Rhizodeposition and roots play opposite but complementary roles for building mineral-associated and particulate organic carbon.
Abstract
Soil organic carbon formation remains poorly understood despite its importance for human livelihoods. Uncertainties remain for the relative contributions of aboveground, root, and rhizodeposition inputs to particulate (POC) and mineral-associated (MAOC) organic carbon fractions. Combining a novel framework with isotope tracer studies, we quantified POC and MAOC formation efficiencies (% of C-inputs incorporated into each fraction). We found that rhizodeposition inputs have the highest MAOC formation efficiency (46%) as compared to roots (9%) or aboveground inputs (7%). In addition, rhizodeposition unexpectedly reduced POC formation, likely because it increased decomposition rates of new POC. Conversely, root biomass inputs have the highest POC formation efficiency (19%). Therefore, rhizodeposition and roots appear to play opposite but complementary roles for building MAOC and POC fractions.
INTRODUCTION
The formation and mineralization of soil organic carbon (SOC) are crucial for climate regulation, food provisioning, cultural heritage, habitat for organisms, and nutrient cycling (1, 2), but they remain poorly understood and quantified, particularly in different soil fractions. Separating SOC into particulate organic carbon (POC) and mineral-associated organic carbon (MAOC) is fundamental to understand the underlying processes that explain SOC formation and mineralization (3, 4). Previous studies have shown that MAOC has a relatively long persistence in the soil but requires large quantities of nitrogen for its formation and has limited storage capacity. In contrast, the POC fraction is more vulnerable to disturbance but has a lower nitrogen demand and can potentially accumulate indefinitely (3). Chemical recalcitrance of soil organic molecules and their location within large aggregates constrain POC mineralization, whereas in the MAOC fraction mineralization is constrained by physical-chemical protection, resulting from carbon (C) occlusion within microaggregates and adsorption onto mineral surfaces (5, 6). Previous research proposes that SOC formation is mainly explained by the amount and quality of plant inputs, where simple compounds probably contribute to MAOC formation and the more complex compounds to POC formation (7).
SOC formation efficiency (SOCFE) is affected not only by many soil factors, including texture, mineralogy, pH, temperature, and microbial biomass and community, but also by vegetation factors, such as plant biomass allocation among shoots, roots, and rhizodeposition (a suite of root exudates, secretions, and detached fine roots sloughed during root elongation and organic compounds from mycorrhizal turnover) (8, 9). This C-input is also defined as gross rhizodeposition, whereas the organic C that remained in the soil after microbial utilization and partial decomposition to CO2 is defined as net rhizodeposition (10). SOCFE is defined as the proportion of C-inputs retained in SOC and, formerly called the humification coefficient, depends on formation and mineralization rates of POC and MAOC fractions. SOCFE is relatively low (from approximately 3 to 30%), because 67 to 97% of total plant biomass that dies each year can be lost through respiration as CO2 (11). Recent analyses in agricultural systems suggest that SOCFE is around five times greater for belowground than for aboveground biomass [belowground-input-SOCFE > aboveground-input-SOCFE (12)]. The importance of roots compared with rhizodeposition in explaining this pattern remains unclear. To our knowledge, the MAOC and POC formation efficiencies (MAOCFE and POCFE, respectively) of roots, rhizodeposition, or aboveground input (shoot biomass) have not been synthesized jointly before, but evidence suggests that C-inputs from these sources may be retained preferentially in different SOC pools and by different mechanisms (13).
By analyzing and comparing different experimental approaches, we estimated SOCFE, POCFE, and MAOCFE of aboveground inputs, roots, and rhizodeposition separately using data from 35 studies and 197 observations that applied 13C as a tracer of plant inputs into the soil (Table 1). On the one hand, we compiled “litter incubation experiments” where roots and aboveground inputs were added to the soil and SOC derived from each input was quantified through time (Fig. 1A). Our database includes litter incubation experiments that added litter and roots from crop and grass species, as well as forest leaves and fine roots, performed either in controlled laboratory conditions or in the field (see Table 1 for details; 61% of the litter incubation experiments were performed in the field). Such experiments have traditionally evaluated SOCFE, although they do not include C-inputs from rhizodeposition and tend to ignore potential protection mechanisms of SOC promoted by living roots (Fig. 1A). On the other hand, “living plant experiments” consider the interactions of living plants with the soil matrix when estimating SOCFE. Our review includes living plant experiments that evaluated crop and grass species growing not only mainly in the field but also under controlled laboratory conditions (see Table 1 for details). In these experiments, rhizodeposition occurs throughout the growing season, whereas root and shoot biomass are incorporated into the soil primarily as a pulse after plant harvest (Fig. 1B). Because these different experimental approaches have important effects on SOCFE estimates, we combined them to estimate the SOCFE of roots, aboveground inputs, and rhizodeposition separately, specifically selecting papers that measured POC and MAOC contents to estimate POCFE and MAOCFE of all C-input sources.
Table 1. Summary of the reviewed articles.
RN, reference number; Ag, aboveground plant residues; NR, net rhizodeposition was estimated; NRm, net rhizodeposition was measured; MAP, mean annual precipitation; MAT, mean annual temperature; NA, not applicable.
| RN |
C-input source |
Experiment type |
SOC fractions* |
Method for new-C estimation |
Environment condition |
Plant species | Soil texture | MAP (mm) | MAT (°C) |
Residue management |
Time (years) |
| (39) | Roots | Litter incubation |
SOC | Labeling | Field | Triticum sp. | Silt loam and silty clay loam |
697 | 10.5 | Mixed with soil | 3 |
| (40) | Ag, roots | Litter incubation |
SOC, POC, and MAOC |
Labeling | Field | Pinus ponderosa |
Sandy loam | 1774 | 17 | Mixed with soil | 1.6 |
| (41) | Roots | Litter incubation |
SOC | Labeling | Field | Triticum sp. | Sandy, clay loam, loamy and silty clay |
528, 1062, 1150, and 1052 |
16.7, 5.1, 4.5, and 4.7 |
Mixed with soil | 1 |
| (7) | Ag | Litter incubation |
SOC, POC, and MAOC |
Labeling | Field | Andropogon gerardii |
Silty clay | 835 | 12.9 | On soil surface | 3 |
| (42) | Ag, roots | Litter incubation |
SOC, POC, and MAOC |
Natural abundance |
Controlled | Eucalyptus sp. | Sandy clay loam |
NA | NA | Mixed with soil | 0.66 |
| (43) | Ag | Litter incubation |
SOC, POC, and MAOC |
Labeling | Field | Picea abies | Clay | 1098 | 3.8 | On soil surface | 1 |
| (44) | Ag | Litter incubation |
SOC | Labeling | Field |
Acer saccharum |
Silt loam | 900 | 9 | On soil surface | 2 |
| (45) | Roots | Litter incubation |
SOC, POC, and MAOC |
Labeling | Field | Triticum sp. | Silty clay and silty clay loam |
1155 and 1062 |
