Abstract
Trait impulsivity is a multifaceted personality characteristic that contributes to maladaptive life outcomes. Although a growing body of neuroimaging studies have investigated the structural correlates of trait impulsivity, the findings remain highly inconsistent and heterogeneous. Herein, we performed a systematic review to depict an integrated delineation of gray matter (GM) substrates of trait impulsivity and a meta‐analysis to examine concurrence across previous whole‐brain voxel‐based morphometry studies. The systematic review summarized the diverse findings in GM morphometry in the past literature, and the quantitative meta‐analysis revealed impulsivity‐related volumetric GM alterations in prefrontal, temporal, and parietal cortices. In addition, we identified the modulatory effects of age and gender in impulsivity‐GM volume associations. The present study advances understanding of brain GM morphometry features underlying trait impulsivity. The findings may have practical implications in the clinical diagnosis of and intervention for impulsivity‐related disorders.
Keywords: attention‐deficit/hyperactivity disorder, borderline personality disorder, gray matter, impulsivity, magnetic resonance imaging, meta‐analysis, psychoradiology, voxel‐based morphometry
To depict an integrated delineation of grey matter substrates underlying trait impulsivity, we performed a systematic review and voxel‐based meta‐analysis to uncover volumetrically correlated regions in prefrontal, temporal, and parietal cortices. The findings may have practical implications in the clinical diagnosis of and intervention for impulsivity‐related disorders.
1. INTRODUCTION
Impulsivity is generally described as a multidimensional construct, referring to the predisposition towards rapid but premature actions and insufficient consideration of potential undesirable consequences (Chamberlain & Sahakian, 2007; Dalley & Robbins, 2017). Previous research indicates a distinction between state impulsivity and trait impulsivity (Ellingson, Potenza, & Pearlson, 2018; Meda et al., 2009; Robbins, Gillan, Smith, de Wit, & Ersche, 2012). State impulsivity derived from the dysregulation of inhibitory processes results in premature responses to intrinsic and extrinsic stimulus, assessed by objective laboratory tasks such as go/no go and stop signal tasks (Dalley, Everitt, & Robbins, 2011; Nguyen, Brooks, Bruno, & Peacock, 2018; Schmitt, Ankeny, Sweeney, & Mosconi, 2016). State impulsivity and associated behaviors are transient, sensitive to environmental stimuli and vary over time within individuals (Bari & Robbins, 2013; Meda et al., 2009). In contrast, trait impulsivity, generally captured by self‐report scales, represents an enduring personality characteristic considered to be a reasonably stable state (Dougherty, Mathias, & Marsh, 2003; MacKillop et al., 2016). The standard measurements designed to evaluate trait impulsivity are self‐reported questionnaires, including the Barratt impulsiveness scale (BIS) (Patton, Stanford, & Barratt, 1995), the urgency, premeditation, perseverance, sensation seeking impulsive behavior scale (UPPS) (Whiteside & Lynam, 2001), and Eysenck's impulsivity scale (EIS) (Eysenck & Eysenck, 1985). These scales capture various aspects of trait impulsivity and reflect somewhat different compositions of personality characteristics (Reynolds, Ortengren, Richards, & de Wit, 2006). Herein, we focused on trait impulsivity instead of state impulsivity, given that trait impulsivity has closer links to mental health (Bari & Robbins, 2013; Dougherty et al., 2003; Meda et al., 2009) and is more likely to interact with brain structure due to its stability over time (Wang et al., 2020).
The negative effect of trait impulsivity is far more significant for an individual's life outcome and mental health (Chamberlain & Sahakian, 2007; Deyoung, 2010). An adaptive range of trait impulsivity allows individuals to take action decisively and seize fleeting opportunities with due regard for predictable behavioral consequences (Block, 2002; Dalley et al., 2011). In contrast, dysfunctional trait impulsivity confers vulnerability to harmful life events (e.g., criminality, Blum, Odlaug, Redden, & Grant, 2018; suicide attempts, Cole, Littlefield, Gauthier, & Bagge, 2019; and risky sexual behavior, Curry et al., 2018). Significant considerations bear on the relevance of impulsivity to various neuropsychiatric disorders, ranging from substance abuse (Moeller, Barratt, Dougherty, Schmitz, & Swann, 2001) to attention‐deficit/hyperactivity disorder (ADHD) (Sudre et al., 2017), borderline personality disorder (Sebastian et al., 2019) and binge eating disorder (Steward et al., 2017). In this regard, few symptoms appear more frequently than impulsivity as a key pathological construct among the diagnostic criteria for psychiatric disorders (Berlin & Hollander, 2014; Whiteside & Lynam, 2001). As high trait impulsivity is closely associated with maladaptive life outcomes, it is important to identify brain features associated with trait impulsivity to enhance understanding of the trait and identify early manifestation of risk for this behavioral pattern (Bari & Robbins, 2013; Gröpper et al., 2016).
Several neuroimaging studies have investigated correlates of trait impulsivity with neuroanatomy (Dalley & Robbins, 2017). In particular, structural MRI has contributed to illuminating gray matter (GM) features related to trait impulsivity using voxel‐based morphometry (VBM) (Matsuo et al., 2009) and surface‐based morphometry (SBM) (Hirjak et al., 2017; Holmes, Hollinshead, Roffman, Smoller, & Buckner, 2016) with indicators of GM volume (GMV), cortical thickness (CTh), surface area (SA), and cortical folding (CF). Similarly, alterations in white matter (WM) microstructure based on diffusion tensor imaging (DTI) also correspond to the extent to impulsivity, although the number of studies is limited (Gruber, Silveri, Dahlgren, & Yurgelun‐Todd, 2011; Ikuta, del Arco, & Karlsgodt, 2018; Myung et al., 2016; Peper et al., 2013). However, there have been heterogeneity and inconsistencies in these previous studies. Several GMV studies revealed positive correlations with trait impulsivity in regions including the anterior cingulate cortex (ACC) (Cho et al., 2013), temporal pole (Schilling et al., 2013), and insula (Charpentier et al., 2016), while others showed opposite patterns in these areas (Grodin, Cortes, Spagnolo, & Momenan, 2017; Muhlert & Lawrence, 2015; Wang, Wen, Cheng, & Li, 2017). Apart from inconsistent patterns in certain areas, the implicated brain regions varied, ranging from cortical (e.g., frontal, Tu, Kuan, Li, & Su, 2017; temporal, Muhlert & Lawrence, 2015; parietal, Schilling et al., 2013; and occipital lobes, Ide, Tung, Yang, Tseng, & Li, 2017) to subcortical structures (e.g., caudate, Dang et al., 2016). The inconsistency and heterogeneity may be attributed to diversity of sample characteristics, behavioral scales, imaging methodologies, and statistical analyses (Hu et al., 2011; Lai et al., 2019).
To mitigate the heterogeneity across previous studies and allow for a comprehensive outline of neurostructural features implicated in trait impulsivity, we performed a systematic review and meta‐analysis based on the existing structural MRI literature. Notably, the current study included only results obtained from healthy individuals to eliminate the pathological features associated with psychiatric disorders (Deserno et al., 2015; Robbins et al., 2012). First, we carried out a systematic review to summarize relevant published work on the relationship between brain GM morphometry and trait impulsivity to characterize the heterogeneity of previous findings. Subsequently, we performed an anisotropic effect‐size seed‐based d mapping (AES‐SDM) meta‐analysis to identify brain areas underlying trait impulsivity. AES‐SDM has proven to be a useful tool in neuroimaging studies to take into account the stable and unbiased selection of brain regions in whole‐brain mapping in published studies (Radua et al., 2012; Radua & Mataix‐Cols, 2012). The method has been widely used in healthy individuals (Lai et al., 2019) and patients (Li et al., 2020). Given the insufficient number of reports examining SBM metrics, we chose to identify the most prominent brain regions associated with trait impulsivity based on whole‐brain VBM findings in GMV. Finally, we explored the modulatory role of demographics (i.e., age and gender) in impulsivity‐GMV associations via meta‐regression analyses.
2. METHODS
2.1. Literature search and study selection
To identify studies correlating GM morphometry with impulsivity up to June 8, 2020, we performed a systematic literature search in the PubMed, Web of Science and Embase databases with the following search terms: (a) impulsivity; impulsiveness; or impulsive behaviors and (b) voxel‐based morphometry; VBM; surface‐based morphometry; SBM; cortical thickness; cortical surface area; cortical folding; gray matter; brain structure; structure MRI; or neuroimaging. The string we employed to conduct the research in those databases was listed in Supplemental Material. In addition, we manually checked the reference lists of the obtained reviews and pertinent articles to identify additional studies to include. A total of 2,403 candidate articles were retrieved from the three databases after excluding duplicates.
Studies were included in the systematic review if they met the following criteria: (a) assessed trait impulsivity as the research variable by self‐reported measures; (b) reported GM correlates of trait impulsivity; (c) reported results in healthy subjects; and (d) used brain structure morphometric measures. Studies were excluded from the systematic review if they (a) were non‐empirical studies; (b) were non‐English articles; and (c) other neuroimaging methods. For studies to be included in the meta‐analysis, the following inclusion criteria were added: (a) used VBM analysis; (b) reported whole‐brain findings with peak coordinates (including null findings); and (c) reported important information including peak coordinates and correlation to calculate effect‐sizes. Two authors (Nanfang and Song) independently assessed each study and extracted data to assure their appropriateness. The third author (Qiyong) resolved inconsistencies. The process of the literature search and eligibility assessment for our analysis is shown based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines (Knobloch, Yoon, & Vogt, 2011) (see Figure 1).
FIGURE 1.
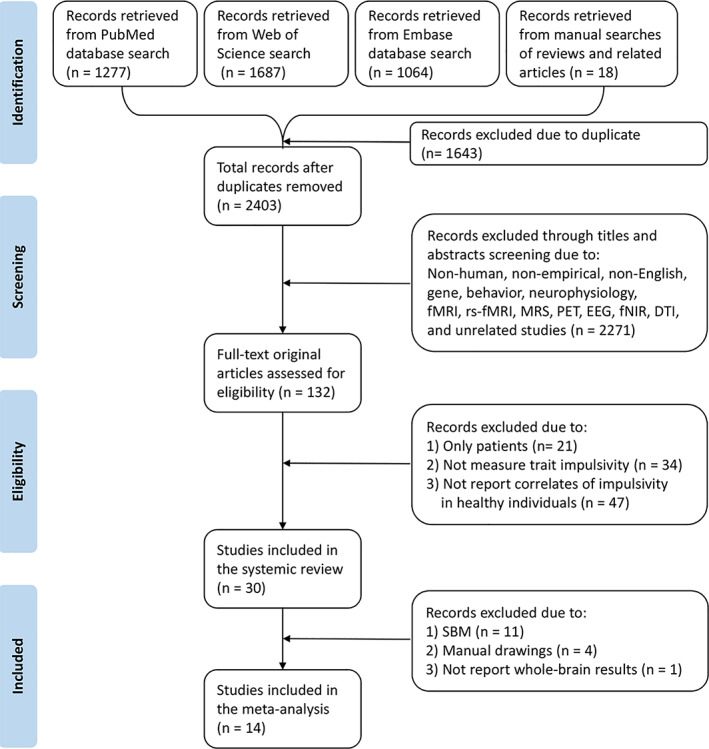
Flowchart of literature search and selection criteria for systematic review and meta‐analysis. fMRI, functional magnetic resonance imaging; rs‐fMRI, resting‐state fMRI; MRS, magnetic resonance spectrum; PET, positron emission tomography; EEG, electroencephalogram; fNIR, functional near‐infrared imaging; DTI, diffusion tensor imaging; VBM, voxel‐based morphometry; GM, gray matter; WM, white matter
2.2. Descriptive analysis
We collected basic information (sample size, gender ratio, mean age, scales of trait impulsivity, measures of GM, nuisance covariates, statistical analyses, and main findings) to detail each study in the systematic review. The results of studies using region‐of‐interest and whole‐brain analysis methods were retrieved and summarized.
2.3. Voxel‐based meta‐analysis
To investigate the correlates of trait impulsivity with GMV at the whole‐brain level, we performed a meta‐analysis with AES‐SDM software (version 5.15; https://www.sdmproject.com/). According to the AES‐SDM manual (Radua et al., 2012; Radua et al., 2014), the following steps were taken. First, we recorded peak coordinates and corresponding t values from correlations of both total scores and subscores of the self‐report measures underlying trait impulsivity in an independent file for each study and set up an SDM table specifying demographic data. Second, identified peak coordinates were used to recreate effect‐size brain maps, whose voxels were permuted to randomly generate Monte Carlo brain maps. Finally, we obtained a mean map by voxel‐wise calculation in which studies with a larger sample or lower variability contributed more based on a random effect model. To identify results from the meta‐analysis, we set widely accepted SDM thresholds (voxel‐wise p < .005, SDM‐Z > 1, and cluster size >10 voxels) to optimally balance sensitivity and specificity (Radua et al., 2012; Yang et al., 2016). Subgroup analyses were conducted for homogenous studies using adult subjects and for those applying BIS scale as a measure to examine the robustness of main findings and control the potential confounding effect of age and scales among included studies. We performed jackknife sensitivity analyses to strengthen the reliability of the main findings (Radua & Mataix‐Cols, 2012), and funnel plots and Egger's test for the identified regions to assess potential publication bias (Egger, Smith, Schneider, & Minder, 1997; Sterne & Egger, 2001). Interstudy diversity was detected through heterogeneity analyses with Q statistics (Radua et al., 2012).
To consider the potential role of demographic factors in relation to heterogeneity among the included studies, we conducted meta‐regression analyses to explore how age and gender modulated the correlates of trait impulsivity with GMV (Radua et al., 2012). Given the exploratory nature of these meta‐regression analyses, the findings of which should be considered with caution, a more rigid threshold (voxel‐wise p < .0005, SDM‐Z > 1, and cluster size >10 voxels) was employed to reduce the likelihood of obtaining spurious results (Lai et al., 2019; Radua & Mataix‐Cols, 2009).
