Abstract
Aims
To develop and internally validate a nomogram to predict the risk of death within 6 months of onset of stroke in Chinese. Identifying risk factors with potentially direct effects on the nomogram will improve the quality of risk assessment and help nurses implement preventive measures based on patient‐specific risk factors.
Design
A retrospective study.
Methods
We performed a least absolute shrinkage and selection operator (LASSO) regression modelling and multivariate logistic regression analysis to establish a prediction model of death risk in stroke patients within 6 months of onset. LASSO and time‐dependent Cox regression models were further used to analyse the 6‐month survival of stroke patients. Data were collected from 21 October 2013–6 May 2019.
Results
The independent predictors of the nomogram were Barthel index (odds ratio (OR) = 0.980, 95% confidence interval (CI) = 0.961–0.998, p = .03), platelet/lymphocyte ratio (OR = 1.005, 95% CI = 1.000–1.010, p = .04) and serum albumin (OR = 0.854, 95% CI = 0.774–0.931, p < .01). This model showed good discrimination and consistency, and its discrimination evaluation C‐statistic was 0.879 in the training set and 0.891 in the internal validation set. The DCA indicated that the nomogram had a higher overall net benefit over most of the threshold probability range. The time‐dependent Cox regression model established the impact of the time effect of the age variable on survival time.
Conclusions
Our results identified three predictors of death within 6 months of stroke in Chinese. These predictors can be used as risk assessment indicators to help caregivers performing clinical nursing work, and in clinical practice, it is suggested that nurses should evaluate the self‐care ability of stroke patients in detail. The constructed nomogram can help identify patients at high risk of death within 6 months, so that intervention can be performed as early as possible.
Keywords: Barthel index, nomogram, nursing, PLR, prediction model, serum albumin, stroke
1. INTRODUCTION
Stroke (including ischaemic and haemorrhagic strokes) is the second leading cause of death worldwide (GBD, 2015 Mortality, & Causes of Death Collaborators, 2016). Every year, 15 million people worldwide suffer a stroke. Among them, 5 million die and another 5 million are permanently disabled (Organización Mundial de la Salud, 2020). An analysis of the 2010 data of Global Burden of Disease (GBD) indicates that stroke mortality has increased by 26% since 1990 (Murray et al., 2012). In China, approximately 1.6 million stroke patients die each year and stroke has become the number‐one cause of death and adult disability in China (Chen, 2008). The poor prognosis of stroke is the result of the concerted action of multiple risk factors. In clinical practice, prognosis can be judged through the evaluation and analysis of risk factors. The construction of tools for predicting the mortality of stroke patients is highly valuable in many ways. These can determine prognosis, provide support for clinical decision‐making and help with patient management.
2. BACKGROUND
Amitrano et al. (2016) developed a model to predict the poor prognosis of patients with acute ischaemic stroke undergoing intravenous thrombolytic therapy (IVT), and the results showed that a National Institutes of Health Stroke Scale (NIHSS) score > 12, hyperdense artery sign (HAS) and age > 70 years could independently predict poor prognosis at 3 months. Counsell et al. (2002) developed and validated a prognostic model for patients with acute and subacute stroke to predict the survival rate within 30 days after the stroke and the disability‐free survival rate within 6 months and the model with six variables (age, living alone, independence in daily life before stroke, the verbal component of the Glasgow Coma Scale, arm strength, walking ability) performed well. The model constructed by Weimar et al. (2004) included age and level of neurological dysfunction at admission (total NIHSS score) and the model accurately predicted 57.9% of the patients who died and 91.5% of those who survived. Inouye (2001) used a prediction model that used age, total functional independence score on admission and onset interval as variables to predict the prognosis of Japanese after their first acute stroke, and this model guided the clinical development of rehabilitation programmes and assessment of the amount of care required for patients at home or after discharge from hospital. Ling et al. (2019) collected the data from 772 patients with acute ischaemic stroke and performed univariate and multivariate logistic regression analysis to establish a prediction model that used age, NIHSS score, level of consciousness, history of stroke or transient ischaemic attack, cancer and blood glucose as independent risk factors to predict poor prognosis at 1 year.
A multitude of risk factors affect the survival and adverse prognosis of stroke patients. For biological risk factors including individual characteristics and complications, studies have shown that age is a predictor of 30‐day mortality in patients with haemorrhagic stroke (Chongruksut et al., 2020). A study conducted in 200 stroke patients shows that gender and smoking are two risk factors for an adverse prognosis (Moalla et al., 2020). A multi‐centre study in Europe on five‐year mortality and related predictors also shows that age, diabetes and atrial fibrillation are independent predictors of increased stroke‐related mortality (De Wit et al., 2012). Another study shows that risk factors including age, atrial fibrillation, coronary heart disease and hypertension are predictors of long‐term mortality (Abdo et al., 2019). In addition, laboratory test results are closely related to the mortality and adverse prognosis of stroke patients. For example, WBCs (Kazmierski et al., 2004; Moalla et al., 2020), Hb (Fabjan et al., 2019), the PLR (Xu et al., 2019), ALT (Fekadu et al., 2020), serum albumin (Babu et al., 2013; Chakraborty et al., 2013), fibrinogen (Frøyshov et al., 2017) and D‐dimer (Nam et al., 2017) are also important risk factors for an adverse prognosis in stroke patients. Additional stroke‐related clinical risk factors include some nursing‐related factors, which can be evaluated and managed to help nurses implement preventive measures and facilitate early intervention. In particular, the BI can be used as a nursing‐dependent indicator and especially assist nurses in estimating the workload involved in physical care (al‐Khawaja et al., 1997). The BI is associated with mortality in stroke patients, and a high BI score at six months is an independent predictor of decreased stroke‐related mortality (De Wit et al., 2012). The Faces Pain Scale was originally developed for children without cognitive impairment (Wong & Baker, 1988). Later, the scale was also used in patients with cerebral palsy (Boldingh et al., 2004) and in elderly patients (Scherder & van Manen, 2005). Studies have shown that the Faces Pain Scale can also be used in stroke patients. When a patient is unable to self‐report, nurses can rely on their own observations to assess the pain level of stroke patients (Benaim et al., 2007; Dogan et al., 2010). Moreover, nutritional status is one of the risk factors closely related to the mortality of stroke patients (Gomes et al., 2016; Maruyama et al., 2018). Nutritional care is an essential component of basic nursing (Kitson et al., 2010) and an important indicator of nursing quality in healthcare settings (Carryer et al., 2017). Nurses can perform nutritional interventions and implement, guide and educate stroke patients on nutritional care. The risk factors discussed above suggest that the mortality of stroke patients is the result of the interaction of many factors.
As mentioned earlier, many tools are available to predict an adverse prognosis in stroke patients, but nursing‐related tools are still lacking. To date, few studies have investigated the effects of nursing‐related risk variables on six‐month mortality following stroke. In the absence of a combination of variables associated with care delivery, the care decisions cannot be well implemented, and rehabilitation plans cannot be developed. In addition, these predictive models and rating systems are mainly designed for Western populations, but mortality from stroke varies from country to country (Yusuf et al., 2001), which may be related to race (Chiu et al., 2010), lifestyle (Tu et al., 2015) and genetic variation (Cheong et al., 2011; L. Wang et al., 2014). Building a risk prediction model for the Chinese population in East Asia is important. Finally, the risks of death from different types of stroke vary widely and most of the traditional mortality prediction models have been developed for specific types of stroke, making it less convenient when the model tools are applied to all types of stroke.
Thus, we hypothesize that six‐month mortality in stroke patients is related to and may change with certain risk factors. Moreover, a new model of risk factors can be built to predict the six‐month mortality of stroke patients and assist nurses in identifying stroke patients at risk of death within 6 months of onset. In addition, the model can be tested in different settings, thereby contributing to better prevention and interventions in stroke patients.
Therefore, we performed joint predictions from the aspects of individual factors, complications, assessment tools related to daily care and laboratory tests. First, we performed a least absolute shrinkage and selection operator (LASSO) regression modelling of the above risk factors to select the risk factors with the best predictive characteristics, using death within 6 months after onset as the outcome variable and we performed multivariate logistic regression analysis to carry out further screening to identify independent risk factors. Then, according to the resulting regression expression, the nomogram of the probability of death was plotted and thus the prediction model of death within 6 months after the onset of stroke in Chinese patients was established. We used the bootstrap technique to perform internal validation. Finally, we used the death outcome and survival time together as the dependent variables to perform survival analysis. For statistically significant risk variables, the proportional hazards (PH) assumption test was performed first to determine whether there were time‐dependent covariates. Time‐dependent covariates that did not meet the PH assumption were also included in the non‐PH time‐dependent Cox model. While performing multivariate analysis, the time‐varying effects of relevant variables on survival time were assessed.
