Abstract
Durvalumab is a humanized monoclonal antibody targeting programmed cell death ligand‐1 (PD‐L1), leading to an antitumor activity, used as consolidation therapy in patients with locally advanced unresectable non‐small cell lung cancer (NSCLC). Several immune‐related adverse events (irAEs) have previously been described in patients following treatment with immune checkpoint inhibitors (ICIs). To the best of our knowledge, we report the first case of immunotherapy‐induced fully reversible bronchiolitis and bronchiectasis, despite the fact that its pathophysiological mechanism has been previously considered to be irreversible.
Keywords: immune‐related adverse event, lung cancer, bronchiectasis, durvalumab, panbronchiolitis
Here we report a case of durvalumab‐induced lesions of bronchiolitis and fully reversible bronchiectasis. This immune‐related adverse event has not previously been described.
INTRODUCTION
Durvalumab is a humanized monoclonal antibody targeting PD‐L1, recently approved as consolidation therapy in locally advanced unresectable NSCLC patients, with nonprogressive disease after two or more cycles of concomitant chemoradiotherapy. 1
Several irAEs have been previously described. 2 Concerning respiratory disorders, the main toxicities reported are diffuse interstitial lung diseases, rated in grade according to their severity, as shown in Tables 1 and 2. 3 , 4
TABLE 1.
Immune‐induced pulmonary toxicities by European Society for Medical Oncology (ESMO) grade 3
| ESMO grade | Symptoms |
|---|---|
| Grade 1 | Radiographic changes only (ex. ground‐glass change, nonspecific interstitial pneumonia) |
| Grade 2 | Mild/moderate new symptoms (ex. dyspnea, cough, chest pain) |
| Grade 3–4 | Severe new symptoms (ex. new/worsening hypoxia, life threatening, difficulty in breathing, acute respiratory distress syndrome) |
| Grade 5 | Death |
TABLE 2.
Immune‐induced pulmonary toxicities by common terminology criteria for adverse events (CTCAE) grade (v5.0), with suggested management by Delaunay et al. 4
| CTCAE grade 5.0 | Definition | Suggested management |
|---|---|---|
| Grade 1 | Asymptomatic | Continuation of IT, monitoring |
| Grade 2 | Symptomatic, limiting instrumental ADL and requiring medical intervention | Stop IT, oral corticosteroids 0,5–1 mg/kg, monitoring, potential rechallenge |
| Grade 3 | Severe symptoms, limiting self‐care ADL and indicated oxygen | Stop IT, oral corticosteroids 2–4 mg/kg, no rechallenge, monitoring |
| Grade 4 | Life‐threatening respiratory compromise and indicated urgent intervention | Stop IT, intravenous corticosteroids 4 mg/kg, no rechallenge, monitoring |
Abbreviations: ADL, activities of daily living; IT, immunotherapy.
Bronchial toxicities induced by anti‐programmed cell death protein‐1 (PD‐1)/PD‐L1 immunotherapy have rarely been reported and rather as an exacerbation of chronic obstructive pulmonary disease (COPD). 5 , 6 Here, we report a case of a patient who developed a pattern of bronchiectasis with bronchiolitis during anti‐PD‐L1 treatment with a favorable outcome after discontinuation of immune checkpoint inhibitors (ICIs).
CASE REPORT
A 77‐year‐old Caucasian man presented with a new episode of respiratory distress and hemoptysis. His relevant medical history was a smoking cessation at 80‐pack‐year, a post‐tobacco COPD and a laryngeal cancer treated with chemoradiotherapy (carboplatin) in 2012. A locally advanced (stage IIIA N2 unresectable) squamous cell carcinoma of the left lower lobe was discovered in May 2018. PD‐L1 immunostaining was <1% (evaluated by the tumor proportion score with DAKO 22C3 antibody). Between June and July 2018, concomitant chemoradiotherapy was implemented, combining seven weekly courses of carboplatin (AUC 2) ‐ paclitaxel (45 mg/m2) and thoracic conformational radiotherapy up to 66 Gy. At the end of this treatment, re‐evaluation with a chest computed tomography (CT) scan showed an objective tumour response. Consolidation therapy with durvalumab (early access program) was initiated from October 12, 2018 (10 mg/kg every 15 days).
