Histamine H1 and H2 receptors regulate multiple health-promoting adaptations to exercise training.
Abstract
Exercise training is a powerful strategy to prevent and combat cardiovascular and metabolic diseases, although the integrative nature of the training-induced adaptations is not completely understood. We show that chronic blockade of histamine H1/H2 receptors led to marked impairments of microvascular and mitochondrial adaptations to interval training in humans. Consequently, functional adaptations in exercise capacity, whole-body glycemic control, and vascular function were blunted. Furthermore, the sustained elevation of muscle perfusion after acute interval exercise was severely reduced when H1/H2 receptors were pharmaceutically blocked. Our work suggests that histamine H1/H2 receptors are important transducers of the integrative exercise training response in humans, potentially related to regulation of optimal post-exercise muscle perfusion. These findings add to our understanding of how skeletal muscle and the cardiovascular system adapt to exercise training, knowledge that will help us further unravel and develop the exercise-is-medicine concept.
INTRODUCTION
Aerobic exercise training is a potent preventive and therapeutic strategy for cardiovascular, metabolic, and other chronic diseases (1). Molecular mechanisms underlying these exercise-induced health-promoting effects may range from autocrine/paracrine signaling within skeletal muscle to whole-body organ cross-talk (2). These events induce adaptations within skeletal muscle such as increased capillarization and mitochondrial capacity, leading to improved metabolic health. Precisely how the beneficial effects of exercise training on skeletal muscle and the cardiovascular system are mediated at a molecular level remains undisclosed. However, a complete understanding of the mechanisms underlying the health-related benefits of exercise training is essential to further unravel its potential clinical impact (3) and develop novel therapeutic and exercise modality strategies (4).
In recent years, histamine has emerged as a potentially important mediator of acute and chronic exercise responses (5). The primordial basis of the histamine system dates back from before the origin of multicellular organisms, with a highly conserved genetic sequence of the histamine-forming enzyme histidine decarboxylase, which also is present in humans (6). Histamine exerts its biological effects via four heterotrimeric guanine nucleotide-binding protein–coupled histamine receptors, H1 to H4, of which H1 and H2 receptors are most frequently studied in relation to exercise (5) and are known to be widely expressed within skeletal muscle (7).
Although the research interest in histamine has mainly been directed to allergic reactions, inflammation, and gastric acid secretion, as early as in 1935, it was shown that muscle contractions induce an increase in venous histamine concentrations in dogs (8). More recently, McCord and Halliwill (9) documented the role of H1/H2 receptors in mediating sustained post-exercise hyperemia in humans. Some studies further addressed the effect of H1/H2 blockade on the acute moderate-intensity exercise response, although the topic remains largely understudied (10–13).
It is currently unknown whether and how the histamine system is relevant for exercise training adaptations. In the current study, we first confirmed that muscle perfusion after acute interval exercise was severely blunted with pharmaceutical H1/H2 blockade. Next, we evaluated the role of histamine signaling for exercise training–induced adaptations by chronic blockade of H1/H2 receptors during a 6-week interval training program in healthy males. We found that the chronic blockade severely impaired multiple clinically relevant adaptations to exercise training, i.e., exercise capacity, glycemic control, and vascular function. Our human histamine blockade strategy provides important insights into the role of H1/H2 signaling in the adaptive response to exercise training and emphasizes translational and therapeutic potential of the histamine pathway.
RESULTS
Post-exercise muscle perfusion is dependent on H1/H2 receptor signaling
To study the effects of H1/H2 blockade on the hemodynamic response during and after interval cycling exercise, healthy adults performed a single exercise session with either placebo (control) or H1/H2 antagonist intake (blockade) on separate days in a randomized and single-blinded design (Fig. 1A and table S1). At rest, acute ingestion of H1/H2 blockers did not alter heart rate, brachial blood pressure, femoral arterial blood flow, or femoral arterial diameter (table S1). The heart rate response during exercise was not different between the placebo and blockade trial (fig. S1A). Muscle perfusion was increased approximately threefold 15 min after exercise and was still ~50% above baseline after 2 hours of passive recovery in the placebo condition. However, the total post-exercise muscle perfusion, expressed as iAUC (incremental area under the curve), was significantly reduced with H1/H2 blockade by ~35% (Fig. 1B and fig. S1B). The brachial arterial blood pressure showed a modest increase during the 2-hour post-exercise period (main effect time) and was, in general, higher after placebo intake (main effect condition) (Fig. 1C). The increase in post-exercise vascular conductance, an important measure of vascular tone, was consequently blunted with H1/H2 blockade (Fig. 1D). Similar to during exercise, post-exercise heart rate recovery was unaffected by H1/H2 blockade (fig. S1C). Collectively, these data demonstrate that histamine H1/H2 receptors are essential for the regulation of sustained elevation of muscle perfusion following interval exercise.
Fig. 1. Acute post-exercise hemodynamics.
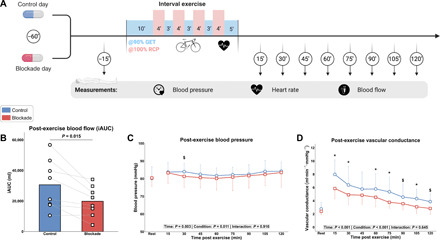
(A) Experimental procedures of the acute exercise study (n = 8). GET, gas exchange threshold; RCP, respiratory compensation point. (B) Total muscle perfusion during the 2-hour post-exercise period, expressed as iAUC. (C) Arterial blood pressure and (D) vascular conductance at baseline and during the 2-hour post-exercise period. Data are presented as means + individual data points (B) or means ± SD (C and D). *P < 0.05 for CON (placebo treated) versus BLOCK (H1/H2 antagonist treated) and $P < 0.10 for CON versus BLOCK. Data were analyzed using a two-tailed paired t test (B) or linear mixed models with Tukey post hoc testing (C and D).