The significance of this study is that it deepens the understanding of the independent relationships between nurse‐assessed risk factors and the mortality of stroke patients and demonstrates the unique contributions of nurse‐assessed risk factors to the six‐month survival of stroke patients. The model provides intervention suggestions, assists nurses in managed care and evaluates nursing outcomes. Ultimately, the model is a useful tool for nurses and policy makers.
3. THE STUDY
3.1. Aims
This study aimed (a) to establish LASSO and multivariate logistic regression models; (b) to identify independent risk factors for death within 6 months of onset from 210 stroke patients; (c) to establish a nomogram that could predict death within 6 months of stroke. Professionals can use this prediction model to help identify stroke patients at higher risk of death within 6 months; and (d) to verify the predictive model of death of stroke patients within 6 months.
3.2. Design
The data for this retrospective study were obtained from a large comprehensive tertiary hospital with 3,000 beds in Jiangsu Province, China, and the Department of Neurology of this hospital has 115 beds.
3.3. Participants
All patients met the following conditions: inclusion criteria: (a) age ≥ 18 years; (b) admission to hospital with a diagnosis of stroke (including ischaemic and haemorrhagic stroke); (c) stroke confirmed by CT or MRI; and (d) time from onset to admission to hospital was less than 14 days. Exclusion criteria were as follows: (a) primary brain tumours, metastatic brain tumours, history of mental illness, infectious encephalitis or secondary epilepsy; (b) time from onset to admission to hospital > 14 days; and (c) loss to follow‐up.
3.4. Data collection
We collected 210 patients with stroke (including ischaemic and haemorrhagic stroke) who were hospitalized in the Department of Neurology from 21 October 2013–6 May 2019.
3.5. Ethical considerations
The study followed the principles of the Declaration of Helsinki and the approval of the ethical norms (NO. 2020‐117‐01) and the waiver of patient consent were obtained from the Ethics Committee of the Nanjing Drum Tower Hospital Affiliated to Nanjing University Medical School.
3.6. Validity, reliability and rigour
The data obtained were saved by the ward staff in a spreadsheet in Microsoft Excel version 2010 and were analysed by descriptive statistics. A total of 19 factors (individual factors: age, sex, disease type, smoking and alcohol use; complications: hypertension, diabetes and heart‐related diseases including atrial fibrillation, coronary heart disease, cardiac insufficiency, cardiac stenting, cardiomyopathy and old myocardial infarction), factors associated with daily care assessment (Barthel index (BI), Wong‐Baker FACES Pain Rating Scale (WBS) and systemic nutritional status assessment) and laboratory values (white blood cells, haemoglobin, platelets, platelet/lymphocyte ratio (PLR), alanine aminotransferase, serum albumin, fibrinogen and D‐dimer) were included in the study as independent variables. Three trained nurses were responsible for the collection of data, and different collecting methods were used according to the participants’ characteristics to guarantee the accuracy of data collection.
The BI, a 10‐item scale evaluating the ability to engage in daily activities, is commonly used in clinical practice. While some researchers have modified the original Barthel scale, Quinn et al strongly recommend the use of a single version of the BI scale in stroke patients to maintain consistency. Specifically, they recommend the use of the 10‐item scale with a total score of 0 to 100 (5‐point increments) as the standard BI for stroke (Quinn et al., 2011). In this study, we used the 10‐item BI scale, including eating, bathing, grooming, dressing, stool control, urine control, using a toilet, bed‐chair transfer, walking on level ground and travelling up and down stairs. A trained nurse rated each patient's ability to perform these 10 items as “completely independent,” “requiring some help” and “requiring a lot of help.” The maximum score was 5 for bathing and grooming, 15 for bed‐chair transfer and walking on level ground and 10 for the remaining items (5‐point increments). The total score ranged between 0–100. A low score indicated a low level of independence and a high level of dependence on nursing.
Previous studies have shown that the BI shows good reliability and is a valid indicator to measure activities of daily living in both stroke (Heuschmann et al., 2005; Kwon et al., 2004; Oveisgharan et al., 2006) and non‐stroke (Sainsbury et al., 2005) patients. According to a meta‐analysis, the weighted kappa of the BI is 0.95 (Quinn et al., 2009). The inter‐rater reliability of the German version of the BI in acute stroke is also very good, with an average weighted kappa of 0.93. The average weighted kappa coefficient of the postal version is 0.79, and the average weighted kappa coefficient of the telephone version is 0.80 (Heuschmann et al., 2005). Haan et al (de Haan et al., 1993) showed excellent consistency and high reliability between the BI total score (average kappa = 0.88; range 0.85–0.90) and individual scores (kappa range = 0.82–1.00) in stroke patients with disability in the Netherlands. The index is very suitable for nursing care and clinical research in stroke patients. A review of several different scales also shows that the BI’s test–retest reliability is better than that of the extended (ADL) scale of basic activities of daily living (Green et al., 2001). In addition, a study of stroke patients in the Middle East by Oveisgharan et al (Oveisgharan et al., 2006) showed that the weighted kappa of the BI’s test–retest reliability was 0.989 and the Spearman correlation coefficient between the BI score and Modified Rankin Scale (MRS) score was −0.912, which confirmed the reliability and validity of stroke clinical trials. The BI is also often used as the gold standard for comparison of the new ADL scale and Kwon et al demonstrates the comparison of the BI and the MRS and the motor component of Functional Independence Measure (M‐FIM) scores further demonstrates the validity of the BI. They were −0.8856 between BI and MRS and 0.9479 between BI and M‐FIM score (Kwon et al., 2004). In addition, the BI’s ability to predict the return of stroke patients to their homes is further evidence of the scale's validity (Hertanu et al., 1984).
Among pain intensity assessment tools, the most commonly used assessment tool is the Faces Pain Scale, which has the most supporting evidence demonstrating that it is an effective means to assess patients’ pain (Edwards et al., 2020). In this study, we used schematic facial depictions to show varying degrees of discomfort in stroke patients and different facial images constitute the Wong‐Baker FACES Pain Rating Scale (WBS). According to pain intensity, facial expressions were divided into 0 (painless), 1 (slight pain), 2 (mild pain), 3 (obvious pain), 4 (severe pain) and 5 (especially severe pain). The nurse can choose the face that best describes the current pain state of a stroke patient to assess pain severity. The WBS does not require the patient to be able to speak. Many studies have shown the validity and reliability of the Faces Pain Scale. In a study of the Faces Pain Scale in acute pain in hospitalized adults, Stuppy (1998) showed a significant positive correlation for the test–retest reliability of the Faces Pain Scale by calculating the correlation between initial and second ratings of pain in the recalled situation of “receiving a shot in the arm.” (r = .704) The scale also has a strong positive correlation with other scales (r = .81 to 0.95), indicating that the scale has sufficient validity. Kim and Buschmann (2006) found a Cohen kappa value of 0.61 for the test–retest reliability of the Faces Pain Scale and a strong correlation between the Faces Pain Scale and the 0–10 numerical rating scale (NRS) (r = .73) and the Visual Analogue Scale (VAS) (r = .73), suggesting the validity of the Faces Pain Scale. The Faces Pain Scale also has good reliability and validity in patients with stroke. When Benaim et al. (2007) assessed the Faces Pain Scale in stroke patients, for inter‐rater reliability, the kappa coefficients of the Faces Pain Scale in left or right hemispheric stroke patients were 0.64 and 0.44, respectively. For intrarater reliability, the kappa coefficients of the Faces Pain Scale in patients with left or right hemispheric stroke were 0.74 and 0.50, respectively. In patients with stroke in the left or right hemispheres, the Faces Pain Scale was highly positively correlated with the VAS and Verbal Rating Scale (VRS), suggesting that the scale has good validity.