3. RESULTS
3.1. Included studies and sample characteristics
After excluding 1,643 duplicate articles that were identified in the search, we examined the remaining 2,403 studies by viewing titles and abstracts, and 2,271 unrelated papers were rejected (see Figure 1). Subsequently, we evaluated the eligibility of the remaining 132 full‐text original articles. The following studies were excluded: studies that examined only patients (n = 21), studies that did not employ a trait impulsivity scale (n = 34), and studies that did not report GM correlates of impulsivity in healthy participants (n = 47). Therefore, the systematic review identified 30 articles that met criteria for inclusion (see Table 1). From the identified studies in the systematic review, 16 studies were unable to be included in the meta‐analysis. The reasons are as follows: SBM (n = 11) (Churchwell & Yurgelun‐todd, 2013; Depping et al., 2018; Grodin et al., 2017; Hirjak et al., 2017; Holmes et al., 2016; Kubera et al., 2018; Merz et al., 2018; Miglin et al., 2019; Qiu et al., 2017; Schilling et al., 2012; Tu et al., 2017), manual drawings (n = 4) (Caravaggio et al., 2018; Dang et al., 2016; Mei et al., 2015; Sala et al., 2011), and not whole‐brain results (n = 1) (Wang et al., 2017) (see Table 1). Finally, a total of 14 whole‐brain VBM studies were included in the meta‐analysis incorporating 1977 healthy subjects and 65 peak coordinates. Table 1 provides the demographic and analytic details and the main findings of the articles in the systemic review.
TABLE 1.
Details of the studies included in the systematic review and meta‐analysis
| Study | Sample | Gender ratio (M/F) (HC) | Mean age ± SD (HC) | Scale | Scanner/FWHM (mm) | Measure of GM | Regions of interest | Nuisance covariate | Statistical analysis/p‐value corr | Main finding about Imp‐GM associations |
|---|---|---|---|---|---|---|---|---|---|---|
| Grodin et al. (2017)b,2,I | HC:49, SUD:60 | 23/26 | 38.77 ± 10.93 | BIS‐11 | 3 T/− |
SBM: CTh; Manual drawings: GMV |
ACC, insula | Age, gender, education, ICV | Correlation/p<.004 (uncorr) |
1.Negative Imp‐GMV: Left ACC; 2.Negative Imp‐CTh: Bilateral ACC |
| Wang et al. (2017)a,1,I | Normal weight:49 | 28/21 | 39.58 ± 1.93 | UPPS‐P | 3 T/8 | VBM: GMV | OFC, AMYG, pallidum | Age, gender, handiness | Correlation/p<.005 (uncorr) | 1. Negative perseverance imp‐GMV: ACC; 2. Negative urgency Imp‐GMV: Insula |
| Mei, Xu, Carroll, and Potenza (2015) b,2,I | HC:41, SUD:41 | 24/17 | 29.7 ± 10.1 | BIS‐11 | 3 T/− | Manual tracing: AMYG and hippocampal volumes | AMYG, hippocampus | Age, gender, ICV | GLM/p<.05 (FDR corr) | NS Imp‐AMYG and hippocampal volumes |
| Caravaggio et al. (2018)c,4,I | HC:88 | 88/0 | 28.16 ± 3.34 | Swedish universities scales of personality impulsivity | 1.5 T/− | Manual tracing: Striatal sub‐region volumes | Putamen | Age, ICV | Correlation/p<.006 (Bonf corr) | NS Imp‐striatal sub‐region volumes |
| Sala et al. (2011)c,4,I | HC:15, BPD:15 | 4/11 | 34.2 ± 8.1 | BIS‐11 | 1.5 T/− | Manual tracing: Hippocampal and dlPFC volumes | Hippocampus, dlPFC | ICV | Spearman's correlation/p<.05 (uncorr) | NS Imp‐GMV: dlPFC |
| Merz, He, and Noble (2018)c,2,III | HC:328 | 172/156 | 13.65 ± 3.62 | UPPS‐P | 3 T/10 | SBM: CTh& SA; manual drawings: GMV | SBM: PFC, subcortex; manual drawings: Subcortex | Age, gender | GLM/p<.05 (FDR corr) |
1. NS Imp‐CTh and SA: vmPFC, medial OFC, lateral OFC, rostral ACC, caudal ACC, pars orbitalis, pars opercularis, pars triangularis, rostral MFG, caudal MFG, SFG, frontal pole; 2.NS Imp‐GMV: AMYG, hippocampus, putamen, caudate, nucleus accumbens, pallidum |
| Kubera et al. (2018)b,2,II | HC:54 | 16/38 | 24.9 ± 4.02 | BIS‐11 | 3 T/15 | SBM: CTh& SA | – | Age, gender | GLM/CTh: p<.003 (Bonf corr); SA: p<.05 (CWP corr) | 1. Negative Imp‐CTh: Left lingual gyrus, left STG, right cuneus, right SPG; 2.NS imp‐SA |
| Holmes et al. (2016)a,2,III | HC:1234 | 564/670 |
21.38 ± 3.13 (n 1 = 1,015) 21.21 ± 3.27 (n 2 = 219) |
BIS‐11 | 3 T/22 |
SBM: CTh; Manual tracing: Subcortical volumes |
SBM: ACC, MFG | Age, gender, IQ | Correlation/p<.05 (Bonf corr) | 1. Negative motor Imp‐CTh: Left pericalcarine cortex, bilateral ACC, bilateral MFG, right SmG; 2.NS motor Imp‐subcortical volumes |
| Tu et al. (2017)b,2,II | HC:59, BD:56 | 22/34 | 33.9 ± 7.4 | BIS‐11 | 3 T/10 | SBM: CTh | – | Age, education | GLM/p<.05 (Monte Carlo corr) | Negative Imp‐CTh: Left IFG, left MFG, left MeFG |
| Churchwell and Yurgelun‐todd (2013)b,2,I | HC:59 | Not report | 10–22 | BIS‐11 | 3 T/− | SBM: CTh | Insula | Age | Correlation/p<.001 (uncorr) | Positive non‐planning Imp‐CTh: Anterior insula |
| Qiu et al.(2017)b,2,II | HC:18, SUD:38 | 18/0 | 24.0 ± 3.08 | BIS‐11 | 1.5 T/10 |
SBM: CTh; Manual tracing: Subcortical volumes |
– | Age, education, ICV | Correlation/p<.05 (Bonf corr) | NS Imp‐CTh& subcortical volumes |
| Dang et al. (2016)c,3,I | HC:71 | 36/35 | 21.7 ± 2.9 | BIS‐11 | 3 T/− | Manual tracing: Caudate volume | Caudate nucleus | Age, gender, handiness | – | Positive attentional Imp‐right relative to left caudate volume ratio |
| Hirjak et al. (2017)b,2,I | HC:54 | 16/38 | 24.9 ± 4.02 | BIS‐11 | 3 T/25 | SBM: CF | Not report | Age, gender, education | GLM/p<.00625 (Bonf corr) | Positive Imp‐LGI: Left isthmus cingulate, bilateral precentral gyrus, right MTG, right SPG |
| Yokoyama et al. (2015)a,1,II* | HC:776 | 432/344 | 20.7 ± 1.8 | Cognitive reflectivity‐impulsiveness questionnaire | 3 T/12 | VBM: GM density | – | Age, gender, ICV | Correlation/p<.05 (nonisotropic adjusted cluster level corr) | Negative cognitive imp‐GM density: vmPFC |
| Matsuo et al. (2009)b,1,III* | HC:62 | 24/38 | 35.4 ± 12.1 | BIS‐11 | 1.5 T/8 | VBM: GMV& WMV | OFC, medial PFC, ACC, AMYG | Age, gender |
Correlation/WBA: p<.005 (uncorr) ROI: p<.05 (FDR‐SVC corr) |
1. Negative Imp‐GMV: Right middle OFC, left superior OFC, left ACC; 2. Negative non‐planning imp‐GMV: Right middle OFC; 3. Negative motor Imp‐GMV: Left superior OFC; 4.NS Imp‐WMV |
| Moreno‐Lopez et al., (2012) a,1,III* | Normal weight:16 | 7/9 | 14.13 ± 1.36 | UPPS‐P | 3 T/8 | VBM: GMV | OFC, dlPFC, striatum, medial temporal lobe, somatosensory cortex | Age, gender, education |
GLM/WBA: p<.05 (FWE corr) ROI: p<.05 (FDR‐SVC corr) |
Negative urgency Imp‐GMV: Left secondary somatosensory cortex |
| Muhlert and Lawrence (2015)a,1,III* | HC:152 | 43/109 | 23.6 ± 5.4 | UPPS‐P | 3 T/8 | VBM: GMV | dmPFC, right temporal pole | Age, gender |
GLM/WBA: p<.05 (FWE corr) ROI: p<.05(FWE‐SVC corr) |
Negative urgency imp‐GMV: Dorsomedial PFC, right temporal pole |
| Dalwani et al. (2011)a,1,II* | HC:19, ASD:25 | 19/0 | 16.59 ± 0.37 | EIS‐junior | 3 T/8 | VBM: GMV | – | Age, IQ, GMV | GLM/p<.05 (FWE corr) | Negative Imp‐GMV: Left PFC, bilaterial OFC, bilaterial MeFG, right frontopolar sup frontal gyrus, left MFG, left IFG |
| Cho et al. (2013)b,1,III* | HC:34 | 23/11 | 23.4 ± 4.3 | BIS‐11 | 1.5 T/8 | VBM: GMV | Medial PFC, ventral striatum | Age, ICV |
GLM/WBA: p<.001 (uncorr) ROI: p<.05(FWE‐SVC corr) |
Positive Imp‐GMV: Left ACC, left MeFG, dorsolateral PFC, MTG, ITG, parahippocampal gyrus |
| Korponay et al.(2017)a,1,II* | HC:105 | 40/65 | 48.6 ± 10.9 | BIS‐11 | 3 T/8 | VBM: GMV | – | Age, gender, ICV | Correlation/p<.05 (FWE corr) |
1. Negative Imp‐GMV: Right medial OFC; 2. Negative non‐planning Imp‐GMV: Paracingulate gyrus |
| Lee, Jerram, Fulwiler, and Gansler (2011)a,1,II* | HC:18, psychiatric patients:35 | 18/0 | 40.5 ± 7.48 | BIS‐11 | 1.5 T/6 | VBM: GMV | – | Age | Correlation/p<.05 (FWE corr) | 1. Negative attentional& motor imp‐GMV: Left STG; 2. Positive non‐planning Imp‐GMV: Left OFC, left lateral frontopolar cortex. |
| Liu and Feng (2017)a,1,II* | HC:169 | 63/106 |
20.53 ± 2.07 (n 1 = 85) 19.51 ± 1.35 (n 2 = 84) |
BIS‐11 | 3 T/8 | VBM: GMV | – | Age, gender, GMV | GLM/p<.05 (AlphaSim corr) |
1. Negative Imp‐GMV: Left dorsolateral PFC, left calcarine gyrus; 2. Positive Imp‐GMV: Right STG |
| Charpentier et al. (2016) a,1,II* | HC:176 | 147/29 | 24.0 ± 2.9 | EIS | 3 T/12 | VBM: gmPVE | – | Age, gender, drinking, ICV | GLM/p<.05 (FWE corr) & p<.001 (uncorr) | Positive imp‐gmPVE: Left inferior frontal/orbital/insula, right inferior frontal/insula |
| Ai, Xin, Luo, Gu, and Xu (2019)a,1,II* | HC:84 | 41/43 | 21.29 ± 1.73 | BIS‐11 | 3 T/8 | VBM: GMV | – | Age, gender, GMV | GLM/p<.05 (FWE corr) |
1. Negative motor imp‐GMV: Right supplementary motor area, paracentral lobule; 2. NS attentional and non‐planning Imp‐GMV |
| Besteher, Gaser, and Nenadic (2019) a,1,II* | HC:85 | 28/57 | 24.06 ± 2.98 | BIS‐11 | 3 T/12 | VBM: GMV | – | Age, gender, ICV | GLM/p<.05 (FWE corr) and p<.001 (uncorr) | 1. Positive Imp‐GMV: Right IPG, postcentral gyrus, SmG; 2. Positive attentional Imp‐GMV: Right IPG, SPG, SmG |
| Schilling et al. (2013) a,1,II* | HC:115 | 45/70 | 14 ± 0.32 | Temperament and character inventory impulsiveness | 3 T/8 | VBM: GMV | – | Age, gender, GMV | Correlation/p<.001 (uncorr) | 1. Negative Imp‐GMV: Bilateral OFC, right IFG, left MFG; 2. Positive Imp‐GMV: Bilateral precentral gyrus, right postcentral gyrus, STG |
| Ide et al., (2017) a,1,II* | HC:113 | 47/66 | 32 ± 14 | BIS‐11 | 3 T/8 | VBM: GMV | – | Age, gender, alcohol use | GLM/p<.05 (FWE corr) and p<.001 (uncorr) | Positive Imp‐GMV: Bilateral parieto‐occipital sulcus |
| Depping et al. (2018) c,2,I | HC:22, MDD:22, BPD:17 | 0/22 | 31.4 ± 11.2 | BIS‐11 | 3 T/25 | SBM: CF | MFG, OFC | – | Correlation/p<.05 (uncorr) | NS imp‐LGI: Caudal MFG, rostral MFG, medial and lateral OFC |
| Miglin et al. (2019)a,2,II | HC:107 | 58/49 | 32.1 ± 9.4 | UPPS‐P | 3 T/10 | SBM: CTh | – | Age, gender, body mass index | GLM/p<.05 (cluster‐wise Monte Carlo corr) |
1. Negative Imp‐CTh: Right pericalcarine/cuneus/occipital pole/SOG/MOG; 2. Positive Imp‐CTh: Left precuneus/SPG/SOG/cuneus, Right paracentral/precuneus/PCG |
| Schilling et al. (2012) a,1,II | HC:32 | 14/18 | 35.2 ± 10.5 | BIS‐11 | 3 T/20 | SBM: CTh | – | Age, gender, smoking | GLM/p<.05 (FDR corr) & p<.001 (uncorr) | Negative Imp‐CTh: Left MFG, left OFC, left SFG |
Note: For studies including both ROI and whole‐brain results, only the whole‐brain results were included in the meta‐analysis. Superscript letters a, b, and c represent peak coordinates reported in MNI space, reported in Talairach space, or others, respectively. Superscript numbers 1, 2, 3, and 4 represent images processed using the VBM toolbox within SPM, using FreeSurfer, using FSL FMRIB or not mentioned, respectively. Superscript roman numerals I, II, and III represent data analysis performed using the ROI approach, the whole‐brain approach, or both, respectively. *Studies included in the meta‐analysis.