4.4 and 5.8 | Mixed with soil | 1 and 3 |
| (46) | Ag, roots | Litter incubation |
SOC, POC, and MAOC |
Labeling | Field |
Pinus ponderosa |
Sandy loam | 1774 | 17 | Mixed with soil | 5 |
| (47) | Ag, roots | Litter incubation |
SOC, POC, and MAOC |
Labeling | Controlled | Zea mays L. | Silty loam | NA | NA | Mixed with soil | 0.23 |
| (48) | Ag, roots | Litter incubation |
SOC | Labeling | Field |
Pinus ponderosa |
Silty clay loam |
1774 | 17 | Mixed with soil | |
| (49) | Ag, roots | Litter incubation |
SOC | Natural abundance |
Controlled |
Populus simonii Carr. |
Loamy sand | NA | NA | Mixed with soil | 0.74 |
| (50) | Ag | Litter incubation |
SOC, POC, and MAOC |
Labeling | Field | Beech forest | ND | 930 | 8.4 | On soil surface | 1 |
| (51) | Ag | Litter incubation |
SOC, POC, and MAOC |
Labeling | Field | Sinapis alba | Silt loam | 803 | 7.4 | Mixed with soil | 1.56 |
| (52) | Ag | Litter incubation |
SOC | Labeling | Field | Populus nigra | Loam | 734 | 15 | On soil surface | 0.92 |
| (53) | Roots | Litter incubation |
SOC, POC, and MAOC |
Labeling | Controlled | Oilseed rape | Sandy loam | NA | NA | Mixed with soil | 0.37 |
| (54) | Roots | Litter incubation |
SOC, POC, and MAOC |
Labeling | Field | Triticum sp. | Silt loam and silty clay loam |
600 | 10.5 | Mixed with soil | 3 |
| (55) | Ag, roots | Litter incubation |
SOC | Labeling | Controlled |
Liquidambar styraciflua L. |
Silty clay loam |
NA | NA | Ag on surface and roots mixed with soil |
0.07 |
| (56) | Roots | Litter incubation |
SOC | Labeling | Field | Acer rubrum | Sand | 838 | 6.8 | Mixed with soil | 1 and 2 |
| (57) | Ag, roots | Litter incubation |
SOC, POC, and MAOC |
Labeling | Controlled | Triticum sp. | Silt | NA | NA | Mixed with soil | 0.18 |
| (58) | Ag, roots | Litter incubation |
SOC | Natural abundance |
Controlled |
Canavalia ensiformis and Gliricidia sepium |
Clay | NA | NA | Mixed with soil | 2 |
| (59) | Ag, roots | Litter incubation |
SOC, POC, and MAOC |
Natural abundance and labeling |
Controlled |
Fagus sylvatica L. and Fraxinus excelsior L. |
Silt loam | NA | NA | Mixed with soil | 0.56 |
| (60) | Ag | Litter incubation |
SOC, POC, and MAOC |
Natural abundance |
Controlled | Zea mays L. | Silt loam | NA | NA | Mixed with soil | 0.13 |
| (61) | Ag, roots, NRm |
Living plant | SOC | Labeling | Field | Secale cereale | Fine-loamy | 1055 | 8.2 | No-Till | 0.42 and 1.42 |
| (62) | Ag, roots, NR | Living plant | SOC, POC, and MAOC |
Natural abundance |
Field | Zea mays L. | Silt loam | 650 | 10.5 | Tillage | 4 |
| (37) | Ag, roots, NR | Living plant | SOC | Treatments | Field | Zea mays L. | Silt loam | 970 | 10 | Tillage | 6, 10 and 12 |
| (63) | Roots, NR | Living plant | SOC | Natural abundance | Field |
Zea mays L., Hordeum vulgare L., Triticum aestivum L., Trifolium pratense L., Phleum pratense L. |
Silt loam | 1200 | 4 | Tillage | 15 |
| (64) | Ag, roots, NR | Living plant | SOC | Natural abundance |
Field | Zea mays L. | silt loam | 820 | 7 | No-Till and tillage |
13 |
| (38) | Ag, roots, NRm |
Living plant | SOC, POC, and MAOC |
Labeling | Controlled |
Triticum aestivum, Brassica napus, Lens culinaris, Pisum sativum |
Sandy loam and loam |
NA | NA | No-Till | Not reported |
| (65) | Ag and roots, NR |
Living plant | SOC | Natural abundance |
Field |
Zea mays L., Triticum sp. |
Clay loam | 551 | 5.7 | Tillage | 16 |
| (66) | Ag, roots, NR | Living plant | SOC, POC, and MAOC |
Labeling | Field |
Vicia dasycarpa Ten. |
Silt loam and silty clay loam |
450 | 16.5 | Tillage | 0.37 |
| (36) | Ag, roots, NR | Living plant | SOC | Treatments | Field | Zea mays L. | Silty clay loam |
906 | 10 | Tillage | 11 |
| (67) | Ag, roots, NR | Living plant | POC | Natural abundance |
Field |
Zea mays L. and Glycine max L. |
Silt loam | 1200 | 17 | No-Till | 2 |
| (68) | Ag, roots, NRm |
Living plant | SOC | Labeling | Field |
Vicia villosa Roth subsp. Villosa |
Clay loam | 1053 | 13 | Tillage | 0.41 |
| (69) | Ag, roots, NRm |
Living plant | SOC | Labeling | Field |
Triticum aestivum L., Pisum sativum L., and Vicia sativa L. |
Sandy loam | 769 | 19.3 | No-Till | 0.49 |
*POC: includes either the fractions with particle size higher than 53 μm or the light fraction separated by density (3). MAOC: includes either the fractions with particle size lower than 53 μm or the heavy fraction separated by density. It was assumed that SOC is equal to the sum of POC plus MAOC; therefore, when the studies reported only one fraction and total SOC, the second fraction was calculated by difference (70).
Fig. 1. Schematic representation of C dynamics in litter incubation experiments and living plant experiments.
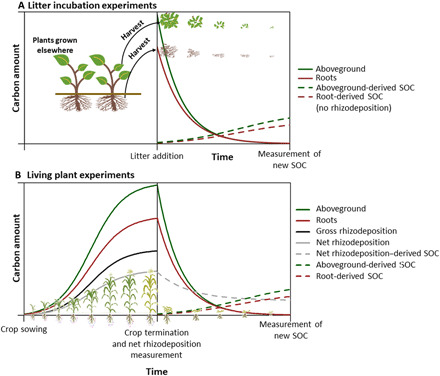
(A) For litter incubation experiments, we illustrate the decrease of root and aboveground C and the formation of new SOC derived from each C-input source. (B) In living plant experiments, we also show C contents in plant biomass and associated gross and net rhizodeposition, as defined by Pausch and Kuzyakov (10). Plant biomass C and gross rhizodeposition are shown as solid lines, and new SOC is shown in dashed lines. The SOCFE of each C-input source is defined as the relation between new SOC derived from a particular C-input source and the amount of C-input added at litter addition (A) or at crop termination (B).
RESULTS AND DISCUSSION
Formation efficiencies of belowground and aboveground inputs into total SOC
Within the reviewed experiments, SOC formation efficiencies (SOCFE) were relatively stable at different C-input levels (Fig. 2), as shown by the linear relationships between C-inputs and C in new SOC in both experimental methods (litter incubations and living plant experiments). Average aboveground-input-SOCFE and root-input-SOCFE were similar in litter incubation experiments (0.31 and 0.36, respectively) (Fig. 2A) but differed markedly in living plant experiments where average aboveground-input-SOCFE was 0.11, one-third the value of average belowground-input-SOCFE of 0.31 (belowground inputs included roots and rhizodeposition in this type of experiments) (Fig. 2B). These results suggest that SOCFE of traditional litter incubation experiments may overestimate real SOCFE occurring under normal field conditions, particularly for aboveground inputs. In addition, the higher belowground-input-SOCFE as compared to aboveground-inputs-SOCFE observed only in experiments with living roots suggests that reasons other than biochemical composition likely explain the higher retention of belowground inputs into bulk SOC. Our estimates agree with recently reported values for aboveground-input-SOCFE but are lower than reported values for belowground-input-SOCFE (12) because our calculations include rhizodeposition amounts plus roots (as belowground inputs) in the SOCFE estimation. Because belowground-input-SOCFE is typically calculated as the ratio between root biomass and belowground-derived-SOC, ignoring rhizodeposition as an input, reported values of belowground-input-SOCFE in living plant experiments may overestimate real formation efficiencies by almost 50% because net rhizodeposition is not included in the denominator (assuming a net rhizodeposition:root ratio of 0.5; see Table 2). Overall, our results suggest that SOCFE values measured in litter incubation experiments may not represent decomposition dynamics under field conditions with living plants and highlight the importance of estimating SOCFE in ways that consider all plant inputs including rhizodeposition.