3.7. Statistical analysis
The collected data were processed and analysed using R software (version 3.6.0) and STATA 12.0 (Stata Corporation). To avoid bias in the selection of the population and information for the model in the study, the R package "rpart" was used to perform data filling for the missing values (Therneau & Atkninson, 2019). The R package "glmnet" (version: 2.0–18) was used to perform penalized regression modelling and screening using LASSO for all risk variable coefficients to select the risk factors with the best predictive characteristics from stroke patients. The variable selection method of continuous coefficient compression was used such that the relatively unimportant independent variable coefficients became zero and thus were excluded from modelling. Then, the magnitude of the variability of the samples was observed. Overdispersion can lead to singular standard error tests and imprecise significance tests, and we used the chi‐squared test to measure overdispersion. The p‐value from the two‐sided test for the null hypothesis “ratio = 1” was calculated. Then, multivariate logistic regression analysis was performed for further optimization and screening of the selected risk variables in the LASSO regression model using the glm function in the R package "robust" to determine the independent risk factors and obtain OR values and 95% confidence intervals. Finally, we used the R package “rms” to construct the nomogram to present the prediction model. Internal validation was done using the bootstrap resampling method of the R package “boot.” We created a validation dataset by searching the data of the 210 stroke patients. Bootstrap resampling is currently the internal validation method advocated by the industry for prognostic models (Gu et al., 2020). Receiver operating characteristic (ROC) curves were plotted using the R package "pROC," and the discrimination of the clinical prediction model was assessed based on the area under the ROC curve (AUC). To assess the consistency between the predicted probability and the actually observed probability, we used the Hosmer–Lemeshow goodness‐of‐fit test for calculation, where a p‐value greater than 0.05 indicated no significant difference between the predicted and observed results. The calibration curve was plotted according to the actual and predicted incidences to assess the calibration of the prediction model. Then, decision curve analysis (DCA) was used to evaluate the model from the perspective of clinical applicability to determine the clinical validity of the nomogram for death within 6 months of onset of stroke. The Cox regression model was used with both mortality outcome and survival time as dependent variables to carry out multivariate analysis of influencing factors. Considering the PH assumption of independent variables, to reduce the bias that neglecting the PH assumption may introduce to the results, we introduced time‐dependent covariates into the Cox model. First, we performed LASSO modelling to screen for significant variables of survival data with time using lambda.min value, then used Stata software to statistically test the PH assumption of significant risk variables, and significant time‐dependent covariates were obtained considering the PH assumption (p < .05). Then, the selected significant risk variables and time‐dependent covariates were further combined to establish a time‐dependent Cox model for multivariate analysis.
4. RESULTS
4.1. Patient characteristics
Table 1 lists the detailed characteristics of the 210 stroke patients, including 186 (88.57%) ischaemic stroke patients and 24 (11.43%) haemorrhagic stroke patients. The mean age was 66.89 ± 14.73 years (mean ± SD). There were 74 female patients (35.24%) and 136 male patients (64.76%). Of these, 49 (23.33%) patients died and 161 (76.67%) patients survived. The remaining detailed baseline information for all patients is shown in Table 1.
TABLE 1.
Demographic and clinical characteristics of stroke patients
| Characteristics | Demographic and clinical values, n (%) or mean ± SD |
|---|---|
| Age (years), mean ± SD | n = 210, 66.89 ± 14.73 |
| Gender (female/ male, n (%)) | 74 (35.24%)/ 136 (64.76%) |
| Disease type (haemorrhagic stroke/ ischaemic stroke, n (%)) | 24 (11.43%)/ 186 (88.57%) |
| Smoking (yes/ no, n (%)) | 51 (24.29%)/ 159 (75.71%) |
| Alcohol use ( yes/ no, n (%)) | 31 (14.76%)/ 179 (85.24%) |
| Hypertension ( yes/ no/ NA, n (%)) | 167 (79.52%)/ 40 (19.05%)/ 3 (1.43%) |
| Diabetes ( yes/ no / NA, n (%)) | 67 (31.90%)/ 142 (67.62%) / 1 (0.48%) |
| Heart‐related diseases ( yes/ no / NA, n (%)) | 61 (29.05%)/ 148 (70.47%) / 1 (0.48%) |
| BI (0–20/ 25–40 / 45–60 / 65–80 / 85–100 / NA, n (%)) | 56 (26.67%)/ 24 (11.43%) / 42 (20.00%) / 33 (15.71%) / 53 (25.24%) / 2 (0.95%) |
| WBS score ( 0/ 1 / 2 / 4 / 5 / NA, n (%)) | 194 (92.38%)/ 3 (1.43%) / 8 (3.80%) / 3 (1.43%) / 1 (0.48%) / 1 (0.48%) |
| SNSA ( poor/ good / NA, n (%)) | 44 (20.95%)/ 137 (65.24%) / 29 (13.81%) |
| WBC (1,000/ml), mean ± SD | n = 188, 8.30 ± 3.79 |
| Haemoglobin (g/dl), mean ± SD | n = 188, 131.24 ± 21.16 |
| Platelets (1,000/ml), mean ± SD | n = 187, 194.95 ± 68.11 |
| PLR, mean ± SD | n = 186, 155.05 ± 93.30 |
| ALT (U/L), mean ± SD | n = 200, 26.74 ± 21.78 |
| Serum albumin (g/L), mean ± SD | n = 203, 38.50 ± 5.53 |
| Fibrinogen (g/L), mean ± SD | n = 181, 3.23 ± 1.16 |
| D‐dimer (mg/L), mean ± SD | n = 179, 2.10 ± 5.55 |
| Survival (yes/ no, n (%)) | 161 (76.67%)/ 49 (23.33%) |
Abbreviations: ALT, alanine aminotransferase; BI, Barthel index; NA, not available; PLR, platelet/lymphocyte ratio; SD, standard deviation; SNSA, systemic nutritional status assessment; WBC, white blood cells; WBS, Wong‐Baker FACES Pain Rating Scale.
4.2. Selection of risk variables
Based on the 210 stroke patients, LASSO model was used to screen for variables from the 19 risk characteristics from individual factors, complications, nursing assessment tools and laboratory tests and 11 significant variables were obtained: age, smoking history, hypertensive disease, heart‐related disease, BI, WBS, systemic nutritional status assessment, white blood cells, PLR, serum albumin and serum D‐dimer. The correlations between regression coefficients of LASSO and lambda are shown in Figure 1a,b.
FIGURE 1.
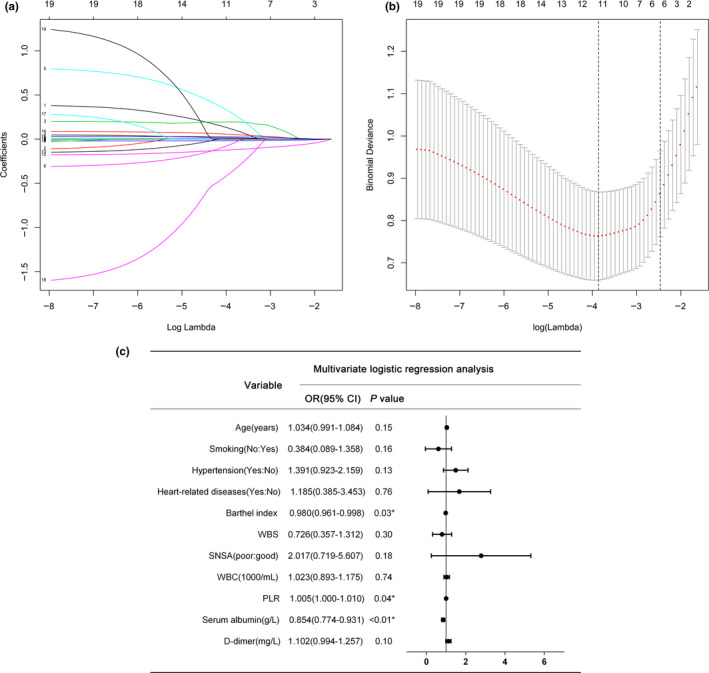
A binomial logistic regression model of LASSO was used to select characteristics. (a) LASSO coefficient profiles of the 19 characteristics of stroke patients. (b) The selected optimal parameter (lambda) in the LASSO model was 10‐fold cross‐validated by the lowest criteria. The partial likelihood deviance (binomial deviation) against log(lambda) was plotted. Dotted vertical lines were drawn at the optimal values by using the minimum criteria and the 1 SE of the minimum criteria (the 1‐SE criteria). (c) Results of multivariate logistic regression analysis for predicting death within 6 months of stroke. Note. LASSO: least absolute shrinkage and selection operator; SE, standard error; WBS, Wong‐Baker FACES Pain Rating Scale; SNSA, systemic nutritional status assessment; WBC, white blood cells; PLR, platelet/lymphocyte ratio
4.3. Development and validation of a prediction model for death within 6 months of stroke patients
A multivariate logistic regression model was further constructed using the risk variables selected by LASSO described above, as shown in Figure 1c. Three independent risk factors (Barthel index (odds ratio (OR) = 0.980, 95% confidence interval (CI) = 0.961–0.998, p = .03), platelet/lymphocyte ratio (OR = 1.005, 95% CI = 1.000–1.010, p = .04) and serum albumin (OR = 0.854, 95% CI = 0.774–0.931, p < .01)) for predicting death within 6 months in stroke patients were finally identified from the 11 variables. Finally, the probability of mortality outcome within 6 months for each study subject was calculated according to the results of logistic multivariate analysis and the resulting regression expression, to establish a nomogram model for predicting the risk of death within 6 months (Figure 2). The logistic regression prediction model can be expressed as p = exp (5.960231 + (−0.0319695) * barthel + 0.005197 * PLR + (−0.1797958) * ALB)/(1 + exp (5.960231 + (−0.0319695) * barthel + 0.005197*PLR + (−0.1797958)*ALB). The result of the overdispersion test was p = .185, indicating that the sample was not overdispersed.