Exercise capacity and mitochondrial function are regulated by H1/H2 receptors
In a chronic 6-week training study, participants were divided in placebo (CON) and H1/H2 blockade (BLOCK) groups in a double-blinded, randomized controlled trial design, matched for similar baseline group characteristics (table S2). Both groups performed the same exercise training program (Fig. 2A and fig. S2A), which elicited similar relative training intensities (fig. S2, B and C) and adherence (table S2). Resting heart rate tended to decrease in CON but not in BLOCK (table S2). Since a high aerobic exercise capacity is an essential protective measure against metabolic dysfunction, we assessed training-induced changes in exercise capacity with an incremental cycling test. Maximal oxygen uptake increased moderately with training (main effect of training), with no statistical differences between CON (+3.9%) and BLOCK (+0.3%) (Fig. 2B and fig. S3A). Functionally, peak power output increased in both groups, with the increase being significantly greater in CON than in BLOCK (+12% versus +7%; Fig. 2B and fig. S3B). Similar to the functional maximal data, both the gas exchange threshold (GET) and respiratory compensation point (RCP) increased substantially with training in both groups (fig. S3, C and D), although the increase in CON (+22 and +19%) was significantly greater than in BLOCK (+11 and +6%) (Fig. 2B). As second functional outcome, we evaluated the capacity to recover after maximal exercise by a time to exhaustion test at a fixed work rate, performed after a 10-min recovery upon cessation of the incremental exercise test. The time to exhaustion improved with training in CON (+81%) and in BLOCK (+31%) (fig. S3E). Adaptations at a submaximal level were further studied via a steady-state exercise test at a constant submaximal power output. Average heart rate during this test decreased in CON (−9%) but not in BLOCK (Fig. 2C). The total energy required to perform this exercise bout, reflecting exercise efficiency, was calculated on the basis of the gas exchange data and was also decreased in CON but not in BLOCK (Fig. 2C). Capillary lactate levels at the end of the exercise bout decreased with training, independent of group (fig. S3F).
Fig. 2. Training-induced changes in exercise capacity and mitochondrial function.
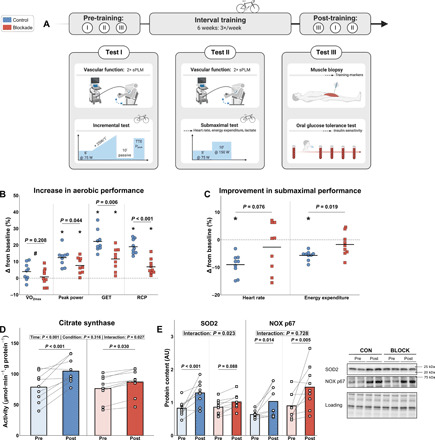
(A) Schematic overview of experimental procedures of the chronic training study (CON = 9 and BLOCK = 9). (B) Changes (in %) from pre-training in exercise capacity outcomes derived from an incremental cycling test. (C) Changes (in %) from pre-training in submaximal exercise performance (average heart rate and total energy expenditure). (D) Effect of training on muscle CS activity. (E) Effect of training on the muscle mitochondrial antioxidant SOD2 and superoxide anion–producing NOX, including representative Western blots. Data are presented as individual data points, accompanied by horizontal lines (B and C) or bars (D and E) as means. *P < 0.05 versus baseline for respective group and #P < 0.05 for main effect of time. Data were analyzed using linear mixed models with Tukey post hoc testing or unpaired t tests. sPLM, single passive leg movement; AU, arbitrary units.
As several training-induced adaptations in exercise capacity were impaired with H1/H2 blockade, we determined the potential link with mitochondrial function. Maximal citrate synthase (CS) activity in skeletal muscle biopsies as marker for mitochondrial capacity increased in both groups, with the increase being significantly greater in CON (+33%) compared to BLOCK (+14%) (Fig. 2D). The mitochondrial antioxidant superoxide dismutase 2 (SOD2) protein content was also markedly increased in CON (+56%) but not in BLOCK (+18%) (Fig. 2E). On the basis of the finding that antioxidant adaptations were blunted in BLOCK, the p67phox subunit of the membrane-bound superoxide-producing NADPH (reduced form of nicotinamide adenine dinucleotide phosphate) oxidase (NOX) protein content was determined. NOX p67phox content increased similarly with training in both groups (+61% versus +63% in CON versus BLOCK) (Fig. 2E). These data indicate an essential role of H1/H2 receptors in regulating improvements in exercise performance related to mitochondrial capacity and antioxidant protein expression.
Improvement in whole-body glucose homeostasis is blunted by H1/H2 blockade
Exercise training is known to improve insulin action, glucose homeostasis, and metabolic flexibility (14). Therefore, whole-body metabolic control was assessed before and after the intensive exercise training program by an oral glucose tolerance test (OGTT) and muscle biopsies in resting conditions. Exercise training decreased fasted blood glucose levels in CON only (table S2). Fasted blood levels of insulin, triglycerides, and cholesterol were unaffected by training (table S2). The total glucose and insulin levels during the OGTT, expressed as AUC, decreased substantially in CON (−11 and −30%) but not in BLOCK (+1 and −3%) (Fig. 3A). Glucose tolerance, expressed as the Matsuda index for whole-body insulin sensitivity, was accordingly significantly improved with training in CON (+26%), but this effect was completely abolished in BLOCK (+1%) (Fig. 3B). Muscle total GLUT4 protein content, the primary insulin-sensitive glucose transporter in skeletal muscle, increased similarly with training in both groups (+17% versus +16% in CON versus BLOCK) (Fig. 3C).
Fig. 3. Metabolic outcomes in response to exercise training.
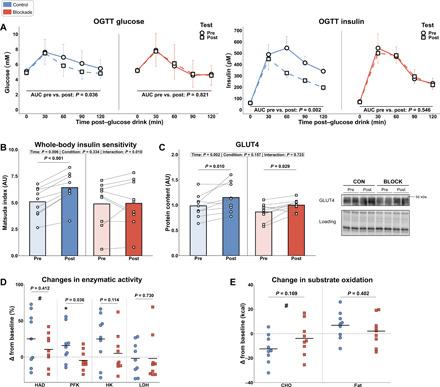
(A) Response curves and changes of AUC of glucose and insulin during the OGTT pre- and post-training. (B) Effect of training on whole-body insulin sensitivity, assessed with the Matsuda index derived from OGTT data. (C) Expression of muscle total GLUT4 content before and after the training intervention, including representative Western blots. (D) Changes (in %) from pre-training in activity of enzymes related to energy delivery pathways. (E) Changes (in kcal) from pre-training in carbohydrate (CHO) and fat oxidation during submaximal cycling. CON = 9 and BLOCK = 9. Data in (A) are presented as means ± SD (glucose) or SEM (insulin, for visual reasons). Data in (B to E) are illustrated as individual data points with mean as bar (B and C) or horizontal line (D and E). *P < 0.05 versus baseline for respective group and #P < 0.05 for main effect of time. Data were analyzed using linear mixed models with Tukey post hoc testing or unpaired t tests.
