Abstract
Novel technology and innovative stimulation paradigms allow for unprecedented spatiotemporal precision and closed-loop implementation of neurostimulation systems. In turn, precise, closed-loop neurostimulation appears to preferentially drive neural plasticity in motor networks, promoting neural repair. Recent clinical studies demonstrate that electrical stimulation can drive neural plasticity in damaged motor circuits, leading to meaningful improvement in users. Future advances in these areas hold promise for the treatment of a wide range of motor systems disorders.
Keywords: electrical stimulation, neural plasticity, motor, close-loop, vagus nerve stimulation (VNS)
Executive Summary
Advances in invasive and non-invasive neural interface technology have increased the spatiotemporal precision of electrical stimulation and allowed for closed-loop systems that respond to changes in physiological state. These innovative devices now allow electrical stimulation drive plasticity within motor circuits, leading to neural repair and restored function.
- The future research and innovation should focus on the directions listed below to improve specificity in stimulation protocols for user-individualized therapy.
- Closed-loop systems that drive stimulation based on patients’ real-time neurophysiological information.
- Increased spatial and temporal precision of stimulation delivery that can harness endogenous neural plasticity mechanisms.
- Improved neural interfaces designed to maximize recording and stimulation effectiveness.
Introduction
Restoring motor function after damage to the nervous system has been an intractable challenge for patients, clinicians and researchers. Motor systems disrupted by traumatic injury, pathological insult or neurodegeneration often do not regain full functionality. Currently, diverse types of electrical stimulation devices are designed to restore or substitute for lost motor functions. These include deep brain stimulation for essential tremor, dystonia and Parkinson’s disease and electrical stimulation of muscles/nerves to facilitate rehabilitation after spinal cord injury or stroke. However, current devices are primarily open-loop systems - controlled by external and arbitrary commands. As such, they lack precision in the delivery of stimulation, limiting the degree of motor control and circuit plasticity that these technologies can bring to users.
Recent advances in neurostimulation research suggests its potential to harness endogenous neural plasticity, broadening the therapeutic possibilities to a diverse range of motor disorders. Early clinical successes hint at the power of this approach. For instance, deep brain stimulation (DBS) applied earlier in disease progression may alter disease trajectory; spinal cord stimulation (SCS) has restored voluntary motor control in a small number of patients; and precisely-timed vagus nerve stimulation (VNS) speeds rehabilitation following stroke. The evolving landscape of innovative neurostimulation technology has been critical to opening up new therapeutic applications.
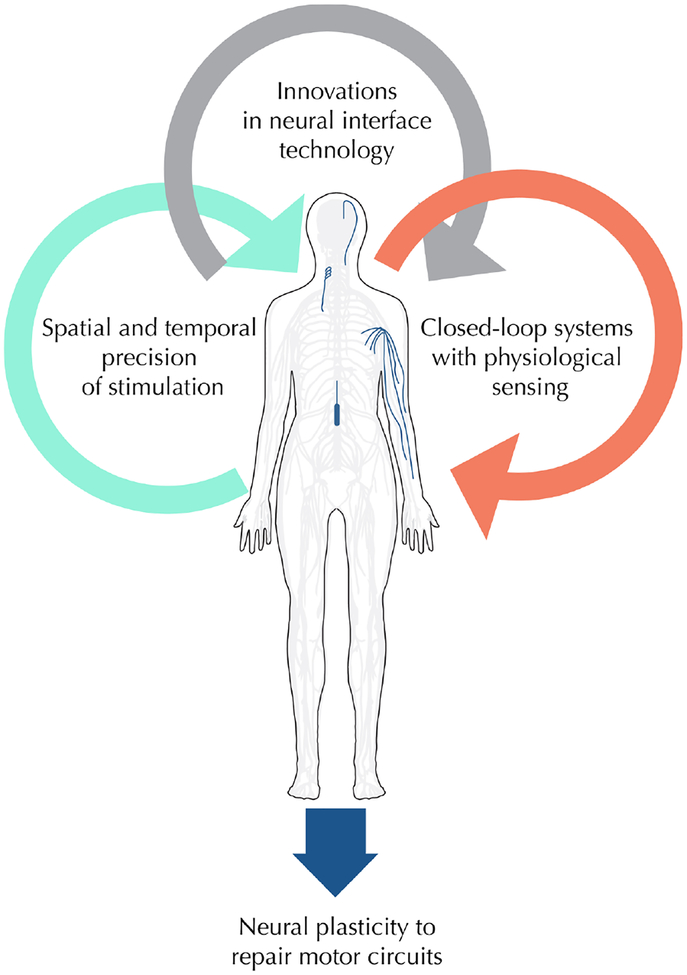
The ability for neuromodulation technology to drive neural plasticity relies on innovations in several key areas: precise timing of stimulation delivery, closed-loop device technology with integrated sensing capabilities and novel invasive and non-invasive neural interfaces to broaden the range of possible targets within the nervous system (Figure 1). Pairing precise electrical stimulation with rehabilitation appears to activate hyperpolarized neural circuits and drive neural plasticity. Closed-loop stimulation devices, which incorporate electrophysiological sensing and feedback stimulation, provide instantaneous targeted delivery - an ultimate form of precision medicine. Novel interface technology, such as smaller implantable electrodes and new non-invasive stimulation devices can reach a wider range of neural structures in the central and peripheral nervous system. Together, the expanding repertoire of medical device technology provides new means by which electrical stimulation can induce neuroplasticity and endogenous repair mechanisms, and is bringing a renewed sense of enthusiasm and hope to researchers, clinicians and patients.
Figure 1.
Innovations in electrical stimulation drive neural plasticity to restore motor function. Advances in invasive and non-invasive neural interface technology have increased the spatiotemporal precision of electrical stimulation and allowed for closed-loop systems that respond to changes in physiological state. These innovative devices now allow electrical stimulation drive plasticity within motor circuits, leading to neural repair and restored function.
Electrical stimulation for acute modulation of motor circuits
The most widely-used neurostimulation therapy for movement disorders is deep brain stimulation (DBS) for the treatment of Parkinson’s disease, essential tremor (ET) and dystonia. As a class, movement disorders effect more than 10 million individuals in the United States [1–4], cost billions of dollars each year in care, and the at-risk population is projected to double over the next three decades [4,5]. For this reason, there has been a focus in the academic and medical community on finding long lasting and effective treatments for these disorders. DBS was first approved by the FDA in 1997 for essential tremor [6–8] and in 2002 for PD. Since being approved for market, DBS implants have been performed at an increasing rate, over 150,000 users have received a DBS system, with over 60% implanted in the last five years [9,10]. These implants have also displayed robust life spans, providing users with significant improvements even after more than five years of use [11–13].
DBS directly modulates motor circuit function DBS is achieved by surgically implanting a stimulating electrode unilaterally or bilaterally into the thalamus or striatum and applying continuous electrical stimulation to those brain structures. DBS produces an acute alleviation of symptoms, and implanted patients revert back to baseline level function minutes after stimulation ends [12–14]. DBS was initially thought to inhibit activity in the basal ganglia circuit as an ‘informational lesion’ [15,16]. However, later research suggests that DBS may serve as a low-pass filter for signal transmission [17,18], disrupting the synchronization of the basal ganglia and downstream structures [19–21] and inducing short-term inhibitory plasticity [22].
