Abstract
The glycocalyx coating the outside of most cells is a polymer meshwork comprising proteins and complex sugar chains called glycans. From a physical perspective, the glycocalyx has long been considered a simple ‘slime’ that protects cells from mechanical disruption or against pathogen interactions, but the great complexity of the structure argues for the evolution of more advanced functionality: the glycocalyx serves as the complex physical environment within which cell-surface receptors reside and operate. Recent studies have demonstrated that the glycocalyx can exert thermodynamic and kinetic control over cell signalling by serving as the local medium within which receptors diffuse, assemble and function. The composition and structure of the glycocalyx change markedly with changes in cell state, including transformation. Notably, cancer-specific changes fuel the synthesis of monomeric building blocks and machinery for production of long-chain polymers that alter the physical and chemical structure of the glycocalyx. In this Review, we discuss these changes and their physical consequences on receptor function and emergent cell behaviours.
Cell-surface receptors serve as the pivotal communication interface between the cell’s external environment and the intracellular signalling networks1. Receptors also mediate specific adhesion between cells and with the tissue matrix2. Over the past four decades, researchers have catalogued a molecular inventory of receptors and associated intermediates whose dysfunction or deregulation is directly linked to cancer progression. Well-known receptors that promote cancer include the family of receptor tyrosine kinases (RTKs), such as epidermal growth factor receptors (EGFR), human epidermal growth receptor 2 (HER2) and c-MET1,3.
Extensive progress has also been made in understanding the chemical biology of receptors, their mechanisms of action and the genetic basis of their perturbation in disease states1. The success of these endeavours is underscored by the fact that therapies targeting dysregulated receptors have been transformative in clinical treatment of multiple cancer types. Notable examples include ten FDA-approved targeted therapies against HER2/EGFR as of 20164 and multiple antibody-based drugs that are currently available to target HER2-positive breast cancers5.
Modern descriptions of receptor behaviour are largely explained through the concepts of solution chemistry, in which chemical affinities, reaction rates and enzyme kinetics govern the activation of downstream cellular processes6. However, physical constraints and spatial organizations imposed on molecules provide another hierarchical level of regulation7. Research over the past decade has provided compelling examples of how disruption of this physical regulatory layer could be causal for tumour progression8,9. A modern quest to develop a comprehensive physical understanding of the cancer cell and the coupled spatiotemporal dynamics of its molecules has now begun.
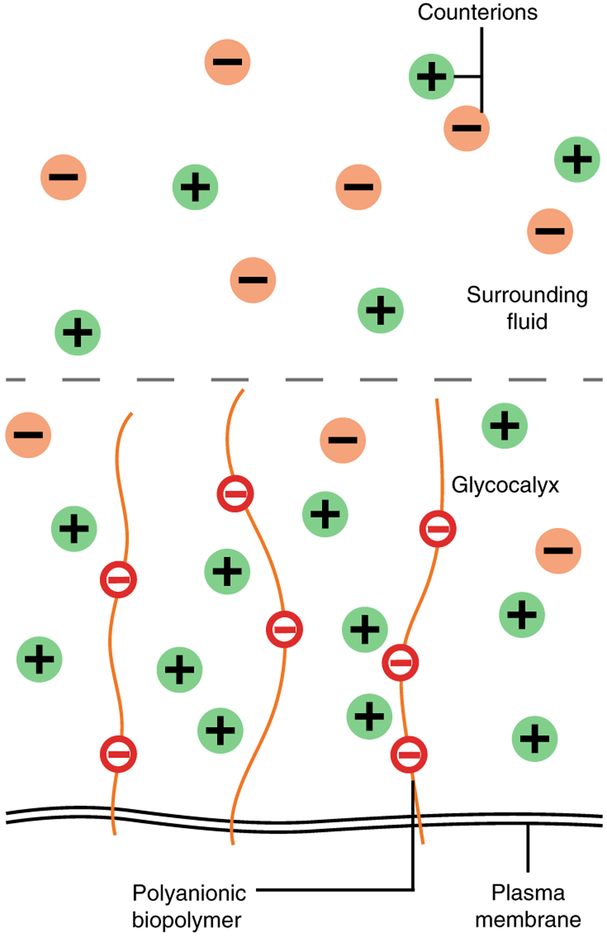
Largely overlooked in this quest is physical consideration of the glycocalyx8. Protein and sugar macromolecules are densely arrayed in the cancer cell glycocalyx, creating a physically crowded network in which embedded receptors must operate10. Emerging theories and experimental results implicate the importance of macromolecular crowding in nearly all aspects of protein function, including folding, conformational stability, mobility, binding of small molecules, enzymatic activity, homotypic assembly and interactions with other proteins11. Architectural details of the glycocalyx also control the spatial positioning of receptors and their binding partners (ligands) on other cells, in the tissue matrix, within the same cell membrane or even free in solution12. As such, the glycocalyx may be ideally positioned to broadly mediate or attenuate receptor function (Fig. 1). Indeed, normal cells engineered to exhibit cancer-like glycosylation can acquire oncogenic features with enhanced cell signalling, survival and invasion8,13.
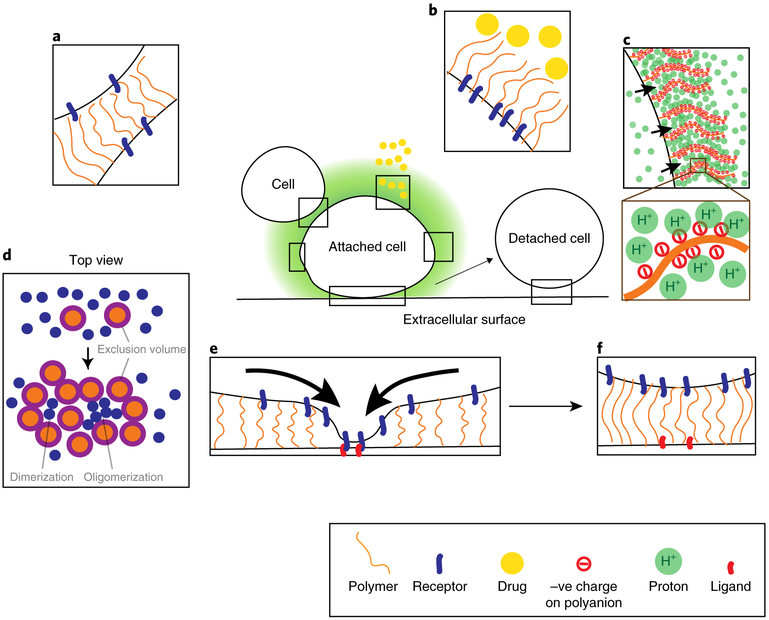
Fig. 1. Overview of large polymers on the cancer cell surface.
Cell surface receptors (blue) are communication devices that direct cancer cell behaviour. These receptors are usually of the order of 20–30 nm and are towered over by glycocalyx polymers (orange). a,b, The presence of large polymers can sterically block the interaction between receptors and their ligands to prevent cell-cell contacts (a) and impart barrier function (b) to block drug molecules (yellow). c, By charge attraction, the presence of polyanions may concentrate protons on the cell surface to decrease local pH. d, Top view of the cell surface. Glycocalyx polymers can occupy large volumes that exclude receptors as they avoid molecular overlaps (purple). This energetically favours the close-packing (oligomerization) of small receptors to reduce the excluded volume. e, A moderately dense glycocalyx can promote the clustering of cell surface receptors and enhance cell adhesion. The black arrows indicate the clustering of receptors towards and into adhesion sites. f, On the other hand, an overly dense glycocalyx can increase the spacing between receptors and their ligands. This can lead to cell detachment.
In this Review, we discuss some of the key physical attributes of the cancer glycocalyx and consider why an altered glycocalyx may be a ‘universal’ feature in cancer. We next describe some of its important physical biology, drawing from existing theories in electrostatics, polymer physics and colloidal science for guidance. A central perspective of this Review is that the glycocalyx and its embedded signalling machinery function as an integrated sensory material whose responses are a product of both its physical structure and biomolecular parts. Tacitly implied in this perspective is that rational disruption of the glycocalyx alone or in combination with traditional therapeutic agents may be a future strategy for cancer intervention.
Molecular architecture of the cancer cell glycocalyx
The glycocalyx architecture varies considerably amongst different cell types. Conceptually, the molecules of the glycocalyx are arranged into two functionally distinct layers: a lower communication interface and a polymer-based structural network that extends through and above it (Fig. 2). The communication interface prominently includes receptors, other integral membrane proteins, and sugar macromolecules called glycans. A high degree of molecular crowding typifies the interface. For instance, integral membrane proteins have an estimated density of 4–5 per 100 nm2 and an average diameter of 5 nm (ref. 14), suggesting that molecules are nearly close-packed in the lower strata of the glycocalyx. Most receptors and proteins extend 30 nm or less from the membrane, although some, like the Notch receptor, can extend further15.
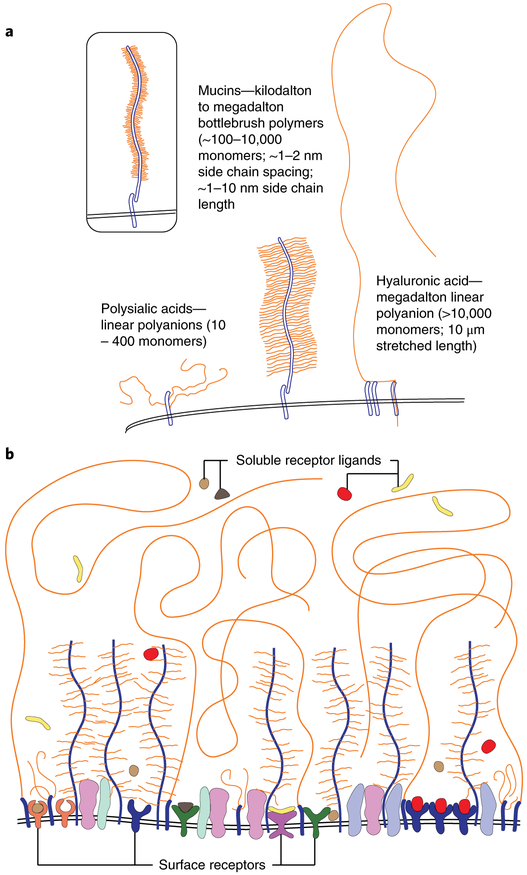
Fig. 2. Glycocalyx architecture and communication interface on the cell surface.
a, Bottlebrush mucin polymers and polyanions such as HA and polysialic acid are common structures in the glycocalyx. In cancer, mucin polymers become decorated with a higher density of short glycan side chains on the polypeptide backbone (insert). b, The glycocalyx as a structural polymer brush and communication interface on the cell surface. A structural network composed of glycopolymers including mucins, HA and polysialic acid acts as a physically crowded layer shielding cell surface molecules. Large polymers are densely arrayed in the cancer cell glycocalyx, creating a crowded network in which embedded cell-surface receptors must operate. The glycocalyx may be ideally positioned to physically mediate or attenuate receptor function on a global level.