Additional metabolic effects of H1/H2 blockade in muscle were explored by assessing metabolic enzymes related to energy delivery. Hydroxyacyl–coenzyme A dehydrogenase (HAD) activity, functionally active in fatty acid oxidation, increased with training, independent of group (+19% versus +11% in CON versus BLOCK) (Fig. 3D). The glycolytic rate-limiting enzyme phosphofructokinase (PFK) increased with training in CON with 13% and was unchanged in BLOCK (Fig. 3D). Hexokinase (HK) and lactate dehydrogenase (LDH) activity were both unaffected by training (Fig. 3D). These effects of the interplay between exercise training and H1/H2 blockade on enzymes related to energy delivery did not affect substrate oxidation during submaximal cycling, although carbohydrate oxidation moderately decreased with training (Fig. 3E). In conclusion, our results demonstrate that exercise training induced H1/H2 receptor–dependent metabolic adaptations related to whole-body insulin action and glycolytic energy delivery pathways.
Blunted training-induced improvement in NO-dependent vascular function and muscle capillarization with H1/H2 blockade
As regular exercise training is a cornerstone strategy for improving cardiovascular health markers, we measured training-induced changes in these outcomes. Resting blood pressure did not change with training (table S2). Leg vascular function was assessed without H1/H2 antagonist intake before and after the training intervention using the single passive leg movement (sPLM) technique (15). The blood flow response upon passive movement was increased in CON but not in BLOCK. More specifically, the total hyperemic response (iAUC), indicative of vascular function, was increased in CON (+37%) but not in BLOCK (−14%) (Fig. 4, A and B). The maximal blood flow increase from baseline and the absolute peak blood flow during sPLM were also increased in CON only (+26 and +17%; table S2). Muscle capillarization, i.e., capillary-fiber ratio and capillary density, also increased with training in CON (+17 and +16%) but not in BLOCK (+5 and −1%) (Fig. 4C). Muscle fiber area was unaffected by exercise training and H1/H2 blockade (table S2). To unravel the mechanistic cause for this blunted increase in NO-dependent vascular function and muscle capillarization, we determined the expression of related proteins. Muscle vascular endothelial growth factor (VEGF) content increased with training, independent of group (+29% versus +23% in CON versus BLOCK; Fig. 4D). Expression of endothelial nitric oxide synthase (eNOS) increased 17% in CON but was unchanged in BLOCK (Fig. 4E). Cyclooxygenase 1 (COX1) increased similarly in both groups (+38 and +23% in CON and BLOCK), whereas prostacyclin synthase (PTGIS) and VEGF receptor 2 (VEGFR-2) were unaffected by training and group (Fig. 4E). In summary, chronic H1/H2 blockade blunted the training-induced improvement in leg vascular function, muscle capillarization, and eNOS content.
Fig. 4. Changes in vascular function and capillarization with exercise training.
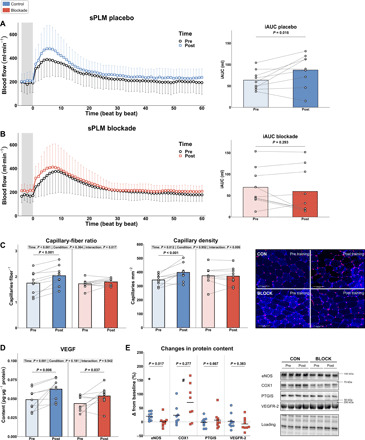
(A and B) Left: Blood flow traces during sPLM before and after training in CON (A) and BLOCK (B). The gray zone denotes baseline blood flow before initiation of passive movement. Right: iAUC of blood flow response before and after training in CON (A) and BLOCK (B). (C) Training-induced changes in capillary-fiber ratio (left) and capillary density (middle), accompanied by representative micrographs (right). (D) Effect of training on VEGF protein content. (E) Training-induced changes (in %) in protein content of vascular-related proteins: eNOS, COX1, PTGIS, and VEGFR-2, including representative Western blots. n = 9 per group, except for COX1 (n = 8 because of technical difficulties). (A) and (B) (left) are means ± SD. All other panels are individual data points with mean as bar (A to D) or horizontal line (E). *P < 0.05 versus baseline for respective group and #P < 0.05 for main effect of time. Data were analyzed using linear mixed models with Tukey post hoc testing or unpaired t tests.
H1/H2 receptor–dependent adaptations to interval training are highly interrelated
As chronic blockade of H1/H2 receptors blunted several different training-induced adaptations, we explored the interrelationship between the various functional and mechanistic outcomes at baseline, as well as their training-induced changes (Fig. 5, A and B). Training-induced changes in markers for maximal exercise capacity, i.e., VO2max and peak power output, were related to each other (fig. S4A) and to the decrease in submaximal exercise heart rate (fig. S4B). The changes in maximal exercise capacity were not related to changes in submaximal ventilatory thresholds GET and RCP (Fig. 5B). On the other hand, the increase in maximal exercise capacity was related to changes in vascular function, i.e., iAUC during sPLM (fig. S4C) and the Matsuda index for whole-body insulin sensitivity (fig. S4D). Furthermore, changes in CS activity were strongly related to all markers of vascular function (Fig. 5B and fig. S4E). The regulatory role of the microvasculature in metabolic control was further supported by the correlation between changes in capillary-fiber ratio and changes in whole-body insulin sensitivity (fig. S4F). These interrelationships indicate the integrative nature of whole-body adaptations to interval exercise training and the regulatory role of H1/H2 receptor signaling herein.
Fig. 5. Training-induced changes via H1/H2 receptors are interrelated.
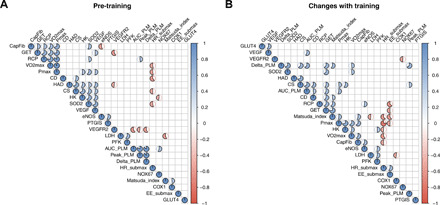
(A) Correlation matrix for different outcomes at baseline (before training). (B) Correlation matrix for training-induced changes in all assessed outcomes. Presence of a circle within cell denotes a significant (P < 0.05) correlation between two variables, whereas the strength of the correlation (r value) is illustrated by size of the filling (e.g., half-filled circle depicts r value of 0.5). n = 18. Blue, positive correlation; red, negative correlation. Data were analyzed with Pearson correlation coefficients. CapFib, capillary-fiber ratio; Pmax, peak power output during incremental test; CD, capillary density; AUC_PLM, iAUC during sPLM; Peak_PLM, peak blood flow during sPLM; Delta_PLM, difference between peak and baseline blood flow during sPLM; HR_submax, average heart rate during submaximal cycling; Matsuda_index, Matsuda index for whole-body insulin sensitivity; EE_submax, energy expenditure during submaximal cycling.