Abbreviations: ACC, anterior cingulate cortex; AMYG, amygdala; BD, bipolar disorder; BIS‐11, Barratt Impulsiveness Scale version 11; Bonf, Bonferroni; BPD, borderline personality disorder; CF, cortical folding; corr, correction; CTh, cortical thickness; CWP, cluster wise probability; dlPFC, dorsolateral PFC; dmPFC, dorsomedial PFC; EIS, Eysenck Impulsiveness Scale; F, female; FDR, false discovery rate; FWE, family‐wise error; FWHM, full width half maximum; GLM, generalized linear model; gmPVE, gray matter partial volume estimates; GMV, gray matter volume; HC, healthy controls or healthy participants; ICV, intracranial volume; IFG, inferior frontal gyrus; Imp, impulsivity; IPG, inferior parietal gyrus; ITG, inferior temporal gyrus; LGI, local gyrification index; M, male; MDD, major depressive disorder; MeFG, medial frontal gyrus; MFG, middle frontal gyrus; MOG, middle occipital gyrus; MTG, middle temporal gyrus; NS, not significant; OFC, orbitofrontal cortex; PCG, posterior cingulate gyrus; PFC, prefrontal cortex; SA, surface area; SBM, surface‐based morphometry; SFG, superior frontal gyrus; SmG, supramarginal gyrus; SOG, superior occipital gyrus; SPG, superior parietal gyrus; STG, superior temporal gyrus; SUD, substance use disorder; SVC, small volume correction; UPPS‐P, urgency, premeditation, perseverance, sensation seeking, positive urgency, impulsive behavior scale; VBM, voxel‐based morphometry; vmPFC, ventromedial PFC; WMV, white matter volume.
3.2. Systematic review of GM correlates
A number of studies have investigated associations between trait impulsivity and GM morphometry based on volumetric and SBM metrics. The findings from the summarized literature suggested that specific morphometric patterns underlying trait impulsivity emerged in the prefrontal cortex (PFC) (e.g., orbitofrontal cortex [OFC], superior frontal gyrus [SFG], inferior frontal gyrus [IFG], middle frontal gyrus [MFG], medial frontal gyrus [MeFG], supplementary motor area, and ACC), temporal gyrus (e.g., middle temporal gyrus [MTG], inferior temporal gyrus [ITG], superior temporal gyrus [STG], and temporal pole), parietal cortex (e.g., secondary somatosensory cortex, postcentral lobule, superior parietal gyrus [SPG], and supramarginal gyrus), occipital lobe (e.g., lingual gyrus and cuneus), and insula. However, these findings were highly heterogeneous (see Table 1 and Supplemental Material). The relation between subcortical GM morphometry and trait impulsivity has been investigated without observing significant correlates. The Supplemental Result provides details about impulsivity‐GM morphometry correlates.
3.3. Meta‐analysis of GMV
3.3.1. Core brain regions linked with trait impulsivity
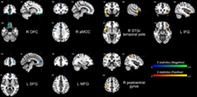
The results of the meta‐analysis performed using AES‐SDM demonstrated that trait impulsivity was negatively correlated with regional GMV in four clusters, including right OFC (i.e., ventromedial PFC, Brodmann area [BA] 11), left SFG (dorsomedial PFC, BA 10/32), right anterior midcingulate cortex (aMCC, BA 32), and left MFG (dorsolateral PFC, BA 9). In contrast, volumetric GM alterations in the right STG/temporal pole (extending to right IFG, BA 38/47/48), left IFG (i.e., pars orbitalis, BA 38/47), and right postcentral gyrus (extending to right inferior parietal gyrus [IPG], BA 2/3) were positively associated with trait impulsivity (see Table 2 and Figure 2).
TABLE 2.
Brain regions where GMV significantly correlated with impulsivity in the meta‐analysis
| Cluster | BA | Number of voxels | MNI coordinate | ||||
|---|---|---|---|---|---|---|---|
| x | y | z | SDM‐Z | p‐value | |||
| Negative correlation | |||||||
| R orbitofrontal cortex | 11 | 901 | 14 | 46 | −26 | −2.452 | .0000 |
| L superior frontal gyrus | 10/32 | 340 | −6 | 54 | 8 | −1.247 | .0010 |
| R anterior midcingulate cortex | 32 | 60 | 10 | 28 | 32 | −1.052 | .0022 |
| L middle frontal gyrus | 9 | 33 | −32 | 38 | 42 | −1.049 | .0022 |
| Positive correlation | |||||||
| R superior temporal gyrus/temporal pole | 38/47/48 | 847 | 56 | 14 | −6 | 1.507 | .0001 |
| R postcentral gyrus | 2/3 | 371 | 42 | −34 | 54 | 1.256 | .0005 |
| L inferior frontal gyrus | 38/47 | 244 | −40 | 24 | −14 | 1.191 | .0008 |
Note: Clusters were identified at voxel‐wise p < .005, SDM‐Z > 1, and cluster size >10 voxels.
Abbreviations: BA, Brodmann area; GMV, gray matter volume; L, left; MNI, Montreal neurological institute; R, right.
FIGURE 2.
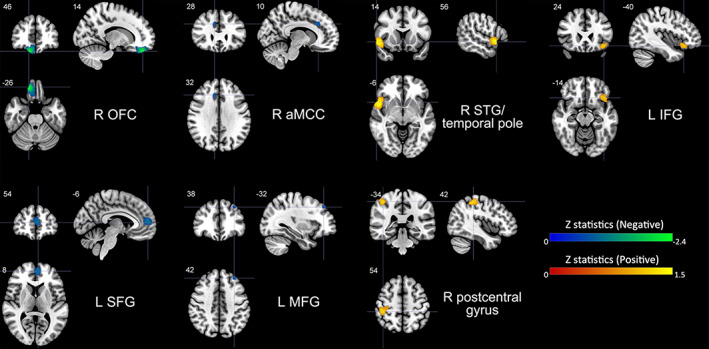
Brain regions where the volumetric GM alterations linked with trait impulsivity. Clusters were exhibited in the sagittal, axial, and coronal planes at voxel‐wise p < .005, z > 1, and cluster size >10 voxels. Regions with negative correlates were shown in blue or green and positive correlates in red or yellow. GM, gray matter; L, left; R, right; OFC, orbitofrontal cortex; SFG, superior frontal gyrus; aMCC, anterior midcingulate cortex; MFG, middle frontal gyrus; STG, superior temporal gyrus; IFG, inferior frontal gyrus
3.3.2. Reliability of main findings
The results of age‐specific subgroup analysis remained largely unchanged except for left SFG and right postcentral gyrus when the analysis was performed and restricted to studies in adult individuals (see Tables S1 and 3). Ancillary analysis within studies applying BIS scale suggested different correlational pattern compared with pooled findings, in which the significant correlation could only be replicated in right OFC, right aMCC, and right STG. The main findings of our study were robust based on jackknife sensitivity analyses, and each identified cluster was replicated in more than 13 compositions (see Table 3). In addition, we conducted a funnel plot and Egger's test for each cluster to evaluate the potential publication biases of the included studies. The funnel plots were found to be symmetric in seven clusters, though Egger's test detected publication bias with the cluster in right OFC (p = .003).
TABLE 3.
Analyses of subgroup and sensitivity analyses
| Discarded study | Negative correlation | Positive correlation | |||||
|---|---|---|---|---|---|---|---|
| R OFC | L SFG | R aMCC | L MFG | R STG | R postcentral gyrus | L IFG | |
| Studies in adult individuals a | Yes | No | Yes | Yes | Yes | No | Yes |
| Studies using BIS scale b | Yes | No | Yes | No | Yes | No | No |
| Jackknife sensitivity analyses, discarded study | |||||||
| Matsuo et al. (2009) | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Moreno‐López, Soriano‐Mas, Delgado‐Rico, Rio‐Valle, and Verdejo‐García (2012) c | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Muhlert and Lawrence (2015) | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Dalwani et al. (2011) | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Cho et al. (2013) | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Korponay et al. (2017) | Yes | No | No | Yes | Yes | Yes | Yes |
| Yokoyama et al. (2015) | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Lee et al. (2011) | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Liu and Feng (2017) I | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Liu and Feng (2017) II | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Charpentier et al. (2016) | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Ai et al. (2019) | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Besteher et al. (2019) | Yes | Yes | Yes | Yes | No | No | Yes |
| Schilling et al. (2013) | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Ide et al. (2017) | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Total | 14/15 | 13/15 | 14/15 | 14/15 | 13/15 | 13/15 | 13/15 |
Note: Yes, the brain region remains a significant correlation with trait impulsivity in the subgroup analysis or sensitivity analysis compared with pooled findings; no, the brain region is no longer significantly correlated.
Abbreviations: aMCC, anterior midcingulate cortex; IFG, inferior frontal gyrus; L, left; MFG, middle frontal gyrus; OFC, orbitofrontal cortex; R, right; SFG, superior frontal gyrus; STG, superior temporal gyrus.
11 studies in adult individuals (age > 18) were included in the subgroup analyses.
8 studies using BIS scale were included in the subgroup analyses.
Studies reported null findings. Superscript roman numerals I and II were used to distinguish two independent samples in one study.
3.3.3. Heterogeneity and meta‐regression analysis
Interstudy diversity, examined by heterogeneity analyses with Q statistics, was observed for several brain areas, including left STG, bilateral IFG, left MFG, right gyrus rectus, left SFG, right aMCC, and right IPG. This finding reflects the heterogeneity observed in our systematic review. To understand sources of the heterogeneity among the included studies, meta‐regression analyses were performed to investigate the potential influences of age and gender in impulsivity‐GMV associations. Age modulated the correlation between trait impulsivity and GMV in left MFG, left OFC, and right aMCC (Table 4 and Figure 3a). The association in left STG/Heschl gyrus, left OFC, and right MTG was modulated by the gender ratio (Table 4 and Figure 3b).
TABLE 4.
The findings of the meta‐regression analyses revealing the modulated role of age and gender
| Meta‐regression | Cluster | Contrast | BA | Number of voxels | MNI coordinate | SDM‐Z | p‐value | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Effect of mean age | L MFG | Positive | 46/10 | 451 | −34 | 48 | 18 | 1.628 | .0001 |
| L OFC | Positive | 11 | 317 | −26 | 36 | −20 | 1.844 | .0000 | |
| R aMCC | Negative | 32 | 13 | 10 | 28 | 32 | −3.697 | .0002 | |
| Effect of gender ratio (female/male) | L STG/Heschl gyrus | Positive | 48 | 1,441 | −54 | −14 | 8 | 2.286 | .0000 |
| −50 | −16 | 4 | 2.232 | .0000 | |||||
| L OFC | Negative | 47 | 327 | −34 | 30 | −18 | −2.430 | .0000 | |
| R MTG/temporal pole | Negative | 36 | 31 | 28 | 18 | −36 | −2.182 | .0000 | |
Note: Clusters were identified at voxel‐wise p < .0005, SDM‐Z > 1, and cluster size >10 voxels.
Abbreviations: aMCC, anterior midcingulate cortex; BA, Brodmann area; GM, gray matter; L, left; MFG, middle frontal gyrus; MNI, Montreal neurological institute; MTG, middle temporal gyrus; OFC, orbitofrontal cortex; R, right; STG, superior temporal gyrus.
FIGURE 3.
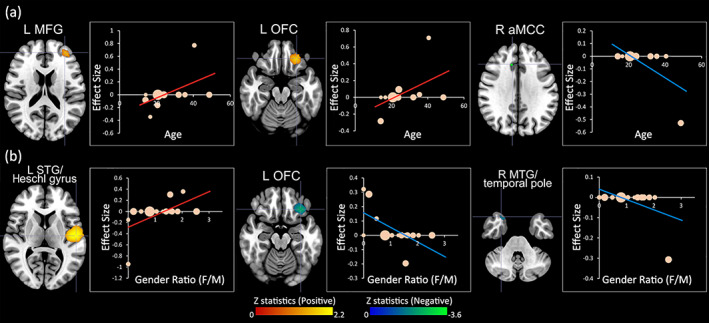
The correlations between the demographics and GMV alteration underlying trait impulsivity. (a) Age modulated impulsivity‐GMV associations; (b) gender modulated impulsivity‐GMV associations. Clusters were displayed in the axial plane at voxel‐wise p < .0005, z > 1, and cluster size >10 voxels. Regions with positive correlates were shown in red or yellow with an upward regression line and negative correlates in blue or green with a downward line. The Z statistics were obtained from the peak of the maximum slope significance of the regression line. In the plot, each study is marked as a dot, and the size of each dot corresponds to the sample size. GMV, gray matter volume; L, left; R, right; MFG, middle frontal gyrus; OFC, orbitofrontal cortex; aMCC, anterior midcingulate cortex; STG, superior temporal gyrus; MTG, middle temporal gyrus
4. DISCUSSION
To the best of our knowledge, this is the first meta‐analytic investigation of the brain structural correlates of trait impulsivity. Our findings provide a comprehensive picture of GM correlates underlying trait impulsivity. Although findings from previous structural MRI studies showed inconsistencies and heterogeneity, our study considering the existing literature together identified seven brain regions whose GMV was linked with trait impulsivity. These findings may shed light on the neural basis of trait impulsivity and within a Research Domain Criteria (RDoC) type framework may provide new information relevant towards understanding, diagnosis, and intervention for impulsivity‐related disorders. Herein, we discuss the potential associations between trait impulsivity and GM structures based on the brake‐propeller metaphor and frontostriatal circuit hypothesis.