FIGURE 2.
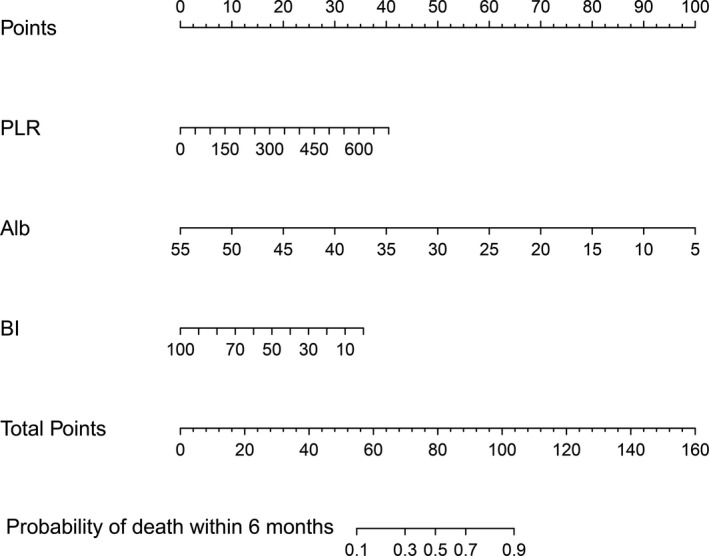
Nomogram for predicting death within 6 months of stroke patients. To use the nomogram, choose a separate value on each variable axis and draw a straight line upward to determine the point value. The sum of all the value is located at the Total Points, and a straight line is drawn downward to obtain the probability of death within 6 months for stroke patients. Note. Alb, serum albumin
The obtained model was then validated internally using the bootstrap resampling method. The AUC of ROC was also calculated, also called the C‐statistic. An assessment of the accuracy of the prediction model in predicting the risk of death within 6 months of stroke, the C‐statistic on the training set was 0.879 (Figure 3a) and on the bootstrapping validation set it was 0.891 (Figure 3b), suggesting that the nomogram had good discrimination. The Hosmer–Lemeshow test showed that the prediction model of death within 6 months of stroke was well calibrated in the training set (X 2 = 7.648, p = .570) and the validation set (X 2 = 9.280, p = .412). The calibration curves showed good consistency between the training and validation sets (Figure 3c,d). The DCAs for the training and validation sets are shown in Figure 3e,f. The DCA showed that the prediction model had a higher overall net benefit over most of the threshold probability range (1% to 2%, 5% to 77% and ≥ 85% in the training sets, 1% to 80% and 83% to 95% in the validation sets, respectively), indicating that the prediction model has high clinical effect and application value.
FIGURE 3.
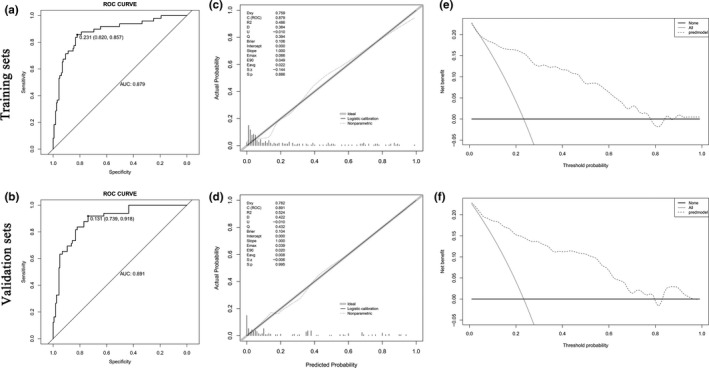
Evaluation of nomogram discrimination, consistency, and clinical applicability by AUC of ROC, calibration plots, and decision curves in the training set and validation set. (a, b) ROC curves of the nomogram predicting death within 6 months of stroke patients in the training and validation sets. (c, d) Calibration curves of the nomogram between the predicted and the actually observed values in the training and validation sets. The calibration curve indicates the goodness of fit of the nomogram. The abscissa of the graph is the predicted probability, which is the prediction of the likelihood of an event with the prediction model. The values 0 to 1 indicate a likelihood of an event of 0 to 100%. The ordinate is the actual probability and represents the actual incidence for the patient. Black solid lines indicate the predictive performance of the nomogram. The p values were .886 and .995 in the training and validation sets, respectively, suggesting that the prediction model was highly calibrated in both datasets. (e, f) DCA of the prediction model in the training and validation sets. The y‐axis represents the net benefit. The thin line represents the assumption that all patients died; the bold line represents the assumption that no patient died; and the dashed line represents the nomogram of death within 6 months. Note. AUC, Area Under Curve; ROC, receiver operating characteristic; DCA, decision curve analysis
4.4. Time‐dependent Cox regression model analysis of the 6‐month prognosis of stroke patients
We used both mortality outcome and survival time as dependent variables for the analysis of influencing factors. First, we used LASSO‐Cox regression to screen for risk factors and a total of seven significant risk factors were identified: age, smoking history, BI, white blood cells, PLR, serum albumin and serum D‐dimer (Figure 4a,b). The effect of risk variables on survival time is not invariable but may change with time. A time‐dependent Cox regression model, as an extended form of the classical Cox regression model, can yield more adequate variable information when the relevant independent variables do not satisfy the PH assumption. In this study, we ran statistical tests on the PH assumption of the seven significant risk variables identified by LASSO regression and the results showed that only the time effect of age (X 2 = 6.97, p = .008) had an effect on survival time. We then used the above seven risk variables and the age time‐dependent covariates together to run a multivariate Cox regression analysis. As in the multivariate logistic regression, BI (hazard ratio (HR) = 0.985, 95% CI = 0.972–0.998, p = .03), PLR (HR = 1.004, 95% CI = 1.002–1.006, p < .01) and serum albumin (HR = 0.922, 95% CI = 0.885–0.961, p < .01) were also independent prognostic factors for the 6‐month survival of stroke patients. In addition, time‐related age (HR = 1.001, 95% CI = 1.000–1.002, p = .01) was an independent prognostic factor (Figure 4c). However, age itself was not an independent prognostic factor for 6‐month survival (p = .652), suggesting that age at onset may not be a key factor for 6‐month survival. However, the protective effect of age may be attenuated with the increase of survival time, suggesting that intervention should be performed as early as possible for stroke patients to reduce the risk of death within 6 months.
FIGURE 4.