Perturbations to the communication interface are well documented for many types of cancer16. Specific changes are selected for based on the microevolutionary advantages that they provide to the tumour16. Receptors are often produced at different levels or with altered functionality (mutated). The interface is reconfigured to ensure that the physical and chemical information from the external environment is perceived in a way that best serves the interest of the tumour cell1. For example, growth-promoting stimuli are amplified, death-inducing cues are ignored and topographical cues from the tissue matrix are recognized as ‘highways’ or escape routes for cell dissemination17,18.
Superimposed over and above the communication interface is a protein and polysaccharide network that forms the structural layer of the glycocalyx. As with the communication interface, specific changes to the structural network seem to be selected for during tumour progression. Notably, acquisition of the most aggressive and lethal cancer phenotypes often correlates with abundant expression of high-molecular-weight biopolymers that form a thick and often dense glycocalyx on the cancer cell surface8. Adopting standard polymer nomenclature, the polymers fall into two categorical types: linear and bottlebrush polyelectrolytes.
Hyaluronic and polysialic acids are linear polyelectrolytes
Linear polyelectrolytes are unbranched polymers whose repeating unit contains a charged group. Negatively charged biopolymers of this type are major structural elements in the glycocalyx of a wide spectrum of metastatic cancer cells19,20. One important example is hyaluronic acid (HA), a very large polymer assembled from repeating disaccharide units that contain glucuronic acid (pKa ≈ 3.3)19,21. Abundant HA production in the tumour often correlates with the lethality of the disease. Increased expression of the machinery associated with HA synthesis and anchorage on the cell surface also provide a signature that can predict poor patient survival in diverse cancer types19,22,23.
A remarkable feature of HA is its massive size. HA synthesis is catalysed and orchestrated at the plasma membrane by a class of membrane-spanning enzymes called HA synthases24. These exquisite machines reliably and repeatedly synthesize polymers with over 10,000 monomers, corresponding to a total polymer length of over 10 μm (ref. 25). The synthases work by processively adding monosaccharides from the cytoplasm to the HA polymer end and simultaneously extruding the growing polymer through the plasma membrane and into the extracellular space24. HA can then be anchored to the cell surface by specific HA receptors, such as CD44, or by remaining associated with the synthases26.
The flexibility of HA is described by an intrinsic property of polymers called persistence length27, which is roughly the length over which a segment of a polymer will look and behave in a rod-like manner28. The persistence length provides a useful description of a polymer’s shape and mechanical behaviour (Fig. 3a). Polymers whose overall contour length is much longer than the persistence length adopt a random-coil configuration and compress more like a spring29. Polymers whose overall size is comparable to or smaller than the persistence length appear rod-like and compress and flex like a beam.
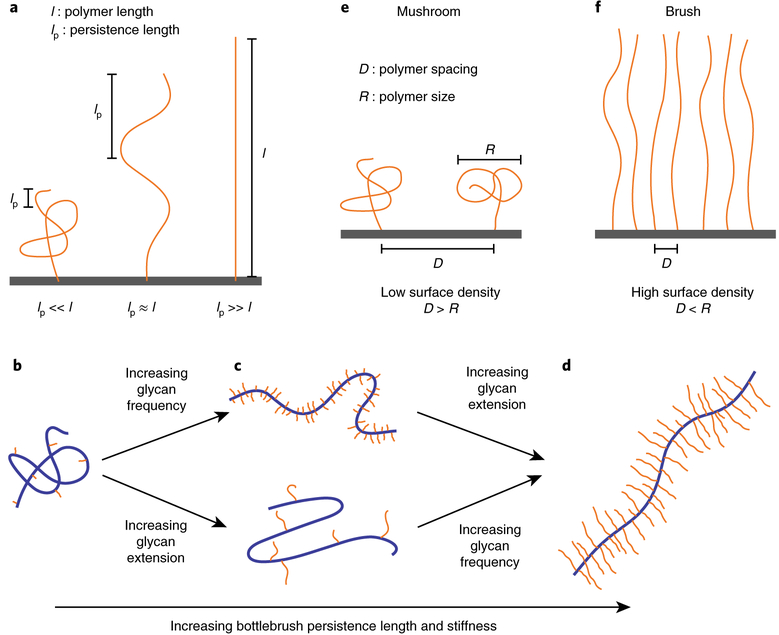
Fig. 3. Physical aspects of polymers.
a, The persistence length, lp, of a polymer is the length scale over which the molecule does not bend. The persistence length is directly proportional to polymer bending stiffness. A flexible polymer with lp≪l adopts a coiled-up shape without any external forces. On the other hand, a polymer with lp≫l does not bend in the absence of external effects and thus is rod-like and stiff. Polymers with comparable length scales lp ≈l fall in between, possessing intermediate stiffness and adopting partially deformed configurations. b–d, Bottlebrush polymers are composed of side chains attached to a polymer backbone, and their structures are controlled by the density and length of the attached side chains. Bottlebrush polymers with short and infrequent side chains adopt flexible structures (b). Moderate increase in glycan frequency or glycan extension stretches out the bottlebrushes partially and enhances the persistence length (c). Heavily glycosylated bottlebrush polymers with high glycan frequency and extension are extended into stiff rod-like structures with a large persistence length (d). e,f, Polymers attached to a substrate with a characteristic polymer size, R, and a spacing between polymers of D exhibit two regimes of physical behaviour. Mushroom regime: at low densities such that D > R, polymers do not sense neighbouring molecules (e). Brush regime: at high surface concentrations, polymers experience steric repulsion with neighbours and, to reduce crowding, they extend out into a brush-like structure (f).
HA has a persistence length of about 5 nm in physiological solutions and adopts a random coil configuration27. The moderately large persistence length can be explained by the rather inflexible linkages between sugar monomers in HA and by additional short-range interactions, including water bridging and hydrogen bonding between nearby monomers. In solvents with low ionic strength, the persistence length of HA increases considerably, owing to large electrostatic repulsions between anionic monomers, causing random coils of HA to expand. However, in physiological conditions, these charges are largely shielded by positively charged ions that concentrate around the anionic monomers, effectively reducing the range of their Coulombic attractions and repulsions to only about 0.7 nm (the Debye length). This picture is complicated by negatively charged proteoglycans, such as versicans that may promote cancer30, which can bind and stretch a HA polymer towards its full length31. It should be noted that each HA disaccharide in solution also organizes ~15 water molecules32, sheathing HA in a ‘watery cloud’ that contributes to its minimal protein-binding behaviour and low fouling33.
Polysialic acid (polySia) is another large polyanion that is often upregulated in the cancer glycocalyx. Composed of repeating units of sialic acid (pKa ≈ 2.6)21, these linear polyanions are covalently anchored on the surface of neural cell adhesion molecule (NCAM) in a number of cancers34. PolySia reaches a degree of polymerization with approximately 400 monomers (~125 kDa) and may adopt either an extended helical structure35 or a random-coil configuration36,37, suggesting that its persistence length may change markedly based on environmental context. Interestingly, some studies suggest that polySia can assemble with specific growth factors to form massive co-polymers and networks38. This system has been proposed to serve as a storage device for excess mitogenic capacity. Cancer regulation of polySia is not as well understood as for HA, but overexpression of its synthetic enzymes also predicts poor patient survival in several cancer types39-41.
Mucins are bottlebrush polymers
Bottlebrush polymers are macromolecules with a linear polymer backbone decorated with polymeric side chains. The unique molecular and particulate features of bottlebrushes are exploited in both synthetic and living systems for use as rheological modifiers, non-fouling surface coatings and stimuli-responsive coatings42. The lethality of several cancer types is strongly correlated with a great increase in the production of a class of bottlebrush biopolymers called mucins. The structure of cell-surface mucins consists of an unstructured central polypeptide backbone with densely grafted polymeric sugar side chains (glycans) and a transmembrane domain that directly anchors one end of the polymer to the plasma membrane. The mucin side chains are frequently terminated with sialic acids (pKa ≈ 2.6)21, making mucins a negatively charged polyelectrolyte bottlebrush at physiological pH.
The mucin bottlebrush domains can be massive, Muc16 being the largest with >22,000 amino acids (AA) in its backbone and an overall molecular weight that can reach up to 20 million daltons, including side chains43. Fully stretched MUC16 is likely to reach ~1,500 nm on the cell surface44. Other cell-surfaced mucins linked to cancer are podocalyxin (~400 AA; ≤165 kDa), Muc1 (1,500 AA; ≤450 kDa) and Muc4 (~7,500 AA; ≤5 MDa). The prevalence of Muc1 in cancer is particularly notable. Muc1 is overexpressed in more than 60% of all cancers diagnosed each year in the United States and in more than 90% of cancers of specific types, including breast45. High Muc1 levels in the tumour can predict metastasis, poor patient response to treatment and low patient survival rate in multiple cancers including those of breast, lung, gastrointestinal and pancreas origins45,46. Normal epithelial cells are usually polarized, maintaining the large mucins on the top, or apical, surface of the cell. A loss of cell polarity is a common feature in cancer and can redistribute the bottlebrush polymers to cover the entire cell surface45. The redistributed mucins interact with receptors residing on the bottom, or basal, cell surface and alter signalling responses such as from RTKs to drive cancer progression47.