DISCUSSION
Exercise training induces health-promoting adaptations to multiple organ systems, orchestrated by an interplay between various exercise factors and signaling events (2). In the present study, we show that histamine H1/H2 signaling is an essential transducer of the adaptive exercise training response with broad clinical relevance: aerobic capacity, glycemic control, and vascular function. These detrimental effects of H1/H2 blockade on functional outcomes were caused by impaired adaptations in key regulatory proteins, illustrating the integrative role of H1/H2 receptors in mediating exercise responses. One potential functional cause of the blunted training adaptations with histamine receptor blockade is a substantially reduced post-exercise muscle perfusion, as also observed in the current study. A summary of our findings is illustrated in Fig. 6.
Fig. 6. Hypothesized working mechanism of H1/H2-dependent regulation of training adaptations.
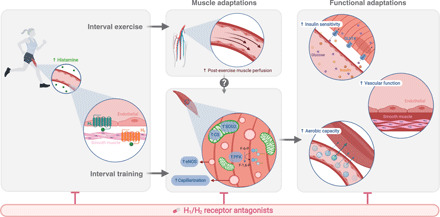
Left: Histamine H1/H2 receptors are mainly located on endothelial (H1) and vascular smooth muscle (H2) cells within skeletal muscle. Both receptors are activated during and following interval exercise, likely via an exercise-induced increase in circulating histamine concentrations. Middle: After acute interval exercise, activation of H1/H2 receptors is essential to regulate the sustained elevation of muscle perfusion. With chronic interval training, H1/H2 receptor signaling during and following exercise mediates peripheral muscle adaptations related to mitochondria (CS activity and antioxidant protein expression), microvasculature (muscle capillarization and eNOS content), and glycolytic energy delivery (PFK activity). Right: These H1/H2-dependent muscle adaptations contribute to key functional adaptations related to overall health: exercise capacity, whole-body insulin sensitivity, and vascular function.
In a chronic training study with H1/H2 blockade, we first observed marked reductions in training-induced effects on aerobic and mitochondrial capacity (Fig. 2 and fig. S3). Maximal exercise performance, i.e., VO2max, peak power output, and time to exhaustion test, was only moderately affected, but aspects related to submaximal performance, i.e., ventilatory thresholds and submaximal heart rate and exercise efficiency, were more severely impaired. These data suggest that H1/H2 receptors are primarily relevant for peripheral, rather than central, adaptations in aerobic capacity. This is in accordance with the rather limited increase in VO2max, which was not statistically different between groups, as VO2max is primarily centrally regulated by the maximal capacity for O2 delivery, which is largely dependent on cardiac output (16). Peripheral adaptations such as increased muscle capillarization and mitochondrial capacity, however, occurred only in the placebo but not in the H1/H2 antagonist group, indicating a potential increase in O2 extraction during exercise, thereby affecting submaximal performance outcomes (17). This discrepancy between maximal and submaximal adaptations is also underpinned by the absence of a correlation between training-induced changes in maximal performance and submaximal ventilatory thresholds (Fig. 5B). Another peripheral adaptation that was negatively affected by histamine antagonism and could have contributed to the reduced effects of training on exercise performance was that of PFK, a rate-limiting enzyme in glycolysis. Future research will need to explore the exact role of H1/H2 receptors in the regulation of mitochondrial function and glycolytic energy production.
An additional important finding in our study was that training-induced adaptations related to glycemic control were impaired with H1/H2 blockade (Fig. 3). More specifically, whole-body insulin sensitivity did not increase in the experimental group treated with H1/H2 antagonists, whereas a substantial improvement was noted in placebo-treated participants. GLUT4 protein content, the primary glucose transporter in skeletal muscle (18), was similarly increased in both groups, suggesting that changes in total GLUT4 expression are not mediated by H1/H2 receptors and up-regulation of total GLUT4 expression per se is not sufficient to induce improvements in whole-body insulin action. However, increased skeletal muscle capillarization was observed only in the placebo-treated group. Skeletal muscle capillarization is associated with muscle insulin sensitivity (19, 20), and thus, the lack of increase in capillarization in the H1/H2 blockade group could, in part, explain the observed effects of a blunted increase in whole-body insulin action. This is in agreement with the observed relationship between training-induced changes in capillary-fiber ratio and whole-body insulin sensitivity (fig. S4F). Similarly, the impaired up-regulation of eNOS content with H1/H2 blockade could also be related, as insulin-stimulated NO production is important for optimal glucose uptake via regulation of muscle perfusion (21, 22). Last, disproportionate mitochondrial reactive oxygen species (ROS) production has been related to insulin resistance (23, 24), implying that the observed impaired up-regulation of SOD2 protein content could have negatively altered mitochondrial redox status and thus affected adaptations in whole-body insulin action. In summary, our data suggest that H1/H2-dependent signaling is involved in the exercise training–induced improvement of whole-body glycemic control. Whether this histaminergic effect is secondary to the observed blunting of angiogenesis and increase in abundance of related proteins or whether there also is a direct role in the up-regulation of the insulin signaling cascade is subject to further research.
A third functional outcome was that NO-dependent vascular endothelial function, as determined by the flow response to passive lower leg movement, was improved by exercise training in the placebo but not in the H1/H2 receptor antagonist group (Fig. 4). Endothelial function is overall highly dependent on NO bioavailability (25), where the bioavailability, in turn, is determined by the balance between NO production and NO scavenging by ROS. Exercise training increased the expression of eNOS and the mitochondrial antioxidant SOD2 in the control group but not in the H1/H2 blockade group, whereas the expression of the p67phox subunit of NOX, a primary source of ROS, increased similarly in both groups. These findings indicate that the training-induced increase in NO bioavailability is blunted when H1/H2 receptors are chronically blocked, consequently leading to impaired adaptations in NO-dependent vascular function.
The histamine H1/H2-mediated training adaptations could be related, in part, to two distinct mechanisms. First, the acute exercise response in the muscle transcriptome related to vascular function, metabolism, inflammation, and other cellular functions is highly dependent on H1/H2 receptor signaling (12). These findings suggest that chronic blockade of H1/H2 receptors could negatively affect the cumulative training response, as observed in the current study. Second, sustained post-exercise muscle perfusion could be a key element for optimal muscle recovery (26) and subsequent training adaptations, as also suggested via post-exercise muscle cooling (27) and heating (28) strategies and blood flow restriction training (29). Although blood flow restriction training reduces muscle perfusion during exercise, it substantially enhances the perfusion after exercise (29). In our study, H1/H2 blockade induced marked reductions in muscle perfusion after interval cycling exercise (Fig. 1 and fig. S1), an effect likely present after each exercise session during the training program. One potential mechanism by which reduced muscle perfusion could impair chronic muscle adaptations is via shear stress, the elevated blood flow in the capillary network during and after exercise. The H1/H2-dependent elevation in post-exercise blood flow and, thus, shear stress possibly mediated the up-regulation of muscle capillarization, as shear stress has been shown to be important for capillary growth (30) by leading to higher expression of angiogenic proteins in endothelial cells (31) and consequent splitting angiogenesis (32, 33). Furthermore, the blunted improvement in NO-dependent vascular function with H1/H2 blockade could be related to shear stress, as exercise training stimulates eNOS via shear stress–induced eNOSSer1177 phosphorylation (34). Other consequences of a reduced muscle perfusion that could have contributed to the impaired adaptations are a reduced delivery of substrates, metabolites, and cytokines, but the exact mechanisms should be addressed in future research.