Future directions to improve DBS therapy
Novel treatment paradigms, stimulation protocols and device technology are driving forward innovations in the treatment of movement disorders with DBS and other forms of electrical stimulation. The application of DBS earlier in disease progression is currently under investigation as a way to alter disease trajectory in Parkinson’s patients by enhancing adaptive plasticity [23–26]. Animal studies suggest that DBS drives markers of plasticity, such as BDNF expression, within motor circuits[27–29]. In addition, a pilot clinical study examining DBS in early stage Parkinson’s disease patients shows promise that DBS may slow the progression of the rest tremor [30,31]
To improve the efficacy and efficiency of DBS, there is a growing focus in the field on adaptive DBS (aDBS) [32]. aDBS utilizes feedback from a user’s unique neurophysiology, typically through recorded local field potentials (LFPs), for real-time information of the user’s state to modify stimulation parameters. During implantation, aDBS strategies use LFPs to help target brain regions more accurately [33–35]. Importantly, LFPs can be reliably detected from DBS users over several years [36], providing critical longitudinal insight into the disease [37–42] and fundamentals of the human motor system [43,44]. Moreover, aDBS may also be used to reduce side effects and extend the longevity of DBS devices [45–47].
Although DBS is the most common electrical stimulation device to treat ET, the FDA recently approved a non-invasive median and ulnar nerve stimulation device that reduces tremor in ET patients [48]. This device is worn on the wrist, and applies transcutaneous electrical stimulation to the nerves, minimizing tremor in some users. Further development of non-invasive techniques could open the door for electrical stimulation devices to be used on a far broader range of patient populations due to their drastically decreased risk profile.
Electrical stimulation to accelerate recovery from injury
While DBS produces instantaneous relief from the symptoms of Parkinson’s disease, electrical stimulation for treating neural injury relies on driving neural plasticity to enhance functional recovery. In this treatment paradigm, chronic electrical stimulation is paired with physical rehabilitation over months to years, and leads to outcomes that surpass physical rehabilitation alone. These stimulation modalities include spinal cord stimulation (SCS), vagus nerve stimulation (VNS), and direct brain stimulation. These paradigms have been applied to treat spinal cord injury and stroke, and may have broader implications for other types of injuries in central and peripheral nervous system.
Epidural spinal cord stimulation to drive neuroplasticity
Spinal cord injury (SCI) is a devastating condition that affects roughly 2.5 million people worldwide[49]. Depending on the location of the injury, patients fully or partially lose motor or sensory functions. After initial diagnosis, patients begin physical rehabilitation programs that aim to maintain muscle tone, reduce muscle atrophy and pain, and may improve prognosis[50]. Physical rehabilitation may induce plasticity in spared spinal cord circuits[51], but has not been able to restore volitional movement to patients with motor complete spinal cord injury.
Historically, electrical stimulation in the spinal cord has been demonstrated to activate motor pools, allowing patients with SCI to initiate some movements, but did not restore function of higher-order movements such as walking[52,53]. A major advance in SCS involved pairing stimulation with ongoing physical rehabilitation. In patients with complete motor injuries and either ‘sensory incomplete’[54,55] or ‘sensory complete’ injuries[56], epidural stimulation paired with rehabilitation led to partial recovery of intentional movement in lower and upper limbs. In addition, EMG activity was detected in the lower limbs during stimulation, suggesting that previously non-functional motor circuits in motor complete SCI patients were re-activated[56]. Several groups have since shown amazing recovery of stepping[57] and over-ground walking[58,59] in patients with SCI, an unprecedented leap forward in treatment for these patients. A perhaps unexpected additional benefit of SCS is the effect on autonomic function, including elevating blood pressure from hypotensive states[60,61] and improving bladder and bowel function[62]. Excitingly, similar results may also be produced by non-invasive transcutaneous electrical stimulation, opening up the possibility of treating a broader range of patients without the need for surgical procedures [63,64].
SCS drives both acute and chronic alterations in motor circuit function
Spinal cord stimulation is thought to aid recovery by: 1) depolarizing motor pools in the spinal cord, reducing the activation threshold of spared motor and sensory tracts and 2) evoking plasticity to remodel the spared motor tracts[65]. Spinal cord injuries typically do not result in a complete “separation” of tissue, rather contusions or lacerations leading to damage and inflammation. Remaining supraspinal connectivity is not sufficient to activate lower motor neuron pools, so SCS is required to acutely activate downstream neural populations. However, for complex voluntary movement, such as over-ground walking, acute SCS must be paired with physical rehabilitation over numerous training sessions, to drive chronic circuit plasticity. Recent clinical reports have suggested that patients with higher likelihood of recovery are those with partial lesions, specifically those with some somatosensory or proprioceptive input[66,67]. Yet, even in the most severe SCI injury, there is evidence that EMG activity can be generated by descending pathways, suggesting some white matter tracts are spared from damage and may allow patients to recover partially through plasticity of spared tracts during SCS paired with rehabilitation[68,69].
Future improvements in technology: Closed-loop SCS
Closed-loop stimulation synchronizes motor intent and SCS, thus inducing more salient plasticity preferentially in connections relevant to higher-order movements[70,71]. Two methods of closed-loop stimulation are the focus for future clinical implementation: cortico-spinal decoding and EMG-based closed loop stimulation. Cortico-spinal decoding records signals in motor cortex to decode movement intentions, and then deliver temporally precise stimulation to the spinal cord below the lesion, leading to greatly improved motor control in rodents[72] and non-human primates [73]. Enhanced plasticity in cortical control was driven by spared ascending sensory afferents in turn increasing the density of cortical fibers projecting to subcortical motor regions[68]. Alternatively, EMG and other physiological measures, can approximate motor intention, allowing for precisely timed delivery of epidural stimulation[74]. In a study of several patients with chronic cervical spinal cord injury, closed-loop stimulation accelerated performance of over–ground walking [59]. Tailoring closed-loop stimulation based on cortical decoding methods may result in different varieties of neuroplasticity in spinal cord circuits, suggesting opportunities for engineered neuroplasticity[75].
Paired VNS for the recovery of motor function after stroke
New advances in VNS therapy gives promise to the recovery of movement capabilities. Currently, VNS is marketed as a treatment for medically-intractable epilepsy and depression. For these therapies, VNS is applied continuously, similar to the stimulation protocol for marketed DBS devices. However, a new VNS stimulation paradigm, where brief bursts of VNS are paired with physical rehabilitation, is thought to drive long-lasting circuit plasticity and speed motor rehabilitation [76–83]. In theory, by inducing plasticity and speeding rehabilitation, paired VNS has potential to improve therapy for a broad range of disorders of motor systems.
Clinical trials are exploring the use of paired VNS to enhance motor rehabilitation in cases of stroke involving upper limb paresis. There are over 795,000 incidents of stroke every year, and approximately 40% of stroke survivors have functional impairment [84–86]. Recovery from stroke depends on structural and functional reorganization of lesioned tissue and intact contralateral structures. A pilot safety and feasibility study of VNS for motor rehabilitation following stroke demonstrated that the device is reasonable and safe [87]. Implantation of the stimulating cuff electrode has low surgical risk and is generally well-tolerated by users, with common adverse events including hoarseness of voice, coughing, and nausea ([88]. Patients receiving paired VNS showed a greater improvement in their Fugl-Meyer Assessment (FMA) compared to those receiving rehabilitation alone, providing justification for a future pivotal trial [89].