In December 2018, after five injections of durvalumab, he presented with the first episode of bronchopneumonia affecting the left lower lobe, undocumented and treated with amoxicillin‐clavulanate for 10 days. Subsequently, chronic purulent bronchorrhea set in. In February 2019, he was hospitalized because of similar respiratory symptoms, treated with cefepime and metronidazole. Bronchoalveolar lavage (BAL) did not identify any organism. Consolidation treatment with durvalumab was maintained. Because of persistent bronchorrhea and the appearance of exertional dyspnea, new bacteriological tests (BAL and sputum culture test) were performed, but they did not isolate any pathogenic bacteria, despite the presence of purulent and very sticky secretions in endobronchial studies. Cytological examination revealed an 85% neutrophil granulocyte count, with 10% macrophages and 5% lymphocytes over 3 500 cells per mm3. Chest CT highlighted the appearance of bronchiolitis lesions (bronchial wall thickening and tree‐in‐bud pattern) and bronchiectasis from January 2019, particularly in the nonirradiated areas (right lower lobe, contralateral to the initial tumour). These images appeared less than two months after durvalumab initiation and were consistent with the appearance of chronic purulent bronchorrhea and exertional dyspnea. Oral corticosteroids were introduced associated with triple long‐acting bronchodilator (beclometasone, formoterol, glycopyrronium), as well as anti‐inflammatory bronchial treatment with azithromycin 250 mg, three times a week, and bronchial drainage respiratory physiotherapy. These therapeutic adaptations did not bring any improvement in respiratory symptomatology. In view of the disabling respiratory symptoms and lack of improvement despite optimal treatment implemented, durvalumab was discontinued after 10 courses of treatment on March 29, 2019.
Chest CT monitoring was performed every three months and during follow‐up, CT lesions seemed to be more extensive at the first re‐evaluation in June 2019, 10 weeks after durvalumab discontinuation, suggestive of prolonged toxicity from this treatment. Thereafter, chest CT monitoring showed spontaneous regression of various lesions observed, especially in September 2019 (Figure 1). Clinically, respiratory symptoms significantly improved three months after durvalumab discontinuation.
FIGURE 1.
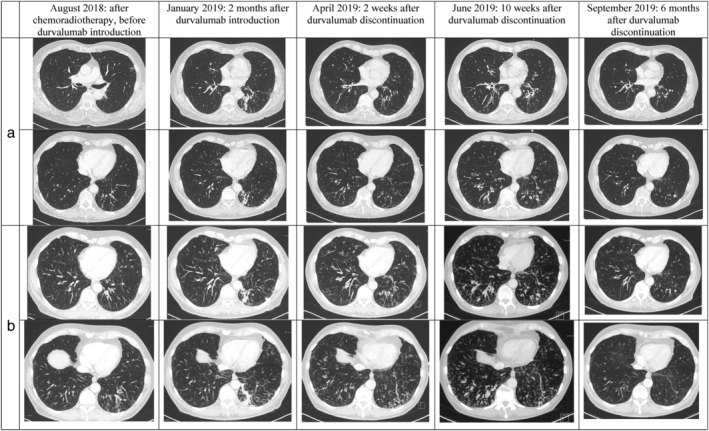
Evolution of bronchiectasis and bronchiolitis lesions before, during and after durvalumab therapy; (a) chest computed tomography (CT) images, and (b) with maximum intensity projection (MIP) 5.0 mm post‐treatment. Lesions appeared to be more extensive in June 2019, 10 weeks after durvalumab discontinuation, and had fully disappeared in September 2019, six months after durvalumab discontinuation
DISCUSSION
To our knowledge, this is the first case reported of ICPI‐induced panbronchiolitis associated with bronchiectasis. Main pulmonary toxicities hitherto described are organizing pneumonia, ground‐glass opacities, interstitial lesions, hypersensitivity pneumonitis, or nonspecific interstitial pneumonitis. 7 Some studies have reported that pulmonary toxicities can be fatal, as in the case of acute respiratory distress syndrome (ARDS), 8 or severe acute asthma. 5 Recently, during durvalumab therapy, Yamakasi et al. described the appearance of a diffuse micronodular pattern, with predominantly ground‐glass micronodules. 9
Lesions of bronchiolitis observed in this case may be similar to the hypersensitivity pneumonitis pattern described by Naidoo et al. 7 and to diffuse panbronchiolitis, which is a complex genetic disease strongly associated with specific human leukocyte antigen, and very rare in Western countries. 10 Diffuse panbronchiolitis is characterized by chronic inflammation in respiratory bronchioles, which can lead to bronchi and bronchiole dilatation, as seen in Figure 1.