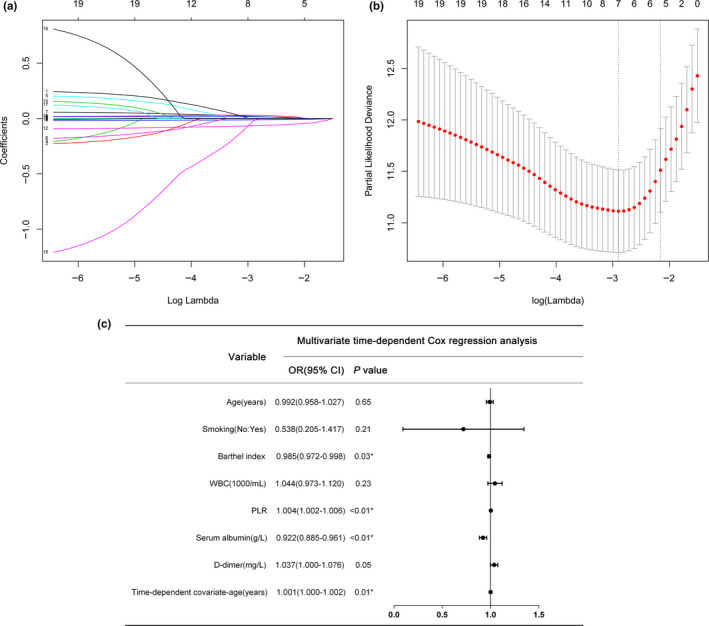
Selection of characteristics using the LASSO‐Cox regression model. (a) LASSO coefficient profiles of the 19 characteristics of 6‐month survival of stroke patients. (b) Ten‐fold cross‐validation of the selected optimal parameter (lambda) in the LASSO model by the lowest criteria. Partial likelihood deviance curves were plotted against log(lambda). Dotted vertical lines were drawn at the optimal values by using the minimum criteria and the 1 SE of the minimum criteria (the 1‐SE criteria). (c) Results of multivariate timecompliant Cox non‐proportional‐hazards analysis
5. DISCUSSION
This retrospective cohort study used the clinical characteristics and 6‐month follow‐up data of 210 patients with different types of stroke from a medical institution in China. The study has proposed a new prediction model by which clinical healthcare workers can use the baseline characteristics of patients to predict the probability of death within 6 months of onset of stroke. In view of the disability and fatal hazards of stroke disease in adults and based on the risk factors for stroke patients, early prediction of the adverse outcomes within 6 months can help guide healthcare workers in performing early intervention and further optimizing the care for stroke patients and can help stroke patients to achieve the best outcomes and quality of life.
In this study, multivariate logistic regression showed that BI, PLR, and serum albumin were independent risk factors for predicting death within 6 months of stroke. In view of this, we constructed a nomogram. These three independent variables were also confirmed in LASSO time‐dependent Cox regression analysis.
PLR is a potential marker of increased inflammation due to the release of many mediators from platelets. Altintas et al. (2016) found that PLR may reflect the pro‐thrombotic inflammatory state in patients with acute ischaemic stroke and an increase in PLR would lead to poor prognosis in patients with ischaemic stroke. The study by Xu et al. (2019) on 286 patients with acute ischaemic stroke who received IVT showed that high PLR was associated with poor prognosis and 3‐month death. These conclusions are like, and support our findings.
Serum albumin is a 65‐kDa protein that has a variety of physiological properties, particularly transporting many endogenous and exogenous substances and drugs. It also has antioxidant, anti‐inflammatory, antiaggregation and anticoagulant effects. In animal models of ischaemic stroke, serum albumin exhibits neuroprotective effects. Hypoproteinaemia is a valuable prognostic marker for stroke. Abubakar et al. (2013) studied 75 hospitalized patients with acute stroke and found that serum albumin was significantly higher in stroke patients who survived than in those who died. Their multivariate regression analysis indicated that low serum albumin after admission was an independent prognostic factor for poor outcome. In a prospective study of 444 patients with ischaemic stroke, Idicula et al. (2009) found that high serum albumin was associated with lower mortality and that high serum albumin may have a neuroprotective effect in patients with ischaemic stroke. Hashem et al. (2018) found that serum albumin was a significant prognostic indicator after ischaemic stroke, and by linear regression analysis, they found that albumin was the only important predictor in their sample. Wang et al. (2019) showed that when serum albumin was higher in patients with acute ischaemic stroke and younger than 60 years, the risk of haemorrhagic transformation was gradually reduced, so it is a marker for improving the prognosis of patients with ischaemic stroke. Our findings are similar and suggest that serum albumin is an independent risk factor for death within 6 months of onset of stroke.
BI is a valid indicator of functional outcome in stroke. The BI is a valid and reliable tool to evaluate the activities of daily living (ADL) dependence of stroke patients (Collin et al., 1988; Wade & Collin, 1988) and has been recommended as the “gold standard” of other ADL rating scales and the benchmark for evaluating the new scale (McDowell & Newell, 1987; Wade, 1992). The ten individual items of BI focus on the assessment of basic daily activities, and the assessment is not time‐consuming. Moreover, BI is highly reliable, effective and sensitive to changes in the disease, making it widely used in clinical practice. There are few studies on BI and mortality outcome of stroke patients. Lee et al. (2013) found that a BI score less than 20 was significantly associated with the 7‐day mortality, 30‐day mortality and in‐hospital mortality of patients with ischaemic stroke. BI was used in constructing the prediction models for the above three mortality rates, all of which had a high predictive power. Thus, BI may be a predictor of mortality outcome of stroke patients. Our findings also confirm that the BI is an independent predictor of mortality in stroke patients, which is consistent with the results of Lee et al. However, at present, there is still a lack of prediction models for death within 6 months for stroke patients (including ischaemic and haemorrhagic stroke) that are constructed with BI as an independent variable.
Our study showed that BI was an independent factor for death within 6 months of stroke. The BI is a powerful predictor of death in stroke patients within 6 months of onset; thus, interventions to improve functional status after stroke may have a protective effect on stroke patients. The BI is a measure of nursing dependence in rehabilitation. Therefore, early nursing interventions and rehabilitation nursing can improve the self‐care ability of stroke patients after stroke, which is of great significance to the survival of stroke patients.
First, the BI is an appropriate tool for predicting the long‐term risk of dependence on nursing care after stroke, showing high prognostic effectiveness (Diederichs et al., 2011). Second, a strong correlation was found between the BI and the global rating of nursing dependence. A strong correlation was also identified between the BI and the Northwick Park nursing dependence score (NPDS), a neurological rehabilitation scale designed for use in neurological rehabilitation, with a correlation coefficient between 0.87–0.95. At the same time, the Spearman correlation coefficient between the BI and the Basic Care Needs section (BCN) in the NPDS was −0.93 and the correlation between the BI and BCN were very strong at each measurement (between −0.85–−0.95), reflecting a good inverse correlation (Post et al., 2002). Similar results were confirmed by Turner‐Stokes et al. (1998). The above results suggest that the mortality of stroke patients is closely related to nursing dependence and that we should provide stroke patients with more nursing care, especially stroke patients requiring basic care. Strengthening the basic nursing care provided to stroke patients, such as care related to mobility, bed transfers, urinary incontinence, dressing, eating, washing and grooming, bathing/showering, skin pressure relief and safety awareness, can improve the BI and reduce the mortality of stroke patients within 6 months of onset. Al‐Khawaja et al. (1997) also found that the correlation between the BI and nursing time may be as high as 0.70. Nurses can use BI scores to determine the approximate workload of physical care needs and then allocate nursing hours according to different types of nursing dependence. Therefore, a lower BI is associated with greater future disability, a longer rehabilitation time and more care needs to promote rehabilitation (Huybrechts & Caro, 2007). Nurses can promote BI improvement by providing more rehabilitation care and increasing nursing time, thus reducing the death rate of stroke patients within 6 months of onset.
In China, due to limited economic conditions and medical resources, the number of stroke wards is far from sufficient. Only approximately 10% of neurology departments have stroke wards and are mainly located in urban areas (Durai Pandian et al., 2007). However, rural areas experience difficulty related to stroke rehabilitation because of long distances and high costs (W. Wang et al., 2017). Chu et al. (2020) suggested that the medical service of a family member‐delivered rehabilitation model for disabled stroke patients can be carried out. Their results show that compared with conventional nursing, this new rehabilitation model provided by nurse training and family members improves BI scores, reduces the incidence and severity of physical disability caused by stroke, promotes physical recovery and improves the work efficiency of nurses.
Moreover, the start time and intensity of rehabilitation are important predictors of BI score at discharge (Hu et al., 2010) and nursing staff can start in‐depth rehabilitation as early as possible to improve patients' self‐care ability and reduce the impact of BI score on poor prognosis after stroke. The organized joint action of medical institutions is more conducive to the rehabilitation of patients (Hsu et al., 2019).
Our predictive model confirmed that the BI, the PLR and serum ALB were independently associated with six‐month mortality in patients with stroke and had independent predictive value. Our research results are consistent with the external literature. As an independent predictor, high serum ALB can reduce the death probability of stroke patients and play a protective role. A higher PLR or a lower BI score corresponds to a higher death probability in stroke patients. Thus, the parameters can be used as predictors of stroke mortality.
Our predictive model is constructed using the BI score of the ADL of stroke patients and the laboratory values of the PLR and serum ALB, which are easily available in clinical practice. The model's execution is simple and can provide evidence for clinical nurses to judge a patient's condition early and evaluate survival within 6 months. Therefore, according to the degree of dependence of a patient's self‐care ability, different nursing measures and nursing times are provided for interventions. Additionally, different nursing service models can be used according to local conditions and rehabilitation activities can be carried out as early as possible and in an organized manner.