VNS drives functional recovery through plasticity in motor circuits
There is a strong basis of animal research to support the use of VNS for stroke rehabilitation. Rat models of ischemic and hemorrhagic stroke that received VNS paired with a forelimb reach showed significant recovery of strength in a forelimb lever pull task. When VNS paired with rehabilitation, the rate of full recovery doubles comparing to rehabilitation only control [80–83], and motor improvements can generalize across motor task [90]. VNS paired with a specific movement causes cortical plasticity, reorganization of motor cortex and increased representation of the corresponding area in healthy[91] and injured animals[92]. There is evidence to suggest that VNS-induced cortical plasticity is mediated cholinergic basal forebrain and noradrenergic locus coeruleus[93]. Neurons in the locus coeruleus are activated and release norepinephrine in response to VNS [94–96]. In SCI, VNS drives plasticity in spared spinal circuits[97], similar to the results in epidural spinal cord stimulation following spinal cord injury.
Future directions for VNS in multiple neurological conditions
VNS holds potential therapeutic efficacy across a rapidly growing range of medical indications, including autoimmune, metabolic and neurological disorders[78,98]. The diverse therapeutic space is reflective of the anatomy of the vagus nerve, which has widespread peripheral afferent and efferent innervation of heart, lungs, gastrointestinal tract and brainstem. In addition to the studies involving recovery from stroke, temporally-precise VNS shows promise in animal models of other motor system injuries, including spinal cord injury, traumatic brain injury and peripheral nerve injury. As with epidural stimulation for spinal cord injury, closed-loop stimulation may enhance the effectiveness of paired VNS [97].
While the implantation surgery for vagus nerve stimulators is relatively low risk, a non-invasive stimulation solution could have a profound impact on the safety, widespread use, and potential therapeutic indications for VNS. Transcutaneous stimulation of vagus afferents can occur at either the cervical or auricular branches. Evidence that non-invasive stimulation can sufficiently activate vagal afferents is accumulating[99–101]. For instance, non-invasive auricular and cervical stimulation showed a vagal sensory evoked potential similar to the one produced with invasive VNS [102]. Moreover, pilot studies of auricular VNS applied for 60 minutes prior to physical rehabilitation [103] or paired with movements during rehabilitation [104] show a small increase in FMA scores compared to sham stimulation.
Cortical stimulation for the recovery of motor function after stroke or spinal cord injury
In addition to VNS, invasive cortical stimulation paired with rehabilitation may also speed motor function recovery [105]. Initial clinical trials for cortical stimulation in patients with hemiparetic stroke showed minor improvements in upper extremity FMA, yet a broader pivotal trial found no increase in recovery over controls [106–108]. However, these trials may not have accounted for variable lesion size [109], lesion location [110] and delivery timing[111,112]. Non-invasive electrical stimulation, such as transcranial direct current stimulation (tDCS), may enhance recovery from stroke[113] and spinal cord injury[114], but large randomized controlled trials are needed to determine effectiveness[115].
Electrical stimulation to directly restore motor and sensory function
In addition to restoring motor function by driving plasticity in damaged neural circuits, electrical stimulation can transmit information directly into, or out of, motor and sensory pathways. Referred generally as neuroprosthetic systems, these devices restore uni- or bi-directional communication between the brain and the user’s peripheral nervous system. By directly activating peripheral motor units in patients with paralysis, electrical stimulation devices can bypass injured motor systems to restore motor function[116]. Alternatively, such systems can restore sensory function through stimulation of remaining peripheral or central sensory pathways.
Direct restoration of motor function through electrical stimulation
Foundational work on neuroprosthetic motor devices for spinal cord injured patients lead to the first implantable motor prosthesis, the Freehand System [117–119]. This device relies on direct stimulation of forearm and hand muscles to produce two hand grasp positions: lateral grasp for small objects and palmar grasp for large objects. Patients controlled the first-generation device through mechanical actuators on the shoulder, and later generations through EMG signals from implanted electrodes in user’s neck and shoulder muscles. The second generation system provide better control of grasp-release, forearm pronation, and elbow extension[120,121]. While the Freehand System is no longer commercially available, advanced work on these functional electrical stimulation systems continues under the direction of the Institute for Functional Restoration at Case Western Reserve University. Newer functional electrical stimulation systems often incorporate muscle-based control commands, allowing for closed-loop control by physiological measurements[122]. These devices provide motor control of the limbs, allowing users to complete activities of daily living[123,124].
The early work on myoelectric neuroprostheses inspired a search for a richer, and perhaps more intuitive set of control signals, leading to the development of brain-controlled neuroprosthetic devices. In these devices, control signals are generated by higher dimension natural cortical activities recorded through microelectrodes in the brain - devices called brain computer interfaces (BCIs)[125–127]. BCIs have been used to control virtual or robotic prosthetic assist devices, providing new movement capabilities to patients with tetraplegia. Moreover, recent work in non-human primates [128] and human patients [129–131] demonstrated that BCI signals can control direct stimulation of paralyzed forelimb muscles to restore voluntary forelimb grasp and reach movements.
Restoration of sensory function with electrical stimulation
Electrical stimulation can also be used to reproduce sensory perceptions in patients who have lost sensory function. As sensory perception is tightly linked to motor systems, restored sensation can have additional benefits to improve motor control in users. In these technologies, sensory input, such as that from tactile sensors on a prosthetic hand, is transmitted to stimulation electrodes in the remaining afferent nerves, allowing users to receive tactile and proprioceptive feedback [132–134]. The addition of sensory feedback improves the amputees performance using myoelectric prosthetic devices, and improves the embodiment of the device[135,136]. Similar systems have been used in pilot investigations to restore somatosensory feedback in SCI patients. Since SCI patients can’t relay peripheral sensation back to brain through damaged spinal cord, the stimulation is delivered directly to somatosensory cortex [137,138], producing cutaneous sensations.
Future directions for neuroprosthetic devices
Information processing in motor systems is highly complex and the circuits can change over short and long timescales. Advances in neuroprosthetic technology will include more robust, reliable interface with high information content. These may include better recording and stimulating electrodes with novel materials, biocompatible coatings, increased channel count and high-throughput wireless connectors[139]. There is also a need for better analytical methods to interpret incoming signals, encode stimulation parameters and calibrate analytical systems. Since both neural circuits and computer algorithms can learn and adapt over time, performance of neuroprosthetic devices needs to be continually optimized based on current benchmarks[140–142]. New computational strategies that incorporate dynamical systems, nonlinear models, rapid control feedback and other strategies to acknowledge network plasticity and account for learning across distributed neural networks may optimize solutions[143–148]. Finally, closed-loop neuroprosthetics systems are complex, and may require individualized configurations to meet each user’s needs. Modular devices, with interconnecting components can allow for personalized solutions, but they require standardized interconnections and may have a more complex regulatory pathway for use in humans[149].