4.1. Brakes on trait impulsivity
Several PFC regions, including right OFC, left SFG, left MFG, and right aMCC, were identified in our meta‐analysis, and their GMV negatively correlated with trait impulsivity. Previous studies proposed a frontostriatal circuit hypothesis and revealed its crucial role in impulsivity, with evidence from the dopamine system and genotypes (Fineberg et al., 2014; Morein‐zamir & Robbins, 2014). The hypothesis elucidated that PFC areas may serve as a brake on impulsive tendencies by exerting inhibitory control and navigating optimal decision‐making, while striatal structures propel the occurrence of impulsive behaviors (Buckholtz & Meyer‐Lindenberg, 2012; Fineberg et al., 2014; Whelan et al., 2012). Consistent with the hypothesis, decreased GMV in the PFC (Gröpper et al., 2016) and increased volumetric patterns in striatal substrates (Kim & Im, 2019; Ziegler et al., 2019) were found to be associated with increased impulsivity. Moreover, functional connectivity between the PFC and striatum underlies the capacity to withhold impulsive tendencies (Diekhof & Gruber, 2010). As the frontostriatal circuit hypothesis asserted, we confirmed that PFC regions, especially OFC, SFG, aMCC, and MFG, were related to trait impulsivity and thus may provide the “brakes” that strengthen inhibitory control and restrain impulsive decision‐making and behaviors.
The OFC plays a critical role in decision‐making referred to as non‐planning impulsivity (Kim & Lee, 2011), and implements a switch between impulsive and reflective behaviors (Maia & McClelland, 2004). Basically, this region represents the reward processing following goal‐directed action to navigate adaptive behaviors (Jonker, Jonker, Scheltens, & Scherder, 2015; Rolls, 2019), and inappropriate anticipation might lead to reduced control over impulsive responses (Rudebeck & Rich, 2018). Our study suggests that lower GMV in the right OFC was related to trait impulsivity in nonclinical subjects (Korponay et al., 2017; Matsuo et al., 2009; Schilling et al., 2013; Yokoyama et al., 2015), paralleling findings in substance abusing (Schwartz et al., 2010) and personality disorder patients (Völlm et al., 2009). In addition, psychopathic individuals who behave impulsively also exhibited decreased GMV in the right OFC (de Oliveira‐Souza et al., 2008; Ly et al., 2012). On the other hand, the impulsive‐antisocial dimension of psychopathy was positively correlated with GMV in the OFC in psychopathic patients, which may indicate a unique neural mechanism of the general population (Korponay & Koenigs, 2020). Functional MRI studies have indicated that OFC activation is associated with hypo‐impulsivity (Whelan et al., 2012), and stronger resting‐state functional connectivity of OFC with striatum is linked with greater impulsivity (Korponay, Dentico, et al., 2017). Human brain networks studies and animal researches indicated that the functional connectivity and neurobiological interactions between the OFC and ventral striatum are crucial aspects of reward‐based decisional impulsivity (Buckholtz & Meyer‐Lindenberg, 2012; Dalley et al., 2011; Hiser & Koenigs, 2018). Studies of brain injury models suggested that lesions to OFC weaken decision‐making capacity via reduced consideration of future consequences, which led to poor impulse control (Bechara, Damasio, & Damasio, 2000; Bechara, Damasio, Damasio, & Anderson, 1994). Thus, our findings demonstrate that in psychiatrically and medically healthy individuals, structural variability in right OFC contributes to trait impulsivity. Based on related behavioral neuroscience research, the association between right OFC GMV and trait impulsivity is likely mediated via impact on reward sensitivity and decision‐making processes.
The ability to regulate impulsive tendencies and make appropriate decisions also relies on SFG (Bari et al., 2011; Etkin, Egner, & Kalisch, 2011), which is sensitive to immediate rewards and decisional impulsivity (McClure, Laibson, Loewenstein, & Cohen, 2004). Our findings in left SFG align with previous GMV studies indicating an inverse relationship between volumetric GM alterations in the left SFG and trait impulsivity (Benegal, Antony, Venkatasubramanian, & Jayakumar, 2007; du et al., 2016; Muhlert & Lawrence, 2015). Intriguingly, externalizing spectrum disorder patients with high‐impulsive traits show reduced cortical thickness and volume in the right SFG (Almeida et al., 2010). In addition to evidence from structural MRI studies, the regional homogeneity patterns of bilateral SFG in resting‐state functional MRI correspond to decisional impulsivity (Lv et al., 2019), indicating the absence of right frontal dominance in SFG. Thus, dysfunction in this region also result in poor impulse control and maladaptive social behaviors through the involvement of alterations in inhibitory processes (du et al., 2016; Zhou et al., 2017).
Regarding cingulate cortex, we identified a cluster in the right aMCC in which lower volumes were associated with high trait impulsivity. The MCC is a critical region responsible for feedback‐mediated decision‐making, supporting rewarded behaviors (Vogt, 2016). Notably, this region serves as a switch for shifting to the reactive inhibitory status from proactive processes (Gavazzi, Giovannelli, Currò, Mascalchi, & Viggiano, 2020), which monitors the external stimuli and transfers appropriate feedback to both top‐down and bottom‐up constructs of inhibitory control (Bari & Robbins, 2013; Gläscher et al., 2012). Therefore, reduced consideration of consequences of behavioral choices and devaluation of delayed rewards (Bechara et al., 1994) may thus contribute to state impulsivity associated with variability of GMV in aMCC (Touroutoglou, Andreano, Dickerson, & Barrett, 2020). Our findings in aMCC is consistent with several studies reporting that impulsivity is negatively correlated with GMV or CTh in aMCC in general and clinical populations (Grodin et al., 2017; Korponay, Dentico, et al., 2017; Qin et al., 2020; Wang et al., 2017). In terms of its neuropsychological significance, the rostral cingulate zone, involving the aMCC, contributed to instantiating behavioral inhibition as suggested by functional MRI findings and electrophysiological recordings (Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004). Clinically, functional and structural abnormalities in the aMCC are known to lead to impairments in inhibitory control (Fletcher, 2001; Luks, Simpson, Feiwell, & Miller, 2002). In that case, the volumetric GM shrinkage in the aMCC accounted for the executive function deficiencies and increase the tendency to behave impulsively.
The lower GMV in left MFG, part of dorsolateral PFC, were also related to trait impulsivity (Dalwani et al., 2011), which is consistent with our findings. However, previous studies have revealed that the right MFG is involved in inhibitory control of executive processes as a component of the brake system (Gavazzi et al., 2020; Sharp et al., 2010), which is related to the behavioral manifestations of impulsivity (Bari & Robbins, 2013), and the impairments in top‐down executive function result in high impulsivity (Nigg, 2017). Basically, the effect of the right lateralization of MFG on response inhibition has captured enormous attention from convergent evidence. To be specific, the right MFG is exclusively engaged in the reactive processes of inhibitory control (Gavazzi et al., 2020; Simmonds, Pekar, & Mostofsky, 2008). When applying neural interventions to the right MFG in healthy subjects, impairments in inhibitory control lead to reinforced decisional impulsivity (Cho et al., 2010; Cho et al., 2013). In functional neuroimaging research focusing on incarcerated adults with psychopathic trait, hyper‐impulsivity was associated with enhanced functional connectivity between the left MFG and right OFC (Korponay, Pujara, et al., 2017), suggesting potential interactions within PFC regions related to behavioral planning and reward value evaluation respectively.
4.2. Propellers of trait impulsivity
In contrast with previously discussed regions where lower GMV pertained to impulsivity, we also identified three clusters with positive impulsivity‐regional GMV associations in the temporal, parietal, and frontal cortex. These cortical areas might propel impulsive tendencies to strengthen trait impulsivity.
With positive associations we found in the STG/temporal pole, previous studies have demonstrated relations to cortical volume and thickness in the STG/temporal pole (Fineberg et al., 2014; Liu & Feng, 2017; Schilling et al., 2013) and other regions of temporal cortex (Cho et al., 2013; Schilling et al., 2012). Intriguingly, Studies investigating surface‐based area indicated the positive correlations with trait impulsivity in this region in community sample but opposite patterns in substance abuse patients (Kaag et al., 2014). The lateral temporal cortex is embedded in the top‐down reflective framework and via that role may be indicative of general impulse control (Bechara, 2005; Lee et al., 2011). Activation of bilateral STG weakens the ability of inhibitory control, thereby contributing to impulsivity tendencies (van Belle, Vink, Durston, & Zandbelt, 2014). WM integrity of STG has also been positively correlated with impulsiveness (Hoptman et al., 2004), perhaps via interactions to frontostriatal loops (Fineberg et al., 2014). The temporal pole plays a critical role in processing perceptual inputs in interaction with the amygdala, and the control of negative emotion expression can impact the perception of action goals related to non‐planning impulsivity (Olson, Plotzker, & Ezzyat, 2007; Van Overwalle & Baetens, 2009). Functional MRI studies indicated that temporal pole activation was associated with social inhibition and negative affectivity implicated in decisional impulsivity (Bornovalova, Lejuez, Daughters, Zachary Rosenthal, & Lynch, 2005; Garon & Moore, 2006). Therefore, it is reasonable that the compensatory effect of the hypertrophy of STG and temporal pole for hindering impulsive tendencies is partially successful. Increased GMV in STG and temporal pole may relate to attentional and non‐planning impulsivity by virtue of their roles in the reflective system and affective systems.
Our findings in right postcentral gyrus (extending to right IPG) indicated a positive association with trait impulsivity. The postcentral gyrus also engages in making choices in the context of intertemporal payoffs, which may account for this effect (Sripada, Gonzalez, Phan, & Liberzon, 2011). In addition, the postcentral gyrus, MeFG, and ventral striatum comprise a valuation system that modulates decisional impulsivity (McClure et al., 2004; McClure, Ericson, Laibson, Loewenstein, & Cohen, 2007). In neuroimaging studies, ADHD patients with hyper‐impulsivity showed enhanced functional activity in the right postcentral gyrus (Robbins et al., 2012; Sörös et al., 2019), and those with substance use disorders exhibit alterations in somatosensory cortex and IPG in relation to dependence severity and hyper‐impulsivity (Jasinska, Stein, Kaiser, Naumer, & Yalachkov, 2014). Evidence from a DTI study showed a positive association between fractional anisotropy in the postcentral gyrus and impulsivity (Hoptman et al., 2004). Thus, in addition to the function of processing somatosensory information (Kropf, Syan, Minuzzi, & Frey, 2019) the postcentral gyrus is also binding to hyper‐impulsivity traits.
Counterintuitive to current understanding of executive function, enlarged bilateral IFG is related to increased trait impulsivity in healthy individuals. Based on previous studies on neural substrates concerning inhibitory control, the right IFG has been viewed as important for preventing detrimental behaviors via its role in executive actions through the frontostriatal circuits, which led to rescheduled steps to withhold impulsive tendencies (Aron, Robbins, & Poldrack, 2014; Kim & Lee, 2011). Meanwhile, the lesion in left IFG disrupts the adaptive inhibitory control in functioning (Swick, Ashley, & Turken, 2008), thus may turn out to enhance trait impulsivity, coupled with evidence that the left IFG may compensate the inhibitory function of the right one in the traumatic lesion (Gavazzi et al., 2019). In addition, our findings suggest that effects of the IFG regarding dysfunctional inhibitory processes may differ from adjacent areas of OFC (Aron et al., 2014; Chikazoe et al., 2009). Compared with the OFC that plays an important role in decision‐making (Groman et al., 2019), the IFG might target the inhibitory response to manipulate trait impulsivity (Bari & Robbins, 2013; Romero‐Garcia et al., 2020). Moreover, the presence of right lateralization of IFG underlying inhibitory control is generally acknowledged in the functional MRI investigation. Specifically, the right IFG engaged in proactive processes plays a more critical role in the suppression of response tendencies (Aron et al., 2014; Gavazzi et al., 2020). Functional MRI studies suggested that participants were apt to slower responses to stop signals when the right IFG was activated (Chikazoe et al., 2009; Swann et al., 2012), highlighting the role of right IFG in response inhibition as a critical component of executive control (Aron et al., 2014). The hyper‐activation of right IFG is linked with enhanced motor impulsivity trait, characterized by the lenient proactive control (Gavazzi et al., 2019). Overall, given that hyper‐impulsive individuals have GM enlargement in bilateral IFG in our findings and the activation pattern of IFG exerts inhibitory control (Qing & Gong, 2016), our findings may reveal its special structure–function relationship and elucidate the inherent neural characteristics of bilateral IFG as its hypertrophy disrupts the normal function of inhibitory control contributing to trait impulsivity.
Notably, the right lateralization of MFG and IFG was absent in the meta‐analysis. First, to reconcile this controversy, we attribute our divergent findings to the distinction from trait impulsivity to response inhibition, except for decisional or non‐planning impulsivity (Bari & Robbins, 2013). Second, previous research on inhibitory control identified different neural markers in varied MRI modalities (Hu et al., 2018), indicating the difference between brain function and structure. On the other hand, enhanced impulsivity is also characterized by increased left hemisphere activity (Hecht, 2011). In this regard, it might be reasonable that the identified brain regions (e.g., left MFG and bilateral IFG) underlying trait impulsivity in the meta‐analysis based on neurostructural substrates is inconsistent with the findings of functional imaging studies on inhibitory control.
In contrast to the hypothesis that the striatum drives the tendencies for impulsive behaviors (Mackey et al., 2017; Morein‐zamir & Robbins, 2014; Robbins et al., 2012), we failed to identify any clusters in the striatum correlated with trait impulsivity in the present meta‐analysis. Likewise, we note that two studies found null correlations between striatal subdivision volumes and trait impulsivity (Caravaggio et al., 2018; Deserno et al., 2015). This varies from findings in clinical populations, where a hypertrophic striatum appeared to be a hallmark feature of psychopathy (Cai et al., 2016; Glenn, Raine, Yaralian, & Yang, 2010; Koehler, Hasselmann, Wüstenberg, Heinz, & Romanczuk‐Seiferth, 2015). We speculate that the relationship between striatum GMV and trait impulsivity might fit with a nonlinear model, with modest striatum hypertrophy not having behavioral impact in impulsive healthy individuals, but patients with more pronounced impulsivity may exhibit marked enlargements in the striatum.
4.3. Potential effects of age gap and scale
Of significant relevance to the current discussions is whether the exclusive impulsivity‐related neural correlates of adolescents affected the main effects regarding the different brain‐behavior patterns between adults and adolescents (Blakemore & Robbins, 2012; Li et al., 2018). The largely unaffected findings of subgroup analysis with studies in adult participants suggested the high robustness of main findings, indicating the shared neural basis underlying trait impulsivity through adolescence and adulthood.