5.1. Limitations
This study has some limitations. First, most patients in this study lived near the hospital. Only a few were from other regions of China, so the sample has limitations in terms of representativeness. Future studies should recruit larger scale and more diverse samples to extend the findings to a larger patient population. Second, the performance of the prediction model needs to be further evaluated in external validation studies.
6. CONCLUSION
The new prediction model can provide a tool for nursing staff engaged in clinical nursing work to accurately predict the risk of death within 6 months of Chinese stroke patients by assessing the risk indicators of the patients after admission, which include the activities of daily life, platelets, lymphocytes and serum albumin. In addition, this study confirmed the time‐varying effect of time‐related age on the survival time by LASSO time‐dependent Cox analysis, based on the judgement results of the exact statistical test of time‐dependent covariates.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors fulfil the criteria of authorship according to the Vancouver Vrules for authorship. LS (Ling Sha) designed the study; LS (Ling Sha), TTX, XJG, LS (Lei Shi), JZ and HMG collected data; LS (Ling Sha) completed the statistics; LS (Ling Sha) drafted the manuscript; LS (Ling Sha) supervised the study. All authors read and approved the final manuscript.
Sha L, Xu T, Ge X, Shi L, Zhang J, Guo H. Predictors of death within 6 months of stroke onset: A model with Barthel index, platelet/lymphocyte ratio and serum albumin. Nurs Open.2021;8:1380–1392. 10.1002/nop2.754
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abdo, R. , Abboud, H. , Salameh, P. , El Hajj, T. , & Hosseini, H. (2019). Mortality and predictors of death poststroke: Data from a multicenter prospective cohort of lebanese stroke patients. Journal of Stroke and Cerebrovascular Diseases, 28(4), 859–868. 10.1016/j.jstrokecerebrovasdis.2018.11.033 [DOI] [PubMed] [Google Scholar]
- Abubakar, S. , Sabir, A. , Ndakotsu, M. , Imam, M. , & Tasiu, M. (2013). Low admission serum albumin as prognostic determinant of 30‐day case fatality and adverse functional outcome following acute ischemic stroke. Pan African Medical Journal, 14, 53. 10.11604/pamj.2013.14.53.1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AI‐Khawaja, I. , Wade, D. T. , & Turner, F. (1997). The Barthel Index and its relationship to nursing dependency in rehabilitation. Clin Rehabil, 11(4), 335–337. 10.1177/026921559701100411 [DOI] [PubMed] [Google Scholar]
- Altintas, O. , Altintas, M. O. , Tasal, A. , Kucukdagli, O. T. , & Asil, T. (2016). The relationship of platelet‐to‐lymphocyte ratio with clinical outcome and final infarct core in acute ischemic stroke patients who have undergone endovascular therapy. Neurological Research, 38(9), 759–765. 10.1080/01616412.2016.1215030 [DOI] [PubMed] [Google Scholar]
- Amitrano, D. , Silva, I. R. , Liberato, B. B. , Batistella, V. , Oliveira, J. , & Nascimento, O. J. (2016). Simple prediction model for unfavorable outcome in ischemic stroke after intravenous thrombolytic therapy. Arquivos De Neuro‐Psiquiatria, 74(12), 986–989. 10.1590/0004-282x20160152 [DOI] [PubMed] [Google Scholar]
- Babu, M. S. , Kaul, S. , Dadheech, S. , Rajeshwar, K. , Jyothy, A. , & Munshi, A. (2013). Serum albumin levels in ischemic stroke and its subtypes: Correlation with clinical outcome. Nutrition, 29(6), 872–875. 10.1016/j.nut.2012.12.015 [DOI] [PubMed] [Google Scholar]
- Benaim, C. , Froger, J. , Cazottes, C. , Gueben, D. , Porte, M. , Desnuelle, C. , & Pelissier, J. Y. (2007). Use of the Faces Pain Scale by left and right hemispheric stroke patients. Pain, 128(1–2), 52–58. 10.1016/j.pain.2006.08.029 [DOI] [PubMed] [Google Scholar]
- Boldingh, E. J. , Jacobs‐van der Bruggen, M. A. , Lankhorst, G. J. , & Bouter, L. M. (2004). Assessing pain in patients with severe cerebral palsy: Development, reliability and validity of a pain assessment instrument for cerebral palsy. Archives of Physical Medicine and Rehabilitation, 85(5), 758–766. 10.1016/j.apmr.2003.06.029 [DOI] [PubMed] [Google Scholar]
- Carryer, J. , Weststrate, J. , Yeung, P. , Rodgers, V. , Towers, A. , & Jones, M. (2017). Prevalence of key care indicators of pressure injuries, incontinence, malnutrition and falls among older adults living in nursing homes in New Zealand. Research in Nursing & Health, 40(6), 555–563. 10.1002/nur.21835 [DOI] [PubMed] [Google Scholar]
- Chakraborty, B. , Vishnoi, G. , Goswami, B. , Gowda, S. H. , Chowdhury, D. , & Agarwal, S. (2013). Lipoprotein(a), ferritin and albumin in acute phase reaction predicts severity and mortality of acute ischemic stroke in North Indian Patients. Journal of Stroke and Cerebrovascular Diseases, 22(7), e159–167. 10.1016/j.jstrokecerebrovasdis.2012.10.013 [DOI] [PubMed] [Google Scholar]
- Chen, Z. (2008). The mortality and death cause of national sample areas. In Chen Z. (Ed.), The Third National Survey on the Cause of Death (pp. 14–15). Peking Union Medical University Press. [Google Scholar]
- Cheong, M. Y. , Bang, O. S. , Cha, M. H. , Park, Y. K. , Kim, S. H. , & Kim, Y. J. (2011). Association of the adiponectin gene variations with risk of ischemic stroke in a Korean population. Yonsei Medical Journal, 52(1), 20–25. 10.3349/ymj.2011.52.1.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu, M. , Austin, P. C. , Manuel, D. G. , & Tu, J. V. (2010). Comparison of cardiovascular risk profiles among ethnic groups using population health surveys between 1996 and 2007. CMAJ, 182(8), E301–310. 10.1503/cmaj.091676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chongruksut, W. , Limpastan, K. , Jetjumnong, C. , Watcharasaksilp, W. , Vaniyapong, T. , Norasetthada, T. , Triamvisit, S. , Ruengorn, C. , Nochaiwong, S. , Nanta, S. , Saengyo, S. , & Rerkasem, K. (2020). Age as a prognostic factor of 30‐day mortality in hemorrhagic stroke patients: A Thai large tertiary care referral center. Asian Journal Surgery, 43(10), 991–995. 10.1016/j.asjsur.2019.11.010 [DOI] [PubMed] [Google Scholar]
- Chu, K. , Bu, X. , Sun, Z. , Wang, Y. , Feng, W. , Xiao, L. I. , Jiang, F. , & Tang, X. (2020). Feasibility of a nurse‐trained, family member‐delivered rehabilitation model for disabled stroke patients in rural chongqing China. Journal of Stroke and Cerebrovascular Diseases, 29(12), 105382. 10.1016/j.jstrokecerebrovasdis.2020.105382 [DOI] [PubMed] [Google Scholar]
- Collin, C. , Wade, D. T. , Davies, S. , & Horne, V. (1988). The Barthel ADL Index: A reliability study. International Disability Studies, 10(2), 61–63. 10.3109/09638288809164103 [DOI] [PubMed] [Google Scholar]
- Counsell, C. , Dennis, M. , McDowall, M. , & Warlow, C. (2002). Predicting outcome after acute and subacute stroke: Development and validation of new prognostic models. Stroke, 33(4), 1041–1047. 10.1161/hs0402.105909 [DOI] [PubMed] [Google Scholar]
- de Haan, R. , Limburg, M. , Schuling, J. , Broeshart, J. , Jonkers, L. , & van Zuylen, P. (1993). Clinimetric evaluation of the Barthel Index, a measure of limitations in dailly activities. Nederlands Tijdschrift Voor Geneeskunde, 137(18), 917–921. [PubMed] [Google Scholar]
- Diederichs, C. , Mühlenbruch, K. , Lincke, H. O. , Heuschmann, P. U. , Ritter, M. A. , & Berger, K. (2011). Predictors of dependency on nursing care after stroke: Results from the Dortmund and Münster stroke registry. Deutsches Aerzteblatt Online, 108(36), 592–599. 10.3238/arztebl.2011.0592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan, S. K. , Ay, S. , Oztuna, D. , Aytur, Y. K. , & Evcik, D. (2010). The utility of the Faces Pain Scale in the assessment of shoulder pain in Turkish stroke patients: Its relation with quality of life and psychologic status. International Journal of Rehabilitation Research, 33(4), 363–367. 10.1097/MRR.0b013e32833cdef3 [DOI] [PubMed] [Google Scholar]
- Durai Pandian, J. , Padma, V. , Vijaya, P. , Sylaja, P. N. , & Murthy, J. M. (2007). Stroke and thrombolysis in developing countries. International Journal of Stroke, 2(1), 17–26. 10.1111/j.1747-4949.2007.00089.x [DOI] [PubMed] [Google Scholar]