Conclusions and future directions
Electrical stimulation is changing the lives of thousands of patients. Deep brain stimulation brings immediate and often remarkable changes to each user’s motor control and quality of life. Implanted functional electrical stimulation and neuroprosthetic systems have restored motor functions to patients with spinal cord injury, stroke and neurodegenerative disorders. Vagus nerve stimulation, spinal cord stimulation, and direct cortical stimulation can accelerate the rehabilitation and induce plasticity in remaining circuits. Yet, each of these technologies faces challenges in implementation, and has exciting new avenues for improvement.
In contrast to pharmaceutical treatment options, electrical stimulation allows for precise, targeted therapy for movement disorders. By nature, stimulation electrodes are placed intentionally in specific targets to provide local treatment. Precisely timed VNS during rehabilitation may enhance cortical plasticity to speed recovery of injured motor circuits. Similarly, spinal cord stimulation for spinal cord injury paired with physical rehabilitation allows users to regain volitional movement. Neuroprosthetic systems deliver temporally and spatially precise stimulation to target muscles or neural tissue, both to re-animate musculature or provide sensation. Improved specificity of device placement, stimulation delivery and stimulation protocols can drive future improvements in user-specific therapy.
Building on the advances brought by spatiotemporal precision, closed-loop stimulation includes a control command that drives stimulation onset. Real-time neurophysiological information recorded by electrodes sensing neural firing or EMG activity are a powerful source of control commands. Adaptive DBS systems use the patient’s neurophysiology to guide stimulation for improvement of both the efficiency, and likely the effectiveness of therapy for movement disorders. Spinal cord stimulation may be improved by pairing stimulation with EMG activity, increasing the speed of recovery from injury. VNS driven by rehabilitation activities that reach a success threshold may lead to increased functional outcomes. And BCI systems that convey sensory information during movement may allow the users to have the most effective control over motor effectors.
The innovations in precise, closed-loop stimulation capitalize on novel small, high density microelectrodes. Increasingly, these electrodes can be effectively targeted to the optimal location within neural tissue, to maximize recording and stimulation effectiveness. In addition, advances in non-invasive stimulation has the benefits of precise, responsive electrical stimulation without the risk of invasive implanted devices. Pushing the boundaries of technology development to create robust yet precise neural interfaces allows for unique and localized electrical stimulation. It also enhances physiological sensing, opening the door for fully closed-loop therapeutic systems.
Advances in neural interface technology, stimulation delivery, closed-loop systems allow devices to respond to each individual’s neurophysiology, optimizing device performance and recovery of motor function. Critically, these new neurostimulation devices are customized to meet each user’s individual needs and respond to real-time physiological cues. The innovative device technology opens up the frontiers for precise and responsive closed-loop neurostimulation systems to harness endogenous neural plasticity mechanisms to repair or compensate for poorly-functioning motor circuits. Moreover, increased accessibility to neural plasticity has implications that transcend the beyond movement disorders: in essence, it can allow injured or poorly-functioning nervous systems to maximize their repair capacity. Electrical stimulation is expanding the boundary of what is considered possible in restoration of motor function.
Financial disclosure:
This work is funded by Biological Technologies Office (BTO) Program: Targeted Neuroplasticity Training (TNT) HR0011–17-2–0051, the NIH R21 EY029458–02 and the Boettcher Foundation Webb-Waring Biomedical Research Awards. The authors declares no conflict of interests.
References
- 1.AANS. Movement Disorders - Classifications, Symptoms and Treatments [Internet]. (2018). Available from: https://www.aans.org/.
- 2.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Movement Disorders. 25(5), 534–541 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Rawlins MD, Wexler NS, Wexler AR, et al. The Prevalence of Huntington’s Disease. NED. 46(2), 144–153 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Suchowersky O Decade in review—movement disorders: Tracking the pathogenesis of movement disorders. Nature Reviews Neurology. 11(11), 618–619 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Bach J-P, Ziegler U, Deuschl G, Dodel R, Doblhammer-Reiter G. Projected numbers of people with movement disorders in the years 2030 and 2050. Movement Disorders. 26(12), 2286–2290 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Benabid AL, Pollak P, Hoffmann D, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. The Lancet. 337(8738), 403–406 (1991). [DOI] [PubMed] [Google Scholar]
- 7.Blond S, Siegfried J. Thalamic stimulation for the treatment of tremor and other movement disorders. Acta Neurochir Suppl (Wien) 52, 109–111 (1991). [DOI] [PubMed] [Google Scholar]
- 8.Kumar R, Lozano AM, Kim YJ, et al. Double-blind evaluation of subthalamic nucleus deep brain stimulation in advanced Parkinson’s disease. Neurology. 51(3), 850 (1998). [DOI] [PubMed] [Google Scholar]
- 9.Gardner J A history of deep brain stimulation: Technological innovation and the role of clinical assessment tools. Soc Stud Sci. 43(5), 707–728 (2013). [Google Scholar]
- 10.Green A Deep Brain Stimulation [Internet]. International Neuromodulation Society; (2018). Available from: https://www.neuromodulation.com/deep-brain-stimulation. [Google Scholar]
- 11.Cury RG, Fraix V, Castrioto A, et al. Thalamic deep brain stimulation for tremor in Parkinson disease, essential tremor, and dystonia. Neurology. 89(13), 1416–1423 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Flora ED, Perera CL, Cameron AL, Maddern GJ. Deep brain stimulation for essential tremor: A systematic review. Movement Disorders. 25(11), 1550–1559 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Zhang K, Bhatia S, Oh MY, Cohen D, Angle C, Whiting D. Long-term results of thalamic deep brain stimulation for essential tremor. Journal of Neurosurgery. 112(6), 1271–1276 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Rizzone MG, Fasano A, Daniele A, et al. Long-term outcome of subthalamic nucleus DBS in Parkinson’s disease: From the advanced phase towards the late stage of the disease? Parkinsonism & Related Disorders. 20(4), 376–381 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Limousin P, Pollak P, Benazzouz A, et al. Effect on parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. The Lancet. 345(8942), 91–95 (1995). [DOI] [PubMed] [Google Scholar]
- 16.Limousin P, Pollak P, Benazzouz A, et al. Bilateral subthalamic nucleus stimulation for severe Parkinson’s disease. Movement Disorders. 10(5), 672–674 (1995). [DOI] [PubMed] [Google Scholar]
