Abstract
Neural crest stem cells (NCSCs) are a transient population of cells that arise during early vertebrate development and harbor stem cell properties, such as self‐renewal and multipotency. These cells form at the interface of non‐neuronal ectoderm and neural tube and undergo extensive migration whereupon they contribute to a diverse array of cell and tissue derivatives, ranging from craniofacial tissues to cells of the peripheral nervous system. Neural crest‐like stem cells (NCLSCs) can be derived from pluripotent stem cells, placental tissues, adult tissues, and somatic cell reprogramming. NCLSCs have a differentiation capability similar to NCSCs, and possess great potential for regenerative medicine applications. In this review, we present recent developments on the various approaches to derive NCLSCs and the therapeutic application of these cells for tissue regeneration.
Keywords: adult stem cells, disease modeling, neural crest stem cells, placental stem cells, regenerative medicine
Neural crest stem cells and neural crest‐like stem cells isolated and derived from various sources offer great potential for regenerative medicine, disease modeling, and drug discovery.
Significance statement.
Neural crest stem cells (NCSCs) are a transient population of cells that arise during early vertebrate development and harbor stem cell‐like properties. The multipotency of NCSCs enables the generation of a diverse population of cells, thereby making NCSCs a valuable cell source for tissue regeneration, disease modeling, and drug discovery. Although NCSC isolation was initially limited to embryonic tissues, neural crest‐like stem cells can be derived from pluripotent stem cells, placental tissues, adult tissues, and somatic cell reprogramming, providing viable cell sources for tissue engineering and regenerative medicine.
1. INTRODUCTION
Neural crest stem cells (NCSCs) are a transient, multipotent cell population that originates along the border of the neural plate during early vertebrate development. 1 , 2 Various signaling molecules, including Wnt, fibroblast growth factor (FGF), bone morphogenic protein (BMP), Notch and retinoic acid (RA), derived from the non‐neural ectoderm, neuroepithelium and underlying mesoderm, activate a cascade of transcription factors that dictate where these cells will form and further develop. 3 These cells undergo an epithelial‐to‐mesenchymal transition, which allows them to acquire a mesenchymal phenotype upon detaching from their neighboring cells and delaminating from the dorsal neuroepithelium. 4 Such an event induces these cells to migrate extensively within the developing embryo whereupon they experience various environmental cues. Accumulative evidence indicates that some cells lose plasticity and confer a fate during or even before migration, although a subpopulation of migratory cells appear to retain their multipotency. 5 , 6 , 7 , 8 After settling in their final sites of differentiation, these cells proceed to give rise to wide array of cell types and tissues, including bone, cartilage, melanocytes, fibroblasts, and smooth muscle cells (SMCs), in addition to neurons and glial cells. 2 , 3 , 4 As NCSCs are responsible for generating a diverse population of cells and tissues, dysregulation of neural crest (NC) development, cell migration, and differentiation can lead to a broad spectrum of human congenital disorders, including cardiovascular defects, melanoma, craniofacial defects, and neuroblastoma, collectively known as neurocristopathies. 9 , 10 Therefore, NCSCs can be used to generate a variety of specific cell types for disease modeling. Furthermore, the multipotency of NCSC differentiation makes NCSCs a valuable cell source for tissue regeneration.
In the past, NCSC isolation was limited to embryonic tissues. NCSCs generally express markers such as Sox10, Slug, Snail, Twist, AP‐2α, p75NTR, and HNK1. Sox10 is critical for neurogenesis and maintenance of multipotency, 11 , 12 , 13 whereas Sox17 functions in the development of definitive endoderm and vasculogenesis. 14 , 15 Sox9, 16 , 17 , 18 Slug, 19 , 20 Snail, 21 , 22 , 23 and Twist 24 , 25 are all found closely related to NC and neural tube development. AP‐2α is required for NC induction and expressed among actively migrating NCSCs. 26 , 27 , 28 p75NTR and HNK1 can be utilized to identify migratory NCSCs, albeit HNK1 only labels a small proportion of migrating human NCSCs. 28 , 29 Recently, the development of pluripotent stem cells (PSCs), especially induced pluripotent stem cells (iPSCs) has enabled the derivation of human neural crest‐like stem cells (NCLSCs), providing an opportunity to not only better understand human development, but also an unlimited cell source for patient‐specific disease modeling and therapy. In addition, significant progress has been made in identifying and isolating NCLSCs from fetal, perinatal, and adult tissues that can potentially serve as viable cell sources for tissue regeneration. In this review, we will present recent findings on available sources of NCLSCs, and discuss their potential application for tissue engineering and regenerative medicine (Figure 1).
FIGURE 1.
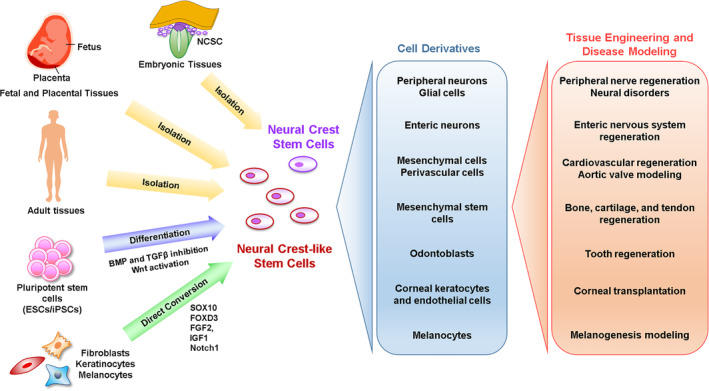
Sources and potential applications of neural crest stem cells (NCSCs) and neural crest‐like stem cells (NCLSCs). NCSCs and NCLSCs can be isolated from embryonic, fetal, placental, and adult tissues. NCLSCs can also be derived in vitro from pluripotent stem cells and mature cells through differentiation and reprogramming strategies, respectively. NCSCs and NCLSCs can be differentiated to yield distinct cell derivatives that are highly valuable for tissue engineering applications and disease modeling
Generally speaking, NCSCs are a cell population in NC‐derived tissues during embryonic development, whereas all other sources of NCSCs, including those derived from PSCs in vitro and those isolated from placental and adult tissues, should be defined as NCLSCs. However, previous studies have interchangeably utilized NCSC to describe NCLSC derived from PSCs and adult tissues, and some NCLSCs have been named differently based on their tissue origin. Furthermore, there is also an argument that some adult NCLSCs are remnants of NCSCs arising during the development. To clearly distinguish all of these NCSCs and NCLSCs, single cell RNA sequencing, in addition to lineage tracing, needs to be performed to classify the cells based on genetic profile, which has not been done in a vast majority of previous studies. Therefore, when presenting results from specific studies, we generally use NCSC or NCLSC as we define it, but sometimes utilize the term as defined by the authors of the study to accommodate their hypothesis and viewpoint and to avoid confusion and controversy.
1.1. Embryonic, fetal, and placental tissue‐derived NCLSCs for tissue regeneration
NCSCs have been identified and isolated from various embryonic and fetal tissues, 30 including the trunk neural tube, sciatic nerve, dorsal root ganglia (DRG), gut, heart, and branchial arches. These NCSCs can self‐renew and differentiate in vitro and in vivo into distinct NC derivatives, 30 such as neurons, glia, myofibroblasts, osteocytes, and SMCs. Recently, a new method to culture premigratory NCSCs as “crestospheres” was developed, which enabled the maintenance of these cells in vitro for an extended period of time without the loss of their stem‐cell characteristics. 31 , 32 Early studies using postmigratory NCSCs isolated from distinct regions within the embryo revealed that spatially distinct NCSCs displayed cell intrinsic differences that regulated their developmental potential in vivo, 33 , 34 suggesting that for tissue regeneration strategies, postmigratory NCSCs should be isolated from locations that match where these cells will be utilized therapeutically.
The boundary cap located at the dorsal root entry zone has also served as a source of embryonic NCSCs, 35 which have shown potential as a therapy for pancreatic and neurodegenerative disorders. Boundary cap NCSCs (bNCSCs) cotransplanted with mouse or human pancreatic islets were found to enhance beta cell proliferation and improve islet graft reinnervation and revascularization in diabetic mice, possibly restoring neural‐islet interactions that consequently improved islet engraftment and function after transplantation. 36 , 37 , 38 Furthermore, in an in vitro coculture system, bNCSCs partially prevented the cell death of human insulin producing cells in response to pro‐inflammatory cytokines, suggesting a potential mechanism by which bNCSCs offer a protective effect that improves islet transplantation and thus, in combination with pancreatic islets, may serve as attractive cell source to treat patients with type 1 diabetes. 39 Additionally, bNCSCs have demonstrated beneficial effects on nerve regeneration. Transplantation of bNCSCs to the site of a dorsal root avulsion injury resulted in cell migration and neuronal differentiation in the host spinal cord as well as differentiation into glia that associated with regenerating sensory axons in the peripheral nervous system. 40 , 41 These bNCSCs not only improved the survival of motor neuron precursors following transplantation into the spinal cord of adult mice, 42 but also prevented the loss of spinal cord neurons and glial activation in acutely injured spinal cord slice cultures through the secretion of brain‐derived neurotrophic factor (BDNF), 43 suggesting these cells exhibit neuroprotective, anti‐apoptotic, glia‐inhibitory, and neurotrophic effects that may aid in neuroregenerative therapies.