- Edwards, S. A. , Ioannou, A. , Carin‐Levy, G. , Cowey, E. , Brady, M. , Morton, S. , Sande, T. A. , Mead, G. , & Quinn, T. J. (2020). Properties of pain assessment tools for use in people living with stroke: Systematic review. Frontiers in Neurology, 11, 792. 10.3389/fneur.2020.00792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabjan, T. H. , Penko, M. , & Hojs, R. (2019). Anemia on admission and long‐term mortality risk in patients with acute ischemic stroke. Advances in Clinical and Experimental Medicine, 28(10), 1419–1424. 10.17219/acem/104540 [DOI] [PubMed] [Google Scholar]
- Fekadu, G. , Chelkeba, L. , Melaku, T. , Tegene, E. , & Kebede, A. (2020). 30‐day and 60‐day rates and predictors of mortality among adult stroke patients: Prospective cohort study. Annals of Medicine and Surgery (London), 53, 1–11. 10.1016/j.amsu.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøyshov, H. M. , Bjørnerem, Å. , Engstad, T. , & Halvorsen, D. S. (2017). Elevated inflammatory markers predict mortality in long‐term ischemic stroke‐survivors: A population‐based prospective study. Aging Clinical and Experimental Research, 29(3), 379–385. 10.1007/s40520-016-0575-9 [DOI] [PubMed] [Google Scholar]
- Gomes, F. , Emery, P. W. , & Weekes, C. E. (2016). Risk of malnutrition is an independent predictor of mortality, length of hospital stay and hospitalization costs in stroke patients. Journal of Stroke and Cerebrovascular Diseases, 25(4), 799–806. 10.1016/j.jstrokecerebrovasdis.2015.12.017 [DOI] [PubMed] [Google Scholar]
- Green, J. , Forster, A. , & Young, J. (2001). A test‐retest reliability study of the Barthel Index, the Rivermead Mobility Index, the Nottingham Extended Activities of Daily Living Scale and the Frenchay Activities Index in stroke patients. Disability and Rehabilitation, 23(15), 670–676. 10.1080/09638280110045382 [DOI] [PubMed] [Google Scholar]
- Gu, X. , Li, H. , Sha, L. , & Zhao, W. (2020). A prognostic model composed of four long noncoding RNAs predicts the overall survival of Asian patients with hepatocellular carcinoma. Cancer Medicine, 9(16), 5719–5730. 10.1002/cam4.3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashem, S. S. , Helmy, S. M. , El‐Fayomy, N. M. , Oraby, M. I. , Menshawy, M. , Dawood, N. A. , & Hashem, H. S. (2018). Predictors of stroke outcome: The role of hemorheology, natural anticoagulants and serum albumin. The Egyptian Journal of Neurology, Psychiatry and Neurosurgery, 54(1), 18. 10.1186/s41983-018-0019-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertanu, J. S. , Demopoulos, J. T. , Yang, W. C. , Calhoun, W. F. , & Fenigstein, H. A. (1984). Stroke rehabilitation: Correlation and prognostic value of computerized tomography and sequential functional assessments. Archives of Physical Medicine and Rehabilitation, 65(9), 505–508. [PubMed] [Google Scholar]
- Heuschmann, P. U. , Kolominsky‐Rabas, P. L. , Nolte, C. H. , Hünermund, G. , Ruf, H. U. , Laumeier, I. , & Berger, K. (2005). The reliability of the german version of the barthel‐index and the development of a postal and telephone version for the application on stroke patients. Fortschritte Der Neurologie‐Psychiatrie, 73(2), 74–82. 10.1055/s-2004-830172 [DOI] [PubMed] [Google Scholar]
- Hsu, Y. C. , Chen, G. C. , Chen, P. Y. , & Lin, S. K. (2019). Postacute care model of stroke in one hospital. Tzu‐Chi Medical Journal, 31(4), 260–265. 10.4103/tcmj.tcmj_95_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, M. H. , Hsu, S. S. , Yip, P. K. , Jeng, J. S. , & Wang, Y. H. (2010). Early and intensive rehabilitation predicts good functional outcomes in patients admitted to the stroke intensive care unit. Disability and Rehabilitation, 32(15), 1251–1259. 10.3109/09638280903464448 [DOI] [PubMed] [Google Scholar]
- Huybrechts, K. F. , & Caro, J. J. (2007). The Barthel Index and modified Rankin Scale as prognostic tools for long‐term outcomes after stroke: A qualitative review of the literature. Current Medical Research and Opinion, 23(7), 1627–1636. 10.1185/030079907x210444 [DOI] [PubMed] [Google Scholar]
- Idicula, T. T. , Waje‐Andreassen, U. , Brogger, J. , Naess, H. , & Thomassen, L. (2009). Serum albumin in ischemic stroke patients: The higher the better. The Bergen Stroke Study. Cerebrovascular Diseases, 28(1), 13–17. 10.1159/000215938 [DOI] [PubMed] [Google Scholar]
- Inouye, M. (2001). Predicting outcomes of patients in Japan after first acute stroke using a simple model. American Journal of Physical Medicine and Rehabilitation, 80(9), 645–649. 10.1097/00002060-200109000-00003 [DOI] [PubMed] [Google Scholar]
- Kazmierski, R. , Guzik, P. , Ambrosius, W. , Ciesielska, A. , Moskal, J. , & Kozubski, W. (2004). Predictive value of white blood cell count on admission for in‐hospital mortality in acute stroke patients. Clinical Neurology and Neurosurgery, 107(1), 38–43. 10.1016/j.clineuro.2004.03.003 [DOI] [PubMed] [Google Scholar]
- Kim, E. J. , & Buschmann, M. T. (2006). Reliability and validity of the Faces Pain Scale with older adults. International Journal of Nursing Studies, 43(4), 447–456. 10.1016/j.ijnurstu.2006.01.001 [DOI] [PubMed] [Google Scholar]
- Kitson, A. , Conroy, T. , Wengstrom, Y. , Profetto‐McGrath, J. , & Robertson‐Malt, S. (2010). Defining the fundamentals of care. International Journal of Nursing Practice, 16(4), 423–434. 10.1111/j.1440-172X.2010.01861.x [DOI] [PubMed] [Google Scholar]
- Kwon, S. , Hartzema, A. G. , Duncan, P. W. , & Min‐Lai, S. (2004). Disability measures in stroke: Relationship among the Barthel Index, the functional independence measure and the modified Rankin scale. Stroke, 35(4), 918–923. 10.1161/01.str.0000119385.56094.32 [DOI] [PubMed] [Google Scholar]
- Lee, J. , Morishima, T. , Kunisawa, S. , Sasaki, N. , Otsubo, T. , Ikai, H. , & Imanaka, Y. (2013). Derivation and validation of in‐hospital mortality prediction models in ischaemic stroke patients using administrative data. Cerebrovascular Diseases, 35(1), 73–80. 10.1159/000346090 [DOI] [PubMed] [Google Scholar]
- Ling, X. , Shen, B. , Li, K. , Si, L. , & Yang, X. (2019). Development of a prediction model for 1‐year poor prognosis in patients with acute ischemic stroke. Journal of Investigative Medicine, 67(6), 957–963. 10.1136/jim-2018-000883 [DOI] [PubMed] [Google Scholar]
- Maruyama, K. , Nakagawa, N. , Koyama, S. , Maruyama, J. I. , & Hasebe, N. (2018). Malnutrition increases the incidence of death, cardiovascular events and infections in patients with stroke after rehabilitation. Journal of Stroke and Cerebrovascular Diseases, 27(3), 716–723. 10.1016/j.jstrokecerebrovasdis.2017.10.002 [DOI] [PubMed] [Google Scholar]
- McDowell, I. , & Newell, C. (1987). Measuring health: A guide to rating scales and questionnaires. Oxford University Press. [Google Scholar]
- Moalla, K. S. , Damak, M. , Chakroun, O. , Farhat, N. , Sakka, S. , Hdiji, O. , & Mhiri, C. (2020). Prognostic factors for mortality due to acute arterial stroke in a North African population. Pan African Medical Journal, 35, 50. 10.11604/pamj.2020.35.50.16287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, C. J. L. , Vos, T. , Lozano, R. , Naghavi, M. , Flaxman, A. D. , Michaud, C. , Ezzati, M. , Shibuya, K. , Salomon, J. A. , Abdalla, S. , Aboyans, V. , Abraham, J. , Ackerman, I. , Aggarwal, R. , Ahn, S. Y. , Ali, M. K. , AlMazroa, M. A. , Alvarado, M. , Anderson, H. R. , … Lopez, A. D. (2012). Disability‐adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. The Lancet, 380(9859), 2197–2223. 10.1016/s0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- Nam, K.‐W. , Kim, C. K. , Kim, T. J. , An, S. J. , Oh, K. , Mo, H. , Kang, M. K. , Han, M.‐K. , Demchuk, A. M. , Ko, S.‐B. , & Yoon, B.‐W. (2017). Predictors of 30‐day mortality and the risk of recurrent systemic thromboembolism in cancer patients suffering acute ischemic stroke. PLoS One, 12(3), e0172793. 10.1371/journal.pone.0172793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- . The atlas of heart disease and stroke. Available online at www.who.int/cardiovascular_diseases/resources/atlas/en/. Accessed 4 February 2020.