- 17.Agnesi F, Muralidharan A, Baker KB, Vitek JL, Johnson MD. Fidelity of frequency and phase entrainment of circuit-level spike activity during DBS. J. Neurophysiol 114(2), 825–834 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimnik AJ, Nora GJ, Desmurget M, Turner RS. Movement-Related Discharge in the Macaque Globus Pallidus during High-Frequency Stimulation of the Subthalamic Nucleus. J. Neurosci 35(9), 3978–3989 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McConnell GC, So RQ, Hilliard JD, Lopomo P, Grill WM. Effective Deep Brain Stimulation Suppresses Low-Frequency Network Oscillations in the Basal Ganglia by Regularizing Neural Firing Patterns. J. Neurosci 32(45), 15657–15668 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moran A, Stein E, Tischler H, Bar-Gad I. Decoupling neuronal oscillations during subthalamic nucleus stimulation in the parkinsonian primate. Neurobiology of Disease. 45(1), 583–590 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Xiao Y, Agnesi F, Bello EM, Zhang S, Vitek JL, Johnson MD. Deep brain stimulation induces sparse distributions of locally modulated neuronal activity. Sci Rep 8(1), 2062 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milosevic L, Kalia SK, Hodaie M, et al. Neuronal inhibition and synaptic plasticity of basal ganglia neurons in Parkinson’s disease. Brain. 141(1), 177–190 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SJ, Udupa K, Ni Z, et al. Effects of subthalamic nucleus stimulation on motor cortex plasticity in Parkinson disease. Neurology. 85(5), 425–432 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lourens MAJ, Schwab BC, Nirody JA, Meijer HGE, Gils SA van. Exploiting pallidal plasticity for stimulation in Parkinson’s disease. J. Neural Eng 12(2), 026005 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Morishita T, Higuchi M, Saita K, Tsuboi Y, Abe H, Inoue T. Changes in Motor-Related Cortical Activity Following Deep Brain Stimulation for Parkinson’s Disease Detected by Functional Near Infrared Spectroscopy: A Pilot Study. Front. Hum. Neurosci 10 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Udupa K, Bahl N, Ni Z, et al. Cortical Plasticity Induction by Pairing Subthalamic Nucleus Deep-Brain Stimulation and Primary Motor Cortical Transcranial Magnetic Stimulation in Parkinson’s Disease. J. Neurosci 36(2), 396–404 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salin P, Manrique C, Forni C, Kerkerian-Le Goff L. High-frequency stimulation of the subthalamic nucleus selectively reverses dopamine denervation-induced cellular defects in the output structures of the basal ganglia in the rat. J. Neurosci 22(12), 5137–5148 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vedam-Mai V, Baradaran-Shoraka M, Reynolds BA, Okun MS. Tissue Response to Deep Brain Stimulation and Microlesion: A Comparative Study. Neuromodulation. 19(5), 451–458 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer DL, Kemp CJ, Cole-Strauss A, et al. Subthalamic Nucleus Deep Brain Stimulation Employs trkB Signaling for Neuroprotection and Functional Restoration. J. Neurosci 37(28), 6786–6796 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hacker ML, DeLong MR, Turchan M, et al. Effects of deep brain stimulation on rest tremor progression in early stage Parkinson disease. Neurology. 91(5), 463–471 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This pilot studies demonstrates the feasibility for DBS during early stage Parkinson’s disease to alter disease trajectory through adaptive neural plasticity.
- 31.Hacker ML, Tonascia J, Turchan M, et al. Deep brain stimulation may reduce the relative risk of clinically important worsening in early stage Parkinson’s disease. Parkinsonism & Related Disorders. 21(10), 1177–1183 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Meidahl AC, Tinkhauser G, Herz DM, Cagnan H, Debarros J, Brown P. Adaptive Deep Brain Stimulation for Movement Disorders: The Long Road to Clinical Therapy. Movement Disorders. 32(6), 810–819 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lozano CS, Ranjan M, Boutet A, et al. Imaging alone versus microelectrode recording-guided targeting of the STN in patients with Parkinson’s disease. Journal of Neurosurgery., 1–6 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Telkes I, Ince NF, Onaran I, Abosch A. Localization of subthalamic nucleus borders using macroelectrode local field potential recordings. In: 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2621–2624 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Thompson JA, Oukal S, Bergman H, et al. Semi-automated application for estimating subthalamic nucleus boundaries and optimal target selection for deep brain stimulation implantation surgery. Journal of Neurosurgery., 1–10 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Abosch A, Lanctin D, Onaran I, Eberly L, Spaniol M, Ince NF. Long-term Recordings of Local Field Potentials From Implanted Deep Brain Stimulation Electrodes. Neurosurgery. 71(4), 804–814 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Hirschmann J, Elben S, et al. High-frequency oscillations in Parkinson’s disease: Spatial distribution and clinical relevance. Movement Disorders. 29(10), 1265–1272 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Wang DD, de Hemptinne C, Miocinovic S, et al. Pallidal deep brain stimulation disrupts pallidal beta oscillations and coherence with primary motor cortex in Parkinson’s disease. J. Neurosci, 0431–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, Zhuang P, Li Y. Altered Neuronal Firing Pattern of the Basal Ganglia Nucleus Plays a Role in Levodopa-Induced Dyskinesia in Patients with Parkinson’s Disease. Front Hum Neurosci 9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanrahan SJ, Nedrud JJ, Davidson BS, et al. Long-Term Task- and Dopamine-Dependent Dynamics of Subthalamic Local Field Potentials in Parkinson’s Disease. Brain Sci 6(4) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malekmohammadi M, Shahriari Y, AuYong N, et al. Pallidal stimulation in Parkinson disease differentially modulates local and network P activity. J Neural Eng. 15(5), 056016 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson JA, Tekriwal A, Felsen G, et al. Sleep patterns in Parkinson’s disease: direct recordings from the subthalamic nucleus. J. Neurol. Neurosurg. Psychiatry. 89(1), 95–104 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Maling N, Lempka SF, Blumenfeld Z, Bronte-Stewart H, McIntyre CC. Biophysical basis of subthalamic local field potentials recorded from deep brain stimulation electrodes. J. Neurophysiol 120(4), 1932–1944 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tankus A, Mirelman A, Giladi N, Fried I, Hausdorff JM. Pace of movement: the role of single neurons in the subthalamic nucleus. Journal of Neurosurgery., 1–6 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Kern K, Naros G, Braun C, Weiss D, Gharabaghi A. Detecting a Cortical Fingerprint of Parkinson’s Disease for Closed-Loop Neuromodulation. Front Neurosci 10 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paek S, Kale RP, Wininger KM, Lujan JL. Physiological Monitoring in Deep Brain Stimulation: Toward Closed-Loop Neuromodulation Therapies [Internet]. In: Emerging Trends in Neuro Engineering and Neural Computation. Bhatti A, Lee KH, Garmestani H, Lim CP (Eds.), Springer Singapore, Singapore, 81–97 (2017). [Google Scholar]
- 47.Swann NC, de Hemptinne C, Thompson MC, et al. Adaptive deep brain stimulation for Parkinson’s disease using motor cortex sensing. J. Neural Eng 15(4), 046006 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; Motor cortex activity is used to drive adaptive DBS to avoid dyskinesia and to increase energy efficiency for the implanted system.