The finding that inhibiting one single piece in the exercise response puzzle leads to blunted improvements in a broad range of molecular and functional systems is notable and contradicts the proposed integrated and redundant regulation of exercise adaptations (35, 36). This indicates a role of H1/H2 receptors as essential molecular transducers or master regulators of exercise responses. It is unclear, however, whether chronic H1/H2 blockade induces a complete inhibition of several key adaptations or rather unfavorably shifts the dose-response exercise curve so that a greater training stimulus is required for a similar response. A dose-response shift would imply that other mechanisms would compensate for the lack of histamine signaling, potentially of more importance if the physiological systems are more profoundly stimulated (e.g., longer training duration). Furthermore, the current intervention consisted of over-the-counter antihistamine drugs. It is of pharmaceutical relevance that combined administration of exercise training and common medication negatively interact and lead to marked impairment of health-promoting adaptations. Other examples of adverse exercise-drug interactions have been reported in recent years, for example, metformin (37), resveratrol (38), and β2 agonists (39), illustrating the huge importance of this emerging research field.
In summary, we found that exercise-induced H1/H2 receptor signaling is essential for the integrative beneficial effects of exercise training on exercise capacity, metabolic control, and vascular function. This is likely related to an impaired post-exercise muscle perfusion and/or transcriptomic response with consequent blunted adaptations in the muscle microvasculature and mitochondrial content and antioxidant function (Fig. 6). Future studies should focus to further unravel the mechanisms underlying the H1/H2-dependent whole-body adaptations and on how the histamine system is affected by chronic diseases. Our data combined with these future endeavors will enable us to discover and develop previously unidentified drug targets and optimize exercise and pharmaceutical prescriptions.
MATERIALS AND METHODS
More details of participants, experimental procedures, and analytical techniques can be found in Supplementary Materials and Methods. The study conforms with the 2013 standards set by the Declaration of Helsinki, was approved by the Ethical Committee of Ghent University Hospital, Belgium (2018/1007 and 2019/1246), and is registered in ClinicalTrials.gov (NCT04450134).
Participants
After medical screening and written informed consent, 8 and 20 healthy adults participated in the acute and chronic study, respectively. For the chronic study, participants were randomly assigned to the placebo- (CON) or H1/H2 blockade–treated (BLOCK) group. One participant per group dropped out during the study period. More details are in the Supplementary Materials.
Acute exercise study design
In a crossover, single-blind (researcher), randomized study design, participants first attended an initial screening visit to determine exercise intensity for subsequent experimental days. The two test days were randomized between placebo and H1/H2 blockade and consisted of a single identical exercise session with pre- and post-exercise measurements (Fig. 1A). Measurements included heart rate during exercise and femoral arterial blood flow, brachial blood pressure, and heart rate before and after exercise. More details are in the Supplementary Materials.
Chronic training study design
In a double-blind, placebo-controlled, and randomized study, participants performed a 6-week training intervention (Fig. 2A). Outcomes related to exercise capacity (maximal and submaximal exercise testing and muscle biopsies), metabolic health (OGTT and muscle biopsies), and NO-dependent vascular function (sPLM and muscle biopsies) were assessed before and after the training intervention. Exercise training consisted of three weekly sessions of cycling interval training (fig. S2A). Furthermore, an intermediate incremental cycling test was performed after 3 weeks of training to adjust exercise intensities during the training sessions. More details are in the Supplementary Materials.
H1/H2 blockade
A double blockade of H1/H2 receptors was performed in all interventions by administration of 540 mg of fexofenadine (H1) and 300 mg of ranitidine or 40 mg of famotidine (H2; see the Supplementary Materials for details). Capsule intake was 1 hour before each training session, and group allocation was blinded for participants and researchers. Capsule intake compliance was reported as 100%. More details are in the Supplementary Materials.
Maximal incremental cycling test
After a 6-min warm-up at 75 W, the work rate increased continuously by 25 W every minute until volitional exhaustion despite strong verbal encouragement. After a 10-min passive rest period, a time to exhaustion test was performed by cycling at 90% of the peak power output during the incremental cycling test until exhaustion. The work rate for this time to exhaustion test was kept constant for the intermediate and post-training testing. Whole-body oxygen uptake was measured breath by breath with a metabolic system (MetaLyzer 3B; Cortex Biophysik GmbH, Leipzig, Germany), and heart rate was measured continuously (H7; Polar, Kempele, Finland). Details concerning determination of peak oxygen uptake, power output, and heart rate as well as ventilatory thresholds can be found in the Supplementary Materials.
Blood pressure and heart rate
Blood pressure and heart rate were measured in the left arm using an automated sphygmomanometer (M3 Comfort; Omron Healthcare, Hoofddorp, The Netherlands). In the acute study, this was measured in triplicate at each time point, whereas an average of five measurements was calculated in the chronic study.
Single passive leg movement
The sPLM technique was used as an indicator of NO-dependent peripheral vascular function, according to standardized methods (40, 41). sPLM was always performed without H1/H2 blockade. Blood flow was determined on a beat-by-beat basis, smoothed by a five-beat average and peak blood flow; change from baseline to peak flow (∆ flow) and iAUC (trapezoidal method) were determined as standard parameters for vascular function. Blood flow was measured in the right femoral artery in both the acute and chronic study using Doppler Ultrasound (Xario 100; Canon Medical Systems Europe, Zoetermeer, The Netherlands). More details are in the Supplementary Materials.
Submaximal exercise test
After a 5-min warm-up at 75 W, participants cycled at 150 W for 10 min. Whole-body oxygen uptake (breath by breath) and heart rate (continuous) were measured. In addition, a capillary blood sample was collected 1 min after the end of exercise and analyzed for lactate concentration (Biosen C-Line; EKF Diagnostics, Cardiff, UK). Breath-by-breath VO2 data were transformed into 10-s averages, and VO2 and heart rate data of the last 8 min at 150 W were analyzed to ensure a metabolic steady state after the abrupt increase in work rate. Substrate oxidation (carbohydrate and fat) and total energy expenditure were calculated from the gas exchange data using stoichiometric equations (42).