Fig. 2. Relationships between C-inputs and new SOC formation in litter incubation experiments and living plant experiments.
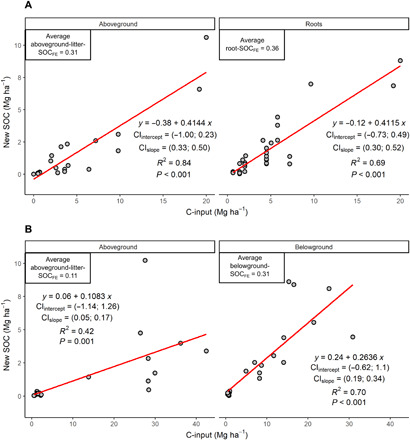
Panel A shows litter incubation experiments and panel B living plant experiments. Average SOC formation efficiencies (SOCFE) are shown for each type of inputs and experimental method. Regression lines are shown in red, and CIintercept and CIslope are the 95% confidence intervals (for the intercept and slope, respectively). Belowground C-inputs in living plant experiments include net rhizodeposition (see Materials and Methods).
Table 2. Mean, SD, and sample size (n) net rhizodeposition-to-root ratios observed in this paper and in Pausch and Kuzyakov review (10).
| Ratio | Land use | Mean | SD | n | Reference |
| Net rhizodeposition/root biomass |
Crops | 0.54 | 0.07 | 99 | Pausch and Kuzyakov (10) |
| Grasses | 0.50 | 0.06 | 128 | ||
| Trees | 0.49 | 0.11 | 9 | ||
| Crops and cover crops | 0.44 | 0.12 | 7 | This review | |
| All | 0.51 | 0.07 | 243 | Weighted average |
Formation efficiency of aboveground, root, and rhizodeposition inputs into POC and MAOC
We found a significant effect of time on POCFE (P < 0.001; Table 4). POCFE decreased during the first year of experimentation, after which time it appears to stabilize, but MAOCFE estimates were not significantly related to time (evaluated using linear and nonlinear models) (Fig. 3). The different formation mechanisms of POC and MAOC could explain these results. POC is largely made up of relatively undecomposed fragments of plant residues, whereas MAOC consists of microscopic fragments of organic material associated with soil minerals (7). Therefore, it is expected that new POC derived from plant inputs follows a decomposition pattern similar to that from litter inputs, and therefore, POCFE decreases across time. However, it is likely that new MAOC has a different decomposition pattern because it is rapidly formed during early stages of litter decomposition and then remains relatively stable because of soil protection (3). Thus, we expected a smaller or lack of correlation of MAOCFE with experimental time. We found these same results when analyzing two particular experiments in our dataset that resampled soils through experimental time and estimated POCFE and MAOCFE variations (see fig. S2).
Table 4. Mean, SD, and sample size (n) of shoot-to-root ratios observed in this paper and Bolinder et al. (75).
| Ratio | Land use | Mean | SD | n | Reference |
| Shoot/root | Small-grain cereals | 7.4 | 3.6 | 59 | Bolinder et al. (75) |
| Corn | 5.6 | 2.8 | 21 | ||
| Soybean | 5.2 | 3.1 | 12 | ||
| Perennial forages | 1.6 | 1.2 | 63 | ||
| Crops and cover crops | 3.3 | 1.5 | 20 | This review | |
| All | 4.5 | 2.4 | 175 | Weighted average |
Fig. 3. Relations between formation efficiencies with experimental time.
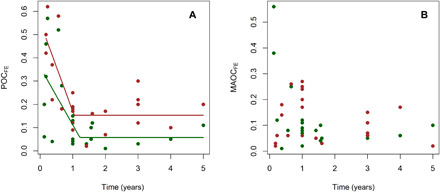
Formation efficiencies of aboveground (green dots) and belowground inputs (roots in litter incubation experiments and root + rhizodeposition in living plant experiments, red dots) into POC [POCFE, (A)] and MAOC [MAOCFE, (B)] with time (after initial input addition). Details of models fitted for POCFE are shown in Table 3.
After correcting for time effects, we found that belowground inputs have substantially higher formation efficiencies than aboveground inputs, not only in total SOC but also in both POC and MAOC fractions, with differential effects of roots, rhizodeposition, and aboveground inputs in the formation efficiencies of each soil fraction. In living plant experiments that reflect real plant growing conditions, belowground-POCFE (roots and rhizodeposition) was ~180% higher than aboveground-input-POCFE, with similar results for MAOC (~170% increase) (Fig. 4). However, different mechanisms seem to explain the observed higher POCFE and MAOCFE of roots and rhizodeposition, as discussed below.
Fig. 4. Formation efficiencies of aboveground, roots, belowground inputs (roots + net rhizodeposition), and net rhizodeposition into particulate organic carbon (POCFE) and mineral-associated organic carbon (MAOCFE) in litter incubation experiments or living plant experiments.
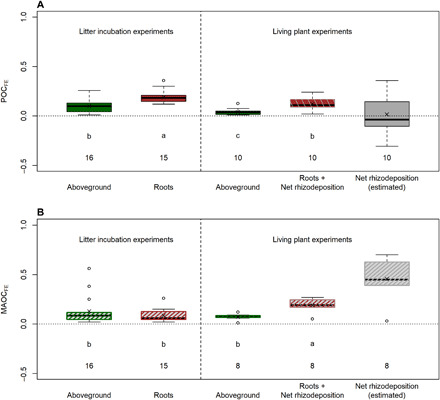
Panel A shows POCFE and panel B shows MAOCFE. Boxes, interquartile distance; line in the box, median; crosses, mean; whiskers, maximum and minimum non-outlier; circles, outliers; the value was considered to be an outlier if it was at least 1.5 interquartile ranges below the first quartile, or at least 1.5 interquartile ranges above the third quartile. Numbers described the sample size, and different letters indicate statistical significance (P < 0.05). Rhizodeposition formation efficiencies in living plant experiments were estimated considering a net rhizodeposition:root ratio of 0.5 ± 0.1 and assuming root-input-POCFE and root-input-MAOCFE as the median of litter incubation experiments (see Eqs. 3 and 4). POCFE measurements made before the break point time in Fig. 3 were corrected to the break point time using the relative decay rates (76% year−1 for aboveground and 79% year−1 for root and belowground inputs; see Table 3 and the Supplementary Materials for details)
Our results for the MAOC fraction suggest that plant rhizodeposition is critical for building SOC in stabilized pools because average net rhizodeposition-MAOCFE in living plant experiments was high (0.46 ± 0.21; Fig. 4B) compared to the MAOCFE of roots or aboveground inputs (~ 0.07; Fig. 4B). Belowground-MAOCFE in living plant experiments (0.19 ± 0.07) was nearly three times higher than MAOCFE of aboveground inputs (0.07 ± 0.03) in the same type of experiment, whereas root-input-MAOCFE did not differ from aboveground-input-MAOCFE in litter experiments (Fig. 4B). These results suggest that the presence of living roots is a more efficient way to increase MAOC. Our calculations show that the majority (more than 75%) of total belowground derived MAOC was contributed by rhizodeposition (Fig. 5). These results assume a net rhizodeposition:root biomass ratio of 0.51 (± 0.07, n = 242) extracted from a literature synthesis (Table 2), but a sensitivity analyses revealed that our estimates remain unchanged for a wide range of net rhizodeposition:root biomass ratio, varying only with net rhizodeposition:root biomass ratios lower than ~0.3, which are uncommon given that 0.3 is two SDs away from the mean (see the Supplementary Materials and fig. S1 for details).