Extraembryonic placental tissues, including early or later gestation chorionic villus tissues, are a unique cell source, yielding robust placental mesenchymal stem/stromal cells (PMSCs) well‐suited for autologous or allogeneic cell therapy and tissue engineering. Interestingly, PMSCs express NCSC transcription factor markers including Sox9, Sox10, Sox17, Slug, Snail, and Twist, 44 , 45 suggesting PMSCs may be NC‐derived and a type of NCLSC. The uniformity of expression of these transcription factors in PMSC cultures may reflect the developmental origins of these cells and could serve as predictors of some related functional properties. PMSCs were found to also express stem cell‐related intracellular structural proteins Nestin, neurofilament medium, and S100β that are often associated with neural lineage phenotypes. Methods have been established to expand PMSCs from various placental tissues. 44 , 45 , 46 , 47 , 48 The in vitro characteristics of PMSCs are also analogous to those of MSCs isolated from various source tissues 49 in terms of surface marker expression and multipotency. 44 , 45 , 46 , 47 , 48
It has been shown that PMSCs display notable immunomodulatory capabilities, 50 , 51 exhibit wound healing capacity, 52 demonstrate neuroprotective effects, 45 , 53 , 54 , 55 , 56 and may exhibit greater immunomodulatory properties and ex vivo expansion potential compared with adult BM‐MSCs. 46 , 51 As quantified by ELISAs, PMSCs secreted significantly higher amounts of BDNF and hepatocyte growth factor (HGF) than adult BM‐MSCs. Both BDNF and HGF are growth‐promoting and chemoattractant for young embryonic cranial motor axons. 57 BDNF is a powerful neurotrophin for neuronal regeneration after injury. 58 HGF is also a potent immunoregulatory 59 and angiogenic factor that has been shown to activate endothelial cell migration and proliferation and may contribute to wound healing in vivo by promoting rapid vascularization. 60 Preclinical studies have shown that PMSCs secrete significant amounts of neuroprotective growth factors and cytokines in vitro, 44 , 45 , 56 and can protect neurons from damage in vivo, 45 , 61 , 62 , 63 suggesting that PMSCs are a potent therapy for developmental or perinatal neurological diseases. Specifically, PMSCs have been used to treat a neurodevelopmental disease, myelomeningocele (MMC), commonly known as spina bifida, which is caused by incomplete neural tube closure during development of the spinal cord. Our preliminary animal research in the rodent model 64 as well as in the well‐established fetal sheep model 45 , 61 , 62 , 63 of MMC has shown that in utero treatment with human PMSCs can functionally cure the paralysis associated with MMC in a dramatic and consistent manner. Treatment of MMC with PMSCs in conjunction with an extracellular matrix (ECM) scaffold as a delivery vehicle (PMSC‐ECM) drastically and significantly improved the locomotor function compared with control animals treated with delivery vehicle alone, and histological analysis demonstrated that PMSC‐ECM consistently and significantly increased neuron survival in the diseased spinal cord. 45 No adverse effects were observed in any of the lambs treated with human PMSC‐ECM. Currently, clinical grade PMSCs produced under current good manufacturing practice 65 are being evaluated in pivotal safety studies and IND‐enabling studies and moving toward a clinical trial for the treatment of MMC in human patients.
Enteric neural crest cell (ENCC) is another type of NCLSC that has been utilized for the treatment of enteric neuropathies. ENCCs have been isolated from embryonic and postnatal murine intestine 66 , 67 and more recently, from fetal and postnatal human gut. 68 , 69 , 70 , 71 In murine studies, ENCCs were capable of colonizing the gut upon migrating and differentiating into enteric neurons and glia. Furthermore, these cells formed neural networks and were able to functionally integrate within the host bowel in vivo without any long‐term safety issues. 72 , 73 A recent study investigated the functional viability of ENCCs derived from fetal human gut following in vivo transplantation into postnatal murine colon. 71 It was demonstrated that these cells displayed engraftment, differentiated into neurons and glia of the enteric nervous system (ENS), and moreover, established functional connectivity with the endogenous ENS. The successful engraftment of transplanted ENCCs provides support for the development and use of ENCC as a cell replacement therapy in enteric neuropathies. Apart from ENCCs, NCLSCs from postnatal DRG were also shown to survive, colonize the appropriate gut layers, and generate functional enteric neurons that could integrate with the endogenous ENS following transplantation into the distal colon of postnatal mice, 74 suggesting that NCLSCs from postnatal tissues besides the gut can undergo ENS differentiation and, therefore, may be another potential candidate for the replacement of ENS cells.
1.2. Adult tissue‐derived NCLSCs for tissue regeneration
As NCSCs proceed through embryonic development, their developmental potential becomes limited and there is a loss of multipotency, although we cannot completely exclude the possibility of NCSC remnants in postnatal tissue. To date, NCLSCs have been discovered in multiple adult tissues, including DRG, 75 skin, 76 , 77 gut, 66 heart, 78 , 79 carotid body, 80 nasal passageways 81 and cavity, 82 adipose tissue, 83 , 84 , 85 bone marrow (BM), 86 , 87 iris, 88 cornea, 89 oral mucosa, 90 palate, 91 , 92 dental pulp, 93 and periodontal ligament. 94 , 95 Adult tissue‐derived NCLSCs express NCSC markers such as p75NTR, Sox10, Sox9, and Snail1/2, and exhibit self‐renewal and multilineage differentiation into various cell types, 30 , 96 , 97 including neurons, glia, cardiomyocytes, adipocytes, smooth muscle, and chondrocytes, although self‐renewal capacity appears to decline with age and these cells demonstrate reduced differentiation potential compared with fetal NCSCs. 66 Despite this, NCLSCs from adult tissues possess stem cell‐like qualities, which make them great potential candidates for tissue regeneration. 97 Most of the studies carried out, thus far, using adult tissue‐derived NCLSCs have primarily focused on blood vessel, nerve, and bone regeneration.
Vascular stem cells (VSCs) play an important role in vascular remodeling and regeneration. 98 NCLSCs, as a type of VSCs, were found in the media and adventitia layers. 99 These Sox10+ NCLSCs not only differentiated into SMCs in the neointima, but also contributed to chondrogenic and osteogenic cell types in the atherosclerotic lesion, providing a novel perspective on the development of vascular diseases. NCLSCs were also identified in the perivascular cells around microvessels throughout the body. 100 , 101 Whether NCSLCs isolated from a variety of vascularized tissues are actually vascular NCLSCs remains to be investigated. When Sox10+ NCLSCs were injected into ischemic limb, these cells formed perivascular cells and promoted angiogenesis. 100
Peripheral nerve injuries (PNIs) are some of the most common types of traumatic lesions affecting the nervous system, which can result in reduced quality of life in affected patients and be a huge social burden. 102 PNI continues to be a major challenge in reconstructive neurosurgery. Owing to huge clinical demand, peripheral nerve regeneration, particularly larger gap injuries, has become a prime focus of basic and clinical research. Accelerating axonal regeneration to promote reinnervation and improve functional recovery after PNI is a clinical necessity and an experimental challenge. Numerous studies have demonstrated the potential utilization of adult tissue‐derived NCLSCs for peripheral nerve regeneration. 102 , 103 , 104 , 105 , 106 Vascular NCLSCs transplanted into nerve conduits enhanced sciatic nerve regeneration. 107 These cells differentiated into perineural cells around the bundles of regenerated myelinated axons, but did not differentiate into Schwann cells. In a recent study, Zhang et al examined the effects of cell cotransplantation on PNI using epidermal NCSC (EPI‐NCSC) and olfactory ensheathing cells (OEC) in a rat sciatic nerve defect model. 105 Their findings indicated that EPI‐NCSC and OEC cotransplantation promoted sciatic nerve regeneration and improved nerve function. Moreover, the mechanism of PNI improvement by EPI‐NCSC and OEC cotransplantation was likely due to an upregulation in the expression of BDNF and nerve growth factor (NGF). Similar findings were observed in the application of BM‐derived NC precursors (BM‐NCPs) for the repair of sciatic nerve defects in adult rats. These BM‐NCPs were capable of repairing nerve defects by promoting axonal regrowth and myelination and preventing muscle atrophy, thereby restoring motor and sensory neuron function, possibly through the secretion of various trophic factors that were distinct from those of BM‐MSCs. 106 In addition to trophic support, it has been suggested that NCLSCs may promote tissue repair through the modulation of the immune system, 104 , 108 suggesting these cells may alter pro‐ and anti‐inflammatory factors that could therefore improve tissue regeneration. The potential mechanisms by which transplanted NCLSCs regulate peripheral nerve repair require further elucidation. Additionally, the application of adult tissue‐derived NCLSCs transplanted alone or with other support cells in preclinical large animal studies will provide further insights into clinical outcomes and the potential of this new therapy for PNI.
Spinal cord injury (SCI) has many distinct factorial aspects including primary mechanical damage, reactive gliosis, secondary cell apoptosis, and the inability of axons to regenerate. 109 , 110 Axon regeneration does not occur due to the nonresponsive environment of the injured spinal cord. Emerging evidence suggests that NCLSCs derived from various adult tissues may serve a promising strategy for SCI. 111 , 112 , 113 Recently, human dental pulp (hDP)‐NCSCs were used to evaluate the effect of hDP‐NCSC delivery on the lesion site and functional recovery after SCI. 111 The data provided a theoretical and experimental basis for hDP‐NCSC transplantation for the treatment of SCI as it was shown that these cells could significant improve motor recovery following spinal cord trauma. Compared with other stem cells, hDP‐NCSCs offer several advantages such as good primitiveness, strong amplification ability, simple acquisition, and weak in vivo rejection, without any damage to the donor. Therefore, hDP‐NCSCs can provide a new cell source and therapy for SCI. 111 Apart from hDP‐NCSCs, transplanted EPI‐NCSCs were shown to not only provide neurotrophic support in an ex vivo SCI contusion model, 112 but also improve motor function after SCI in rats, in particular demonstrating beneficial synergistic effects when delivered in combination with the potent antioxidant Astaxanthin. 113 These findings suggest that further research into combinatorial strategies with EPI‐NCSCs and pharmaceutical agents may prove to be valuable for the treatment of SCI. Indeed, valproic acid has been proposed as a potential candidate as it can enhance the expression level of various trophic factors, such as BDNF and glial cell line‐derived neurotrophic factor (GDNF), and promote SCI recovery. 112 , 114 The purpose of neuroregenerative medicine is to replace, regenerate, or arrest the loss of cells and tissues due to neurodegenerative and neurological disorders. Fortino et al successfully induced periodontal ligament stem cells (PDLSCs) derived from the NC into neural‐like cells using a combination of basic FGF and epidermal growth factor. 115 The ease of sourcing and expansion, their embryologic NC origin, and the lack of ethical implications in their use make PDLSCs an attractive cell source for neuroregenerative medicine.
Previous studies have shown that adult tissue‐derived NCLSCs are multipotent and, thus, can differentiate into various NC derivatives, including bone cells. For instance, Ono et al used double transgenic (P0‐Cre/CAG‐CAT‐EGFP) mice to investigate the precise distribution and properties of neural crest‐derived stem cells (NCDCs) in adult oral tissues. They found that these NCDCs widely reside throughout different adult oral tissues, such as the buccal mucosa, gingiva, tongue, and palate. 116 In addition, NCDCs were found to proliferate and differentiate into osteoblastic cells in vitro. In another study, Wnt pathway activator lithium chloride (LiCl) was used to investigate whether it could promote odontoblast differentiation of hair follicle neural crest cells (hfNCCs). The results showed that LiCl activated canonical Wnt signaling and promoted the proliferation and odontogenic differentiation of hfNCCs, suggesting that hfNCCs may be a good candidate for tooth regeneration. 117 As adult tissue‐derived NCLSCs can undergo osteogenic differentiation in vitro, they are a promising cell source for bone regeneration. 118 , 119 NCLSCs isolated from excised human oral mucosa could generate spheres that were multipotent and capable of self‐renewal in vitro. 118 Subcutaneous implantation of composites of osteogenic‐induced NCLSCs and multiporous polylactic scaffolds into immunocompromised mice for 10 weeks revealed these cells were capable of generating ectopic bone tissue in vivo. Similarly, human palate‐derived NCLSCs incorporated into an allogen bone substitute were found to contribute to the formation of new bone tissue during peri‐implant bone repair and induced extended peri‐implant bone remodeling with good biocompability, 119 indicating these stem cell‐supported allogen bone scaffolds may be beneficial for bone regeneration, although the long‐term effects of these implants has yet to be fully evaluated. A recent study demonstrated that adult manidular skeletal stem cells activated an embryonic NC cell‐like gene regulatory program that is dependent on the focal adhesion kinase pathway during distraction osteogenesis, 120 enabling these cells to acquire a more plastic, developmental state that aids in jaw bone regeneration. In addition, alveolar bone‐derived MSCs 121 display high osteogenic potential and promote ectopic bone formation in vivo, 122 , 123 , 124 , 125 providing a more accessible MSC source compared with the iliac crest.