Bronchiectasis is a chronic inflammatory lung disease whose pathophysiological mechanism is not clearly identified, but seems to be based on a vicious circle in which chronic bronchial infection is responsible for persistent inflammation, causing an impaired mucociliary clearance and a priori irreversible structural damages, which in turn favors infection (Figure 2). 11
FIGURE 2.
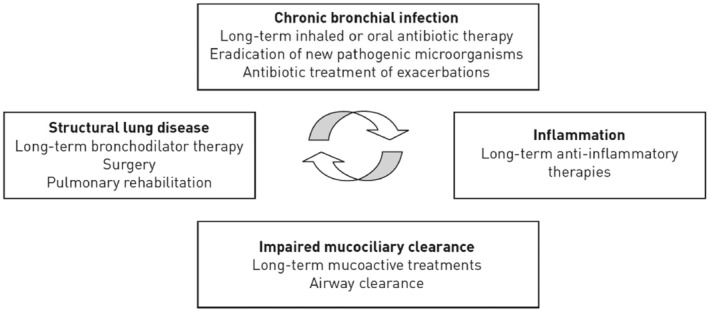
Bronchiectasis pathophysiological mechanism as reported by Polverino et al. 11
They occur in immune deficiency cases, or systemic inflammatory disease. 12 Neutrophils appear to play a key role, but it has also been shown that T cells, particularly via T helper 17 (Th17) subtype and interleukin‐17 secretion, participate in neutrophil recruitment and in a proinflammatory airway environment maintenance. 13
However, blocking the PD‐1/PD‐L1 interaction could induce a reorientation of the lymphocyte response towards Th17 differentiation, as demonstrated in mouse models. 14 As in the case of other irAE, Th17 cells appear to play the main role, via their ability to activate different actors of the immune system, such as macrophages, dendritic cells, or CD8+ cytotoxic T cells. 15
Finally, it could be hypothesized that blocking the PD‐1/PD‐L1 cosignal with an ICI such as durvalumab could direct the T cell differentiation towards Th17 cell subtype, inducing proinflammatory cytokine secretion, neutrophils activation, and airways proinflammatory environment creation that could favor bronchitis, bronchiolitis and then bronchiectasis development. This mechanism may also explain the COPD exacerbation reported cases, or immunotherapy‐induced asthma. 6 Treatment discontinuation would allow a return to a physiological immune response and disappearance of lesions. However, a complete regression of bronchiectasis is rare during adulthood, especially with high‐resolution CT, because of a suspected irreversible pathophysiology mechanism. Only a few case reports have previously been published without clear explanation concerning the mechanisms, alerting us to the possibility of pseudobronchiectasis due to pneumonia, 16 , 17 but there was no evidence on chest CT scan of pneumonia lesions in our patient at any time.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
Domblides M, Geier M, Decroisette C, Descourt R. Durvalumab‐induced lesions of bronchiolitis and fully reversible bronchiectasis in a patient with non‐small cell lung cancer: A case report. Thoracic Cancer. 2021;12:1240–1243. 10.1111/1759-7714.13862
REFERENCES
- 1. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III Non–small‐cell lung cancer. N Engl J Med. 2017;377(20):1919–29. 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 2. Zubiri L, Allen IM, Taylor MS, Guidon AC, Chen ST, Schoenfeld SR, et al. Immune‐related adverse events in the setting of PD‐1/L1 inhibitor combination therapy. Oncologist. 2020;25(3):e398–8. 10.1634/theoncologist.2018-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2017;28(suppl_4):iv119–42. 