Fig. 5. Contributions of roots and net rhizodeposition to new POC and MAOC as a percentage of total belowground inputs.
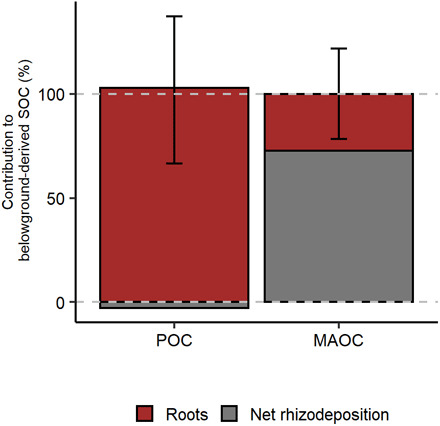
Contributions were calculated for each belowground input values reported in the literature in living plant experiments, assuming root-POCFE and root-MAOCFE as the median of litter incubation experiments and considering a net rhizodeposition:root ratio of 0.5 ± 0.1 (from Eqs. 6 to 9). Gray dashed lines indicate the range between 0 and 100%. Negative contribution of net rhizodeposition in the POC fraction shows that this C-input can reduce POC formation.
Although several studies show that rhizodeposition promotes SOC decomposition (i.e., priming) (14, 15), our results suggest that in the MAOC fraction it may have the opposite result—MAOC formation. This result may occur because rhizodeposition is rich in simple carbohydrates (16) that are easily consumed by microbial activity that produce microbial necromass and microbial-derived compounds. These compounds are recovered in the dissolved organic C fraction, which is the dominant and more efficient pathway of MAOC formation (7, 13). In addition, sorption of other low–molecular weight compounds (e.g., organic acids) from rhizodeposition could be another pathway of MAOC formation. When fine minerals of soil (clay + silt) are nonsaturated with organic compounds (17), these simple molecules have a strong capacity to interact with minerals and contribute to MAOC formation (13, 18). Furthermore, MAOCFE of roots and aboveground inputs was not different in litter incubation experiments (~0.11), therefore suggesting that biochemical composition is less important for retaining C in this stable SOC fraction (Fig. 4B).
Mycorrhizal contribution to rhizodeposition is unclear but could partly explain the high net rhizodeposition-MAOCFE found in our study. Substantial quantities of rhizosphere exudates come from extraradical hyphae secretions, and, in contrast, death and turnover of mycorrhizal tissues may also contribute substantially as C-inputs to soil decomposers, therefore increasing microbial necromass and microbial-derived compounds (19). As discussed above, these two pathways (increasing the microbial necromass or producing low–molecular weight compounds) are key C-inputs for MAOC formation. Most of the data analyzed in our study came from croplands (Table 1), where fertilization practices or tillage could have reduced mycorrhizal abundance. Therefore, MAOC formation rates from rhizodeposition could be potentially higher in nonmanaged and more natural ecosystems with abundant mycorrhizal colonization.
In contrast to MAOC formation, root biomass was the more efficient C-source for forming POC because root-input-POCFE was highest in litter incubation experiments (0.19 ± 0.07), even higher than belowground-input-POCFE in living plant experiments (0.12 ± 0.06) where both root + rhizodeposition inputs are occurring jointly (Fig. 4A). This differential stabilization into POC is likely attributable to the chemical recalcitrance of roots because root-input-POCFE was higher than aboveground-input-POCFE (0.10 ± 0.07) in litter experiments (Fig. 4A). In such experiments, root and aboveground inputs are incubated under similar soil conditions without rhizodeposition, the main difference being their chemical composition. Our estimates also showed that the majority of belowground-derived-POC was contributed by roots (Fig. 5). However, because POC is usually a smaller fraction of total SOC in croplands (20), the chemical recalcitrance mechanism would usually play a minor role in the overall SOC formation (21, 22), with POC formation becoming relatively more important in forest and coarser textured soils (23). It was unclear from our data how root stimulation of soil aggregation promotes POC accumulation, as suggested previously (24, 25). The larger differences between aboveground-input-POCFE and belowground-input-POCFE in living plant experiments as compared to the difference between aboveground-input-POCFE and root-input-POCFE in litter incubation experiments may also suggest that root stimulation of soil aggregation could increase root conservation in POC in addition to its chemical recalcitrance.
Our results show that rhizodeposition increased MAOC formation (as explained above) but reduced POC formation, likely because it increased decomposition rates of new POC. Rhizodeposition seems to decrease POCFE, based on observations comparing average root-input-POCFE in litter experiments (0.19 ± 0.07) with belowground-input-POCFE (root + rhizodeposition) in living plant experiments (0.12 ± 0.07) (Fig. 4A). Even assuming that rhizodeposition does not form POC because this soil fraction contains coarse plant materials and not simpler molecules of low molecular weight through rhizodeposition (9), and considering an average net rhizodeposition:root ratio of 0.5 ± 0.1 (Table 2), root-input-POCFE in living plant experiments would be 0.18, still lower than the observed in litter incubation experiments (0.19 ± 0.07). This lower root-input-POCFE in living plant experiments may be explained if root decomposition is increased by rhizodeposition inputs (26). This increased decomposition rate could explain the negative values of net rhizodeposition-POCFE (Fig. 4A) and the negative contribution of net rhizodeposition to belowground-derived-POC (Fig. 5). Other research suggests that rhizodeposition induces greater microbial activity and increases SOC mineralization (i.e., priming effect) (14), either by contributing compounds that serve as cometabolites or by facilitating the release of mineral protected C into more accessible pools (26). These mechanisms not only could explain the increased decomposition rates of new POC but also could be operating on preexisting POC (i.e., “old POC”) producing a priming effect (not accounted for in our study). Therefore, in the presence of rhizodeposition, a higher proportion of roots and aboveground inputs may be respired due to an increase in POC decomposition rates (both new and old), as observed for new POC only in living plant experiments. Our results strongly suggest that rhizodeposition increases decomposition rates only for roots entering the POC fraction, where recalcitrance limits its decomposition (7), reconciling previous evidence showing that rhizodeposition may either induce SOC decomposition (in the POC fraction) or increase its formation (in the MAOC fraction).
Shoot:root effects on C-input contributions to POC and MAOC formation
When considering a shoot:root ratio of 1, 81% of the new SOC formed annually was derived from belowground inputs (root + rhizodeposition), but belowground contributions were 43% when the shoot:root ratio was 6 (Fig. 6A). SOC formation depends on both input formation efficiencies and total input amounts from vegetation. High aboveground inputs explain why even with shoot:root ratios of 6, our estimates show that SOC is mainly aboveground-derived, despite aboveground inputs having a relatively low POCFE and MAOCFE (Fig. 4). The relative contributions of aboveground and belowground inputs to SOC formation are therefore controlled by the shoot:root ratio, which varied between 1 and 6 in our dataset (Fig. 6A and Table 3). To estimate C-input contributions to POC and MAOC formation, we combined three hypothetical shoot:root ratios (1, 3, and 6) with POC and MAOC formation efficiencies of aboveground input, roots, and rhizodeposition in living plant experiments (Fig. 4). For shoot:root ratios of 1 and 3, POC and MAOC were mainly formed from root and rhizodeposition inputs, respectively. However, when the shoot:root ratio increased to 6, both fractions were formed more from aboveground inputs (Fig. 6A). Overall, when considering all the experiments reviewed, SOCFE decreased with increasing shoot:root ratios (Fig. 6B). Therefore, when shoot:root ratios increased from 1 to 6, SOCFE decreased from 25 to 16%, representing a 36% decrease in total SOC formation efficiencies (Fig. 6A). These results confirm earlier studies, suggesting that increasing C allocation to roots (and rhizodeposition) may be an important tool for increasing SOC storage (13). Our estimates of root:shoot ratios do not consider crop harvesting, which would remove substantial amounts of aboveground biomass (grains) decreasing the proportion of POC and MAOC formed from aboveground inputs.