Dental pulp stem cells (DPSCs) are also a source of NCLSCs that have multilineage differentiation potential, immunomodulatory properties, and high regenerative capability. 126 , 127 As a result, DPSCS can be utilized to treat a broad spectrum of disorders, 126 , 127 including PNI, 128 , 129 retinal injury, diabetes, cerebral ischemia, myocardial infarction, muscular dystrophy, and neurological diseases. 128 , 130 , 131 Indeed, in several case studies and preliminary clinical trials, the transplantation of DPSCs has proven to be a safe and effective therapeutic strategy, 127 indicating that DPSCs are a promising cell source for tissue regeneration and the treatment of various diseases.
1.3. PSC‐derived NCLSCs for tissue regeneration
PSCs, including embryonic stem cells (ESCs) and iPSCs, offer the advantage that they can proliferate extensively and give rise to cells from all three germ layers, thus making them powerful and instrumental for regenerative cell therapy, disease modeling, and drug discovery. 132 , 133 , 134 , 135 Utilizing existing knowledge of NCSC specification during development, researchers have created protocols to generate NCLSCs from mouse and human PSCs in vitro. 30 Although initial protocols relied on deriving NCLSCs through a neural progenitor cell population or coculture with stromal cells, direct and specific NCSC induction protocols have been developed that are based on using small molecule activators of Wnt signaling and inhibitors of BMP and Activin/Nodal signaling. 136 , 137 , 138 This direct induction approach was further modified and refined into a completely defined system utilizing inhibition of transforming growth factor beta and glycogen synthase kinase 3 to generate NCLSCs, thereby improving the potential translation of these cells for clinical application. 139 Moreover, inclusion of additional factors, such as BMP‐4, RA, and FGF‐2, and modulation of Wnt signal 140 has enabled researchers to induce NCLSCs with distinct regional identity (eg, cranial, 141 trunk, 142 and vagal 138 , 143 , 144 NCLSCs), and as a result provides an approach to not only study regional identity in vitro, but also the possibility to generate NC derivatives that may closely resemble the in vivo counterparts and be utilized for tissue regeneration. As these NCLSCs can be differentiated in vitro into various cell types, 30 including peripheral neurons, glial cells, chondrocytes, osteocytes, myofibroblasts, melanocytes, SMCs, and adipocytes, this had led researchers to investigate the in vivo regenerative potential of NCLSCs derived from mouse and human PSCs.
As NCSCs give rise to peripheral nervous tissue in humans, it comes as no surprise that human ESC/iPSC‐derived NCLSCs can be differentiated into peripheral neurons and Schwann cells 30 and as such, have shown great promise for the treatment of PNI. Results from several studies where these derived NCLSCs were suspended in different types of hydrogels and transplanted in conjunction with biomaterials, such as nanofibrous tubular scaffolds and polymeric tubular conduits, to generate tissue‐engineered nerve conduits for peripheral nerve repair have been encouraging. 145 , 146 , 147 , 148 In rat and mice sciatic nerve injury models, grafted NCLSCs were not only able to survive, but also promoted axonal regrowth and myelination, 145 , 146 , 147 enhanced angiogenesis, 147 and secreted neurotrophic factors 146 , 148 (eg, BDNF and NGF), thereby providing trophic support to stimulate peripheral nerve regeneration. More importantly, NCLSCs accelerated functional recovery as assessed through electrophysiological and behavioral analysis. 146 , 147 Interestingly, the application of physical stimulation, such as low‐intensity pulsed ultrasound 149 , 150 and electrical stimulation, 151 after NCLSC transplantation, can also further improve nerve regeneration and functional recovery following PNI. Apart from PNI, these PSC‐derived NCLSCs can also be utilized to treat spinal cord damage in patients with spina bifida. 152 Findings from these studies suggest that PSC‐derived NCLSCs are a potent therapy for neural regeneration and repair.
Cumulative evidence has also shown that ENCCs and enteric‐like neurons can be generated from ESC/iPSC‐derived NCLSCs and, thus, potentially serve as a therapeutic cell source for ENS disorders and regeneration. 143 , 144 , 153 , 154 , 155 Grafted ENCC precursors were able to repopulate the adult mouse colon and capable of targeted migration into the gut region. 143 In addition, these ENCCs rescued disease‐related mortality in an Ednrbs‐1/s‐1 Hirschsprung (HSCR) mice model, 143 demonstrating these cells were functional in vivo. Moreover, iPSC‐derived NCLSCs transplanted into the hindgut of severe combined immunodeficiency (SCID) mice were not only capable of migrating towards myenteric and submucosal regions, but also differentiated into glial cells and mature enteric neurons in vivo. 155 Similar findings were observed in human intestinal organoids after the inclusion of human PSC‐derived NCLSCs, 144 , 154 enabling the development of human tissue‐engineered intestines that are potentially useful for the treatment of enteric neuropathies and the study of human gastrointestinal tract mobility disorders.
PSC‐derived NCLSCs have also been differentiated into various cell types, including MSCs, osteocytes, and chondrocytes, that are beneficial for bone, cartilage, and tendon regeneration. 30 , 156 , 157 , 158 , 159 , 160 Xu et al examined whether iPSC‐derived NCLSCs could repair a rat patellar tendon window defect. Transplantation of these NCLSCs in a fibrin gel promoted the host endogenous repair process, resulting in a significant improvement in tendon healing and repair. 156 Moreover, MSC‐like cells generated from iPSC‐derived NCLSCs have been utilized to repair rat femoral osteochondral defects and regenerate mouse craniofacial bone in vivo, respectively. In comparison to human BM‐MSCs, tissue engineered constructs of MSCs from iPSC‐derived NCLSCs did not undergo chondrogenesis in vivo nor did they effectively repair osteochondral defects. 158 On the other hand, mouse iPSC‐NCLSC‐MSCs transplanted into calvarial defects differentiated into osteoblasts, produced no tumors, and contributed to bone regeneration. 159 These studies highlight how these derived cells may behave differently in vivo. However, a direct comparison cannot be made as the cells used in these studies were from different species and distinct defect models were implemented. Further investigation of craniofacial bone repair using MSCs from human iPSC‐derived NCLSCs would reveal whether these cells could be a potential cell source for clinical application.
Melanocytes, which produce melanin and play a critical role in skin homeostasis and protection, have also been derived from ESCs and iPSCs after proceeding through a NC stage. 138 , 161 , 162 , 163 Notably, human ESC‐derived melanocytes were able to localize to the appropriate layer after being introduced into a human skin xenograft model. Engraftment of these skin reconstructs into SCID mice revealed that these cells survived and were functional for over 4 weeks. 161 Furthermore, these melanocytes were capable of producing melanin and functionally integrated into a reconstituted pluristratified epidermis in vitro. 162 Altogether, this in vitro system provides an opportunity to study melanocyte developmental biology and may serve as a potential cellular therapy for hypopigmentation disorders.
Corneal scarring or blindness resulting from damage to the corneal stroma or corneal endothelial (CE) dysfunction is currently being treated with surgical corneal transplantation. 164 However, limitations, such as graft failure and shortage of donor cornea, still exist, prompting researchers to search for more suitable alternatives, such as an NCLSC‐based cell therapy. Using a two‐step induction process, several studies have derived corneal endothelial cells (CECs) 165 , 166 , 167 and corneal keratocytes 168 , 169 from ESCs and iPSCs after first generating an NCLSC population. Transcriptomic analysis of CEC‐like cells from human ESC‐derived NCLSCs using RNA sequencing has revealed that the transcriptome of these cells closely resembles that of adult CECs. 167 Yet, whether these cells would integrate and aid in tissue regeneration in vivo remains unknown. Preliminary studies using CECs generated from human ESCs that proceeded through a periocular mesenchymal phase, rather than an NCSC stage, have shown promising results in improving CE dysfunction in rabbit models, 170 suggesting the possibility that keratocytes and CECs generated from ESC/iPSC‐derived NCLSCs may yield similar beneficial effects. Although further investigation into the functionality of these cells in vivo is still required, these NCLSC‐derived corneal cells may serve a promising alternative for corneal repair.
Mouse iPSC‐derived NCLSCs can also undergo differentiation into odontoblast‐like cells upon cotransfection of Pax9 and BMP4 expression plasmids. 171 Interestingly, transplantation of control and transfected NCLSCs did not give rise to teratomas after they were subcutaneously injected into mice, suggesting these cells were not tumorigenic and, thus, a safe cell source for tooth regeneration. Another study also showed that mouse iPSC‐derived NCLSCs could differentiate into odontogenic mesenchymal cells. 172 Culturing these NCLSCs with conditioned medium from mouse dental epithelium further promoted their differentiation into odontoblasts. However, whether PSC‐derived NCLSCs can be differentiated into other dental cell types (eg, dental pulp and dental follicle cells) and aid in dental tissue regeneration has yet to be determined.
In addition to tissue regeneration, numerous studies have demonstrated the potential of using iPSCs from patients affected by neurocristopathies for modeling human disease in vitro. Various diseases have been studied, thus far, including CHARGE syndrome, 173 , 174 Ewing sarcomas, 175 familial dysautonomia, 176 , 177 pigmentation disorders (eg, Hermansky‐Pudlak and Chediak‐Higashi syndromes), 138 HSCR disease, 143 , 178 Bardet‐Biedl syndrome, 179 Treacher Collins syndrome, 180 and cardiovascular malformations, such as bicuspid aortic valves. 181 Findings from these studies have not only provided demonstration of disease‐related phenotypes, but also evidence on the origin of certain neurocristopathies, which appear to arise from defects in NCSCs, rather than MSCs. 174 , 175 , 181 Additionally, this technology has served as a platform to identify potential drug candidates that are capable of restoring impaired function and possibly serve as therapeutic agents. 143 , 176 , 182
Apart from deriving NCLSCs from PSCs through directed differentiation, it has also been shown the NCLSCs can be generated using direct reprogramming, a process that bypasses the iPSC stage during the conversion of somatic cells into distantly related cell types. 183 , 184 In contrast to directed differentiation, which can take up to several weeks, 137 reprogramming can yield NCLSCs in 10 to 14 days. 177 , 185 Therefore, this approach provides a rapid method of obtaining NCLSCs that can be potentially administered clinically. To date, NCLSCs have been derived from human and mouse fibroblasts, 177 , 186 , 187 , 188 keratinocytes, 189 , 190 and melanocytes 191 via the introduction of transcription factors, such as SOX10 and FOXD3, specific growth factors, or forced expression of Notch1 signaling, respectively. It has been reported that the derived NCLSCs are functional in vivo when they were investigated for neural repair in a zebrafish model 186 and migration capability in a chick embryo model system 177 , 191 ; however, more detailed and comprehensive analysis of in vivo functional outcomes is still necessary. Furthermore, this reprogramming approach has been applied to generate NCLSCs from fibroblasts isolated from patients with familial autonomic dystrophy, enabling the production of patient‐specific cells, which holds great promise for personalized medicine and disease modeling. 177 Further elucidation on whether reprogrammed‐derived NCLSCs can be used for the various aforementioned tissue regeneration applications will greatly expand their therapeutic utility and clinical applicability.