The noticeable heterogeneity across BIS and other impulsivity scales may lead to the non‐reproducible pooled results in ancillary analysis. The preliminary construct of BIS questionnaire is derived from the scale of anxiety to distinguish incarcerated psychiatric patients and healthy populations (Barratt, 1959; Barratt, 1965). In contrast, Eysenck's Impulsiveness scale captures trait impulsivity based on the differentiation of extraversion, and the construct of UPPS scale relies on the factor analytic approach (Eysenck & Eysenck, 1985; Whiteside & Lynam, 2001). Therefore, the ancillary analysis of studies applying BIS questionnaire may not depict an integrated outline of trait impulsivity without accounting for its multifaceted nature.
4.4. Modulatory effects of age and gender
Factors such as age and gender appear to contribute to contradictory findings from previous studies (Jones, Steele, & Nagel, 2017; Stewart & McDermott, 2004). In meta‐regression analyses, we identified a modulatory role of age in the correlations between trait impulsivity and GMV in several PFC regions (i.e., left MFG, left OFC, and right aMCC). The GMV in left MFG and OFC were negatively associated with trait impulsivity in children and adolescents (Dalwani et al., 2011; Liu & Feng, 2017; Schilling et al., 2013), but the pattern changed to a positive relationship at an elderly age (Lee et al., 2011). In a period of adolescence, the shrinkage in prefrontal regions, especially in the medial prefrontal gyrus, co‐occurred with increased impulsivity (Merz et al., 2018; Ziegler et al., 2019), potentially indicating delayed maturation of MFG that is known to occur during adolescence in high‐impulsive individuals (Luna et al., 2001; Luna, Garver, Urban, Lazar, & Sweeney, 2004). Likewise, the reduction in decisional impulsivity in response to increased age was associated with activation in the OFC through the frontostriatal network, suggesting potential developmental diversity in neurons of the OFC between hyper‐ and hypo‐impulsive individuals (Christakou, Brammer, & Rubia, 2011). However, the CTh of bilaterial MFG was negatively associated with trait impulsivity in studies using adults (Holmes et al., 2016; Schilling et al., 2012), which might be explained by the distinct property between GMV and CTh. Intriguingly, the neural correlates of OFC underlying impulsivity in adult individuals remain controversial (Korponay & Koenigs, 2020), given the findings of inverse correlation of right OFC (Korponay, Pujara, et al., 2017; Matsuo et al., 2009). In this regard, the left‐lateralized neural activity associated with impulsivity may partially induce the unique developmental trajectory of left OFC (Hecht, 2011). Moreover, inverse patterns of trait impulsivity with GMV in the right aMCC were discovered only in late adulthood (Korponay, Dentico, et al., 2017; Matsuo et al., 2009). Related evidence revealed that decreased activation in cingulate cortex was associated with hypo‐impulsivity with increases in age (Christakou et al., 2011). Overall, complex changes in anatomic patterns in relation to impulsivity appear to occur with development and normal aging which may be a promising area for future research.
Furthermore, we found that the association of trait impulsivity with GM structures in OFC and temporal cortex varied across genders. Gender differences in the propensity for impulsive maladaptive behaviors are well‐known, with males more likely to engage in aggressive and criminal behaviors (Cross, Copping, & Campbell, 2011). Males exhibit a relation between reduced GMV in left STG/Heschl gyrus (Lee et al., 2011) and increased GMV in left OFC binding to trait impulsivity (Cho et al., 2013; Lee et al., 2011), while the opposite findings in the left STG and OFC were observed in the female‐dominated neuroimaging studies (Schilling et al., 2013). The OFC navigates temporal discounting of reward for goal‐directed actions in relation to non‐planning or decisional impulsivity (Rolls, 2019), and females showed better inhibitory control capacities and higher sensitivity for incoming reward or punishment to allow for a preferred consequence regarding the diversity of GM features in OFC across genders (Chowdhury et al., 2019; Liu, Xiao, & Shi, 2013). Biological variation in rodent OFC neurons based on sex had an impact on inhibitory control abilities and impulsivity‐related behaviors (Bayless & Daniel, 2015). Thus, the distinct morphometric patterns in the OFC might account for gender differences observed in trait impulsivity. The only negative association between trait impulsivity and MTG volume was found in a study with more female subjects (Muhlert & Lawrence, 2015). Moreover, given that the STG and MTG are involved in decision‐making (Owens et al., 2017) and performance in delay discounting tasks varies across genders (Mei, Tian, Xue, & Li, 2017), our findings in the STG and MTG might shed light on gender distinctions in brain structural variations related to decisional impulsivity.
5. LIMITATIONS AND FUTURE DIRECTIONS
Several limitations of our study need to be taken into account. First, the current study focused on general trait impulsivity rather than distinct component features of impulsivity. This was necessary given the nature of existing literature, but is an area requiring more nuanced future investigation. Second, we did not investigate brain function at rest or in task‐based studies, regarding the inconsistency of findings obtained across varied modalities underlying the difference between brain function and structure (Hu et al., 2018), of which the impact could hardly be examined. Besides, the functional MRI decoding cognitive processes based on a specific task may not be appropriate to capture an entire set of a personality trait given its multidimensional nature. The structural MRI may depict stable and unbiased neural substrates of a personality construct, and studies investigating relations of anatomic and functional changes are warranted. Third, we focused on GM morphometry rather than WM, given the lack of relevant studies. Neural correlates of WM in the frontostriatal circuitry implicated in trait impulsivity could be elaborated through a well‐rounded meta‐analysis as more DTI studies emerge. Besides, our meta‐analysis included only VBM studies. Regarding the limited number of whole‐brain studies with SBM metrics (e.g., CTh) in healthy individuals, the prerequisite of a SBM meta‐analysis is not available. Nevertheless, we summarized the findings of SBM studies on trait impulsivity in the systematic review. Although GMV is determined by CTh and SA together and may reflect their properties (Winkler et al., 2010), SBM parameters are distinct with unique developmental trajectories (Wierenga, Langen, Oranje, & Durston, 2014). Further investigation of impulsivity‐related surface‐based features remains an area where more work is needed. Finally, the funnel plot illustrated that publication bias existed within a reasonable boundary, but Egger's test detected bias in one cluster. Publication bias is nearly inescapable since we could not include unpublished findings, although we made efforts to embrace null findings. In addition, since raw images were not accessible, a voxel‐wise meta‐analysis may be less accurate than direct image‐based studies (Radua & Mataix‐Cols, 2012). However, compared with image‐based meta‐analysis, a coordinate‐based voxel‐wise AES‐SDM meta‐analysis allows for the expansion of the inclusive examination of diverse studies to obtain a more comprehensive conclusion (Radua et al., 2014; Radua & Mataix‐Cols, 2012).
6. CONCLUSIONS AND FUTURE DIRECTIONS
Taken together, our findings based on a systematic review and meta‐analysis, for the first time provide an integrated depiction of brain GM morphometry underlying trait impulsivity in healthy individuals revealing its unique neurostructural substrates. Age and gender may play modulatory roles in the neural bases of trait impulsivity that merit much more extensive examination. More studies are needed to examine similarities and differences of brain behavior relations with regard to trait impulsivity in community and clinical populations. In addition, our study may advance the understanding of “psychoradiology" (https://radiopaedia.org/articles/psychoradiology) (Gong, 2020), the application of clinical imaging to both psychiatry and psychology, and aid in the clinical diagnosis of and intervention for impulsivity‐related disorders (Lui et al., 2016; Sun et al., 2018).
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Nanfang Pan, Song Wang, and Qiyong Gong conceptualized the project. Nanfang Pan and Song Wang designed the study and drafted the manuscript. Nanfang Pan, Han Lai, Kun Qin, and Jingguang Li contributed to literature search, data collection and analysis, as well as data interpretation. Song Wang, Yajun Zhao, Han Lai, Bharat B. Biswal, John A. Sweeney, and Qiyong Gong critically revised the paper. All authors approved the final version of the paper.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLDGMENTS
This study was funded by the National Natural Science Foundation of China (Grant Nos. 31800963, 81621003, 82027808 and 81820108018), the China Postdoctoral Science Foundation (Grant No. 2019M653421). Drs. Sweeney and Gong received support from National Natural Science Foundation (No. 81820108018). We thank all the authors of the included studies who responded to our requests for further information.
Pan N, Wang S, Zhao Y, et al. Brain gray matter structures associated with trait impulsivity: A systematic review and voxel‐based meta‐analysis. Hum Brain Mapp. 2021;42:2214–2235. 10.1002/hbm.25361
Funding information China Postdoctoral Science Foundation, Grant/Award Number: 2019M653421; National Natural Science Foundation of China, Grant/Award Numbers: 31800963, 81621003, 81820108018, 82027808
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Ai, H. , Xin, Y. , Luo, Y.‐j. , Gu, R. , & Xu, P. (2019). Volume of motor area predicts motor impulsivity. The European Journal of Neuroscience, 49, 1470–1476. 10.1111/ejn.14339 [DOI] [PubMed] [Google Scholar]
- Almeida, L. G. , Ricardo‐Garcell, J. , Prado, H. , Barajas, L. , Fernández‐Bouzas, A. , Ávila, D. , & Martínez, R. B. (2010). Reduced right frontal cortical thickness in children, adolescents and adults with ADHD and its correlation to clinical variables: A cross‐sectional study. Journal of Psychiatric Research, 44, 1214–1223. 10.1016/j.jpsychires.2010.04.026 [DOI] [PubMed] [Google Scholar]
- Aron, A. R. , Robbins, T. W. , & Poldrack, R. A. (2014). Inhibition and the right inferior frontal cortex: One decade on. Trends in Cognitive Sciences, 18, 177–185. 10.1016/j.tics.2013.12.003 [DOI] [PubMed] [Google Scholar]
- Bari, A. , Mar, A. C. , Theobald, D. E. , Elands, S. A. , Oganya, K. C. N. A. , Eagle, D. M. , & Robbins, T. W. (2011). Prefrontal and monoaminergic contributions to stop‐signal task performance in rats. The Journal of Neuroscience, 31, 9254–9263. 10.1523/JNEUROSCI.1543-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari, A. , & Robbins, T. W. (2013). Inhibition and impulsivity: Behavioral and neural basis of response control. Progress in Neurobiology, 108, 44–79. 10.1016/j.pneurobio.2013.06.005 [DOI] [PubMed] [Google Scholar]
- Barratt, E. S. (1959). Anxiety and impulsiveness related to psychomotor efficiency. Perceptual and Motor Skills, 9, 191–198. [Google Scholar]
- Barratt, E. S. (1965). Factor analysis of some psychometric measures of impulsiveness and anxiety. Psychological Reports, 16, 547–554. [DOI] [PubMed] [Google Scholar]
- Bayless, D. W. , & Daniel, J. M. (2015). Sex differences in myelin‐associated protein levels within and density of projections between the orbital frontal cortex and dorsal striatum of adult rats: Implications for inhibitory control. Neuroscience, 300, 286–296. 10.1016/j.neuroscience.2015.05.029 [DOI] [PubMed] [Google Scholar]
- Bechara, A. (2005). Decision making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nature Neuroscience, 8, 1458–1463. 10.1038/nn1584 [DOI] [PubMed] [Google Scholar]
- Bechara, A. , Damasio, A. R. , Damasio, H. , & Anderson, S. W. (1994). Insensitivity to future consequences following damage to human prefrontal cortex. Cognition, 50, 7–15. 10.1016/0010-0277(94)90018-3 [DOI] [PubMed] [Google Scholar]
- Bechara, A. , Damasio, H. , & Damasio, A. R. (2000). Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex, 10, 295–307. 10.1093/cercor/10.3.295 [DOI] [PubMed] [Google Scholar]
- Benegal, V. , Antony, G. , Venkatasubramanian, G. , & Jayakumar, P. N. (2007). Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addiction Biology, 12, 122–132. 10.1111/j.1369-1600.2006.00043.x [DOI] [PubMed] [Google Scholar]
- Berlin, G. S. , & Hollander, E. (2014). Compulsivity, impulsivity, and the DSM‐5 process. CNS Spectrums, 19, 62–68. [DOI] [PubMed] [Google Scholar]
- Besteher, B. , Gaser, C. , & Nenadic, I. (2019). Brain structure and trait impulsivity: A comparative VBM study contrasting neural correlates of traditional and alternative concepts in healthy subjects. Neuropsychologia, 131, 139–147. 10.1016/j.neuropsychologia.2019.04.021 [DOI] [PubMed] [Google Scholar]
- Blakemore, S. J. , & Robbins, T. W. (2012). Decision‐making in the adolescent brain. Nature Neuroscience, 15, 1184–1191. [DOI] [PubMed] [Google Scholar]
- Block, J. (2002). Personality as an affect‐processing system: Toward an integrative theory. In Psychology Press. Abington, England: Lawrence Erlbaum Associates. [Google Scholar]