The conformation and physical properties of the brush-like macromolecules are controlled by the steric repulsion between their densely grafted side chains48 (Fig. 3b-d). Increase in grafting density or length of the side chains—that is, an increase in the frequency or extent of glycosylation—enhances the crowding of side chains on the bottlebrush backbone. The consequently stronger steric repulsions between side chains in the crowded environment can stretch the bottlebrush into a stiffer structure48,49. For high densities of side chain grafting, the persistence length of a bottlebrush polymer is approximately the same as the length of the glycan side chains48. The length of glycan side chains is estimated to be 5–10 nm for mucins44,50, and, correspondingly, mucins are known to have persistence lengths of about 10 nm as well51,52.
Altered glycosylation in cancer can affect the length and grafting density of side chains on mucins. Glycan side chains are initiated by the addition of a single sugar N-acetylgalactosamine (GalNAc) to the −OH group of serines and threonines on the mucin polypeptide backbone. Hence, the side chains of mucins are called O-linked glycans, or simply O-glycans. The sugar chains are elongated by the sequential addition of other sugar monomers in the Golgi apparatus, a series of membrane-enclosed chemical reactors inside the cell. The length and grafting density of the glycan side chains are controlled by enzymes called glycosyltransferases, which are organized sequentially in sub-compartments of the Golgi. Oncogenes and other drivers for carcinogenesis can change the cellular location and overall composition of glycosyltransferases and the availability of sugar monomer substrates for glycan extension53. These changes often produce cancer mucins with short and sialylated glycan side chains but at a higher grafting density53,54. Future studies are needed to unravel how these cancer-associated changes affect the molecular properties, including flexibility, of individual mucins.
Glycocalyx behaviour from a polymer brush perspective
How high-molecular-weight polymers behave as an ensemble in the glycocalyx is still under investigation. However, mucins and other glycopolymers are anchored to the membrane in such a way that long polymer chains or polymer loops extend from the cell surface. This network approximately resembles a well-studied structure in polymer physics called a polymer brush, composed of polymers grafted on one end to a surface (Fig. 3e,f). Pioneering efforts by Alexander and de Gennes led to the development of elegant relations capturing the physical behaviour of polymer brushes55,56. These scaling relations or ‘scaling laws’ are simple, yet useful in illustrating important aspects of how the glycocalyx structural layer is expected to behave, at least to a first approximation57.
Notably, brush theory provides insight into the balance of opposing forces at play in an anchored polymer network, like the glycocalyx58,59. For densely crowded polymers, steric repulsion between the polymer chains drives stretching of the molecules away from the surface (that is, the membrane). An opposing elastic energy arises from the decreased entropy associated with the smaller number of configurations that the stretched molecules can wiggle into and explore. More simply stated, a polymer behaves as an elastic spring and tries to recoil itself when stretched. For charged polymers, additional forces arise from (1) the electrostatic repulsions between chains and (2) the osmotic pressure associated with recruitment of oppositely charged ions from solution to the polymers60. However, theory and experiment indicate that at high physiological salt concentrations, the osmotic and electrostatic forces may be small compared with the elastic and steric forces58.
Balancing of forces leads to the scaling relations describing the behaviour of the network. For example, Alexander balanced the elastic and steric forces and found the brush height, h, to scale as σ1/3, where σ is the density of polymers on the surface. In other words, how far polymers extend and stretch out from the surface varies in a very predictable way based on how densely the polymers are grafted. Theories have now been developed to describe several relevant behaviours of the glycocalyx including thickness and compression of polymer and polyelectrolyte brushes60,61; diffusion and equilibrium distributions of soluble particles and macromolecules in the brush62; and shearing of brush bilayers60.
Some of the key polymer brush behaviours have now been observed in cells and biomimetic systems that recapitulate the glycocalyx. For instance, Muc1 polymers appear to extend considerably from their random coils when densely crowded on the surface of epithelial cells8. HA polymers similarly extend into a brush configuration with the help of negatively charged proteoglycans31,63. Rubinstein and colleagues demonstrated that mucins in the glycocalyx of lung airway epithelium probably form a dense polymer brush that lifts and suspends mucus above cells64. Notably, brush theory successfully predicted conditions in cystic fibrosis where the mucin polymer brush would collapse, eventually slowing down the beating motions of cilia and stopping mucosal clearance64. Atomic force microscopy measurements of the endothelial glycocalyx, a structure rich in HA and other anionic glycopolymers, are consistent with brush expectations for compression65. Studies in biomimetic systems have also shown that reconstructed HA and mucin brushes follow expected scaling laws for brush extension, compressibility and response to changes in solution ionic strength66,67.
Of course, brush theory probably does not accurately describe behaviour close to the membrane, because many of the crowded proteins and macromolecules in the receptor interface are too rigid to accurately model as flexible polymers. Some studies have instead modelled the more rigid macromolecules as hard disks, successfully replicating some aspects of membrane behaviour, such as bending68. Another complicating factor is the potential for crosslinking in the network, for example through multivalent sugar binding proteins called lectins and HA binding proteins (such as TSG-6)69. Finally, the structure will be permeated with other macromolecules (‘nanoinclusions’) and unanchored glycopolymers that can be shed from the membrane70.
Cancer metabolism and glycopolymer synthesis
In the 1920s, Otto Warburg famously observed that tumour cells take up large amounts of glucose and ferment it into lactate, even in the presence of sufficient oxygen to run oxidative phosphorylation, which is a much more efficient energy production process from the perspective of cellular energetics71,72. How tumour cells actually benefit from the ‘Warburg effect’ has remained mysterious until recently, when intense research over the past decade has uncovered at least one explanation73. By rewiring their metabolism, cancer cells can shunt more glucose and nutrients into making building blocks to meet the anabolic demands of rapid cell growth74. An important consequence of the reprogrammed metabolism is a diversion of nutrient flux into the anabolic pathways that generate building blocks for the synthesis of glycans74-76 (Fig. 4).
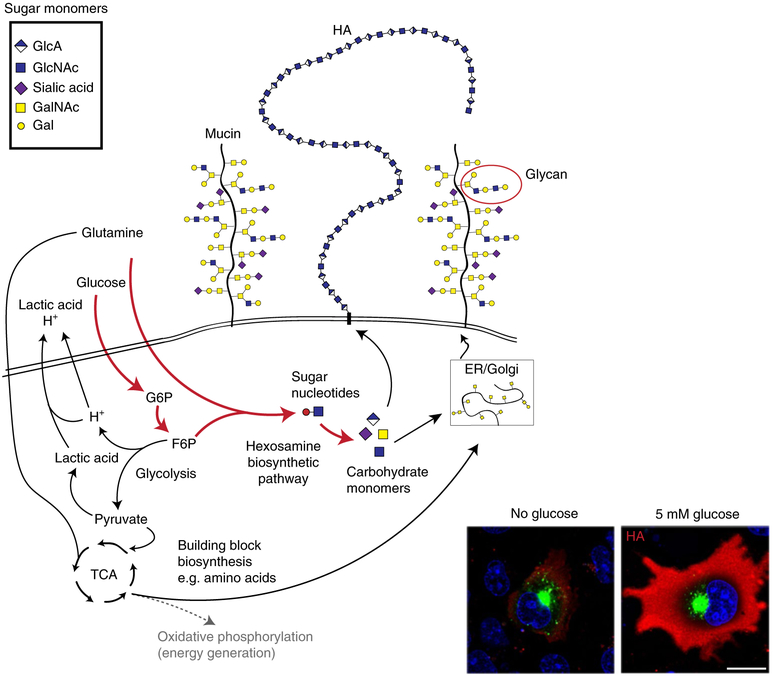
Fig. 4. Cancer cell metabolism is rewired to produce large polymers in the glycocalyx.
Normal cells use nutrients such as glucose to run oxidative phosphorylation that efficiently produces energy (dotted grey line). In cancer cells, these nutrients are instead diverted into the hexosamine biosynthetic pathway to produce sugar nucleotide monomers and enhance the production of glycocalyx polymers such as mucins and HA. Cancer cells also produce large amounts of lactic acid and H+ that must be pumped out of the cell to avoid toxicity. Supplementing glucose can greatly increase the amount of HA (stained red in the inset) on the cell surface (fluorescence images reproduced from ref. 167, American Society for Biochemistry and Molecular Biology). ER, endoplasmic reticulum; TCA, tricarboxylic acid.
The Warburg effect funnels more glucose and other nutrients into the hexosamine biosynthetic pathway (HBP), a sequence of reactions that generates the sugar nucleotide monomers for glycan chain construction77. HA provides an illustrative example of how glucose uptake, metabolic programming and polymer synthesis are tightly coupled. Simply feeding cells in culture more glucose or glucosamine, which is converted to N-acetylglucosamine intermediate in HBP, greatly increases HA content in the cellular glycocalyx78 (Fig. 4). HA production and intracellular sugar nucleotide availability are so tightly coupled that HA synthesis has been proposed as a rheostat controlling acceptable ranges of intracellular sugar nucleotides79. For instance, recent reports indicate that high levels of HA sugar nucleotide signal to suppress the internalization and lysosomal destruction of HA synthases, providing a mechanism to dump out excess intracellular sugar through the construction and extracellular extrusion of HA polymers80. Coupled metabolic reprogramming and HA production may have a role in the initiation of cancer. For example, increased expression of glutamine fructose-6-phosphate amidotransferase (GFAT), the rate-limiting enzyme of HBP, correlates with HA content in the early stages of melanoma80.
Metabolic flux through the HBP is also associated with downstream mucin-type O-glycosylation, sialylation and polysialylation in cancer cells, suggesting broad consequences of the Warburg effect on glycocalyx structures81-83. HBP flux can be increased by metabolic pathways that indirectly connect to the HBP. For example, upregulated folic acid metabolic pathway can increase HBP flux for sialic acid synthesis84. The loss of a glycogen debranching enzyme, AGL, also enhances the Warburg effect and diverts its use for HA synthesis85,86. Metabolic reprogramming into the HBP is now thought to be an immediate consequence of an oncogenic mutation, such as by KRAS in pancreatic cancer83, or as an adaptive response to sustained environmental stresses, such as low oxygen conditions (hypoxia) in the centre of tumours87, as well as in the transition into aggressive behaviour88-90. Importantly, recent genetic and pharmacological studies have conclusively demonstrated that the Warburg effect is a requirement for tumour growth91,92, arguing that an altered glycocalyx structure is a hallmark of cancer.