Fig. 6. Changes in SOCFE and C-inputs retained in SOC fractions with varying shoot:root ratios.
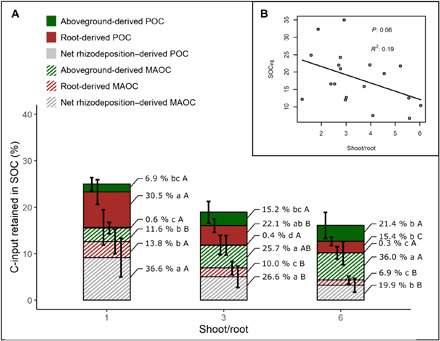
Estimated contributions to POC and MAOC formation of different C-input sources under varying shoot:root ratios (A) and relation between soil organic C formation efficiency (SOCFE) and shoot:root ratio of analyzed experiments (B). For each shoot:root ratio, we assumed the same total input (100%) that was distributed into aboveground, root, and rhizodeposition, considering a root:net rhizodeposition ratio of 0.5 (Table 2). We then multiplied each C-input by POC and MAOC formation efficiency of our database to estimate the C-input retained from each source in both soil fractions (see Materials and Methods). The percentages indicated to the right of each bar correspond to the contribution of each fraction to the total SOC, and error bars are the SDs. Different letters indicate significant differences (P < 0.05) between fractions within each shoot:root ratio (lowercase letters) and between shoot:root ratios within each fraction (uppercase letters). The error bars correspond, from left to right, to aboveground-derived POC, root-derived POC, net rhizodeposition–derived POC, aboveground-derived MAOC, root-derived MAOC, and net rhizodeposition–derived MAOC.
Table 3. Summary of bilinear models with no preestablished break point fitted for the relationship between the particulate organic carbon formation efficiency (POCFE) with time (Fig. 3).
Equation fitted: POCFE ~ A + B * (T − C < 0) * (T − C), where T is the time (years), C is the year where the break occurs, A is mean POCFE after T = C, and B is the change rate of POCFE (POCFE year−1) before T = C. These nonlinear models were fitted with the nls function of the R software (73).
| Aboveground | Belowground | |||||
| Parameters | Estimated | SE | P | Estimated | SE | P |
| A | 0.06 | 0.04 | 0.2042 | 0.15 | 0.03 | 4.03 × 10–5 |
| B* | −0.25 | 0.09 | 0.0076 | −0.38 | 0.08 | 6.25 × 10–5 |
| C | 1.22 | 0.29 | 0.0003 | 1.04 | 0.13 | 3.99 × 10–8 |
*The change rate B in relative terms (percentage of change respecting to the initial POCFE) is −76% year−1 for aboveground and −79% year−1 for belowground.
Predicting SOC dynamics is important for developing sustainable land use strategies in the context of global change. The latest discoveries in SOC dynamics are not typically included in traditional simulation models, and a new generation of models based on measurable SOC fractions is needed (3, 27, 28). Our findings on formation efficiencies of different C sources and shoot:root ratios could directly inform these models and improve management of the C cycle and soil C sequestration. We showed that roots and rhizodeposition are highly efficient C sources for POC and MAOC formation, respectively. Thus, the inclusion of plants with higher C allocation to belowground biomass may increase SOC stocks, especially in croplands where SOC stock depletion has occurred worldwide (29, 30). However, plant breeding has traditionally selected crops for aboveground C allocation because of its relation to harvestable products (31). Therefore, potential trade-offs between production and SOC formation (12), and multiple-objective breeding programs (32, 33), could emerge. A good strategy that would contribute to reducing these trade-offs could be the inclusion of service crops (cover crops) in agricultural rotations, with high root production and elevated rhizodeposition (34, 35). Our work shows that root production and rhizodeposition, often overlooked in agronomy and plant breeding programs, should be evaluated for novel C cycle management options.
MATERIALS AND METHODS
Literature search and database building
We searched for peer-reviewed studies that evaluated SOC formation from aboveground and/or belowground inputs in Scopus (www.scopus.com). The following combination of terms was used: (“decomposition” OR “humification” OR “mineralization” OR “stabilization”) AND (“soil organic carbon” OR “soil organic matter” OR “SOM” OR “SOC”) AND (“root” OR “litter” OR “belowground” OR “aboveground”) AND (“isotopes” OR “label” OR “labelled” OR “labeling” OR “labeled”). The search resulted in 248 articles. To build a database with the papers, a two-step selection process was carried out. First, titles and abstracts of all articles were examined to exclude those that clearly do not focus on SOC formation. Second, only full papers that met the above criteria were reviewed and the papers where SOC, POC, or MAOC formation efficiency was reported, or could be calculated from data, were kept. When a study had measurements through time, we emphasize the final (i.e., longest) sampling time. The isotopic techniques used as tracers included the isotopic labeling of plants or the use of plant species with a different natural abundance of 13C (C3 versus C4 species). In addition to the selected papers, two older articles were also included in our review, because they were pioneer works in the topic, and although they did not use isotope tracers, their experimental setup allowed us to estimate separately belowground from aboveground SOC formation efficiencies (36, 37). The final database included 35 articles with 197 observations (Table 1).
Experiments were divided into litter incubation experiments and living plant experiments (Table 1), as explained in Introduction (Fig. 1). In litter incubation experiments, roots and aboveground plant tissues were added to the soil and, after a time period, the amount of new SOC formed from these tissues was measured (Fig. 1A). These experiments were carried out under controlled conditions in the laboratory or in the field (in situ) and assessed crops and forest litter. We included forest and grass treatments because no statistical differences were found between vegetation types (fig. S3). The living plant experiments have two phases. The first phase included the crop’s growing period, from sowing to crop termination. During this period of crop growth, C accumulates in aboveground and roots tissues, while gross rhizodeposition occurs and net rhizodeposition can be measured at crop termination in the soil (Fig. 1B). C accumulated at crop termination in roots and net rhizodeposition is considered as the total belowground inputs, and C accumulated in aboveground litter as the total aboveground inputs (Fig. 1B). In the second phase of living plant experiments, aboveground, roots, and net rhizodeposition begin to decompose and form new SOC (similar to the litter incubation experiments; Fig. 1A). All the living plant experiments were carried out under field conditions, excepting Comeau et al. (38), where plants were grown under greenhouse conditions.
Data analysis
The data collected from the reviewed papers included experimental method (litter incubation or living plant experiment), soil texture, soil depth, experiment time, initial SOC, total new SOC, new SOC in each soil fraction (POC or MAOC), and C-input sources [aboveground, root, or net rhizodeposition (when available)]. When net rhizodeposition was neither measured nor estimated, it was assumed as 50% of root biomass (Table 2). Because data distribution was not normal [tested with Shapiro test (71)], differences between groups (Fig. 4) were assessed with Wilcoxon test (72). All statistical analyses were performed with R software version 4.0.2 with stats package (73).