- Blum, A. W. , Odlaug, B. L. , Redden, S. A. , & Grant, J. E. (2018). Stealing behavior and impulsivity in individuals with kleptomania who have been arrested for shoplifting. Comprehensive Psychiatry, 80, 186–191. 10.1016/j.comppsych.2017.10.002 [DOI] [PubMed] [Google Scholar]
- Bornovalova, M. A. , Lejuez, C. W. , Daughters, S. B. , Zachary Rosenthal, M. , & Lynch, T. R. (2005). Impulsivity as a common process across borderline personality and substance use disorders. Clinical Psychology Review, 25, 790–812. 10.1016/j.cpr.2005.05.005 [DOI] [PubMed] [Google Scholar]
- Buckholtz, J. W. , & Meyer‐Lindenberg, A. (2012). Psychopathology and the human Connectome: Toward a transdiagnostic model of risk for mental illness. Neuron, 74, 990–1004. 10.1016/j.neuron.2012.06.002 [DOI] [PubMed] [Google Scholar]
- Cai, C. , Yuan, K. , Yin, J. , Feng, D. , Bi, Y. , Li, Y. , … Tian, J. (2016). Striatum morphometry is associated with cognitive control deficits and symptom severity in internet gaming disorder. Brain Imaging and Behavior, 10, 12–20. 10.1007/s11682-015-9358-8 [DOI] [PubMed] [Google Scholar]
- Caravaggio, F. , Plavén‐Sigray, P. , Matheson, G. J. , Plitman, E. , Chakravarty, M. M. , Borg, J. , … Cervenka, S. (2018). Trait impulsivity is not related to post‐commissural putamen volumes: A replication study in healthy men. PLoS One, 13, 1–16. 10.1371/journal.pone.0209584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain, S. R. , & Sahakian, B. J. (2007). The neuropsychiatry of impulsivity. Current Opinion in Psychiatry, 20, 255–261. 10.1097/YCO.0b013e3280ba4989 [DOI] [PubMed] [Google Scholar]
- Charpentier, J. , Dzemidzic, M. , West, J. , Oberlin, B. G. , Eiler, W. J. A., II , Saykin, A. J. , & Kareken, D. A. (2016). Externalizing personality traits, empathy, and gray matter volume in healthy young drinkers. Psychiatry Research: Neuroimaging, 248, 64–72. 10.1016/j.pscychresns.2016.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe, J. , Jimura, K. , Hirose, S. , Yamashita, K. I. , Miyashita, Y. , & Konishi, S. (2009). Preparation to inhibit a response complements response inhibition during performance of a stop‐signal task. The Journal of Neuroscience, 29, 15870–15877. 10.1523/JNEUROSCI.3645-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, S. S. , Ko, J. H. , Pellecchia, G. , van Eimeren, T. , Cilia, R. , & Strafella, A. P. (2010). Continuous theta burst stimulation of right dorsolateral prefrontal cortex induces changes in impulsivity level. Brain Stimulation, 3, 170–176. 10.1016/j.brs.2009.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, S. S. , Pellecchia, G. , Aminian, K. , Ray, N. , Segura, B. , Obeso, I. , & Strafella, A. P. (2013). Morphometric correlation of impulsivity in medial prefrontal cortex. Brain Topography, 26, 479–487. 10.1007/s10548-012-0270-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury, T. G. , Wallin‐Miller, K. G. , Rear, A. A. , Park, J. , Diaz, V. , Simon, N. W. , & Moghaddam, B. (2019). Sex differences in reward‐ and punishment‐guided actions. Cognitive, Affective, & Behavioral Neuroscience, 19, 1404–1417. 10.3758/s13415-019-00736-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou, A. , Brammer, M. , & Rubia, K. (2011). Maturation of limbic corticostriatal activation and connectivity associated with developmental changes in temporal discounting. NeuroImage, 54, 1344–1354. 10.1016/j.neuroimage.2010.08.067 [DOI] [PubMed] [Google Scholar]
- Churchwell, J. C. , & Yurgelun‐todd, D. A. (2013). Age‐related changes in insula cortical thickness and impulsivity: Significance for emotional development and decision‐making. Developmental Cognitive Neuroscience, 6, 80–86. 10.1016/j.dcn.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, A. B. , Littlefield, A. K. , Gauthier, J. M. , & Bagge, C. L. (2019). Impulsivity facets and perceived likelihood of future suicide attempt among patients who recently attempted suicide. Journal of Affective Disorders, 257, 195–199. 10.1016/j.jad.2019.07.038 [DOI] [PubMed] [Google Scholar]
- Cross, C. P. , Copping, L. T. , & Campbell, A. (2011). Sex differences in impulsivity: A meta‐analysis. Psychological Bulletin, 137, 97–130. 10.1037/a0021591 [DOI] [PubMed] [Google Scholar]
- Curry, I. , Luk, J. W. , Trim, R. S. , Hopfer, C. J. , Hewitt, J. K. , Stallings, M. C. , … Wall, T. L. (2018). Impulsivity dimensions and risky sex behaviors in an at‐risk young adult sample. Archives of Sexual Behavior, 47, 529–536. 10.1007/s10508-017-1054-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley, J. W. , Everitt, B. J. , & Robbins, T. W. (2011). Impulsivity, compulsivity, and top‐down cognitive control. Neuron, 69, 680–694. 10.1016/j.neuron.2011.01.020 [DOI] [PubMed] [Google Scholar]
- Dalley, J. W. , & Robbins, T. W. (2017). Fractionating impulsivity: Neuropsychiatric implications. Nature Reviews. Neuroscience, 18, 158–171. 10.1038/nrn.2017.8 [DOI] [PubMed] [Google Scholar]
- Dalwani, M. , Sakai, J. T. , Mikulich‐Gilbertson, S. K. , Tanabe, J. , Raymond, K. , McWilliams, S. K. , … Crowley, T. J. (2011). Reduced cortical gray matter volume in male adolescents with substance and conduct problems. Drug and Alcohol Dependence, 118, 295–305. 10.1016/j.drugalcdep.2011.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang, L. C. , Samanez‐Larkin, G. R. , Young, J. S. , Cowan, R. L. , Kessler, R. M. , & Zald, D. H. (2016). Caudate asymmetry is related to attentional impulsivity and an objective measure of ADHD‐like attentional problems in healthy adults. Brain Structure & Function, 221, 277–286. 10.1007/s00429-014-0906-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira‐Souza, R. , Hare, R. D. , Bramati, I. E. , Garrido, G. J. , Azevedo Ignácio, F. , Tovar‐Moll, F. , & Moll, J. (2008). Psychopathy as a disorder of the moral brain: Fronto‐temporo‐limbic grey matter reductions demonstrated by voxel‐based morphometry. NeuroImage, 40, 1202–1213. 10.1016/j.neuroimage.2007.12.054 [DOI] [PubMed] [Google Scholar]
- Depping, M. S. , Thomann, P. A. , Wolf, N. D. , Vasic, N. , Sosic‐Vasic, Z. , Schmitgen, M. M. , … Wolf, R. C. (2018). Common and distinct patterns of abnormal cortical gyrification in major depression and borderline personality disorder. European Neuropsychopharmacology, 28, 1115–1125. 10.1016/j.euroneuro.2018.07.100 [DOI] [PubMed] [Google Scholar]
- Deserno, L. , Wilbertz, T. , Reiter, A. , Horstmann, A. , Neumann, J. , Villringer, A. , … Schlagenhauf, F. (2015). Lateral prefrontal model‐based signatures are reduced in healthy individuals with high trait impulsivity. Translational Psychiatry, 5, e659. 10.1038/tp.2015.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyoung, C. G. (2010). Impulsivity as a personality trait. Handbook of self‐regulation: Research, theory, and applications, 485–502). New York, United States: Guilford Press. [Google Scholar]
- Diekhof, E. K. , & Gruber, O. (2010). When desire collides with reason: Functional interactions between anteroventral prefrontal cortex and nucleus accumbens underlie the human ability to resist impulsive desires. The Journal of Neuroscience, 30, 1488–1493. 10.1523/JNEUROSCI.4690-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty, D. M. , Mathias, C. W. , & Marsh, D. M. (2003). Laboratory measures of impulsivity. Aggression: Psychiatric Assessment and Treatment (pp. 247–266). New York, NY: Marcel Dekker Publishers. [Google Scholar]
- du, X. , Qi, X. , Yang, Y. , du, G. , Gao, P. , Zhang, Y. , … Zhang, Q. (2016). Altered structural correlates of impulsivity in adolescents with internet gaming disorder. Frontiers in Human Neuroscience, 10, 4. 10.3389/fnhum.2016.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger, M. , Smith, G. D. , Schneider, M. , & Minder, C. (1997). Bias in meta‐analysis detected by a simple, graphical test measures of funnel plot asymmetry. BMJ, 315, 629–634. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson, J. M. , Potenza, M. N. , & Pearlson, G. D. (2018). Methodological factors as a potential source of discordance between self‐report and behavioral measures of impulsivity and related constructs. Addictive Behaviors, 84, 126–130. 10.1016/j.addbeh.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin, A. , Egner, T. , & Kalisch, R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15, 85–93. 10.1016/j.tics.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck, H. , Eysenck, M. (1985): Personality and individual differences: A natural science approach. Berlin, Germany: Springer. [Google Scholar]
- Fineberg, N. A. , Chamberlain, S. R. , Goudriaan, A. E. , Stein, D. J. , Vanderschuren, L. J. M. J. , Gillan, C. M. , … Potenza, M. N. (2014). New developments in human neurocognition: Clinical, genetic, and brain imaging correlates of impulsivity and compulsivity. CNS Spectrums, 19, 69–89. 10.1017/S1092852913000801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher, P. C. (2001). Frontal lobes and human memory: Insights from functional neuroimaging. Brain, 124, 849–881. 10.1093/brain/124.5.849 [DOI] [PubMed] [Google Scholar]
- Garon, N. , & Moore, C. (2006). Negative affectivity predicts individual differences in decision making for preschoolers. The Journal of Genetic Psychology, 167, 443–462. 10.3200/GNTP.167.4.443-462 [DOI] [PubMed] [Google Scholar]
- Gavazzi, G. , Giovannelli, F. , Currò, T. , Mascalchi, M. , & Viggiano, M. P. (2020). Contiguity of proactive and reactive inhibitory brain areas: A cognitive model based on ALE meta‐analyses. Brain Imaging and Behavior, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavazzi, G. , Lenge, M. , Bartolini, E. , Bianchi, A. , Agovi, H. , Mugnai, F. , … Mascalchi, M. (2019). Left inferior frontal cortex can compensate the inhibitory functions of right inferior frontal cortex and pre‐supplementary motor area. Journal of Neuropsychology, 13, 503–508. [DOI] [PubMed] [Google Scholar]
- Gavazzi, G. , Rossi, A. , Orsolini, S. , Diciotti, S. , Giovannelli, F. , Salvadori, E. , … Viggiano, M. P. (2019). Impulsivity trait and proactive cognitive control: An fMRI study. The European Journal of Neuroscience, 49, 1171–1179. [DOI] [PubMed] [Google Scholar]
- Gläscher, J. , Adolphs, R. , Damasio, H. , Bechara, A. , Rudrauf, D. , Calamia, M. , … Tranel, D. (2012). Lesion mapping of cognitive control and value‐based decision making in the prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America, 109, 14681–14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn, A. L. , Raine, A. , Yaralian, P. S. , & Yang, Y. (2010). Increased volume of the striatum in psychopathic individuals. Biological Psychiatry, 67, 52–58. 10.1016/j.biopsych.2009.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, Q. (2020). Psychoradiology. Neuroimaging Clinics of North America, 30(1–123). New York: Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- Grodin, E. N. , Cortes, C. R. , Spagnolo, P. A. , & Momenan, R. (2017). Structural deficits in salience network regions are associated with increased impulsivity and compulsivity in alcohol dependence. Drug and Alcohol Dependence, 179, 100–108. 10.1016/j.drugalcdep.2017.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman, S. M. , Keistler, C. , Keip, A. J. , Hammarlund, E. , DiLeone, R. J. , Pittenger, C. , … Taylor, J. R. (2019). Orbitofrontal circuits control multiple reinforcement‐learning processes. Neuron, 103, 734–746. 10.1016/j.neuron.2019.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröpper, S. , Spengler, S. , Stuke, H. , Gawron, C. K. , Parnack, J. , Gutwinski, S. , … Bermpohl, F. (2016). Behavioral impulsivity mediates the relationship between decreased frontal gray matter volume and harmful alcohol drinking: A voxel‐based morphometry study. Journal of Psychiatric Research, 83, 16–23. 10.1016/j.jpsychires.2016.08.006 [DOI] [PubMed] [Google Scholar]
- Gruber, S. A. , Silveri, M. M. , Dahlgren, M. K. , & Yurgelun‐Todd, D. (2011). Why so impulsive? White matter alterations are associated with impulsivity in chronic marijuana smokers. Experimental and Clinical Psychopharmacology, 19, 231–242. 10.1037/a0023034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht, D. (2011). An inter‐hemispheric imbalance in the psychopath's brain. Personality and Individual Differences, 51(1), 3–10. [Google Scholar]
- Hirjak, D. , Thomann, A. K. , Kubera, K. M. , Wolf, R. C. , Jeung, H. , Maier‐Hein, K. H. , & Thomann, P. A. (2017). Cortical folding patterns are associated with impulsivity in healthy young adults. Brain Imaging and Behavior, 11, 1592–1603. 10.1007/s11682-016-9618-2 [DOI] [PubMed] [Google Scholar]
- Hiser, J. , & Koenigs, M. (2018). The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biological Psychiatry, 83, 638–647. 10.1016/j.biopsych.2017.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes, A. J. , Hollinshead, M. O. , Roffman, J. L. , Smoller, J. W. , & Buckner, R. L. (2016). Individual differences in cognitive control circuit anatomy link sensation seeking, impulsivity, and substance use. The Journal of Neuroscience, 36, 4038–4049. 10.1523/JNEUROSCI.3206-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman, M. J. , Ardekani, B. A. , Butler, P. D. , Nierenberg, J. , Javitt, D. C. , & Lim, K. O. (2004). DTI and impulsivity in schizophrenia: A first voxelwise correlational analysis. NeuroReport, 15, 2467–2470. 10.1097/00001756-200411150-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, S. , Ide, J. S. , Chao, H. H. , Castagna, B. , Fischer, K. A. , Zhang, S. , & Li C shan, R. (2018). Structural and functional cerebral bases of diminished inhibitory control during healthy aging. Human Brain Mapping, 39, 5085–5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, X. , Erb, M. , Ackermann, H. , Martin, J. A. , Grodd, W. , & Reiterer, S. M. (2011). Voxel‐based morphometry studies of personality: Issue of statistical model specification‐effect of nuisance covariates. NeuroImage, 54, 1994–2005. 10.1016/j.neuroimage.2010.10.024 [DOI] [PubMed] [Google Scholar]
