in the perioperative treatment of patients with T1‐3N0M0 non‐small cell lung cancer: A systematic review and meta‐analysis. J Thorac Dis. 2018;10(4):2186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Z, Yang R, Shao F, Pan Y. Modified procedure of uniportal video‐assisted thoracoscopic lobectomy with muscle sparing incision. Ann Transl Med. 2016;4(19):367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shen Y, Wang H, Feng M, Xi Y, Tan L, Wang Q. Single‐ versus multiple‐port thoracoscopic lobectomy for lung cancer: A propensity‐matched studydagger. Eur J Cardiothorac Surg. 2016;49(Suppl 1):i48–53. [DOI] [PubMed] [Google Scholar]
- 26. Wang X, Wang L, Zhang H, Li K, Gong X. Feasibility and application of single‐hole video‐assisted thoracoscope in pulmonary peripheral tumors. Oncol Lett. 2016;12(6):4957–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yao Y, Shen H, Zhou Y, Yang Z, Huang H. Efficacy of thoracoscopic surgery in the treatment of lung cancer in the perioperative period and its effects on serum D‐dimer. Oncol Lett. 2018;15(4):4397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Detillon D, Veen EJ. Postoperative outcome after pulmonary surgery for non‐small cell lung cancer in elderly patients. Ann Thorac Surg. 2018;105(1):287–93. [DOI] [PubMed] [Google Scholar]
- 29. Murakami J, Ueda K, Hayashi M, Kobayashi T, Kunihiro Y, Hamano K. Size‐capacity mismatch in the lung: A novel predictor for complications after lung cancer surgery. J Surg Res. 2017;209:131–8. [DOI] [PubMed] [Google Scholar]
- 30. French DG, Thompson C, Gilbert S. Transition from multiple port to single port video‐assisted thoracoscopic anatomic pulmonary resection: Early experience and comparison of perioperative outcomes. Ann Cardiothorac Surg. 2016;5(2):92–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McElnay PJ, Molyneux M, Krishnadas R, Batchelor TJ, West D, Casali G. Pain and recovery are comparable after either uniportal or multiport video‐assisted thoracoscopic lobectomy: An observation study. Eur J Cardiothorac Surg. 2015;47(5):912–5. [DOI] [PubMed] [Google Scholar]