Estimates of formation efficiencies of aboveground, roots, and rhizodeposition into POC and MAOC
Formation efficiencies of a given C-input (aboveground, root, and belowground) were estimated by dividing the amount of input by the amount of new SOC (POC or MAOC) formed from that C-input (Eq. 1)
| (1) |
To differentiate POC and MAOC formation efficiencies originated from rhizodeposition or roots, two steps were made. First, we considered that belowground-input-POCFE and belowground-input-MAOCFE are a weighted average of net rhizodeposition and root formation efficiencies (Eqs. 2 and 3)
| (2) |
| (3) |
where Belowground-input-POCFE is the formation efficiency of belowground input to POC, fnr is the fraction of belowground input that is net rhizodeposition, net rhizodeposition-POCFE is the formation efficiency of net rhizodeposition to POC, root-POCFE is the formation efficiency of roots to POC, Belowground-input-MAOCFE is the formation efficiency of belowground inputs to MAOC, net rhizodeposition-MAOCFE is the formation efficiency of net rhizodeposition to MAOC, and root-MAOCFE is the formation efficiency of roots to MAOC. It is important to note that in previous works that estimate SOC formation efficiencies [see review by Jackson et al. (12)], estimates are based only on root biomass, ignoring rhizodeposition inputs, and therefore, SOC formation efficiencies of roots are overestimated and will be lower if rhizodeposition is considered as an input.
Second, we solved Eqs. 2 and 3 to estimate net rhizodeposition-POCFE and net rhizodeposition-MAOCFE (Eqs. 4 and 5) for each living plant experiment
| (4) |
| (5) |
where
1) Belowground-POCFE and belowground-MAOCFE were taken from the observed values in living plant experiments.
2) Root-POCFE and root-MAOCFE were assumed to be the median of observed values in the litter incubation experiments (0.18 and 0.06, respectively) because in these experiments roots are added to the soil manually and therefore no rhizodeposition exists. We used the median instead of the average for this assumption because median is a measure of central tendency not distorted by skewed data (74).
3) The net rhizodeposition:root ratio was assumed to be 0.5, and therefore, the fraction of net rhizodeposition (fnr) used was 0.33 of total belowground inputs (see Table 2).
Estimates of the relative contributions of root and net rhizodeposition to the total belowground-derived SOC pool
After solving Eqs. 4 and 5, we estimated the contributions of roots and net rhizodeposition to new POC and new MAOC as a percentage of total belowground inputs (Fig. 5), with Eqs. 6 to 9 as follows
| (6) |
| (7) |
| (8) |
| (9) |
Estimates of new POC and MAOC formation under varying shoot:root ratios
The contribution of different C-inputs to SOC pools depends not only on formation efficiencies but also on the relative amount of inputs added to the soil, which depends on plant allocation patterns (i.e., shoot:root ratio). To evaluate the impact of different shoot:root ratios on SOC formation, we performed an additional analysis (Fig. 6). We varied the relative contributions of C-inputs assuming shoot:root ratios of 1, 3, and 6 (which are within the reported range in the reviewed experiments; see Table 4) and multiplied them by the aboveground-input-POCFE, root-POCFE, net rhizodeposition-POCFE, aboveground-input-MAOCFE, root-MAOCFE, or the net rhizodeposition-MAOCFE estimated for each experiment to calculate the relative contributions (in percentage) of each C-input to the different pools of new SOC formed, as follows
| (10) |
| (11) |
| (12) |
where fa, fr, and fnr are the fractions of total input (fa + fr + fnr = 1) that is aboveground, root, or net rhizodeposition, respectively. These fractions vary with the different shoot:root ratios considered.
Acknowledgments
We are grateful to K Georgiou, A. Malhotra, and M. Oesterheld for providing comments on previous manuscript drafts. Funding: This work was supported by grants from PICT-2018-03786, Argentina; Gordon and Betty Moore Foundation (Grant GBMF5439); CSIC I+D 321; and INNOVAGRO FSA 148819 and FSA 148651 from ANII, Uruguay. R.B.J. acknowledges support from the Gordon and Betty Moore Foundation (GBMF5439 “Advancing Understanding of the Global Methane Cycle” to Stanford University). Author contributions: All authors contributed to conceive the ideas and to manuscript development. S.H.V. collected the data and performed all the statistical analyses with substantial intellectual and methodological inputs from P.P., R.B.J., and G.P. S.H.V. wrote the first draft of the manuscript, and all authors contributed to subsequent revisions. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/16/eabd3176/DC1
REFERENCES AND NOTES
- 1.Adhikari K., Hartemink A. E., Linking soils to ecosystem services—A global review. Geoderma 262, 101–111 (2016). [Google Scholar]
- 2.K. Lorenz, R. Lal, Soil Organic Carbon—An Appropriate Indicator to Monitor Trends of Land and Soil Degradation Within the SDG Framework? (Umweltbundesamt, 2016); www.umweltbundesamt.de/en/publikationen/soil-organic-carbon-an-appropriate-indicator-to.
- 3.Lavallee J. M., Soong J. L., Cotrufo M. F., Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Chang. Biol. 26, 261–273 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Cotrufo M. F., Ranalli M. G., Haddix M. L., Six J., Lugato E., Soil carbon storage informed by particulate and mineral-associated organic matter. Nat. Geosci. 12, 989–994 (2019). [Google Scholar]
- 5.M. Kleber, K. Eusterhues, M. Keiluweit, C. Mikutta, R. Mikutta, P. S. Nico, Advances in Agronomy (Elsevier, 2015), vol. 130, pp. 1–140. [Google Scholar]
- 6.Kögel-Knabner I., Guggenberger G., Kleber M., Kandeler E., Kalbitz K., Scheu S., Eusterhues K., Leinweber P., Organo-mineral associations in temperate soils: Integrating biology, mineralogy, and organic matter chemistry. J. Plant Nutr. Soil Sci. 171, 61–82 (2008). [Google Scholar]
- 7.Cotrufo M. F., Soong J. L., Horton A. J., Campbell E. E., Haddix M. L., Wall D. H., Parton W. J., Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 8, 776–779 (2015). [Google Scholar]
- 8.Rasse D. P., Rumpel C., Dignac M.-F., Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 269, 341–356 (2005). [Google Scholar]
- 9.Oburger E., Jones D. L., Sampling root exudates—Mission impossible? Rhizosphere 6, 116–133 (2018). [Google Scholar]
- 10.Pausch J., Kuzyakov Y., Carbon input by roots into the soil: Quantification of rhizodeposition from root to ecosystem scale. Glob. Chang. Biol. 24, 1–12 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Castellano M. J., Mueller K. E., Olk D. C., Sawyer J. E., Six J., Integrating plant litter quality, soil organic matter stabilization, and the carbon saturation concept. Glob. Chang. Biol. 21, 3200–3209 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Jackson R. B., Lajtha K., Crow S. E., Hugelius G., Kramer M. G., Piñeiro G., The ecology of soil carbon: Pools, vulnerabilities, and biotic and abiotic controls. Annu. Rev. Ecol. Evol. Syst. 48, 419–445 (2017). [Google Scholar]