Oral glucose tolerance test
After an overnight fast, an intravenous catheter was inserted and blood samples were obtained before and after (30, 60, 90, and 120 min) consumption of 75 g of glucose (dissolved in 300 ml of drinking water). The Matsuda insulin sensitivity index, a widely used method to assess whole-body insulin sensitivity, was calculated as described previously (43). More details are in the Supplementary Materials.
Muscle biopsies
After local anesthesia (0.5 ml of Xylocaïne, 1% without epinephrine; Aspen Netherlands B.V., Gorinchem, The Netherlands) and a small incision (3 mm), a biopsy was taken from the right m. vastus lateralis using the percutaneous Bergstrom needle biopsy technique with suction (44). One part of the muscle sample was immediately frozen in liquid nitrogen (enzyme activity, Western blot, and VEGF), whereas the other part was mounted in embedding medium (OCT Compound Tissue-Tek; Sakura Finetek, Zoeterwoude, The Netherlands), frozen in liquid nitrogen–cooled isopentane, and subsequently frozen in liquid nitrogen (histochemical analysis). All muscle samples were stored at −80°C until further analysis. Before analysis, non-embedded muscle samples were freeze-dried (48 hours); dissected free from blood, fat, and connective tissue; and divided into two pieces for Western blotting/VEGF (6 to 8 mg) and enzyme activities (2 to 3 mg).
Enzyme activity
Freeze-dried muscle samples (2 to 3 mg) were homogenized in phosphate/bovine serum albumin (BSA) buffer twice for 40 s at 28.5 Hz (QIAGEN TissueLyser II; Retsch GmbH, Haan, Germany), and the total protein concentration was determined by a BSA standard kit (Pierce Reagents, Rockford, IL, USA). Maximal enzyme activity of CS, HAD, HK, PFK, and LDH was quantified on the homogenates using standard fluorometric methods (Fluoroskan Ascent; Thermo Fisher Scientific, Waltham, MA, USA) (45). Analysis was performed in triplicate (CS, HAD, HK, and PFK) or quadruplicate (LDH), averaged, and expressed relative to the total protein content.
Western blotting
Western blotting on muscle lysates was performed as described previously (46), with details in the Supplementary Materials.
Muscle VEGF
VEGF protein levels were determined in the muscle lysates as previously described (47) using a human VEGF kit according to the manufacturer’s instructions (catalog no. K151RHG-2, Human VEGF kit; Meso Scale Discovery, Rockville, MD, USA). Samples were analyzed in duplicate, measured with QuickPlex SQ 120 (catalog no. AI0AA-0, Meso Scale Discovery), and analyzed using Discovery Workbench Software. VEGF is expressed relative to total protein content in the muscle lysates.
Immunohistochemical analysis
Analysis of capillary-fiber ratio, capillary density, and mean cross-sectional fiber area was performed by immunohistochemistry as described previously (48). Details can be found in the Supplementary Materials.
Statistical analysis
Researchers analyzing the data were blinded to the condition of the participants. Data were analyzed with R (version 4.0.0) in the RStudio interface (version 1.2.5033) with two-sided statistical tests. Changes over time in the acute and chronic study were analyzed using a linear mixed model approach with the lme4 package. The model was checked for homogeneity of variance and normality of the residuals using residual plots/Levene’s tests and Q-Q plots/Shapiro-Wilk’s tests. In case of violations of these assumptions, data were log-transformed before analysis, followed by rechecking of the model. For the acute study, fixed factors were condition (control or blockade) and time (nine time points), and for the chronic study, fixed factors were group (placebo or blockade) and time (before, halfway, and after the intervention). Participants were always specified as random intercept in the model. In case of significant main or interaction effects, analysis was followed by post hoc pairwise comparisons with Tukey adjustments for multiple comparisons (emmeans package). Differences in iAUC between conditions in the acute study were analyzed with paired t tests. For the chronic study, differences in changes from before to after the intervention between groups were analyzed with unpaired t tests. In case of non-normally distributed data (Shapiro-Wilk and Q-Q plots), a Wilcoxon signed-rank test was conducted instead of unpaired t tests. Relationships between variables were investigated with Pearson correlation coefficients, after verifying normality and log transform if necessary. For all analyses, statistical outliers were maintained in the dataset if physiologically plausible, although we conducted statistical analyses with and without the extreme outliers. These analyses did not alter interpretation of the results, verifying validity of the results. The significance level was set at P < 0.05, and results are presented as means ± SD unless otherwise noted.
Acknowledgments
We thank all volunteers who participated in these studies. We appreciate the help of G. De Leenheer and A. Volkaert (Ghent University) during data collection. We thank G. Kroos, K. Olsen, J. J. Nielsen, A. Tamariz-Ellemann, and J. B. Birk (Copenhagen University) for skilled technical assistance. Figures 1A, 2A, and 6 and fig. S2A were created with BioRender.com. Funding: This study was funded by Research Foundation Flanders (FWO 11B4220N, 1216819N, and 1503720N). Author contributions: T.V.d.S., L.B., I.E., C.V., L.G., Y.H., and W.D. designed the studies. T.V.d.S., L.B., F.S., and R.V.T. performed research. T.V.d.S., L.B., F.S., L.G., Y.H., and W.D. analyzed data. T.V.d.S., L.B., F.S., I.E., L.G., Y.H., and W.D. wrote the paper. All authors approved the final version of the paper. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/16/eabf2856/DC1
REFERENCES AND NOTES
- 1.Pedersen B. K., Saltin B., Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 25, 1–72 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Murphy R. M., Watt M. J., Febbraio M. A., Metabolic communication during exercise. Nat. Metab. 2, 805–816 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Sanford J. A., Nogiec C. D., Lindholm M. E., Adkins J. N., Amar D., Dasari S., Drugan J. K., Fernández F. M., Radom-Aizik S., Schenk S., Snyder M. P., Tracy R. P., Vanderboom P., Trappe S., Walsh M. J.; Molecular Transducers of Physical Activity Consortium , Molecular transducers of physical activity consortium (MoTrPAC): Mapping the dynamic responses to exercise. Cell 181, 1464–1474 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Findeisen M., Allen T. L., Henstridge D. C., Kammoun H., Brandon A. E., Baggio L. L., Watt K. I., Pal M., Cron L., Estevez E., Yang C., Kowalski G. M., O’Reilly L., Egan C., Sun E., Thai L. M., Krippner G., Adams T. E., Lee R. S., Grötzinger J., Garbers C., Risis S., Kraakman M. J., Mellet N. A., Sligar J., Kimber E. T., Young R. L., Cowley M. A., Bruce C. R., Meikle P. J., Baldock P. A., Gregorevic P., Biden T. J., Cooney G. J., Keating D. J., Drucker D. J., Rose-John S., Febbraio M. A., Treatment of type 2 diabetes with the designer cytokine IC7Fc. Nature 574, 63–68 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Luttrell M. J., Halliwill J. R., The intriguing role of histamine in exercise responses. Exerc. Sport Sci. Rev. 45, 16–23 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hegyesi H., Szalai C., Falus A., Csaba G., The histidine decarboxylase (HDC) gene of tetrahymena pyriformis is similar to the mammalian one. A study of HDC expression. Biosci. Rep. 19, 73–79 (1999). [DOI] [PubMed] [Google Scholar]