Altered receptor function regulated by cell surface acidity
Acidic extracellular pH is important in tumour progression. Cancer cells generate and pump out increased amounts of H+ ions and lactic acid as a consequence of their reprogrammed metabolism93. Extracellular acidification stimulates cells through specialized acidsensing ion channels and proton-sensing receptors94. It also broadly alters the function of receptors and other cell surface machinery, which have typically evolved to work optimally at a near-neutral pH in normal healthy tissues.
The anionic polymers and glycans in the cancer cell glycocalyx attract H+ and other positively charged ions from the surrounding fluid, affecting local pH. The situation is similar to a classic thermodynamics problem originally described by Frederic Donnan a century ago95,96. The problem considers the redistribution of ions between an electrolyte reservoir and a solution of entrapped polyelectrolytes that are blocked from passing into the reservoir by a semi-permeable membrane. The glycocalyx is analogous to the polyelectrolyte solution (Fig. 5). In this case, the structural integrity of the glycocalyx network functions as the barrier to diffusion between the glycocalyx solution and the extracellular fluid. Cations and protons migrate from the fluid reservoir into the anionic network and concentrate until the charge is neutralized and the ‘Donnan equilibrium’ is reached. Although the Donnan formalism is simplistic, it successfully predicts the distribution of ions in entangled mucin networks97 and bio-polyelectrolyte brushes58. However, more detailed solutions to the Poisson–Boltzmann equation that include additional effects of charge ionogenicity, steric exclusion and charge distribution within the glycocalyx are available and could provide more accurate descriptions of the glycocalyx, particularly when its molecules have a highly non-uniform distribution98.
Fig. 5. Donnan equilibrium between the glycocalyx and surrounding fluid.
Acting as a semi-permeable polyelectrolyte network, the glycocalyx attracts and traps cations from the surrounding fluid. This increases the osmotic pressure or concentration of ions in the glycocalyx and reduces the local pH.
Notably, these models show that when distributed near the membrane, even a modest sialic acid density of ~2.5 × 105 molecules per square micrometre in the glycocalyx can lower the cell surface pH by approximately 1 unit below the bulk extracellular pH98. In comparison, sialic acid surface densities are reported to range from 1.5 × 105 to 5 × 106 molecules per square micrometre on cells99, and increased sialylation of the glycocalyx is a common feature of many human cancers100. Recent studies using optical sensors have confirmed that the pH near cancer cell surfaces can be fairly acidic101. Indeed, the pH immediately above the cell membrane measures between pH 6.1 and 6.4 in the tested cancer models101. To what extent the lowered pH can be attributed to the glycocalyx charge density rather than to other contributing factors is still pending resolution.
Acidification of the cell surface has important implications for cell receptor function and may provide a selective pressure for tumour evolution. Cancer cells that have adapted to an acidic environment are more motile and aggressive102. Prominent arginine (pKa ≈ 12) to histidine (pKa ≈ 6.5) mutations in cancer are proposed to give mutant proteins the ability to sense pH103. EGFRs that carry these mutations become activated by tumour pH changes, even in the absence of ligand stimulation104. Other receptors that have evolved specifically to sense protons can become activated in the acidic conditions of the tumour. For instance, the protonation of extracellular histidines on a G-protein-coupled receptor results in receptor activation and signalling for cancer invasion94,105. Acidity also has large, non-specific effects on the affinity and rates of certain receptor–ligand interactions. For example, a cell surface pH of 6.1 can cause 70% or more of integrin adhesion receptors to disengage their extracellular matrix (ECM) ligands106, possibly favouring faster modes of cell migration107.
Physical forces in the tumour can enhance the acidification effects by the glycocalyx. Polymer brushes and networks, like the glycocalyx, are soft materials that can deform under moderate loads. Theory shows that cell surface potential is enhanced and pH is reduced when the anionic structure of the glycocalyx is denser and distributed more closely to the membrane98. Compressive stresses on the glycocalyx would have this effect. Using a genetically encoded force sensor, recent studies indicate that the negatively charged mucins in the glycocalyx are compressed when the cell adheres and contracts against the ECM8. Cancer cells are known to generate considerably higher contractile forces than their normal counterparts and can pull harder against the rigid ECM of tumours108. Cancer cells also experience compressive forces when they are disseminating through tightly confined tissue spaces, where the ensuing forces can deform even the comparatively rigid cytoskeletal network and nucleus109. In yet another physical perturbation in the tumour, cancer cells endure large solid stresses, up to 10 kPa, that result from the growth and physical expansion of tumours in the confined tissue spaces of the body110. Because glycocalyx compliance is estimated to be 200 Pa (elastic modulus) or less65, forces of this magnitude are expected to impose considerable strains on the glycocalyx structure.
Macromolecular crowding in the glycocalyx
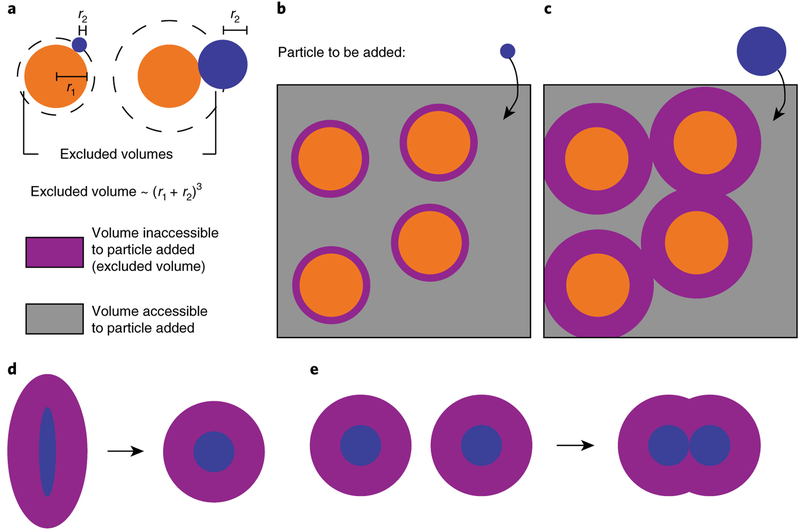
One of the most intuitive laws of nature is that two finite-sized objects cannot occupy the same space at the same time. Each molecule in a system thus excludes another molecule from a fraction of the total system volume (Fig. 6). Although this physical principle is simple, the consequences on reaction rates and equilibria in a crowded system can be striking11,111,112. The ‘excluded volume effects’ are particularly important in tissues, cells and subcellular structures, like the glycocalyx, that are densely packed with macromolecules11,113. This section considers the significance of macromolecular crowding in the glycocalyx, using signalling through soluble growth factors as an illustrative example. These factors convey information across short or long distances to target cells, where they bind and stimulate specific cell-surface receptors114. Communicating in this way, one cell can regulate another cell’s growth, proliferation, death, nutrient uptake, differentiation or migration115.
Fig. 6. Excluded volumes depict crowding in the glycocalyx.
a, Molecules have excluded volumes where the centre of another molecule cannot exist. The excluded volume of two spheres of size r1 and r2 is proportional to (r1 + r2)3. b,c, Addition of a particle (blue) to a box filled with other particles (orange) depicts the implications of excluded volume (purple) for molecular transport, as expected for diffusion of soluble ligands through the glycocalyx. A small particle encounters small excluded volumes, enjoying access to considerable space and several positional states, and thus requires less energy to diffuse to the cell surface (b). On the other hand, a large particle finds it difficult to enter and diffuse through a crowded environment owing to large excluded volumes and consequently requires higher energy for transport (c). d,e, A crowded environment energetically favours changes that decrease the total excluded volume of molecules, such as conformational shape changes in a protein (d) or the clustering and association of proteins (e) on the cell membrane.
The excluded volume effect is non-specific and based solely on steric exclusion116-118. Consider a soluble test molecule that is introduced into a crowded system. Macromolecular crowding restricts the number of unique positions in which the test molecule can be placed in the system, reducing the molecule’s entropy and increasing the system’s free energy. Any reaction or process that increases the volume available to molecules lowers the free energy and is favoured, regardless of the type of molecules or the process that transforms them. Folding of macromolecules into more compact states and molecular associations reduces the excluded volume in the system (Fig. 6). Consistent with expectations, binding interactions between receptors and ligands are favoured in the cell-surface glycocalyx, as recently shown119.
The strength of the excluded volume effect on a process depends on the extent of macromolecular crowding and the magnitude of the volume change. The free energy difference associated with an excluded volume change, Δv, is given by ΔG = ΠΔv, where Π is the osmotic pressure of the medium. The osmotic pressure is related to the crowding and can be thought of as an amplification factor that magnifies the effect of any arbitrary volume change, Δv. Notably, the osmotic pressure varies in a highly nonlinear way with the number of crowding agents in the system120,121. For instance, the osmotic pressure increases quadratically with polymer surface density in a de Gennes brush122.
Excluded volume effects in the cancer cell glycocalyx were first reported by Chinard and colleagues nearly 25 years ago123. Novikoff hepatocarcinoma cells poses a dense, mucin-rich glycocalyx. Centrifuging the cells through oil to remove “all extracellular water except that intimately associated with the cell,” Chinard found that glycocalyx volumes accessible to solutes are inversely related to the solute size, similar to a gel chromatographic matrix. Remarkably, solute partitioning in the Novikoff glycocalyx matches expectations for an ideal system that is crowded with inert, non-interacting agents.
The large glycopolymers in the cancer glycocalyx are expected to behave as ideal crowding agents in many respects. Polymers that have a large and predominantly repulsive interaction with proteins tend to induce macromolecular associations and compaction in accord with crowding theory124-126. In fact, the effect of macromolecular crowding on a particular reaction in solution is generally studied by measuring changes in reaction rates or equilibrium in the presence of ‘inert’ sugar or synthetic polymers11. In contrast, protein crowding elements can disrupt protein structure and function through weak interactions, negating the benefits of crowding127,128. These crowding-effect observations are consistent with fundamental theory predictions in the literature on Brownian suspensions112.