- 13.Sokol N. W., Bradford M. A., Microbial formation of stable soil carbon is more efficient from belowground than aboveground input. Nat. Geosci. 12, 46–53 (2019). [Google Scholar]
- 14.Huo C., Luo Y., Cheng W., Rhizosphere priming effect: A meta-analysis. Soil Biol. Biochem. 111, 78–84 (2017). [Google Scholar]
- 15.Jilling A., Keiluweit M., Contosta A. R., Frey S., Schimel J., Schnecker J., Smith R. G., Tiemann L., Grandy A. S., Minerals in the rhizosphere: Overlooked mediators of soil nitrogen availability to plants and microbes. Biogeochemistry 139, 103–122 (2018). [Google Scholar]
- 16.Derrien D., Marol C., Balesdent J., The dynamics of neutral sugars in the rhizosphere of wheat. An approach by13C pulse-labelling and GC/C/IRMS. Plant and Soil 267, 243–253 (2004). [Google Scholar]
- 17.Stewart C. E., Paustian K., Conant R. T., Plante A. F., Six J., Soil carbon saturation: Concept, evidence and evaluation. Biogeochemistry 86, 19–31 (2007). [Google Scholar]
- 18.Mikutta R., Turner S., Schippers A., Gentsch N., Meyer-Stüve S., Condron L. M., Peltzer D. A., Richardson S. J., Eger A., Hempel G., Kaiser K., Klotzbücher T., Guggenberger G., Microbial and abiotic controls on mineral-associated organic matter in soil profiles along an ecosystem gradient. Sci. Rep. 9, 10294 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frey S. D., Mycorrhizal fungi as mediators of soil organic matter dynamics. Annu. Rev. Ecol. Evol. Syst. 50, 237–259 (2019). [Google Scholar]
- 20.Christensen B. T., Physical fractionation of soil and structural and functional complexity in organic matter turnover. Eur. J. Soil Sci. 52, 345–353 (2001). [Google Scholar]
- 21.Dungait J. A., Hopkins D. W., Gregory A. S., Whitmore A. P., Soil organic matter turnover is governed by accessibility not recalcitrance. Glob. Chang. Biol. 18, 1781–1796 (2012). [Google Scholar]
- 22.Schmidt M. W., Torn M. S., Abiven S., Dittmar T., Guggenberger G., Janssens I. A., Kleber M., Kogel-Knabner I., Lehmann J., Manning D. A., Nannipieri P., Rasse D. P., Weiner S., Trumbore S. E., Persistence of soil organic matter as an ecosystem property. Nature 478, 49–56 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Villarino S. H., Studdert G. A., Baldassini P., Cendoya M. G., Ciuffoli L., Mastrángelo M., Piñeiro G., Deforestation impacts on soil organic carbon stocks in the Semiarid Chaco Region, Argentina. Sci. Total Environ. 575, 1056–1065 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Tisdall J. M., Oades J. M., Organic matter and water-stable aggregates in soils. J. Soil Sci. 33, 141–163 (1982). [Google Scholar]
- 25.Poirier V., Roumet C., Angers D. A., Munson A. D., Species and root traits impact macroaggregation in the rhizospheric soil of a Mediterranean common garden experiment. Plant Soil 424, 289–302 (2018). [Google Scholar]
- 26.Keiluweit M., Bougoure J. J., Nico P. S., Pett-Ridge J., Weber P. K., Kleber M., Mineral protection of soil carbon counteracted by root exudates. Nat. Clim. Chang. 5, 588–595 (2015). [Google Scholar]
- 27.Abramoff R., Xu X., Hartman M., O’Brien S., Feng W., Davidson E., Finzi A., Moorhead D., Schimel J., Torn M., The Millennial model: In search of measurable pools and transformations for modeling soil carbon in the new century. Biogeochemistry 137, 51–71 (2018). [Google Scholar]
- 28.Robertson A. D., Paustian K., Ogle S., Wallenstein M. D., Lugato E., Cotrufo M. F., Unifying soil organic matter formation and persistence frameworks: The MEMS model. Biogeosciences 16, (2019). [Google Scholar]
- 29.Smith P., House J. I., Bustamante M., Sobocká J., Harper R., Pan G., West P. C., Clark J. M., Adhya T., Rumpel C., Global change pressures on soils from land use and management. Glob. Chang. Biol. 22, 1008–1028 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Guo L. B., Gifford R., Soil carbon stocks and land use change: A meta analysis. Glob. Chang. Biol. 8, 345–360 (2002). [Google Scholar]
- 31.Milla R., García-Palacios P., Matesanz S., Looking at past domestication to secure ecosystem services of future croplands. J. Ecol. 105, 885–889 (2017). [Google Scholar]
- 32.Kell D. B., Breeding crop plants with deep roots: Their role in sustainable carbon, nutrient and water sequestration. Ann. Bot. 108, 407–418 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crews T. E., Rumsey B. E., What agriculture can learn from native ecosystems in building soil organic matter: A review. Sustainability 9, 578 (2017). [Google Scholar]
- 34.Pinto P., Long M. E. F., Piñeiro G., Including cover crops during fallow periods for increasing ecosystem services: Is it possible in croplands of Southern South America? Ecosyst. Environ. 248, 48–57 (2017). [Google Scholar]
- 35.Garcia L., Damour G., Gary C., Follain S., Le Bissonnais Y., Metay A., Trait-based approach for agroecology: Contribution of service crop root traits to explain soil aggregate stability in vineyards. Plant Soil 435, 1–14 (2019). [Google Scholar]
- 36.Larson W., Clapp C., Pierre W., Morachan Y., Effects of increasing amounts of organic residues on continuous corn: II. Organic carbon, nitrogen, phosphorus, and sulfur 1. Agron. J. 64, 204–209 (1972). [Google Scholar]
- 37.Barber S. A., Corn residue management and soil organic matter 1. Agron. J. 71, 625–627 (1979). [Google Scholar]
- 38.Comeau L.-P., Lemke R., Knight J., Bedard-Haughn A., Carbon input from 13C-labeled crops in four soil organic matter fractions. Biol. Fertil. Soils 49, 1179–1188 (2013). [Google Scholar]
- 39.Baumann K., Sanaullah M., Chabbi A., Dignac M.-F., Bardoux G., Steffens M., Kögel-Knabner I., Rumpel C., Changes in litter chemistry and soil lignin signature during decomposition and stabilisation of 13C labelled wheat roots in three subsoil horizons. Soil Biol. Biochem. 67, 55–61 (2013). [Google Scholar]
- 40.Bird J. A., Kleber M., Torn M. S., 13C and 15N stabilization dynamics in soil organic matter fractions during needle and fine root decomposition. Org. Geochem. 39, 465–477 (2008). [Google Scholar]
- 41.Casals P., Garcia-Pausas J., Montané F., Romanyà J., Rovira P., Root decomposition in grazed and abandoned dry Mediterranean dehesa and mesic mountain grasslands estimated by standard labelled roots. Agr. Ecosyst. Environ. 139, 759–765 (2010). [Google Scholar]
- 42.de Sales Moreira Demolinari M., de Sousa R. N., da Silva I. R., da Silva Teixeira R., Neves J. C. L., de Oliveira Mendes G., Effect of mineral nitrogen on transfer of 13C-carbon from eucalyptus harvest residue components to soil organic matter fractions. Rev. Bras. Ciênc. Solo 41, (2017). [Google Scholar]
- 43.Egli M., Hafner S., Derungs C., Ascher-Jenull J., Camin F., Sartori G., Raab G., Bontempo L., Paolini M., Ziller L., Decomposition and stabilisation of Norway spruce needle-derived material in Alpine soils using a 13C-labelling approach in the field. Biogeochemistry 131, 321–338 (2016). [Google Scholar]