- 7.Thangam E. B., Jemima E. A., Singh H., Baig M. S., Khan M., Mathias C. B., Church M. K., Saluja R., The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: The hunt for new therapeutic targets. Front. Immunol. 9, 1873 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anrep G. V., Barsoum G. S., Appearance of histamine in the venous blood during muscular contraction. J. Physiol. 85, 409–420 (1935). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCord J. L., Halliwill J. R., H1 and H2 receptors mediate postexercise hyperemia in sedentary and endurance exercise-trained men and women. J. Appl. Physiol. 101, 1693–1701 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Pellinger T. K., Simmons G. H., Maclean D. A., Halliwill J. R., Local histamine H(1-) and H(2)-receptor blockade reduces postexercise skeletal muscle interstitial glucose concentrations in humans. Appl. Physiol. Nutr. Metab. 35, 617–626 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Pellinger T. K., Dumke B. R., Halliwill J. R., Effect of H1- and H2-histamine receptor blockade on postexercise insulin sensitivity. Physiol. Rep. 1, e00033 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romero S. A., Hocker A. D., Mangum J. E., Luttrell M. J., Turnbull D. W., Struck A. J., Ely M. R., Sieck D. C., Dreyer H. C., Halliwill J. R., Evidence of a broad histamine footprint on the human exercise transcriptome. J. Physiol. 594, 5009–5023 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ely M. R., Romero S. A., Sieck D. C., Mangum J. E., Luttrell M. J., Halliwill J. R., A single dose of histamine-receptor antagonists before downhill running alters markers of muscle damage and delayed-onset muscle soreness. J. Appl. Physiol. 122, 631–641 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodnick K. J., Haskell W. L., Swislocki A. L., Foley J. E., Reaven G. M., Improved insulin action in muscle, liver, and adipose tissue in physically trained human subjects. Am. J. Physiol. -Endocrinol. Metab. 253, E489–E495 (1987). [DOI] [PubMed] [Google Scholar]
- 15.Mortensen S. P., Askew C. D., Walker M., Nyberg M., Hellsten Y., The hyperaemic response to passive leg movement is dependent on nitric oxide: A new tool to evaluate endothelial nitric oxide function. J. Physiol. 590, 4391–4400 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lundby C., Montero D., Joyner M., Biology of VO2 max: Looking under the physiology lamp. Acta Physiol. 220, 218–228 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Hellsten Y., Nyberg M., Cardiovascular adaptations to exercise training. Compr. Physiol. 6, 1–32 (2015). [DOI] [PubMed] [Google Scholar]
- 18.James D. E., Brown R., Navarro J., Pilch P. F., Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature 333, 183–185 (1988). [DOI] [PubMed] [Google Scholar]
- 19.Bonner J. S., Lantier L., Hasenour C. M., James F. D., Bracy D. P., Wasserman D. H., Muscle-specific vascular endothelial growth factor deletion induces muscle capillary rarefaction creating muscle insulin resistance. Diabetes 62, 572–580 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akerstrom T., Laub L., Vedel K., Brand C. L., Pedersen B. K., Lindqvist A. K., Wojtaszewski J. F. P., Hellsten Y., Increased skeletal muscle capillarization enhances insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 307, E1105–E1116 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Vincent M. A., Barrett E. J., Lindner J. R., Clark M. G., Rattigan S., Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am. J. Physiol. -Endocrinol. Metab. 285, E123–E129 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Vincent M. A., Clerk L. H., Lindner J. R., Klibanov A. L., Clark M. G., Rattigan S., Barrett E. J., Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 53, 1418–1423 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Haber C. A., Lam T. K. T., Yu Z., Gupta N., Goh T., Bogdanovic E., Giacca A., Fantus I. G., N-acetylcysteine and taurine prevent hyperglycemia-induced insulin resistance in vivo: Possible role of oxidative stress. Am. J. Physiol. Endocrinol. Metab. 285, E744–E753 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Anderson E. J., Lustig M. E., Boyle K. E., Woodlief T. L., Kane D. A., Lin C.-T., Price J. W., Kang L., Rabinovitch P. S., Szeto H. H., Houmard J. A., Cortright R. N., Wasserman D. H., Neufer P. D., Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Invest. 119, 573–581 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tousoulis D., Kampoli A.-M., Tentolouris C., Papageorgiou N., Stefanadis C., The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 10, 4–18 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Borne R., Hausswirth C., Bieuzen F., Relationship between blood flow and performance recovery: A randomized, placebo-controlled study. Int. J. Sports Physiol. Perform. 12, 152–160 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Roberts L. A., Raastad T., Markworth J. F., Figueiredo V. C., Egner I. M., Shield A., Cameron-Smith D., Coombes J. S., Peake J. M., Post-exercise cold water immersion attenuates acute anabolic signalling and long-term adaptations in muscle to strength training. J. Physiol. 593, 4285–4301 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hesketh K., Shepherd S. O., Strauss J. A., Low D. A., Cooper R. J., Wagenmakers A. J. M., Cocks M., Passive heat therapy in sedentary humans increases skeletal muscle capillarization and eNOS content but not mitochondrial density or GLUT4 content. Am. J. Physiol. Heart Circ. Physiol. 317, H114–H123 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Christiansen D., Eibye K. H., Rasmussen V., Voldbye H. M., Thomassen M., Nyberg M., Gunnarsson T. G. P., Skovgaard C., Lindskrog M. S., Bishop D. J., Hostrup M., Bangsbo J., Cycling with blood flow restriction improves performance and muscle K+ regulation and alters the effect of anti-oxidant infusion in humans. J. Physiol. 597, 2421–2444 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egginton S., Hudlická O., Brown M. D., Walter H., Weiss J. B., Bate A., Capillary growth in relation to blood flow and performance in overloaded rat skeletal muscle. J. Appl. Physiol. 85, 2025–2032 (1998). [DOI] [PubMed] [Google Scholar]