- 48.Lin PT, Ross EK, Chidester P, et al. Noninvasive neuromodulation in essential tremor demonstrates relief in a sham-controlled pilot trial. Movement Disorders. 33(7), 1182–1183 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thuret S, Moon LDF, Gage FH. Therapeutic interventions after spinal cord injury. Nat. Rev. Neurosci 7(8), 628–643 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Nas K, Yazmalar L, Şah V, Aydin A, Öneş K. Rehabilitation of spinal cord injuries. World J Orthop 6(1), 8–16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ying Z, Roy RR, Edgerton VR, Gomez-Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp. Neurol 193(2), 411–419 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Dimitrijevic MR, Gerasimenko Y, Pinter MM. Evidence for a spinal central pattern generator in humans. Ann. N. Y. Acad. Sci 860, 360–376 (1998). [DOI] [PubMed] [Google Scholar]
- 53.Gerasimenko YP, Avelev VD, Nikitin OA, Lavrov IA. Initiation of locomotor activity in spinal cats by epidural stimulation of the spinal cord. Neurosci. Behav. Physiol 33(3), 247–254 (2003). [DOI] [PubMed] [Google Scholar]
- 54.Harkema S, Gerasimenko Y, Hodes J, et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 377(9781), 1938–1947 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu DC, Edgerton VR, Modaber M, et al. Engaging Cervical Spinal Cord Networks to Reenable Volitional Control of Hand Function in Tetraplegic Patients. Neurorehabil Neural Repair. 30(10), 951–962 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain. 137(Pt 5), 1394–1409 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gill ML, Grahn PJ, Calvert JS, et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat. Med 24(11), 1677–1682 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Angeli CA, Boakye M, Morton RA, et al. Recovery of Over-Ground Walking after Chronic Motor Complete Spinal Cord Injury. N. Engl. J. Med 379(13), 1244–1250 (2018). [DOI] [PubMed] [Google Scholar]; Patients with motor complete spinal cord injury demonstrate voluntary over-ground walking following spinal cord stimulation paired with rehabilitation training.
- 59.Wagner FB, Mignardot J-B, Le Goff-Mignardot CG, et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature. 563(7729), 65–71 (2018). [DOI] [PubMed] [Google Scholar]; Closed-loop spinal cord stimulation optimizes the recovery of voluntary walking in patients with spinal cord stimulation.
- 60.Aslan SC, Legg Ditterline BE, Park MC, et al. Epidural Spinal Cord Stimulation of Lumbosacral Networks Modulates Arterial Blood Pressure in Individuals With Spinal Cord Injury-Induced Cardiovascular Deficits. Front Physiol 9, 565 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harkema SJ, Wang S, Angeli CA, et al. Normalization of Blood Pressure With Spinal Cord Epidural Stimulation After Severe Spinal Cord Injury. Front Hum Neurosci 12, 83 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herrity AN, Williams CS, Angeli CA, Harkema SJ, Hubscher CH. Lumbosacral spinal cord epidural stimulation improves voiding function after human spinal cord injury. Sci Rep 8(1), 8688 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gad P, Lee S, Terrafranca N, et al. Non-Invasive Activation of Cervical Spinal Networks after Severe Paralysis. J. Neurotrauma 35(18), 2145–2158 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Inanici F, Samejima S, Gad P, Edgerton VR, Hofstetter CP, Moritz CT. Transcutaneous Electrical Spinal Stimulation Promotes Long-Term Recovery of Upper Extremity Function in Chronic Tetraplegia. IEEE Trans Neural Syst Rehabil Eng. 26(6), 1272–1278 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taccola G, Sayenko D, Gad P, Gerasimenko Y, Edgerton VR. And yet it moves: Recovery of volitional control after spinal cord injury. Prog. Neurobiol 160, 64–81 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scivoletto G, Tamburella F, Laurenza L, Torre M, Molinari M. Who is going to walk? A review of the factors influencing walking recovery after spinal cord injury. Front Hum Neurosci. 8, 141 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Formento E, Minassian K, Wagner F, et al. Electrical spinal cord stimulation must preserve proprioception to enable locomotion in humans with spinal cord injury. Nat. Neurosci 21(12), 1728–1741 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Asboth L, Friedli L, Beauparlant J, et al. Cortico-reticulo-spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nat. Neurosci 21(4), 576–588 (2018). [DOI] [PubMed] [Google Scholar]
- 69.Heald E, Hart R, Kilgore K, Peckham PH. Characterization of Volitional Electromyographic Signals in the Lower Extremity After Motor Complete Spinal Cord Injury. Neurorehabil Neural Repair. 31(6), 583–591 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grahn PJ, Mallory GW, Berry BM, Hachmann JT, Lobel DA, Lujan JL. Restoration of motor function following spinal cord injury via optimal control of intraspinal microstimulation: toward a next generation closed-loop neural prosthesis. Front Neurosci 8, 296 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moritz CT. Now is the Critical Time for Engineered Neuroplasticity. Neurotherapeutics. 15(3), 628–634 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bonizzato M, Pidpruzhnykova G, DiGiovanna J, et al. Brain-controlled modulation of spinal circuits improves recovery from spinal cord injury. Nat Commun. 9(1), 3015 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Capogrosso M, Milekovic T, Borton D, et al. A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature. 539(7628), 284–288 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McPherson JG, Miller RR, Perlmutter SI. Targeted, activity-dependent spinal stimulation produces long-lasting motor recovery in chronic cervical spinal cord injury. Proc. Natl. Acad. Sci. U.S.A 112(39), 12193–12198 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nishimura Y, Perlmutter SI, Eaton RW, Fetz EE. Spike-timing-dependent plasticity in primate corticospinal connections induced during free behavior. Neuron. 80(5), 1301–1309 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ganzer PD, Darrow MJ, Meyers EC, et al. Closed-loop neuromodulation restores network connectivity and motor control after spinal cord injury. eLife 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hays SA. Enhancing Rehabilitative Therapies with Vagus Nerve Stimulation. Neurotherapeutics. 13(2), 382–394 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hays SA, Rennaker RL, Kilgard MP. Targeting Plasticity with Vagus Nerve Stimulation to Treat Neurological Disease. Prog Brain Res. 207, 275–299 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hays SA, Khodaparast N, Ruiz A, et al. The timing and amount of vagus nerve stimulation during rehabilitative training affect post-stroke recovery of forelimb strength. Neuroreport. 25(9), 682–688 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hays SA, Khodaparast N, Hulsey DR, et al. Vagus Nerve Stimulation during Rehabilitative Training Improves Functional Recovery after Intracerebral Hemorrhage. Stroke. 45(10), 3097–3100 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khodaparast N, Hays SA, Sloan AM, et al. Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiology of Disease. 60, 80–88 (2013). [DOI] [PubMed] [Google Scholar]
- 82.Khodaparast N, Hays SA, Sloan AM, et al. Vagus Nerve Stimulation Delivered During Motor Rehabilitation Improves Recovery in a Rat Model of Stroke. Neurorehabil Neural Repair. 28(7), 698–706 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khodaparast N, Kilgard MP, Casavant R, et al. Vagus Nerve Stimulation during Rehabilitative Training Improves Forelimb Recovery after Chronic Ischemic Stroke in Rats. Neurorehabil Neural Repair. 30(7), 676–684 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; Temporally-precise delivery of vagus nerve stimulation promotes recovery from stroke in rats.
- 84.Stroke Facts| cdc.gov [Internet]. (2017). Available from: https://www.cdc.gov/stroke/facts.htm.