- 44.Fahey T. J., Yavitt J. B., Sherman R. E., Maerz J. C., Groffman P. M., Fisk M. C., Bohlen P. J., Earthworm effects on the incorporation of litter C and N into soil organic matter in a sugar maple forest. Ecol. Appl. 23, 1185–1201 (2013). [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Pausas J., Casals P., Rovira P., Vallecillo S., Sebastià M.-T., Romanyà J., Decomposition of labelled roots and root-C and -N allocation between soil fractions in mountain grasslands. Soil Biol. Biochem. 49, 61–69 (2012). [Google Scholar]
- 46.Hatton P. J., Castanha C., Torn M. S., Bird J. A., Litter type control on soil C and N stabilization dynamics in a temperate forest. Glob. Chang. Biol. 21, 1358–1367 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Helfrich M., Ludwig B., Potthoff M., Flessa H., Effect of litter quality and soil fungi on macroaggregate dynamics and associated partitioning of litter carbon and nitrogen. Soil Biol. Biochem. 40, 1823–1835 (2008). [Google Scholar]
- 48.Pries C. E. H., Bird J. A., Castanha C., Hatton P.-J., Torn M. S., Long term decomposition: The influence of litter type and soil horizon on retention of plant carbon and nitrogen in soils. Biogeochemistry 134, 5–16 (2017). [Google Scholar]
- 49.Hu Y.-L., Zeng D.-H., Ma X.-Q., Chang S. X., Root rather than leaf litter input drives soil carbon sequestration after afforestation on a marginal cropland. For. Ecol. Manage. 362, 38–45 (2016). [Google Scholar]
- 50.Kammer A., Hagedorn F., Mineralisation, leaching and stabilisation of 13C-labelled leaf and twig litter in a beech forest soil. Biogeosciences 8, 2195–2208 (2011). [Google Scholar]
- 51.Kölbl A., von Lützow M., Rumpel C., Munch J. C., Kögel-Knabner I., Dynamics of 13C-labeled mustard litter (Sinapis alba) in particle-size and aggregate fractions in an agricultural cropland with high- and low-yield areas. J. Plant Nutr. Soil Sci. 170, 123–133 (2007). [Google Scholar]
- 52.Rubino M., Dungait J., Evershed R., Bertolini T., De Angelis P., D’Onofrio A., Lagomarsino A., Lubritto C., Merola A., Terrasi F., Carbon input belowground is the major C flux contributing to leaf litter mass loss: Evidences from a 13C labelled-leaf litter experiment. Soil Biol. Biochem. 42, 1009–1016 (2010). [Google Scholar]
- 53.Sall S., Bertrand I., Chotte J.-L., Recous S., Separate effects of the biochemical quality and N content of crop residues on C and N dynamics in soil. Biol. Fertil. Soils 43, 797–804 (2007). [Google Scholar]
- 54.Sanaullah M., Chabbi A., Leifeld J., Bardoux G., Billou D., Rumpel C., Decomposition and stabilization of root litter in top- and subsoil horizons: What is the difference? Plant Soil 338, 127–141 (2011). [Google Scholar]
- 55.Sánchez-de León Y., Lugo-Pérez J., Wise D. H., Jastrow J. D., González-Meler M. A., Aggregate formation and carbon sequestration by earthworms in soil from a temperate forest exposed to elevated atmospheric CO2: A microcosm experiment. Soil Biol. Biochem. 68, 223–230 (2014). [Google Scholar]
- 56.Santos F., Nadelhoffer K., Bird J., Rapid fine root C and N mineralization in a northern temperate forest soil. Biogeochemistry 128, 187–200 (2016). [Google Scholar]
- 57.Shahbaz M., Kuzyakov Y., Heitkamp F., Decrease of soil organic matter stabilization with increasing inputs: Mechanisms and controls. Geoderma 304, 76–82 (2017). [Google Scholar]
- 58.Sierra J., Motisi N., Shift in C and N humification during legume litter decomposition in an acid tropical Ferralsol. Soil Res. 50, 380–389 (2012). [Google Scholar]
- 59.Steffens C., Helfrich M., Joergensen R. G., Eissfeller V., Flessa H., Translocation of 13C-labeled leaf or root litter carbon of beech (Fagus sylvatica L.) and ash (Fraxinus excelsior L.) during decomposition—A laboratory incubation experiment. Soil Biol. Biochem. 83, 125–137 (2015). [Google Scholar]
- 60.Wachendorf C., Potthoff M., Ludwig B., Joergensen R. G., Effects of addition of maize litter and earthworms on C mineralization and aggregate formation in single and mixed soils differing in soil organic carbon and clay content. Pedobiologia 57, 161–169 (2014). [Google Scholar]
- 61.Austin E. E., Wickings K., McDaniel M. D., Robertson G. P., Grandy A. S., Cover crop root contributions to soil carbon in a no-till corn bioenergy cropping system. GCB Bioenergy 9, 1252–1263 (2017). [Google Scholar]
- 62.Balesdent J., Balabane M., Major contribution of roots to soil carbon storage inferred from maize cultivated soils. Soil Biol. Biochem. 28, 1261–1263 (1996). [Google Scholar]
- 63.Bolinder M., Angers D., Giroux M., Laverdiere M., Estimating C inputs retained as soil organic matter from corn (Zea mays L.). Plant Soil 215, 85–91 (1999). [Google Scholar]
- 64.Clapp C. E., Allmaras R. R., Layese M. F., Linden D. R., Dowdy R. H., Soil organic carbon and 13C abundance as related to tillage, crop residue, and nitrogen fertilization under continuous corn management in Minnesota. Soil Tillage Res. 55, 127–142 (2000). [Google Scholar]
- 65.Ghafoor A., Poeplau C., Kätterer T., Fate of straw- and root-derived carbon in a Swedish agricultural soil. Biol. Fertil. Soils 53, 257–267 (2017). [Google Scholar]
- 66.Kong A. Y., Six J., Tracing root vs. residue carbon into soils from conventional and alternative cropping systems. Soil Sci. Soc. Am. J. 74, 1201–1210 (2010). [Google Scholar]
- 67.Mazzilli S. R., Kemanian A. R., Ernst O. R., Jackson R. B., Piñeiro G., Greater humification of belowground than aboveground biomass carbon into particulate soil organic matter in no-till corn and soybean crops. Soil Biol. Biochem. 85, 22–30 (2015). [Google Scholar]
- 68.Puget P., Drinkwater L., Short-term dynamics of Root- and shoot-derived carbon from a leguminous green manure. Soil Sci. Soc. Am. J. 65, 771–779 (2001). [Google Scholar]
- 69.Tahir M. M., Recous S., Aita C., Schmatz R., Pilecco G. E., Giacomini S. J., In situ roots decompose faster than shoots left on the soil surface under subtropical no-till conditions. Biol. Fertil. Soils 52, 853–865 (2016). [Google Scholar]
- 70.Cambardella C., Elliott E., Particulate soil organic-matter changes across a grassland cultivation sequence. Soil Sci. Soc. Am. J. 56, 777–783 (1992). [Google Scholar]
- 71.Royston P., Remark AS R94: A remark on algorithm AS 181: The W-test for normality. J. R. Stat. Soc. Ser. C. Appl. Stat. 44, 547–551 (1995). [Google Scholar]
- 72.F. Wilcoxon, Breakthroughs in Statistics (Springer, 1992), pp. 196–202. [Google Scholar]
- 73.R. Core Team, R: A Language and Environment for Statistical Computing (R Foundation for statistical computing, 2013).
- 74.Manikandan S., Measures of central tendency: Median and mode. J. Pharmacol. Pharmacother. 2, 214–215 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bolinder M., Janzen H., Gregorich E., Angers D., VandenBygaart A., An approach for estimating net primary productivity and annual carbon inputs to soil for common agricultural crops in Canada. Agr. Ecosyst. Environ. 118, 29–42 (2007). [Google Scholar]
- 76.Soong J. L., Vandegehuchte M. L., Horton A. J., Nielsen U. N., Denef K., Shaw E. A., de Tomasel C. M., Parton W., Wall D. H., Cotrufo M. F., Soil microarthropods support ecosystem productivity and soil C accrual: Evidence from a litter decomposition study in the tallgrass prairie. Soil Biol. Biochem. 92, 230–238 (2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/16/eabd3176/DC1