Assembly of cell-surface receptors into clusters has emerged as a general principle for signal modulation7. Transduction of soluble cues by RTKs is an important example. Ligand-induced assembly of RTKs into homodimers or higher-order oligomers is often crucial for generating competent signalling receptors from auto-inhibited monomers114. For instance, the EGFR can assemble into dimers, tetramers, hexamers and higher-order oligomers129,130. Studies suggest that the dynamics of EGFR signalling response depends on an intricate balance of EGFR dimers and oligomers modulated by ligand concentration and binding affinity129. The same principle is likely to hold true for other family members of human EGFR (HER2 and HER3) as they assemble to form higher-order oligomers131.
Macromolecular crowding can strongly favour oligomerized states and in a highly nonlinear manner. For example, physiological crowding can increase the equilibrium association constant 10-fold for two 40-kDa monomers that form a dimer132,133. If the dimers can form a homotetramer, the association constant increases 10,000-fold. Such effects strongly argue that crowding could be a major determinant of RTK oligomer status, although the possibility has not been tested directly.
The effects of crowding on the rates of association between species are complex11. Crowding affects the overall association rate in two important ways. First, it is expected to effectively lower the activation energy barrier for reaction and increase the association rate constant by a factor similar to the increase in the equilibrium association constant134. Second, it controls the reaction frequency by influencing how often reacting partners encounter each other through diffusion135. Fast intrinsic reactions are limited by the diffusion rate136, which has a complex dependence on the number, size and mobility of the crowding elements as now discussed.
When crowding elements are low in concentration (volume fraction), their hindrance to diffusion is linear in the (small) volume fraction. However, as concentration grows, multi-body interactions exert a stronger influence, and diffusivity can drop precipitately, vanishing quadratically with volume fraction relative to maximum packing121. Reduction of available volume due to the presence of fixed objects, such as tethered mucins, can give rise to spatially heterogeneous dynamics112,137,138. A particle attempting to diffuse through a fixed network will diffuse locally within a ‘pore’ or ‘cage’ of nearest neighbours. If multiple particles are diffusing through such an environment, the presence of these tethered cages can sequester the diffusers closer together and for longer periods of time, increasing the likelihood of encounter (or influencing protein stability)139.
Polydispersity, combined with crowding, can also greatly increase the frequency of encounters between smaller particles (E. Gonzalez, C. Aponte-Rivera and R.N.Z., manuscript in preparation). As a volume becomes more crowded, it is entropically favourable for smaller particles to locate preferentially in the interstitial volume between larger particles. This well-known phenomenon in bulk suspensions underlies the suppression of the formation of crystal structure. But in locally confined pockets, this effect can be even more pronounced, leading to the formation of regions in which larger particles are nearly excluded and smaller particles are caged into a nano-environment where their encounters with one another are markedly more favourable than encounters with larger particles.
Coupling of chemistry and mechanics by the glycocalyx
The ability of cells to adhere and communicate through close physical contact with each other and with the ECM is essential for a broad range of processes, including stem cell differentiation, coordinated cell migration, tissue patterning and tumorigenesis140. These physical interactions are often mediated by specific receptors that bind ligands tethered to the ECM or to the membrane of nearby cells. The finite sizes of receptors and ligands are key physical constraints on the probability of forming successful bonds when each binding partner is tethered to a structure (such as membrane or tissue matrix). Indeed, the dynamics and stability of bonds depend on how far the molecules must stretch to reach each other and how much force is loaded on the ensuing bond141. The glycocalyx can have a large effect on these dynamics by setting the physical spacing between cells and with the ECM, and by controlling the effective compliance of these interfaces10.
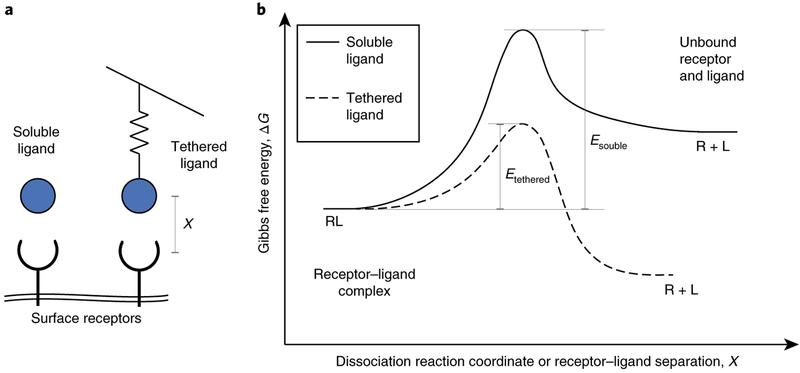
To illustrate, consider a tethered molecule that must stretch like a spring to bind another tethered molecule across a gap. This spring exerts a tensile force on the receptor–ligand pair. Conceptually, this force on the stretched molecules tilts the bond’s energy landscape, changing the relative heights of the activation energy barriers and energy minima (Fig. 7). For an interaction described by a single activation barrier, the tensile force lowers the energy barrier to dissociation, thereby speeding up dissociation (Fig. 7)142. Most receptor–ligand bonds behave this way under tension and are called ‘slip’ bonds143. However, bonds with more complex energy landscapes can actually become stronger under load141,144. These bonds are called ‘catch’ bonds and probably evolved to withstand high loading forces145.
Fig. 7. Tethered ligand binding.
a, Whereas soluble ligands can bind receptors relatively freely, ligands tethered to stiff molecules and substrates in the cellular environment can be considerably impeded by a spring-like resistance from the tether. b, Typical energy curves for the dissociation of a receptor–ligand (RL) complex for soluble and tethered ligands, indicating the effects of the tether. Soluble ligands prefer binding receptors and encounter large activation energies for dissociation. Tethering the ligand tilts the energy landscape because of the force pulling the ligand. Tethered ligands thus encounter smaller activation energies and are more likely to dissociate from receptors.
In terms of general receptor interactions, the glycocalyx is a potent means to independently regulate the time and length scales of the binding process of the specific ligand–receptor type or affinity146,147. Perturbations to the glycocalyx that increase cell–cell or cell–ECM spacing or that stiffen the glycocalyx will generally distort the energy landscape for all interactions across the spacing10. In other words, a thick or less compliant glycocalyx generally makes it harder for any receptor to reach ligands on an opposing surface and tends to pull apart bonds when they form. The presence of polysialic acids, HA and mucins can change the gap thickness by tens of nanometres or more148,149. Such distances can alter affinities by several orders of magnitude8,10,150.
The glycocalyx in our discussion so far may seem to be a simple physical barrier that resists adhesion and intercellular interactions. But molecules in the glycocalyx are mobile, as they can rearrange owing to chemical, mechanical and thermodynamic forces150-152. In particular, the mismatch in size between short receptor–ligand pairs and long glycopolymers can lead to their spatial segregation on the membrane. The physical basis of this segregation is understood; if a receptor–ligand pair and larger macromolecules are in close proximity, the cell membrane must deform locally with an associated energy cost153. To relieve this penalty, the different-sized molecules must segregate. This process of molecular organization has been termed ‘kinetic segregation’ and is proposed to underlie the exquisite molecular organization of the immunological synapse that forms between an antigen-presenting cell and an effector T cell145. Similarly, cancer-associated mucins and HA are larger than most receptor–ligand pairs, suggesting that multiple receptor types could be spatially sorted in the cancer glycocalyx.
The principle of kinetic segregation as an organizing force in the cancer cell glycocalyx has recently been demonstrated for integrin adhesion receptors8. These single-molecule studies revealed that the kinetic segregation of large cancer mucins is intertwined with integrin association rates with ligands on the ECM substrate. The larger mucins are squeezed out of the integrin bond sites where the cell membrane is pulled close to the substrate. Bond formation therefore creates a region favouring further receptor–ligand interactions, causing a chain reaction of cooperative receptor binding events in close spatial proximity. This integrin bond clustering can trigger the assembly of larger complexes with intracellular adaptor proteins and signalling proteins to stimulate cell growth and resistance to programmed cell death.
Applied loads are known to trigger activation of many types of receptors by inducing force-dependent conformational changes154. Compression of the glycocalyx by short molecular bonds with an opposing surface must, in turn, generate an equal-and-opposite reaction force on the bonds8. For adhesion receptors that are activated by mechanical changes, such as integrin155, the conformational changes imposed by this pulling force can strengthen receptor attachment and adhesion signalling. Here, the glycocalyx harnesses chemical energy from favourable binding interactions and uses this energy to conduct mechanical work to induce the conformational change.
An interesting case arises if the glycocalyx of the cancer cell is so thick that it disrupts most receptor-mediated interactions between cells and with the ECM. Membrane-tethered mucins and HA have long been considered as anti-adhesion molecules that promote cell rounding and detachment156,157. For instance, excessive MUC1 expression inhibits integrin–ECM interaction and abrogates cell adhesion in multiple cell types, often causing cells to ‘pop off’ from their underlying substrate158. MUC4 and MUC21 have also been shown to specifically disrupt receptor-mediated adhesion in a manner that is dependent on its backbone polymer length159,160.
Complete detachment of cells from the tissue matrix may promote more efficient invasion of tissues by cancer cells. Cells can adopt different modes of migration based on their three-dimensional environment and the strength of attachment to other cells and to the ECM107. In the past, it had been thought that efficient tissue invasion was through a mesenchymal migration mode that involves strong integrin-dependent adhesion to generate a high traction force; essentially, cells grip hard and pull themselves forward107. However, recent studies have revealed that once detached from their collective mass, single cancer cells can adopt a fast-moving, amoeboid motility that operates independently of integrin adhesion receptors161-163. Cancer cells instead migrate by ‘chimneying’ through confined three-dimensional spaces, relying on nonspecific interactions, potentially mediated by the glycocalyx, to provide friction against extracellular structures164.
Outlook
Electrostatics, excluded volume effects and chemo-mechanical coupling are important physical principles that underlie the biology of the glycocalyx. Examples of cell-surface pH, signalling through soluble factors and tethered molecular interactions are meant to illustrate these principles, but are by no means exhaustive. Indeed, chemical affinities, chemical rates, molecular transport and equilibrium configurations at the cell surface are broadly dependent on the precise physical structure and charge distribution of the glycocalyx. Adaptive changes in the glycocalyx structure are a common feature of cell state transitions, including differentiation and transformation, and are associated with metabolic reprogramming. This Review has focused on perturbations in the cancer cell glycocalyx, in part because these changes are comparatively well described. However, the dynamic nature of the glycocalyx suggests a role in mediating specialized behaviours in a wide range of cell types.