- Ide, J. S. , Tung, H. C. , Yang, C.‐T. , Tseng, Y. C. , & Li, C. S. R. (2017). Barratt impulsivity in healthy adults is associated with higher Gray matter concentration in the parietal occipital cortex that represents peripheral visual field. Frontiers in Human Neuroscience, 11, 222. 10.3389/fnhum.2017.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta, T. , del Arco, A. , & Karlsgodt, K. H. (2018). White matter integrity in the fronto‐striatal accumbofrontal tract predicts impulsivity. Brain Imaging and Behavior, 12, 1524–1528. 10.1007/s11682-017-9820-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska, A. J. , Stein, E. A. , Kaiser, J. , Naumer, M. J. , & Yalachkov, Y. (2014). Factors modulating neural reactivity to drug cues in addiction: A survey of human neuroimaging studies. Neuroscience and Biobehavioral Reviews, 38, 1–16. 10.1016/j.neubiorev.2013.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, S. A. , Steele, J. S. , & Nagel, B. J. (2017). Binge drinking and family history of alcoholism are associated with an altered developmental trajectory of impulsive choice across adolescence. Addiction, 112, 1184–1192. 10.1111/add.13823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker, F. A. , Jonker, C. , Scheltens, P. , & Scherder, E. J. A. (2015). The role of the orbitofrontal cortex in cognition and behavior. Reviews in the Neurosciences, 26, 1–11. 10.1515/revneuro-2014-0043 [DOI] [PubMed] [Google Scholar]
- Kaag, A. M. , Crunelle, C. L. , van Wingen, G. , Homberg, J. , van den Brink, W. , & Reneman, L. (2014). Relationship between trait impulsivity and cortical volume, thickness and surface area in male cocaine users and non‐drug using controls. Drug and Alcohol Dependence, 144, 210–217. 10.1016/j.drugalcdep.2014.09.016 [DOI] [PubMed] [Google Scholar]
- Kim, B. S. , & Im, H. I. (2019). The role of the dorsal striatum in choice impulsivity. Annals of the New York Academy of Sciences, 1451, 92–111. 10.1111/nyas.13961 [DOI] [PubMed] [Google Scholar]
- Kim, S. , & Lee, D. (2011). Prefrontal cortex and impulsive decision making. Biological Psychiatry, 69, 1140–1146. 10.1016/j.biopsych.2010.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch, K. , Yoon, U. , & Vogt, P. M. (2011). Preferred reporting items for systematic reviews and meta‐analyses (PRISMA) statement and publication bias. Journal of Cranio‐Maxillofacial Surgery, 39, 91–92. 10.1016/j.jcms.2010.11.001 [DOI] [PubMed] [Google Scholar]
- Koehler, S. , Hasselmann, E. , Wüstenberg, T. , Heinz, A. , & Romanczuk‐Seiferth, N. (2015). Higher volume of ventral striatum and right prefrontal cortex in pathological gambling. Brain Structure & Function, 220, 469–477. 10.1007/s00429-013-0668-6 [DOI] [PubMed] [Google Scholar]
- Korponay, C. , Dentico, D. , Kral, T. , Ly, M. , Kruis, A. , Goldman, R. , … Davidson, R. J. (2017). Neurobiological correlates of impulsivity in healthy adults: Lower prefrontal gray matter volume and spontaneous eye‐blink rate but greater resting‐state functional connectivity in basal ganglia‐thalamo‐cortical circuitry. NeuroImage, 157, 288–296. 10.1016/j.neuroimage.2017.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korponay, C , Koenigs, M (2020): Gray matter correlates of impulsivity in psychopathy and in the general population differ by kind, not by degree: A comparison of systematic reviews. PsyArXiv Prepr :1–28. [DOI] [PMC free article] [PubMed]
- Korponay, C. , Pujara, M. , Deming, P. , Philippi, C. , Decety, J. , Kosson, D. S. , … Koenigs, M. (2017). Impulsive‐antisocial psychopathic traits linked to increased volume and functional connectivity within prefrontal cortex. Social Cognitive and Affective Neuroscience, 12, 1169–1178. 10.1093/scan/nsx042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropf, E. , Syan, S. K. , Minuzzi, L. , & Frey, B. N. (2019). From anatomy to function: The role of the somatosensory cortex in emotional regulation. Revista Brasileira de Psiquiatria, 41, 261–269. 10.1590/1516-4446-2018-0183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubera, K. M. , Schmitgen, M. M. , Maier‐Hein, K. H. , Thomann, P. A. , Hirjak, D. , & Wolf, R. C. (2018). Differential contributions of cortical thickness and surface area to trait impulsivity in healthy young adults. Behavioural Brain Research, 350, 65–71. 10.1016/j.bbr.2018.05.006 [DOI] [PubMed] [Google Scholar]
- Lai, H. , Wang, S. , Zhao, Y. , Zhang, L. , Yang, C. , & Gong, Q. (2019). Brain gray matter correlates of extraversion: A systematic review and meta‐analysis of voxel‐based morphometry studies. Human Brain Mapping, 40, 4038–4057. 10.1002/hbm.24684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, A. K. W. W. , Jerram, M. , Fulwiler, C. , & Gansler, D. A. (2011). Neural correlates of impulsivity factors in psychiatric patients and healthy volunteers: A voxel‐based morphometry study. Brain Imaging and Behavior, 5, 52–64. 10.1007/s11682-010-9112-1 [DOI] [PubMed] [Google Scholar]
- Li, Q. , Zhao, Y. , Chen, Z. , Long, J. , Dai, J. , Huang, X. , … Gong, Q. (2020). Meta‐analysis of cortical thickness abnormalities in medication‐free patients with major depressive disorder. Neuropsychopharmacology, 45, 703–712. 10.1038/s41386-019-0563-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Zhao, Y. , Kong, F. , Du, S. , Yang, S. , & Wang, S. (2018). Psychometric assessment of the short grit scale among chinese adolescents. Journal of Psychoeducational Assessment, 36(3), 291–296. 10.1177/0734282916674858. [DOI] [Google Scholar]
- Liu, P. , & Feng, T. (2017). The overlapping brain region accounting for the relationship between procrastination and impulsivity: A voxel‐based morphometry study. Neuroscience, 360, 9–17. 10.1016/j.neuroscience.2017.07.042 [DOI] [PubMed] [Google Scholar]
- Liu, T. , Xiao, T. , & Shi, J. (2013). Response inhibition, preattentive processing, and sex difference in young children: An event‐related potential study. NeuroReport, 24, 126–130. 10.1097/WNR.0b013e32835d846b [DOI] [PubMed] [Google Scholar]
- Lui, S. , Zhou, X. , Sweeney, John A. , & Gong, Q. (2016). Psychoradiology: The frontier of neuroimaging in Psychiatry. Radiology, 281(2), 357–372. 10.1148/radiol.2016152149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luks, T. L. , Simpson, G. V. , Feiwell, R. J. , & Miller, W. L. (2002). Evidence for anterior cingulate cortex involvement in monitoring preparatory attentional set. NeuroImage, 17, 792–802. 10.1016/S1053-8119(02)91210-3 [DOI] [PubMed] [Google Scholar]
- Luna, B. , Garver, K. E. , Urban, T. A. , Lazar, N. A. , & Sweeney, J. A. (2004). Maturation of cognitive processes from late childhood to adulthood. Child Development, 75, 1357–1372. 10.1111/j.1467-8624.2004.00745.x [DOI] [PubMed] [Google Scholar]
- Luna, B. , Thulborn, K. R. , Munoz, D. P. , Merriam, E. P. , Garver, K. E. , Minshew, N. J. , … Sweeney, J. A. (2001). Maturation of widely distributed brain function subserves cognitive development. NeuroImage, 13, 786–793. 10.1006/nimg.2000.0743 [DOI] [PubMed] [Google Scholar]
- Lv, C. , Wang, Q. , Chen, C. , Qiu, J. , Xue, G. , & He, Q. (2019). The regional homogeneity patterns of the dorsal medial prefrontal cortex predict individual differences in decision impulsivity. NeuroImage, 200, 556–561. 10.1016/j.neuroimage.2019.07.015 [DOI] [PubMed] [Google Scholar]
- Ly, M. , Motzkin, J. C. , Philippi, C. L. , Kirk, G. R. , Newman, J. P. , Kiehl, K. A. , & Koenigs, M. (2012). Cortical thinning in psychopathy. The American Journal of Psychiatry, 169, 743–749. 10.1176/appi.ajp.2012.11111627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey, S. , Chaarani, B. , Kan, K. J. , Spechler, P. A. , Orr, C. , Banaschewski, T. , … IMAGEN Consortium . (2017). Brain regions related to impulsivity mediate the effects of early adversity on antisocial behavior. Biological Psychiatry, 82, 275–282. 10.1016/j.biopsych.2015.12.027 [DOI] [PubMed] [Google Scholar]
- MacKillop, J. , Weafer, J. , Gray, C. , Oshri, A. , Palmer, A. , & de Wit, H. (2016). The latent structure of impulsivity: Impulsive choice, impulsive action, and impulsive personality traits. Psychopharmacology, 233, 3361–3370. 10.1007/s00213-016-4372-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia, T. V. , & McClelland, J. L. (2004). A reexamination of the evidence for the somatic marker hypothesis: What participants really know in the Iowa gambling task. Proceedings of the National Academy of Sciences of the United States of America, 101, 16075–16080. 10.1073/pnas.0406666101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo, K. , Nicoletti, M. , Nemoto, K. , Hatch, J. P. , Peluso, M. A. M. , Nery, F. G. , & Soares, J. C. (2009). A voxel‐based morphometry study of frontal gray matter correlates of impulsivity. Human Brain Mapping, 30, 1188–1195. 10.1002/hbm.20588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure, S. M. , Ericson, K. M. , Laibson, D. I. , Loewenstein, G. , & Cohen, J. D. (2007). Time discounting for primary rewards. The Journal of Neuroscience, 27, 5796–5804. 10.1523/JNEUROSCI.4246-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure, S. M. , Laibson, D. I. , Loewenstein, G. , & Cohen, J. D. (2004). Separate neural systems value immediate and delayed monetary rewards. Science (80‐), 306, 503–507. 10.1126/science.1100907 [DOI] [PubMed] [Google Scholar]
- Meda, S. A. , Stevens, M. C. , Potenza, M. N. , Pittman, B. , Gueorguieva, R. , Andrews, M. M. , … Pearlson, G. D. (2009). Investigating the behavioral and self‐report constructs of impulsivity domains using principal component analysis. Behavioural Pharmacology, 20, 390–399. 10.1097/FBP.0b013e32833113a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei, S. , Xu, J. , Carroll, K. M. , & Potenza, M. N. (2015). Self‐reported impulsivity is negatively correlated with amygdalar volumes in cocaine dependence. Psychiatry Research, 233, 212–217. 10.1016/j.pscychresns.2015.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei, X. , Tian, L. , Xue, Z. , & Li, X. (2017). A working memory task reveals different patterns of impulsivity in male and female college students. Behavioural Processes, 138, 127–133. 10.1016/j.beproc.2017.02.023 [DOI] [PubMed] [Google Scholar]
- Merz, E. C. , He, X. , & Noble, K. G. (2018). Anxiety, depression, impulsivity, and brain structure in children and adolescents. NeuroImage Clinicl, 20, 243–251. 10.1016/j.nicl.2018.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miglin, R. , Bounoua, N. , Goodling, S. , Sheehan, A. , Spielberg, J. M. , & Sadeh, N. (2019). Cortical thickness links impulsive personality traits and risky behavior. Brain Sciences, 9(12), 373. 10.3390/brainsci9120373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller, F. G. , Barratt, E. S. , Dougherty, D. M. , Schmitz, J. M. , & Swann, A. C. (2001). Psychiatric aspects of impulsivaity. The American Journal of Psychiatry, 158, 1783–1793. 10.1176/appi.ajp.158.11.1783 [DOI] [PubMed] [Google Scholar]
- Morein‐zamir, S. , & Robbins, T. W. (2014). Fronto‐striatal circuits in response‐inhibition: Relevance to addiction. Brain Research, 1628, 1–13. 10.1016/j.brainres.2014.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno‐López, L. , Soriano‐Mas, C. , Delgado‐Rico, E. , Rio‐Valle, J. S. , & Verdejo‐García, A. (2012). Brain structural correlates of reward sensitivity and impulsivity in adolescents with Normal and excess weight. PLoS One, 7, 1–8. 10.1371/journal.pone.0049185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlert, N. , & Lawrence, A. D. (2015). Brain structure correlates of emotion‐based rash impulsivity. NeuroImage, 115, 138–146. 10.1016/j.neuroimage.2015.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung, W. , Han, C. E. , Fava, M. , Mischoulon, D. , Papakostas, G. I. , Heo, J. Y. , … Jeon, H. J. (2016). Reduced frontal‐subcortical white matter connectivity in association with suicidal ideation in major depressive disorder. Translational Psychiatry, 6, e835. 10.1038/tp.2016.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, R. , Brooks, M. , Bruno, R. , & Peacock, A. (2018). Behavioral measures of state impulsivity and their psychometric properties: A systematic review. Personality and Individual Differences, 135, 67–79. 10.1016/j.paid.2018.06.040 [DOI] [Google Scholar]
- Nigg, J. T. (2017). Annual research review: On the relations among self‐regulation, self‐control, executive functioning, effortful control, cognitive control, impulsivity, risk‐taking, and inhibition for developmental psychopathology. The Journal of Child Psychology and Psychiatry and Allied Disciplines, 58, 361–383. 10.1111/jcpp.12675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, I. R. , Plotzker, A. , & Ezzyat, Y. (2007). The enigmatic temporal pole: A review of findings on social and emotional processing. Brain, 130, 1718–1731. 10.1093/brain/awm052 [DOI] [PubMed] [Google Scholar]