2. CONCLUSIONS AND FUTURE DIRECTIONS
Current progress in the derivation and therapeutic application of NCLSCs suggests that these cells have great potential for regenerative cell therapy, disease modeling, and drug discovery. NCLSCs isolated from various fetal and adult tissues have demonstrated beneficial effects in several tissue engineering paradigms. However, acquisition and isolation of cells from fetal tissue are still somewhat controversial and adult NCLSCs may display limited multipotentiality. 30 As such, PSCs may serve as a promising alternative for the derivation of NCLSCs. Although ESC‐based cell therapies are currently undergoing clinical trials, 134 , 135 the discovery of iPSCs has generated new excitement in the field of personalized regenerative medicine as these cells lack the ethical controversy associated with ESCs and are easier to obtain from the primary source of tissue. The source of iPSCs is an important factor to consider when generating NCLSCs as it has been shown that NCLSCs derived from iPSCs generated from NC tissue, such as periodontal ligament, are more equivalent to their in vivo counterparts compared with fibroblast‐derived iPSC‐NCLSCs, 192 suggesting these cells may have improved therapeutic efficacy. Nonetheless, several challenges still exist and need to be overcome before there is effective clinical translation of iPSC‐based cell therapies. 193
Although NCLSC therapy shows great promise for tissue regeneration, the safety and potential risks associated with these cells, such as tumorigenicity, need to be overcome before these cells can serve as a viable therapeutic option. In several studies where the therapeutic effects of human PSC‐derived NCLSCs were investigated in animal models, no tumors were present, even up to 1 year after transplantation. 145 , 158 , 171 However, transplantation of a specific subset of NCLSCs derived from PSCs resulted in tumors and unwanted grafts. 194 Thus, the differentiation stage of the transplanted cells is an important factor to be considered. In addition to the formation of teratomas or tumors of NC origin, including neuroblastoma and melanomas, the immunogenic response of transplanted NCLSCs is an important parameter that should also be considered and closely monitored. A recent study has shown that iPSC‐derived NCLSCs exhibit a nonimmunogenic phenotype, rather than an immunosuppressive one, as demonstrated by low levels of immune‐related antigens in a noninflammatory environment and no induction of T‐cell proliferation or pro‐inflammatory cytokine production. 195 In contrast, another study proposed that PSC‐derived NCLSCs exhibited immunosuppressive properties. 196 More work on the immunogenic properties of these cells will aid to provide more clarity on the issue. Overall, careful consideration of these concerns will ensure these cells are clinically safe and effective upon transplantation.
To facilitate the therapeutic applications of NCLSCs in the clinical setting, current NCLSC isolation, expansion, and differentiation protocols may require further optimization to consider good manufacturing practices. 197 Furthermore, during the development of favorable scale‐up procedures to achieve large numbers for cell transplantation, certain aspects, such as passage number, should be taken into account as increased cell passaging can potentially diminish the therapeutic effects of these cells 198 and result in chromosomal defects that may lead to tumorigenesis. 199 In stem cell‐based therapies, often times materials are used in combination with stem cells. 200 , 201 These materials can provide a scaffold that not only promotes cell survivability and the regeneration process, but also greatly influences cell fate and function in vivo. Future developments of next‐generation biomaterials that are biocompatible and able to further improve NCSC expansion and differentiation in vitro and in vivo will be highly desirable for regenerative therapies.
Although NCSC and NCLSC appear to share common characteristics, such as multipotency, NC marker expression (eg, Sox10, p75NTR, AP‐2, and Nestin) and pluripotent gene expression, and a molecular signature representative of EPI‐NCSC and embryonic NCSC has been defined, 202 there also are some apparent differences. For instance, long SAGE‐transcriptome profiling revealed that human NCSCs exhibited a unique NC molecular signature, expressed pluripotent genes (Nanog, POU5F1. and Sox2), and were found to have global molecular profile similar to ESCs. 203 On the other hand, mouse epidermal NCLSCs only partially share the gene expression pattern of PSCs in that they express Myc, Klf4, and Sox2 but significantly lower levels of Nanog and Oct‐4 when compared with mouse ESCs, 204 an attribute that can potentially reduce their tumorigenicity potential. A recent study performed transcriptome profiling at different time points during the induction of cranial neural crest cells (cNCCs) from mouse iPSCs and found that cNCCs exhibited gene expression profiles that were only partially similar to those previously reported. 205 Interestingly, they observed that these cNCCs did not express certain NC specifier genes (eg, FoxD3, Gbx2, Msx1, Dlx3, Zic2, and Zic3) during the derivation process and other markers, such as Sox10, were only expressed at day 14, indicating that cNCCs take longer to acquire a migratory phenotype in vitro in comparison to mouse embryos in vivo. 205 These findings suggest that the molecular network that governs gene expression during iPSC‐derived cNCC induction may potentially differ to the in vivo NCSC gene regulatory network. Moreover, although there is substantial overlap among human, mouse, and avian NCSC transcriptomes, there exists a specific subset of genes that are only expressed by human cells, 203 highlighting the importance of human NCLSCs derivation and their translational potential for regenerative medicine. Further genome and epigenome profiling of NCSCs and NCLSCs from various sources will reveal new molecular insights into the unique attributes of each cell type and the similarities they share.
Advancements in high‐throughput omics technologies and drug screening platforms will provide new mechanistic insights into the signaling pathways and gene expression profiles of NCLSCs during development 206 , 207 , 208 and tissue repair, and furthermore, facilitate the development of therapeutics that can be used to treat patients with neurocristopathies. Moreover, iPSC technology in conjunction with gene‐editing platforms, such as CRISPR‐Cas9, 209 , 210 , 211 will aid to broaden our understanding of disease pathogenesis and provide an approach to correct genetic mutations in patient‐derived NCLSCs, thereby enabling the therapeutic utilization of these cells upon restoring normal cell function. Additionally, the systemic delivery of gene editing components provides an opportunity to modulate disease‐causing alleles in vivo without the need for cell isolation. 210 , 211 Taken together, NCLSCs, which can be isolated and derived from multiple sources, are a promising cell source for regenerative medicine.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
J.S., X.D., A.W.: wrote and edited the manuscript; S.L.: conceived overall review content and edited the manuscript.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the National Institute of Health (HL121450 and R56DE029157 to Song Li), the UCLA Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research Innovation Award (to Song Li), and the National Natural Science Foundation of China (32000968 to Xili Ding).
Soto J, Ding X, Wang A, Li S. Neural crest‐like stem cells for tissue regeneration. STEM CELLS Transl Med. 2021;10:681–693. 10.1002/sctm.20-0361
Funding information National Natural Science Foundation of China, Grant/Award Number: 32000968; UCLA Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research Innovation Award; National Institutes of Health, Grant/Award Numbers: R56DE029157, HL121450
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Achilleos A, Trainor PA. Neural crest stem cells: discovery, properties and potential for therapy. Cell Res. 2012;22:288‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martik ML, Bronner ME. Regulatory logic underlying diversification of the neural crest. Trends Genet. 2017;33:715‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mayor R, Theveneau E. The neural crest. Dev. 2012;140:2247‐2251. [DOI] [PubMed] [Google Scholar]
- 4. Szabó A, Mayor R. Mechanisms of neural crest migration. Annu Rev Genet. 2018;52:43‐63. [DOI] [PubMed] [Google Scholar]
- 5. Ito K, Sieber‐Blum M. In vitro clonal analysis of quail cardiac neural crest development. Dev Biol. 1991;148:95‐106. [DOI] [PubMed] [Google Scholar]
- 6. Henion PD, Weston JA. Timing and pattern of cell fate restrictions in the neural crest lineage. Development. 1997;124:4351‐4359. [DOI] [PubMed] [Google Scholar]
- 7. Krispin S, Nitzan E, Kassem Y, Kalcheim C. Evidence for a dynamic spatiotemporal fate map and early fate restrictions of premigratory avian neural crest. Development. 2010;137:585‐595. [DOI] [PubMed] [Google Scholar]
- 8. Soldatov R, Kaucka M, Kastriti ME, et al. Spatiotemporal structure of cell fate decisions in murine neural crest. Science. 2019;364:eaas9536. [DOI] [PubMed] [Google Scholar]
- 9. Vega‐Lopez GA, Cerrizuela S, Tribulo C, Aybar MJ. Neurocristopathies: new insights 150 years after the neural crest discovery. Dev Biol. 2018;444:S110‐S143. [DOI] [PubMed] [Google Scholar]
- 10. Ahsan K, Treffy RW, Nacke LM, Green‐Saxena A, Rocha M. Neural crest and cancer: divergent travelers on similar paths. Mech Dev. 2017;148:89‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Britsch S, Goerich DE, Riethmacher D, et al. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001;15:66‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kelsh RN. Sorting outSox10 functions in neural crest development. Bioessays. 2006;28:788‐798. [DOI] [PubMed] [Google Scholar]
- 13. Kim J, Lo L, Dormand E, Anderson DJ. SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron. 2003;38:17‐31. [DOI] [PubMed] [Google Scholar]
- 14. Lee S‐H, Lee S, Yang H, et al. Notch pathway targets proangiogenic regulator Sox17 to restrict angiogenesis. Circ Res. 2014;115:215‐226. [DOI] [PubMed] [Google Scholar]
- 15. Tam PPL, Kanai‐Azuma M, Kanai Y. Early endoderm development in vertebrates: lineage differentiation and morphogenetic function. Curr Opin Genet Dev. 2003;13:393‐400. [DOI] [PubMed] [Google Scholar]
- 16. Cheung M, Briscoe J. Neural crest development is regulated by the transcription factor Sox9. Development. 2003;130:5681‐5693. [DOI] [PubMed] [Google Scholar]