- Oveisgharan, S. , Shirani, S. , Ghorbani, A. , Soltanzade, A. , Baghaei, A. , Hosseini, S. , & Sarrafzadegan, N. (2006). Barthel index in a Middle‐East country: Translation, validity and reliability. Cerebrovascular Disease, 22(5–6), 350–354. 10.1159/000094850 [DOI] [PubMed] [Google Scholar]
- Post, M. W. , Visser‐Meily, J. M. , & Gispen, L. S. (2002). Measuring nursing needs of stroke patients in clinical rehabilitation: A comparison of validity and sensitivity to change between the Northwick Park Dependency Score and the Barthel Index. Clin Rehabil, 16(2), 182–189. 10.1191/0269215502cr474oa [DOI] [PubMed] [Google Scholar]
- Quinn, T. J. , Dawson, J. , Walters, M. R. , & Lees, K. R. (2009). Reliability of the modified Rankin Scale: A systematic review. Stroke, 40(10), 3393–3395. 10.1161/strokeaha.109.557256 [DOI] [PubMed] [Google Scholar]
- Quinn, T. J. , Langhorne, P. , & Stott, D. J. (2011). Barthel index for stroke trials: Development, properties and application. Stroke, 42(4), 1146–1151. 10.1161/strokeaha.110.598540 [DOI] [PubMed] [Google Scholar]
- Sainsbury, A. , Seebass, G. , Bansal, A. , & Young, J. B. (2005). Reliability of the Barthel Index when used with older people. Age and Ageing, 34(3), 228–232. 10.1093/ageing/afi063 [DOI] [PubMed] [Google Scholar]
- Scherder, E. , & van Manen, F. (2005). Pain in Alzheimer's disease: Nursing assistants' and patients' evaluations. Journal of Advanced Nursing, 52(2), 151–158. 10.1111/j.1365-2648.2005.03577.x [DOI] [PubMed] [Google Scholar]
- Stuppy, D. J. (1998). The faces pain scale: Reliability and validity with mature adults. Applied Nursing Research, 11(2), 84–89. 10.1016/s0897-1897(98)80229-2 [DOI] [PubMed] [Google Scholar]
- Therneau, T. , & Atkninson, E. (2019). An introduction to recursive partitioning using the RPART routines. Mayo foundation technical report. Mayo Clinic. [Google Scholar]
- Tu, J. V. , Chu, A. , Rezai, M. R. , Guo, H. , Maclagan, L. C. , Austin, P. C. , Booth, G. L. , Manuel, D. G. , Chiu, M. , Ko, D. T. , Lee, D. S. , Shah, B. R. , Donovan, L. R. , Sohail, Q. Z. , & Alter, D. A. (2015). The incidence of major cardiovascular events in immigrants to Ontario, Canada: The CANHEART Immigrant Study. Circulation, 132(16), 1549–1559. 10.1161/circulationaha.115.015345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner‐Stokes, L. , Tonge, P. , Nyein, K. , Hunter, M. , Nielson, S. , & Robinson, I. (1998). The Northwick Park Dependency Score (NPDS): A measure of nursing dependency in rehabilitation. Clin Rehabil, 12(4), 304–318. 10.1191/026921598669173600 [DOI] [PubMed] [Google Scholar]
- Wade, D. T. (1992). Measurement in neurological rehabilitation. Oxford University Press. [PubMed] [Google Scholar]
- Wade, D. T. , & Collin, C. (1988). The Barthel ADL Index: A standard measure of physical disability? International Disability Studies, 10(2), 64–67. 10.3109/09638288809164105 [DOI] [PubMed] [Google Scholar]
- Wang, C. , Deng, L. , Qiu, S. , Bian, H. , Wang, L. U. , Li, Y. , Liu, M. , & Wu, B. O. (2019). Serum albumin is negatively associated with hemorrhagic transformation in acute ischemic stroke patients. Cerebrovascular Diseases, 47(1–2), 88–94. 10.1159/000498855 [DOI] [PubMed] [Google Scholar]
- GBD 2015 Mortality and Causes of Death Collaborators (2016). Global, regional and national life expectancy, all‐cause mortality and cause‐specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the global burden of disease study 2015. Lancet, 388(10053), 1459–1544. 10.1016/s0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Zhao, C. , Xia, Q. X. , & Qiao, S. J. (2014). Association between 12p13 SNP rs11833579 and ischemic stroke in Asian population: An updated meta‐analysis. Journal of the Neurological Sciences, 345(1–2), 198–201. 10.1016/j.jns.2014.07.047 [DOI] [PubMed] [Google Scholar]
- Wang, W. , Jiang, B. , Sun, H. , Ru, X. , Sun, D. , Wang, L. , Wang, L. , Jiang, Y. , Li, Y. , Wang, Y. , Chen, Z. , Wu, S. , Zhang, Y. , Wang, D. , Wang, Y. , & Feigin, V. L. (2017). Prevalence, incidence and mortality of stroke in China: Results from a nationwide population‐based survey of 480 687 adults. Circulation, 135(8), 759–771. 10.1161/circulationaha.116.025250 [DOI] [PubMed] [Google Scholar]
- Weimar, C. , Konig, I. R. , Kraywinkel, K. , Ziegler, A. , & Diener, H. C. (2004). Age and national institutes of health stroke scale score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: Development and external validation of prognostic models. Stroke, 35(1), 158–162. 10.1161/01.str.0000106761.94985.8b [DOI] [PubMed] [Google Scholar]
- Wit, L. , Putman, K. , Devos, H. , Brinkmann, N. , Dejaeger, E. , Weerdt, W. , Jenni, W. , Lincoln, N. , Schuback, B. , Schupp, W. , & Lesaffre, E. (2012). Five‐year mortality and related prognostic factors after inpatient stroke rehabilitation: A European multi‐centre study. Journal of Rehabilitation Medicine, 44(7), 547–552. 10.2340/16501977-0991 [DOI] [PubMed] [Google Scholar]
- Wong, D. L. , & Baker, C. M. (1988). Pain in children: Comparison of assessment scales. Pediatr Nurs, 14(1), 9–17. [PubMed] [Google Scholar]
- Xu, J. H. , He, X. W. , Li, Q. , Liu, J. R. , Zhuang, M. T. , Huang, F. F. , & Bao, G. S. (2019). Higher platelet‐to‐lymphocyte ratio is associated with worse outcomes after intravenous thrombolysis in acute ischaemic stroke. Frontiers in Neurology, 10, 1192. 10.3389/fneur.2019.01192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf, S. , Reddy, S. , Ounpuu, S. , & Anand, S. (2001). Global burden of cardiovascular diseases: Part I: General considerations, the epidemiologic transition, risk factors and impact of urbanization. Circulation, 104(22), 2746–2753. 10.1161/hc4601.099487 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