- 85.Winstein CJ, Stein J, Arena R, et al. Guidelines for Adult Stroke Rehabilitation and Recovery. Stroke. (2016). [Google Scholar]
- 86.Young J, Forster A. Review of stroke rehabilitation. BMJ [Internet]. 334(7584), 86–90 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dawson J, Pierce D, Dixit A, et al. Safety, Feasibility, and Efficacy of Vagus Nerve Stimulation Paired With Upper-Limb Rehabilitation After Ischemic Stroke. Stroke. 47(1), 143–150 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Labar D, Nikolov B, Tarver B, Fraser R. Vagus Nerve Stimulation for Symptomatic Generalized Epilepsy: A Pilot Study. Epilepsia. 39(2), 201–205 (1998). [DOI] [PubMed] [Google Scholar]
- 89.Kimberley TJ, Pierce D, Prudente CN, et al. Vagus Nerve Stimulation Paired With Upper Limb Rehabilitation After Chronic Stroke. Stroke. 49(11), 2789–2792 (2018). [DOI] [PubMed] [Google Scholar]; Pilot clinical study demonstrating recovery of motor function in stroke patients with VNS.
- 90.Meyers EC, Solorzano BR, James J, et al. Vagus Nerve Stimulation Enhances Stable Plasticity and Generalization of Stroke Recovery. Stroke. 49(3), 710–717 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Porter BA, Khodaparast N, Fayyaz T, et al. Repeatedly Pairing Vagus Nerve Stimulation with a Movement Reorganizes Primary Motor Cortex. Cereb Cortex. 22(10), 2365–2374 (2012). [DOI] [PubMed] [Google Scholar]
- 92.Khodaparast N, Hays SA, Sloan AM, et al. Vagus nerve stimulation delivered during motor rehabilitation improves recovery in a rat model of stroke. Neurorehabil Neural Repair. 28(7), 698–706 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hulsey DR, Hays SA, Khodaparast N, et al. Reorganization of Motor Cortex by Vagus Nerve Stimulation Requires Cholinergic Innervation. Brain Stimul 9(2), 174–181 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Groves DA, Bowman EM, Brown VJ. Recordings from the rat locus coeruleus during acute vagal nerve stimulation in the anaesthetised rat. Neurosci. Lett 379(3), 174–179 (2005). [DOI] [PubMed] [Google Scholar]
- 95.Hulsey DR, Riley JR, Loerwald KW, Rennaker RL, Kilgard MP, Hays SA. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp. Neurol 289, 21–30 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Manta S, Dong J, Debonnel G, Blier P. Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. J Psychiatry Neurosci. 34(4), 272–280 (2009). [PMC free article] [PubMed] [Google Scholar]
- 97.Ganzer PD, Darrow MJ, Meyers EC, et al. Closed-loop neuromodulation restores network connectivity and motor control after spinal cord injury. Elife. 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tracey KJ. Neurons Are the Inflammatory Problem. Cell. 173(5), 1066–1068 (2018). [DOI] [PubMed] [Google Scholar]
- 99.Frangos E, Ellrich J, Komisaruk BR. Non-invasive Access to the Vagus Nerve Central Projections via Electrical Stimulation of the External Ear: fMRI Evidence in Humans. Brain Stimul 8(3), 624–636 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frangos E, Komisaruk BR. Access to Vagal Projections via Cutaneous Electrical Stimulation of the Neck: fMRI Evidence in Healthy Humans. Brain Stimul 10(1), 19–27 (2017). [DOI] [PubMed] [Google Scholar]
- 101.Mourdoukoutas AP, Truong DQ, Adair DK, Simon BJ, Bikson M. High-Resolution Multi-Scale Computational Model for Non-Invasive Cervical Vagus Nerve Stimulation. Neuromodulation. 21(3), 261–268 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nonis R, D’Ostilio K, Schoenen J, Magis D. Evidence of activation of vagal afferents by non-invasive vagus nerve stimulation: An electrophysiological study in healthy volunteers. Cephalalgia. 37(13), 1285–1293 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Capone F, Miccinilli S, Pellegrino G, et al. Transcutaneous Vagus Nerve Stimulation Combined with Robotic Rehabilitation Improves Upper Limb Function after Stroke. Neural Plast 2017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Redgrave JN, Moore L, Oyekunle T, et al. Transcutaneous Auricular Vagus Nerve Stimulation with Concurrent Upper Limb Repetitive Task Practice for Poststroke Motor Recovery: A Pilot Study. J Stroke Cerebrovasc Dis. 27(7), 1998–2005 (2018). [DOI] [PubMed] [Google Scholar]
- 105.Plow EB, Machado A. Invasive neurostimulation in stroke rehabilitation. Neurotherapeutics. 11(3), 572–582 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang M, Harvey RL, Stoykov ME, et al. Cortical stimulation for upper limb recovery following ischemic stroke: a small phase II pilot study of a fully implanted stimulator. Top Stroke Rehabil 15(2), 160–172 (2008). [DOI] [PubMed] [Google Scholar]
- 107.Levy R, Ruland S, Weinand M, Lowry D, Dafer R, Bakay R. Cortical stimulation for the rehabilitation of patients with hemiparetic stroke: a multicenter feasibility study of safety and efficacy. J. Neurosurg 108(4), 707–714 (2008). [DOI] [PubMed] [Google Scholar]
- 108.Levy RM, Harvey RL, Kissela BM, et al. Epidural Electrical Stimulation for Stroke Rehabilitation: Results of the Prospective, Multicenter, Randomized, Single-Blinded Everest Trial. Neurorehabil Neural Repair. 30(2), 107–119 (2016). [DOI] [PubMed] [Google Scholar]
- 109.Plow EB, Carey JR, Nudo RJ, Pascual-Leone A. Invasive cortical stimulation to promote recovery of function after stroke: a critical appraisal. Stroke. 40(5), 1926–1931 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boychuk JA, Schwerin SC, Thomas N, et al. Enhanced Motor Recovery After Stroke With Combined Cortical Stimulation and Rehabilitative Training Is Dependent on Infarct Location. Neurorehabil Neural Repair. 30(2), 173–181 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ramanathan DS, Guo L, Gulati T, et al. Low-frequency cortical activity is a neuromodulatory target that tracks recovery after stroke. Nat. Med 24(8), 1257–1267 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zanos S, Rembado I, Chen D, Fetz EE. Phase-Locked Stimulation during Cortical Beta Oscillations Produces Bidirectional Synaptic Plasticity in Awake Monkeys. Curr. Biol 28(16), 2515–2526.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cunningham DA, Varnerin N, Machado A, et al. Stimulation targeting higher motor areas in stroke rehabilitation: A proof-of-concept, randomized, double-blinded placebo-controlled study of effectiveness and underlying mechanisms. Restor. Neurol. Neurosci 33(6), 911–926 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Potter-Baker KA, Janini DP, Lin Y-L, et al. Transcranial direct current stimulation (tDCS) paired with massed practice training to promote adaptive plasticity and motor recovery in chronic incomplete tetraplegia: A pilot study. J Spinal Cord Med. 41(5), 503–517 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grefkes C, Fink GR. Noninvasive brain stimulation after stroke: it is time for large randomized controlled trials! Curr. Opin. Neurol 29(6), 714–720 (2016). [DOI] [PubMed] [Google Scholar]
- 116.Peckham PH, Kilgore KL. Challenges and opportunities in restoring function after paralysis. IEEE Trans Biomed Eng. 60(3), 602–609 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Keith MW, Kilgore KL, Peckham PH, Wuolle KS, Creasey G, Lemay M. Tendon transfers and functional electrical stimulation for restoration of hand function in spinal cord injury. J Hand Surg Am. 21(1), 89–99 (1996). [DOI] [PubMed] [Google Scholar]
- 118.FDA C for D and R. Neurocontrol Freehand (PMA) [Internet]. (1997). Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P950035.