- 17. Mori‐Akiyama Y, Akiyama H, Rowitch DH, De Crombrugghe B. Sox9 is required for determination of the chondrogenic cell lineage in the cranial neural crest. Proc Natl Acad Sci USA. 2003;100:9360‐9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sahar DE, Longaker MT, Quarto N. Sox9 neural crest determinant gene controls patterning and closure of the posterior frontal cranial suture. Dev Biol. 2005;280:344‐361. [DOI] [PubMed] [Google Scholar]
- 19. Ros MA, Sefton M, Nieto MA. Slug, a zinc finger gene previously implicated in the early patterning of the mesoderm and the neural crest, is also involved in chick limb development. Development. 1997;124:1821‐1829. [DOI] [PubMed] [Google Scholar]
- 20. Vernon AE, LaBonne C. Slug stability is dynamically regulated during neural crest development by the F‐box protein Ppa. Development. 2006;133:3359‐3370. [DOI] [PubMed] [Google Scholar]
- 21. Aybar MJ, Nieto MA, Mayor R. Snail precedes slug in the genetic cascade required for the specification and migration of the Xenopus neural crest. Development. 2003;130:483‐494. [DOI] [PubMed] [Google Scholar]
- 22. Labonne C, Bronner‐Fraser M. Snail‐related transcriptional repressors are required in Xenopus for both the induction of the neural crest and its subsequent migration. Dev Biol. 2000;221:195‐205. [DOI] [PubMed] [Google Scholar]
- 23. York JR, Zehnder K, Yuan T, Lakiza O, McCauley DW. Evolution of snail‐mediated regulation of neural crest and placodes from an ancient role in bilaterian neurogenesis. Dev Biol. 2019;453:180‐190. [DOI] [PubMed] [Google Scholar]
- 24. Ishii M, Merrill AE, Chan YS, et al. Msx2 and twist cooperatively control the development of the neural crest‐derived skeletogenic mesenchyme of the murine skull vault. Development. 2003;130:6131‐6142. [DOI] [PubMed] [Google Scholar]
- 25. Soo K, O'Rourke MP, Khoo PL, et al. Twist function is required for the morphogenesis of the cephalic neural tube and the differentiation of the cranial neural crest cells in the mouse embryo. Dev Biol. 2002;247:251‐270. [DOI] [PubMed] [Google Scholar]
- 26. Luo T, Lee YH, Saint‐Jeannet JP, Sargent TD. Induction of neural crest in Xenopus by transcription factor AP2α. Proc Natl Acad Sci USA. 2003;100:532‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li W, Cornell RA. Redundant activities of Tfap2a and Tfap2c are required for neural crest induction and development of other non‐neural ectoderm derivatives in zebrafish embryos. Dev Biol. 2007;304:338‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Betters E, Liu Y, Kjaeldgaard A, Sundström E, García‐Castro MI. Analysis of early human neural crest development. Dev Biol. 2010;344:578‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tucker GC, Aoyama H, Lipinski M, Tursz T, Thiery JP. Identical reactivity of monoclonal antibodies HNK‐1 and NC‐1: conservation in vertebrates on cells derived from the neural primordium and on some leukocytes. Cell Differ. 1984;14:223‐230. [DOI] [PubMed] [Google Scholar]
- 30. Liu JA, Cheung M. Neural crest stem cells and their potential therapeutic applications. Dev Biol. 2016;419:199‐216. [DOI] [PubMed] [Google Scholar]
- 31. Kerosuo L, Nie S, Bajpai R, Bronner ME. Crestospheres: long‐term maintenance of multipotent, premigratory neural crest stem cells. Stem Cell Rep. 2015;5:499‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mohlin S, Kunttas E, Persson CU, et al. Maintaining multipotent trunk neural crest stem cells as self‐renewing crestospheres. Dev Biol. 2019;447:137‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bixby S, Kruger GM, Mosher JT, Joseph NM, Morrison SJ. Cell‐intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron. 2002;35:643‐656. [DOI] [PubMed] [Google Scholar]
- 34. Mosher JT, Yeager KJ, Kruger GM, et al. Intrinsic differences among spatially distinct neural crest stem cells in terms of migratory properties, fate determination, and ability to colonize the enteric nervous system. Dev Biol. 2007;303:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hjerling‐Leffler J, Marmigère F, Heglind M, et al. The boundary cap: a source of neural crest stem cells that generate multipe sensory neuron subtypes. Development. 2005;132:2623‐2632. [DOI] [PubMed] [Google Scholar]
- 36. Olerud J, Kanaykina N, Vasilovska S, et al. Neural crest stem cells increase beta cell proliferation and improve islet function in co‐transplanted murine pancreatic islets. Diabetologia. 2009;52:2594‐2601. [DOI] [PubMed] [Google Scholar]
- 37. Lau J, Vasylovska S, Kozlova EN, Carlsson P‐O. Surface coating of pancreatic islets with neural crest stem cells improves engraftment and function after intraportal transplantation. Cell Transplant. 2015;24:2263‐2272. [DOI] [PubMed] [Google Scholar]
- 38. Grapensparr L, Vasylovska S, Li Z, et al. Co‐transplantation of human pancreatic islets with post‐migratory neural crest stem cells increases β‐cell proliferation and vascular and neural regrowth. J Clin Endocrinol Metab. 2015;100:E583‐E590. [DOI] [PubMed] [Google Scholar]
- 39. Ngamjariyawat A, Vasylovska S. Co‐culture of insulin producing human Endoc‐Βh1 cells with boundary cap neural crest stem cells protects partially against cytokine‐induced cell death. J Stem Cell Res Ther. 2016;6(6). 10.4172/2157-7633.1000343. [DOI] [Google Scholar]
- 40. Trolle C, Konig N, Abrahamsson N, Vasylovska S, Kozlova EN. Boundary cap neural crest stem cells homotopically implanted to the injured dorsal root transitional zone give rise to different types of neurons and glia in adult rodents. BMC Neurosci. 2014;15:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Konig N, Trolle C, Kapuralin K, et al. Murine neural crest stem cells and embryonic stem cell‐derived neuron precursors survive and differentiate after transplantation in a model of dorsal root avulsion. J Tissue Eng Regen Med. 2017;11:129‐137. [DOI] [PubMed] [Google Scholar]
- 42. Aggarwal T, Hoeber J, Ivert P, Vasylovska S, Kozlova EN. Boundary cap neural crest stem cells promote survival of mutant SOD1 motor neurons. Neurotherapeutics. 2017;14:773‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schizas N, König N, Andersson B, et al. Neural crest stem cells protect spinal cord neurons from excitotoxic damage and inhibit glial activation by secretion of brain‐derived neurotrophic factor. Cell Tissue Res. 2018;372:493‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lankford L. Early gestation chorionic villi‐derived stromal cells for fetal tissue engineering. World J Stem Cells. 2015;7:195‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang A, Brown EG, Lankford L, et al. Placental mesenchymal stromal cells rescue ambulation in ovine myelomeningocele. Stem Cells Translational Medicine. 2015;4:659‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Poloni A, Rosini V, Mondini E, et al. Characterization and expansion of mesenchymal progenitor cells from first‐trimester chorionic villi of human placenta. Cytotherapy. 2008;10:690‐697. [DOI] [PubMed] [Google Scholar]
- 47. Abumaree MH, al Jumah MA, Kalionis B, et al. Phenotypic and functional characterization of mesenchymal stem cells from chorionic villi of human term placenta. Stem Cell Rev Rep. 2013;9:16‐31. [DOI] [PubMed] [Google Scholar]
- 48. Igura K, Zhang X, Takahashi K, Mitsuru A, Yamaguchi S, Takahashi TA. Isolation and characterization of mesenchymal progenitor cells from chorionic villi of human placenta. Cytotherapy. 2004;6:543‐553. [DOI] [PubMed] [Google Scholar]
- 49. Dominici M, le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315‐317. [DOI] [PubMed] [Google Scholar]
- 50. Vellasamy S. Isolation and characterisation of mesenchymal stem cells derived from human placenta tissue. World J Stem Cells. 2012;4:53‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lee JM, Jung J, Lee HJ, et al. Comparison of immunomodulatory effects of placenta mesenchymal stem cells with bone marrow and adipose mesenchymal stem cells. Int Immunopharmacol. 2012;13:219‐224. [DOI] [PubMed] [Google Scholar]
- 52. Jones GN, Moschidou D, Puga‐Iglesias TI, et al. Ontological differences in first compared to third trimester human fetal placental chorionic stem cells. PLoS One. 2012;7:e43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Calzarossa C, Bossolasco P, Besana A, et al. Neurorescue effects and stem properties of chorionic villi and amniotic progenitor cells. Neuroscience. 2013;234:158‐172. [DOI] [PubMed] [Google Scholar]
- 54. Hsieh JY, Wang HW, Chang SJ, et al. Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PLoS One. 2013;8:e72604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yust‐Katz S, Fisher‐Shoval Y, Barhum Y, et al. Placental mesenchymal stromal cells induced into neurotrophic factor‐producing cells protect neuronal cells from hypoxia and oxidative stress. Cytotherapy. 2012;14:45‐55. [DOI] [PubMed] [Google Scholar]
- 56. Kumar P, Becker JC, Gao K, et al. Neuroprotective effect of placenta‐derived mesenchymal stromal cells: role of exosomes. FASEB J. 2019;33:5836‐5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Naeem A, Abbas L, Guthrie S. Comparison of the effects of HGF, BDNF, CT‐1, CNTF, and the branchial arches on the growth of embryonic cranial motor neurons. J Neurobiol. 2002;51:101‐114. [DOI] [PubMed] [Google Scholar]
- 58. Lu B, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb Exp Pharmacol. 2015;220:223‐250. [DOI] [PubMed] [Google Scholar]
- 59. Najar M, Raicevic G, Kazan HF, et al. Immune‐related antigens, surface molecules and regulatory factors in human‐derived Mesenchymal stromal cells: the expression and impact of inflammatory priming. Stem Cell Rev Rep. 2012;8:1188‐1198. [DOI] [PubMed] [Google Scholar]
- 60. Bussolino F, di Renzo MF, Ziche M, et al. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kabagambe S, Keller B, Becker J, et al. Placental mesenchymal stromal cells seeded on clinical grade extracellular matrix improve ambulation in ovine myelomeningocele. J Pediatr Surg. 2018;53:178‐182. [DOI] [PubMed] [Google Scholar]
- 62. Galganski LA, Kumar P, Vanover MA, et al. In utero treatment of myelomeningocele with placental mesenchymal stromal cells—selection of an optimal cell line in preparation for clinical trials. J Pediatr Surg. 2019;55:1941‐1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vanover M, Pivetti C, Lankford L, et al. High density placental mesenchymal stromal cells provide neuronal preservation and improve motor function following in utero treatment of ovine myelomeningocele. J Pediatr Surg. 2019;54:75‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chen YJ, Chung K, Pivetti C, et al. Fetal surgical repair with placenta‐derived mesenchymal stromal cell engineered patch in a rodent model of myelomeningocele. J Pediatr Surg. 2018;53:183‐188. [DOI] [PubMed] [Google Scholar]
- 65. Lankford L, Chen YJ, Saenz Z, et al. Manufacture and preparation of human placenta‐derived mesenchymal stromal cells for local tissue delivery. Cytotherapy. 2017;19:680‐688. [DOI] [PubMed] [Google Scholar]