The glycocalyx is implicated in regulating molecular behaviours beyond receptors. For instance, studies with model membranes suggest that specific sugar polymer structures can impose order on membrane lipids165. Organization of glycoproteins may also co-organize lipids through their embedded transmembrane domains. Macromolecular crowding on the membrane may also provide an entropic force to drive membrane bending166. In addition, recent spatially resolved modelling of the intracellular milieu has shown that hydrodynamic interactions coupled with crowded structure underlie previously misunderstood macromolecular physical dynamics111,112. If such effects play a role in the glycocalyx, this could provide further understanding of its function. Future studies must address the codependent molecular organizations and morphologies of the glycocalyx, plasma membrane and adjacent cytoplasm/cytoskeleton.
Approximating the structure of the cancer cell glycocalyx as a basic polymer brush or network is undoubtedly reductionist, but it provides useful physical insights for the early stages of hypothesis generation and testing. New details on the dynamic molecular organization and connectivity of the glycocalyx are necessary to develop a more comprehensive framework of understanding, including on an individual molecular level. For instance, bottlebrush polymers demonstrate unique and fascinating ensemble behaviours that are being actively considered in the polymer field. Cancer mucins are often decorated with glycan side chains of varying structure and grafting density, which could contribute to unexpected physical behaviours in the assembled glycocalyx. Addressing such a question would challenge the state-of-the-art in both polymer physics and glycobiology, calling for a broader partnership between the disciplines. The improved molecular and physical understanding is likely to lead to new therapies that can specifically target the altered biopolymers in the cancer glycocalyx.
Acknowledgements
This work was supported by the National Institute of Health New Innovator DP2 GM229133 (M.J.P) and the Center on the Physics of Cancer Metabolism through award no. 1U54CA210184-01 from the National Cancer Institute (M.J.P). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Competing interests
The authors declare no competing interests
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sever R & Brugge JS Signal transduction in cancer. Cold Spring Harb. Perspect. Med 5, a006098 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Multhaupt HAB, Leitinger B, Gullberg D & Couchman JR Extracellular matrix component signaling in cancer. Adv. Drug Deliv. Rev 97, 28–40 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Casaletto JB & McClatchey AI Spatial regulation of receptor tyrosine kinases in development and cancer. Nat. Rev. Cancer 12, 387–400 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Twomey JD, Brahme NN & Zhang B Drug-biomarker co-development in oncology—20 years and counting. Drug Resist. Updat 30, 48–62 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Swain SM et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med 372, 724–734 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baron R & McCammon JA Molecular recognition and ligand association. Annu. Rev. Phys. Chem 64, 151–175 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Manz BN & Groves JT Spatial organization and signal transduction at intercellular junctions. Nat. Rev. Mol. Cell Biol 11, 342–352 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paszek MJ et al. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature 511, 319–325 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salaita K et al. Restriction of receptor movement alters cellular response: physical force sensing by EphA2. Science 327, 1380–1385 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paszek MJ, Boettiger D, Weaver VM & Hammer DA Integrin clustering is driven by mechanical resistance from the glycocalyx and the substrate. PLoS Comput. Biol 5, e1000604 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou H-X, Rivas G & Minton AP Macromolecular crowding and confinement: biochemical, biophysical, and potential physiological consequences. Annu. Rev. Biophys 37, 375–397 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostrowski PP, Grinstein S & Freeman SA Diffusion barriers, mechanical forces, and the biophysics of phagocytosis. Dev. Cell 38, 135–146 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Radhakrishnan P et al. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc. Natl Acad. Sci. USA 111, E4066–E4075 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stamos J, Sliwkowski MX & Eigenbrot C Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J. Biol. Chem 277, 46265–46272 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Springer TA Adhesion receptors of the immune system. Nature 346, 425–434 (1990). [DOI] [PubMed] [Google Scholar]
- 16.Giancotti FG Deregulation of cell signaling in cancer. FEBS Lett. 588, 2558–2570 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanahan D & Weinberg RA Hallmarks of cancer: the next generation. Cell 144, 646–74 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Wyckoff JB et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 67, 2649–2656 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Turley EA, Wood DK & McCarthy JB Carcinoma cell hyaluronan as a ‘portable’ cancerized prometastatic microenvironment. Cancer Res. 76, 2507–2512 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniel L et al. A nude mice model of human rhabdomyosarcoma lung metastases for evaluating the role of polysialic acids in the metastatic process. Oncogene 20, 997–1004 (2001). [DOI] [PubMed] [Google Scholar]
- 21.Nyström B et al. Characterization of polyelectrolyte features in polysaccharide systems and mucin. Adv. Colloid Interface Sci 158, 108–118 (2010). [DOI] [PubMed] [Google Scholar]
- 22.McAtee CO, Barycki JJ & Simpson MA Emerging roles for hyaluronidase in cancer metastasis and therapy. Adv. Cancer Res 123, 1–34 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bohaumilitzky L et al. A trickster in disguise: hyaluronan’s ambivalent roles in the matrix. Front. Oncol 7, 242 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weigel PH & DeAngelis PL Hyaluronan synthases: a decade-plus of novel glycosyltransferases. J. Biol. Chem 282, 36777–36781 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Cowman MK, Lee H-G, Schwertfeger KL, McCarthy JB & Turley EA The content and size of hyaluronan in biological fluids and tissues. Front. Immunol 6, 261 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toole BP Hyaluronan: from extracellular glue to pericellular cue. Nat. Rev. Cancer 4, 528–539 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Berezney JP & Saleh OA Electrostatic effects on the conformation and elasticity of hyaluronic acid, a moderately flexible polyelectrolyte. Macromolecules 50, 1085–1089 (2017). [Google Scholar]
- 28.Skolnick J & Fixman M Electrostatic persistence length of a wormlike polyelectrolyte. Macromolecules 10, 944–948 (1977). [Google Scholar]
- 29.Flory PJ The configuration of real polymer chains. J. Chem. Phys 17, 303–310 (1949). [Google Scholar]
- 30.Wight TN Provisional matrix: a role for versican and hyaluronan. Matrix Biol. 60–61, 38–56 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang PS et al. Cell surface access is modulated by tethered bottlebrush proteoglycans. Biophys. J 110, 2739–2750 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jouon N, Rinaudo M, Milas M & Desbrières J Hydration of hyaluronic acid as a function of the counterion type and relative humidity. Carbohydr. Polym 26, 69–73 (1995). [Google Scholar]
- 33.Morra M & Cassineli C Non-fouling properties of polysaccharide-coated surfaces. J. Biomater. Sci. Polym. Ed 10, 1107–1124 (1999). [DOI] [PubMed] [Google Scholar]
- 34.Sato C & Kitajima K Disialic, oligosialic and polysialic acids: distribution, functions and related disease. J. Biochem. (Tokyo) 154, 115–136 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Battistel MD, Shangold M, Trinh L, Shiloach J & Freedberg DI Evidence for helical structure in a tetramer of α2-8 sialic acid: unveiling a structural antigen. J. Am. Chem. Soc 134, 10717–10720 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henderson TJ, Venable RM & Egan W Conformational flexibility of the group B meningococcal polysaccharide in solution. J. Am. Chem. Soc 125, 2930–2939 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Nagae M et al. Crystal structure of anti-polysialic acid antibody single chain Fv fragment complexed with octasialic acid: insight into the binding preference for polysialic acid. J. Biol. Chem 288, 33784–33796 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toikka J, Aalto J, Häyrinen J, Pelliniemi LJ & Finne J The polysialic acid units of the neural cell adhesion molecule N-CAM form filament bundle networks. J. Biol. Chem 273, 28557–28559 (1998). [DOI] [PubMed] [Google Scholar]
- 39.Figarella-Branger DF, Durbec PL & Rougon GN differential spectrum of expression of neural cell adhesion molecule isoforms and L1 adhesion molecules on human neuroectodermal tumors. Cancer Res. 50, 6364–6370 (1990). [PubMed] [Google Scholar]
- 40.Glüer S, Schelp C, von Schweinitz D & Gerardy-Schahn R Polysialylated neural cell adhesion molecule in childhood rhabdomyosarcoma. Pediatr. Res 43, 145–147 (1998). [DOI] [PubMed] [Google Scholar]
- 41.Tanaka F et al. Expression of polysialic acid and STX, a human polysialyltransferase, is correlated with tumor progression in non-small cell lung cancer. Cancer Res 60, 3072–3080 (2000). [PubMed] [Google Scholar]
- 42.Verduzco R, Li X, Pesek SL & Stein GE Structure, function, self-assembly, and applications of bottlebrush copolymers. Chem. Soc. Rev 44, 2405–2420 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Hattrup CL & Gendler SJ Structure and function of the cell surface (tethered) mucins. Annu. Rev. Physiol 70, 431–457 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Kesimer M et al. Molecular organization of the mucins and glycocalyx underlying mucus transport over mucosal surfaces of the airways. Mucosal Immunol. 6, 379–392 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kufe DW Mucins in cancer: function, prognosis and therapy. Nat. Rev. Cancer 9, 874–85 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaur S, Kumar S, Momi N, Sasson AR & Batra SK Mucins in pancreatic cancer and its microenvironment. Nat. Rev. Gastroenterol. Hepatol 10, 607–620 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh PK & Hollingsworth MA Cell surface-associated mucins in signal transduction. Trends Cell Biol. 16, 467–476 (2006). [DOI] [PubMed] [Google Scholar]