- Owens, M. M. , Gray, J. C. , Amlung, M. T. , Oshri, A. , Sweet, L. H. , & MacKillop, J. (2017). Neuroanatomical foundations of delayed reward discounting decision making. NeuroImage, 161, 261–270. 10.1016/j.neuroimage.2017.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton, J. H. , Stanford, M. S. , & Barratt, E. S. (1995). Factor structure of the barratt impulsiveness scale. Journal of Clinical Psychology, 51, 768–774. [DOI] [PubMed] [Google Scholar]
- Peper, J. S. , Mandl, R. C. W. , Braams, B. R. , de Water, E. , Heijboer, A. C. , Koolschijn, P. C. M. P. , & Crone, E. A. (2013). Delay discounting and frontostriatal fiber tracts: A combined DTI and MTR study on impulsive choices in healthy young adults. Cerebral Cortex, 23, 1695–1702. 10.1093/cercor/bhs163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, K. , Zhang, F. , Chen, T. , Li, L. , Li, W. , Suo, X. , … Gong, Q. (2020). Shared gray matter alterations in individuals with diverse behavioral addictions: A voxel‐wise meta‐analysis. Journal of Behavioral Addictions, 9, 44–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing, Z. , & Gong, G. (2016). Size matters to function: Brain volume correlates with intrinsic brain activity across healthy individuals. NeuroImage, 139, 271–278. 10.1016/j.neuroimage.2016.06.046 [DOI] [PubMed] [Google Scholar]
- Qiu, Y.‐w. , Lv, X. F. , Jiang, G.‐h. , Su, H.‐H. , Ma, X.‐f. , Tian, J.‐z. , & Zhuo, F.‐z. (2017). Potential gray matter unpruned in adolescents and young adults dependent on dextromethorphan‐containing cough syrups: Evidence from cortical and subcortical study. Brain Imaging and Behavior, 11, 1470–1478. 10.1007/s11682-016-9628-0 [DOI] [PubMed] [Google Scholar]
- Radua, J. , & Mataix‐Cols, D. (2009). Voxel‐wise meta‐analysis of grey matter changes in obsessive‐compulsive disorder. The British Journal of Psychiatry, 195, 393–402. 10.1192/bjp.bp.108.055046 [DOI] [PubMed] [Google Scholar]
- Radua, J. , & Mataix‐Cols, D. (2012). Meta‐analytic methods for neuroimaging data explained. Biology of Mood & Anxiety Disorders, 2, 6 10.1186/2045-5380-2-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua, J. , Mataix‐Cols, D. , Phillips, M. L. , el‐Hage, W. , Kronhaus, D. M. , Cardoner, N. , & Surguladze, S. (2012). A new meta‐analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. European Psychiatry, 27, 605–611. 10.1016/j.eurpsy.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Radua, J. , Rubia, K. , Canales‐Rodríguez, E. J. , Pomarol‐Clotet, E. , Fusar‐Poli, P. , & Mataix‐Cols, D. (2014). Anisotropic kernels for coordinate‐based meta‐analyses of neuroimaging studies. Frontiers in Psychiatry, 5, 1–8. 10.3389/fpsyt.2014.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, B. , Ortengren, A. , Richards, J. B. , & de Wit, H. (2006). Dimensions of impulsive behavior: Personality and behavioral measures. Personality and Individual Differences, 40, 305–315. 10.1016/j.paid.2005.03.024 [DOI] [Google Scholar]
- Ridderinkhof, K. R. , Ullsperger, M. , Crone, E. A. , & Nieuwenhuis, S. (2004). The role of the medial frontal cortex in cognitive control. Science (80‐), 306, 443–447. 10.1126/science.1100301 [DOI] [PubMed] [Google Scholar]
- Robbins, T. W. , Gillan, C. M. , Smith, D. G. , de Wit, S. , & Ersche, K. D. (2012). Neurocognitive endophenotypes of impulsivity and compulsivity: Towards dimensional psychiatry. Trends in Cognitive Sciences, 16, 81–91. 10.1016/j.tics.2011.11.009 [DOI] [PubMed] [Google Scholar]
- Rolls, E. T. (2019). The orbitofrontal cortex and emotion in health and disease, including depression. Neuropsychologia, 128, 14–43. 10.1016/j.neuropsychologia.2017.09.021 [DOI] [PubMed] [Google Scholar]
- Romero‐Garcia, R. , Hook, R. W. , Tiego, J. , Bethlehem, R. A. I. , Goodyer, I. M. , Jones, P. B. , … Chamberlain, S. R. (2020). Brain micro‐architecture and disinhibition: A latent phenotyping study across 33 impulsive and compulsive behaviours. Neuropsychopharmacology, 46, 423–431. 10.1038/s41386-020-00848-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck, P. H. , & Rich, E. L. (2018). Orbitofrontal cortex. Current Biology, 28, R1083–R1088. 10.1016/j.cub.2018.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian, A. , Retz, W. , Tüscher, O. , & Turner, D. (2019). Violent offending in borderline personality disorder and attention deficit/hyperactivity disorder. Neuropharmacology, 156, 107565. 10.1016/j.neuropharm.2019.03.008. [DOI] [PubMed] [Google Scholar]
- Sala, M. , Caverzasi, E. , Lazzaretti, M. , Morandotti, N. , de Vidovich, G. , Marraffini, E. , … Brambilla, P. (2011). Dorsolateral prefrontal cortex and hippocampus sustain impulsivity and aggressiveness in borderline personality disorder. Journal of Affective Disorders, 131, 417–421. 10.1016/j.jad.2010.11.036 [DOI] [PubMed] [Google Scholar]
- Schilling, C. , Kühn, S. , Romanowski, A. , Banaschewski, T. , Barbot, A. , Barker, G. J. , … the IMAGEN consortium . (2013). Common structural correlates of trait impulsiveness and perceptual reasoning in adolescence. Human Brain Mapping, 34, 374–383. 10.1002/hbm.21446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling, C. , Kühn, S. , Romanowski, A. , Schubert, F. , Kathmann, N. , & Gallinat, J. (2012). Cortical thickness correlates with impulsiveness in healthy adults. NeuroImage, 59, 824–830. 10.1016/j.neuroimage.2011.07.058 [DOI] [PubMed] [Google Scholar]
- Schmitt, L. M. , Ankeny, L. D. , Sweeney, J. A. , & Mosconi, M. W. (2016). Inhibitory control processes and the strategies that support them during hand and eye movements. Frontiers in Psychology, 7, 1927. 10.3389/fpsyg.2016.01927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, D. L. , Mitchell, A. D. , Lahna, D. L. , Luber, H. S. , Huckans, M. S. , Mitchell, S. H. , & Hoffman, W. F. (2010). Global and local morphometric differences in recently abstinent methamphetamine‐dependent individuals. NeuroImage, 50, 1392–1401. 10.1016/j.neuroimage.2010.01.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp, D. J. , Bonnelle, V. , de Boissezon, X. , Beckmann, C. F. , James, S. G. , Patel, M. C. , & Mehta, M. A. (2010). Distinct frontal systems for response inhibition, attentional capture, and error processing. Proceedings of the National Academy of Sciences of the United States of America, 107, 6106–6111. 10.1073/pnas.1000175107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds, D. J. , Pekar, J. J. , & Mostofsky, S. H. (2008). Meta‐analysis of Go/No‐go tasks demonstrating that fMRI activation associated with response inhibition is task‐dependent. Neuropsychologia, 46, 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sörös, P. , Hoxhaj, E. , Borel, P. , Sadohara, C. , Feige, B. , Matthies, S. , … Philipsen, A. (2019). Hyperactivity/restlessness is associated with increased functional connectivity in adults with ADHD: A dimensional analysis of resting state fMRI. BMC Psychiatry, 19, 43. 10.1186/s12888-019-2031-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada, C. S. , Gonzalez, R. , Phan, K. L. , & Liberzon, I. (2011). The neural correlates of intertemporal decision‐making: Contributions of subjective value, stimulus type, and trait impulsivity. Human Brain Mapping, 32, 1637–1648. 10.1002/hbm.21136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne, J. A. , & Egger, M. (2001). Funnel plots for detecting bias in meta‐analysis: Guidelines on choice of axis. Journal of Clinical Epidemiology, 54, 1046–1055. 10.1016/s0895-4356(01)00377-8 [DOI] [PubMed] [Google Scholar]
- Steward, T. , Mestre‐Bach, G. , Vintró‐Alcaraz, C. , Agüera, Z. , Jiménez‐Murcia, S. , Granero, R. , & Fernández‐Aranda, F. (2017). Delay discounting of reward and impulsivity in eating disorders: From anorexia nervosa to binge eating disorder. European Eating Disorders Review, 25, 601–606. 10.1002/erv.2543 [DOI] [PubMed] [Google Scholar]
- Stewart, A. J. , & McDermott, C. (2004). Gender in psychology. Annual Review of Psychology, 55, 519–544. 10.1146/annurev.psych.55.090902.141537 [DOI] [PubMed] [Google Scholar]
- Sudre, G. , Choudhuri, S. , Szekely, E. , Bonner, T. , Goduni, E. , Sharp, W. , & Shaw, P. (2017). Estimating the heritability of structural and functional brain connectivity in families affected by attention‐deficit/hyperactivity disorder. JAMA Psychiatry, 74, 76–84. 10.1001/jamapsychiatry.2016.3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, H. , Chen, Y. , Huang, Q. , Lui, S. , Huang, X. , Shi, Y. , … Gong, Q. (2018). Psychoradiologic utility of MR imaging for diagnosis of attention deficit hyperactivity disorder: A radiomics analysis. Radiology, 287(2), 620–630. 10.1148/radiol.2017170226. [DOI] [PubMed] [Google Scholar]
- Swann, N. C. , Cai, W. , Conner, C. R. , Pieters, T. A. , Claffey, M. P. , George, J. S. , … Tandon, N. (2012). Roles for the pre‐supplementary motor area and the right inferior frontal gyrus in stopping action: Electrophysiological responses and functional and structural connectivity. NeuroImage, 59, 2860–2870. 10.1016/j.neuroimage.2011.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick, D. , Ashley, V. , & Turken, A. U. (2008). Left inferior frontal gyrus is critical for response inhibition. BMC Neuroscience, 9, 102. 10.1186/1471-2202-9-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou, A. , Andreano, J. , Dickerson, B. C. , & Barrett, L. F. (2020). The tenacious brain: How the anterior mid‐cingulate contributes to achieving goals. Cortex, 123, 12–29. 10.1016/j.cortex.2019.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu, P. C. , Kuan, Y. H. , Li, C. T. , & Su, T. P. (2017). Structural correlates of trait impulsivity in patients with bipolar disorder and healthy controls: A surface‐based morphometry study. Psychological Medicine, 47, 1292–1299. 10.1017/S0033291716003299 [DOI] [PubMed] [Google Scholar]
- van Belle, J. , Vink, M. , Durston, S. , & Zandbelt, B. B. (2014). Common and unique neural networks for proactive and reactive response inhibition revealed by independent component analysis of functional MRI data. NeuroImage, 103, 65–74. 10.1016/j.neuroimage.2014.09.014 [DOI] [PubMed] [Google Scholar]
- Van Overwalle, F. , & Baetens, K. (2009). Understanding others' actions and goals by mirror and mentalizing systems: A meta‐analysis. NeuroImage, 48, 564–584. 10.1016/j.neuroimage.2009.06.009 [DOI] [PubMed] [Google Scholar]
- Vogt, B. A. (2016). Midcingulate cortex: Structure, connections, homologies, functions and diseases. Journal of Chemical Neuroanatomy, 74, 28–46. 10.1016/j.jchemneu.2016.01.010 [DOI] [PubMed] [Google Scholar]
- Völlm, B. A. , Zhao, L. , Richardson, P. , Clark, L. , Deakin, J. F. W. , Williams, S. , & Dolan, M. C. (2009). A voxel‐based morphometric MRI study in men with borderline personality disorder: Preliminary findings. Criminal Behaviour and Mental Health, 19, 64–72. 10.1002/cbm.716 [DOI] [PubMed] [Google Scholar]
- Wang, H. , Wen, B. , Cheng, J. , & Li, H. (2017). Brain structural differences between normal and obese adults and their links with lack of perseverance, negative urgency, and sensation seeking. Scientific Reports, 7, 40595. 10.1038/srep40595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. , Zhao, Y. , Li, J. , Lai, H. , Qiu, C. , Pan, N. , & Gong, Q. (2020). Neurostructural correlates of hope: dispositional hope mediates the impact of the SMA gray matter volume on subjective well‐being in late adolescence. Social Cognitive and Affective Neuroscience, 15(4), 395–404. 10.1093/scan/nsaa046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan, R. , Conrod, P. J. , Poline, J. B. , Lourdusamy, A. , Banaschewski, T. , Barker, G. J. , … Garavan, H. (2012). Adolescent impulsivity phenotypes characterized by distinct brain networks. Nature Neuroscience, 15, 920–925. 10.1038/nn.3092 [DOI] [PubMed] [Google Scholar]
- Whiteside, S. P. , & Lynam, D. R. (2001). The five factor model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences, 30, 669–689. 10.1016/S0191-8869(00)00064-7 [DOI] [Google Scholar]
- Wierenga, L. M. , Langen, M. , Oranje, B. , & Durston, S. (2014). Unique developmental trajectories of cortical thickness and surface area. NeuroImage, 87, 120–126. 10.1016/j.neuroimage.2013.11.010 [DOI] [PubMed] [Google Scholar]
- Winkler, A. M. , Kochunov, P. , Blangero, J. , Almasy, L. , Zilles, K. , Fox, P. T. , … Glahn, D. C. (2010). Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. NeuroImage, 53, 1135–1146. 10.1016/j.neuroimage.2009.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. , Tian, F. , Zhang, H. , Zeng, J. , Chen, T. , Wang, S. , … Gong, Q. (2016). Cortical and subcortical gray matter shrinkage in alcohol‐use disorders: A voxel‐based meta‐analysis. Neuroscience and Biobehavioral Reviews, 66, 92–103. 10.1016/j.neubiorev.2016.03.034 [DOI] [PubMed] [Google Scholar]
- Yokoyama, R. , Nozawa, T. , Takeuchi, H. , Taki, Y. , Sekiguchi, A. , Nouchi, R. , … Kawashima, R. (2015). Regional gray matter density associated with cognitive reflectivity‐impulsivity: Evidence from voxel‐based morphometry. PLoS One, 10, e0122666. 10.1371/journal.pone.0122666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, T. , Zhu, H. , Fan, Z. , Wang, F. , Chen, Y. , Liang, H. , … Hu, H. (2017). History of winning remodels thalamo‐PFC circuit to reinforce social dominance. Science (80‐), 357, 162–168. 10.1126/science.aak9726 [DOI] [PubMed] [Google Scholar]
- Ziegler, G. , Hauser, T. U. , Moutoussis, M. , Bullmore, E. T. , Goodyer, I. M. , Fonagy, P. , … Dolan, R. J. (2019). Compulsivity and impulsivity traits linked to attenuated developmental frontostriatal myelination trajectories. Nature Neuroscience, 22, 992–999. 10.1038/s41593-019-0394-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author upon reasonable request.