- 66. Kruger GM, Mosher JT, Bixby S, Joseph N, Iwashita T, Morrison SJ. Neural crest stem cells persist in the adult gut but undergo changes in self‐renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35:657‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bondurand N, Natarajan D, Thapar N, Atkins C, Pachnis V. Neuron and glia generating progenitors of the mammalian enteric nervous system isolated from foetal and postnatal gut cultures. Development. 2003;130:6387‐6400. [DOI] [PubMed] [Google Scholar]
- 68. Almond S, Lindley RM, Kenny SE, Connell MG, Edgar DH. Characterisation and transplantation of enteric nervous system progenitor cells. Gut. 2007;56:489‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Metzger M, Caldwell C, Barlow AJ, Burns AJ, Thapar N. Enteric nervous system stem cells derived from human gut mucosa for the treatment of Aganglionic gut disorders. Gastroenterology. 2009;136:2214‐2225.e3. [DOI] [PubMed] [Google Scholar]
- 70. Binder E, Natarajan D, Cooper J, et al. Enteric neurospheres are not specific to neural crest cultures: implications for neural stem cell therapies. PLoS One. 2015;10:e0119467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cooper JE, Natarajan D, McCann CJ, et al. In vivo transplantation of fetal human gut‐derived enteric neural crest cells. Neurogastroenterol Motil. 2017;29:e12900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hotta R, Stamp LA, Foong JPP, et al. Transplanted progenitors generate functional enteric neurons in the postnatal colon. J Clin Invest. 2013;123:1182‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cooper JE, McCann CJ, Natarajan D, et al. In vivo transplantation of enteric neural crest cells into mouse gut; engraftment, functional integration and long‐term safety. PLoS One. 2016;11:e0147989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hu H, Ding Y, Mu W, et al. DRG‐derived neural progenitors differentiate into functional enteric neurons following transplantation in the postnatal colon. Cell Transplant. 2019;28:157‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Li H‐Y, Say EHM, Zhou X‐F. Isolation and characterization of neural crest progenitors from adult dorsal root ganglia. Stem Cells. 2007;25:2053‐2065. [DOI] [PubMed] [Google Scholar]
- 76. Toma JG, Akhavan M, Fernandes KJL, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778‐784. [DOI] [PubMed] [Google Scholar]
- 77. Wong CE, Paratore C, Dours‐Zimmermann M'..T, et al. Neural crest‐derived cells with stem cell features can be traced back to multiple lineages in the adult skin. J Cell Biol. 2006;175:1005‐1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tomita Y, Matsumura K, Wakamatsu Y, et al. Cardiac neural crest cells contribute to the dormant multipotent stem cell in the mammalian heart. J Cell Biol. 2005;170:1135‐1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tang W, Martik ML, Li Y, Bronner ME. Cardiac neural crest contributes to cardiomyocytes in amniotes and heart regeneration in zebrafish. eLife. 2019;8. 10.7554/eLife.47929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pardal R, Ortega‐Sáenz P, Durán R, López‐Barneo J. Glia‐like stem cells sustain physiologic neurogenesis in the adult mammalian carotid body. Cell. 2007;131:364‐377. [DOI] [PubMed] [Google Scholar]
- 81. Hauser S, Widera D, Qunneis F, et al. Isolation of novel multipotent neural crest‐derived stem cells from adult human inferior turbinate. Stem Cells Dev. 2012;21:742‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Schürmann M, Brotzmann V, Bütow M, et al. Identification of a novel high yielding source of multipotent adult human neural crest‐derived stem cells. Stem Cell Rev Rep. 2018;14:277‐285. [DOI] [PubMed] [Google Scholar]
- 83. Fu M, Xu L, Chen X, et al. Neural crest cells differentiate into brown adipocytes and contribute to periaortic arch adipose tissue formation. Arterioscler Thromb Vasc Biol. 2019;39:1629‐1644. [DOI] [PubMed] [Google Scholar]
- 84. Qi Y, Miao X, Xu L, et al. Isolation, culture, and adipogenic induction of neural crest original adipose‐derived stem cells from periaortic adipose tissue. J Vis Exp. 2020;2020:e60691. [DOI] [PubMed] [Google Scholar]
- 85. Zhang K, Cui X, Zhang B, Song X, Liu Q, Yang S. Multipotent stem cells with neural crest stem cells characteristics exist in bovine adipose tissue. Biochem Biophys Res Commun. 2020;522:819‐825. [DOI] [PubMed] [Google Scholar]
- 86. Nagoshi N, Shibata S, Kubota Y, et al. Ontogeny and multipotency of neural crest‐derived stem cells in mouse bone marrow, dorsal root ganglia, and whisker pad. Cell Stem Cell. 2008;2:392‐403. [DOI] [PubMed] [Google Scholar]
- 87. Coste C, Neirinckx V, Sharma A, et al. Human bone marrow harbors cells with neural crest‐associated characteristics like human adipose and dermis tissues. PLoS One. 2017;12:e0177962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kikuchi M, Hayashi R, Kanakubo S, et al. Neural crest‐derived multipotent cells in the adult mouse iris stroma. Genes Cells. 2011;16:273‐281. [DOI] [PubMed] [Google Scholar]
- 89. Yoshida S, Shimmura S, Nagoshi N, et al. Isolation of multipotent neural crest‐derived stem cells from the adult mouse cornea. Stem Cells. 2006;24:2714‐2722. [DOI] [PubMed] [Google Scholar]
- 90. Davies LC, Locke M, Webb RDJ, et al. A multipotent neural crest‐derived progenitor cell population is resident within the oral mucosa lamina propria. Stem Cells Dev. 2010;19:819‐830. [DOI] [PubMed] [Google Scholar]
- 91. Widera D, Zander C, Heidbreder M, et al. Adult palatum as a novel source of neural crest‐related stem cells. Stem Cells. 2009;27:1899‐1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zeuner M‐T, Didenko NN, Humphries D, et al. Isolation and characterization of neural crest‐derived stem cells from adult ovine palatal tissue. Front Cell Dev Biol. 2018;6:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Janebodin K, Horst OV, Ieronimakis N, et al. Isolation and characterization of neural crest‐derived stem cells from dental pulp of neonatal mice. PLoS One. 2011;6:e27526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149‐155. [DOI] [PubMed] [Google Scholar]
- 95. Coura GS, Garcez RC, de Aguiar CBNM, Alvarez‐Silva M, Magini RS, Trentin AG. Human periodontal ligament: a niche of neural crest stem cells. J Periodontal Res. 2008;43:531‐536. [DOI] [PubMed] [Google Scholar]
- 96. Greenberg JM, Lumbreras V, Pelaez D, Rajguru SM, Cheung HS. Neural crest stem cells can differentiate to a cardiomyogenic lineage with an ability to contract in response to pulsed infrared stimulation. Tissue Eng Part C Methods. 2016;22:982‐990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mehrotra P, Tseropoulos G, Bronner ME, Andreadis ST. Adult tissue‐derived neural crest‐like stem cells: sources, regulatory networks, and translational potential: concise review. Stem Cells Translational Medicine. 2019;9:328‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wang D, Li LK, Dai T, Wang A, Li S. Adult stem cells in vascular remodeling. Theranostics. 2018;8:815‐829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tang Z, Wang A, Yuan F, et al. Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat Commun. 2012;3:875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wang D, Wang A, Wu F, et al. Sox10+ adult stem cells contribute to biomaterial encapsulation and microvascularization. Sci Rep. 2017;7:40295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wang D, Wu F, Yuan H, et al. Sox10+ cells contribute to vascular development in multiple organs ‐ brief report. Arterioscler Thromb Vasc Biol. 2017;37:1727‐1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pisciotta A, Bertoni L, Vallarola A, Bertani G, Mecugni D, Carnevale G. Neural crest derived stem cells from dental pulp and tooth‐associated stem cells for peripheral nerve regeneration. Neural Regen Res. 2020;15:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. El‐Hashash A. Neural Crest Stem Cells: A Therapeutic Hope Machine for Neural Regeneration. in 233–250 (Humana Press, Cham, Switzerland, 2016). doi: 10.1007/978-3-319-33270-3_11 [DOI] [Google Scholar]
- 104. Li Y, Yao D, Zhang J, et al. The effects of epidermal neural crest stem cells on local inflammation microenvironment in the defected sciatic nerve of rats. Front Mol Neurosci. 2017;10:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhang L, Li B, Liu B, Dong Z. Co‐transplantation of epidermal neural crest stem cells and olfactory ensheathing cells repairs sciatic nerve defects in rats. Front Cell Neurosci. 2019;13. 10.3389/fncel.2019.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Shi H, Li X, Yang J, et al. Bone marrow‐derived neural crest precursors improve nerve defect repair partially through secreted trophic factors. Stem Cell Res Ther. 2019;10:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Huang CW, Hsueh YY, Huang WC, Patel S, Li S. Multipotent vascular stem cells contribute to neurovascular regeneration of peripheral nerve. Stem Cell Res Ther. 2019;10:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Peng CK, Wu SY, Tang SE, et al. Protective effects of neural crest‐derived stem cell‐conditioned media against ischemia‐reperfusion‐induced lung injury in rats. Inflammation. 2017;40:1532‐1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Teng YD, Yu D, Ropper AE, et al. Functional multipotency of stem cells: a conceptual review of neurotrophic factor‐based evidence and its role in translational research. Curr Neuropharmacol. 2011;9:574‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Teng YD, Kabatas S, Wakeman DR, Li J, Snyder EY, Sidman RL. Functional multipotency of neural stem cells and its therapeutic implications. Perspectives of Stem Cells. Dordrecht, the Netherlands: Springer; 2010:255‐270. 10.1007/978-90-481-3375-8_16. [DOI] [Google Scholar]
- 111. Kabatas S, Demir CS, Civelek E, et al. Neuronal regeneration in injured rat spinal cord after human dental pulp derived neural crest stem cell transplantation. Bratislava Med J. 2018;119:143‐151. [DOI] [PubMed] [Google Scholar]
- 112. Pandamooz S, Salehi MS, Zibaii MI, Ahmadiani A, Nabiuni M, Dargahi L. Epidermal neural crest stem cell‐derived glia enhance neurotrophic elements in an ex vivo model of spinal cord injury. J Cell Biochem. 2018;119:3486‐3496. [DOI] [PubMed] [Google Scholar]
- 113. Mohaghegh Shalmani L, Valian N, Pournajaf S, Abbaszadeh F, Dargahi L, Jorjani M. Combination therapy with astaxanthin and epidermal neural crest stem cells improves motor impairments and activates mitochondrial biogenesis in a rat model of spinal cord injury. Mitochondrion. 2020;52:125‐134. [DOI] [PubMed] [Google Scholar]