- 48.Paturej J, Sheiko SS, Panyukov S & Rubinstein M Molecular structure of bottlebrush polymers in melts. Sci. Adv 2, e1601478 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Subbotin A, Saariaho M, Ikkala O & ten Brinke G Elasticity of comb copolymer cylindrical brushes. Macromolecules 33, 3447–3452 (2000). [Google Scholar]
- 50.McMaster TJ, Berry M, Corfield AP & Miles MJ Atomic force microscopy of the submolecular architecture of hydrated ocular mucins. Biophys. J 77, 533–541 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bansil R & Turner BS Mucin structure, aggregation, physiological functions and biomedical applications. Curr. Opin. Colloid Interface Sci 11, 164–170 (2006). [Google Scholar]
- 52.Cao X et al. pH-dependent conformational change of gastric mucin leads to sol–gel transition. Biophys. J 76, 1250–1258 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pinho SS & Reis CA Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer 15, 540–555 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Gill DJ, Clausen H & Bard F Location, location, location: new insights into O-GalNAc protein glycosylation. Trends Cell Biol. 21, 149–58 (2011). [DOI] [PubMed] [Google Scholar]
- 55.Alexander S Polymer adsorption on small spheres. A scaling approach. J. Phys 38, 977–981 (1977). [Google Scholar]
- 56.de Gennes PG Conformations of polymers attached to an interface. Macromolecules 13, 1069–1075 (1980). [Google Scholar]
- 57.Gennes P-G Scaling Concepts in Polymer Physics (Cornell Univ. Press, Ithaca, NY, 1979). [Google Scholar]
- 58.Bracha D, Karzbrun E, Shemer G, Pincus PA & Bar-Ziv RH Entropy-driven collective interactions in DNA brushes on a biochip. Proc. Natl. Acad. Sci. USA 110, 4534–4538 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen W-L, Cordero R, Tran H & Ober CK 50th anniversary perspective: polymer brushes: novel surfaces for future materials. Macromolecules 50, 4089–4113 (2017). [Google Scholar]
- 60.Zhulina EB & Rubinstein M Lubrication by polyelectrolyte brushes. Macromolecules 47, 5825–5838 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Milner ST Polymer brushes. Science 251, 905–914 (1991). [DOI] [PubMed] [Google Scholar]
- 62.Kim JU & O’Shaughnessy B Nanoinclusions in dry polymer brushes. Macromolecules 39, 413–425 (2006). [Google Scholar]
- 63.Lee GM, Johnstone B, Jacobson K & Caterson B The dynamic structure of the pericellular matrix on living cells. J. Cell Biol 123, 1899–1907 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Button B et al. Periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 337, 937–941 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Targosz-Korecka M et al. AFM-based detection of glycocalyx degradation and endothelial stiffening in the db/db mouse model of diabetes. Sci. Rep 7, 15951 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zappone B, Ruths M, Greene GW, Jay GD & Israelachvili JN Adsorption, lubrication, and wear of lubricin on model surfaces: polymer brush-like behavior of a glycoprotein. Biophys. J 92, 1693–1708 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Attili S, Borisov OV & Richter RP Films of end-grafted hyaluronan are a prototype of a brush of a strongly charged, semiflexible polyelectrolyte with intrinsic excluded volume. Biomacromolecules 13, 1466–1477 (2012). [DOI] [PubMed] [Google Scholar]
- 68.Stachowiak JC et al. Membrane bending by protein-protein crowding. Nat. Cell Biol 14, 944–949 (2012). [DOI] [PubMed] [Google Scholar]
- 69.Rugg MS et al. Characterization of complexes formed between TSG-6 and inter-α-inhibitor that act as intermediates in the covalent transfer of heavy chains onto hyaluronan. J. Biol. Chem 280, 25674–25686 (2005). [DOI] [PubMed] [Google Scholar]
- 70.Levitin F et al. The MUC1 SEA module is a self-cleaving domain. J. Biol. Chem 280, 33374–33386 (2005). [DOI] [PubMed] [Google Scholar]
- 71.Warburg O The metabolism of carcinoma cells. J. Cancer Res 9, 148–163 (1925). [Google Scholar]
- 72.Warburg O On the origin of cancer cells. Science 123, 309–314 (1956). [DOI] [PubMed] [Google Scholar]
- 73.Liberti MV & Locasale JW The Warburg effect: how does it benefit cancer cells? Trends Biochem. Sci 41, 211–218 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pavlova NN & Thompson CB The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abdel Rahman AM, Ryczko M, Pawling J & Dennis JW Probing the hexosamine biosynthetic pathway in human tumor cells by multitargeted tandem mass spectrometry. ACS Chem. Biol 8, 2053–2062 (2013). [DOI] [PubMed] [Google Scholar]
- 76.Wellen KE et al. The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev. 24, 2784–2799 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Locasale JW & Cantley LC metabolic flux and the regulation of mammalian cell growth. Cell Metab. 14, 443–451 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Siiskonen H et al. Hyaluronan synthase 1 (HAS1) produces a cytokine-and glucose-inducible, CD44-dependent cell surface coat. Exp. Cell Res 320, 153–163 (2014). [DOI] [PubMed] [Google Scholar]
- 79.Hascall VC et al. The dynamic metabolism of hyaluronan regulates the cytosolic concentration of UDP-GlcNAc. Matrix Biol. 35, 14–17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Deen AJ et al. UDP-sugar substrates of HAS3 regulate its O-GlcNAcylation, intracellular traffic, extracellular shedding and correlate with melanoma progression. Cell. Mol. Life Sci 73, 3183–3204 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kohnz RA et al. Protein sialylation regulates a gene expression signature that promotes breast cancer cell pathogenicity. ACS Chem. Biol 11, 2131–2139 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schreiber SC et al. Polysialylated NCAM represses E-cadherin-mediated cell-cell adhesion in pancreatic tumor cells. Gastroenterology 134, 1555–1566 (2008). [DOI] [PubMed] [Google Scholar]
- 83.Ying H et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149, 656–670 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gupta R, Yang Q, Dogra SK & Wajapeyee N Serine hydroxymethyl transferase 1 stimulates pro-oncogenic cytokine expression through sialic acid to promote ovarian cancer tumor growth and progression. Oncogene 36, 4014–4024 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guin S et al. Loss of glycogen debranching enzyme AGL drives bladder tumor growth via induction of hyaluronic acid synthesis. Clin. Cancer Res 22, 1274–1283 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guin S et al. Role in tumor growth of a glycogen debranching enzyme lost in glycogen storage disease. J. Natl Cancer Inst 106, dju062 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guillaumond F et al. Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma. Proc. Natl Acad. Sci. USA 110, 3919–3924 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shaul YD et al. Dihydropyrimidine accumulation is required for the epithelial-mesenchymal transition. Cell 158, 1094–1109 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lucena MC et al. Epithelial mesenchymal transition induces aberrant glycosylation through hexosamine biosynthetic pathway activation. J. Biol. Chem 291, 12917–12929 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maupin KA et al. Glycogene expression alterations associated with pancreatic cancer epithelial–mesenchymal transition in complementary model systems. PLoS One 5, e130002 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fantin VR, St-Pierre J & Leder P Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 9, 425–434 (2006). [DOI] [PubMed] [Google Scholar]
- 92.Shim H, Chun YS, Lewis BC & Dang CV A unique glucose-dependent apoptotic pathway induced by c-Myc. Proc. Natl Acad. Sci. USA 95, 1511–1516 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Corbet C & Feron O Tumour acidosis: from the passenger to the driver’s seat. Nat. Rev. Cancer 17, 577 (2017). [DOI] [PubMed] [Google Scholar]
- 94.Damaghi M, Wojtkowiak JW & Gillies RJ pH sensing and regulation in cancer. Front. Physiol 4, 370 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Donnan FG Theorie der Membrangleichgewichte und Membranpotentiale bei Vorhandensein von nicht dialysierenden Elektrolyten. Ein Beitrag zur physikalisch-chemischen Physiologie. Z. Elektrochem. Angew. Phys. Chem 17, 572–581 (1911). [Google Scholar]
- 96.Donnan FG The theory of membrane equilibria. Chem. Rev 1, 73–90 (1924). [Google Scholar]
- 97.Tam PY & Verdugo P Control of mucus hydration as a Donnan equilibrium process. Nature 292, 340–342 (1981). [DOI] [PubMed] [Google Scholar]
- 98.Schnitzer JE Glycocalyx electrostatic potential profile analysis: ion, pH, steric, and charge effects. Yale J. Biol. Med 61, 427–446 (1988). [PMC free article] [PubMed] [Google Scholar]
- 99.Born GV & Palinski W Unusually high concentrations of sialic acids on the surface of vascular endothelia. Br. J. Exp. Pathol 66, 543–549 (1985). [PMC free article] [PubMed] [Google Scholar]
- 100.Pearce OMT & Läubli H Sialic acids in cancer biology and immunity. Glycobiology 26, 111–128 (2016). [DOI] [PubMed] [Google Scholar]
- 101.Anderson M, Moshnikova A, Engelman DM, Reshetnyak YK & Andreev OA Probe for the measurement of cell surface pH in vivo and ex vivo. Proc. Natl Acad. Sci. USA 113, 8177–8181 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Damaghi M & Gillies R Phenotypic changes of acid-adapted cancer cells push them toward aggressiveness in their evolution in the tumor microenvironment. Cell Cycle 16, 1739–1743 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Szpiech ZA et al. Prominent features of the amino acid mutation landscape in cancer. PLoS One 12, e0183273 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.White KA et al. Cancer-associated arginine-to-histidine mutations confer a gain in pH sensing to mutant proteins. Sci. Signal 10, eaam9931 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ludwig M-G et al. Proton-sensing G-protein-coupled receptors. Nature 425, 93 (2003). [DOI] [PubMed] [Google Scholar]
- 106.Kharitidi D et al. Interplay of endosomal pH and ligand occupancy in integrin α5β1 ubiquitination, endocytic sorting, and cell migration. Cell Rep. 13, 599–609 (2015). [DOI] [PubMed] [Google Scholar]
- 107.Friedl P & Wolf K Plasticity of cell migration: a multiscale tuning model. J. Cell Biol 188, 11–19 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Paszek MJ et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005). [DOI] [PubMed] [Google Scholar]