- 31.Wragg J. W., Durant S., McGettrick H. M., Sample K. M., Egginton S., Bicknell R., Shear stress regulated gene expression and angiogenesis in vascular endothelium. Microcirculation 21, 290–300 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Zhou A. L., Egginton S., Brown M. D., Hudlicka O., Capillary growth in overloaded, hypertrophic adult rat skeletal muscle: An ultrastructural study. Anat. Rec. 252, 49–63 (1998). [DOI] [PubMed] [Google Scholar]
- 33.Rivilis I., Milkiewicz M., Boyd P., Goldstein J., Brown M. D., Egginton S., Hansen F. M., Hudlicka O., Haas T. L., Differential involvement of MMP-2 and VEGF during muscle stretch- versus shear stress-induced angiogenesis. Am. J. Physiol. Heart Circ. Physiol. 283, H1430–H1438 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Hambrecht R., Adams V., Erbs S., Linke A., Kränkel N., Shu Y., Baither Y., Gielen S., Thiele H., Gummert J. F., Mohr F. W., Schuler G., Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 107, 3152–3158 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Hawley J. A., Hargreaves M., Joyner M. J., Zierath J. R., Integrative biology of exercise. Cell 159, 738–749 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Joyner M. J., Dempsey J. A., Physiological redundancy and the integrative responses to exercise. Cold Spring Harb. Perspect. Med. 8, a029660 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konopka A. R., Laurin J. L., Schoenberg H. M., Reid J. J., Castor W. M., Wolff C. A., Musci R. V., Safairad O. D., Linden M. A., Biela L. M., Bailey S. M., Hamilton K. L., Miller B. F., Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell 18, e12880 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gliemann L., Schmidt J. F., Olesen J., Biensø R. S., Peronard S. L., Grandjean S. U., Mortensen S. P., Nyberg M., Bangsbo J., Pilegaard H., Hellsten Y., Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J. Physiol. 591, 5047–5059 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hostrup M., Onslev J., Jacobson G. A., Wilson R., Bangsbo J., Chronic β2-adrenoceptor agonist treatment alters muscle proteome and functional adaptations induced by high intensity training in young men. J. Physiol. 596, 231–252 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gifford J. R., Richardson R. S., CORP: Ultrasound assessment of vascular function with the passive leg movement technique. J. Appl. Physiol. 123, 1708–1720 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venturelli M., Layec G., Trinity J., Hart C. R., Broxterman R. M., Richardson R. S., Single passive leg movement-induced hyperemia: A simple vascular function assessment without a chronotropic response. J. Appl. Physiol. 122, 28–37 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeukendrup A. E., Wallis G. A., Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int. J. Sports Med. 26, S28–S37 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Matsuda M., DeFronzo R. A., Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 22, 1462–1470 (1999). [DOI] [PubMed] [Google Scholar]
- 44.Bergstrom J., Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand. J. Clin. Lab. Invest. 35, 609–616 (1975). [PubMed] [Google Scholar]
- 45.O. Lowry, J. Passonneau, in A Flexible System of Enzymatic Analysis (Academic Press, 1972), pp. 237–249. [Google Scholar]
- 46.Rytter N., Piil P., Carter H., Nyberg M., Hellsten Y., Gliemann L., Microvascular function is impaired after short-term immobilization in healthy men. Med. Sci. Sports Exerc. 52, 2107–2116 (2020). [DOI] [PubMed] [Google Scholar]
- 47.Olsen L. N., Hoier B., Hansen C. V., Leinum M., Carter H. H., Jorgensen T. S., Bangsbo J., Hellsten Y., Angiogenic potential is reduced in skeletal muscle of aged women. J. Physiol. 598, 5149–5164 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Nyberg M., Fiorenza M., Lund A., Christensen M., Rømer T., Piil P., Hostrup M., Christensen P. M., Holbek S., Ravnholt T., Gunnarsson T. P., Bangsbo J., Adaptations to speed endurance training in highly trained soccer players. Med. Sci. Sports Exerc. 48, 1355–1364 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Caen K., Vermeire K., Bourgois J. G., Boone J., Exercise thresholds on trial: Are they really equivalent? Med. Sci. Sports Exerc. 50, 1277–1284 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Russell T., Stoltz M., Weir S., Pharmacokinetics, pharmacodynamics, and tolerance of single- and multiple-dose fexofenadine hydrochloride in healthy male volunteers. Clin. Pharmacol. Ther. 64, 612–621 (1998). [DOI] [PubMed] [Google Scholar]
- 51.Roberts C. J., Clinical pharmacokinetics of ranitidine. Clin. Pharmacokinet. 9, 211–221 (1984). [DOI] [PubMed] [Google Scholar]
- 52.Garg D. C., Eshelman F. N., Weidler D. J., Pharmacokinetics of ranitidine following oral administration with ascending doses and with multiple-fixed doses. J. Clin. Pharmacol. 25, 437–443 (1985). [DOI] [PubMed] [Google Scholar]
- 53.Kroemer H., Klotz U., Pharmacokinetics of famotidine in man. Int. J. Clin. Pharmacol. Ther. Toxicol. 25, 458–463 (1987). [PubMed] [Google Scholar]
- 54.Berardi R. R., Tankanow R. M., Nostrant T. T., Comparison of famotidine with cimetidine and ranitidine. Clin. Pharm. 7, 271–284 (1988). [PubMed] [Google Scholar]
- 55.Beaver W. L., Wasserman K., Whipp B. J., A new method for detecting anaerobic threshold by gas exchange. J. Appl. Physiol. 60, 2020–2027 (1986). [DOI] [PubMed] [Google Scholar]
- 56.Binder R. K., Wonisch M., Corra U., Cohen-Solal A., Vanhees L., Saner H., Schmid J.-P., Methodological approach to the first and second lactate threshold in incremental cardiopulmonary exercise testing. Eur. J. Cardiovasc. Prev. Rehabil. 15, 726–734 (2008). [DOI] [PubMed] [Google Scholar]
- 57.Wasserman K., Mcilroy M. B., Detecting the threshold of anaerobic metabolism in cardiac patients during exercise. Am. J. Cardiol. 14, 844–852 (1964). [DOI] [PubMed] [Google Scholar]
- 58.Boone J., Koppo K., Bouckaert J., The response to submaximal ramp cycle exercise: Influence of ramp slope and training status. Respir. Physiol. Neurobiol. 161, 291–297 (2008). [DOI] [PubMed] [Google Scholar]
- 59.Caen K., Boone J., Bourgois J. G., Colosio A. L., Pogliaghi S., Translating ramp into constant power output: A novel strategy that minds the gap. Med. Sci. Sports Exerc. 52, 2020–2028 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/16/eabf2856/DC1