- 114. Pandamooz S, Salehi MS, Safari A, et al. Enhancing the expression of neurotrophic factors in epidermal neural crest stem cells by valproic acid: a potential candidate for combinatorial treatment. Neurosci Lett. 2019;704:8‐14. [DOI] [PubMed] [Google Scholar]
- 115. Fortino VR, Chen RS, Pelaez D, Cheung HS. Neurogenesis of neural crest‐derived periodontal ligament stem cells by EGF and bFGF. J Cell Physiol. 2014;229:479‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ono M, Suzawa T, Takami M, et al. Localization and osteoblastic differentiation potential of neural crest‐derived cells in oral tissues of adult mice. Biochem Biophys Res Commun. 2015;464:1209‐1214. [DOI] [PubMed] [Google Scholar]
- 117. Shan T, Zhou C, Yang R, et al. Lithium chloride promotes the odontoblast differentiation of hair follicle neural crest cells by activating Wnt/β‐catenin signaling. Cell Biol Int. 2015;39:35‐43. [DOI] [PubMed] [Google Scholar]
- 118. Abe S, Yamaguchi S, Sato Y, Harada K. Sphere‐derived multipotent progenitor cells obtained from human Oral mucosa are enriched in neural crest cells. Stem Cells Translational Medicine. 2016;5:117‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Grimm WD. Effect of human neural crest‐related stem cell homing (hncscs homing) on the mineralization of newly formed alveolar bone using an allogen bone substitute. Biomed J Sci Tech Res. 2019;17:12732‐12743. 10.26717/BJSTR.2019.17.002987. [DOI] [Google Scholar]
- 120. Ransom RC, Carter AC, Salhotra A, et al. Mechanoresponsive stem cells acquire neural crest fate in jaw regeneration. Nature. 2018;563:514‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Liu J, Yu F, Sun Y, et al. Concise reviews: characteristics and potential applications of human dental tissue‐derived mesenchymal stem cells. Stem Cells. 2015;33:627‐638. [DOI] [PubMed] [Google Scholar]
- 122. Steinhardt Y, Aslan H, Regev E, et al. Maxillofacial‐derived stem cells regenerate critical mandibular bone defect. Tissue Eng Part A. 2008;14:1763‐1773. [DOI] [PubMed] [Google Scholar]
- 123. Wang X, Xing H, Zhang G, et al. Restoration of a critical mandibular bone defect using human alveolar bone‐derived stem cells and porous nano‐HA/collagen/PLA scaffold. Stem Cells Int. 2016;2016:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Buduru SD, Gulei D, Zimta AA, Tigu AB, Cenariu D, Berindan‐Neagoe I. The potential of different origin stem cells in modulating Oral bone regeneration processes. Cell. 2019;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Liu Y, Wang H, Dou H, et al. Bone regeneration capacities of alveolar bone mesenchymal stem cells sheet in rabbit calvarial bone defect. J Tissue Eng. 2020;11:204173142093037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Verma K, Bains R, Bains VK, Rawtiya M, Loomba K, Srivastava SC. Therapeutic potential of dental pulp stem cells in regenerative medicine: an overview. Dent Res J (Isfahan). 2014;11:302‐308. [PMC free article] [PubMed] [Google Scholar]
- 127. Yamada Y, Nakamura‐Yamada S, Kusano K, Baba S. Clinical potential and current progress of dental pulp stem cells for various systemic diseases in regenerative medicine: a concise review. Int J Mol Sci. 2019;20:1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Luo L, He Y, Wang X, et al. Potential roles of dental pulp stem cells in neural regeneration and repair. Stem Cells Int. 2018;2018:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sayad‐Fathi S, Nasiri E, Zaminy A. Advances in stem cell treatment for sciatic nerve injury. Expert Opin Biol Ther. 2019;19:301‐311. [DOI] [PubMed] [Google Scholar]
- 130. Kanada S, Makino E, Nakamura N, et al. Direct comparison of therapeutic effects on diabetic polyneuropathy between transplantation of dental pulp stem cells and administration of dental pulp stem cell‐secreted factors. Int J Mol Sci. 2020;21:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hata M, Omi M, Kobayashi Y, et al. Transplantation of human dental pulp stem cells ameliorates diabetic polyneuropathy in streptozotocin‐induced diabetic nude mice: the role of angiogenic and neurotrophic factors. Stem Cell Res Ther. 2020;11:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Grskovic M, Javaherian A, Strulovici B, Daley GQ. Induced pluripotent stem cells—opportunities for disease modelling and drug discovery. Nat Rev Drug Discov. 2011;10:915‐929. [DOI] [PubMed] [Google Scholar]
- 133. Sayed N, Liu C, Wu JC. Translation of human‐induced pluripotent stem cells: from clinical trial in a dish to precision medicine. J Am Coll Cardiol. 2016;67:2161‐2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ilic D, Ogilvie C. Concise review: human embryonic stem cells‐what have we done? What are we doing? Where are we going? Stem Cells. 2017;35:17‐25. [DOI] [PubMed] [Google Scholar]
- 135. Cubillo JE, Ngo SM, Juarez A, Gagan J, Lopez GD, Stout DA. Embryonic stem cell therapy applications for autoimmune, cardiovascular, and neurological diseases: a review. AIMS Cell Tissue Eng. 2018;1:191‐223. [Google Scholar]
- 136. Menendez L, Yatskievych TA, Antin PB, Dalton S. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc Natl Acad Sci USA. 2011;108:19240‐19245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Menendez L, Kulik MJ, Page AT, et al. Directed differentiation of human pluripotent cells to neural crest stem cells. Nat Protoc. 2013;8:203‐212. [DOI] [PubMed] [Google Scholar]
- 138. Mica Y, Lee G, Chambers SM, Tomishima MJ, Studer L. Modeling neural crest induction, melanocyte specification, and disease‐related pigmentation defects in hESCs and patient‐specific iPSCs. Cell Rep. 2013;3:1140‐1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Hackland JOS, Frith TJR, Thompson O, et al. Top‐down inhibition of BMP signaling enables robust induction of hPSCs into neural crest in fully defined, xeno‐free conditions. Stem Cell Rep. 2017;9:1043‐1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Gomez GA, Prasad MS, Wong M, et al. WNT/β‐catenin modulates the axial identity of embryonic stem cell‐derived human neural crest. Development. 2019;146:dev175604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Mimura S, Suga M, Okada K, Kinehara M, Nikawa H, Furue MK. Bone morphogenetic protein 4 promotes craniofacial neural crest induction from human pluripotent stem cells. Int J Dev Biol. 2016;60:21‐28. [DOI] [PubMed] [Google Scholar]
- 142. Huang M, Miller ML, McHenry LK, et al. Generating trunk neural crest from human pluripotent stem cells. Sci Rep. 2016;6. 10.1038/srep19727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Fattahi F, Steinbeck JA, Kriks S, et al. Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature. 2016;531:105‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Schlieve CR, Fowler KL, Thornton M, et al. Neural crest cell implantation restores enteric nervous system function and alters the gastrointestinal transcriptome in human tissue‐engineered small intestine. Stem Cell Rep. 2017;9:883‐896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Wang A, Tang Z, Park IH, et al. Induced pluripotent stem cells for neural tissue engineering. Biomaterials. 2011;32:5023‐5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Huang C‐W, Huang WC, Qiu X, et al. The differentiation stage of transplanted stem cells modulates nerve regeneration. Sci Rep. 2017;7:17401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kimura H, Ouchi T, Shibata S, et al. Stem cells purified from human induced pluripotent stem cell‐derived neural crest‐like cells promote peripheral nerve regeneration. Sci Rep. 2018;8:10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Jones I, Novikova LN, Novikov LN, et al. Regenerative effects of human embryonic stem cell‐derived neural crest cells for treatment of peripheral nerve injury. J Tissue Eng Regen Med. 2018;12:e2099‐e2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Lv Y, Nan P, Chen G, Sha Y, Xia B, Yang L. In vivo repair of rat transected sciatic nerve by low‐intensity pulsed ultrasound and induced pluripotent stem cells‐derived neural crest stem cells. Biotechnol Lett. 2015;37:2497‐2506. [DOI] [PubMed] [Google Scholar]
- 150. Xia B, Chen G, Zou Y, Yang L, Pan J, Lv Y. Low‐intensity pulsed ultrasound combination with induced pluripotent stem cells‐derived neural crest stem cells and growth differentiation factor 5 promotes sciatic nerve regeneration and functional recovery. J Tissue Eng Regen Med. 2019;13:625‐636. [DOI] [PubMed] [Google Scholar]
- 151. Du J, Zhen G, Chen H, et al. Optimal electrical stimulation boosts stem cell therapy in nerve regeneration. Biomaterials. 2018;181:347‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Saadai P, Wang A, Nout YS, et al. Human induced pluripotent stem cell‐derived neural crest stem cells integrate into the injured spinal cord in the fetal lamb model of myelomeningocele. J Pediatr Surg. 2013;48:158‐163. [DOI] [PubMed] [Google Scholar]
- 153. Hotta R, Pepdjonovic L, Anderson RB, et al. Small‐molecule induction of neural crest‐like cells derived from human neural progenitors. Stem Cells. 2009;27:2896‐2905. [DOI] [PubMed] [Google Scholar]
- 154. Workman MJ, Mahe MM, Trisno S, et al. Engineered human pluripotent‐stem‐cell‐derived intestinal tissues with a functional enteric nervous system. Nat Med. 2017;23:49‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Li W, Huang L, Zeng J, et al. Characterization and transplantation of enteric neural crest cells from human induced pluripotent stem cells. Mol Psychiatry. 2018;23:499‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Xu W, Wang Y, Liu E, et al. Human iPSC‐derived neural crest stem cells promote tendon repair in a rat patellar tendon window defect model. Tissue Eng Part A. 2013;19:2439‐2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Ouchi T, Morikawa S, Shibata S, et al. LNGFR+THY‐1+ human pluripotent stem cell‐derived neural crest‐like cells have the potential to develop into mesenchymal stem cells. Differentiation. 2016;92:270‐280. [DOI] [PubMed] [Google Scholar]
- 158. Chijimatsu R, Ikeya M, Yasui Y, et al. Characterization of mesenchymal stem cell‐like cells derived from human iPSCs via neural crest development and their application for osteochondral repair. Stem Cells Int. 2017;2017:1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Kikuchi K, Masuda T, Fujiwara N, et al. Craniofacial bone regeneration using iPS cell‐derived neural crest like cells. J Hard Tissue Biol. 2018;27:1‐10. [Google Scholar]
- 160. Kawano E, Toriumi T, Iguchi S, Suzuki D, Sato S, Honda M. Induction of neural crest cells from human dental pulp‐derived induced plu‐ripotent stem cells. Biomed Res. 2017;38:135‐147. [DOI] [PubMed] [Google Scholar]
- 161. Fang D, Leishear K, Nguyen TK, et al. Defining the conditions for the generation of melanocytes from human embryonic stem cells. Stem Cells. 2006;24:1668‐1677. [DOI] [PubMed] [Google Scholar]