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 4. Delaunay M, Prévot G, Collot S, Guilleminault L, Didier A, Mazières J. Management of pulmonary toxicity associated with immune checkpoint inhibitors. Eur Respir Rev. 2019;28(154):190012. 10.1183/16000617.0012-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ogawa T, Miyata J, Maehara J, Inoue T, Betsuyaku T. Fatal airway inflammation induced by pembrolizumab in a patient With NSCLC. J Thorac Oncol. 2019;14(1):e9–e10. 10.1016/j.jtho.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 6. Maeno K, Fukuda S, Oguri T, Niimi A. Nivolumab‐induced asthma in a patient with non‐small‐cell lung cancer. Ann Oncol. 2017;28(11):2891. 10.1093/annonc/mdx455. [DOI] [PubMed] [Google Scholar]
- 7. Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in patients treated with anti‐programmed death‐1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35(7):709–17. 10.1200/JCO.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nishino M, Sholl LM, Hodi FS. Anti–PD‐1–related pneumonitis during cancer immunotherapy. N Engl J Med. 2015;373(3):288–90. 10.1056/NEJMc1505197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yamasaki M, Taniwaki M, Kawamoto K, Matsumoto N, Hashimoto K, Nabeshima S, et al. Durvalumab‐induced organizing pneumonia with a diffuse micronodular pattern in a patient with lung cancer. Am J Respir Crit Care Med. 2020;201(8):e52–3. 10.1164/rccm.201907-1310IM. [DOI] [PubMed] [Google Scholar]
- 10. Keicho N, Hijikata M. Genetic predisposition to diffuse panbronchiolitis: panbronchiolitis‐related genes. Respirology. 2011;16(4):581–8. 10.1111/j.1440-1843.2011.01946.x. [DOI] [PubMed] [Google Scholar]
- 11. Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3):1700629. 10.1183/13993003.00629-2017. [DOI] [PubMed] [Google Scholar]
- 12. King PT. The role of the immune response in the pathogenesis of bronchiectasis. Biomed Res Int. 2018;2018:1–12. 10.1155/2018/6802637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chalmers JD, Hill AT. Mechanisms of immune dysfunction and bacterial persistence in non‐cystic fibrosis bronchiectasis. Mol Immunol. 2013;55(1):27–34. 10.1016/j.molimm.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 14. McAlees JW, Lajoie S, Dienger K, Sproles AA, Richgels PK, Yang Y, et al. Differential control of CD4 + T‐cell subsets by the PD‐1/PD‐L1 axis in a mouse model of allergic asthma: cellular immune response. Eur J Immunol. 2015;45(4):1019–29. 10.1002/eji.201444778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anderson R, Theron AJ, Rapoport BL. Immunopathogenesis of immune checkpoint inhibitor‐related adverse events: roles of the intestinal microbiome and Th17 cells. Front Immunol. 2019;10:2254. 10.3389/fimmu.2019.02254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kucuk C, Turkkani MH, Arda K. A case report of reversible bronchiectasis in an adult: pseudobronchiectasis. Respir Med Case Rep. 2019;26:315–6. 10.1016/j.rmcr.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yap VL, Metersky ML. Reversible bronchiectasis in an Adult: A case report. J Bronchology Interv Pulmonol. 2012;19(4):336–7. 10.1097/LBR.0b013e31826ca79d. [DOI] [PubMed] [Google Scholar]