- 119.Peckham PH, Kilgore KL, Keith MW, Bryden AM, Bhadra N, Montague FW. An advanced neuroprosthesis for restoration of hand and upper arm control using an implantable controller. J Hand Surg Am. 27(2), 265–276 (2002). [DOI] [PubMed] [Google Scholar]
- 120.Kilgore KL, Hart RL, Montague FW, et al. An implanted myoelectrically-controlled neuroprosthesis for upper extremity function in spinal cord injury. Conf Proc IEEE Eng Med Biol Soc. 1, 1630–1633 (2006). [DOI] [PubMed] [Google Scholar]
- 121.Kilgore KL, Hoyen HA, Bryden AM, Hart RL, Keith MW, Peckham PH. An implanted upper-extremity neuroprosthesis using myoelectric control. J Hand Surg Am. 33(4), 539–550 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moss CW, Kilgore KL, Peckham PH. A novel command signal for motor neuroprosthetic control. Neurorehabil Neural Repair. 25(9), 847–854 (2011). [DOI] [PubMed] [Google Scholar]
- 123.Memberg WD, Polasek KH, Hart RL, et al. Implanted neuroprosthesis for restoring arm and hand function in people with high level tetraplegia. Arch Phys Med Rehabil. 95(6), 1201–1211.e1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kilgore KL, Bryden A, Keith MW, et al. Evolution of Neuroprosthetic Approaches to Restoration of Upper Extremity Function in Spinal Cord Injury. Top Spinal Cord Inj Rehabil. 24(3), 252–264 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hochberg LR, Serruya MD, Friehs GM, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 442(7099), 164–171 (2006). [DOI] [PubMed] [Google Scholar]
- 126.Hochberg LR, Bacher D, Jarosiewicz B, et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 485(7398), 372–375 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Collinger JL, Wodlinger B, Downey JE, et al. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet. 381(9866), 557–564 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ethier C, Oby ER, Bauman MJ, Miller LE. Restoration of grasp following paralysis through brain-controlled stimulation of muscles. Nature. 485(7398), 368–371 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bouton CE, Shaikhouni A, Annetta NV, et al. Restoring cortical control of functional movement in a human with quadriplegia. Nature. (2016). [DOI] [PubMed] [Google Scholar]
- 130.Ajiboye AB, Willett FR, Young DR, et al. Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: a proof-of-concept demonstration. Lancet. 389(10081), 1821–1830 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; Cortical neural activity drives closed-loop control of functional electrical stimulation to restore upper limb function in patient with tetraplegia.
- 131.Colachis SC, Bockbrader MA, Zhang M, et al. Dexterous Control of Seven Functional Hand Movements Using Cortically-Controlled Transcutaneous Muscle Stimulation in a Person With Tetraplegia. Front Neurosci 12, 208 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Raspopovic S, Capogrosso M, Petrini FM, et al. Restoring Natural Sensory Feedback in Real-Time Bidirectional Hand Prostheses. Science Translational Medicine. 6(222), 222ra19–222ra19 (2014). [DOI] [PubMed] [Google Scholar]
- 133.Tan DW, Schiefer MA, Keith MW, Anderson JR, Tyler J, Tyler DJ. A neural interface provides long-term stable natural touch perception. Science Translational Medicine. 6(257), 257ra138–257ra138 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Davis TS, Wark H a. C, Hutchinson DT, et al. Restoring motor control and sensory feedback in people with upper extremity amputations using arrays of 96 microelectrodes implanted in the median and ulnar nerves. J Neural Eng. 13(3), 036001 (2016). [DOI] [PubMed] [Google Scholar]
- 135.Schiefer M, Tan D, Sidek SM, Tyler DJ. Sensory feedback by peripheral nerve stimulation improves task performance in individuals with upper limb loss using a myoelectric prosthesis. J Neural Eng. 13(1), 016001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; Restoration of sensation improves performance in patients with upper limb amputations who are using myoelectric prosthetic devices.
- 136.Page DM, George JA, Kluger DT, et al. Motor Control and Sensory Feedback Enhance Prosthesis Embodiment and Reduce Phantom Pain After Long-Term Hand Amputation. Front Hum Neurosci 12, 352 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Flesher SN, Collinger JL, Foldes ST, et al. Intracortical microstimulation of human somatosensory cortex. Sci Transl Med. 8(361), 361ra141 (2016). [DOI] [PubMed] [Google Scholar]
- 138.Hiremath SV, Tyler-Kabara EC, Wheeler JJ, et al. Human perception of electrical stimulation on the surface of somatosensory cortex. PLoS ONE. 12(5), e0176020 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Seymour JP, Wu F, Wise KD, Yoon E. State-of-the-art MEMS and microsystem tools for brain research. Microsystems & Nanoengineering. 3, 16066 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Orsborn AL, Moorman HG, Overduin SA, Shanechi MM, Dimitrov DF, Carmena JM. Closed-loop decoder adaptation shapes neural plasticity for skillful neuroprosthetic control. Neuron. 82(6), 1380–1393 (2014). [DOI] [PubMed] [Google Scholar]
- 141.Balasubramanian K, Vaidya M, Southerland J, et al. Changes in cortical network connectivity with long-term brain-machine interface exposure after chronic amputation. Nat Commun. 8(1), 1796 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Brandman DM, Hosman T, Saab J, et al. Rapid calibration of an intracortical brain-computer interface for people with tetraplegia. J Neural Eng. 15(2), 026007 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hiremath SV, Chen W, Wang W, et al. Brain computer interface learning for systems based on electrocorticography and intracortical microelectrode arrays. Front Integr Neurosci. 9, 40 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kao JC, Ryu SI, Shenoy KV. Leveraging neural dynamics to extend functional lifetime of brain-machine interfaces. Sci Rep. 7(1), 7395 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Orsborn AL, Pesaran B. Parsing learning in networks using brain-machine interfaces. Curr. Opin. Neurobiol 46, 76–83 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pandarinath C, Nuyujukian P, Blabe CH, et al. High performance communication by people with paralysis using an intracortical brain-computer interface. Elife. 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pandarinath C, Ames KC, Russo AA, et al. Latent Factors and Dynamics in Motor Cortex and Their Application to Brain-Machine Interfaces. J. Neurosci 38(44), 9390–9401 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shanechi MM, Orsborn AL, Moorman HG, Gowda S, Dangi S, Carmena JM. Rapid control and feedback rates enhance neuroprosthetic control. Nat Commun. 8, 13825 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bowsher K, Civillico EF, Coburn J, et al. Brain-computer interface devices for patients with paralysis and amputation: a meeting report. J Neural Eng. 13(2), 023001 (2016). [DOI] [PubMed] [Google Scholar]
- 150.Hacker ML, DeLong MR, Turchan M, et al. Effects of deep brain stimulation on rest tremor progression in early stage Parkinson disease. Neurology. 91(5), e463–e471 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]