- 109.Denais CM et al. Nuclear envelope rupture and repair during cancer cell migration. Science 352, 353–358 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Helmlinger G, Netti PA, Lichtenbeld HC, Melder RJ & Jain RK Solid stress inhibits the growth of multicellular tumor spheroids. Nat. Biotechnol 15, 778–783 (1997). [DOI] [PubMed] [Google Scholar]
- 111.Aponte-Rivera C & Zia RN Simulation of hydrodynamically interacting particles confined by a spherical cavity. Phys. Rev. Fluids 1, 023301 (2016). [Google Scholar]
- 112.Aponte-Rivera C, Su Y & Zia RN Equilibrium structure and diffusion in concentrated hydrodynamically interacting suspensions confined by a spherical cavity. J. Fluid Mech 836, 413–450 (2018). [Google Scholar]
- 113.Rivas G & Minton AP Macromolecular crowding in vitro, in vivo, and in between. Trends Biochem. Sci 41, 970–981 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lemmon MA & Schlessinger J Cell signaling by receptor tyrosine kinases. Cell 141, 1117–1134 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Blume-Jensen P & Hunter T Oncogenic kinase signalling. Nature 411, 355–365 (2001). [DOI] [PubMed] [Google Scholar]
- 116.Minton AP Excluded volume as a determinant of protein structure and stability. Biophys. J 32, 77–79 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Minton AP Effect of a concentrated ‘inert’ macromolecular cosolute on the stability of a globular protein with respect to denaturation by heat and by chaotropes: a statistical-thermodynamic model. Biophys. J 78, 101–109 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Batchelor GK Brownian diffusion of particles with hydrodynamic interaction. J. Fluid Mech 74, 1–29 (1976). [Google Scholar]
- 119.Chapanian R et al. Enhancement of biological reactions on cell surfaces via macromolecular crowding. Nat. Commun 5, 4683 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Minton AP Effective hard particle model for the osmotic pressure of highly concentrated binary protein solutions. Biophys. J 94, L57–L59 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zia RN Active and passive microrheology: theory and simulation. Annu. Rev. Fluid Mech 50, 371–405 (2018). [Google Scholar]
- 122.Halperin A Polymer brushes that resist adsorption of model proteins: design parameters. Langmuir 15, 2525–2533 (1999). [Google Scholar]
- 123.Polefka TG, Garrick RA, Redwood WR, Swislocki NI & Chinard FP Solute-excluded volumes near the Novikoff cell surface. Am. J. Physiol 247, C350–C356 (1984). [DOI] [PubMed] [Google Scholar]
- 124.Laurent TC The interaction between polysaccharides and other macromolecules. 5. The solubility of proteins in the presence of dextran. Biochem. J 89, 253–257 (1963). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sasahara K, McPhie P & Minton AP Effect of dextran on protein stability and conformation attributed to macromolecular crowding. J. Mol. Biol 326, 1227–1237 (2003). [DOI] [PubMed] [Google Scholar]
- 126.Christiansen A & Wittung-Stafshede P Synthetic crowding agent dextran causes excluded volume interactions exclusively to tracer protein apoazurin. FEBS Lett. 588, 811–814 (2014). [DOI] [PubMed] [Google Scholar]
- 127.Harada R, Tochio N, Kigawa T, Sugita Y & Feig M Reduced native state stability in crowded cellular environment due to protein–protein interactions. J. Am. Chem. Soc 135, 3696–3701 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mittal S, Chowhan RK & Singh LR Macromolecular crowding: macromolecules friend or foe. Biochim. Biophys. Acta 1850, 1822–1831 (2015). [DOI] [PubMed] [Google Scholar]
- 129.Needham SR et al. EGFR oligomerization organizes kinase-active dimers into competent signalling platforms. Nat. Commun 7, 13307 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huang Y et al. Molecular basis for multimerization in the activation of the epidermal growth factor receptor. eLife 5, e14107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van Lengerich B, Agnew C, Puchner EM, Huang B & Jura N EGF and NRG induce phosphorylation of HER3/ERBB3 by EGFR using distinct oligomeric mechanisms. Proc. Natl Acad. Sci. USA 114, E2836–E2845 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zimmerman SB & Trach SO Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J. Mol. Biol 222, 599–620 (1991). [DOI] [PubMed] [Google Scholar]
- 133.Ellis RJ in Molecular Aspects of the Stress Response: Chaperones, Membranes and Networks 1–13 (Springer, New York, NY, 2007). [Google Scholar]
- 134.Minton AP Effects of excluded surface area and adsorbate clustering on surface adsorption of proteins. II. Kinetic models. Biophys. J 80, 1641–1648 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Roosen-Runge F et al. Protein self-diffusion in crowded solutions. Proc. Natl Acad. Sci. USA 108, 11815–11820 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhou HX & Szabo A Theory and simulation of the time-dependent rate coefficients of diffusion-influenced reactions. Biophys. J 71, 2440–2457 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zia RN, Swan JW & Su Y Pair mobility functions for rigid spheres in concentrated colloidal dispersions: force, torque, translation, and rotation. J. Chem. Phys 143, 224901 (2015). [DOI] [PubMed] [Google Scholar]
- 138.Su Y, Swan JW & Zia RN Pair mobility functions for rigid spheres in concentrated colloidal dispersions: stresslet and straining motion couplings. J. Chem. Phys 146, 124903 (2017). [DOI] [PubMed] [Google Scholar]
- 139.Bolis D, Politou AS, Kelly G, Pastore A & Andrea Temussi P Protein stability in nanocages: a novel approach for influencing protein stability by molecular confinement. J. Mol. Biol 336, 203–212 (2004). [DOI] [PubMed] [Google Scholar]
- 140.Haeger A, Wolf K, Zegers MM & Friedl P Collective cell migration: guidance principles and hierarchies. Trends Cell Biol. 25, 556–566 (2015). [DOI] [PubMed] [Google Scholar]
- 141.Dembo M, Torney DC, Saxman K & Hammer D The reaction-limited kinetics of membrane-to-surface adhesion and detachment. Proc. R. Soc. Lond. B 234, 55–83 (1988). [DOI] [PubMed] [Google Scholar]
- 142.Bustamante C, Chemla YR, Forde NR & Izhaky D Mechanical processes in biochemistry. Annu. Rev. Biochem 73, 705–748 (2004). [DOI] [PubMed] [Google Scholar]
- 143.Bell GI Models for the specific adhesion of cells to cells. Science 200, 618–627 (1978). [DOI] [PubMed] [Google Scholar]
- 144.Beste MT & Hammer DA Selectin catch–slip kinetics encode shear threshold adhesive behavior of rolling leukocytes. Proc. Natl Acad. Sci. USA 105, 20716–20721 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Marshall BT et al. Direct observation of catch bonds involving cell-adhesion molecules. Nature 423, 190–193 (2003). [DOI] [PubMed] [Google Scholar]
- 146.Jeppesen C et al. Impact of polymer tether length on multiple ligand–receptor bond formation. Science 293, 465–468 (2001). [DOI] [PubMed] [Google Scholar]
- 147.Wong JY, Kuhl TL, Israelachvili JN, Mullah N & Zalipsky S Direct measurement of a tethered ligand–receptor interaction potential. Science 275, 820–822 (1997). [DOI] [PubMed] [Google Scholar]
- 148.Johnson CP, Fujimoto I, Rutishauser U & Leckband DE Direct evidence that neural cell adhesion molecule (NCAM) polysialylation increases intermembrane repulsion and abrogates adhesion. J. Biol. Chem 280, 137–145 (2005). [DOI] [PubMed] [Google Scholar]
- 149.Shurer CR et al. Genetically encoded toolbox for glycocalyx engineering: tunable control of cell adhesion, survival, and cancer cell behaviors. ACS Biomater. Sci. Eng 4, 388–399 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bell GI, Dembo M & Bongrand P Cell adhesion. Competition between nonspecific repulsion and specific bonding. Biophys. J 45, 1051–1064 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ward MD & Hammer DA Morphology of cell-substratum adhesion. Cell Biophys. 20, 177–222 (1992). [DOI] [PubMed] [Google Scholar]
- 152.Torney DC, Dembo M & Bell GI Thermodynamics of cell adhesion. II. Freely mobile repellers. Biophys. J 49, 501–507 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Coombs D & Goldstein B Effects of the geometry of the immunological synapse on the delivery of effector molecules. Biophys. J 87, 2215–2220 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang X & Ha T Defining single molecular forces required to activate integrin and notch signaling. Science 340, 991–994 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sun Z, Guo SS & Fässler R Integrin-mediated mechanotransduction. J. Cell Biol 215, 445–456 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hilkens J, Ligtenberg MJL, Vos HL & Litvinov SV Cell membrane-associated mucins and their adhesion-modulating property. Trends Biochem. Sci 17, 359–363 (1992). [DOI] [PubMed] [Google Scholar]
- 157.Brecht M, Mayer U, Schlosser E & Prehm P Increased hyaluronate synthesis is required for fibroblast detachment and mitosis. Biochem. J 239, 445–450 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wesseling J, van der Valk SW, Vos HL, Sonnenberg A & Hilkens J Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J. Cell Biol 129, 255–265 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yi Y et al. Mucin 21/epiglycanin modulates cell adhesion. J. Biol. Chem 285, 21233–21240 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Komatsu M, Carraway CA, Fregien NL & Carraway KL Reversible disruption of cell-matrix and cell-cell interactions by overexpression of sialomucin complex. J. Biol. Chem 272, 33245–33254 (1997). [DOI] [PubMed] [Google Scholar]
- 161.Lämmermann T & Sixt M Mechanical modes of ‘amoeboid’ cell migration. Curr. Opin. Cell Biol 21, 636–644 (2009). [DOI] [PubMed] [Google Scholar]
- 162.Liu Y-J et al. Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell 160, 659–672 (2015). [DOI] [PubMed] [Google Scholar]
- 163.Paul CD, Mistriotis P & Konstantopoulos K Cancer cell motility: lessons from migration in confined spaces. Nat. Rev. Cancer 17, 131–140 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bergert M et al. Force transmission during adhesion-independent migration. Nat. Cell Biol 17, 524–529 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Subramaniam AB, Guidotti G, Manoharan VN & Stone HA Glycans pattern the phase behaviour of lipid membranes. Nat. Mater 12, 128–133 (2013). [DOI] [PubMed] [Google Scholar]
- 166.Busch DJ et al. Intrinsically disordered proteins drive membrane curvature. Nat. Commun 6, 7875 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rilla K et al. Hyaluronan synthase 1 (HAS1) requires higher cellular UDP-GlcNAc concentration than HAS2 and HAS3. J. Biol. Chem 288, 5973–5983 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]