- 162. Nissan X, Larribere L, Saidani M, et al. Functional melanocytes derived from human pluripotent stem cells engraft into pluristratified epidermis. Proc Natl Acad Sci USA. 2011;108:14861‐14866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ohta S, Imaizumi Y, Akamatsu W, Okano H, Kawakami Y. Generation of human melanocytes from induced pluripotent stem cells. Methods Mol Biol. 2013;989:193‐215. [DOI] [PubMed] [Google Scholar]
- 164. Wilson SL, El Haj AJ, Yang Y. Control of scar tissue formation in the cornea: strategies in clinical and corneal tissue engineering. J Funct Biomater. 2012;3:642‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Chen P, Chen JZ, Shao CY, et al. Treatment with retinoic acid and lens epithelial cell‐conditioned medium in vitro directed the differentiation of pluripotent stem cells towards corneal endothelial cell‐like cells. Exp Ther Med. 2015;9:351‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. McCabe KL, Kunzevitzky NJ, Chiswell BP, Xia X, Goldberg JL, Lanza R. Efficient generation of human embryonic stem cell‐derived corneal endothelial cells by directed differentiation. PLoS One. 2015;10:e0145266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Song Q, Yuan S, An Q, et al. Directed differentiation of human embryonic stem cells to corneal endothelial cell‐like cells: a transcriptomic analysis. Exp Eye Res. 2016;151:107‐114. [DOI] [PubMed] [Google Scholar]
- 168. Hertsenberg AJ, Funderburgh JL. Generation of corneal keratocytes from human embryonic stem cells. Methods Mol Biol. 2015;1341:285‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Zhu Q, Li M, Yan C, et al. Directed differentiation of human embryonic stem cells to neural crest stem cells, functional peripheral neurons, and corneal keratocytes. Biotechnol J. 2017;12:1700067. [DOI] [PubMed] [Google Scholar]
- 170. Zhang K, Pang K, Wu X. Isolation and transplantation of corneal endothelial cell‐like cells derived from in‐vitro‐differentiated human embryonic stem cells. Stem Cells Dev. 2014;23:1340‐1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Seki D, Takeshita N, Oyanagi T, et al. Differentiation of odontoblast‐like cells from mouse induced pluripotent stem cells by Pax9 and Bmp4 transfection. Stem Cells Translational Medicine. 2015;4:993‐997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Otsu K, Kishigami R, Oikawa‐Sasaki A, et al. Differentiation of induced pluripotent stem cells into dental mesenchymal cells. Stem Cells Dev. 2012;21:1156‐1164. [DOI] [PubMed] [Google Scholar]
- 173. Bajpai R, Chen DA, Rada‐Iglesias A, et al. CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature. 2010;463:958‐962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Okuno H, Renault Mihara F, Ohta S, et al. CHARGE syndrome modeling using patient‐iPSCs reveals defective migration of neural crest cells harboring CHD7 mutations. eLife. 2017;6. 10.7554/eLife.21114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. von Levetzow C, Jiang X, Gwye Y, et al. Modeling initiation of Ewing sarcoma in human neural crest cells. PLoS One. 2011;6:e19305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Lee G, Papapetrou EP, Kim H, et al. Modelling pathogenesis and treatment of familial dysautonomia using patient‐specific iPSCs. Nature. 2009;461:402‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Kim YJ, Lim H, Li Z, et al. Generation of multipotent induced neural crest by direct reprogramming of human postnatal fibroblasts with a single transcription factor. Cell Stem Cell. 2014;15:497‐506. [DOI] [PubMed] [Google Scholar]
- 178. Lai FPL, Lau ST, Wong JKL, et al. Correction of Hirschsprung‐associated mutations in human induced pluripotent stem cells via clustered regularly interspaced short palindromic repeats/Cas9, restores neural crest cell function. Gastroenterology. 2017;153:139‐153.e8. [DOI] [PubMed] [Google Scholar]
- 179. Barrell WB, Griffin JN, Harvey JL, et al. Induction of neural crest stem cells from Bardet–Biedl syndrome patient derived hiPSCs. Front Mol Neurosci. 2019;12:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Serrano F, Bernard WG, Granata A, et al. A novel human pluripotent stem cell‐derived neural crest model of Treacher Collins Syndrome shows defects in cell death and migration. Stem Cells Dev. 2019;28:81‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Jiao J, Xiong W, Wang L, et al. Differentiation defect in neural crest‐derived smooth muscle cells in patients with aortopathy associated with bicuspid aortic valves. EBioMedicine. 2016;10:282‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Lee G, Ramirez CN, Kim H, et al. Large‐scale screening using familial dysautonomia induced pluripotent stem cells identifies compounds that rescue IKBKAP expression. Nat Biotechnol. 2012;30:1244‐1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Xu J, Du Y, Deng H. Direct lineage reprogramming: strategies, mechanisms, and applications. Cell Stem Cell. 2015;16:119‐134. [DOI] [PubMed] [Google Scholar]
- 184. Morris SA, Daley GQ. A blueprint for engineering cell fate: current technologies to reprogram cell identity. Cell Res. 2013;23:33‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Motohashi T, Kunisada T. Direct conversion of mouse embryonic fibroblasts into neural crest cells. Methods Mol Biol. 2018;1879:307‐321. [DOI] [PubMed] [Google Scholar]
- 186. Tseng TC, Hsieh FY, Dai NT, Hsu SH. Substrate‐mediated reprogramming of human fibroblasts into neural crest stem‐like cells and their applications in neural repair. Biomaterials. 2016;102:148‐161. [DOI] [PubMed] [Google Scholar]
- 187. Ho L, Hsu S. huiCell reprogramming by 3D bioprinting of human fibroblasts in polyurethane hydrogel for fabrication of neural‐like constructs. Acta Biomater. 2018;70:57‐70. [DOI] [PubMed] [Google Scholar]
- 188. Motohashi T, Watanabe N, Nishioka M, et al. Gene array analysis of neural crest cells identifies transcription factors necessary for direct conversion of embryonic fibroblasts into neural crest cells. Biol Open. 2016;5:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Bajpai VK, Kerosuo L, Tseropoulos G, et al. Reprogramming postnatal human epidermal keratinocytes toward functional neural crest fates. Stem Cells. 2017;35:1402‐1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Moghadasi Boroujeni S, Koontz A, Tseropoulos G, et al. Neural crest stem cells from human epidermis of aged donors maintain their multipotency in vitro and in vivo. Sci Rep. 2019;9:9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Zabierowski SE, Baubet V, Himes B, et al. Direct reprogramming of melanocytes to neural crest stem‐like cells by one defined factor. Stem Cells. 2011;29:1752‐1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Tomokiyo A, Hynes K, Ng J, et al. Generation of neural crest‐like cells from human periodontal ligament cell‐derived induced pluripotent stem cells. J Cell Physiol. 2017;232:402‐416. [DOI] [PubMed] [Google Scholar]
- 193. Doss MX, Sachinidis A. Current challenges of ipsc‐based disease modeling and therapeutic implications. Cells. 2019;8:403. 10.3390/cells8050403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Lee DR, Yoo JE, Lee JS, et al. PSA‐NCAM‐negative neural crest cells emerging during neural induction of pluripotent stem cells cause mesodermal tumors and unwanted grafts. Stem Cell Rep. 2015;4:821‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Mehler VJ, Burns CJ, Stauss H, Francis RJ, Moore ML. Human iPSC‐derived neural crest stem cells exhibit low immunogenicity. Mol Ther Methods Clin Dev. 2020;16:161‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Fujii S, Yoshida S, Inagaki E, et al. Immunological properties of neural crest cells derived from human induced pluripotent stem cells. Stem Cells Dev. 2019;28:28‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Greiner JF, Grunwald LM, Müller J, et al. Culture bag systems for clinical applications of adult human neural crest‐derived stem cells. Stem Cell Res Ther. 2014;5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Du J, Chen H, Zhou K, Jia X. Quantitative multimodal evaluation of passaging human neural crest stem cells for peripheral nerve regeneration. Stem Cell Rev Rep. 2018;14:92‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Wislet‐Gendebien S, Poulet C, Neirinckx V, et al. In vivo tumorigenesis was observed after injection of in vitro expanded neural crest stem cells isolated from adult bone marrow. PLoS One. 2012;7:e46425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Uto K, Arakawa CK, DeForest CA. Next‐generation biomaterials for culture and manipulation of stem cells. Cold Spring Harb Perspect Biol. 2019;12:a035691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Xu Y, Chen C, Hellwarth PB, Bao X. Biomaterials for stem cell engineering and biomanufacturing. Bioact Mater. 2019;4:366‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Hu YF, Zhang Z‐J, Sieber‐Blum M. An epidermal neural crest stem cell (EPI‐NCSC) molecular signature. Stem Cells. 2006;24:2692‐2702. [DOI] [PubMed] [Google Scholar]
- 203. Thomas S, Thomas M, Wincker P, et al. Human neural crest cells display molecular and phenotypic hallmarks of stem cells. Hum Mol Genet. 2008;17:3411‐3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Sieber‐Blum M, Hu Y. Epidermal neural crest stem cells (EPI‐NCSC) and pluripotency. Stem Cell Rev. 2008;4:256‐260. [DOI] [PubMed] [Google Scholar]
- 205. Odashima A, Onodera S, Saito A, Ogihara Y, Ichinohe T, Azuma T. Stage‐dependent differential gene expression profiles of cranial neural crest‐like cells derived from mouse‐induced pluripotent stem cells. Med Mol Morphol. 2020;53:28‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Simoes‐Costa M, Bronner ME. Reprogramming of avian neural crest axial identity and cell fate. Science. 2016;352:1570‐1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Lau ST, Li Z, Pui‐Ling Lai F, et al. Activation of hedgehog signaling promotes development of mouse and human enteric neural crest cells, based on single‐cell transcriptome analyses. Gastroenterology. 2019;157:1556‐1571.e5. [DOI] [PubMed] [Google Scholar]
- 208. Williams RM, Candido‐Ferreira I, Repapi E, et al. Reconstruction of the global neural crest gene regulatory network in vivo. Dev Cell. 2019;51:255‐276.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Adli M. The CRISPR tool kit for genome editing and beyond. Nat Commun. 2018;9:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Wang HX, Li M, Lee CM, et al. CRISPR/Cas9‐based genome editing for disease modeling and therapy: challenges and opportunities for nonviral delivery. Chem Rev. 2017;117:9874‐9906. [DOI] [PubMed] [Google Scholar]
- 211. Savić N, Schwank G. Advances in therapeutic CRISPR/Cas9 genome editing. Transl Res. 2016;168:15‐21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


